Abstract
Microglia has been reported to be able to regulate the proliferation, differentiation and survival of adult neural stem/progenitor cells (NSPCs) by modulating the microenvironment, which results in different consequences of adult neurogenesis. However, whether the microglial activation is beneficial or harmful to NSPCs is still controversial because of the complexity and variability of microglial activation phenotypes. In this study, we systematically explored the activation phenotypes of IFN-γ- or IL-4-induced microglia at different time after stimulation, and investigated the effects of the secretome of different phenotype of microglia on the process of proliferation, differentiation and survival of NSPCs. Moreover, the possible molecular pathways of secretory influence on NSPCS were further explored using western blotting. The result showed that IFN-γ and IL-4 differently regulate microglial phenotypes, IL-4 induced a M2-like phenotype, while IFN-γ induced a M1-like phenotype. These phenotypes of microglia can only be maintained for 24 h after removal of IFN-γ or IL-4 intervention. The secretome from IFN-γ- or IL-4-induced microglia also had opposite effects on NSPCs proliferation, differentiation and survival. The secretome from the IL-4-treated microglia promoted NSPCs proliferation, survival and differentiation into neurons and oligodendrocytes, while factors secreted by the INF-γ-treated microglia stimulated the NSPCs differentiation into astrocyte, inhibited the neurogenesis and oligodendrogliogenesis, and induced NSPCs apoptosis. Furthermore, the PI3K-Akt pathway mediates the effects of the secretome from IFN-γ- or IL-4-induced microglia on NSPC proliferation, differentiation, and survival. In conclusion, our results suggested that the secretome of microglia induced by IL-4 of IFN-γ differently regulate proliferation, differentiation and survival of adult neural stem/progenitor cell by targeting the PI3K-Akt pathway. These findings will help further study the biological mechanism of microglia regulating neurogenesis, and provide a therapeutic strategy for neurological diseases by regulating microglial phenotypes to affect neurogenesis.
Keywords: Microglia; Interferon-gamma, Interleukin-4, Neural stem/progenitor cell; Neurogenesis; PI3K/Akt pathway
Introduction
Neural stem/progenitor cells (NSPCs) are multipotent cells that renew themselves and could differentiate into neurons and macro glia of the nervous system during embryonic development (Toni et al. 2008). They are associated with learning, memory, and emotional behavior and play an important role in the neuropathological progression of mammals, including humans (Chu et al. 2015). Therefore, regulating the function of adult NSPCs has great potential for the treatment of various neurodegenerative diseases. However, the neurobiological behaviors of NSPCs, such as proliferation or differentiation are affected by the environment where they reside. Thus, it is important to find effective ways to promote NSPCs’ proliferation, differentiation and survival.
Microglia are central nervous system (CNS)-resident immune cells and play a crucial role in the CNS by participating in local inflammatory reaction, regulating homeostasis of brain and microenvironment of neurogenesis, and modulating synapse formation (Paolicelli et al. 2011). It had been reported that microglia regulate proliferation, differentiation and survival of NSPCs by modulating the microenvironment where they reside (Li and Barres 2018). Microglia are known to polarize into two reciprocate forms in response to external pro-inflammatory (M1) state and anti-inflammatory (M2) state (Taylor et al. 2014). M1 microglia disrupts the internal environment by release a variety of inflammatory factors, such as tumor necrosis factor alpha (TNF-α) and interferon γ (IFN-γ) (Kawabori and Yenari 2015). In contrast, M2 microglia plays a neuroprotective role by secreting anti-inflammatory mediators and neurotrophic factors involved in homeostasis (Zhao et al. 2013). Modulation of microglial phenotype is an appealing neurotherapeutics.
However, the effects of M1 and M2 microglia on NSPCs are controversial. IFN-γ signaling synergizes microglia derived from human induced pluripotent stem cells (Panagiotakopoulou et al. 2020). LPS-activated microglia enhance neurogenesis via trypsinogen secretion (Nikolakopoulou et al. 2013). Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects (Zhang et al. 2020). Interferon-γ differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice (Mastrangelo et al. 2009). This discrepancy may reflect the complexity and variability of the microglia activation phenotype (Hu et al. 2012). Our previous work revealed that IFN-γ- or IL-4-induced microglia is extremely plastic in morphology, molecular properties and function (Jiang et al. 2021). These phenotypes of microglia did not maintain a long time after removal of the stimulus intervention. We hypothesized that this might be why microglia with the same phenotype has opposite effects on NSPCs.
In this study, we systematically explored the activation phenotypes of IFN-γ- or IL-4-induced microglia at different time after stimulation, and investigated the effect of the secretome of different phenotype of microglia on the process of proliferation, differentiation and survival of NSPCs. And the possible molecular pathways of secretory influence on NSPCS were further explored.
Materials and methods
Microglia culture
Primary cultures of microglia cells were performed, as described previously (Yuan et al. 2017). In brief, the cerebral hemispheres were obtained from of neonatal (P0–P3) C57BL/6 J mice, minced tissue was enzymatically dissociated to a single cell suspension using 0.125% pancreatin, and the suspension was filtered with a 70 µm cell strainer. Filtrate after centrifugation prepared a cell suspension in DMEM/F12 containing 1% double antibodies and 10% FBS, incubated at 37 °C, 5% CO2. Individual microglia were collected on the 14th day by briefly shaking the mixed glial cultures.
Adult NSPCs culture
Adult NSPCs were obtained from the SVZ of 8-weeks-old C57BL/6 J male mice accordingly to a protocol previously described (Matsui and Mori 2018). Briefly, the entire SVZ region from mice was dissected and the lateral wall of the lateral ventricles was carefully removed from the surrounding brain tissue into PBS. These tissues were chopped into 1 mm cubes and digested in 0.125% trypsin for 5 min, stopped from digestion with soybean trypsin inhibitor. The cells were re-suspended in DMEM/F12 (Gibco, Grand Island, NY, USA) complete medium containing 20 ng/mL recombinant murine FGF (PeproTech, NJ, USA), 20 ng/mL recombinant murine EGF (PeproTech, Rocky Hill, NJ, USA), 1% N2 (Gibico, CA, USA) and 2% B-27 supplement (Gibico, CA, USA). After culture for 7 days, the neurospheres were isolated by centrifugation (600×g), enzymatically dissociated to a single cell suspension using 0.25% pancreatin, and plated at a density of 5 × 104 cells/cm2 in the proliferation medium. To permit serial cell passages, this pancreatin-dissociation process was repeated every 3 to 4 days.
