Abstract
Objective:
This study aimed to evaluate the effects of an antenatal behavioral lifestyle intervention on total gestational weight gain (GWG) and perinatal outcomes.
Methods:
Pregnant women with overweight and obesity in South Carolina were recruited into a theory-based randomized controlled trial (n = 112 intervention, n = 105 standard care), which was designed to target weight self-monitoring, increased physical activity, and improved dietary practices.
Results:
Participants were racially/ethnically diverse (44% African American). Intervention and standard care participants had similar total GWG at delivery (12.9 ± 6.9 vs. 12.4 ± 8.3 kg, respectively), but intervention participants had a smaller standard deviation (P = 0.04) in total GWG. The treatment effects were moderated by race/ethnicity and prepregnancy BMI. Among African American participants with overweight, intervention participants gained 4.5 kg less, whereas, among African American women with obesity, intervention participants gained 4.1 kg more than standard care participants. Total GWG among White participants was similar regardless of weight status and group assignment. Fewer intervention participants than standard care participants had adverse pregnancy outcomes (P ≤ 0.01).
Conclusions:
The behavioral lifestyle intervention favorably impacted GWG in African American participants with overweight but not African American participants with obesity. The intervention’s overall favorable impact on perinatal outcomes suggests that the mechanisms beyond total GWG may drive these outcomes.
Introduction
The proportion of women entering pregnancy with overweight or obesity (BMI ≥ 25 kg/m2) has been rising over the past 3 decades (1,2). Half of pregnant women in the United States exceed the Institute of Medicine (IOM)’s recommended weight gain (3). Women with overweight and obesity are two to three times more likely to exceed IOM gestational weight gain (GWG) guidelines than women with normal weight (4), and the trend of gaining excessive weight during pregnancy appears to be rising over time (5–7). Women with overweight and obesity who exceed IOM guidelines further increase their risk for adverse perinatal outcomes (8). Higher maternal GWG is also associated with higher offspring weight at birth, which persists from childhood to young adulthood (9). Limiting GWG among women with overweight and obesity holds promise to prevent obesity and to improve health status in both mothers (10) and their offspring (9).
Trials testing behavioral lifestyle interventions on limiting GWG in pregnant women with overweight and obesity have increased over the past decade. On average, these lifestyle interventions result in 1.8 kg lower GWG than comparison groups (11). A majority of these trials were conducted outside of the US, typically in countries with universal health care (11,12). In the US, African American women are disproportionately affected by overweight and obesity (13) and are also more likely to exceed weight gain recommendations during pregnancy (3,14). However, much of the extant literature targets White women (12), and, to our knowledge, only two trials have focused on African American women with overweight and obesity (15,16). Few published lifestyle intervention trials in pregnancy have been conducted in the southeastern states, where overweight and obesity rates are high (17), maternal and child health indicators are the poorest, and health disparities are most striking (18). Furthermore, several recent large trials have shown success in reducing maternal weight gain but not in reducing adverse pregnancy outcomes (APOs) (15,19–22).
The overall goal of this trial was to examine the impact of a behavioral lifestyle intervention on total GWG in White and African American women with overweight or obesity. We hypothesized that women receiving the behavioral lifestyle intervention would have less total weight gain (primary outcome), be less likely to exceed weight gain recommendations during pregnancy, be more physically active, and have lower total caloric intake (secondary outcomes) than women receiving standard care. We tested moderation by race/ethnicity and weight status in intervention effectiveness. Finally, we evaluated the intervention’s effects on perinatal outcomes.
Methods
The Health in Pregnancy and Postpartum (HIPP) study was a randomized controlled trial conducted in South Carolina. The Institutional Review Boards at participating institutes approved the study protocol. Participants provided written informed consent.
Participants
By design, we only enrolled White and African American women in order to examine racial/ethnic differences; these two groups account for the 95% of the South Carolina population (23). Potentially eligible women completed a brief screening form at obstetrician-gynecologists’ offices or via the website between January 2015 and December 2018. This initial screening assessed the following eligibility criteria: 18 to 44 years of age, gestational age ≤ 16 weeks, self-identified as a Black/African American or White individual, English-speaking, and prepregnancy BMI ≥ 25 and weight ≤ 370 lb (maximum weight assessed by scale). Study staff called initially eligible women to assess additional exclusion criteria: multiple gestation, contraindications to aerobic exercise during pregnancy (24), hospitalization for a mental health or substance abuse disorder in the past 6 months, physical disability that prevents exercise, doctor’s advice not to exercise during pregnancy, and current or previous eating disorder. Intervention-related exclusions included inconsistent phone access and unwillingness to be randomized or take part in weekly phone calls.
All participants were assessed at baseline (≤16 weeks’ gestation) and at 32 weeks’ gestation. At both measurement visits, all participants were systematically screened for new symptoms, conditions, or adverse events. If new symptoms, conditions, or adverse events were disclosed by participants outside measurement visits, a symptom form was completed. Symptoms were reviewed by the study medical monitor to determine safety of continued participation. Participants were withdrawn from the study if they had a miscarriage, still birth, or discovery of multiple gestation after randomization.
Randomization
Those who completed baseline measurement activities before 18 weeks’ gestation were randomly assigned within delivery hospital sites and by racial/ethnic group. With each stratum, for every four participants, two were randomized to the behavioral lifestyle group and two to the standard care group (allocation ratio = 1:1). A randomization list was generated by the statistician. The study coordinator randomized participants and forwarded the group assignment to intervention staff.
Behavioral lifestyle intervention
Participants in the behavioral lifestyle intervention group were encouraged to attend clinic visits with their prenatal care providers. Intervention participants were advised to follow GWG (5), physical activity (PA) (25), and dietary intake (26) guidelines for pregnant women. Specifically, GWG goals were consistent with the 2009 IOM recommendations (5). They were also advised to accumulate 150 min/wk of moderate-intensity PA (25) and to eat a diet high in fruits, vegetables, and whole grains and low in saturated and trans fats while also balancing energy intake to match, but not exceed, dietary needs for pregnancy (26). The “MyPlate Daily Checklist for Moms” (formerly the “Daily Food Plan for Moms”) was used to help participants select a balanced diet (27), and customized calorie goals were provided.
