Abstract
Early life is a dynamic period for skin microbial colonization and immune development. We postulate that microbial exposures in this period durably alter the skin immune trajectory and later disease susceptibility. Bacteria contribute to infant skin immune imprinting via interactions with microbes as well as with cutaneous epithelial and immune cells. Excellent research is underway at the skin microbiome–immune interface, both in deciphering basic mechanisms and implementing their therapeutic applications. As emphasized herein, focusing on the unique opportunities and challenges presented by microbial immune modulation in early life will be important. In our view, only through dedicated study of skin–microbe crosstalk in this developmental window can we elucidate the molecular underpinnings of pivotal events that contribute to sustained host–microbe symbiosis.
Host–microbe interactions in neonatal skin: a unique opportunity for discovery awaits
Our understanding of the mammalian skin microbiome (see Glossary), the cutaneous immune system, and the interactions between these two entities is based largely on studies of adult humans and animals. However, the composition and function of skin microbes and immune cells differ substantially in neonates versus adults (Box 1). In addition, the neonatal period has been shown to be a particularly formative window wherein host–microbe interactions can have life-long impact. We argue that only through separate, dedicated study of skin–microbe crosstalk during early development can we understand the biology of pivotal events that sustain cutaneous host–microbe symbiosis and durably shape skin immune function. Furthermore, by understanding factors that disrupt early-life microbial tuning of skin immune function and their consequences, we may identify ways to better support and restore optimal crosstalk so as to proactively correct the trajectory and maintain or restore skin immune health.
Box 1. Age-dependent changes in the skin microbiome and immune system.
Skin microbiome
The composition of the skin microbiota is distinctly different in human infants and children compared to adults. Birth mode influences the bacterial genera found on the skin in the first hours to days of life. Compared to cesarean delivery, vaginal birth is associated with a greater predominance of maternal vaginal versus skin-derived strains [84,85]. These early differences are largely undetectable after several weeks, by which time the human infant skin microbiota demonstrates a relative predominance of Firmicutes, including Staphylococci and Streptococci [86–88]. During late puberty, increasing skin sebum production and other hormonal changes facilitate another shift towards a more Actinobacteria-dominated skin microbial community [89]. Few metagenomic studies have been performed on infant skin, limiting our insight into the functional capacity of pioneer species and whether or how this differs from those that dominate cutaneous niches in later life.
Skin immune system
Early life is an equally dynamic period for the skin immune system. However, our current understanding of how the composition and function of skin immune cells evolves during infancy and childhood relies heavily on murine studies. Direct extrapolation from mice to humans is fundamentally flawed given the presence of species-specific cell types [90,91] and profound differences in timing of thymic [92–95], skin barrier [96,97], and hair follicle [98,99] development. However, two general principles derived from mice appear to be applicable to humans. First, there is a layered transition during which key tissue immune functions initially performed by innate-type cells are eventually superseded by an expanding population of tissue-resident memory αβ T cells [100]. Populations enriched early in life include commensal-responsive cell types such as mucosal invariant T (MAIT) cells [74] and other PLZF+ lymphocytes [101]. Although the timing of this transition and the specific cell types involved are better defined in mice [71], analogous principles are thought to apply in humans, although many remain to be fully demonstrated [19,79,80,102]. Second, studies on human tissues have consistently revealed an increased early-life propensity for immune regulation and immune tolerance [103,104]. This may extend to human skin, based on the observations that Tregs appear in skin during the second trimester of human fetal life [78] and are enriched in pediatric versus adult skin [105], as well as the finding that skin dendritic cells from human fetal versus adult skin have an increased capacity to promote Tregs while limiting effector T cell proliferation [81].
Early-life interactions with our commensal microbes influence the development of the immune system and have life-long implications for skin health and disease [1,2]. The ability of microbes to tune immune function is not merely transient. They can have enduring effects that are often referred to as immune imprinting (Figure 1). Although interactions with bacteria in adult mouse skin have demonstrated lasting changes in immune composition and function [3–5], early-life interactions can result in unique immune outputs [6]. An often-cited example of this phenomenon is the ‘hygiene hypothesis’, wherein children that grow up exposed to antigens abundant in farmlands with animals are less susceptible in the long-term to atopic conditions such as asthma versus those from urban areas [7–9]. Immune imprinting encompasses concepts such as tissue memory, which can refer to epigenetic changes in epithelial or stromal cells that alter their behavior [10], or the idea that cumulative antigen exposure establishes a population of tissue-resident memory lymphocytes [11] that will change the coordinated tissue immune response to subsequent antigen encounter. We use immune imprinting here as a term that can also extend to other mechanisms such as alterations in bacterial community composition or cytokine-mediated recruitment of polyclonal or innate-type lymphocyte populations that, when occurring in a time-limited neonatal window, have the potential for unique and enduring effects.
Figure 1. Model of microbial immune imprinting.
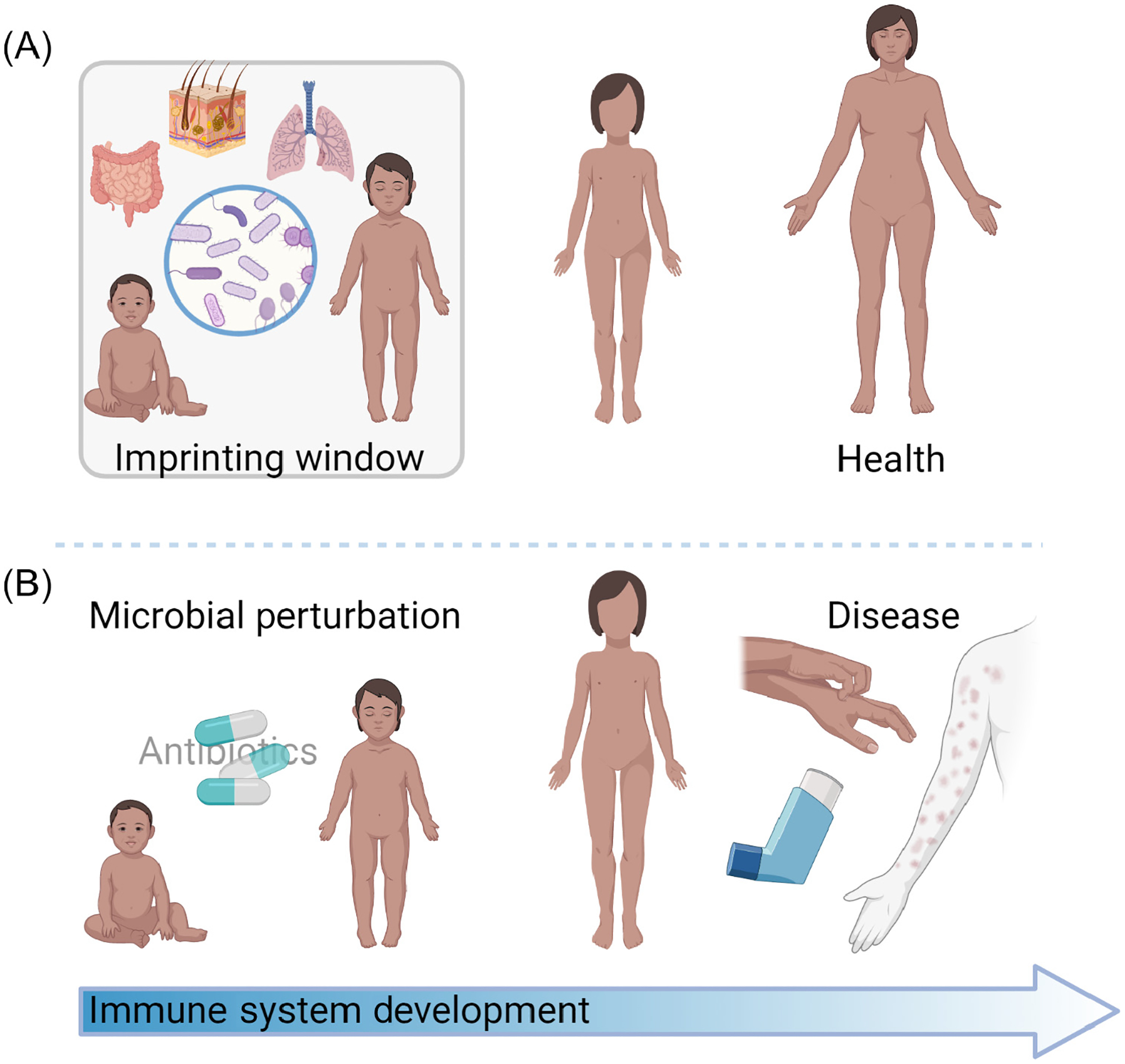
(A) Microbial exposure via barrier tissues such as the skin, gut, and lung in the early developmental window can imprint healthy immune function through to adulthood [6]. (B) Microbial perturbations such as antibiotic exposure in the early developmental window have been associated with chronic inflammatory conditions that affect barrier tissues, such as food allergies, asthma, and psoriasis [14–17].
