Abstract
Introduction
Preeclampsia (PE) is an important complication of pregnancy that can lead to chronic kidney disease. Soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), the sFlt-1/PlGF ratio and endoglin are biomarkers for the differential diagnosis of PE and other diseases. We aimed to explore the correlation of these biomarkers with long-term renal function, blood pressure and the urine albumin/creatinine ratio (UACR) in PE patients.
Methods
34 patients with PE were enrolled. Blood samples for sFlt-1, PlGF, endoglin and the urine albumin/creatinine ratio (UACR) were collected at the time of PE diagnosis (at 35–40 weeks’ gestational age (GA) (87.50% of cases). After delivery, the patients were followed up at three months and one year to assess blood pressure, renal function and the UACR.
Results
Thirty-four PE patients were included, and 17 completed the study. The estimated glomerular filtration rate (eGFR) decreased significantly at three months and one year after follow-up (128.20 ± 10.34 to 120.75 ± 10.166 ml/min/1.73 m2 (p = 0.001) at three months and 126.71 ± 9.948 to 114.29 ± 11.274 ml/min/1.73 m2 (p < 0.001) at one year). The endoglin level correlated significantly with the eGFR level during PE, but there was no correlation of any biomarker with eGFR, blood pressure, or the UACR at one year.
Conclusion
Women with PE have a reduction of eGFR at three months and one year after the diagnosis of PE. Only endoglin is correlated with eGFR antepartum; however, it is not correlated with long-term renal function, blood pressure or the UACR.
Keywords: Endoglin, Placental growth factor (PlGF), Preeclampsia, Renal failure, Soluble fms-like tyrosine kinase-1 (sFlt-1)
Highlights
-
•
Preeclampsia has been suggested to increase the risk of kidney problems.
-
•
The soluble endoglin level and sFlt-1:PlGF ratio can predict early and late-onset preeclampsia.
-
•
Analysis of angiogenic factors may be associated with long term renal function.
1. Introduction
Preeclampsia (PE) is a considerable obstetric problem and a significant source of long-term cardiovascular problems [1,2]. In general, black women and women with lower socioeconomic status are at increased risk for PE [3,4]. Because low socioeconomic status is a marked risk factor for obesity, metabolic syndrome, hypertension and cardiovascular disease, socioeconomic status is also likely to be associated with maternal hypertension [5]. PE affects approximately 4–8% of pregnancies [6]. Moreover, it has been shown that women with PE have an increased risk of hypertensive disorders and acute kidney injury, though renal function might improve at 6 weeks postpartum. PE has been shown to increase the risk of future chronic kidney disease (CKD) [7]. McDonald et al. [8] published a systematic review and meta-analysis of 7 cohort studies involving 273 patients with PE. They found a 4-fold increased risk of microalbuminuria. Furthermore, Khashan et al. [9] reported that women with PE have a 5-fold increased risk of end-stage renal disease (ESRD) compared with those without PE.
Soluble fms-like tyrosine kinase 1 (sFlt-1) is a key factor in the pathogenesis of PE and has the most prominent role in the pathology. Circulating levels of sFlt-1 correlate with PE severity, and its high levels lead to widespread maternal vascular endothelial dysfunction and hypertension or proteinuria [10,11]. Endoglin, a coreceptor for transforming growth factor β1 and β3, is upregulated in PE [12]. sFlt binds two proangiogenic proteins, namely, placental growth factor (PlGF) and vascular endothelial growth factor (VEGF). The serum levels of free PlGF and free VEGF decrease before PE develops [10,13,14]. Urinary PlGF has been explored as another positive screening test for the diagnosis of PE [15], and PlGF levels have been demonstrated to be decreased in PE both in urine and in serum [[16], [17], [18], [19]]. This is most likely due to its binding to circulating sFlt-1, whose levels are elevated [20]. The levels of sFlt-1 begin to rise in the early second trimester and as early as 10–11 weeks of gestation [21,22]. Excess sFlt-1 in patients with PE causes endothelial dysfunction and produces proteinuria, hypertension and kidney damage [20]. In a single study, Katela et al. demonstrated that antepartum sFlt-1 levels correlated with impaired renal function parameters and that a reduced glomerular filtration rate (GFR) persisted up to 6 months postpartum [23]. A significant correlation was found between a high level of serum endoglin and severe PE. A positive correlation was also found between the serum level of soluble endoglin and intrauterine growth retardation (IUGR) [24]. The soluble endoglin level and log-transformed sFlt-1:PlGF ratio tended to track together and were each associated with PE [10].