Polarization of microglia
The microglia were plated at a density of 5 × 10 5 cells/cm2 and treated with either IFN-γ (50 ng/mL, Sigma, MO, USA), IL-4 (20 ng/mL, Sigma, MO, USA) or PBS. Microglia were treated by IFN-γ or IL-4 for 24 h, 48 h and 72 h, and the area of each microglia was measured by image J software (version 1.45 J; National Institutes of Health, Bethesda, MD, USA). Activated microglia were stained by immunofluorescence, and the genes and protein expression levels of anti-inflammatory markers (Arg-1, TGF-β, YM-1, CD206 and IL-10) and pro-inflammatory markers (INF-α, iNOS, BDNF, IL-1β and CCR2) were detected at 24 h, 48 h and 72 h after treatment.
NSPCs conditional culture and co-culture with microglia
The microglia were plated at a density of 5 × 105 cells/cm2 and treated with either IFN-γ or IL-4 for 24 h, microglia cells were washed by PBS twice then added DMEM/F12 (Gibco, Grand Island, NY, USA) + GlutaMax culture medium for 24 h. The microglial medium supernatant was collected and made conditioned medium for differentiation and proliferation of NSPCs. NSPCs cultured in different conditioned medium from PBS-, IL-4- or IFN-γ-treated microglia and control medium added PBS, IFN-γ and IL-4. The proliferation, differentiation and survival of NSPCs were detected by immunofluorescence staining. The neurosphere co-cultured with microglia for 24 h by Transwell as previous described (Zhang et al. 2020).
Immunofluorescence
The cells (including microglia, neurosphere, proliferative NSPCs and differential NSPCs) were plated at a density of 1 × 10 5 cells/cm2 and fixed with 4% paraformaldehyde (PFA; pH 7.2) for 30 min. The cells were permeabilized with 0.5% Triton X-100 in PBS for 15 min. Then the cells were blocked in 10% donkey serum for 2 h and incubated with primary antibodies (goat anti-Iba1, 1:400, Abcam, FCE, UK; rabbit anti-Arg-1, 1:400, Abcam; rabbit anti-iNOS, 1:50, Santa Cruz Biotechnology, Santa Cruz, USA; mouse anti-BrdU, 1:400, Cell Signaling Technology, BOS, USA; mouse anti-MAP2, 1:400, Cell Signaling Technology; mouse anti-NG2, 1:400, Cell Signaling Technology; mouse anti-GFAP, 1:400, Cell Signaling Technology; anti-CC3, 1:150, Cell Signaling Technology) overnight at 4℃, and further incubated with fluorescent-dye-conjugated secondary antibodies (Dy Light 549-conjugate donkey anti-goat, 1:300, Jackson Immuno Research; Dy Light 488-conjugate donkey anti-mouse, 1:300, Jackson Immuno Research) for 2 h at room temperature. Subsequently, the cells were incubated with DAPI (1:10,000, Roche Life Science, Basel, Switzerland) for 5 min and imaged using a fluorescent microscope (Olympus BX51). The Iba1+ area was quantized by Image J software (version 1.45 J; National Institutes of Health, Bethesda, MD, USA).
Real-time polymerase chain reaction (RT-PCR)
Cell plates of each group were selected and washed with PBS. Total ribonucleic acid (RNA) was isolated by using the Trizol reagent (Life Technologies, CA, USA), according to the standard program. RT-qPCR was performed using a First Strand cDNA Synthesis Kit (TaKaRa, Shiga, Japan), according to the manufacturer’s instructions. The threshold amplification cycle number (Ct) was determined for each reaction by Quantitative RT-PCR (Bio-Rad CFX 96, Bio-Rad Laboratories, CA, USA) in the linear phase of the amplification plot. Each sample was tested in triplicate, and an analysis of gene expression was performed using the -ΔΔCt method. The values were normalized against the housekeeping genes β-actin. The primer sequences of each gene are described in Table 1.
Table 1.
Primers used in quantitative RT-PCR
| Primer name | Sequence |
|---|---|
| β-actin forward | 5′-CCGTGAAAAGATGACCCAGATC-3′ |
| β-actin reverse | 5′-GGACATGCTGAAGCGCACTT-3′ |
| IL-1β forward | 5′-GGCAACTGTTCCTGAACTCAACTG-3′ |
| IL-1β reverse | 5′-CCATTGAGGTGGAGAGCTTTCAGC-3′ |
| TNF-α forward | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ |
| TNF-α reverse | 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ |
| iNOS forward | 5′-CAAGAGTTTGACCAGAGGACC-3′ |
| iNOS reverse | 5′-TGGAACCACTCGTACTTGGGA-3′ |
| CCR2 forward | 5′-CTCAGTTCATCCACGGCATAC-3′ |
| CCR2 reverse | 5′-GACAAGGCTCACCATCATCG-3′ |
| IL-10 forward | 5′-TGGCCCAGAAATCAAGGAGC-3′ |
| IL-10 reverse | 5′-CAGCAGACTCAATACACACT-3′ |
| TGF-β forward | 5′-GACCGCAACAACGCCATCTA-3′ |
| TGF-β reverse | 5′-GGCGTATCAGTGGGGGTCAG-3′ |
| Arg-1forward | 5′-AGACAGCAGAGGAGGTGAAGAG-3′ |
| Arg-1reverse | 5′-CGAAGCAAGCCAAGGTTAAAGC-3′ |
| CD206 forward | 5′-AGTTGGGTTCTCCTGTAGCCCAA-3′ |
| CD206 reverse | 5′-ACTACTACCTGAGCCCACACCTGCT-3′ |
| YM1 forward | 5′-CATTCAGTCAGTTATCAGATTCC-3′ |
| YM1 reverse | 5′-AGTGAGTAGCAGCCTTGG-3′ |
| BDNF forward | 5′-GAGCTGAGCGTGTGTGACAG-3′ |
| BDNF reverse | 5′-CGCCAGCCAATTCTCTTTTTGC-3′ |
ELISA
Enriched microglia were cultured in 6-well culture plate at 5 × 105 cells/cm2, and treated with either IFN-γ, IL-4 or PBS. 24 h or 48 h after continuous stimulation or cessation of stimulation, the medium was collected, and the enriched microglia were fractured by a cell lysis buffer (Solarbio, Beijing, China), and the lysates were centrifuged at 1000×g for 30 min. The supernatants were used to examine the cytokine concentration. The concentrations of Arg-1, iNOS, TNF-α, TGF-β, IL-10 and IL-1β were quantified using ELISA kits (QuantiCyto, Shenzhen, China) according to the manufacturer’s protocol. The detection limit for them was 8 pg/mL.