Growing evidence in humans and mice suggests that unique molecular interactions between commensal microbes and cells of the neonatal immune system have the potential to tip the balance between health and disease in adult life. There are several such examples where bacteria in the neonatal gut shape immune function in a way that regulates later susceptibility to inflammatory disease. For instance, a recent study in human newborns used longitudinal systems-immunology analyses and metagenomic profiling of the infant gut microbiome to uncover a negative correlation between the prevalence of specific gut bacteria, Bifidobacteria, and markers of systemic or intestinal inflammation, for example increased blood neutrophils and concentrations of tumor necrosis factor α, interleukin (IL)-17A, IL-1α, and IL-13 [12]. Bifidobacteria are known to metabolize human breast milk oligosaccharides to generate organic compounds with immunomodulatory properties. In a small cohort of infants, Bifidobacterium infantis supplementation was sufficient to reduce systemic inflammatory markers. Moreover, fecal water from these infants skewed the in vitro differentiation of helper T cells towards a type 1 T helper (Th1) cell rather than a type 2 (Th2) or type 17 (Th17) cellular fate [12]; this in turn showed the potential impact of these bacteria on influencing future infant susceptibility to allergic or other immune-mediated diseases. Similarly, bacteria in the developing lung and intestine of mice have been shown to limit the accumulation of invariant natural killer T (iNKT) cells, thereby reducing subsequent susceptibility to oxazolone-induced colitis or ovalbumin-driven asthma [13]. Other studies have reported that early, antibiotic-mediated perturbation of the developing microbial diversity can be associated with a significantly altered later-life risk of developing inflammatory conditions such as food allergies [14,15], asthma [16], or psoriasis [17]. Although there are emerging examples of how in utero exposure to microbes or their products might tune the function of developing human intestinal immune cells [18,19], we focus here on the potential role of postnatal microbial exposures.
Inclusive of hair follicles, skin represents arguably the largest body surface that interfaces with microbes [20]. Our understanding of early microbial imprinting at this large external body site still lags behind that for the gut. However, it is a field ripe with opportunity to generate meaningful insights into the bacterial or host molecules and pathways that could be targeted for early correction of the microbe–skin–immune trajectory, saving years of disease and morbidity down the road. We are likely to see accelerated work in this area in coming years owing to renewed interest in neonatal immune development and tissue immunology [21,22], as well as the increasing availability of high-throughput system-level tools to study immune cells and bacterial communities [23,24]. Recent work has already begun to delineate the mechanistic categories by which skin bacteria can shape the composition and function of the developing cutaneous immune system. These include: bacteria–bacteria interactions, bacteria–epithelial cell interactions, and bacteria–immune cell interactions (Figure 2). We address each in turn, summarizing recent studies and highlighting areas for continued mechanistic investigation.
Figure 2. Potential mechanisms of bacterial immune imprinting.
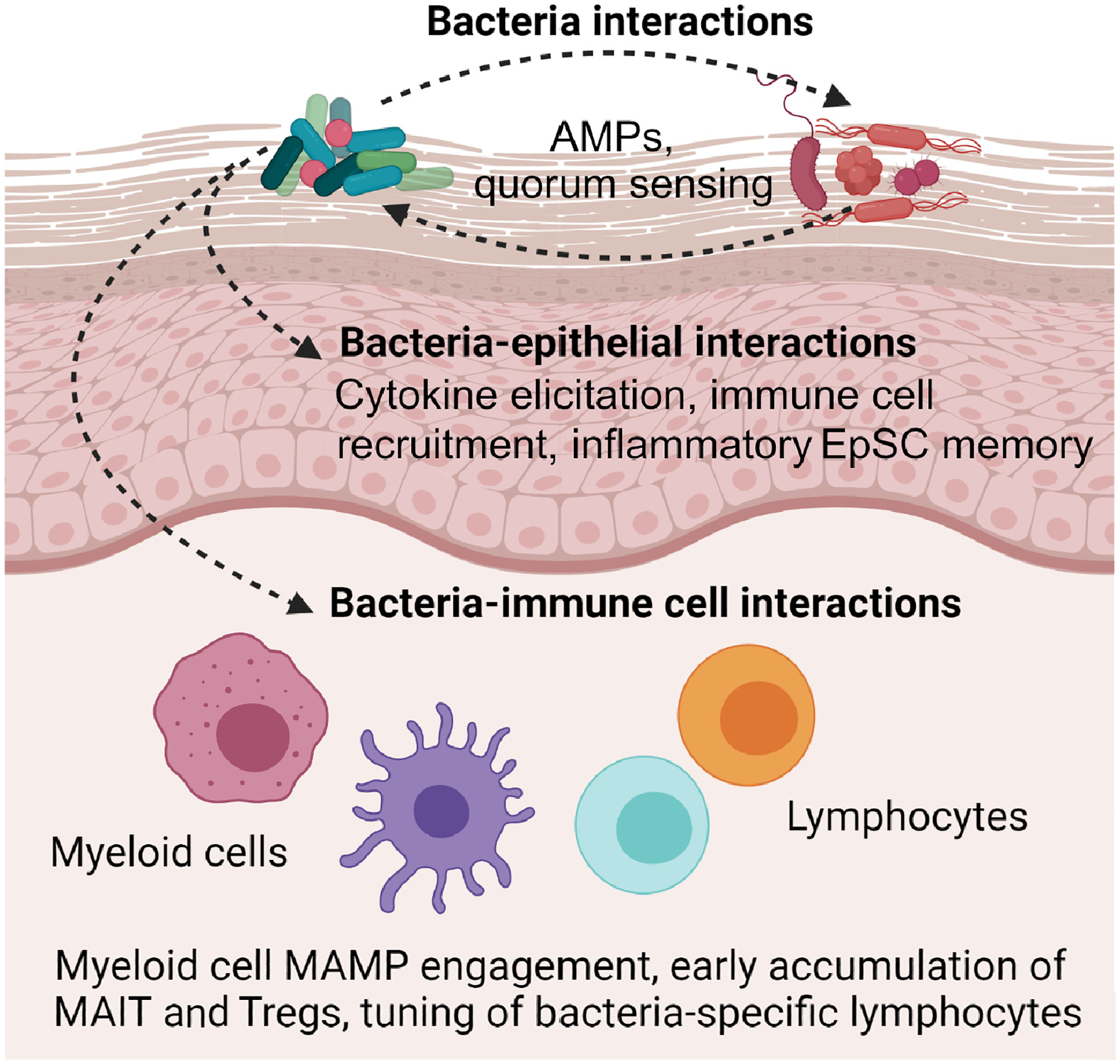
Although much additional work will be necessary to elucidate how skin bacteria can mediate immune imprinting, the current literature would suggest the possibility of at least three mechanistic categories: (i) bacteria–bacteria interactions, (ii) bacteria–epithelial interactions, and (iii) bacteria–immune cell interactions. As discussed herein, bacteria–bacteria interactions could influence the composition and function of the neonatal skin microbiome, for example via bacteria-derived antimicrobial peptides (AMPs) [32,33] or inter-species inhibition of quorum-sensing pathways, as has been shown in vitro and in mice [35]. Bacterial interactions with human or murine keratinocytes could alter epithelial production of cytokines, thereby influencing the recruitment of skin immune cells, as seen in mice [57,61]. This might also occur by conferring epigenetically mediated inflammatory memory in murine or human epithelial stem cells (EpSCs) that could influence downstream wound healing [10]. Bacterial molecules could, as demonstrated in murine systems, interact with microbe-associated molecular patterns (MAMPs) on myeloid cells or lymphocytes [76,77,111], thereby changing their behavior or directly expanding bacteria-specific CD8+ T cells [3,4], mucosa-associated invariant T cells (MAIT) [71,75], and regulatory T cells (Tregs) [58].
