Abstract
Background
Adenomyosis can cause symptoms like dysmenorrhea, dyspareunia, pelvic pain and bleeding disorders and is related to subfertility and obstetrical complications. The disease is probably underestimated and underdiagnosed because of difficulties in reliable clinical examination and imaging results. The age-related prevalence of adenomyosis still remains unclear. In this retrospective analysis we describe the rate of adenomyosis in two independent cohorts of patients undergoing hysterectomy for benign diseases (2011–2013 and 2015–2018) and its correlation to presurgical symptoms respectively indications for hysterectomy.
Materials and methods
All surgeries have been performed in the same department of minimally invasive gynecological surgery by a total of two experienced surgeons following a surgical internal standard for the indication bleeding disorder, dysmenorrhea. We analyzed the overall rate of patients with adenomyosis in both cohorts and related the histological presence of adenomyosis to presurgical symptoms. We also analyzed a subgroup of postmenopausal patients with uterine prolapse.
Results
In 307 patients we detected 42.0% of cases with histologically proven adenomyosis. In the group of patients with bleeding disorders and dysmenorrhea as indication for surgery we found the highest rate of adenomyosis (59.3%, cohort 1). 81,1% patients with adenomyosis (cohort 1) reported symptoms. In the subgroup of 42 postmenopausal patients, we found 23.8% of cases with adenomyosis.
Conclusion
Our data shows that a positive anamnesis regarding the symptoms bleeding disorders and dysmenorrhea is suspicious for adenomyosis. In hysterectomy specimen adenomyosis can be found in more than 40%. The role of adenomyosis-related symptoms requires further investigation, especially in adolescent and postmenopausal patients.
Keywords: Adenomyosis, Dysmenorrhea, Bleeding disorders, Laparoscopic hysterectomy, Pelvic pain, Endometriosis, Enzian classification
Highlights
-
•
In 307 patients undergoing hysterectomy we detected 42.0% of cases with adenomyosis.
-
•
In patients with bleeding disorders and dysmenorrhea we found the highest rate of adenomyosis (59.3%).
-
•
81,1% of patients with adenomyosis reported symptoms.
-
•
In 58.9% of patients with adenomyosis we found additional fibroids.
-
•
In 42 postmenopausal patients we found adenomyosis in 23.8% of cases.
1. Introduction
Adenomyosis affects the central reproductive organ in the central female pelvis, has an important impact on womens health in reproductive age and can also cause symptoms or irregular imaging findings in postmenopausal women, especially when they are under hormon replacement therapy or endocrine treatment for breast cancer (Tamoxifen). Most of the patients with adenomyosis are symptomatic [1]. Typical symptoms in adenomyosis are dysmenorrhea, bleeding disorders, dyspareunia and pelvic pain. Adenomyosis can have a negative impact on fertility. It is related to higher abortion rates, reduced pregnancy rates and reduced birth rates, the success rate in assisted reproduction is lower in patients with adenomyosis [[2], [3], [4], [5], [6], [7]]. Adenomyosis can also be the cause for obstetrical complications like premature birth, rupture of membrane, uterine rupture and postpartum hemorrhage [[8], [9], [10]]. With the combination of anamnesis, clinical examination and imaging (transvaginal ultrasound and/or MRI) adenomyosis can be diagnosed and included to the treatment of patients with endometriosis [11,12]. The first diagnostic step is a complete and comprehensive anamnesis, as adenomyosis is related to typical symptoms. Adenomyosis might also be the reason for persistent symptoms after surgical interventions for peritoneal and deep endometriosis [13], as in almost 50% of deep endometriosis an additional adenoymosis can be found [14]. Various typical ultrasound patterns in adenomyosis have been reported in the last years: subendometrial microcysts, myometrial cysts, question mark sign, heterogenious myometrium, uterine asymmetry, hyperechoic myometrial lesions, subendometrial thickening, disrupture of the junctional zone, subendometrial linear striae and uterine enlargement [15]. It remains unclear which of these ultrasound signs have the highest importance and if a certain combination of ultrasound signs is related to a reliable prediction of adenomyosis. In a 10-year meta-analysis the pooled sensitivity (83.8%) and pooled specificity (63.9%) showed a good accuracy of the transvaginal ultrasound in the hands of the skilled examiner [16]. Additional sonographic techniques like doppler-ultrasound, elastography and 3D transvaginal ultrasound can enhance the diagnostic reliability [17,18]. These diagnostic criteria might be missing in adolescents and young women [19,20]. In order to differentiate focal and diffuse adenomyosis and to presurgically localize and measure the affected uterine tissue, MR imaging is a potential diagnostic tool with high accuracy [21]. The most important diagnostic sign in MR imaging seems to be the irregularity of the junctional zone [22], followed by focal or diffuse thickening of the junctional zone, a JZ (max) to myometrial thickness ratio >40%, areas of myometrial low-signal-intensity and high-signal-intensity spots in the T2-weighted technique [[23], [24], [25]]. Transvaginal, hysteroscopic and laparoscopic biopsy techniques can help to determine the diagnosis by obtaining a histological proof [26]. However, the prevalence of this important benign disease is not yet known exactly. The few available data report the incidence of adenomyosis in hysterectomy specimen. In our analysis we describe the prevalence in hysterectomy specimen and relate the results to the presurgical indication and age including postmenopausal patients.
