Abstract
Bourbon virus (BRBV) was first isolated from a blood sample collected from a male patient living in Bourbon county, Kansas, during the spring of 2014. The patient later died due to complications associated with multiorgan failure. Currently, several BRBV infection-caused deaths have been reported in the United States, and misdiagnosed cases are often undercounted. BRBV is a member of the genus Thogotovirus of the Orthomyxoviridae family, and is transmitted through the Lone Star tick, Amblyomma Americanum, in North America. Currently, there are no specific antivirals or vaccinations available to treat or prevent BRBV infection. Several small molecular compounds have been identified to effectively inhibit BRBV infection of in vitro cell cultures at a single- or sub-micromolar level. Favipiravir, an RNA-dependent RNA polymerase inhibitor, prevented the death of Type I interferon receptor knockout mice infected with BRBV infection.
Keywords: Bourbon virus, Lone Star tick, infection, antivirals
Introduction
Bourbon virus (BRBV) is a member of the genus Thogotovirus of the Orthomyxoviridae family, a family of segmented negative-strand RNA viruses [1]. There are 7 genera in the family of Orthomyxoviridae, including four types of influenza viruses (influenza virus A, B, C, and D), Quaranjavirus, Isavirus and Thogotovirus (Fig. 1). Many viruses in the Orthomyxoviridae family are important pathogens to humans or animals. The epidemic/pandemic influenza A viruses have caused hundreds of thousands of human deaths and are still discretely circulating, continuing to pose huge threat to human lives.
Figure 1. Classification of Orthomyxoviridae.
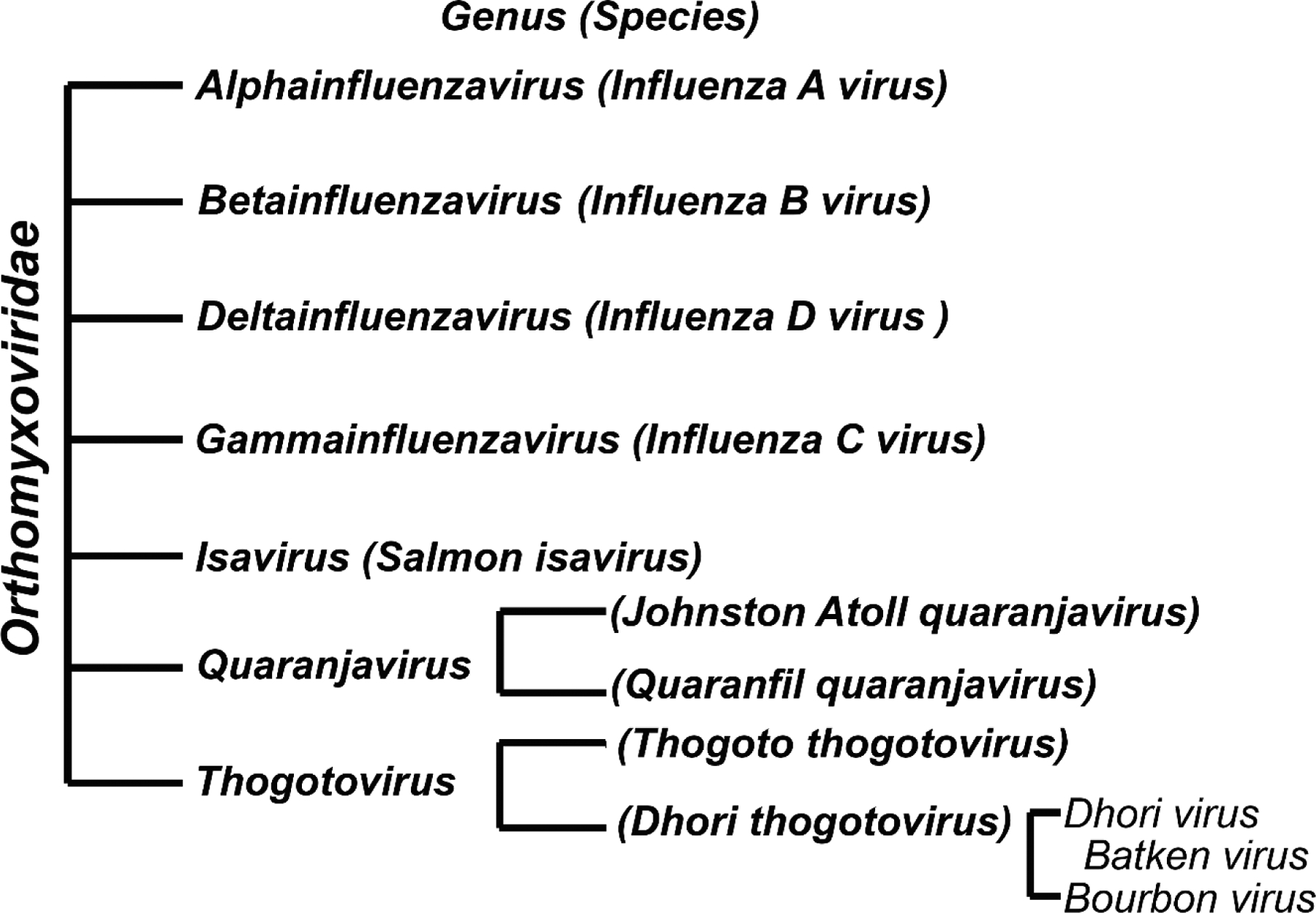
Seven genera of the Orthomyxoviridae family are listed with species shown in parenthesis, which is based on the revised classification of Orthomyxoviridae (ICTV 2017) [44].
In contrast to influenza viruses, thogotoviruses are transmitted mainly through tick vectors and thus are also called “tick-borne viruses” [2,3]. Two thogotoviruses, Thogoto virus and Dhori virus, have been reported to infect humans and cause death [4,5]. Thogoto virus largely circulates in domestic animals, such as sheep, cattle, and camels, causing neurological diseases and abortion [6]. Human antibodies against Thogoto virus and Dhori virus have been identified in Europe, Asia, and Africa [6,7]. Importantly, Dhori virus has been reported to cause human infections in a vector-free manner, possibly by an aerosol route [4], which highlights the potential to infect humans in a large population.