Although these antiangiogenic factors are reported to be predictive of progression to CKD, there have been no longitudinal studies to specifically examine the effects of these factors on renal function [25]. Moreover, there are still controversies regarding the predictive potential of these biomarkers as biomarkers of progression of renal function [26]. PE patients require long-term follow-up of albuminuria and renal function. The aims of this study were to investigate the relationships of sFlt-1, PlGF and endoglin with long-term renal function, blood pressure and proteinuria in women with PE.
2. Materials and methods
This was a prospective observational study conducted at the Department of Medicine and Department of Obstetrics and Gynecology, Faculty of Medicine, Navamindradhiraj University, from October 2019 to December 2020. All the participants provided informed consent, and the study was approved by the local ethics committee (Approval date December 19, 2018, No 177/2561). This work has been reported in accordance with STROCSS 2021 guidelines [27]. Also, it is registered at ClinicalTrials.Gov Identifier: NCT04940260.
The study subjects included 34 pregnant women aged 18 years or higher at 24 or more weeks of gestation, who were diagnosed with PE per the criteria recommended by the American College of Obstetricians and Gynecologists (ACOG) [28] between March 2019 and December 2020. The exclusion criteria were chronic hypertension, CKD according to the KDIGO criteria [29], twin pregnancy, or underlying diabetes mellitus. Of the 34 women enrolled in this trial, 2 patients were excluded for twin pregnancy or a history of diabetes mellitus. Fifteen women were lost to follow-up. The remaining 17 patients completed the study.
2.1. Data collection and laboratory measurements
The following baseline demographic and clinical data were recorded: age, GA at which the PE was diagnosed, blood pressure, medication history, and parity. Laboratory data included the complete blood count, blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, alkaline phosphate, and lactate dehydrogenase (LDH). We also measured 24-h urine protein excretion and calculated the random urine protein-creatinine ratio. The estimated GFR (eGFR) was determined by the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation [30]. Enzyme-linked immunosorbent assays (ELISAs) for human soluble endoglin, sFlt1, and free PlGF were conducted in duplicate (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions.
We then calculated the ratio of sFlt-1:PIGF, which is an index of antiangiogenic activity that reflects both increased sFlt-1 and decreased PlGF in women with PE and better predicts its occurrence than either protein alone [31]. We measured the sFlt-1/PlGF ratio and endoglin at the time of PE diagnosis in every patient, mostly at a GA of 35–40 weeks (87.50% of cases). All the participants were followed up at 3 months and at 1 year. At each follow-up visit, the patient's blood pressure, UACR and serum creatinine were recorded.
2.2. Investigation of study outcome
The primary outcome was the ratio of sFlt-1/PlGF together with endoglin and eGFR at 1 year postpartum. Because this was an observational study with infrequent visits, namely, only two visits in one year, we did not record the time to doubling of serum creatinine as an outcome marker. The secondary outcomes were the correlation of these biomarkers with systemic blood pressure and the UACR at 3 and 12 months.
2.3. Statistical analysis
Values are expressed as the mean ± SD or median (interquartile range) as appropriate unless otherwise indicated. Categorical variables are expressed as percentages. The comparison between eGFR at various time points (0, 3, 12 months) in the same patient was examined with the paired t-test. Pearson's correlation coefficient was calculated between individual biomarker proteins, blood pressure, and eGFR at 3 and 12 months. The biomarker level data were stratified into 4 quartiles to examine the correlation between these biomarkers and the UACR by means of the Kruskal-Wallis test. A p value less than 0.05 was regarded as representing a statistically significant difference. STATA Software, version 12 (Stata Corp, College Station, TX), was used for all calculations.
3. Results
The clinical and biochemical characteristics of the study participants are presented in Table 1. Forty-two patients who were diagnosed with PE were recruited. Eight patients withdrew from the study for personnel reasons. Two were excluded due to twin pregnancy or previous history of diabetic kidney disease with preexisting proteinuria. The remaining 32 patients were included in the study. The mean age was 28.14 ± 6.00 years, and 13 patients (40.6%) were primigravid. The mean gestational age was 37.23 ± 2.28 months (range 35–40 weeks). The mean systolic and diastolic blood pressures were 144.44 ± 12.61 mmHg and 90.28 ± 8.87 mmHg, respectively. The median UACR at the diagnosis point of PE was 70.8 (41.63–141.8) mg/g creatinine. The mean fetal weight was 2777.03 ± 407.20 g. The UACR values were above 20 mg/g creatinine in 30 (93.75%) of the participants. The renal function tests were within normal limits: the median creatinine level was 0.58 mg/dl (0.49–0.66), and the mean eGFR value was 127.00 ± 11.02 mL/min/1.73 m2. The CBC and liver function tests of all participants, except one who had severe PE with hemolysis, elevated liver enzymes and low platelet count (HELLP syndrome), were within the normal ranges. In total, 20 participants were followed up at 3 months, but only 17 patients remained in the study at 12 months. The reason was that almost all of them returned to their hometown in rural areas and due to COVID-19 pandemic with caused travel restriction in our country.