2. Aim of the study
To describe the prevalence of adenomyosis in hysterectomy specimen in patients with benign symptomatic diseases and its relation to the respective indication for hysterectomy.
3. Material and methods
All surgeries have been carried out by three skilled gynecological surgeons following the same internal standard procedures in a department of gynecology in a German public hospital. In both cohorts, all patients signed an informed consent. Cohort 1 included total laparoscopic hysterectomies, laparoscopic subtotal hysterectomies, vaginal hysterectomies assisted by laparoscopy and vaginal hysterectomies. Cohort 2 only included laparoscopic supracervical hysterectomies. The work has been reported in line with the STROCCS criteria [27]. The study has been retrospectively registered in Research Registry under the UIN 7619.
3.1. Cohort 1
Retrospective single center analysis including 153 laparoscopic hysterectomies in benign uterine pathologies from 2011 to 2013. We included total laparoscopic hysterectomies, laparoscopic subtotal hysterectomies, vaginal hysterectomies assisted by laparoscopy and vaginal hysterectomies. We did not include abdominal hysterectomies as we did not realize any open procedures for benign diseases in that period. We excluded all cases suspicious for uterine malignancy or with histological proven malignancy. All hysterectomy specimen underwent standard pathological examination. We analyzed the incidence of adenomyosis in this cohort by histological proof of adenomyosis by the pathologist. Within the included patients we found one case of occult endometrial cancer in an endometrial hyperplasia related to adenomyosis and one case of endometrial hyperplasia with irregular cells. In this cohort we included patients with the indications bleeding disorders, dysmenorrhea, the combination of both and we also included patients with hysterectomies for the indication uterine descensus or prolapse in a subgroup of postmenopausal women (n = 42). The mean age in this cohort therefore was 54.9 years. The mean uterine weight was 186.9 g (with a minimum weight of 20 g and a maximum weight of 1565 g). The mean duration of the surgical procedure was 109.2 min (Table 1).
Table 1.
General information on patients of cohort 1 and 2.
| Cohort | total number of patients | median age of patients | mean uterine weight | mean surgical time |
|---|---|---|---|---|
| 1 | 153 | 54.9 years | 186.9 g | 109.2 min |
| 2 | 154 | 44.5 years | 172.8 g | 103.5 min |
3.2. Cohort 2
Retrospective single center analysis including 154 laparoscopic supracervical hysterectomies from 2015 to 2018. In this cohort, we included all patients with indication for laparoscopic supracervical hysterectomies for benign pathologies. In this cohort we did not collect the data on indication for hysterectomy. Thus, this analysis is limited to cohort 1. The uterine tissue has been evaluated by our pathological institute. No occult malignancy has been revealed by histological examination.
4. Results
In 307 patients who underwent hysterectomy in two independent analysis from 2011 to 2013 (cohort 1) and 2015–2018 (cohort 2) the histological examination revealed a total of 129 cases of adenoymosis (42.0%). The main indication for hysterectomy in both groups have been bleeding disorders without or in combination with dysmenorrhea. The mean age in cohort 1 was higher due to the fact of inlusion of postmenopausal women with the indication of pelvic floor defect (Fig. 1, Fig. 2, Fig. 3).
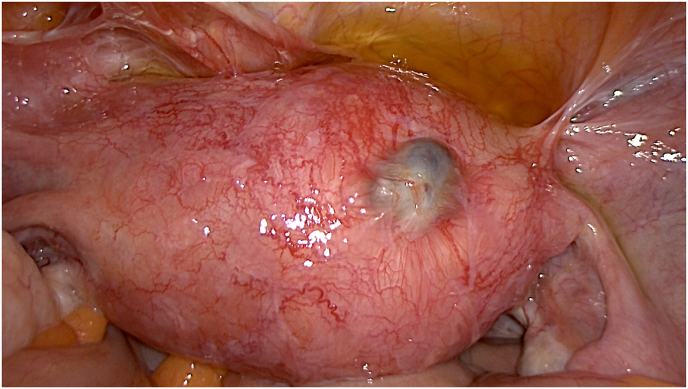
Fig. 1.
Laparoscopic appearance of adenomyotic uterus with subserous cystic adenomyosis and hypervascularization.
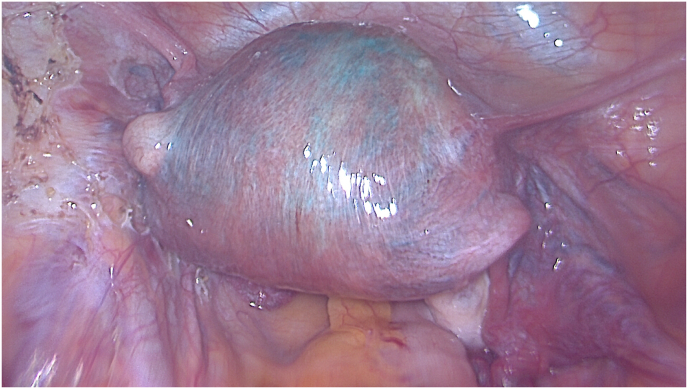
Fig. 2.