Based on the available genomic sequences, thogotoviruses have been subdivided into two clusters [8–11], Thogoto-like and Dhori-like thogotoviruses. BRBV is phylogenetically closely related to Dhori-like thogotoviruses, e.g., Batken virus, which have been known to emerge in regions throughout Africa, Asia and Europe [6,7,12,13]. Dhori and Batken viruses have been isolated from Hyalomma ticks, and antibodies against Dhori virus have been identified in camels, goats, horses, cattle and humans [6,7,12,13]. Interestingly, Batken virus has also been isolated from several mosquito species [13]. BRBV replicates at a high level in a variety of invertebrate and vertebrate cell lines and produces relatively high titers derived from all mammalian cells that are compatible with the known susceptibility of the human host to BRBV infection and disease [10].
Bourbon virus infection causes human diseases
The first case of BRBV infection was found in Kansas, USA. The virus was isolated from a blood sample collected from a male patient who lived in Bourbon County, Kansas, and hospitalized in The University of Kansas Hospital, Kansas City, Kansas, in the spring of 2014 [14]. The patient was a >50 years old healthy man who was bitten by several ticks prior to hospitalization. During the beginning of the illness, symptoms included nausea, weakness, and diarrhea. Later, after 2 days, the patient had fever, anorexia, chills, headache, myalgia, and arthralgia. He was hospitalized after 4 days. The lab tests showed the patient had leukopenia, lymphopenia, thrombocytopenia, hyponatremia, and increased levels of aspartate aminotransferase and alanine aminotransferase. He died 11 days after the initial onset of symptoms [14]. The patient had a papular rash identified on his trunk that initiated serological and molecular testing for several known tick-borne pathogens that were all found negative [14]. However, when a serum sample was inoculated on Vero cells, at 3 days post-infection, obvious plaques were observed in a plaque assay. The supernatants of the inoculated culture were observed containing filamentous and spherical virus-like particles under transmission electron microscopy, typical morphologies of virions in the family Orthomyxoviridae, and novel viral sequences that share high similarity with Dhori and Batken viruses were identified [3,15]. This novel Thogotovirus was named Bourbon virus (BRBV), based on the county where the patient lived. As the high level of viremia and unique identification of the virus in the serum of the patient, BRBV was believed to be the cause of the illness and the death of the patient.
Up to date, a limited number of BRBV disease cases have been identified in the Midwest and southern United States, with some people who have been infected later died. In 2015, a patient, who was a resident of Payne County, Oklahoma, tested positive for neutralization antibodies to BRBV before fully recovering [16]. In June 2017, a 58-year-old woman from Missouri died from an infection of BRBV after 23 days in hospital. The patient had symptoms and laboratory test results similar to those described in the first case in Kansas approximately one week after tick bites [17]. Virus was isolated from inoculation of a serum sample collected from the patient in Vero cells, and viral RNA was sequenced by next generation sequencing, which had an average of 104-fold coverage with BRBV [17].
Taken together, BRBV is the first species of the genus Thogotovirus to be identified as a human pathogen in North America.
Lone Star tick is the vector to transmit BRBV
The United States Centers for Disease Control and Prevention (CDC) carried out several epidemiologic studies on BRBV infection in Amblyomma Americanum ticks (Fig. 2) and animals using real time PCR with BRBV-specific primer/probe sets targeting nucleoprotein (NP) and polymerase (PB1) genes, respectively. In 2013, BRBV was found in 3 pools of Amblyomma Americanum ticks from retrospective tests in 39,096 ticks from northwestern Missouri, located 240 km from Bourbon county, Kansas [16]. The BRBV infection rate (IR) per 1,000 adult ticks for all sites was 0.32. The BRBV strain isolated from tick pools share >99.0% sequence at the amino acid level and 95.0% identity at the RNA sequence level, compared to the first human BRBV strain [16]. In the summer of 2015, CDC continued tick surveillance in Bourbon county and adjacent southern Linn county, Kansas. A total of 20,639 host-seeking ticks representing four species were collected from 12 sites. BRBV was detected at an IR of 0.25 in the abundant and aggressive human-biting tick Amblyomma Americanum in Bourbon county. This survey supports the contention that Amblyomma Americanum is a vector of BRBV [18]. In June 2016, the same group collected a total of 14,193 ticks representing four species from four sites in eastern Kansas, including the sites where BRBV was detected in 2015. Surprisingly, all pools tested negative of BRBV [19]. Thus, it is possible that Amblyomma Americanum encounters viremic vertebrate hosts of BRBV infrequently, or that BRBV is inefficiently passed vertically from infected female ticks to their offspring.
Figure 2. Geographical distribution of Amblyomma americanum, the Lone Star tick, in the United States.