Intervention components, guided by the Social Cognitive Theory (28), have been described in detail elsewhere (29). In brief, the intervention began with an in-depth counseling session (≤18 weeks’ gestation) at which the interventionist shared the participant’s printed report of her dietary intake and PA (based on the dietary recalls and objective assessment of PA) and a personalized weight-gain-tracking graph. Participants set a PA and diet goal. Participants also received a binder of study handouts (referenced during pregnancy calls 1-10), a pedometer, and a bathroom scale.
Based on our formative work (30), we initially included 10 weekly group sessions after the in-depth counseling session. However, owing to the challenges of recruiting adequate women at one time to form a group and the less-than-ideal attendance at sessions, these 10 group sessions were replaced with 10 individual phone counseling calls with all of the content retained. Only one intervention group was conducted prior to the protocol change (n = 6). These participants were retained in analyses.
After the in-depth counseling session, participants received 10 weekly content-based phone calls and 10 weekly podcasts with content complementary to the calls. During each call, participants plotted their weight on the graph provided in the counseling session, and the interventionist engaged the participant in a discussion of a diet or PA topic along with at least one behavioral strategy. After the first 10 pregnancy counseling calls, participants received shorter weekly or biweekly counseling calls throughout their pregnancy. The total number of the shorter calls delivered varied by participants and depended on when the participant enrolled and delivered. The calls included the continued plotting of weight and an assessment of any changes in health status, discussion of progress toward PA and healthy eating goals set in the previous call, problem-solving regarding barriers to reaching goals when needed, and behavioral goal setting for the new week. In addition, all intervention participants were encouraged to join a private Facebook group (Facebook, Inc., Menlo Park, California), which was designed to allow study participants to support each other. Messages were posted each weekday to reinforce intervention content.
Standard care
Participants in this group were encouraged to attend clinic visits with their prenatal care providers. In order to enhance retention and keep participants engaged, this group received 6 monthly mailings and 10 weekly podcasts (all publicly available) focused on a healthy pregnancy or fetal development. The podcasts were matched for duration and frequency to the intervention group. Neither the mailings nor the podcasts discussed weight, PA, or diet.
Measures
At measurement visits, trained and blinded research staff assessed weight and height in duplicate to the nearest 0.1 kg or 0.1 cm by using a calibrated Seca scale and stadiometer (Seca, Hamburg, Germany). The participant wore lightweight clothing without shoes. Staff were recertified every 6 months. Staff also conducted medical chart reviews within 2 months after delivery.
GWG outcomes
Total GWG (primary outcome) was calculated as the difference between the medically abstracted weight recorded at delivery and self-reported prepregnancy weight reported at initial screening. When delivery room weight was not available, weight at the last prenatal care visit was used (n = 56), such that the mean gestational age was 38.5 weeks, an average of 4.8 days earlier than gestational age at delivery. Self-reported prepregnancy weight was highly correlated with clinic prepregnancy weight measured within a year prior to this pregnancy (Pearson correlation coefficient r = 0.95, n = 112).
The weekly rate of weight gain at delivery was calculated as the change in weight from the baseline to delivery (or last prenatal weight) divided by the number of gestational weeks between the two time points. In order to verify our method, we also calculated weekly rate of weight gain in the second and third trimesters based on methods from Lifestyle Interventions for Expectant Moms trials (21) and the weekly rate of weight gain from baseline to 32 weeks’ gestation. The alternative measures yielded similar results. Owing to space limits, only the weekly rate of weight gain at delivery was presented. Participants were also categorized as above (excessive), below (inadequate), or within (adequate) the 2009 IOM recommendations for total GWG (5).
Maternal and infant health outcomes.
Health outcomes were abstracted from medical records. Maternal health outcomes included the diagnosis of gestational diabetes, gestational hypertension, preeclampsia, cesarean delivery, and long hospital stay (>4 days for cesarean deliveries and >2 days for vaginal deliveries). The infant birth outcomes included preterm delivery (gestational age <37 weeks), low birth weight (<2,500 g), macrosomia (≥4,000 g), and low 1-minute Apgar score (<7). We further calculated APOs, which were defined as the occurrence of gestational hypertension, preeclampsia, preterm birth, or small-for-gestational-age birth (31). APOs are linked to a lifetime of higher cardiovascular disease risk for mothers (32,33).
PA.
The SenseWear Armband (CamNTech, Fenstanton, UK) was used to assess minutes per week of moderate-to-vigorous physical activity (MVPA) at baseline and 32 weeks’ gestation. The device, worn on the upper left arm, has been validated with pregnant women (34–36). The proprietary algorithms use the accelerometer and sensor data to classify intensity of activity by metabolic equivalents. If participants did not meet the wear criteria (≥5 days, ≥1 weekend day, ≥21 hours/day) or experienced an equipment failure, they were given the opportunity to re-wear the monitor.
Dietary intake.
Each participant completed two unannounced dietary recalls (1 weekday and 1 weekend day) at baseline and at 32 weeks’ gestation using the validated Automated Self-Administered 24-hour dietary recall (37). The first recall was completed at the measurement visit. Participants were notified to complete the second dietary recall. Staff-administered recall was offered to participants unable to complete the recall on their own. Data from the two recalls were averaged. Because GWG is our primary outcome, we reported energy intake (kilocalories) in this paper.
Statistical analyses
The study’s target sample size was 400 participants (200 participants/group, 200 White and 200 African American) to detect a 2.0 kg difference in total GWG between intervention and standard care participants, corresponding to a small effect size of 0.28 and assuming a two-sided type I error rate of 0.05 and 80% power.