Laparoscopic blue sign in adenomyosis during test of fallopian tube permeability with blue dye. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
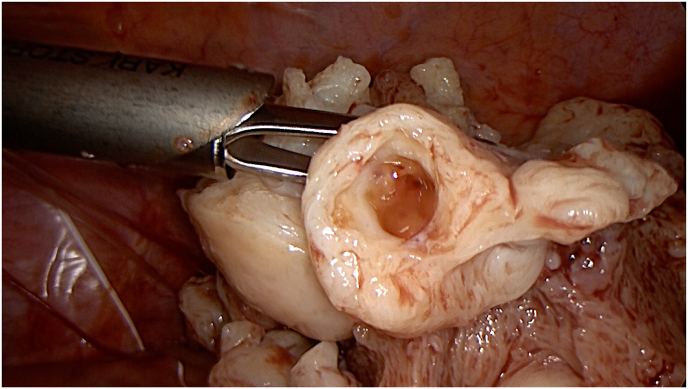
Fig. 3.
Adenomyotic uterine tissue during laparoscopic subtotal hysterectomy with laparoscopic in-bag morcellation.
4.1. Cohort 1
In this cohort of 153 patients who underwent hysterectomy for the indications dysmenorrhoa, bleeding disorders, the combination of both and uterine descensus or prolapse we found 53 cases of adenomyosis (53/153) (34.6%). Without the subgroup of patients with the indication uterine descensus or prolapse (postmenopausal patients), adenomyosis was found in 43/111 cases (38.7%). In 40/53 (75.5%) of the patients with adenomyosis an additional uterine myomatosis was diagnosed by histological examination. The mean age of all patients included was 54.9 years (29–88 years). We analyzed the incidence of adenomyosis in relation to the indication for hysterectomy (Table 2). 48.4% of the included patients presented with bleedings disorders as main indication for hysterectomy, while 17.7% presented with bleeding disorders and dysmenorrhea and 6.5% indicated pain as the main symptom. In the subgroup of patients with a combination of both symptoms (bleedings disorders and dysmenorrhea), adenomyosis was diagnosed in 59.3% of cases. Analyzing the symptoms caused by adenomyosis, the data shows that 43/53 (81.1%) patients with adenomyosis were symptomatic, reporting bleeding disorders, dysmenorrhea or a combination of both. Additional endometriosis was found in 11% of the patients with adenomyosis.
Table 2.
Indications for hysterectomy in cohort 1 and relation to adenomyosis. N = 153.
| Bleeding disorders | Dysmenorrhea | Bleeding disorders and dysmenorrhea | Uterine deszensus | |
|---|---|---|---|---|
| Indication for hysterectomy | 74/153 (48.4%) | 10/153 (6.5%) | 27/153 (17.7%) | 42/153 (27.5%) |
| Adenomyosis | 25/74 (33.8%) | 2/10(20.0%) | 16/27 (59.3%) | 10/42 (23.8%) |
4.2. Subgroup of hysterectomies with indication “bleeding disorder” (n = 74)
The mean age in this subgroup was 50.2 years. The mean uterine weight was 272.9 g and the mean surgical time 119.9 min. In most of the patients in this subgroup uterine fibroids were the main pathological finding. Pathology also revealed adenomyosis in 25 patients in this group (25/74) (33.8%). In 23/25 (92.0%) cases of patients with adenomyosis and the symptom bleeding disorder we found additional uterine myomatosis.
4.3. Subgroup of hysterectomies with indication “dysmenorrhea” (n = 10)
The mean age in this subgroup was 48.1 years. The mean uterine weight was 185.7 g and the mean surgical time 126.0 min. Pathology revealed 2 cases of adenomyosis in this group (2/10) (20.0%).
4.4. Subgroup of hysterectomies with indication “bleeding disorder and dysmenorrhea” (n = 27)
The mean age in this subgroup was 47.2 years. The mean uterine weight was 168.9 g and the mean surgical time 98.7 min. Pathology revealed 16 cases of adenomyosis in this group (16/27) (59.3%). In 11/16 (68.8%) cases of adenomyosis in this group we found additional uterine myomatosis.
4.5. Subgroup of hysterectomies with indication “uterine deszensus” (n = 42)
27.5% of the patients in cohort 1 required hysterectomy for pelvic floor defect. The mean age in this subgroup was 68.5 years. All patients in this subgroup were postmenopausal at the moment of surgery. The mean uterine weight was 52.2 g and the mean surgical time 94.5 min. Pathology revealed 10 cases of postmenopausal adenomyosis in this group (10/42) (23.8%).
4.6. Cohort 2
In this cohort of 154 patients who underwent laparoscopic supracervical hysterectomy for benign uterine pathologies, the histopathological examination revealed adenomyosis in almost every second patient (76/154; 49.4%) and uterine myomatosis in 68.2% (105/154) of the patients. In 36/76 patients, pathology reported a combination of adenomyosis and uterine myomatosis (47.4%). In 5 patients a disseminated uterine leiomyomatosis was found (3.25%) and in 4 patients any pathology was detected (2.6%). No occult malignant lesions were found in the extracted tissue (0/154).