Bacteria–bacteria interactions
Independently of their interaction with host cells, bacteria impact the skin environment via mechanisms that favor the outgrowth and niche establishment of particular bacteria over others. This has been well described in the context of the neonatal gut microbiome in which the early presence of so-called founder species helps to cultivate conditions that enable new strains to enter the niche and establish long-term residence [25,26]. This microbial succession is guided via various bacterially mediated processes, including the generation of metabolic byproducts, alteration of the pH or oxygen availability, and the production of small molecules that can influence the growth or behavior of other bacterial species. Intra-individual longitudinal tracking of skin bacteria has been studied in healthy human adults [27–30] and preliminarily in some disease states such as atopic dermatitis [31]. However, many early findings still require robust validation, and a more granular understanding will be necessary to determine which are the key founder species on infant skin and how they might support the evolution of the cutaneous microbiome over the first years of life.
Interactions among skin bacterial species have been studied mostly in the context of atopic dermatitis, in which particular strains can limit the growth or pathogenicity of the atopic dermatitis-associated pathobiont, Staphylococcus aureus. Coagulase-negative Staphylococci in particular, as well as other genera, have been shown to produce antimicrobial peptides such as lantibiotics and oleic acids that limit the growth of S. aureus [32,33]. Many of the same strains can produce small molecules such as short-chain fatty acids that inhibit pathogenicity by limiting biofilm-forming capacity [34] or by autoinducing peptides that limit the production of quorum-sensing-dependent toxins [35]. Although the field is already in the process of exploring the use of particular commensal strains as potential topical therapeutics to treat atopic dermatitis flares by reducing the cutaneous burden of S. aureus [36], it is intriguing to consider how similar interactions between bacterial species might play out even earlier – namely in neonates – before atopic dermatitis and its accompanying skin inflammation appear. Both sequencing- and cultivation-based studies of the skin microbiome in infants at risk for atopic dermatitis have documented an altered skin microbiome composition before disease onset, characterized by reduced Staphylococcus epidermidis prevalence or heightened S. aureus prevalence – especially S. aureus strains with a functional Agr quorum-sensing system [37–39]. Presumably, inherited host factors such as compromised barrier function and exposure of epidermal molecules for bacterial adhesion can augment the propensity for S. aureus skin colonization [40–42], but failure to establish a ‘protective’ microbiome might also be an important factor that allows this pathobiont to establish a skin niche.
Ultimately, the successful potential use of live bacteria or bacterially derived therapies to promote skin health or to treat particular skin diseases would rely on being able to safely introduce key species as stable members of the skin microbial community. Thus, it is important to seek a holistic understanding of the direct and indirect effects of such candidate therapies on skin microbial community composition. Preliminary work and clinical trials have shown promise for the use of these strains in the treatment of atopic dermatitis [36,43], and this is garnering excitement for further validation. Continuing this push in parallel with studies that probe interaction networks among skin commensals – especially for those bacteria that occupy the niche in infants – can help to clarify inter-species bacteria interactions that contribute to shaping the development and long-term maintenance of the skin microbial community composition. Such efforts can lay the foundation for opportunities to intervene in the host–microbe relationship to not only treat but perhaps even prevent disease.
Bacteria–epithelial cell interactions
Although bacteria and their byproducts can be found below the human skin surface [44], their density is greatest in the epidermis, both between and within hair follicles [45]. This results in a myriad of opportunities for bacteria–keratinocyte interactions that help to modulate the core physical and antimicrobial barrier functions of the epidermis. Studies using murine models and primary human keratinocytes have demonstrated that commensal activation of the aryl hydrocarbon receptor pathway in keratinocytes augments their expression of key skin differentiation genes to limit transepidermal water loss in the skin tape-stripping mouse model [46]. Conversely, proteases such as EcpA and staphopains expressed by S. epidermidis and S. aureus species, respectively, can contribute to epidermal barrier breakdown in murine and human skin under some pathogenic circumstances [47]. In addition to these effects on the physical properties of the epidermal barrier, skin bacteria can also augment the human and murine skin antimicrobial barrier and protection against pathogen assault by bolstering keratinocyte expression of antimicrobial peptides [48–50]. Collectively, these studies demonstrate the ability of commensal microbes to interact with epithelial cells and directly influence skin barrier health.
Keratinocytes, however, are also instrumental in coordinating cutaneous immunity. Under homeostatic conditions these cells – especially those in the hair follicle – produce chemokines and cytokines, such as IL-7, IL-15, CCL2, CCL8, or CCL20, that influence the recruitment and behavior of mouse innate lymphoid cells, dendritic cells (DCs), and T cells [51–54]. Additional murine studies have illustrated that skin bacterial dysbiosis associated with epidermal deletion of metalloproteinases ADAM10 or ADAM17 can modulate keratinocyte cytokine production, augment immune cell recruitment, and hasten skin pathology [55,56]. These interactions remain comparatively understudied in the neonatal period, but work in mice suggests that commensal-augmented production of the chemokine CCL20 in developing hair follicle keratinocytes can facilitate the recruitment of regulatory T cells (Tregs) into skin [57]. These Tregs support long-lasting immune tolerance to bacteria and other antigens, and this is a pivotal step in promoting healthy skin development [58]. Nevertheless, the extent to which keratinocyte-derived signals might coordinate the early recruitment or function of other cutaneous immune cell types remains an outstanding question with potential implications for neonatal immune imprinting.