Intent-to-treat analyses were conducted. Group (intervention vs. standard care) differences in total GWG and weekly rate of GWG were compared using multiple linear regression models that adjusted for maternal age, gestational age at baseline (continuous), race/ethnicity, Medicaid status, parity, marital status, and prepregnancy BMI category. Multiple logistic regression models were used to examine the effect of the intervention on the odds of participants exceeding or gaining weight within IOM recommendations for total GWG after adjusting for the covariates. We also examined whether treatment effects differed across categories of prepregnancy BMI and race/ethnicity by including two- and three-way interaction terms between treatment, BMI, and race/ethnicity. The log likelihood-ratio test statistic for total GWG outcome indicated that the model with interaction terms fitted the data better compared with the model without interaction (χ2 4 degrees of freedom = 9.8, P = 0.04). Considering the very different treatment effects in subgroups as well as model-fitting statistics, treatment effects were examined in four subgroups based on race/ethnicity and prepregnancy BMI (i.e., White participants with overweight, White participants with obesity, African American participants with overweight, and African American participants with obesity).
In order to examine the impacts of the intervention on changes in MVPA and energy intake from baseline to 32 weeks’ gestation and pregnancy outcomes, multiple linear or logistic regression models were used. We further examined whether the total number of content-based phone calls delivered varied by race/ethnicity, prepregnancy BMI category, and by categories of meeting IOM recommendations using t tests or ANOVA models among intervention participants. A multiple linear regression model was used to examine the relationship between the total content-based phone calls delivered and total GWG.
Results
The HIPP study randomized 228 eligible participants. Nine participants were withdrawn by the study because of medical reasons after randomization, and two participants’ medical abstractions were not completed, resulting in a final analytical sample of 217 participants (Figure 1).
Figure 1.
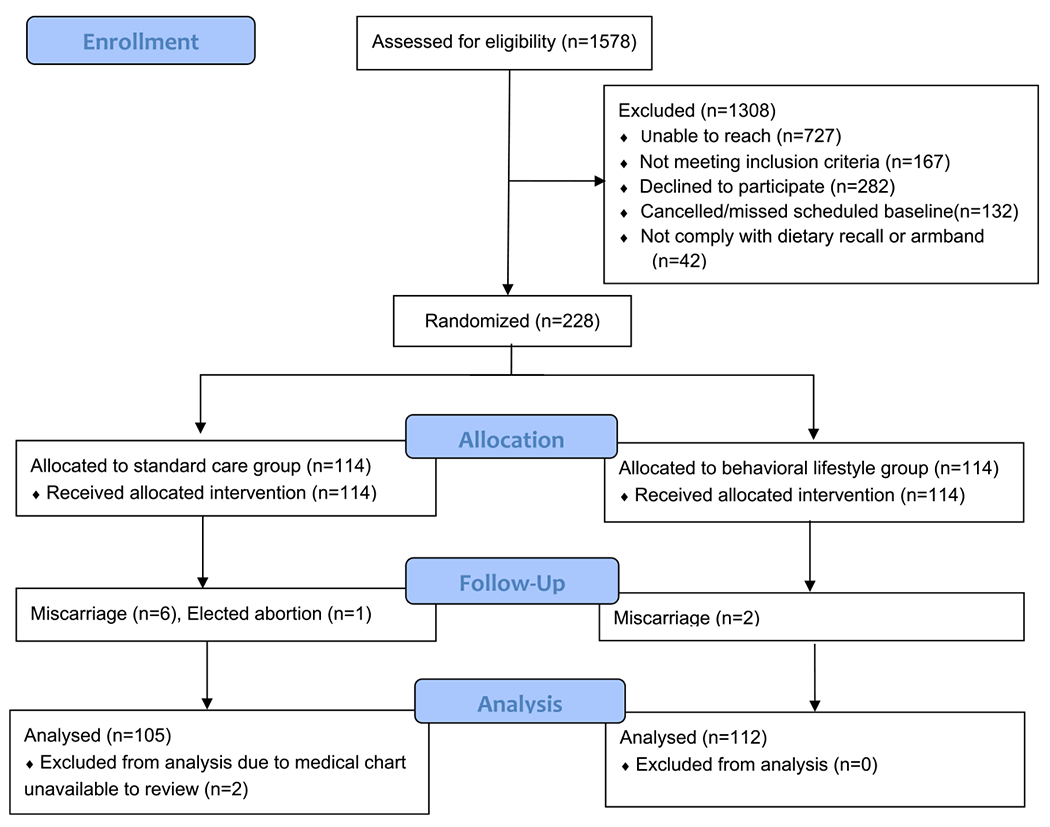
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Table 1 describes participant characteristics, which were well balanced between randomized groups at baseline. Participants were racially/ethnically diverse (55.3% White, 44.7% African American), with a mean prepregnancy BMI of 32.3 and 12.6 weeks’ gestation at baseline, and over half (51.6%) of participants had obesity prior to their pregnancy. Participant characteristics in race/ethnicity and prepregnancy BMI subgroups are shown in Supporting Information Table S1.
TABLE 1.
HIPP study participants’ baseline characteristics
| Total (n = 217) | Behavioral intervention (n = 112) | Standard care (n = 105) | |
|---|---|---|---|
| Maternal age (y), mean ± SD | 29.7 ± 5.0 | 30.4 ± 5.1 | 29.1 ± 4.8 |
| Maternal age (%) | |||
| 18-24 years | 15.7 | 12.5 | 19.1 |
| 25-29 years | 28.6 | 27.7 | 29.5 |
| 30-34 years | 37.3 | 39.3 | 35.2 |
| ≥35 years | 18.4 | 20.5 | 16.2 |
| Race/ethnicity, % | |||
| White | 55.3 | 58.0 | 52.4 |
| African American | 44.7 | 42.0 | 47.6 |
| Marital status, % | |||
| Married | 67.7 | 75.0 | 60.0 |
| Education, % | |||
| ≤12th grade or high school graduate | 40.6 | 40.2 | 40.9 |
| College, 1-3 years or more | 59.4 | 59.8 | 59.1 |
| Employed full-time during pregnancy, % | |||
| Yes | 61.3 | 61.6 | 60.9 |
| Family income, % | |||
| <$35,000 | 29.2 | 23.4 | 35.2 |
| $35,000-$49,999 | 13.9 | 15.3 | 12.4 |
| $50,000-$74,999 | 18.9 | 18.0 | 20.0 |
| ≥$75,000 | 37.9 | 43.2 | 32.4 |
| Medicaid recipient, % | |||
| Yes | 30.4 | 29.5 | 31.4 |
| Primiparous, % | 42.9 | 43.8 | 41.9 |
| Prepregnancy BMI status, % | |||
| Obesity | 51.6 | 50.0 | 53.3 |
| Prepregnancy BMI (kg/m2), mean ± SD | 32.3 ± 5.9 | 31.9 ± 5.9 | 32.7 ± 5.9 |
| Baseline weight (kg), mean ± SD | 86.9 ± 17.6 | 85.7 ± 17.5 | 88.3 ± 17.7 |
| Baseline gestational age (wk), mean ± SD | 12.6 ± 2.3 | 12.6 ± 2.3 | 12.6 ± 2.3 |
| MVPA at baseline (min/d), mean ± SD | 36.7 ± 22.4 | 38.0 ± 21.4 | 35.2 ± 23.4 |
| Total energy intake at baseline (kcal/d), mean ± SD | 1,933 ± 622 | 1,857 ± 490 | 2,014 ± 730 |
HIPP, Health in Pregnancy and Postpartum study; MVPA, moderate-to-vigorous physical activity.