Western blotting
The NSPCs were lysed by RIPA lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) then centrifuged at 1000×g for 30 min. The concentration of total protein was measured by the BCA method. Equal amount of protein was resolved using 12% SDS polyacrylamide gel. Fractionated proteins were transferred onto PVDF membranes at 300 mA for 30 min, then the membrane was washed in TBST, blocked in skim milk for 30 min, and incubated overnight on a shaker at 4 °C with primary antibody: GAPDH (1:1000, Abcam, FCE, UK), PI3K (1:800, Abcam, FCE, UK), Akt (1:800, Abcam, FCE, UK) or phosphorylated Akt (p-TrkB) (1:400, Abcam, FCE, UK). Then the membrane was washed three times with TBST, incubated with secondary antibody (1:10,000; Abcam, FCE, UK) for 30 min and washed with TBST again. Finally, bands were visualized using the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics GmbH, Mannheim, Germany). The ChemiDoc Touch system (Bio-rad, CA, USA) was used to analyze and photograph the gels, and the Alpha software processing system (version 1.45 J; National Institutes of Health, Bethesda, MD, USA) was used to analyze the optical density of target bands.
Statistical analysis
All of the data were expressed as means ± SEMs. The statistical analyses were performed using Graghpad prism (version 5; GraphPad). Individual comparisons were assessed using Student's two-tailed t test, and multiple comparisons were performed with two-way or one-way analysis of variance and Tukey’s post-hoc tests. Statistically significant differences were defined as P < 0.05.
Result
IFN-γ and IL-4 differently regulate microglial phenotypes
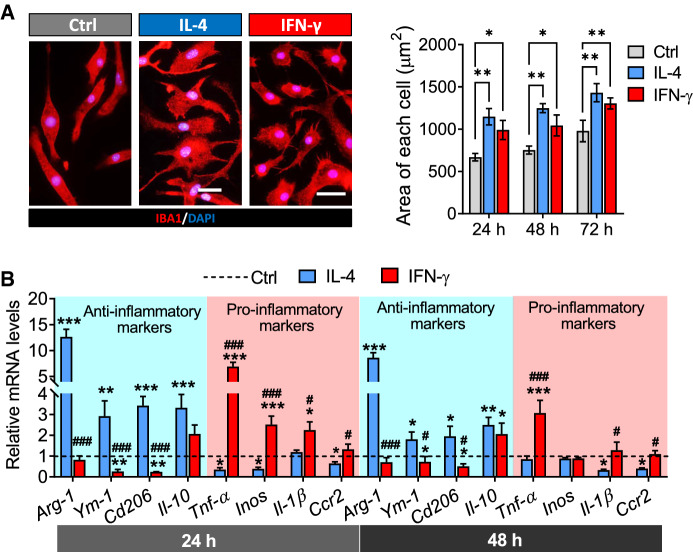
First, we assessed the shape and area of microglia with different phenotypes. The untreated microglia were fusiform, while the microglia induced by IFN-γ exhibited oval shape and thorny convexity, and the microglia activated by IL-4 were multiramose. In addition, the results showed that the area of microglia stimulated with IL-4 or IFN-γ for 24 h, 48 h and 72 h were larger than the area of untreated microglia (Fig. 1A).
Fig. 1.
Characterization of microglia under IFN-γ or IL-4 intervention conditions. A The fluorescence micrographs of microglia exposed in PBS, IFN-γ or IL-4 for 24 h. Microglia were stained with IBA1 (red) by immunocytochemistry and nuclei were stained with DAPI (blue). Scale bar is 10 μm. And the histogram shows the area of each microglia. B Levels of mRNA encoding anti-inflammatory markers (Arg-1, YM-1, CD206 and IL-10) and pro-inflammatory markers (TNF-α, iNOS, IL-1β and CCR2) in microglia after treatment with PBS, IFN-γ or IL-4 for 24 h and 48 h. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01, ***P < 0.001 vs Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs IL-4 group
The result of gene expression showed that the expression of anti-inflammatory markers (Arg-1, YM-1, CD206 and IL-10) markedly increased while the expression of pro-inflammatory markers (TNF-α, iNOS, IL-1β and CCR2) decreased after treated by IL-4 for 24 h and 48 h in microglia. In contrast, we found that the expression of anti-inflammatory makers (Arg-1, YM-1 and CD206) was decreased while the expression of pro-inflammatory markers (TNF-α, iNOS and IL-1β) was increased after treated by IFN-γ for 24 h and 48 h in microglia (Fig. 1B).
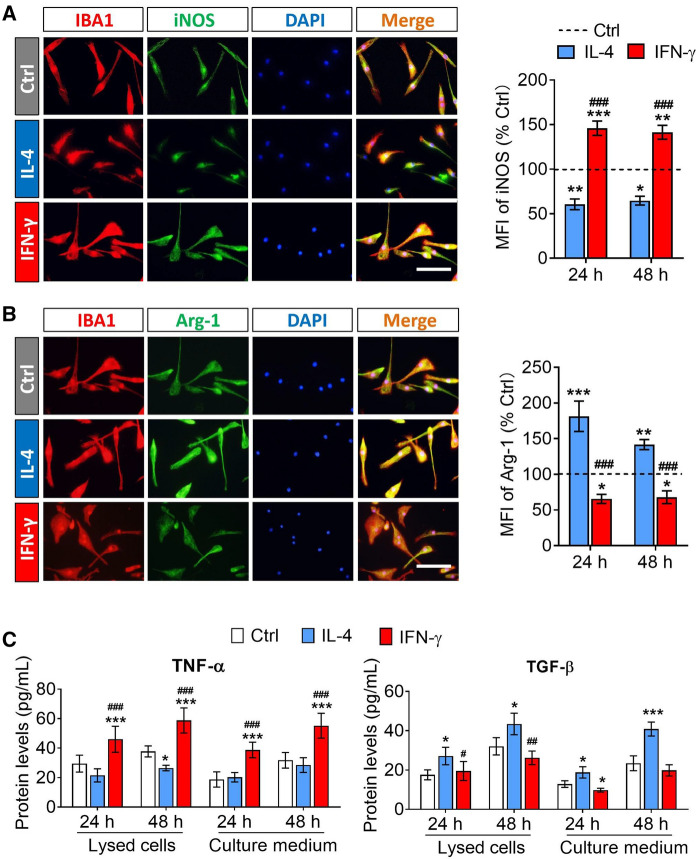
The result of protein expression showed that the IL-4 treatment decreased the expression of iNOS in microglia, but increased the expression of Arg-1. Meanwhile, an opposite trend was observed in IFN-γ-activated microglia (Figs. 2A and B). The result of ELISA indicated that the intracellular and extracellular protein concentrations of TNF-α in IFN-γ-activated microglia were significantly higher than those in untreated microglia at 24 h or 48 h after stimulation. While the protein concentrations of TGF-β in IL-4-treated microglia were significantly higher than those in untreated microglia at 24 h or 48 h after stimulation (Fig. 2C).