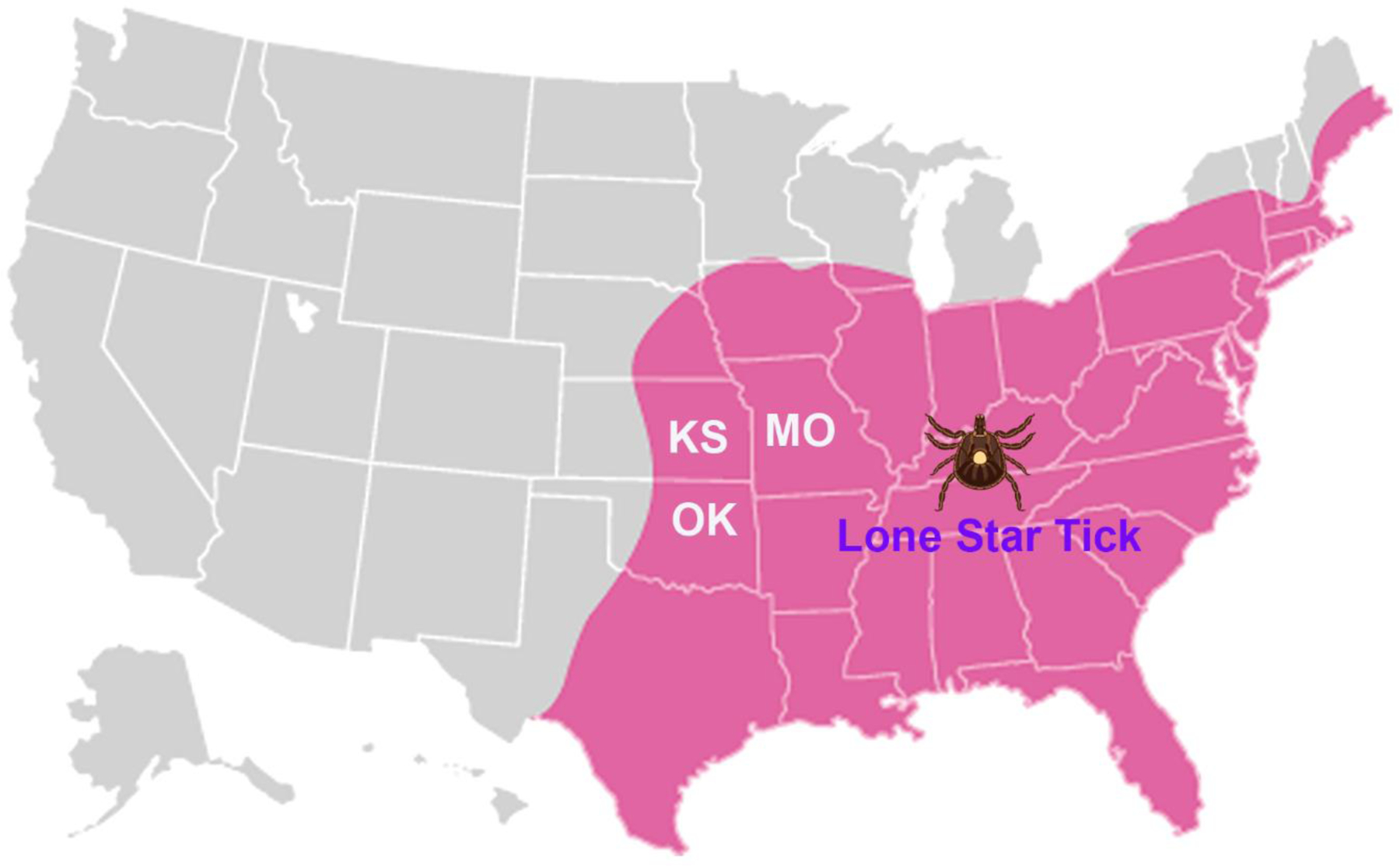
The area highlighted in pink indicates the distribution of Lone Star ticks in North America up to the upper midwestern and northeastern regions of the United States and eastern Canada. KS, Kansas; MO, Missouri; and OK, Oklahoma of the United Sates.
Moreover, in a surveillance of animals for evidence of BRBV infection. Plaque-reduction neutralization test (PRNT) was used to detect BRBV antibodies in animal sera and plasma specimens of white-tailed deer (Odocoileus virginianus), raccoon (Procyon lotor), Virginia opossum (Didelphis virginiana), and various other mammals and birds from northwest Missouri. A high seroprevalence in wild animals, raccoons (50%) and white-tailed deer (86%), as well as a low rate in domestic dogs (seroprevalence 15%) and horses (seroprevalence 4%) were detected [20]. In North Carolina where the Lone Star tick is abundant, 18 out of 32 (56%) white-tailed deer were detected BRBV-specific PRNT positive [21]. This study indicates that BRBV is widely spread among animals as previously recognized and implicates raccoons and white-tailed deer might be potential zoonotic reservoirs of BRBV.
BRBV replicates in cell lines derived from hard ticks Amblyomma, Hyalomma and Rhipicephalus [10]. Experimental infection of Amblyomma Americanum with BRBV evidenced transstadial transmission of BRBV from the larval to nymphal stages and from the nymphal to adult stages of the tick [22]. Importantly, a high rate of transmission from infected to uninfected ticks through cofeeding was observed. The study suggests that cofeeding may be an important mechanism for viral maintenance in the environment and is in line with the finding of nonviremic or cofeeding transmission of Thogoto virus by Rhipicephalus Appendiculatus [23]. While vertical transmission to their progeny occurred at a low rate. Rabbits fed on by BRBV infected ticks at active life stages or needle-inoculated with BRBV developed high titers (1:320 to 1:2,560) of 90% PRNT to BRBV. Interestingly, BRBV injected rabbits tested negative for virus by both RT-PCR and plaque assay, although serologic conversion was observed at 42 days post-inoculation [22].
Together with the geographic location of the BRBV infection and the geographic distribution of Amblyomma Americanum ticks [16,18], these studies strongly suggest that the Lone Star ticks (Amblyomma Americanum) widely carry BRBV and are a competent vector to transmit BRBV. Lone Star tick is a species that is aggressive, feeds on humans, and is abundant in the states of Kansas, Missouri, and Oklahoma (Fig. 2). Importantly, the distribution of Lone Star ticks in North America has geographically expanded to the upper midwestern and northeastern regions of the United States and eastern Canada due to climatic change and land use patterns [24]. Moreover, Lone Star ticks have become the most commonly encountered tick in Delaware [25]. Importantly, BRBV was detected in pools of tick Haemaphysalis Longicornis (Ixodidae), the Asian long horned tick, in several counties of Virginia [26]. Apparently, future epidemic surveillance of BRBV in ticks and animals are necessary to assess geographic distribution of BRBV and the natural host of BRBV.