GWG
Total GWG at delivery was similar in intervention versus standard care participants (12.9 ± 6.9 vs. 12.6 ± 8.3 kg), but intervention participants had a smaller SD in total GWG (unequal variance P = 0.04) (Supporting Information Figure S1). Participants in the intervention and standard care groups also had similar weekly rates of GWG at delivery (0.39 ± 0.20 vs. 0.38 ± 0.27 kg/wk; unequal variance P = 0.002). Irrespective of group, most participants exceeded the IOM guidelines (66.1% intervention group vs. 63.8% standard care group). Multiple linear or logistic regression models also showed nonsignificant treatment effects on GWG and meeting IOM guidelines (Tables 2–3). However, within the category of meeting IOM guidelines, total GWG for participants in the intervention group was more favorable (i.e., tighter distribution and right direction) than that of the standard care group (Supporting Information Figure S2).
TABLE 2.
Interactive effects of behavioral intervention, race/ethnicity, and prepregnancy BMI on GWG and behavioral outcomes
| Behavioral intervention |
Standard care |
Intervention effect |
|
|---|---|---|---|
| Outcomes | Least squares mean (95% CI)a | ||
| Total GWG at delivery (kg) b | |||
| All participants | 13.2 (11.7 to 14.6) | 12.8 (11.4 to 14.4)c | 0.3 (−1.6 to 2.3) |
| With overweight, African American | 13.1 (9.7 to 16.5) | 17.6 (14.5 to 20.6) | −4.5 (−9.9 to 0.0) |
| With obesity, African American | 12.6 (10.0 to 15.2) | 8.5 (5.8 to 11.2) | 4.1 (0.3 to 7.9) |
| With overweight, White | 14.6 (12.1 to 17.0) | 15.6 (12.7 to 18.6) | −1.0 (−4.6 to 2.5) |
| With obesity, White | 11.6 (8.8 to 14.5) | 10.0 (7.3 to 12.7) | 1.6 (−2.2 to 5.4) |
| Rate of weight gain from baseline to delivery (kg/wk) d | |||
| All participants | 0.39 (0.35 to 0.44) | 0.38 (0.34 to 0.43)c | 0.01 (−0.05 to 0.07) |
| With overweight, African American | 0.39 (0.29 to 0.50) | 0.57 (0.48 to 0.66) | −0.17 (−0.31 to −0.04) |
| With obesity, African American | 0.35 (0.27 to 0.43) | 0.24 (0.15 to 0.32) | 0.12 (0.00 to 0.23) |
| With overweight, White | 0.44 (0.37 to 0.52) | 0.44 (0.35 to 0.53) | 0.01 (−0.10 to 0.11) |
| With obesity, White | 0.36 (0.27 to 0.44) | 0.30 (0.22 to 0.38) | 0.06 (−0.06 to 0.17) |
| Change in daily moderate-to-vigorous physical activity from baseline to 32 weeks’gestation (min) | |||
| All participants | −2.6 (−7.5 to 2.4) | −7.7 (−12.7 to −2.7) | 5.1 (−1.9 to 12.1) |
| With overweight, African American | −7.0 (−19.7 to 5.6) | −8.6 (−20.1 to 2.9) | 1.6 (−15.5 to 18.7) |
| With obesity, African American | −3.4 (−13.0 to 6.2) | −7.7 (−17.6 to 2.2) | 4.3 (−9.5. 18.1) |
| With overweight, White | 0.2 (−8.1 to 8.5) | −10.0 (−19.9 to −0.2) | 10.2 (−2.6 to 23.1) |
| With obesity, White | −2.7 (−13.1 to 7.8) | −5.1 (−14.2 to 4.0) | 2.4 (−11.4 to 16.2) |
| Change in daily energy intake from baseline to 32 weeks’ gestation (kcal) | |||
| All participants | 123 (1 to 244) | 83 (−36 to 202) | 40 (−131 to 210) |
| With overweight, African American | 273 (−23 to 569) | 325 (73 to 576) | −51 (−440 to 337) |
| With obesity, African American | 258 (22 to 494) | 163 (−72 to 396) | 95 (−238 to 428) |
| With overweight, White | 34 (−167 to 235) | −50 (−277 to 177) | 84 (−220 to 387) |
| With obesity, White | 1 (−242 to 245) | −46 (−264 to 172) | 47 (−280 to 374) |
Least squares means from linear regression models including treatment group, prepregnancy BMI (with overweight or with obesity), race/ethnicity, gestation age at baseline, age, Medicaid status, parity, marital status, and interaction terms (race/ethnicity × BMI, BMI × treatment, race/ethnicity × treatment, race/ethnicity × BMI × treatment).
Total GWG was based on self-reported prepregnancy weight and measured height and was defined as the difference between self-reported prepregnancy weight and weight measured at delivery room or weight measured at the last prenatal care visit. The mean gestational age at last prenatal care visit was 38.4 ± 1.7 weeks for standard care participants and 38.5 ± 2.1 weeks for intervention participants.
P value for the variance being unequal < 0.05.