Table 1.
Baseline Characteristics of patients.
| Baseline characteristic | n | Mean ± SD, Median (IQR) |
|---|---|---|
| Age (years) | 32 | 28.41 ± 6.50 |
| Primigravid | 13 | 40.63 |
| Parity (weeks) | 32 | 37.34 ± 2.28 |
| 30 - 34 | 2 | 6.25 |
| 35 - 40 | 28 | 87.5 |
| More than 40 | 2 | 6.25 |
| Preterm | 7 | 21.88 |
| Systolic blood pressure (mmHg) | 32 | 144.44 ± 12.61 |
| Diastolic blood pressure (mmHg) | 32 | 90.28 ± 8.87 |
| BUN (mg/dl) | 32 | 9.16 ± 4.01 |
| Creatinine (mg/dl) | 32 | 0.58 (0.49–0.66) |
| Estimate glomerular filtration rate (eGFR) | 32 | 127.00 ± 11.02 |
| Urine microalbumin (mg/L) | 32 | 69.5 (33.95–185) |
| Urine creatinine (mg/dL) | 32 | 90.29 (56.83–127.94) |
| UACR (mg/g) | 32 | 70.8 (41.63–141.8) |
| Fetal weight (g) | 32 | 2777.03 ± 407.28 |
| AST (U/L) | 32 | 17 (15–23) |
| ALT (U/L) | 32 | 17 (12.5–26) |
| ALP (U/L) | 32 | 139 (128–164) |
| Albumin (g/dl) | 32 | 2.68 ± 0.49 |
| Hemoglobin (g/dL) | 32 | 12.55 (11.45–12.85) |
| White blood cell count (/uL) | 32 | 10070 (8245–12230) |
| Platelet count (/uL) | 32 | 246000 (217500–269500) |
| LDH (u/l) | 32 | 173 (156.5–188) |
BUN; blood urea nitrogen, UACR; urine albumin/creatinine ratio; AST; aspartate aminotransferase, ALT; alanine aminotransferase ALP; alkaline phosphatase, LDH; lactate dehydrogenase.
3.1. Biomarker levels
3.1.1. Endoglin
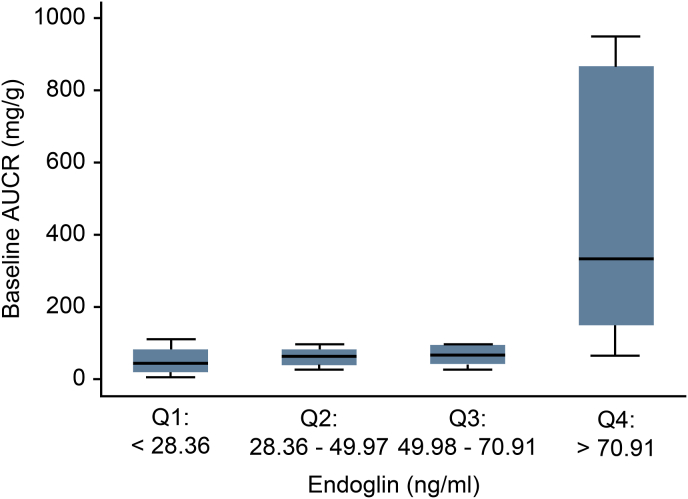
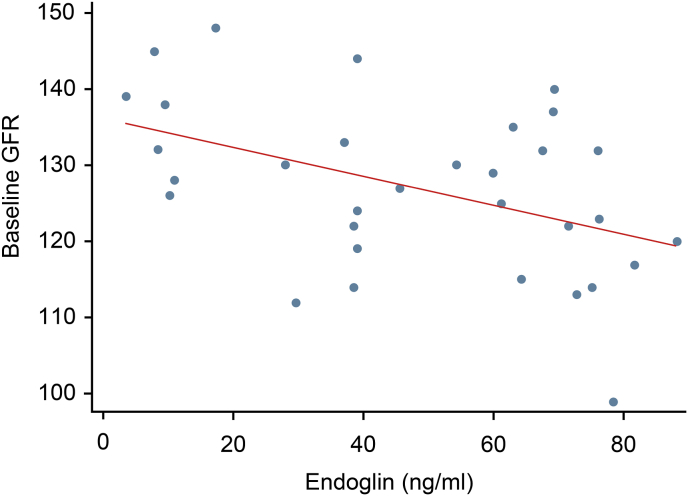
Among all 32 participants, the mean serum endoglin was 47.84 ± 26.02 ng/ml (95% CI, 38.46 to 57.22). After stratifying serum endoglin into quartiles, we found that the serum endoglin level was significantly negatively correlated with eGFR at the time of the PE diagnosis (p = 0.003) but did not show a correlation at any other time point. Serum endoglin did not show any correlation with UACR at any time point (Fig. 1, Fig. 2).