Fig. 2.
The expression of pro-inflammatory markers and anti-inflammatory markers in microglia after treatment with IFN-γ or IL-4 for 24 h and 48 h. A and B Fluorescence micrographs of the expression of iNOS and Arg-1 when microglia were exposed in PBS, IFN-γ or IL-4 for 24 h. iNOS and Arg-1 was stained with antibody (green). The microglia were stained IBA1 (red) and the nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histograms represent quantification of the mean fluorescence intensity (MFI) of iNOS or Arg-1 when microglia were exposed in PBS, IFN-γ or IL-4 for 24 h and 48 h. C The histograms show the intracellular and extracellular protein concentrations of TNF-α and TGF-β in microglia were exposed in PBS, IFN-γ or IL-4 for 24 h and 48 h. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01, ***P < 0.001 vs Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs IL-4 group
These results demonstrated that IL-4 and IFN-γ had opposite effects on the activation of microglia. And the M1 or M2 phenotype of microglia induced by IFN-γ or IL-4 respectively was temporarily stable between 24 and 48 h after stimulation.
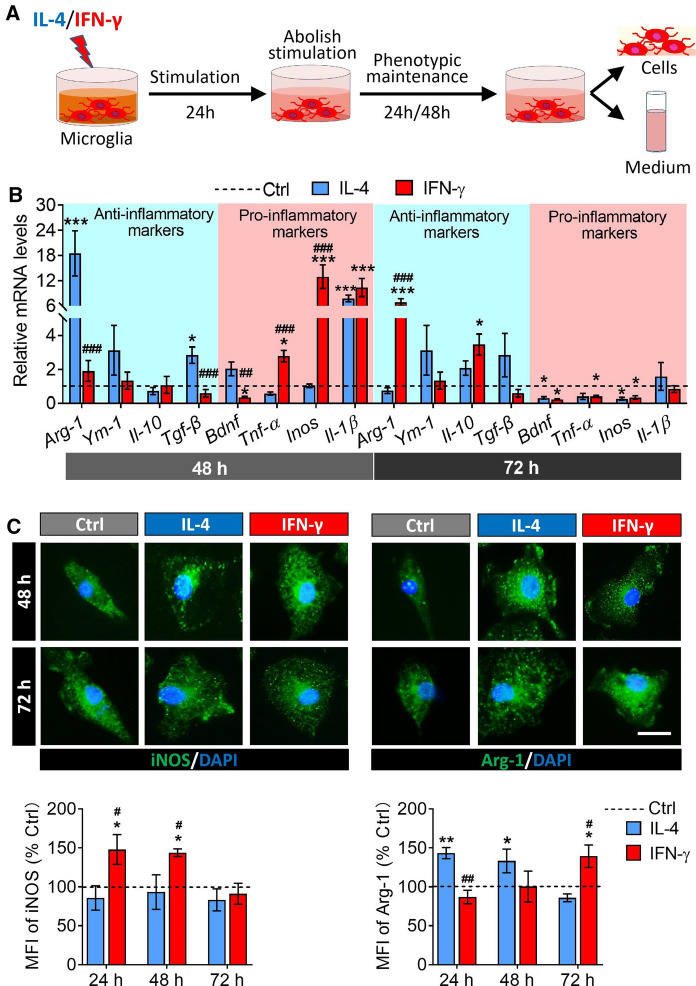
Phenotypic maintenance of microglia after removal of IFN-γ or IL-4 intervention
Then we assessed the gene expression of anti-inflammatory markers and pro-inflammatory markers in activated microglia at 24 h and 48 h after the intervening factors were removed (Fig. 3A). The results showed that IL-4-treated microglia had higher anti-inflammatory makers expression (Arg-1, and TGF-β) at 24 h compared to untreated microglia, but the expression of anti-inflammatory markers fell back to normal levels at 48 h after removal of intervention. In INF-γ-treated microglia, an increase in the expression of pro-inflammatory markers (TNF-α, iNOS and IL-1β) and anti-inflammatory markers (Arg-1) was recorded at 24 h and 48 h post-intervention, respectively, relative to untreated microglia (Fig. 3B). Meanwhile, the results of immunofluorescence indicated that the protein expression of Arg-1 increased in IL-4-induced microglia and iNOS increased in IFN-γ treated microglia at 24 h after removal of intervention, respectively. But after another 24 h, the expression of iNOS fell back to normal while Arg-1 still remained highly expressed in IFN-γ-activated microglia (Fig. 3C).
Fig. 3.
Phenotype maintenance of M1 and M2 microglia after removing IFN-γ or IL-4 intervention. A Experimental scheme to monitor phenotype maintenance of microglia after removal of IFN-γ or IL-4 intervention. B Levels of mRNA encoding anti-inflammatory markers (Arg-1, YM-1, IL-10 and TGF-β) and pro-inflammatory markers (BDNF, TNF-α, iNOS and IL-1β) in microglia at 24 h and 48 h after the removal of the intervening factors. C Fluorescence micrographs of the expression of iNOS and Arg-1 at 24 h and 48 h after the removal of the intervening factors in microglia. The iNOS and Arg-1 was stained with antibody (green) and the nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histograms represent quantification of the mean fluorescence intensity (MFI) of iNOS or Arg-1. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01, ***P < 0.001 vs Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs IL-4 group
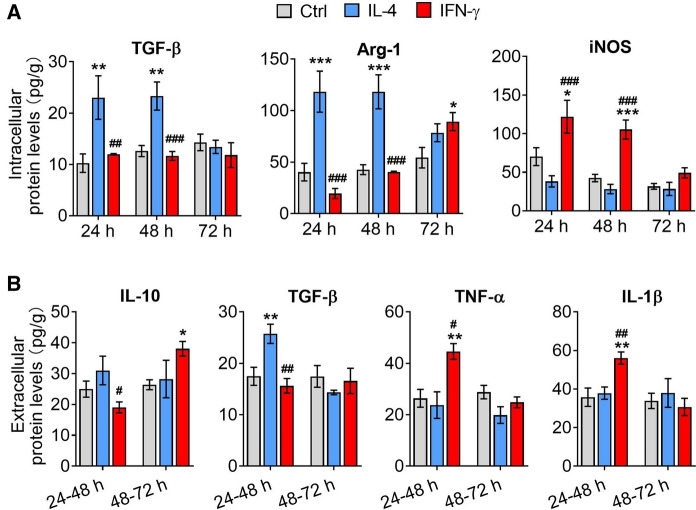
In addition, the intracellular protein expression (TGF-β, Arg-1 and iNOS) and extracellular protein (IL-10, TGF-β, TNF-α and IL-1β) in IFN-γ- and IL-4-treated microglia were assessed at 24 h and 48 h after removal of IL-4 or IFN-γ intervention by ELISA. The results were consistent with gene expression level (Figs. 4A and B).