The rate of weight gain was defined as the weight difference from baseline to delivery divided by the number of weeks between observations. The mean ± SD number of weeks from baseline until the delivery date or last prenatal care visit was 26.6 ± 2.8 weeks and from baseline until 32 weeks’ gestation was 20.4 ± 2.6 weeks.
Bold indicates that P values for the intervention effects or changes from baseline to 32 weeks’ gestation were less than 0.05.
GWG, gestational weight gain.
TABLE 3.
Interactive effects of behavioral intervention, race/ethnicity, and prepregnancy BMI on meeting IOM guidelines in GWG
| Behavioral intervention |
Standard care |
Intervention effect |
|
|---|---|---|---|
| Outcomesa | Predicted percentages (95% CI)b | AOR (95% CI)b | |
| Total GWG exceeding IOM guidelines c | |||
| All participants | 66.1 (55.6-75.2) | 63.8 (53.0-73.1) | 1.11 (0.62-1.99) |
| With overweight, African American | 61.2 (36.2-81.4) | 90.3 (68.1-97.6) | 0.17 (0.03-1.00) |
| With obesity, African American | 71.6 (52.7-85.1) | 43.8 (26.3-63.1) | 3.23 (1.02-10.17) |
| With overweight, White | 67.5 (49.2-81.7) | 67.2 (45.1-83.6) | 1.02 (0.34-3.03) |
| With obesity, White | 55.4 (35.1-74.0) | 53.8 (34.5-72.1) | 1.06 (0.36-3.18) |
| Total GWG within IOM guidelines | |||
| All participants | 20.3 (13.2-30.0) | 16.7 (10.4-25.8) | 1.27 (0.64-2.55) |
| With overweight, African American | 30.4 (13.0-56.2) | 9.7 (2.4-32.2) | 4.07 (0.67-24.84) |
| With obesity, African American | 13.0 (4.8-30.6) | 22.8 (10.5-42.7) | 0.50 (0.12-2.13) |
| With overweight, White | 23.6 (11.8-41.5) | 13.9 (4.8-33.8) | 1.92 (0.52-7.02) |
| With obesity, White | 18.1 (7.2-38.5) | 19.2 (8.3-38.6) | 0.93 (0.24-3.58) |
The number of participants below IOM guidelines was insufficient to allow for estimation of BMI and race/ethnicity-specific intervention effects.
Predicted percentages, adjusted odds ratios, and their 95% CI were from multiple logistic regression models adjusting for treatment group, prepregnancy BMI (with overweight or with obesity), race/ethnicity, age, gestation age at baseline, Medicaid status, parity, marital status, and interaction terms (race/ethnicity × BMI, BMI × treatment, race/ethnicity × treatment, race/ethnicity × BMI × treatment).
IOM recommends the amount of total GWG at 40 weeks’ gestation is 6.8 to 11.3 kg for women with overweight and 5.0 to 9.0 kg for women with obesity.
Bold indicates that the P values for the intervention effects were less than 0.05.
AOR, adjusted odds ratio; GWG, gestational weight gain; IOM, Institute of Medicine.
Different treatment effects in GWG and behavioral outcomes
Table 2 shows that treatment effects were moderated by prepregnancy BMI and race/ethnicity. The treatment effect for total GWG among African American participants with overweight was in the expected direction (intervention participants gained 4.5 kg less than standard care participants), whereas, among African American participants with obesity, the treatment effect was in the opposite and unexpected direction (intervention participants gained 4.1 kg more than standard care participants). The same patterns were found for rate of weight gain at delivery. In contrast, among White participants, total GWG and rate of weight gain was similar regardless of weight status and intervention group assignment.
African American participants with overweight assigned to the intervention group had lower predicted percentage of exceeding IOM guidelines than standard care participants (61.2% vs. 90.3%), whereas the opposite effect was observed among African American participants with obesity (71.6% vs. 43.8%). For meeting IOM guidelines, the treatment effect among African American participants with overweight was also in the expected direction (30.4% vs. 9.7%), whereas the treatment effect was in the opposite direction (13.0% vs. 22.8%) among African American participants with obesity. Again, the treatment effects in meeting or exceeding IOM guidelines were not different among White participants (Table 3).
Table 2 also shows the reduction in MVPA minutes was smaller among intervention participants than standard care participants in each subgroup, but none were statistically significant. The increase in total energy intake from baseline to 32 weeks’ gestation was seen among African American participants. The difference between intervention and standard care participants was in the expected direction among African American participants with overweight but was in the opposite direction among African American participants with obesity, although neither difference was significant. Among White participants, standard care participants reduced total energy intake at 32 weeks’ gestation (−50 kcal for participants with overweight, −46 kcal for participants with obesity). White intervention participants had a small mean increase in energy intake (34 kcal) among participants with overweight and no change (1 kcal) among participants with obesity. The treatment effects on energy intake in subgroups were not significant.
Intervention dose-response analyses
All but seven participants in the intervention group attended the introductory in-depth counseling session. On average, they received 7.9 ± 3.7 calls of the 10 possible content-based phone calls during pregnancy, with call completion similar within racial/ethnic groups: African American participants with overweight (7.8 ± 3.8 calls) and African American participants with obesity (7.3 ± 4.2 calls); White participants with overweight (8.0 ± 3.4 calls) and White participants with obesity (8.5 ± 3.4 calls). On average, participants whose total GWG was within IOM guidelines received 8.7 ± 3.1 calls, which did not differ significantly from participants whose weight gain exceeded IOM guidelines (7.6 ± 3.8 calls). In the linear regression model, each additional phone call received was associated with a 0.41 kg reduction in total GWG (95% CI: −0.78 to −0.04, P = 0.03) after covariates adjustment. In each subgroup, each additional phone call was associated with a nonsignificant reduction in total GWG, ranging from −1.2 kg among African American participants with overweight (P = 0.07) to −0.18 kg among White participants with overweight (data not shown).