Fig. 1.
Correlation of serum endoglin with UACR at baseline.
Fig. 2.
Correlation of serum endoglin with baseline eGFR.
3.1.2. Changes in renal function, UACR and blood pressure
During the 12-month follow-up period, eGFR declined significantly from baseline (Table 2). At the end of 12 months, eGFR dropped from 126.71 ± 9.94 mL/min/1.73 m2 to 114.29 ± 11.29 mL/min/1.73 m2 (p < 0.001). At 3 months after the diagnosis of PE, eGFR significantly decreased from 128.20 ± 10.34 mL/min/1.73 m2 to 120.75 ± 10.6 mL/min/1.73 m2 (p < 0.001). Only 8 patients still had UACR above 20 mg/g creatinine at 3 months (Table 3), whereas 6 patients (35.29%) had persistent albuminuria above 20 m/g at 1 year. At 3 months, 3 patients had blood pressure greater than 140/90 mmHg (15%) and remained hypertensive at the end of 12 months. Of note, one patient in this group also had persistent albuminuria, while 14 in 17 patients at one year (82.35%) were normotensive and 11 patients (64.71%) were normoalbuminuric. At one year, one of the remaining 17 patients (5.88%) still had both albuminuria and hypertension. There was no correlation between biomarker levels and blood pressure at baseline. However, the sFLt-1 and sFLt-1/PlGF ratio had negative correlation with diastolic blood pressure at 3 months (p = 0.008 and p = 0.002 respectively) (Table 4).
Table 2.
Changes in eGFR from baseline to 3 months and 1 year.
| 3 month (n = 20) |
p-value | 1 year (n = 17) |
p-value | |||
|---|---|---|---|---|---|---|
| Baseline Mean ± SD |
3 month Mean ± SD |
Baseline Mean ± SD |
1 year Mean ± SD |
|||
| eGFR | 128.20 ± 10.34 | 120.75 ± 10.16 | 0.001 | 126.71 ± 9.94 | 114.29 ± 11.27 | <0.001 |
The baseline eGFR at each time point denoted the value of theremaining patients at that time.
Table 3.
Follow-up blood pressure and UACR from baseline to 3 and 6 months.
| Baseline |
3 month |
1 year |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Blood pressure (mmHg) | 32 (100) | 20 | 17 |
| <140/90 | 17 (85.00) | 14 (82.35) | |
| ≥140/90 | 3 (15.00) | 3 (17.65) | |
| UACR (mg/g) | 32 (100) | 20 (100) | 17 (100) |
| ≤20 | 2 (6.25) | 12 (60.00) | 11 (64.71) |
| >2 | 30 (93.75) | 8 (40.00) | 6 (35.29) |
| Blood pressure and UACR | 32 (100) | 20 | 17 |
| BP < 140/90 and UACR ≤ 20 | 18 (90.00) | 16 (94.12) | |
| BP ≥ 140/90 and UACR>20 | 2 (10.00) | 1 (5.88) |
UACR; urine albumin/creatinine ratio, BP; blood pressure.
Table 4.
Correlation between blood pressure and biomarkers at different time point.