BRBV genome and replicon construction
BRBV harbors six segments of negative-sense (−)RNA genome, PB2, PB1, PA, NP, GP, and M, that have a size of 2,381, 2,200, 1,961, 1,480, 1,588, and 9,57 nucleotides, respectively (Fig. 3A). These segments encode genes for the RNA-dependent polymerase (RdRP), consisting of the polymerase basic protein 2 (PB2), PB1, and the polymerase acidic protein (PA), a nucleoprotein (NP) that encapsidates the -RNA genome, a glycoprotein (GP) that is involved in virus attachment and fusion, and a matrix protein (M) linking the viral envelope with the virus core [27,28].
Figure 3. BRBV replicon construction. (A) Schematic representation of the method for the construction of the plasmids to replicate the BRBV genome.
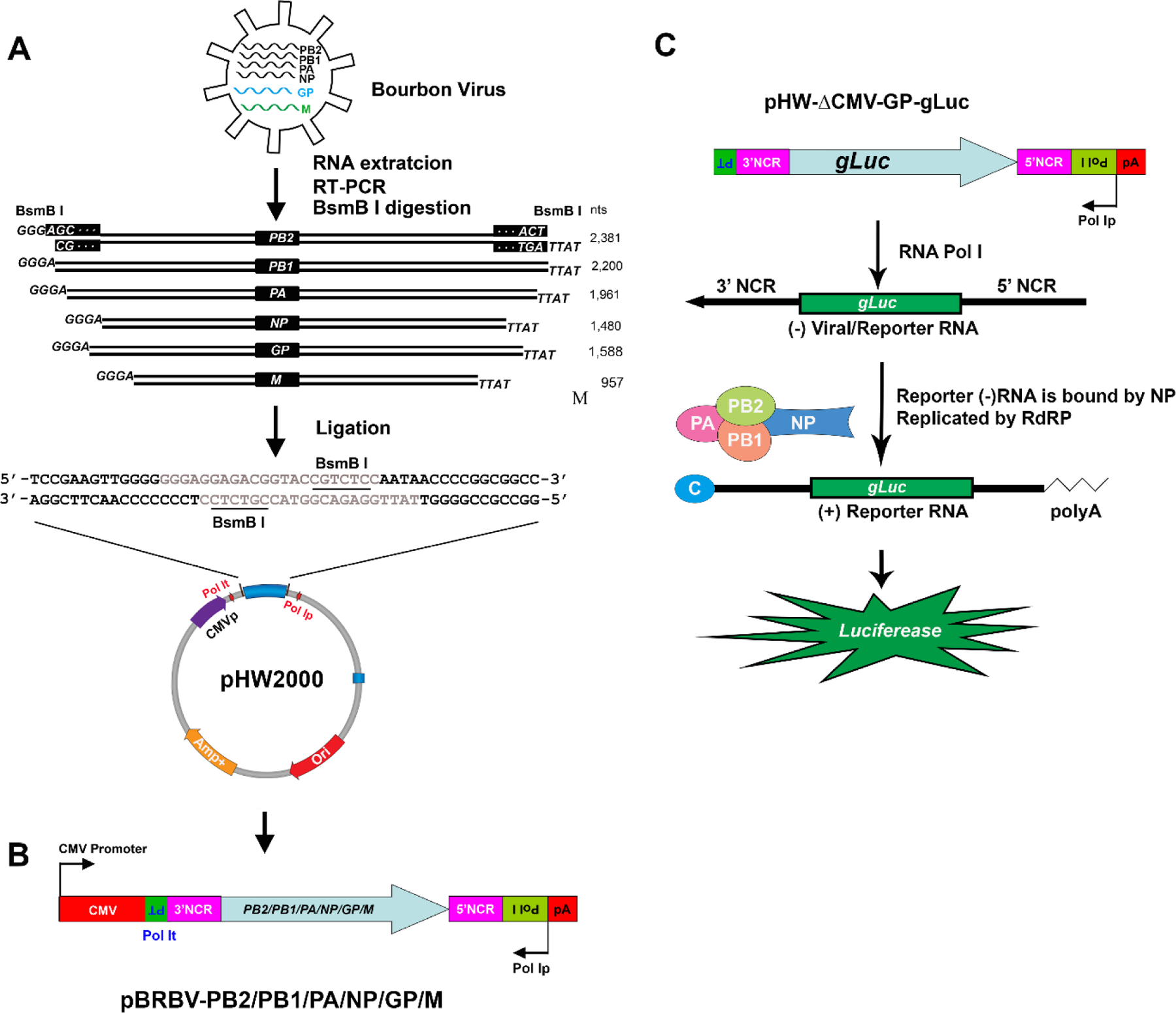
Viral RNA was extracted from virus particles. Reverse transcription(RT)-PCR was performed with primers containing segment-specific nucleotides and sequences for restriction enzyme BsmB I. The six viral RT-PCR fragments were digested with BsmB I and inserted into pHW2000 (linearized with BsmB I). This insertion resulted in 6 expression constructs where the viral cDNAs are precisely fused to the polymerase I promoter (Pol Ip) and the terminator (Pol It). The viral terminal sequences (AGC… and …ACT) are only shown in the PB2 segment in the black rectangles. (B) pBRBV-viral cDNA. The diagram shows the expression plasmid with a Pol Ip and a CMV pol II promoter containing viral cDNA of the PB2, PB1, PA, NP, GP, or M segment. The open reading frame for the 6 viral proteins are flanked by the segment-specific 3’ and 5’ noncoding regions (3’ NCR and 5’ NCR, respectively; pink boxes). (C) BRBV RNA replicon reporter system. pBRBV-gLuc reporter. Gaussia luciferase (gLuc) gene flanked by the 3’ and 5’ NCR of BRBV is cloned into pHW2000 that lacks the CMV pol II promoter. Thus, only replication of the gLuc gene by BRBV RNA-dependent RNA polymerase (RdRP) and NP protein produces gLuc +RNA for gLuc protein expression.