From another angle, skin bacteria have the potential to influence essential skin functions via their effects on epithelial stem cells (EpSCs). EpSCs and the cytokines IL-1α and IL-1β are crucially involved in skin wound healing and hair growth [59]. S. aureus and coagulase-negative Staphylococci can elicit production of IL-1α and IL-1β [3,60], and have been shown to accelerate wound healing and post-wounding hair regrowth in mice via activation of the IL-1 receptor (IL-1R) on keratinocytes [61]. To further demonstrate the importance of bacterial signals in wound healing, the same investigators performed a small human cohort study that demonstrated delayed closure of skin biopsy wound sites treated with topical antibiotic ointment versus placebo [61]. Separate studies in adult mice showed that epidermal inflammation resulting from topical exposure to imiquimod, calcipotriol, physical abrasion, or Candida albicans led to persistent chromatin changes in loci within EpSCs, subsequently augmenting keratinocyte migration and wound closure in a model of biopsy-induced skin wounding [10]. This putative EpSC ‘inflammatory memory’ might represent an epigenetic [62] form of immune imprinting, as shown by recent work looking at the intestinal epithelium. Specifically, in a mouse model of maternal Yersinia sp. infection, increased concentrations of IL-6 induced epigenetic changes in fetal intestinal EpSCs, as determined from assays of transposase-accessible chromatin with high-throughput sequencing (ATAC-seq), relative to uninfected mice [63]. This study demonstrated persistent heritable differences in the transcriptional and epigenetic profiles of intestinal EpSCs in the mouse offspring, as well as heightened Th17-associated inflammation in the intestinal lamina propria of the mice relative to controls [63]. The data raise the intriguing possibility that microbially mediated activation of innate cytokines in neonatal skin might induce persistent epigenetic changes in EpSCs, with long-term implications for tissue immune tone. However, robust future investigations are warranted to examine if such hypotheses are true.
Bacteria–immune cell interactions
As discussed above, there are many ways that skin bacteria can shape skin immune function without interacting directly with immune cells. However, there is also significant evidence to suggest that early bacteria–immune cell communication might affect imprinting of cutaneous immune function.
Myeloid cells
Antigen-presenting cells (APCs), especially DCs, have a sentinel function in sensing and integrating signals from skin bacteria. These cells are densely decorated with an array of pattern recognition receptors (PRRs) that respond to microbe-associated molecular patterns (MAMPs) [64,65] and can be influenced by microbially derived metabolites [66]. Skin APCs actively phagocytose bacteria, even extending their dendrites across the intact skin epithelium, as has been shown for Langerhans cells [67]. Although much remains to be untangled regarding how specific skin APC subsets help to coordinate responses to skin bacteria, Langerhans cells, as well as type 1 and type 2 conventional DCs (cDC1, cDC2), have risen to the forefront as central quarterbacks in detecting and responding to cutaneous microbes [3,60,68]. However, homeostatic interactions between skin bacteria and non-DC subsets such as monocytes and macrophages remain largely understudied. Nevertheless, recent work has reported that early-life microbial exposure can influence the numbers of intestinal macrophages, thereby modulating colonic iNKT seeding [69]; indeed, this suggests another area of research focus for further understanding the microbial influences on cutaneous myeloid cell functions.
Lymphocytes
Lymphocytes, especially T cells, are central players in skin health and disease [70]. Lymphocytes that recognize and respond to commensal bacteria may be particularly important for skin homeostasis; For instance, these T cells have been demonstrated to exhibit enhanced Gata3 expression – a presumed reparative transcriptional signature – and to accelerate wound healing in mice [4,5,71]. There are now several examples of how skin bacteria-derived molecules can directly influence skin lymphocyte numbers and functions. This has been studied for skin CD8+ T cells in adult mice and non-human primates; in these models, colonization by specific strains of S. epidermidis, which produce N-formyl methionine-containing (fMet) peptides, expand fMet-specific, IL-17A-producing CD8+ T cells via non-classical antigen presentation [4]. Likewise, mycolic acids produced by Corynebacterium spp. have been shown to expand the numbers of murine Vγ4+ dermal γδ T cells and to augment their IL-17A production – an effect that was attenuated in IL-23-deficient (Il23r−/−) mice or upon colonization with a Corynebacterium accolens mutant lacking the gene for mycolic acid synthetase [72]. These studies highlight how molecules produced by commensal microbes can substantially influence the composition and function of the cutaneous lymphocyte compartment.
The introduction of skin commensal bacteria in neonatal life, however, can elicit distinct immune effects that cannot be recapitulated by adult colonization. Two key examples include the preferential early-life expansion of cutaneous mucosa-associated invariant T (MAIT) cells and antigen-specific Tregs by S. epidermidis, as shown in mice (Figure 3). MAIT cells are innate lymphocytes that are enriched in barrier tissues, such as the gut and skin, and express semi-invariant αβ T cell receptors that recognize microbial products [73], including riboflavin metabolites produced by S. epidermidis and other bacteria [74]. Colonization of neonatal but not adult mice with riboflavin-producing bacteria can induce thymic accumulation of this metabolite and increase generation of MAIT cells, which then populate peripheral tissues, including the skin [75]. Subsequent S. epidermidis re-exposure in adult mice can further activate and expand these cutaneous MAIT cells [71]. Similarly, colonizing murine skin with S. epidermidis in the first 2 weeks of life leads to substantial enrichment of Tregs among the antigen-specific CD4+ T cell population. By contrast, analogous colonization of adult mice primarily generates antigen-specific proinflammatory effector CD4+ T cells [58]. Furthermore, the enduring effects of ‘missing’ this microbial imprinting in early life has been reported in relation to both MAITs and Tregs. For example, delaying the exposure of murine skin to MAIT-promoting S. epidermidis bacteria until adulthood resulted in a failure to generate MAIT cells and a consequent loss of their reparative benefits, as evidenced by delayed closure of biopsy-inducing skin wounds [71]. Similarly, in a tape-stripping model of epidermal injury, adult murine skin exposed to S. epidermidis – without undergoing prior neonatal S. epidermidis colonization to induce antigen-specific Tregs – displayed significantly increased tissue pathology and skin neutrophil numbers relative to the skin of mice exposed to this bacterium as neonates [58]. In both of these examples, beneficial interactions with cutaneous bacteria occurred preferentially during a time-limited window of early life in mice, emphasizing the importance of efforts to identify and dissect analogous or additional mechanisms in human infant skin.
Figure 3. Bacterial colonization of neonatal skin can shape the composition and function of the cutaneous immune system.