| Baseline |
At 3 mo |
At 1 yr |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | r | p-value | n | r | p-value | n | r | p-value | |
| systolic (mmHg) | |||||||||
| sFLt-1 (pg/ml) | 32 | −0.078 | 0.67 | 20 | 0.086 | 0.719 | 17 | 0.040 | 0.880 |
| PlGf (pg/ml) | 32 | 0.106 | 0.564 | 20 | −0.158 | 0.504 | 17 | 0.190 | 0.466 |
| sFlt-1/PlGF | 32 | −0.169 | 0.355 | 20 | 0.069 | 0.771 | 17 | −0.264 | 0.307 |
| Endoglin (ng/ml) | 32 | −0.269 | 0.136 | 20 | 0.056 | 0.814 | 17 | −0.263 | 0.308 |
| diastolic (mmHg) | |||||||||
| sFLt-1 (pg/ml) | 32 | −0.070 | 0.704 | 20 | −0.576 | 0.008 | 17 | −0.170 | 0.515 |
| PlGf (pg/ml) | 32 | 0.017 | 0.928 | 20 | 0.173 | 0.465 | 17 | −0.154 | 0.556 |
| sFlt-1/PlGF | 32 | −0.116 | 0.526 | 20 | −0.646 | 0.002 | 17 | −0.085 | 0.746 |
| Endoglin (ng/ml) | 32 | 0.016 | 0.932 | 20 | −0.299 | 0.201 | 17 | −0.429 | 0.086 |
Pearson Correlation CoefficientSignificant if p < 0.05sFlt-1; soluble fms-like tyrosine kinase-1, PlGF; placental growth factor.
3.1.3. sFlt1/PIGF ratios
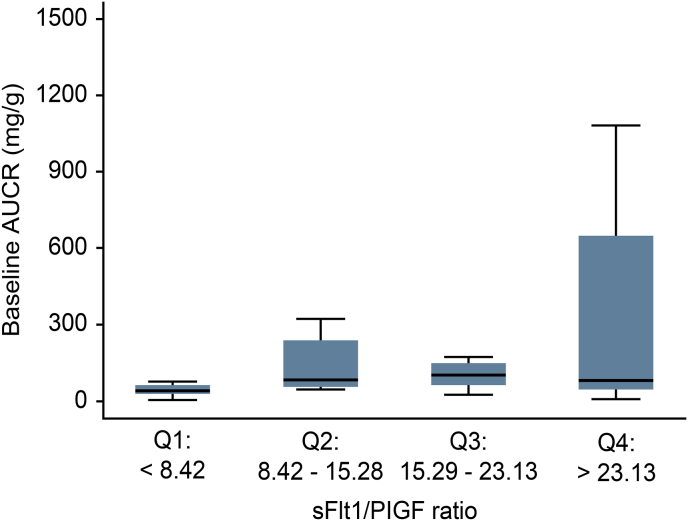
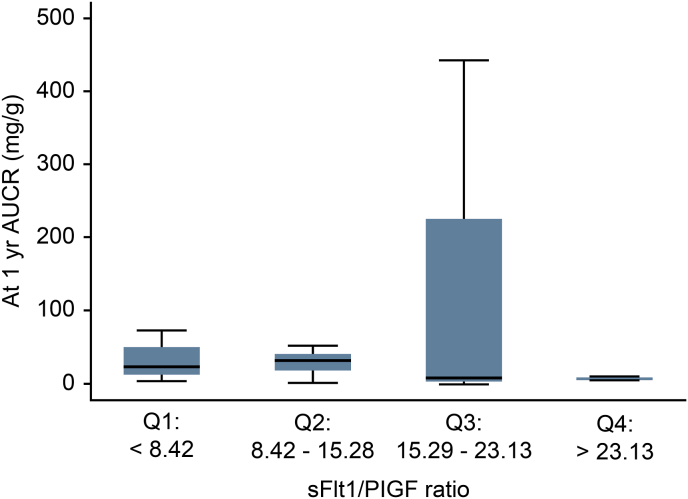
The median sFlt-1 level was 3096.35 pg/mL (range 2271.51–3978.48 pg/mL), while the PlGF level was 219.58 pg/mL (range 767.08–373.04 pg/mL). The sFtl-1/PlGF ratio was 15.70 ± 9.01 (95% CI, 12.46–18.95) (Table 5). When the sFtl-1/PlGF ratios were classified into 4 quartiles, they did not show any correlation with the UACR or eGFR (Fig. 3, Fig. 4). Each of the following quartiles comprised 25% of the patients in the study: a sFLt1/PlGF ratio below 9.14 (Q1), within 9.14–15.28 (Q2), within 15.29–22.85 (Q3) or above 22.85 (Q4). As the sFtl-1/PlGF ratio increased from quartiles 1 to 3, the UACR tended to rise but did not reach statistical significance (Fig. 3).
Table 5.