Similar to influenza virus, BRBV RNA replication follows the model of viruses in the family Orthomyxoviridae. The viral polymerase (P) subunits—PB1, PB2, and PA compose the viral RNA replicase complex [29]. The viral RNA replication elements in cis are located at the 3’ and 5’ noncoding regions (NCR) of each viral genome [29]. With expression of the BRBV replicase complex (PB1, PB2, and PA), the nucleocapsid (NP) and a reporter (GFP or Gaussia luciferase, gLuc)-coding gene flanked by BRBV 3’ and 5’ NCR will replicate and express GFP or gLuc. This replicon reporter has been independently established in three labs and used for testing or screening of antivirals in a biosafety level 2 setting [17,30,31].
In principle, one of the 6 viral replication-essential genes (PB2, PB1, PA, NP, GP, and M) is amplified from viral RNA extracted from infected cells and cloned in plasmid pHW2000 to generate pBRBV-PA/PB1/PB2/NP/GP/M (Fig. 3B). The pHW2000 was widely used for the development of the reverse genetic system of influenza viruses [32]. Next, a reporter (GFP or gLuc) cDNA is cloned into pHW2000 but without the cytomegalovirus immediate-early enhancer and promoter (CMVp), which is named pBRBV-ΔCMV-GP-gLuc (Fig. 3C). Then, four CMVp driven PA, PB1, PB2, and NP-expressing plasmids (i.e., pcDNA-PA/PB1/PB2/NP), and pBRBV-gLuc are co-transfected into HEK293T cells. At 2 days post-infection, transfected cells are detected for gLuc expression. Expression of PA, PB1, PB2, and NP replicates the BRBV-gLuc RNA genome to become a capped viral positive sense +RNA genome, which is capable of translation. Because the gLuc gene in pBRBV-gLuc is not driven by a polymerase II promoter (e.g., a CMVp), only replication of the BRBV genome generates viral +RNA for gLuc expression (Fig. 3C).
If all the 6 pHW2000 based plasmids, pBRBV-PB2, PB1, PA, NP, GP, and M (Fig. 3B) are co-transfected into cells, recombinant BRBV is expected to be generated. Similar reverse genetic systems for influenza virus and Thogoto virus have been established and widely used to study virus replication and pathogenesis [32–37].
In vitro culture of BRBV
Vero cells (ATCC #CCL-81), kidney epithelial cells of the African green monkey, are mostly permissive to BRBV infection. A production level of 108 plaque forming units (pfu)/ml can be readily obtained (Fig. 4), when Vero cells were infected with the BRBV strain (#NR-50132, the Biodefense and Emerging Infections Research Resources Repository (BEI), NIAID, NIH), originally isolated in Bourbon county. A wide range of human cells, including human embryonic kidney cell line HEK293T (ATCC #CRL-11268), human hepatocyte cell line Huh7, and human cervical epithelial cell line HeLa (ATCC #CCL-2), human lung epithelial A549 cells (ATCC #CCL-185), are permissive to productive infection of BRBV [10,30]. HEK293T and Huh7 cells exhibited growth kinetics similar to those in Vero cells, but both A549 and HeLa cells had a decrease of ~2 log in virus production [10,30,38]. The virus also can infect polarized human airway epithelia at a level of ~1 × 106 to 107 genome copies per ml of the apical washes at one week post-infection. Of note, human foreskin fibroblast (HFF; ATCC #SCRC-1041) cells were not infected by BRBV, even though humans are supposed to be infected by tick bites on skins. These cells may have a strong interferon response upon virus infection [39], and therefore are resistant to BRBV infection. BRBV infection has been proven to be sensitive to treatment of interferon (IFN)-α2a and IFN-γ, and BRBV infection-induced IFN elicited an antiviral immunity that suppressed virus propagation [38]. BRBV replicates in the cell lines derived from the hard ticks Amblyomma, Hyalomma and Rhipicephalus, but at a level of >4 log less than that in Vero or Huh7 cells. Mosquito cell lines are poorly infected with only a yield less than 104 pfu/ml [10].
Figure 4. BRBV plaque assay.
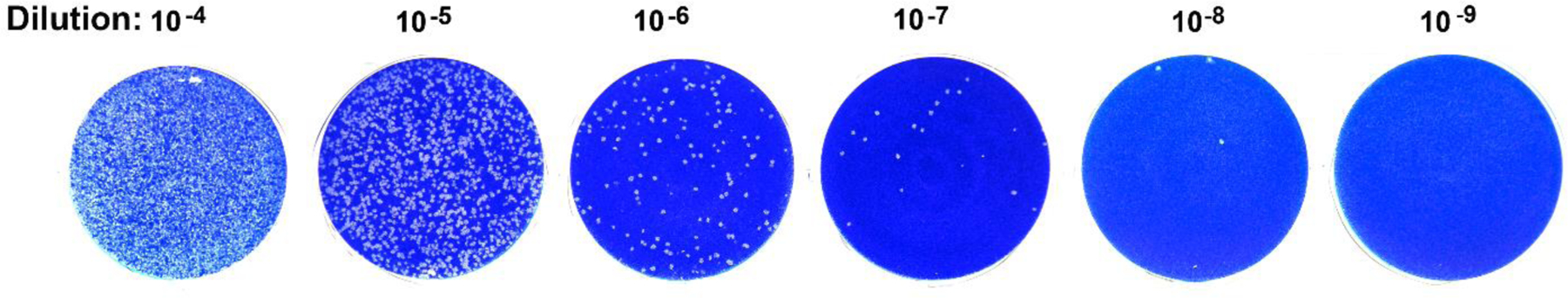
A BRBV stock was diluted from 10 −4 to 10−9 in Dulbecco’s Modified Eagle’s medium (DMEM) media. 1 ml of each diluted virus were added in one well of a 6-well plate of Vero cells. After incubation of 1 hour at 37°C, diluted virus-containing media were removed from each well. 2 ml of DMEM with 10% fetal calf serum and 1% methylcellulose overlay were added to each well. After 3 days post-infection, the media was aspirated from each well, and the cells were stained with crystal violet [30]. Plaques, foci of infected cells, are shown as white dots in the stained cell sheet (blue background).