4.7. Prediction of adenomyosis by presurgical transvaginal ultrasound
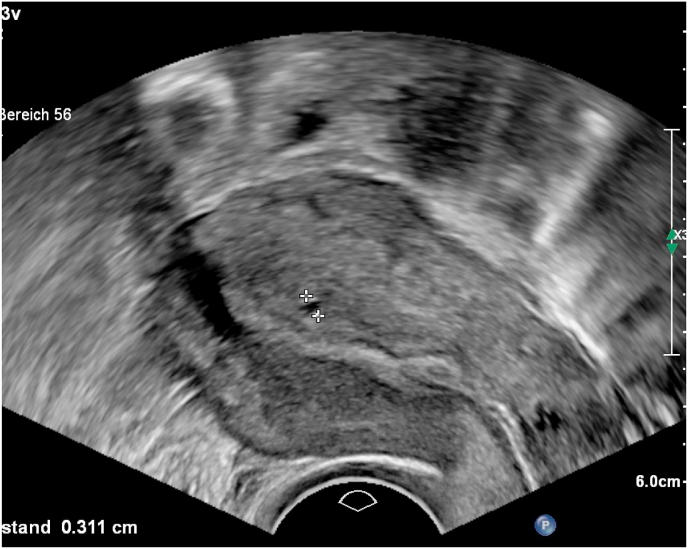
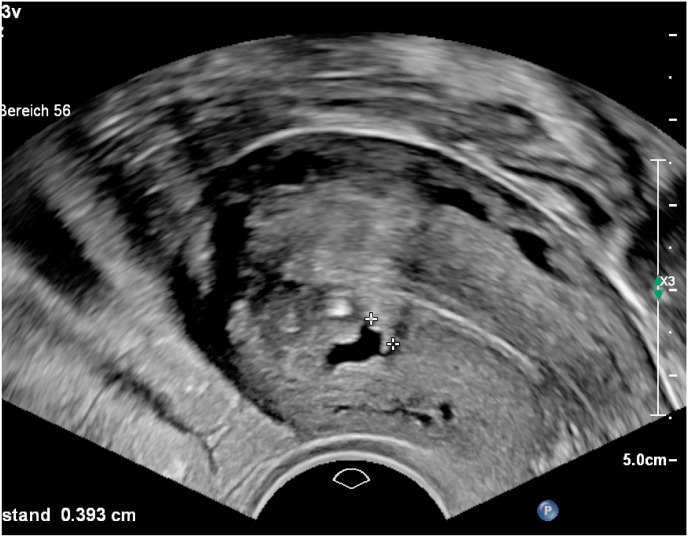
In 56/76 patients of cohort 2 we predicted adenomyosis by transvaginal 2D ultrasound examination using the sonographic patterns subendometrial microcysts, myometrial cysts, question mark sign, heterogenious myometrium, uterine asymmetry, hyperechoic myometrial lesions, subendometrial thickening, disrupture of junctional zone, subendometrial linear striae and uterine enlargement. This is a prediction rate of 73.7%. There was no false positive prediction, but 20/76 cases with adenomyosis have not been detected or suspected before surgery (Fig. 4, Fig. 5, Fig. 6).
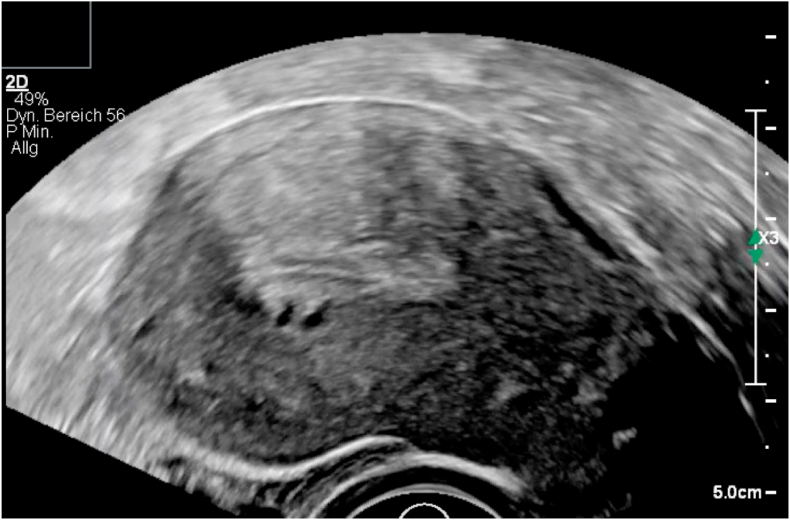
Fig. 4.
Transvaginal ultrasound in adenomyotic uterus with subendometrial cysts, hyperechoic spots, irregular myometrium.
Fig. 5.
Subendometrial microcysts in uterus with adenomyosis.
Fig. 6.
Myometrial cystic lesion in uterus with adenomyosis.
As the ultrasound prediction of adenomyosis was not the aim of the study group in cohort 2, we cannot present more detailed data on the combination of ultrasound signs in each case. From 2011 to 2013 we did not yet include presurgical transvaginal sonography prediction of adenomyosis to our standard ultrasound protocol.