Mean and median sFLt-1, PlGF, sFlt-1/PlGF and endoglin levels.
| Measurement | n | Mean ± SD | 95%CI | Median (IQR) |
|---|---|---|---|---|
| sFlt-1 (pg/ml) | 32 | – | – | 3096.35 (2271.56–3978.44) |
| PlGF (pg/ml) | 32 | – | – | 219.58 (167.08–373.04) |
| sFlt-1/PlGF | 32 | 15.70 ± 9.01 | 12.46–18.95 | – |
| Endoglin (ng/ml) | 32 | 47.84 ± 26.02 | 38.46–57.22 | – |
Fig. 3.
sFlt-1/PIGF ratio divided into 4 quartiles plotted against the UACR at baseline and one year.
Fig. 4.
Correlation between serum endoglin and eGFR at baseline and one year.
4. Discussion
In this study, the majority of PE patients were diagnosed from 35 to 40 weeks (87.50%), and most of them had near-term or term deliveries, with only 7 cases delivering before 37 weeks. This indicated that their preeclamptic symptoms were mild or moderate in severity. The mean systolic and diastolic values of the patients in this study were in the moderate range of hypertension. The endoglin and sFtl-1 levels observed in our preeclamptic pregnancies were higher than the levels found in normal pregnancies (9.8–17.8 ng/ml for endoglin and 1130–1500 pg/mL for sFtl-1) as reported by other authors [15,31,32]. Also, the sFtl-1/PlGF ratio observed in preeclamptic pregnant women in this study was above 10, which was the cutoff level suggesting PE [33].
Our findings are consistent with the notion that these biomarker changes are a consequence of PE, making these indicators a prognostic factor for developing impaired kidney function in the long term. As PE is a generalized endothelial dysfunction syndrome that leads to hypertension, glomerular endotheliosis results in proteinuria and coagulopathy seen in the maternal syndrome of PE. The anti-angiogenic states from excessive levels of circulating anti-endothelial factors produced by the diseased placenta, such as sFlt-1 and endoglin, can cause generalized endothelial dysfunction. The consequences of endothelial dysfunction in PE are progressive proteinuria and CKD [26]. A Taiwanese study compared 26,651 women who experienced hypertensive disorders during pregnancy compared with 213,397 women with normal pregnancies.They found that women with high blood pressure had a 5.38-fold increased risk of CKD and a 12.4-fold increased risk of ESRD [34]. In a meta-analysis, McDonald et al. [8] included7 cohort studies involving 273 patients with preeclampsia and 333 uncomplicated pregnancies.They showed that at 7.1 years postpartum, women who had PE developed microalbuminuria 4.31 times as often as women with uncomplicated pregnancies (31% vs. 7%). In line with the findings of McDonald et al., we found a reduction in eGFR at 12 months postpartum in women with PE. The levels of sFlt1-1 and endoglin were moderately elevated in our study, and serum endoglin was the only marker to negatively correlate with eGFR at the time of the PE diagnosis. This is in agreement with a previous study by Cui et al. [35] who found that serum endoglin in 172 pregnant women including 110 subjects with preeclampsia and 62 normotensive pregnancies correlated with the occurrence and severity of PE. Serum creatinine was found to be significantly correlated with PE. We did not find the correlation between blood pressure and biomarkers except at 3 months postpartum, when we found that the diastolic blood pressure negatively correlated with sFLt-1 and sFLt-1/PlGF ratio. The mean blood pressure was also observed to be lowest at 3 months postpartum. This could be because of the continuation of hypertensive medications among these women.
It has been reported that serum endoglin increases at early stages of atherogenesis due to damage to endothelial cells [36]. Endoglin expression has been associated with plague neoangiogenesis, collagen production and atherosclerotic lesion stabilization [37]. Serum endoglin has been shown to have profibrotic effects in kidney disease [38]. Our study showed that endoglin had a correlation with eGFR only at the time of the PE diagnosis, since these biomarkers returned to normal postpartum. The sFlt1/PlGF ratio had no correlation with long-term renal function in our study. In contrast to sFlt-1, soluble endoglin does not interfere with VEGF-A signaling in endothelial cells but does inhibit TGF-β signaling, leading to decreased endothelial nitric oxide synthase activity and vasoconstriction in isolated rat blood vessels [12]. Since TGF-β plays a central role in renal fibrosis, endoglin should have a pivotal role in CKD progression.
Our study has several limitations. First, we did not have a control group. We used only the cutoff levels previously reported from other trials, which led to concordant results. Second, the sample size was small and decreased in size even more before completion of the study, with only 17 patients remaining in the end. This was due to the remote area of the patients’ residences, so they were reluctant to attend follow-up visits. Third, we did not repeat the biomarker measurements again at the end of the study. Thus, trials with larger sample sizes are warranted.