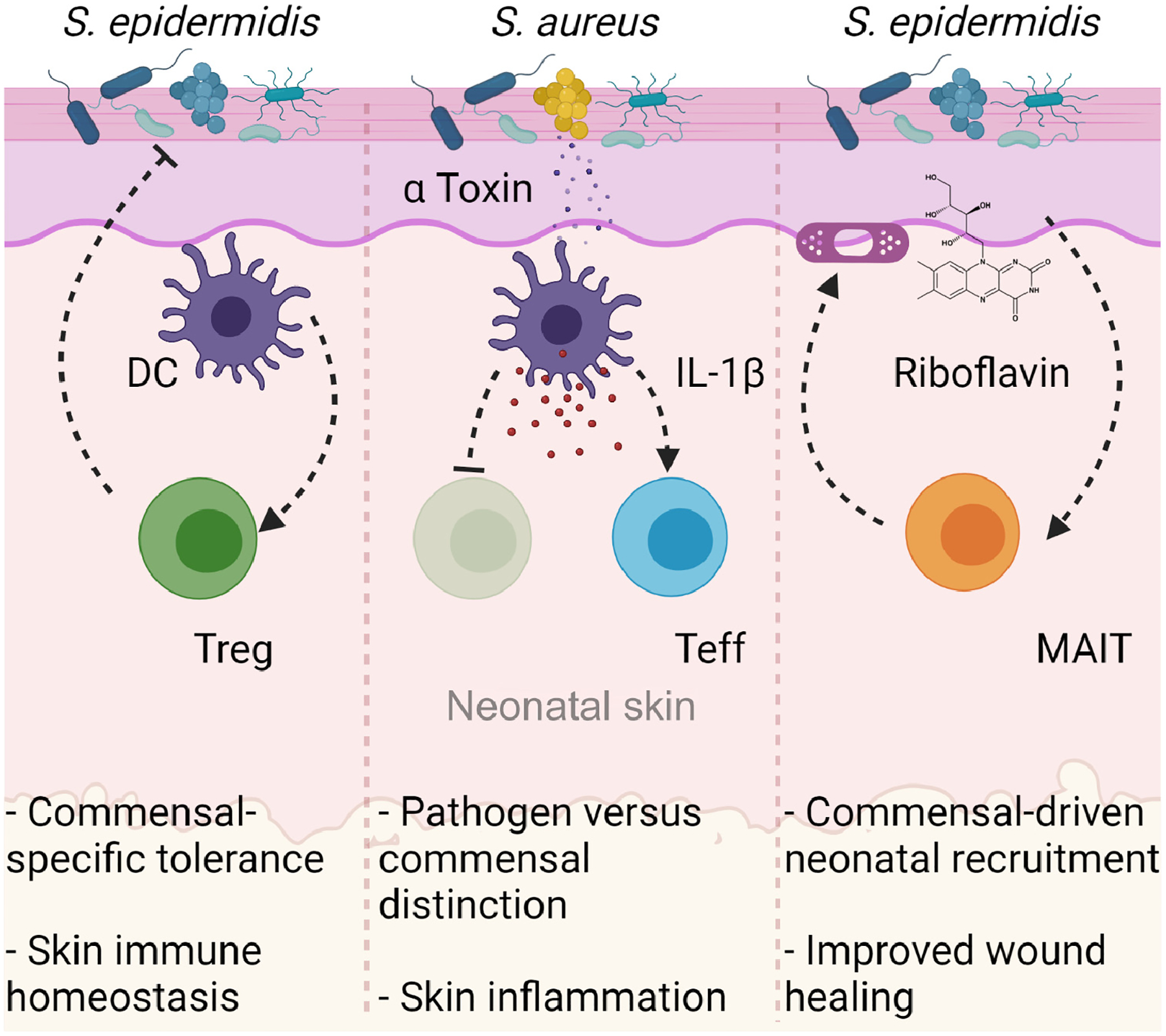
(Left) Colonization of neonatal murine skin by the commensal Staphylococcus epidermidis is detected by cutaneous dendritic cells (DCs). These DCs migrate to the draining lymph nodes and present S. epidermidis antigens to T cells. Antigen-specific regulatory T cells (Tregs) then accumulate in neonatal murine skin where they drive tolerance to commensal bacteria and establish healthy skin homeostasis [58,60]. (Center) Neonatal colonization of mice by the pathogenic strain, Staphylococcus aureus, generates a robust effector T cell (Teff) and a weak Treg response, primarily due to the release of the bacterial α-toxin which drives IL-1β release. This mechanism facilitates an immunological distinction of pathogen from commensal, resulting in failure to establish tolerance to pathogens and leading to robust skin inflammation upon subsequent re-exposure [60]. (Right) Exposure of neonatal mice to riboflavin-synthesizing bacteria such as S. epidermidis drives the accumulation of this metabolite in the thymus, leading to increased output of mucosa-associated invariant T (MAIT) cells and their subsequent accumulation in the skin. MAIT cells contribute to homeostatic functions in murine skin, including augmented wound closure after biopsy-induced wounding [71,75].
Molecular tuning of commensal-specific responses and considerations for early life
Although a significant amount of work will be necessary to decipher the molecular language by which bacteria tune skin cells and the ensuing immune responses, recent work has begun to identify bacterial molecules and receptors that may play a role. For example, there is preliminary evidence that S. epidermidis-mediated expansion of IL-17A-producing CD8+ T cells in adult mice [4] is supported by the recognition of S. epidermidis cell-envelope components by Toll-like receptor (TLR) 2 and the C-type lectin receptor, Dectin-1, on epithelial cells, and potentially on myeloid cells [76]. Although TLR2 signaling has also been implicated in expansion of colonic Tregs following exposure to intestinal bacteria [77], it remains to be seen whether this same pathway plays a role in mediating the expansion of S. epidermidis-specific Tregs following neonatal skin colonization [58]. Of note, myeloid cells, in particular cDC2s, have been implicated in early-life generation of S. epidermidis-specific Tregs based on the fact that they are a dominant subset of DCs that capture bacteria in neonatal murine skin [60]. Whether S. epidermidis or other commensal bacteria provide specific tolerizing signals to these cDC2s to promote their Treg generation is unknown, but interactions with α-toxin-producing S. aureus strains in neonatal mice can induce cDC2 production of IL-1β, thereby limiting S. aureus-specific Tregs, unlike S. epidermidis Tregs [60]. In addition to bacteria-directed signals, several host factors should be considered in future efforts to mechanistically dissect the basis of differential responses to colonization of neonate versus adult skin. These include the distinct composition and function of immune cells in neonatal skin (Box 1), for example; the predominance of different lymphocyte subsets [78–80] and potentially the higher tolerogenic capacity of neonatal skin APCs, as observed in human fetal skin [81]. Similarly, age-dependent differences in the expression of PRR receptors [82] on epidermal or immune cells, or the propensity for cytokines to lead to epigenetic modifications in immune and/or skin cells [83] – together with a heritable altered function of these cell types – might support neonatally restricted imprinting of skin immune function.
Concluding remarks
It is an exciting time for the investigation of skin microbiome–immune interactions, both in terms of deciphering basic mechanisms and identifying potential therapeutic applications. As emphasized herein, it will be important to focus on the unique opportunities and challenges afforded by an understanding of early-life immune imprinting by skin microbes. We posit that this area holds great potential for learning how early-life interventions could possibly be used to prevent or mitigate the severity of inflammatory skin diseases such as atopic dermatitis. Although microbially directed therapy holds great potential for treating established skin diseases [36], we should simultaneously explore its application in the context of early ‘course-correction’ in at-risk infants [37]. This strategy has merit in that targeting the skin–microbe–immune axis might ideally occur in infancy during a ‘window of opportunity’, presumably before the stable establishment of microbial communities and immune cell populations. These approaches, however, will need to consider the possibility that inherited host factors that increase the risk of developing a particular skin disease might themselves alter early immune responses to skin bacteria. Thus, principles gleaned from the study of healthy infants and neonatal animals may not directly apply, and will need special consideration in each disease context.
Exciting work is expanding in the academic and biotech spheres, and identification of microbially directed therapeutics or prophylactics will benefit from a better understanding of the molecular underpinnings and the immune and microbial sequelae of potential interventions. This is especially important for the pediatric population, where well-intended interventions may have inadvertent and undesirable long-term consequences. Given the complexity of the unresolved issues (see Outstanding questions), a combined approach that incorporates complementary studies in both mice and humans will be important to move the field forward. Ultimately, the future is bright for baby’s skin bacteria – so enjoy bath time, but go easy on the soap.