Maternal and infant health outcomes
Table 4 shows that participants in the intervention group had significantly fewer adverse birth outcomes (i.e., low-birth-weight babies, gestational hypertension, and APOs) than participants in the standard care group. There were no differences in other pregnancy outcomes. Owing to sample sizes, treatment effects among race/ethnicity and BMI subgroups were only examined for APOs. Treatment effects in APOs were evident among participants with obesity, White participants, and White participants with obesity (Supporting Information Table S2). Among participants who exceeded IOM’s GWG guidelines, participants in the intervention group had significantly lower percentages of adverse birth outcomes (Supporting Information Table S3).
TABLE 4.
Effect of behavioral lifestyle intervention on pregnancy outcomes
| Behavioral interventionb | Standard careb | P valuec | Intervention effect | |
|---|---|---|---|---|
| Categorical outcomes a | AOR (95% CI)d | |||
| Preterm delivery (<37 weeks) | 3 (2.7) | 8 (7.6) | 0.13 | 0.25 (0.06 to 1.10) |
| Low birth weight (<2,500 g) | 2 (1.8) | 11 (10.5) | 0.009 | 0.08 (0.01 to 0.47) |
| Macrosomia (≥4,000 g) | 12 (10.9) | 9 (8.6) | 0.65 | 1.10 (0.42 to 2.86) |
| Small for gestational age e | 9 (8.1) | 11 (10.5) | 0.64 | 0.59 (0.21 to 1.64) |
| Gestational diabetes | 8 (7.1) | 13 (12.5) | 0.25 | 0.50 (0.19 to 1.33) |
| Gestational hypertension f | 10 (8.9) | 22 (21.2) | 0.01 | 0.27 (0.11 to 0.68) |
| Adverse pregnancy outcomes g | 19 (16.9) | 33 (31.4) | 0.02 | 0.36 (0.17 to 0.74) |
| Cesarean delivery | 45 (40.5) | 40 (38.1) | 0.78 | 1.17 (0.66 to 2.07) |
| Apgar score at 1 minute <7 | 7 (6.4) | 7 (6.8) | 1.00 | 0.86 (0.27 to 2.78) |
| Long hospital stay h | 75 (68.2) | 76 (73.7) | 0.45 | 0.75 (0.40 to 1.41) |
| Continuous outcomes a | Adj. coefficient (95% CI) d | |||
| Birth weight (g) | 3,415.8 ± 535.4 | 3,285.7 ± 594.8 | 0.09 | 123.4 (−30.3 to 277.2) |
| Gestational age at delivery (wk) | 38.7 ± 1.5 | 38.5 ± 1.4 | 0.18 | 0.27 (−0.13 to 0.68) |
Numbers in bold face indicate that the P values for the intervention effects were less than 0.05.
Sample size for all outcomes was 216, except that the sample sizes for birth-weight-related outcomes (low birth weight, macrosomia, and small for gestational age) were 215.
Unadjusted proportions or means ± SD are presented.
P values represent the results of t tests for continuous variables and Fisher exact tests for categorical variables to compare the differences by treatment group.
All models were adjusted for gestation age at baseline, age, race/ethnicity, Medicaid status, parity, marital status, and prepregnancy BMI category. Reference group was standard care.
Birth weight was below the 10th percentile for babies of the same gestational age at birth and same gender.
Gestational hypertension included pregnancy-induced hypertension and preeclampsia in this pregnancy.
Adverse pregnancy outcomes included any of these three outcomes: preterm delivery, small for gestational age, and gestational hypertension.
Long hospital stay at delivery was defined as ≥ 3 days for vaginal delivery and ≥ 5 days for cesarean delivery.
adj., adjusted; AOR, adjusted odds ratio.
Discussion
Contrary to hypotheses, this theory-based behavioral lifestyle intervention did not alter total GWG, the weekly rate of weight gain, or the percentage of exceeding IOM guidelines among the full sample of pregnant women with overweight and obesity. The treatment effect, however, was modified by race/ethnicity and prepregnancy BMI status. Among African American women with overweight, the treatment effects were in the expected direction in that intervention participants gained less weight than standard care participants; however, among African American women with obesity, intervention participants gained more weight than standard care participants. The opposite treatment effects on weight gain measures observed in African American participants were consistent with the direction of the nonsignificant change in total energy intake from baseline to 32 weeks’ gestation. Furthermore, the treatment effects on GWG, diet energy intake, and MVPA were not seen among White participants regardless of their prepregnancy BMI. Despite the nonsignificant treatment effect in White participants and the opposite effect in African American participants with overweight and obesity, intervention participants had significantly better perinatal outcomes than standard care women.
The HIPP trial was developed based on formative work among African American individuals (81.3% having overweight before pregnancy) (30). Our pilot study included evidence-based behavioral change strategies delivered through traditional intervention channels (i.e., in-person and telephone-based), and more innovative intervention channels (i.e., podcast and social media support) were added in this larger trial. Similar to two other trials among African American women with overweight or obesity (15,16), the HIPP trial’s intervention messages were also tailored to unique barriers and enablers of our study population and emphasized both PA and dietary change. Intervention participants completed, on average, 79% of the content-based telephone calls, indicating moderately high adherence. The average length of phone calls was 25 min/call (range = 11-42), which was less intensive than Cahill et al.’s trial (9 home visits, with 53 min/visit [range = 44-60]) (15). In the HIPP trial, adherence to the telephone calls was unrelated to BMI status and race/ethnicity. Furthermore, participants who completed more intervention contacts gained less weight than those who did not. These results are promising and consistent with the effectiveness of these calls in helping women to control their weight gain.
Recently, researchers (22,38) have questioned the IOM’s general pregnancy guidelines of increasing daily energy intake by 340 to 450 kcal during the second and third trimesters (5,39), indicating these values might be too high for pregnant women with overweight or obesity to meet the IOM GWG guidelines (40). It is possible that the myplate.gov recommendations for pregnant women were derived from the IOM energy guidelines, which would have contributed to the higher energy intake and, in turn, weight gain in our intervention participants and null results in White participants. The myplate.gov may consider providing culturally tailored food recommendations for African American women and White women with overweight and obesity who live in the South. We call for future studies to examine the energy recommendations in relation to meeting IOM-recommended GWG for women with overweight and obesity during pregnancy. Such an energy recommendation should take into account the well-documented decline in PA during pregnancy (41).