In conclusion, the sFlt-1/PlGF ratio and endoglin were elevated at the time of the PE diagnosis but did not correlate with long-term renal function or blood pressure. Endoglin was correlated with eGFR at the time of diagnosis. Hypertension and albuminuria were resolved in the majority of PE cases. Nevertheless, kidney function declined in these cases. Therefore, PE has a meaningful effect on long-term renal function.The present study provides a good framework for future study to explore the new biomarkers to better correlate with kidney outcomes.
Statements
This article has been presented as poster at 19th Asian Pacific Congress of Nephrology in August 2021 [39].
Statement of ethics
This study was approved by the Ethics Committee of the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University. (COA 177/2561), and this research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all participants.
Funding sources
This work was supported by grant no. 52/2562 from Navamindradhiraj University research fund.
Author contributions
T. Thitivichienlert recruited and followed up the patients, interpreted the data and wrote the manuscript.T. Trakarnvanich conceived the study and wrote the manuscript.C. Phaloprakan analyzed the data and conducted the statistical analysis.
Registration of research studies
-
1
Name of the registry: http://ClinicalTrials.Gov
-
2
ClinicalTrials.Gov Identifier: NCT04940260
-
3
Hyperlink to the registration: https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S000B2CY&selectaction=Edit&uid=U0000VTF&ts=2&cx=-emjiny
Data availability statement
All data generated and/or analyzed in this study are included in this published article.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
None of the authors have declared any competing financial interests.
Acknowledgments
The authors thank the obstetrics and gynecology nurses and staff for their help in recruiting patients and obtaining the samples and Ms Worachane Imjaijit for her assistance with statistical analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103818.
Contributor Information
Thanaphan Thitivichienlert, Email: http://ClinicalTrials.Gov.
Thananda Trakarnvanich, Email: thananda@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bellamy L., Casas J.-P., Hingorani A.D., Williams D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magee L.A., von Dadelszen P. Pre-eclampsia and increased cardiovascular risk. BMJ. 2007;335:945–946. doi: 10.1136/bmj.39337.427500.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steegers E.A., von Dadelszen P., Duvekot J.J., Pijnenborg R. Pre-eclampsia. Lancet. 2010 Aug 21;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 4.Silva L.M., Coolman M., Steegers E.A., Jaddoe V.W., Moll H.A., Hofman A., Mackenbach J.P., Raat H. Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J. Hypertens. 2008;26:1200–1208. doi: 10.1097/HJH.0b013e3282fcc36e. [DOI] [PubMed] [Google Scholar]
- 5.Silva L., Coolman M., Steegers E., Jaddoe V., Moll H., Hofman A., Mackenbach J., Raat H. Maternal educational level and risk of gestational hypertension: the Generation R Study. J. Hum. Hypertens. 2008;22:483–492. doi: 10.1038/jhh.2008.22. [DOI] [PubMed] [Google Scholar]
- 6.Rudra C.B., Williams M.A. Monthly variation in preeclampsia prevalence: Washington state, 1987–2001. J. Matern. Fetal Neonatal Med. 2005;18:319–324. doi: 10.1080/14767050500275838. [DOI] [PubMed] [Google Scholar]
- 7.Vikse B.E., Irgens L.M., Leivestad T., Skjaerven R., Iversen B.M. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 8.McDonald S.D., Han Z., Walsh M.W., Gerstein H.C., Devereaux P.J. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am. J. Kidney Dis. 2010;55:1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Khashan A.S., Evans M., Kublickas M., McCarthy F.P., Kenny L.C., Stenvinkel P., et al. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine R.J., Maynard S.E., Qian C., Lim K.-H., England L.J., Yu K.F., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 11.Chaiworapongsa T., Romero R., Kim Y.M., Kim G.J., Kim M.R., Espinoza J., et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J. Matern. Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesha S., Toporsian M., Lam C., Hanai J.I., Mammoto T., Kim Y.M., et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 13.Taylor R.N., Grimwood J., Taylor R.S., McMaster M.T., Fisher S.J., North R.A. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am. J. Obstet. Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 14.Tidwell S.C., Ho H.N., Chiu W.H., Torry R.J., Torry D.S. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am. J. Obstet. Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 15.Lam C., Lim K.H., Karumanchi S.A. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 16.Torry D.S., Mukherjea D., Arroyo J., Torry R.J. Expression and function of placenta growth factor: implications for abnormal placentation. J. Soc. Gynecol. Invest. 