Fig. 4.
The intracellular and extracellular protein expression of microglia after removal of IL-4 or IFN-γ intervention. A The intracellular protein expression (TGF-β, Arg-1 and iNOS) in IL-4- and IFN-γ-treated microglia at 0 h, 24 h and 48 h after removal of intervention. B The extracellular protein (IL-10, TGF-β, TNF-α and IL-1β) in IL-4- and IFN-γ-treated microglia at 0 h, 24 h and 48 h after removal of intervention. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01, ***P < 0.001 vs Ctrl group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs IL-4 group
The above results indicated that after the removal of stimulation, IL-4-induced microglia continued to secrete anti-inflammatory factors at first 24 h, then cease to do so for the next 48 h. Meanwhile, IFN-γ-induced microglia ceased to secrete pro-inflammatory factors instead secrete anti-inflammatory factors at 48 h after removal of intervening factors.
Effects of the secretome from IFN-γ- or IL-4-induced microglia on proliferation of NSPCs
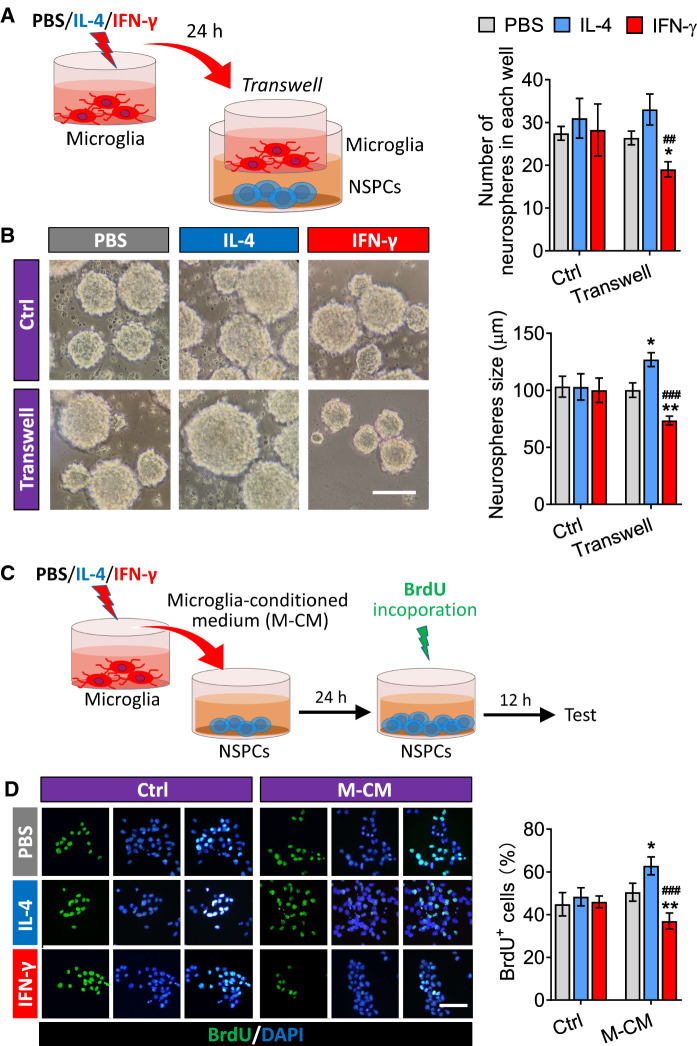
The NSPCs of adult mice were treated with PBS, IL-4 or IFN-γ, respectively, or co-cultured with PBS-, IFN-γ- or IL-4-treated microglia for 24 h (Fig. 5A). There was no significant difference in the number of neurospheres in each well and the neurosphere size when NSPCs were exposed to PBS, IL-4 and IFN-γ alone. However, compared to those co-cultured with PBS-treated microglia, the neurosphere size increased when adult NSPCs co-cultured with IL-4-induced microglia, while both the number of neurospheres in each well and the neurosphere size decreased when co-cultured with IFN-γ-induced microglia (Fig. 5B).
Fig. 5.
Effects of the secretome from IL-4- or IFN-γ-treated microglia on proliferation of NSPCs. A Experimental scheme to monitor microglia-conditioned medium on neurosphere size of adult NSPCs. B Micrographs of adult NSPCs treated with PBS, IL-4 or IFN-γ or co-cultured with PBS-, IFN-γ- or IL-4-treated microglia for 24 h. The histograms represent the number of neurospheres in each well and the neurosphere size. C Experimental scheme to monitor microglia-conditioned medium on proliferation of adult NSPCs. D Fluorescence micrographs of BrdU+ cells from NSPCs exposed to PBS, IL-4 or IFN-γ or microglia-conditioned medium for 24 h. The BrdU+ cells were stained with antibody (green) and nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histogram represents quantification of percentage of BrdU+ cells from adult NSPCs. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01 vs PBS group, ##P < 0.01, ###P < 0.001 vs IL-4 group
To further confirm the effects of IL-4 or IFN-γ activated microglia on the proliferation of adult NSPCs, the NSPCs were separately cultured in the conditioned medium (CM) collected from PBS, IFN-γ or IL-4 treated microglia for 24 h. The proliferation of NSPCs was assessed by labeled with BrdU (Fig. 5C). According to the results of immunofluorescence, there was no significant change in the percentage of BrdU+ cells when adult NSPCs were exposed to PBS, IL-4 or IFN-γ alone. However, the percentage of BrdU+ cells increased when the adult NSPCs were cultured in the CM from IL-4-treated microglia, but decreased when cultured in the CM from INF-γ-treated microglia (Fig. 5D).