Outstanding questions.
How does the number and function of human skin myeloid and lymphoid cells evolve between birth and adolescence?
What are the key bacterial founder species on infant skin, how do they support the establishment of the cutaneous microbiome over the first years of life, and how do they differ functionally from strains that supplant them in adult life?
To what extent do keratinocyte-derived signals coordinate the early recruitment or function of cutaneous immune cell types in developing human skin?
Do neonatal skin microbes modulate epithelial stem cell ‘inflammatory memory’ via epigenetic modifications or other mechanisms in a way that alters later skin reparative capacity?
What are the key myeloid cell populations in neonatal skin that sample microbial antigens and integrate their signals to support the development of tolerance and homeostasis in the presence of commensals?
Which innate-type and/or classical T cell populations in developing human skin are particularly dependent on microbial signals for their abundance and/or functions?
What are the key host receptors and microbial molecules that govern bacterially mediated imprinting of skin immune function in early life?
How do genetic host factors that mediate susceptibility to pediatric inflammatory diseases, for example filaggin mutations in atopic dermatitis, alter the quantity and quality of early-life interactions between skin microbes and immune cells?
Highlights.
The composition and function of skin bacteria and immune cells differ substantially in mammalian neonates versus adults.
Although much remains to be understood about early-life skin immunity in humans, this period is demarcated by an increased capacity for tolerogenic function and a layered transition from innate-type lymphocytes to classical memory populations.
The ability of microbes to durably tune immune composition or function is often referred to as ‘immune imprinting’ – this phenomenon is preferentially active early in life and, if missed, some immune education events cannot be reproduced later on.
Growing evidence suggests that unique molecular interactions between commensal microbes and cells of the neonatal immune system have the potential to tip the balance between skin health and disease in adult life.
Mechanistically, early life bacterial exposure can influence skin immune function via bacteria–bacteria interactions, bacteria–epithelial cell interactions, and/or bacteria–immune cell interactions.
Only through separate, dedicated study of skin–bacteria crosstalk in the early developmental window can we understand the biology of pivotal events that impact on host–microbe symbiosis and skin immune function.
The safe and successful use of live bacteria or bacteria-derived therapies to promote skin health or treat skin disease relies upon the ability to stably introduce key species and understand their direct and indirect effects on the composition of the skin microbial community.
The strategy of targeting skin microbe–immune interactions in early life has the advantage of ‘correcting course’ before the stable establishment of microbial communities and/or immune cell populations, thereby not only treating but perhaps preventing skin disease.
Clinician’s corner.
Neonatal skin conditions, such as erythema toxicum neonatorum, acne neonatorum, and neonatal cephalic pustulosis, may reflect early-life sequelae of normal skin immune–microbe interactions [86,106,107].
Seborrheic dermatitis and infantile acne in young children likely represent more pathologic interactions with skin bacteria and fungi [108,109].
Atopic dermatitis is the poster child disease for disrupted early life skin–microbe crosstalk [110]. Emerging evidence indicates that this breakdown occurs well before profound flares of the disease are apparent [37–39], suggesting that early interventions to ‘correct course’ might help to mitigate disease severity or even prevent its onset, although this remains to be rigorously tested.
Even in the absence of any visible skin inflammation, early subclinical interactions between skin microbes and the developing cutaneous immune system may help set the stage for lifelong skin health and disease susceptibility [58,60].
Acknowledgments
We thank our colleagues in the fields of microbe–immune skin crosstalk and early-life immunity whose work inspired this Opinion, and apologize to any whose contributions we could not highlight owing to space constrains. Figures were generated using BioRender. M.O.D. is supported by grant K99AR079554 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). T.C.S. is supported by a Sun Pharma Award from the Dermatology Foundation and grant DP2AI44968 from the National Institute of Allergy and Infectious Diseases (NIAID).
Glossary
- Actinobacteria
bacteria that are commonly found on skin; this group includes genera such as Corynebacterium and Cutibacterium. The prevalence of these bacteria on sebaceous sites such as the face, chest, and back increases during late puberty
- Atopic dermatitis
a common inflammatory, allergic skin disorder with onset often in early life and that is characterized by flares typified by itching and redness, often associated with increases in S. aureus
- Biofilm
a community of microbes that grow on surfaces encased in a tough matrix which is not easily removable from the surface
- Epidermis
the outermost layer of the skin that comprises several layers of keratinocytes which terminally differentiate to form the stratum corneum
- Epigenetic
reversible changes in genetic information that are not encoded in the DNA sequence but are instead mediated by DNA methylation or histone modifications. These types of changes can occur in response to various environmental factors including microbes and alter the function of the cells in which they occur
- Epithelial stem cells
these reside in the basal layer of the epidermis and the hair follicle bulge region; they help to maintain skin homeostasis and hair regeneration, and participate in repair of the epidermis after injury
- Firmicutes
bacteria that are commonly found on the skin. They are especially dominant in early life and include genera such as Staphylococcus and Streptococcus
- Founder species
species of microorganisms that establish a niche, early in life, at a given anatomical site in the body, which then influences the future composition of the microbiome at that site
- Immune imprinting
the concept that specific immune interactions (e.g., with microbes) can durably alter the composition and/or function of the immune system in a way that alters subsequent immune responses. This has classically been intertwined with the molecular mechanism of epigenetic modification, but there are examples of imprinting that do not rely on this mechanism
- Keratinocytes
epithelial cells of the skin that form the epidermis, including that portion which lines the hair follicles
- Microbe-associated molecular patterns (MAMPs)
molecules that are conserved and expressed across several species of microbes and that are recognized by pattern recognition receptors
- Microbiome
the collective microbial composition, including but not restricted to, bacteria, viruses, and fungi, in any given anatomical niche
- Mucosa-associated invariant T (MAIT) cells
these are resident mainly in mucosal tissues, have a semi-invariant αβ T cell receptor, and are characterized by their innate-like properties
- Pattern recognition receptors
proteins that recognize and bind to conserved molecular patterns typically expressed by microbes. These proteins generally initiate signal transduction pathways upon binding their cognate molecules
- Quorum sensing
a method of communication between bacteria via secreted molecules that in turn govern gene expression and foster community behavior. In Staphylococcus aureus, the accessory gene regulator (Agr) system is a key regulator of quorum-sensing behavior and related toxin production
- Regulatory T cells (T regs)
a subset of CD4+ T cells defined by the transcription factor FoxP3; they play a key role in suppressing inflammation and preventing autoimmunity
- Staphylococcus aureus
Gram-positive skin bacteria of the Firmicutes phylum; these can asymptomatically colonize nares and skin, but are also a major cause of skin bacterial infections and are associated with flares of atopic dermatitis
- Staphylococcus epidermidis
one of several coagulase-negative Staphylococcus species that are commonly found on human skin
- Type 1 T helper (Th1) cells
a subset of CD4+ effector T cells that are traditionally studied for their role in mounting immune responses to fight infection by intracellular pathogens and viruses via the production of IFN-γ
- Type 2 T helper (Th2) cells
a subset of CD4+ effector T cells that are traditionally studied for their role in mounting immune responses to fight infection by extracellular parasites such as helminths; they are known producers of IL-4, IL-5, and IL-13. Overt production of these cytokines has been associated with allergic immune responses
- Type 17 T helper (Th17) cells
a subset of CD4+ effector T cells that are deemed to have both protective and pathogenic roles; they are known producers of IL-17A and have a protective role against cutaneous bacterial infections (e.g., S. aureus). Th17-mediated inflammation has also been linked to disease pathology in skin conditions such as psoriasis
Footnotes
Declaration of interests
T.C.S. is a Scientific Advisory Board member for Concerto biosciences. M.O.D. has no conflicts to disclose.