Animal models
Mice (CD-1) were susceptible to BRBV infection as evidenced by seroconversion but the infection did not cause any disease symptoms or death of animals [10]. C57BL/6 mice were intraperitoneally infected at a high dose of 105 pfu per animal, but BRBV did not cause severe disease without or only a transient reduction in body weight [11].
Mice of type I IFN deficient (lacking IFN-α/β receptor expression) (IFNαAR−/−), both type I and III deficient (IFNαAR−/− IL28R−/− double knockout) mice, and both type I and II IFN deficient (STAT1 knockout) (STAT1−/−) mice supported growth of BRBV-KS (the original isolate in Kansas) in liver, lung, spleen, and kidney cells [38], with the latter growing to higher virus titers. In the clinical and pathologic changes, STAT1−/− mice (intraperitoneal (IP) inoculation of 100 pfu) had the most severe clinical score and died, whereas IFNαAR−/− mice were less severely affected and recovered [38]. The clinical and pathologic changes observed in STAT1−/− animals were compatible with the clinical manifestations, such as liver damage and acute respiratory complications, as described in BRBV-infected patients [14,17]. Thus, STAT1−/− mice should be an ideal model to better understand the replication and pathogenesis of BRBV and for the evaluation of antiviral strategies. However, in another study, with IP inoculation of a higher titer (4 × 102 pfu) of BRBV STL strain that was isolated in St. Louis, IFNAR−/− mice not only resulted in substantial weight loss but also 100% mortality after infection [17].
Prevention and treatment
No specific antivirals or vaccines have been developed to treat BRBV-infection caused diseases. IFN treatment significantly decreased virus yields in BRBV infected cell cultures. A combination of IFN-α and IFN-γ reduced virus titer by >103-fold [38]. Treatment with a guanosine analog (ribavirin) and a guanine analog (favipiravir [T705]) reduced virus titer by > 1 × 106 -fold in cell culture. Treatment of IFNαAR −/− or STAT1−/− mice with ribavirin (40 mg/kg) reduced BRBV replication in organs and weight loss which resulted in a significant delay of death of STAT1−/− mice. However, in another study, favipiravir protected IFNαAR−/− mice from lethal BRBV infection. In IFNαAR−/− mice, administration of 150 mg/kg of favipiravir twice daily at 3 days post-infection protected all the animals from death of BRBV infection [17]. However, in sera of these treated animals, favipiravir reached a concentration of 1.28 mM, questioning the practical use of favipiravir to treat BRBV-infected patients. The half-maximal inhibitory concentration (IC50) of favipiravir against BRBV infection of Vero cells was 310 μM [17], and in HEK293T cells was 64.48 μM [31].
In a campaign to identify antivirals against BRBV infection, myricetin, a flavonoid, was identified to effectively inhibit BRBV infection with a IC50 of 4.6 μM in HEK293T and of 20 μM in Vero cells [30], suggesting that myricetin may be a more potent inhibitor of BRBV infection than favipiravir. Myricetin is a common plant-derived flavonoid, which exhibits a wide range of activities, such as antioxidant, anticancer, antidiabetic, anti-inflammatory activities, and antimicrobial activities [40]. Notably, myricetin was an active inhibitor of the helicase activity of nsp13 of the severe acute respiratory syndrome (SARS) coronavirus (IC50=2.71 μM) [41,42], and was a strong inhibitor of the reverse transcriptase of retroviruses [43]. More importantly, myricetin does not exert obvious cytotoxicity in Vero or HEK239T cells [30], as well as in normal breast epithelial cells [41,42]. The half cytotoxic concentrations (CC50) of myricetin in HEK293T and Vero cells were 537 μM and >1 μM, respectively [30].
Using the BRBV replicon reporter, screening of an antiviral compound library of 596 bioactive antiviral compounds identified two dihydroorotate dehydrogenase (DHODH) inhibitors, hDHODH–IN–4 and brequinar, that have a high potency in inhibition of BRBV infection in HEK293T cells, which have an IC50 of 0.33 and 0.07 μM, respectively [31]. hDHODH–IN–4 and brequinar also have a pan-inhibition on replicon replication of other orthomyxoviruses, including human influenza A, swine influenza D virus, and Thogoto virus in a nanomolar range. Unfortunately, both compounds were not examined for their cytotoxicity.
Overall, potent antiviral compounds against BRBV infection have much promise to treat BRBV infection of patients. It will be curious to examine the antiviral activity of myricetin, hDHODH–IN–4, and brequinar in IFNαAR−/− or STAT1−/− mice using different BRBV isolates.
Future directions
Since the identification of BRBV in 2014, molecular epidemiology studies have confirmed Lone Star tick is the vector to transmit BRBV. The Lone Star ticks (Amblyomma Americanum) are present in North America up to the upper midwestern and northeastern regions. In the future, surveys of BRBV-close or related viruses in hard ticks in the genus Amblyomma should be carried out in other regions, in Eurasia, Africa, Australia, and Asian (https://web.archive.org/web/20100922170634/http://www.kolonin.org/4.html). A new Dhori-like thogotovirus, Oz virus, has been recently isolated from the hard tick (Amblyomma Testudinarium) in Ehime, Japan [9]. Phylogenetic analyses indicated that Oz virus is most closely related to BRBV in glycoprotein (GP) and matrix protein (M) coding genes. Interestingly, like BRBV, infection of Oz virus in C57BL/6 mice did not cause severe disease, in contrast to other thogotoviruses [11]. Human infection of Oz virus has not been reported.
With the establishment of the BRBV replicon, high throughput screen of small compounds against replication of the replicon, further validation of the candidates in virus infection cell model and animal models are the next step to develop antivirals against BRBV infection. Structure of effective compounds with the RNA-dependent RNA polymerase will be necessary to for further structure-based design of antivirals.