2003;10:178–188. doi: 10.1016/s1071-5576(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 17.Torry D.S., Wang H.S., Wang T.H., Caudle M.R., Torry R.J. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am. J. Obstet. Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 18.Taylor R.N., Grimwood J., Taylor R.S., McMaster M.T., Fisher S.J., North R.A. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am. J. Obstet. Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 19.Reuvekamp A., Velsing-Aarts F.V., Poulina I.E., Capello J.J., Duits A.J. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 20.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersinger N.A., Odegard R.A. Second- and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational age pregnancies. Acta Obstet. Gynecol. Scand. 2004;83:37–45. [PubMed] [Google Scholar]
- 22.Chappell L.C., Seed P.T., Briley A., Kelly F.J., Hunt B.J., Charnock-Jones D.S., Mallet A.I., Poston L. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am. J. Obstet. Gynecol. 2002;187:127–136. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 23.Kaleta T., Stock A., Panayotopoulos D., Vonend O., Niederacher D., Neumann M., Fehm T., Kaisers W., Fleisch M. Predictors of impaired postpartum renal function in women after preeclampsia: results of a prospective single center study. Dis. Markers. 2016;2016:7861919. doi: 10.1155/2016/7861919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Dayem T., Elagwan A., Khamis M., Kamha E., Hassan A. Correlation of serum soluble endoglin to the severity of pre-eclampsia and its effect on the pregnancy outcome. Int J Reprod Contracept Obstet Gynecol. 2016;5(5):1593–1600. [Google Scholar]
- 25.Tanabe K., Sato Y., Wada J. Endogenous antiangiogenic factors in chronic kidney disease: potential biomarkers of progression. Int. J. Mol. Sci. 2018;19:1859. doi: 10.3390/ijms19071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Balen V., Spaan J., Cornelis T., Spaanderman M. Prevalence of chronic kidney disease after preeclampsia. J. Nephrol. 2017;30:403–409. doi: 10.1007/s40620–016–0342–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew G., Agha R., for the STROCSS Group Strocss 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96:106165. doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 28.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstet. Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 29.Inker L.A., Astor B.C., Fox C.H., Isakova T., Lash J.P., Peralta C.A., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 30.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine R.J., Lam C., Qian C., Yu K.F., Maynard S.E., Sachs B.P., Sibai B.M., Epstein F.H., Romero R., Thadhani R., Karumanchi S.A., CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. 7. [DOI] [PubMed] [Google Scholar]
- 32.Leaños-Miranda A., Navarro-Romero C.S., Sillas-Pardo L.J., Ramírez-Valenzuela K.L., Isordia-Salas I., Jiménez-Trejo L.M. Soluble endoglin as a marker for preeclampsia, its severity, and the occurrence of adverse outcomes. Hypertension. 2019;74(4):991–997. doi: 10.1161/HYPERTENSIONAHA.119.13348. [DOI] [PubMed] [Google Scholar]
- 33.Hirashima C., Ohkuchi A., Arai F., Takahashi K., Suzuki H., Watanabe T., Kario K., Matsubara S., Suzuki M. Establishing reference values for both total soluble Fms-like tyrosine kinase 1 and free placental growth factor in pregnant women. Hypertens. Res. 2005;28(9):727–732. doi: 10.1291/hypres.28.727. [DOI] [PubMed] [Google Scholar]
- 34.Wang I.K., Muo C.H., Chang Y.C., Liang C.C., Chang C.T., Lin S.Y., et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ (Can. Med. Assoc. J.) 2013;185:207–213. doi: 10.1503/cmaj.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui L., Shu C., Liu Z., Tong W., Cui M., Wei C., et al. Serum protein marker panel for predicting preeclampsia. Pregnancy Hypertens. 2018;14:279–285. doi: 10.1016/j.preghy.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Li C.G., Bethell H., Wilson P.B., Bhatnagar D., Walker M.G., Kumar S. The significance of CD105, TGFβ and CD105/TGFβ complexes in coronary artery disease. Atherosclerosis. 2000;152:249–256. doi: 10.1016/s0021-9150(99)00476-1. [DOI] [PubMed] [Google Scholar]
- 37.Nachtigal P., Zemankova L., Rathouska J., Strasky Z. The role of endoglin in atherosclerosis. Atherosclerosis. 2012;224:4–11. doi: 10.1016/j.atherosclerosis.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe K., Sato Y., Wada J. Endogenous antiangiogenic factors in chronic kidney disease: potential biomarkers of progression. Int. J. Mol. Sci. 2018 Oct;19:1859. doi: 10.3390/ijms19071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poster presentations P 020. Nephrology. 2021;21(suppl1):32–55. doi: 10.1111/nep.13944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and/or analyzed in this study are included in this published article.