5. Discussion
Our data shows that the rate of adenomyosis in patients with indication for hysterectomy is high (42%) and that adenomyosis plays an important role as a factor for uterine symptomatology leading to hysterectomy in women older than 40 years. The rate of adenomyosis is higher in cohort 2 compared to cohort 1. The reason for this difference might be the analyzed surgical approach. Laparoscopic subtotal hysterectomy with laparoscopic in-bag morcellation plays an important role in the treatment of symptomatic adenomyosis in our department. But also in cohort 1, excluding the postmenopausal patients, the rate of histologically proven adenomyosis reaches 38.7%. However, these rates do not represent the overall prevalence of adenomyosis in the female population at fertile age as the factor hysterectomy is a bias per se. In a recent large cohort-study Yu et al. described an overall adenomyosis incidence of 28.9 per 10.000 woman-years. The incidence was highest for women aged 41–45 years [28]. Taking a closer look at the symptoms, our results support the available data. In a retrospective analysis, Chen et al. reported a rate of 71.8% of the patients with adenomyosis having symptoms [29]. Our data shows that approximately 80% of pastients (cohort 1) present with symptoms. The combination of bleeding disorders and dysmenorrhea might be a predictor for the presence of adenomyosis, as in this subgroup the rate of adenomyosis was the highest in this data collection. The probability of presence of adenomyosis in patients with dysmenorrhea, and/or bleeding disorders is higher in comparison to asymptomatic patients. Li et al. recently reported a positive relation of history of cesarian section and dysmenorrhea in patients with adenomyosis [30]. Zannoni et al. described dysmenorrhea and also dyspareunia as risk factors for adenomyosis in young women [31]. Interestingly, the rate of patients with dysmenorrhea alone was relatively low in the present cohorts. A detailed anamnesis considering all typical symptoms of adenomyosis is indispensable and can easily lead to the right diagnostic decisions in order to detect adenomyosis such as transvaginal ultrasound considering the described sonographic patterns. The evaluation of additional symptoms in future studies in patients with adenomyosis like brownish irregular spotting or dyspareunia could be useful. As the biopsy techniques are not reliable enough [26], it will be difficult to design a study on the incidence of adenomyosis in adolescents and women between 20 and 35 years of age, unless a highly sensitive imaging technique is able to detect adenomyosis without a relevant false-positive or false-negative failure rate in these patient groups. In 2010, Dietrich stated that adenomyosis may be present during adolescence and she concluded that treatment should aim to preserve patients' fertility [32]. Juvenile cystic adenomyotic lesions in adolescents and its surgical and medical treatment has been described in various publications [[33], [34], [35]]. However, the role of adenomyosis in adolescents with dysmenorrhea remains unclear. Zannoni et al. reported a prevalence of 46% of adenomyosis in young women (14–24 years) in a cross-sectional study including patients with a history of pelvic pain. Young women showed a higher incidence than adolescents [30]. In a retrospective observational study, Exacoustos et al. described ultrasound features and correlation to symptoms in 43 adolescents (12–20 years) with adenomyosis. Dysmenorrhea, dyspareunia, heavy menstrual bleeding were the most reported symptoms. These results underline that adenomyosis is not just a pathology of adult life [36]. Medical and surgical treatment options of adenomyosis are able to reduce symptoms and can have a positive effect on fertility outcome [[37], [38], [39], [40]]. Which treatment of adenomyosis in adolescents and young women would be the right choice and if early diagnosis and treatment of adenomyosis can prevent symptoms and worsening of the disease in fertile life can't be answered.
Our data shows a relatively high coexistence of adenomyosis and fibroids in 58.9% of adenomyosis cases. Previous publications reported a rate of 47.6% of co-occurrence of adenomyosis and fibroids [28]. In ultrasound diagnosis of adenomyosis this fact plays an important role, as the presence of fibroids might be the reason for false-positive prediction by transvaginal ultrasound when the examiner focuses on general ultrasound patterns like uterine enlargement, asymmetry and heterogeneous myometrium. In these combined cases, doppler ultrasound is a helpful tool in order to differentiate adenomyosis from fibroids, as fibroids usually show a circular vascularization while adenomyosis presents with central vascularization.
In cohort 1 the pathological examination revealed adenomyosis in 23.8% of hysterectomy specimen in postmenopausal patients. This might be of importance in patients using hormone replacement therapy or endocrine treatment of breast cancer [41]. These treatments are able to cause an activation of the adenomyotic lesions with irregular myometrial findings in transvaginal ultrasound. Several publications show that adenomyosis and endometrial cancer can co-exist and endometrial cancer can arise in adenomyotic lesions [[42], [43], [44]]. Hermens et al. described an increased incidence of endometrial cancer in patients with endometriosis and adenomyosis in a large retrospective cohort study [45]. Adenomyosis as an estrogen-dependent disease might be a potential risk factor for myometrial or endometrial neoplasms. In clinical and sonographic examination, the differentiation between adenomyosis, endometrial cancer arising in adenomyosis and endometrial cancer coexisting with adenomyosis might be difficult in postmenopausal patients. The risk of transformation of adenomyosis probably should be discussed as an indication for hysterectomy in this subgroup. However, another recent systematic review does not support an association between adenomyosis and endometrial cancer [46].
The data of our analysis is limited to patients with an indication for hysterectomy. Women with ongoing family planning (adolescents (12–19), young women (20–40)) are not considered in this data collection. Nevertheless, our data adds to the existing literature and shows that adenomyosis plays an important role causing uterine changes including symptoms like bleeding disorders and dysmenorrhea and may probably lead to subfertility, obstetrical complications and finally to organ loss. The high prevalence is these cohorts is alarming as it shows that adenomyosis plays an important role in uterine pathology. Reliable diagnostic tools are needed in order to detect adenomyosis as early as possible. The group of postmenopausal patients with adenomyosis also require further investigations as the role of adenomyosis in the development of endometrial cancer is not yet fully understood. Finally, a consensus on adenomyosis classification would be useful in order to differentiate different types of adenomyosis and correlate them to symptoms, severity and therapeutical approach and make further investigations comparable.
6. Conclusion
In patients undergoing hysterectomy for uterine symptomatology adenomyosis can be found in approximately 40% of cases. In women with uterine bleeding disorders and dysmenorrhea adenomyosis should be suspected. Further investigations are needed in order to evaluate the incidence and impact of adenomyosis in adolescents, young women and postmenopausal women.