In addition, it is critical to understand the tropism of the virus infection through identification of the host receptor or entry factors that mediate BRBV entry. Apparently, much have to study on the life cycle of BRBV in the future.
Acknowledgements
A part of the BRBV work was supported by PHS grant AI144564 from NIAID, NIH. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We thank Dr. Wenjun Ma (University of Missouri-Columbia) for providing the pHW2000 plasmid and Dr. Zekun Wang for help in some experiments of this study. The Bourbon Virus, Original (NR-50132) was obtained through BEI Resources, NIAID, NIH.
Biography

Jianming Qiu received his Veterinary Medicine (B.S.) and Biochemistry (M.S.) degrees from Zhejiang University and his Ph.D. in Virology from China CDC, China. He did postdoctoral trainings at the Hematology Branch, NIH, Bethesda, MD, and the Department of Molecular Microbiology and Immunology, University of Missouri, Columbia, MO, USA. He currently is Professor at the Department of Microbiology, Molecular Genetics and Immunology, University of Kansas Medical Center, Kansas City, KS, USA. His research interests have focused on molecular virology, viral pathogenesis, and viral vector. He is a elected fellow of the American Academy of Microbiology.
Footnotes
Conflicts of Interest
The authors declared no conflicts of interest exist.
References
- 1.Kawaoka Y, Palese P (2006) Family Orthomyxoviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball L, editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier Academic. pp. 681–693. [Google Scholar]
- 2.HAIG DA, WOODALL JP, DANSKIN D (1965) Thogoto virus: A hitherto underscribed agent isolated from tocks in kenya. J Gen Microbiol 38: 389–394. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CR, Casals J (1973) Dhori virus, a new agent isolated from Hyalomma dromedarii in India. Indian J Med Res 61: 1416–1420. [PubMed] [Google Scholar]
- 4.Butenko AM, Leshchinskaia EV, Semashko IV, Donets MA, Mart’ianova LI (1987) [Dhori virus--a causative agent of human disease. 5 cases of laboratory infection]. Vopr Virusol 32: 724–729. [PubMed] [Google Scholar]
- 5.Lledó L, Giménez-Pardo C, Gegúndez MI (2020) Epidemiological Study of Thogoto and Dhori Virus Infection in People Bitten by Ticks, and in Sheep, in an Area of Northern Spain. Int J Environ Res Public Health 17: 2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubalek Z, Rudolf I (2012) Tick-borne viruses in Europe. Parasitol Res 111: 9–36. [DOI] [PubMed] [Google Scholar]
- 7.Filipe AR, Calisher CH, Lazuick J (1985) Antibodies to Congo-Crimean haemorrhagic fever, Dhori, Thogoto and Bhanja viruses in southern Portugal. Acta Virol 29: 324–328. [PubMed] [Google Scholar]
- 8.Bussetti AV, Palacios G, Travassos da RA, Savji N, Jain K, Guzman H, Hutchison S, Popov VL, Tesh RB, Lipkin WI (2012) Genomic and antigenic characterization of Jos virus. J Gen Virol 93: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejiri H, Lim CK, Isawa H, Fujita R, Murota K, Sato T, Kobayashi D, Kan M, Hattori M, Kimura T, Yamaguchi Y, Takayama-Ito M, Horiya M, Posadas-Herrera G, Minami S, Kuwata R, Shimoda H, Maeda K, Katayama Y, Mizutani T, Saijo M, Kaku K, Shinomiya H, Sawabe K (2018) Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: A significant phylogenetic relationship to Bourbon virus. Virus Res 249: 57–65. [DOI] [PubMed] [Google Scholar]
- 10.Lambert AJ, Velez JO, Brault AC, Calvert AE, Bell-Sakyi L, Bosco-Lauth AM, Staples JE, Kosoy OI (2015) Molecular, serological and in vitro culture-based characterization of Bourbon virus, a newly described human pathogen of the genus Thogotovirus. J Clin Virol 73: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs J, Lamkiewicz K, Kolesnikova L, Hölzer M, Marz M, Kochs G (2022) Comparative Study of Ten Thogotovirus Isolates and Their Distinct In Vivo Characteristics. J Virol 96: e0155621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, David-West TS, Kemp GE (1975) Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol 69: 49–64. [DOI] [PubMed] [Google Scholar]
- 13.Lvov DK, Karas FR, Tsyrkin YM, Vargina SG, Timofeev EM, Osipova NZ, Veselovskaya OV, Grebenyuk YI, Gromashevski VL, Fomina KB (1974) Batken virus, a new arbovirus isolated from ticks and mosquitoes in Kirghiz S.S.R. Arch Gesamte Virusforsch 44: 70–73. [DOI] [PubMed] [Google Scholar]
- 14.Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, Hunt DC, Staples JE (2015) Novel thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis 21: 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frese M, Weeber M, Weber F, Speth V, Haller O (1997) Mx1 sensitivity: Batken virus is an orthomyxovirus closely related to Dhori virus. J Gen Virol 78: 2453–2458. [DOI] [PubMed] [Google Scholar]
- 16.Savage HM, Burkhalter KL, Godsey MS Jr., Panella NA, Ashley DC, Nicholson WL, Lambert AJ (2017) Bourbon Virus in Field-Collected Ticks, Missouri, USA. Emerg Infect Dis 23: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bricker TL, Shafiuddin M, Gounder AP, Janowski AB, Zhao G, Williams GD, Jagger BW, Diamond MS, Bailey T, Kwon JH, Wang D, Boon ACM (2019) Therapeutic efficacy of favipiravir against Bourbon virus in mice. PLoS Pathog 15: e1007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage HM, Godsey MS Jr., Panella NA, Burkhalter KL, Manford J, Trevino-Garrison IC, Straily A, Wilson S, Bowen J, Raghavan RK (2018) Surveillance for Tick-Borne Viruses Near the Location of a Fatal Human Case of Bourbon Virus (Family Orthomyxoviridae: Genus Thogotovirus) in Eastern Kansas, 2015. J Med Entomol 55: 701–705. [DOI] [PubMed] [Google Scholar]