Several lifestyle interventions effective in reducing GWG among pregnant women with overweight and obesity have included dietary components, such as partial meal replacement (20) or individually prescribed calorie goals based on height, preconception weight, PA level, and energy needs for the restricted rate of weight gain per week for the second and third trimesters (22). In the HIPP study, calorie intake was included on a handout provided at the in-depth counseling session. However, unless participants chose calorie intake as a behavioral goal, calorie intake was not tracked. A recent systematic review of intervention strategies for preventing excessive GWG among women with overweight and obesity concluded that healthy eating had a larger effect than combined healthy eating/PA in limiting GWG. The authors recommended that healthy eating with prescribed daily calorie and macronutrient goals can reduce GWG by more than 4 kg (11).
Several recent lifestyle interventions that showed success in reducing GWG among women with overweight and obesity did not show the benefits of reduced risks for adverse perinatal outcomes (15,19–22). In contrast, the HIPP trial showed significantly lower proportions of low birth weight, gestational hypertension, and APOs (including preterm births) in intervention versus standard care women. Prior interventions designed to reduce excessive weight gain led to the reduction in macrosomia but did not reduce the risk of low birth weight and small for gestational age (42). Our findings of a lower prevalence of gestational hypertension are consistent with prior lifestyle interventions showing reduced systolic and diastolic blood pressures (43). We speculate that our intervention’s impacts on reducing variability in weight gain in the intervention group may have contributed to more favorable pregnancy outcomes. The behavioral intervention tightened the distribution of weight gain by reducing both tails of extreme values that might lead to APOs. Finally, we cannot exclude the possibility that intervention participants might have been more motivated than standard care participants to improve their pregnancy outcomes. Lost to follow-up is an unlikely explanation for the better pregnancy outcomes in intervention participants because few women were lost to follow-up in this trial.
To our knowledge, this is one of the first lifestyle intervention trials in pregnant women with overweight and obesity with a high proportion of African American women living in a southeastern state of the US. The intervention targeted barriers and enablers identified in our formative work (44), including dispelling myths about the risks of exercise during pregnancy and developing content to target situations that made healthy eating difficult. This study also had nearly complete follow-up at delivery (99%). Furthermore, this study applied some innovative channels (i.e., podcasts and social media support). One limitation is that this study was underpowered to detect the differences in total GWG and racial/ethnic and BMI differences because of the difficulties in recruiting the target sample size (N = 400) in early pregnancy. Also, HIPP participants were more educated or with a lower proportion of participants on Medicaid than the general population in South Carolina (23). Therefore, our findings may not fully generalize to pregnant women with overweight or obesity in South Carolina.
Conclusion
This study did not find an intervention main effect for total GWG or the proportion of women meeting IOM-recommended weight gain, although we did find evidence for treatment moderation such that intervention effects operated in the expected direction for African American participants with overweight but in the opposite direction for African American participants with obesity. The intervention was also successful in reducing APOs, which has not been shown in published trials that were successful in reducing GWG. Furthermore, women who received a greater dose of the intervention telephone calls showed significantly more favorable weight gain outcomes. Future studies are needed to identify effective intervention strategies for healthy GWG, particularly for African American women with obesity.
Supplementary Material
Study Importance.
What is already known?
Women with overweight and obesity are two to three times more likely to exceed Institute of Medicine gestational weight gain (GWG) guidelines than women with normal weight. Women with overweight and obesity who exceed Institute of Medicine guidelines further increase their risk for adverse perinatal outcomes, and the trend of gaining excessive weight during pregnancy in this group appears to be rising over time.
In the United States, African American women are disproportionately affected by overweight and obesity and are also more likely to exceed GWG recommendations.
What does this study add?
In a diverse sample of pregnant women with overweight and obesity, the treatment effect of this theory-based behavioral lifestyle intervention was modified by race/ethnicity and prepregnancy weight status. Intervention effects operated in the expected direction for African American participants with overweight but in the opposite direction for African American participants with obesity.
The intervention was successful in reducing adverse pregnancy outcomes, which has not been shown in published trials that were successful in reducing GWG.
How might these results change the direction of research or the focus of clinical practice?
Future studies are needed to identify effective intervention strategies for healthy GWG, particularly for African American pregnant women with obesity.
Behavioral lifestyle interventions have the potential to reduce adverse pregnancy outcomes.
Acknowledgments
We thank the participating clinics for their invaluable assistance with recruiting participants. We also thank the many staff and students at the University of South Carolina who contributed to the study. Finally, we thank each of the participants who took time out of their busy lives to take part in the program.
The deidentified participant data that underlie the results reported in the article, including data dictionaries and study protocol, will be shared with the researchers who provide a methodologically sound proposal beginning 9 months and ending 36 months following article publication. Proposals should be directed to jliu@mailbox.sc.edu or wilcoxs@mailbox.sc.edu. Data requestors will need to sign a data access agreement.
Funding agencies:
The Health in Pregnancy and Postpartum (HIPP) study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award number R01HD078407. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure: The authors declared no conflict of interest.
Clinical trial registration: ClinicalTrials.gov identifier NCT02260518.