These results demonstrated that the secretome from IFN-γ- or IL-4-induced microglia had opposite effects on NSPCs proliferation: the factors secreted by the IL-4-treated microglia promoted NSPCs proliferation, while factors secreted by the INF-γ-treated microglia inhibited NSPCs proliferation.
Effects of the secretome from IFN-γ- or IL-4-induced microglia on differentiation of NSPCs
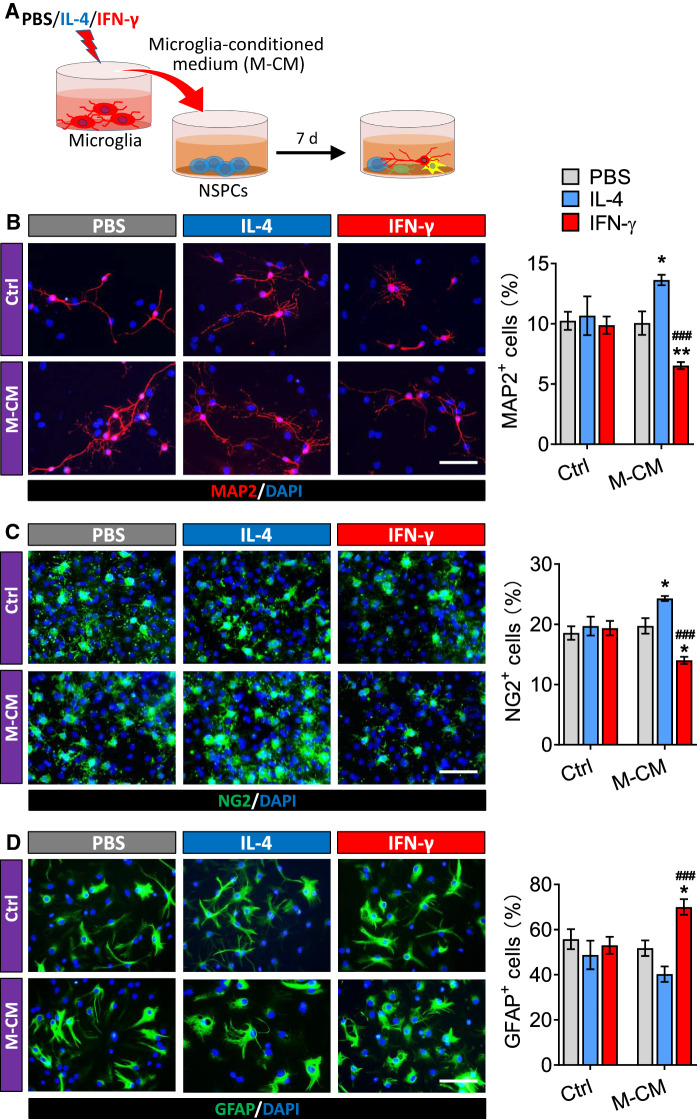
NSPCs were allowed to differentiate for seven days in conditioned medium from microglia treated with PBS, IFN-γ or IL-4 (Fig. 6A). Then the percentages of cells that were developmentally committed to becoming neurons, oligodendrocytes, or astrocytes were analyzed by immunofluorescence. The results showed that PBS, IL-4 and IFN-γ alone treatment did not affect the percentages of neurons, oligodendrocytes, or astrocytes differentiated from NSPCs (Fig. 6B–D). However, the conditioned medium from microglia treated with IFN-γ reduced the differentiation of NSPCs into neurons (MAP2+ cells), but such differentiation was promoted by conditioned medium from microglia treated with IL-4 (Fig. 6B). Similar results were observed for differentiation of NSPCs into oligodendrocytes (NG2+ cells) (Fig. 6C). Conditioned medium from IFN-γ-treated microglia promoted differentiation of NSPCs into astrocytes (GFAP+ cells) (Fig. 6D).
Fig. 6.
Effects of the secretome from IL-4- or IFN-γ-treated microglia on differentiation of NSPCs. A Experimental scheme to monitor microglia-conditioned medium on differentiation of adult NSPCs. B Fluorescence micrographs of MAP2+ cells from NSPCs differentiate in PBS, IL-4 or IFN-γ or microglia-conditioned medium for 7 days. The MAP2+ cells were stained with antibody (red) and nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histogram represents quantification of percentage of MAP2+ cells from adult NSPCs. C Fluorescence micrographs of NG2+ cells from NSPCs differentiate in PBS, IL-4 or IFN-γ or microglia-conditioned medium for 7 days. The NG2+ cells were stained with antibody (green) and nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histogram represents quantification of percentage of NG2+ cells from adult NSPCs. D Fluorescence micrographs of GFAP+ cells from NSPCs differentiate in PBS, IL-4 or IFN-γ or microglia-conditioned medium for 7 days. The GFAP+ cells were stained with antibody (green) and nucleus is labeled by DAPI (blue). Scale bar is 10 μm. The histogram represents quantification of percentage of GFAP+ cells from adult NSPCs. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01 vs PBS group, ###P < 0.001 vs IL-4 group
These results demonstrated that the secretome from IFN-γ- or IL-4-induced microglia had opposite effects on NSPCs differentiation: the factors secreted by the IL-4-treated microglia promoted NSPCs differentiation into neurons and oligodendrocytes but not astrocyte, while factors secreted by the INF-γ-treated microglia promoted NSPCs differentiation into astrocyte but inhibited the neurogenesis and oligodendrogliogenesis.
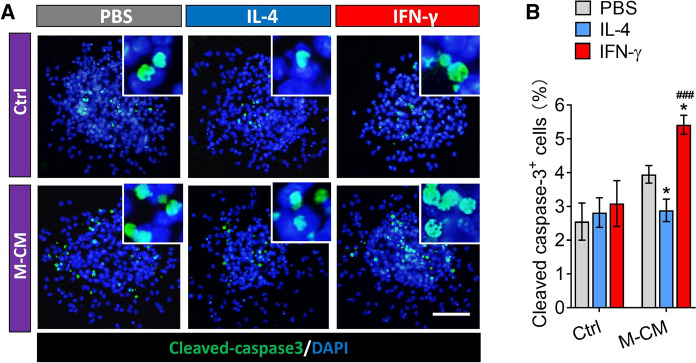
Effects of the secretome from IFN-γ- or IL-4-induced microglia on survival of NSPCs
NSPCs were allowed to differentiate for seven days in differentiation medium in the presence or absence of conditioned medium from microglia. Then, the percentages of apoptotic cells were analyzed by immunofluorescence. The results showed that PBS, IL-4 and IFN-γ alone treatment did not affect the percentages of apoptotic NSPCs (CC-3+ cells) (Fig. 7A and B). However, the conditioned medium from microglia treated with IFN-γ promoted the apoptosis of NSPCs into, but such CC-3+ cells was reduced by conditioned medium from microglia treated with IL-4 (Fig. 7A and B). These observations suggest that the secretome from IL-4-induced microglia can prevent the apoptosis of NSPCs during differentiation, while factors secreted by the INF-γ-treated microglia promoted apoptosis of NSPCs.