Ethical approval
The internal ethics board decided that an approval is not necessary due to the retrospective data collection design of the study.
Sources of funding
There is no funding.
Author contribution
Both authors contributed equally to the study design, the data analysis and the writing. HK was mainly responsible for the data collection.
Research registration Unique Identifying number (UIN)
Name of the registry: Research Registry.
Unique Identifying number or registration ID: 7619.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://researchregistry.knack.com/research-registry#home/registrationdetails/62002bb120a3a0001e151e1c/
Guarantor
HK.
Ethics approval
The local IRB decided that the manuscript does not require ethics approval as it is a retrospective data collection (April 20, 2020).
Data availability
The clinical data used to support the findings of this study are stored at Clinic of Gynecology, Obstetrics and Gynecological Oncology, Bethesda Hospital Duisburg, Academic Teaching Hospital, Duisburg, Germany and are available from corresponding author upon request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103809.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li X., Liu X., Guo S.W. Clinical profiles of 710 premenopausal women with adenomyosis who underwent hysterectomy. J. Obstet. Gynaecol. Res. 2014 Feb;40(2):485–494. doi: 10.1111/jog.12211. Epub 2013 Oct 22. PMID: 24148010. [DOI] [PubMed] [Google Scholar]
- 2.Younes G., Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil. Steril. 2017 Sep;108(3):483–490. doi: 10.1016/j.fertnstert.2017.06.025. e3. PMID: 28865548. [DOI] [PubMed] [Google Scholar]
- 3.Salim R., Riris S., Saab W., Abramov B., Khadum I., Serhal P. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod. Biomed. Online. 2012 Sep;25(3):273–277. doi: 10.1016/j.rbmo.2012.05.003. Epub 2012 May 23. PMID: 22832421. [DOI] [PubMed] [Google Scholar]
- 4.Tremellen K., Thalluri V. Impact of adenomyosis on pregnancy rates in IVF treatment. Reprod. Biomed. Online. 2013 Mar;26(3):299–300. doi: 10.1016/j.rbmo.2013.01.013. PMID: 23472864. [DOI] [PubMed] [Google Scholar]
- 5.Thalluri V., Tremellen K.P. Ultrasound diagnosed adenomyosis has a negative impact on successful implantation following GnRH antagonist IVF treatment. Hum. Reprod. 2012 Dec;27(12):3487–3492. doi: 10.1093/humrep/des305. Epub 2012 Sep 20. PMID: 22997247. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S., Bathwal S., Agarwal N., Chattopadhyay R., Saha I., Chakravarty B. Does presence of adenomyosis affect reproductive outcome in IVF cycles? A retrospective analysis of 973 patients. Reprod. Biomed. Online. 2019 Jan;38(1):13–21. doi: 10.1016/j.rbmo.2018.09.014. Epub 2018 Oct 26. PMID: 30446308. [DOI] [PubMed] [Google Scholar]
- 7.Stanekova V., Woodman R.J., Tremellen K. The rate of euploid miscarriage is increased in the setting of adenomyosis. Hum. Reprod Open. 2018 Jul 4;2018(3) doi: 10.1093/hropen/hoy011. Erratum in: Hum Reprod Open. 2019 Jan 29;2019(1):hoy026. PMID: 30895252; PMCID: PMC6276689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buggio L., Monti E., Gattei U., Dridi D., Vercellini P. Adenomyosis: fertility and obstetric outcome. A comprehensive literature review. Minerva Ginecol. 2018 Jun;70(3):295–302. doi: 10.23736/S0026-4784.17.04163-6. Epub 2017 Nov 7. PMID: 29115118. [DOI] [PubMed] [Google Scholar]
- 9.Vlahos N.F., Theodoridis T.D., Partsinevelos G.A. Myomas and adenomyosis: I mpact on reproductive outcome. BioMed Res. Int. 2017;2017:5926470. doi: 10.1155/2017/5926470. Epub 2017 Nov 6. PMID: 29234680; PMCID: PMC5694987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada T., Khine Y.M., Kaponis A., Nikellis T., Decavalas G., Taniguchi F. The impact of adenomyosis on women's fertility. Obstet. Gynecol. Surv. 2016 Sep;71(9):557–568. doi: 10.1097/OGX.0000000000000346. PMID: 27640610; PMCID: PMC5049976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krentel H., Cezar C., Becker S., et al. From clinical symptoms to MR imaging: diagnostic steps in adenomyosis. BioMed Res. Int. 2017;2017:1514029. doi: 10.1155/2017/1514029. Epub 2017 Dec 4. PMID: 29349064; PMCID: PMC5733957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapron C., Vannuccini S., Santulli P., et al. Diagnosing adenomyosis: an integrated clinical and imaging approach. Hum. Reprod. Update. 2020 Apr 15;26(3):392–411. doi: 10.1093/humupd/dmz049. PMID: 32097456. [DOI] [PubMed] [Google Scholar]