Supporting information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev Med 2013;56:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol 2015;125:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deputy NP, Sharma AJ, Kim SY. Gestational weight gain—United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep 2015;64:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen KM, Yaktine AL, eds.; Institute of Medicine and National Research Council to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. National Academies Press; 2009. [PubMed] [Google Scholar]
- 6.Johnson JL, Farr SL, Dietz PM, Sharma AJ, Barfield WD, Robbins CL. Trends in gestational weight gain: the Pregnancy Risk Assessment Monitoring System, 2000-2009. Am J Obstet Gynecol 2015;212:e801–e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wende ME, Liu J, McLain AC, Wilcox S. Gestational weight gain disparities in South Carolina: temporal trends, 2004–2015. Pediatr Perinat Epidemiol 2021;35:37–46. doi: 10.1111/ppe.12706 [DOI] [PubMed] [Google Scholar]
- 8.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 2017;317:2207–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau EY, Liu J, Archer E, McDonald SM, Liu J. Maternal weight gain in pregnancy and risk of obesity among offspring: a systematic review. J Obes 2014;2014:524939. doi: 10.1155/2014/524939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siega-Riz AM, Evenson KR, Dole N. Pregnancy-related weight gain—a link to obesity? Nutr Rev 2004;62:S105–S111. [DOI] [PubMed] [Google Scholar]
- 11.Shieh C, Cullen DL, Pike C, Pressler SJ. Intervention strategies for preventing excessive gestational weight gain: systematic review and meta-analysis. Obes Rev 2018;19:1093–1109. [DOI] [PubMed] [Google Scholar]
- 12.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med 2012;10:47. doi: 10.1186/1741-7015-10-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497. [DOI] [PubMed] [Google Scholar]
- 14.Olson CM. Achieving a healthy weight gain during pregnancy. Annu Rev Nutr 2008;28:411–423. [DOI] [PubMed] [Google Scholar]
- 15.Cahill AG, Haire-Joshu D, Cade WT, et al. Weight control program and gestational weight gain in disadvantaged women with overweight or obesity: a randomized clinical trial. Obesity (Silver Spring) 2018;26:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: a randomized clinical trial. Obesity (Silver Spring) 2016;24:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Adult obesity facts. Updated February 11, 2021. Accessed August 1, 2020. https://www.cdc.gov/obesity/data/adult.html
- 18.United Health Foundation; America’s Health Rankings. Health of Women and Children Report 2019. Published September 2019. Accessed January 28, 2020. https://www.americashealthrankings.org/learn/reports/2019-health-of-women-and-children-report
- 19.Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep 2020;20:11. doi: 10.1007/s11892-020-1296-1 [DOI] [PubMed] [Google Scholar]
- 20.Phelan S, Wing RR, Brannen A, et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. Am J Clin Nutr 2018;107:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peaceman AM, Clifton RG, Phelan S, et al. Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE-moms prospective meta-analysis. Obesity (Silver Spring) 2018;26:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Horn L, Peaceman A, Kwasny M, et al. Dietary approaches to stop hypertension diet and activity to limit gestational weight: maternal offspring metabolics family intervention trial, a technology enhanced randomized trial. Am J Prev Med 2018;55:603–614. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau. QuickFacts South Carolina. Population estimates, July 1, 2019. Accessed August 1, 2020. https://www.census.gov/quickfacts/SC
- 24.Canadian Society for Exercise Physiology. PARmed-X for Pregnancy: Physical Activity Readiness Medical Examination. Canadian Society for Exercise Physiology; 2002. [Google Scholar]
- 25.US Department of Health and Human Services. 2008. Physical Activity Guidelines for Americans. Published October 2008. Accessed January 21, 2021. https://health.gov/sites/default/files/2019-09/paguide.pdf
- 26.Procter SB, Campbell CG. Position of the Academy of Nutrition and Dietetics: nutrition and lifestyle for a healthy pregnancy outcome. J Acad Nutr Diet 2014;114:1099–1103. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Agriculture. MyPlate plan. Accessed January 21, 2021. https://www.myplate.gov/myplate-plan
- 28.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. 1st ed. Prentice-Hall; 1986. [Google Scholar]
- 29.Wilcox S, Liu J, Addy CL, et al. A randomized controlled trial to prevent excessive gestational weight gain and promote postpartum weight loss in overweight and obese women: Health in Pregnancy and Postpartum (HIPP). Contemp Clin Trials 2018;66:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Wilcox S, Whitaker K, Blake C, Addy C. Preventing excessive weight gain during pregnancy and promoting postpartum weight loss: a pilot lifestyle intervention for overweight and obese African American women. Matern Child Health J 2015;19:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. doi: 10.1186/1471-2431-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas DM, Parker CB, Marsh DJ, et al. Association of adverse pregnancy outcomes with hypertension 2 to 7 years postpartum. J Am Heart Assoc 2019;8:e013092. doi: 10.1161/JAHA.119.013092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol 2016;215:e481–e484. [DOI] [PubMed] [Google Scholar]
- 34.Berntsen S, Stafne SN, Morkved S. Physical activity monitor for recording energy expenditure in pregnancy. Acta Obstet Gynecol Scand 2011;90:903–907. [DOI] [PubMed] [Google Scholar]
- 35.Johannsen DL, Calabro MA, Stewart J, Franke W, Rood JC, Welk GJ. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc 2010;42:2134–2140. [DOI] [PubMed] [Google Scholar]
- 36.Smith KM, Lanningham-Foster LM, Welk GJ, Campbell CG. Validity of the SenseWear(R) Armband to predict energy expenditure in pregnant women. Med Sci Sports Exerc 2012;44:2001–2008. [DOI] [PubMed] [Google Scholar]
- 37.Kirkpatrick SI, Subar AF, Douglass D, et al. Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. Am J Clin Nutr 2014;100:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Invest 2019;129:4682–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Obstetricians and Gynecologists. Nutrition during pregnancy. Published June 2020. Accessed August 1, 2020. https://www.acog.org/patient-resources/faqs/pregnancy/nutrition-during-pregnancy
- 40.Committee Opinion No. 650 Summary: Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol 2015;126:1326–1327. [DOI] [PubMed] [Google Scholar]
- 41.Pereira MA, Rifas-Shiman SL, Kleinman KP, Rich-Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project Viva. Am J Prev Med 2007;32:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett CJ, Walker RE, Blumfield ML, et al. Attenuation of maternal weight gain impacts infant birthweight: systematic review and meta-analysis. J Dev Orig Health Dis 2019;10:387–405. [DOI] [PubMed] [Google Scholar]
- 43.Vinter CA, Jørgensen JS, Ovesen P, Beck-Nielsen H, Skytthe A, Jensen DM. Metabolic effects of lifestyle intervention in obese pregnant women. Results from the randomized controlled trial ’Lifestyle in Pregnancy’ (LiP). Diabet Med 2014;31:1323–1330. [DOI] [PubMed] [Google Scholar]
- 44.Goodrich K, Cregger M, Wilcox S, Liu J. A qualitative study of factors affecting pregnancy weight gain in African American women. Matern Child Health J 2013;17:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