References
- 1.Tamburini S et al. (2016) The microbiome in early life: implications for health outcomes. Nat. Med 22, 713–722 [DOI] [PubMed] [Google Scholar]
- 2.Hornef MW and Torow N (2020) ‘Layered immunity’ and the ‘neonatal window of opportunity’ – timed succession of non-redundant phases to establish mucosal host–microbial homeostasis after birth. Immunology 159, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naik S et al. (2015) Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520, 104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linehan JL et al. (2018) Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell 172, 784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison OJ et al. (2019) Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science 363, eaat6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Nabhani Z and Eberl G (2020) Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 13, 183–189 [DOI] [PubMed] [Google Scholar]
- 7.House JS et al. (2017) Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J. Allergy Clin. Immunol 140, 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojwang V et al. (2020) Early exposure to cats, dogs and farm animals and the risk of childhood asthma and allergy. Pediatr. Allergy Immunol 31, 265–272 [DOI] [PubMed] [Google Scholar]
- 9.Ege MJ (2017) The hygiene hypothesis in the age of the microbiome. Ann. ATS 14, S348–S353 [DOI] [PubMed] [Google Scholar]
- 10.Naik S et al. (2017) Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Y and Kupper TS (2018) Metabolic reprogramming and longevity of tissue-resident memory T cells. Front. Immunol 9, 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrick BM et al. (2021) Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898 [DOI] [PubMed] [Google Scholar]
- 13.Olszak T et al. (2012) Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashir MEH et al. (2004) Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J. Immunol 172, 6978–6987 [DOI] [PubMed] [Google Scholar]
- 15.Lee KH et al. (2020) The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds LA and Finlay BB (2017) Early life factors that affect allergy development. Nat. Rev. Immunol 17, 518–528 [DOI] [PubMed] [Google Scholar]
- 17.Zanvit P et al. (2015) Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat. Commun 6, 8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra A et al. (2021) Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rackaityte E et al. (2020) Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med 26, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallo RL (2017) Human skin is the largest epithelial surface for interaction with microbes. J. Invest. Dermatol 137, 1213–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scanlon ST (2020) The immune system’s first steps. Science 368, 598–599 [DOI] [PubMed] [Google Scholar]
- 22.Jennewein MF et al. (2018) Neonate-omics: charting the unknown immune response in early life. Cell 174, 1051–1053 [DOI] [PubMed] [Google Scholar]
- 23.Grogan MD et al. (2019) Research techniques made simple: profiling the skin microbiota. J. Invest. Dermatol 139, 747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y et al. (2020) Single-cell RNA sequencing in Immunology. Curr. Genomics 21, 564–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.York A (2019) Microbiota succession in early life. Nat. Milestones Microbiota Res, S8–S9 Published online June 2019. https://media.nature.com/original/magazine-assets/d42859-019-00010-6/d42859-019-00010-6.pdf [Google Scholar]
- 26.Litvak Y and Bäumler AJ (2019) The founder hypothesis: a basis for microbiota resistance, diversity in taxa carriage, and colonization resistance against pathogens. PLoS Pathog. 15, e1007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh J et al. (2014) Biogeography and individuality shape function in the human skin metagenome. Nature 514, 59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh J et al. (2016) Temporal stability of the human skin microbiome. Cell 165, 854–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W et al. (2020) Host-specific evolutionary and transmission dynamics shape the functional diversification of Staphylococcus epidermidis in human skin. Cell 180, 454–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conwill A et al. (2021) Anatomy promotes neutral coexistence of strains in the human skin microbiome. BioRxiv Published online May 14, 2021. 10.1101/2021.05.12.443817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Key FM et al. (2021) On-person adaptive evolution of Staphylococcus aureus during atopic dermatitis increases disease severity. BioRxiv Published online March 24, 2021. 10.1101/2021.03.24.436824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakatsuji T et al. (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med 9, eaah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C-H et al. (2011) An innate bacteriidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: a therapy concordant with evolutionary medicine. J. Microbiol. Biotechnol 21, 391–399 [PubMed] [Google Scholar]
- 34.Nakamura K et al. (2020) Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep 10, 21237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams MR et al. (2019) Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med 11, eaat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakatsuji T et al. (2021) Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat. Med 27, 700–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y et al. (2020) Staphylococcus Agr virulence is critical for epidermal colonization and associates with atopic dermatitis development. Sci. Transl. Med 12, eaay4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meylan P et al. (2017) Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J. Investig. Dermatol 137, 2497–2504 [DOI] [PubMed] [Google Scholar]
- 39.Kennedy EA et al. (2017) Skin microbiome before development of atopic dermatitis: early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol 139, 166–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miajlovic H et al. (2010) Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J. Allergy Clin. Immunol 126, 1184–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towell AM et al. (2021) Staphylococcus aureus binds to the N-terminal region of corneodesmosin to adhere to the stratum corneum in atopic dermatitis. Proc. Natl. Acad. Sci. U. S. A 118, e2014444118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogonowska P et al. (2020) Colonization with Staphylococcus aureus in atopic dermatitis patients: attempts to reveal the unknown. Front. Microbiol 11, 567090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatsuji T et al. (2021) Use of autologous bacteriotherapy to treat Staphylococcus aureus in patients with atopic dermatitis: a randomized double-blind clinical trial. JAMA Dermatol. 157, 978–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsuji T et al. (2013) The microbiome extends to subepidermal compartments of normal skin. Nat. Commun 4, 1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lange-Asschenfeldt B et al. (2011) Distribution of bacteria in the epidermal layers and hair follicles of the human skin. Skin Pharmacol. Physiol 24, 305–311 [DOI] [PubMed] [Google Scholar]
- 46.Uberoi A et al. (2021) Commensal microbiota regulates skin barrier function and repair via signaling through the aryl hydrocarbon receptor. Cell Host & Microbe 29 (8), 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams MR et al. (2020) Interplay of staphylococcal and host proteases promotes skin barrier disruption in netherton syndrome. Cell Rep. 30, 2923–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakatsuji T and Gallo RL (2012) Antimicrobial peptides: old molecules with new ideas. J. Investig. Dermatol 132, 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanford JA and Gallo RL (2013) Functions of the skin microbiota in health and disease. Semin. Immunol 25, 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai Y et al. (2010) Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J. Investig. Dermatol 130, 2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mansfield K and Naik S (2020) Unraveling immune–epithelial interactions in skin homeostasis and injury. Yale J. Biol. Med 93, 133–143 [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi T et al. (2019) Choreographing immunity in the skin epithelial barrier. Immunity 50, 552–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagao K et al. (2012) Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol 13, 744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adachi T et al. (2015) Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med 21, 1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi T et al. (2015) Dysbiosis and Staphyloccus aureus colonization drives inflammation in atopic dermatitis. Immunity 42, 756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakamoto K et al. (2021) Disruption of the endopeptidase ADAM10–Notch signaling axis leads to skin dysbiosis and innate lymphoid cell-mediated hair follicle destruction. Immunity 54, 2321–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharschmidt TC et al. (2017) Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe 21, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharschmidt TC et al. (2015) A wave of regulatory T cells into neonatal skin mediates tolerance to commensal microbes. Immunity 43, 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nosenko MA et al. (2019) Proinflammatory cytokines and skin wound healing in mice. Mol. Biol 53, 653–664 [DOI] [PubMed] [Google Scholar]