Fig. 7.
Effect of secretome from from IL-4- or IFN-γ-treated microglia on survival and proliferation via PI3K/Akt pathway in adult NSPCs. A Fluorescence micrographs of apoptotic cells from NSPCs differentiate in PBS, IL-4 or IFN-γ or microglia-conditioned medium for 7 days. The apoptotic cells were stained with Cleaved-caspase 3 (green) by immunocytochemistry and nucleus is labeled by DAPI (blue). Scale bar is 10 μm. B The histogram represents quantification of percentage of CC-3+ cells. Data are showed Mean ± SEM, n = 4–6, *P < 0.05 vs PBS group, ###P < 0.001 vs IL-4 group
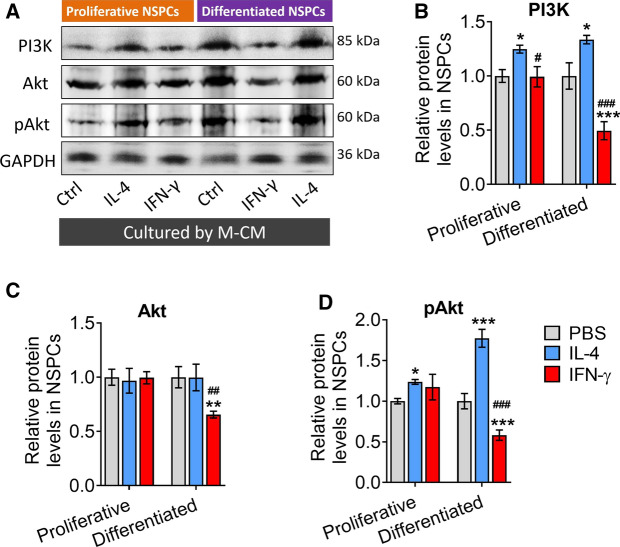
Effects of the secretome from IFN-γ- or IL-4-induced microglia on PI3K/Akt signaling pathway during NSPCs proliferation or differentiation
Since phosphatidylinositol 3 kinase/serine-threonine kinase (PI3K/Akt) signaling pathway plays a critical role in neurogenesis regulation of NSPCs (Zhang et al. 2018), we next analyzed the effects of the secretome from IFN-γ- or IL-4-induced microglia on PI3K/Akt signaling pathway in proliferative or differentiated NSPCs. We found that the secretome from IL-4-induced microglia upregulated the levels of PI3K and p-Akt either in proliferative or differentiated NSPCs. While conditioned medium from microglia treated with IFN-γ reduced levels of PI3K, AKT, and p-AKT either in proliferative or differentiated NSPCs (Fig. 8A–D).
Fig. 8.
Effects of the secretome from IFN-γ- or IL-4-induced microglia on PI3K/Akt signaling pathway during NSPCs proliferation or differentiation. A–D Western blotting showing the levels of PI3K, Akt, and phospho-Akt (p-Akt) in NSPCs under different treatment conditions. Levels of PI3K and Akt were normalized to those of β-actin, and levels of p-Akt was normalized to those of Akt. Data are showed Mean ± SEM, n = 4–6, *P < 0.05, **P < 0.01, ***P < 0.001 vs PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001 vs IL-4 group
These results suggest that the PI3K-Akt pathway mediates the effects of the secretome from IFN-γ- or IL-4-induced microglia on NSPC proliferation, differentiation and survival.
Discussion
The effect of microglia with different phenotypes on NSPCs is treacherous, and the molecular mechanism are hard to understand. In this study, we demonstrated that the secretome from IFN-γ- or IL-4-induced microglia had opposite effects on NSPCs proliferation, differentiation and survival. The secretome secreted by the IL-4-treated microglia promoted NSPCs proliferation, survival and differentiation into neurons and oligodendrocytes, while factors secreted by the INF-γ-treated microglia stimulated the NSPCs differentiation into astrocyte, inhibited the neurogenesis and oligodendrogliogenesis, and induced NSPCs apoptosis. Furthermore, the PI3K-Akt pathway mediates the effects of the secretome from IFN-γ- or IL-4-induced microglia on NSPC proliferation, differentiation, and survival. To our knowledge, this is the first time to identify and provide a clear result and mechanism for the influence of different phenotypic microglia on the proliferation, neural differentiation and survival of adult NSPCs.
Microglia showed strictly stimuli dependent and related to the pathological conditions (Wang et al. 2013). In this study, microglia stimulated continuously by IL-4 for 24 h and 48 h showed multiramose, higher expression level of anti-inflammatory markers and lower expression level of pro-inflammatory markers compared to the untreated microglia. In contrast, those treated continuously with INF-γ for 24 h and 48 h showed oval shape and thorny convexity, significant higher expression level of pro-inflammatory markers and low expression level of anti-inflammatory markers. These results suggest that microglia can polarize to M1 microglia or M2 by INF-γ and IL-4, respectively. And their activation can persist for a long time as a double-edged sword. However, whether microglia treated by IL-4 or IFN-γ can maintain M1 or M2 phenotype after removal of the stimulus intervention is less well-defined. Previous research showed that 24 h-LPS-treatment induced M1 microglia, whereas 72 h-LPS-treatment induced M2 microglia in vitro, which indicated that the activation state of microglia was significantly dependent on the stimulation duration (Cacci et al. 2008). In the present study, expression of anti-inflammatory markers (Arg-1 and TGF-β) in IL-4-treated microglia increased during the first 24 h after stimulus removal, but fell back to normal within the next 24 h. Nevertheless, compared to the untreated microglia, higher expression of pro-inflammatory marker (TNF-α, iNOS and IL-1β) and anti-inflammatory marker (Arg-1) was observed in INF-γ-treated microglia at 24 h and 48 h after removing the stimulation, respectively. These results suggest that microglia maintain a relatively stable phenotype (M1 or M2) within 24 h after removal of stimulus. However, microglia of different phenotypes showed different patterns of change over the next 24 h.