- 13.Ferrero S., Camerini G., Menada M.V., Biscaldi E., Ragni N., Remorgida V. Uterine adenomyosis in persistence of dysmenorrhea after surgical excision of pelvic endometriosis and colorectal resection. J. Reprod. Med. 2009 Jun;54(6):366–372. PMID: 19639926. [PubMed] [Google Scholar]
- 14.Lazzeri L., Di Giovanni A., Exacoustos C., et al. Preoperative and postoperative clinical and transvaginal ultrasound findings of adenomyosis in patients with deep infiltrating endometriosis. Reprod. Sci. 2014 Aug;21(8):1027–1033. doi: 10.1177/1933719114522520. Epub 2014 Feb 14. PMID: 24532217. [DOI] [PubMed] [Google Scholar]
- 15.Van den Bosch T., Dueholm M., Leone F.P., et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015 Sep;46(3):284–298. doi: 10.1002/uog.14806. Epub 2015 Aug 10. PMID: 25652685. [DOI] [PubMed] [Google Scholar]
- 16.Andres M.P., Borrelli G.M., Ribeiro J., Baracat E.C., Abrão M.S., Kho R.M. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2018 Feb;25(2):257–264. doi: 10.1016/j.jmig.2017.08.653. Epub 2017 Aug 30. PMID: 28864044. [DOI] [PubMed] [Google Scholar]
- 17.Benagiano G., Brosens I., Habiba M. Adenomyosis: a life-cycle approach. Reprod. Biomed. Online. 2015 Mar;30(3):220–232. doi: 10.1016/j.rbmo.2014.11.005. Epub 2014 Nov 20. PMID: 25599903. [DOI] [PubMed] [Google Scholar]
- 18.Krentel H., De Wilde R.L. Adenomyosis: diagnostics and treatment. Gynäkologe. 2020;53:683–688. doi: 10.1007/s00129-020-04655-7. [DOI] [Google Scholar]
- 19.Hung Y.C., Westfal M.L., Chang D.C., Kelleher C.M. Lack of data-driven treatment guidelines and wide variation in management of chronic pelvic pain in adolescents and young adults. J. Pediatr. Adolesc. Gynecol. 2020 Aug;33(4):349–353. doi: 10.1016/j.jpag.2020.03.009. e1. Epub 2020 Apr 4. PMID: 32259629. [DOI] [PubMed] [Google Scholar]
- 20.Myszko O., Al-Husayni N., Talib H.J. Painful periods in the adolescent girl. Pediatr. Ann. 2020 Apr 1;49(4):e176–e182. doi: 10.3928/19382359-20200318-01. PMID: 32275762. [DOI] [PubMed] [Google Scholar]
- 21.Agostinho L., Cruz R., Osório F., Alves J., Setúbal A., Guerra A. MRI for adenomyosis: a pictorial review. Insights Imaging. 2017 Dec;8(6):549–556. doi: 10.1007/s13244-017-0576-z. Epub 2017 Oct 4. PMID: 28980163; PMCID: PMC5707223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tellum T., Matic G.V., Dormagen J.B., et al. Diagnosing adenomyosis with MRI: a prospective study revisiting the junctional zone thickness cutoff of 12 mm as a diagnostic marker. Eur. Radiol. 2019 Dec;29(12):6971–6981. doi: 10.1007/s00330-019-06308-3. Epub 2019 Jul 1. PMID: 31264010. [DOI] [PubMed] [Google Scholar]
- 23.Bazot M., Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018 Mar;109(3):389–397. doi: 10.1016/j.fertnstert.2018.01.024. PMID: 29566851. [DOI] [PubMed] [Google Scholar]
- 24.Bazot M., Cortez A., Darai E., et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum. Reprod. 2001 Nov;16(11):2427–2433. doi: 10.1093/humrep/16.11.2427. PMID: 11679533. [DOI] [PubMed] [Google Scholar]
- 25.Novellas S., Chassang M., Delotte J., et al. MRI characteristics of the uterine junctional zone: from normal to the diagnosis of adenomyosis. AJR Am. J. Roentgenol. 2011 May;196(5):1206–1213. doi: 10.2214/AJR.10.4877. PMID: 21512093. [DOI] [PubMed] [Google Scholar]
- 26.Movilla P., Morris S., Isaacson K. A systematic review of tissue sampling techniques for the diagnosis of adenomyosis. J. Minim. Invasive Gynecol. 2020 Feb;27(2):344–351. doi: 10.1016/j.jmig.2019.09.001. Epub 2019 Sep 6. PMID: 31499191. [DOI] [PubMed] [Google Scholar]
- 27.Mathew G., Agha R., for the Strocss Group STROCSS 2021: strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int. J. Surg. 2021;96:106165. doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 28.Yu O., Schulze-Rath R., Grafton J., Hansen K., Scholes D., Reed S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am. J. Obstet. Gynecol. 2020 Jul;223(1):94. doi: 10.1016/j.ajog.2020.01.016. e1-94.e10, Epub 2020 Jan 15. PMID: 31954156. [DOI] [PubMed] [Google Scholar]
- 29.Chen Q., Li Y.W., Wang S., et al. Clinical manifestations of adenomyosis patients with or without pain symptoms. J. Pain Res. 2019 Nov 14;12:3127–3133. doi: 10.2147/JPR.S212117. PMID: 31814754; PMCID: PMC6861517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Q., Huang J., Zhang X.Y., Feng W.W., Hua K.Q. Dysmenorrhea in patients with adenomyosis: a clinical and demographic study. J. Gynecol. Obstet. Hum. Reprod. 2021 Mar;50(3):101761. doi: 10.1016/j.jogoh.2020.101761. Epub 2020 Apr 20. PMID: 32325268. [DOI] [PubMed] [Google Scholar]