- 60.Leech JM et al. (2019) Toxin-triggered interleukin-1 receptor signaling enables early-life discrimination of pathogenic versus commensal skin bacteria. Cell Host Microbe 26, 795–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang G et al. (2021) Bacteria induce skin regeneration via IL-1β signaling. Cell Host Microbe 29, 777–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aristizabal MJ et al. (2020) Biological embedding of experience: a primer on epigenetics. PNAS 117, 23261–23269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lim AI et al. (2021) Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 373, eabf3002. [DOI] [PubMed] [Google Scholar]
- 64.Geijtenbeek TBH and Gringhuis SI (2016) C-type lectin receptors in the control of T helper cell differentiation. Nat. Rev. Immunol 16, 433–448 [DOI] [PubMed] [Google Scholar]
- 65.Sun L et al. (2019) The role of Toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J Immunol Res 2019, 1824624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castro CN et al. (2015) Microbe-associated immunomodulatory metabolites: Influence on T cell fate and function. Mol. Immunol 68, 575–584 [DOI] [PubMed] [Google Scholar]
- 67.Kubo A et al. (2009) External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med 206, 2937–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Aar AMG et al. (2013) Langerhans cells favor skin flora tolerance through limited presentation of bacterial antigens and induction of regulatory T cells. J. Invest. Dermatol 133, 1240–1249 [DOI] [PubMed] [Google Scholar]
- 69.Gensollen T et al. (2021) Embryonic macrophages function during early life to determine invariant natural killer T cell levels at barrier surfaces. Nat. Immunol 22, 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cruz MS et al. (2018) Human αβ and γδ T cells in skin immunity and disease. Front. Immunol 9, 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Constantinides MG et al. (2019) MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridaura VK et al. (2018) Contextual control of skin immunity and inflammation by Corynebacterium. J. Exp. Med 215, 785–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinks TSC and Zhang X-W (2020) MAIT cell activation and functions. Front. Immunol 11, 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tastan C et al. (2018) Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 11, 1591–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Legoux F et al. (2019) Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366, 494–499 [DOI] [PubMed] [Google Scholar]
- 76.Chen YE et al. (2019) Decoding commensal–host communication through genetic engineering of Staphylococcus epidermidis. BioRxiv Published online June 10, 2019. 10.1101/664656 [DOI] [Google Scholar]
- 77.Round JL et al. (2011) The Toll-like receptor pathway establishes commensal gut colonization. Science 332, 974–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhariwala MO et al. (2020) Developing human skin contains lymphocytes demonstrating a memory signature. Cell Reports Med. 1, 100132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reynolds G et al. (2021) Developmental cell programs are co-opted in inflammatory skin disease. Science 371, eaba6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reitermaier R et al. (2021) αβγδ T cells play a vital role in fetal human skin development and immunity. J. Exp. Med 218, e20201189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McGovern N et al. (2017) Human fetal dendritic cells promote prenatal T-cell immune suppression through arginase-2. Nature 546, 662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu JC et al. (2018) Innate immunity of neonates and infants. Front. Immunol 9, 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lynch SV and Vercelli D (2021) Microbiota, epigenetics, and trained immunity. Convergent drivers and mediators of the asthma trajectory from pregnancy to childhood. Am. J. Respir. Crit. Care Med 203, 802–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu DM et al. (2017) Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med 23, 314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dominguez-Bello MG et al. (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci 107, 11971–11975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoch JJ et al. (2019) The infantile cutaneous microbiome: a review. Pediatr. Dermatol 36, 574–580 [DOI] [PubMed] [Google Scholar]
- 87.Capone KA et al. (2011) Diversity of the human skin microbiome early in life. J. Investig. Dermatol 131, 2026–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Younge NE et al. (2018) Early-life skin microbiota in hospitalized preterm and full-term infants. Microbiome 6, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oh J et al. (2012) Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 4, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shay T et al. (2013) Conservation and divergence in the transcriptional programs of the human and mouse immune systems. PNAS 110, 2946–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjornson-Hooper ZB et al. (2019) A comprehensive atlas of immunological differences between humans, mice and non-human primates. BioRxiv Published online March 11, 2019. 10.1101/574160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.den Braber I et al. (2012) Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 36, 288–297 [DOI] [PubMed] [Google Scholar]
- 93.Park J-E et al. (2020) Prenatal development of human immunity. Science 368, 600–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mold JE and McCune JM (2012) Immunological tolerance during fetal development: from mouse to man. Adv. Immunol 115, 73–111 [DOI] [PubMed] [Google Scholar]
- 95.Pellicci DG et al. (2020) Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat. Rev. Immunol 20, 756–770 [DOI] [PubMed] [Google Scholar]
- 96.Hardman MJ et al. (1999) Barrier formation in the human fetus is patterned. J. Investig. Dermatol 113, 1106–1113 [DOI] [PubMed] [Google Scholar]
- 97.Stamatas GN et al. (2011) Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. Int. J. Cosmet. Sci 33, 17–24 [DOI] [PubMed] [Google Scholar]
- 98.Saxena N et al. (2019) An updated classification of hair follicle morphogenesis. Exp. Dermatol 28, 332–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Houschyar KS et al. (2020) Molecular mechanisms of hair growth and regeneration: current understanding and novel paradigms. Dermatology 236, 271–280 [DOI] [PubMed] [Google Scholar]
- 100.Semmes EC et al. (2020) Understanding early-life adaptive immunity to guide interventions for pediatric health. Front. Immunol 11, 595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ennamorati M et al. (2020) Intestinal microbes influence development of thymic lymphocytes in early life. PNAS 117, 2570–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen P et al. (2019) Circulating mucosal-associated invariant T cells in a large cohort of healthy Chinese individuals from newborn to elderly. Front. Immunol 10, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thome JJC et al. (2015) Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med 22, 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rackaityte E and Halkias J (2020) Mechanisms of fetal T cell tolerance and immune regulation. Front. Immunol 11, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cordoro KM et al. (2017) Skin-infiltrating, interleukin-22-producing T cells differentiate pediatric psoriasis from adult psoriasis. J. Am. Acad. Dermatol 77, 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schneider AM and Nelson AM (2019) Skin microbiota: friend or foe in pediatric skin health and skin disease. Pediatr. Dermatol 36, 815–822 [DOI] [PubMed] [Google Scholar]
- 107.Chadha A and Jahnke M (2019) Common neonatal rashes. Pediatr. Ann 48, e16–e22 [DOI] [PubMed] [Google Scholar]
- 108.Maroñas-Jiménez L and Krakowski AC (2016) Pediatric acne: clinical patterns and pearls. Dermatol. Clin 34, 195–202 [DOI] [PubMed] [Google Scholar]
- 109.Hammond AM et al. (2021) The role of the pediatric cutaneous and gut microbiomes in childhood disease: a review. Semin. Perinatol 45, 151452. [DOI] [PubMed] [Google Scholar]
- 110.Paller AS et al. (2019) The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol 143, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chandran SS et al. (2009) TLR2 engagement on dendritic cells promotes high frequency effector and memory CD4 T cell responses. J. Immunol 183, 7832–7841 [DOI] [PubMed] [Google Scholar]