- 19.Savage HM, Godsey MS Jr., Tatman J, Burkhalter KL, Hamm A, Panella NA, Ghosh A, Raghavan RK (2018) Surveillance for Heartland and Bourbon Viruses in Eastern Kansas, June 2016. J Med Entomol 55: 1613–1616. [DOI] [PubMed] [Google Scholar]
- 20.Jackson KC, Gidlewski T, Root JJ, Bosco-Lauth AM, Lash RR, Harmon JR, Brault AC, Panella NA, Nicholson WL, Komar N (2019) Bourbon Virus in Wild and Domestic Animals, Missouri, USA, 2012–2013. Emerg Infect Dis 25: 1752–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komar N, Hamby N, Palamar MB, Staples JE, Williams C (2020) Indirect Evidence of Bourbon Virus (Thogotovirus, Orthomyxoviridae) Infection in North Carolina. N C Med J 81: 214–215. [DOI] [PubMed] [Google Scholar]
- 22.Godsey MS, Rose D, Burkhalter KL, Breuner N, Bosco-Lauth AM, Kosoy OI, Savage HM (2021) Experimental Infection of Amblyomma americanum (Acari: Ixodidae) With Bourbon Virus (Orthomyxoviridae: Thogotovirus). J Med Entomol 58: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones LD, Davies CR, Steele GM, Nuttall PA (1987) A novel mode of arbovirus transmission involving a nonviremic host. Science 237: 775–777. [DOI] [PubMed] [Google Scholar]
- 24.Molaei G, Little EAH, Williams SC, Stafford KC (2019) Bracing for the Worst - Range Expansion of the Lone Star Tick in the Northeastern United States. N Engl J Med 381: 2189–2192. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy AC, Marshall E (2021) Lone Star Ticks (Amblyomma americanum):: An Emerging Threat in Delaware. Dela J Public Health 7: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cumbie A, Trimble R, Eastwood G (2022) Pathogen spillover to an invasive tick species: First detection of Bourbon virus in Haemaphysalis longicornis in the United States. Preprints (www preprints org) doi: 10.20944/preprints202203.0387.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerx JP, Fuller F, Bishop DH (1983) Tick-borne viruses structurally similar to Orthomyxoviruses. Virology 127: 205–219. [DOI] [PubMed] [Google Scholar]
- 28.Portela A, Jones LD, Nuttall P (1992) Identification of viral structural polypeptides of Thogoto virus (a tick-borne orthomyxo-like virus) and functions associated with the glycoprotein. J Gen Virol 73: 2823–2830. [DOI] [PubMed] [Google Scholar]
- 29.Shaw MH, Palese P (2018) Orthomyxoviridae. In: Knipe DM, Howley PM, editors. Fields VIROLOGY. Philadelphia.Baltimore.New York: Wolters Kluwer/Lippincott Williams & Wilkins. pp. 1151–1185. [Google Scholar]
- 30.Hao S, Ning K, Wang X, Wang J, Cheng F, Ganaie SS, Tavis JE, Qiu J (2020) Establishment of a replicon system of the emerging tick-borne Bourbon Virus and use it for evaluation of antivirals. Front Microbiol 11: 572631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaoka S, Weisend CM, Swenson VA, Ebihara H (2022) Development of accelerated high-throughput antiviral screening systems for emerging orthomyxoviruses. Antiviral Res 200:105291. doi: 10.1016/j.antiviral.2022.105291.: 105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann E, Mahmood K, Yang CF, Webster RG, Greenberg HB, Kemble G (2002) Rescue of influenza B virus from eight plasmids. Proc Natl Acad Sci U S A 99: 11411–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogales A, Perez DR, Santos J, Finch C, Martinez-Sobrido L (2017) Reverse Genetics of Influenza B Viruses. Methods Mol Biol 1602: 205–238. [DOI] [PubMed] [Google Scholar]
- 34.Nogales A, Martinez-Sobrido L (2016) Reverse Genetics Approaches for the Development of Influenza Vaccines. Int J Mol Sci 18: ijms18010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye S, Evans JG, Stambas J (2014) Influenza reverse genetics: dissecting immunity and pathogenesis. Expert Rev Mol Med 16: e2. [DOI] [PubMed] [Google Scholar]
- 36.Engelhardt OG (2013) Many ways to make an influenza virus--review of influenza virus reverse genetics methods. Influenza Other Respir Viruses 7: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner E, Engelhardt OG, Gruber S, Haller O, Kochs G (2001) Rescue of recombinant Thogoto virus from cloned cDNA. J Virol 75: 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuchs J, Straub T, Seidl M, Kochs G (2019) Essential Role of Interferon Response in Containing Human Pathogenic Bourbon Virus. Emerg Infect Dis 25: 1304–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryu JH, Kim CH, Yoon JH (2010) Innate immune responses of the airway epithelium. Mol Cells 30: 173–183. [DOI] [PubMed] [Google Scholar]
- 40.Semwal DK, Semwal RB, Combrinck S, Viljoen A (2016) Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu MS, Lee J, Lee JM, Kim Y, Chin YW, Jee JG, Keum YS, Jeong YJ (2012) Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg Med Chem Lett 22: 4049–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keum YS, Jeong YJ (2012) Development of chemical inhibitors of the SARS coronavirus: viral helicase as a potential target. Biochem Pharmacol 84: 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono K, Nakane H, Fukushima M, Chermann JC, Barre-Sinoussi F (1990) Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur J Biochem 190: 469–476. [DOI] [PubMed] [Google Scholar]
- 44.Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB (2018) Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res 46: D708–D717. [DOI] [PMC free article] [PubMed] [Google Scholar]