- 31.Zannoni L., Del Forno S., Raimondo D., et al. Adenomyosis and endometriosis in adolescents and young women with pelvic pain: prevalence and risk factors. Minerva Pediatr. 2020 Jun 16 doi: 10.23736/S0026-4946.20.05842-9. Epub ahead of print. PMID: 32549030. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich J.E. An update on adenomyosis in the adolescent. Curr. Opin. Obstet. Gynecol. 2010 Oct;22(5):388–392. doi: 10.1097/GCO.0b013e32833cefaf. PMID: 20613517. [DOI] [PubMed] [Google Scholar]
- 33.Ho M.L., Ratts V., Merritt D. Adenomyotic cyst in an adolescent girl. J. Pediatr. Adolesc. Gynecol. 2009 Jun;22(3):e33–e38. doi: 10.1016/j.jpag.2008.05.011. PMID: 19539194. [DOI] [PubMed] [Google Scholar]
- 34.Mansouri R., Santos X.M., Bercaw-Pratt J.L., Dietrich J.E. Regression of adenomyosis on magnetic resonance imaging after a course of hormonal suppression in adolescents: a case series. J. Pediatr. Adolesc. Gynecol. 2015 Dec;28(6):437–440. doi: 10.1016/j.jpag.2014.12.009. Epub 2014 Dec 29. PMID: 26233288. [DOI] [PubMed] [Google Scholar]
- 35.Brosens I., Gordts S., Habiba M., Benagiano G. Uterine cystic adenomyosis: a disease of younger women. J. Pediatr. Adolesc. Gynecol. 2015 Dec;28(6):420–426. doi: 10.1016/j.jpag.2014.05.008. Epub 2014 May 28. PMID: 26049940. [DOI] [PubMed] [Google Scholar]
- 36.Exacoustos C., Lazzeri L., Martire F.G., et al. Ultrasound findings of adenomyosis in adolescents: type and grade of the disease. J. Minim. Invasive Gynecol. 2021 Aug 28:S1553–S4650. doi: 10.1016/j.jmig.2021.08.023. (21)00409-X, Epub ahead of print. PMID: 34464760. [DOI] [PubMed] [Google Scholar]
- 37.Vannuccini S., Luisi S., Tosti C., Sorbi F., Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil. Steril. 2018 Mar;109(3):398–405. doi: 10.1016/j.fertnstert.2018.01.013. PMID: 29566852. [DOI] [PubMed] [Google Scholar]
- 38.Pontis A., D'Alterio M.N., Pirarba S., de Angelis C., Tinelli R., Angioni S. Adenomyosis: a systematic review of medical treatment. Gynecol. Endocrinol. 2016 Sep;32(9):696–700. doi: 10.1080/09513590.2016.1197200. Epub 2016 Jul 5. PMID: 27379972. [DOI] [PubMed] [Google Scholar]
- 39.Sunkara S.K., Khan K.S. Adenomyosis and female fertility: a critical review of the evidence. J. Obstet. Gynaecol. 2012 Feb;32(2):113–116. doi: 10.3109/01443615.2011.624208. PMID: 22296416. [DOI] [PubMed] [Google Scholar]
- 40.Dueholm M. Uterine adenomyosis and infertility, review of reproductive outcome after in vitro fertilization and surgery. Acta Obstet. Gynecol. Scand. 2017 Jun;96(6):715–726. doi: 10.1111/aogs.13158. PMID: 28556124. [DOI] [PubMed] [Google Scholar]
- 41.Cohen I., Beyth Y., Shapira J., et al. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol. Obstet. Invest. 1997;44(3):200–205. doi: 10.1159/000291520. PMID: 9359649. [DOI] [PubMed] [Google Scholar]
- 42.Yuan H., Zhang S. Malignant transformation of adenomyosis: literature review and meta-analysis. Arch. Gynecol. Obstet. 2019 Jan;299(1):47–53. doi: 10.1007/s00404-018-4991-2. Epub 2018 Dec 5. PMID: 30519753. [DOI] [PubMed] [Google Scholar]
- 43.Mao X., Zheng W., Mao W. Malignant changes in adenomyosis in patients with endometrial adenocarcinoma: a case series. Medicine (Baltim.) 2017 Oct;96(43) doi: 10.1097/MD.0000000000008336. PMID: 29069006; PMCID: PMC5671839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habiba M., Pluchino N., Petignat P., Bianchi P., Brosens I.A., Benagiano G. Adenomyosis and endometrial cancer: literature review. Gynecol. Obstet. Invest. 2018;83(4):313–328. doi: 10.1159/000487320. Epub 2018 Jun 6. PMID: 29874641. [DOI] [PubMed] [Google Scholar]
- 45.Hermens M., van Altena A.M., Velthuis I., et al. Endometrial cancer incidence in endometriosis and adenomyosis. Cancers. 2021 Sep 13;13(18):4592. doi: 10.3390/cancers13184592. PMID: 34572823; PMCID: PMC8464914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raffone A., Seracchioli R., Raimondo D., et al. Prevalence of adenomyosis in endometrial cancer patients: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 2021 Jan;303(1):47–53. doi: 10.1007/s00404-020-05840-8. Epub 2020 Oct 23. PMID: 33098006; PMCID: PMC7854401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data used to support the findings of this study are stored at Clinic of Gynecology, Obstetrics and Gynecological Oncology, Bethesda Hospital Duisburg, Academic Teaching Hospital, Duisburg, Germany and are available from corresponding author upon request.