NSPCs undergo a series of developmental processes before giving rise to newborn neurons, astrocytes and oligodendrocytes in adult neurogenesis (Cacci et al. 2008). It has been proven that microglia regulate the proliferation, differentiation and survival of adult NSPCs in different ways, which results in the varying consequences of adult neurogenesis (Xu et al. 2016; Berger et al. 2020). However, the effects of M1 and M2 microglia on neural stem cells are controversial, due to the complexity of the microglia phenotype, especially after removal of stimulus intervention. A recent study had demonstrated that unstimulated BV2 microglia and M2-polarized primary microglia secrete molecules supportive of NSPC proliferation (Matsui and Mori 2018). And it has also been reported that the conditioned medium from M2 microglia was found to promote survival and migration, but suppress the astrocytic differentiation of NSPCs compared to the conditioned medium collected from the M1 subtype (Osman et al. 2019). In this study, we first excluded the effect of direct treatment with IFN-γ or IL-4 on the proliferation, differentiation and survival of NSPCs. Then our results indicated that NSPCs kept in conditioned medium (CM) from the M2 microglial had batter proliferation, decreased survival, and higher neurons and polydendrocytes differentiation. In contrast, CM from M1 microglia showed an opposite effect on NSPC proliferation and survival. And it increased the percentage of cells positive for astrocytes differentiation. The results of our experiments are largely the same as those previously reported, but differ in some respects, possibly because the activation state of microglia is dependent on the duration of stimulation. In fact, the study by Masaya Nakanishi, Tetsuhiro Niidome et al. concluded that activated microglia are not neurogenic or anti-neurogenic per se. It is the balance between pro- and anti-inflammatory factors that is critical to the behavior of activated microglia toward neurogenesis (Nakanishi et al. 2007). So, at the first 24 h, M1 microglia suppress the proliferation, promote astrocytes differentiation and survival of NSPCs through releasing pro-inflammatory markers such as NO, IL-1β, TNF-α, etc. Then at 48 h after the cessation of stimulation, M2 microglia become immune-inactivated, while M1 microglia may transform into M2 microglia. And M2 microglia can opposite affect the aforementioned processes of NSPCs by secreting anti-inflammatory markers including BDNF, TGF-β, IGF-1, etc.
In addition, we also explored the potential pathways by which microglia-condition medium regulate NSPCs. The PI3K/Akt pathway regulates a wide range of cellular activities including cell survival, proliferation, metabolism, neuroscience, motility and cancer progression (Zhang et al. 2018). In the current study, we found that the phosphorylation levels of PI3K and Akt are promoted and inhibited in differentiated NSPCs by CM from M2 and M1 microglia, respectively. However, in proliferative NSPCs the phosphorylation levels of PI3K and Akt was promoted only by CM from M2 microglia. Therefore, we speculate that PI3K/Akt signaling pathway may play a key role in the differentiation of NSPCs by condition medium. Whereas, further studies should be designed to confirm the specificity of using PI3K/Akt signaling pathway to interpret the mechanism of regulation of the proliferation of NSPCs by condition medium.
In conclusion, our results suggested that the secretome of microglia induced by IL-4 of IFN-γ differently regulate proliferation, differentiation and survival of adult neural stem/progenitor cell by targeting the PI3K-Akt pathway. These findings will help further study the biological mechanism of microglia regulating neurogenesis, and provide a therapeutic strategy for neurological diseases by regulating microglial phenotypes to affect neurogenesis.
Acknowledgements
We acknowledge Professor Tao Zhou at the Resource Center of the Chinese Academy of Traditional Chinese Medicine and Zili You at University of Electronic Science and Technology of China for giving us guidance on the experiment and writing.
Author contributions
JX, and ZJ designed the conceptual idea for this study and wrote the manuscript. YS, LQ and ZJ performed the experiments. ZJ analyzed these data. All the authors participated in the discussion and approved the manuscript as submitted.
Funding
This work was supported by the National Natural Science Foundation of China (81960811, 82060726), the PhD Start-up Fund of the Guizhou University of Traditional Chinese Medicine ([2019]31), Guizhou Science and Technology Plan Project ([2019]5611), the Department of Science and Technology of Guizhou High-level Innovative Talents ([2018]5638-2), and the Department of Science and Technology of Guizhou basic research ([2019]1026).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Conflict interest
Author declares that there is no conflict of interest in this research.
Ethical approval and consent to participate
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee, University of Electronic Science and Technology of China.
Consent for publication
All authors agree to the publication of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xue Jiang, Email: 1017036604@qq.com.
Saini Yi, Email: 945328088@qq.com.
Qin Liu, Email: 1297499413@qq.com.
Jinqiang Zhang, Email: 552450374@qq.com.
References
- Berger T, Lee H, Young AH, Aarsland D, Thuret S. Adult hippocampal neurogenesis in major depressive disorder and Alzheimer's Disease. Trends Mol Med. 2020;26:803–818. doi: 10.1016/j.molmed.2020.03.010. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Chu W, Yuan J, Huang L, Xiang X, Zhu H, Chen F, Feng H. Valproic acid arrests proliferation but promotes neuronal differentiation of adult spinal NSPCs from SCI rats. Neurochem Res. 2015;40:1472–1486. doi: 10.1007/s11064-015-1618-x. [DOI] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/strokeaha.112.659656. [DOI] [PubMed] [Google Scholar]
- Jiang X, He H, Mo L, Liu Q, Yang F, Zhou Y, Zhang J. Mapping the plasticity of morphology, molecular properties and function in mouse primary microglia. Front Cell Neurosci. 2021 doi: 10.3389/fncel.2021.811061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M, Yenari MA. The role of the microglia in acute CNS injury. Metab Brain Dis. 2015;30(2):381–392. doi: 10.1007/s11011-014-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MA, Sudol KL, Narrow WC, Bowers WJ. Interferon-{gamma} differentially affects Alzheimer's disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am J Pathol. 2009;175:2076–2088. doi: 10.2353/ajpath.2009.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui TK, Mori E. Microglia support neural stem cell maintenance and growth. Biochem Biophys Res Commun. 2018;503:1880–1884. doi: 10.1016/j.bbrc.2018.07.130. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nikolakopoulou AM, Dutta R, Chen Z, Miller RH, Trapp BD. Activated microglia enhance neurogenesis via trypsinogen secretion. Proc Natl Acad Sci USA. 2013;110:8714–8719. doi: 10.1073/pnas.1218856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman AM, Rodhe J, Shen X, Dominguez CA, Joseph B, Blomgren K. The secretome of microglia regulate neural stem cell function. Neuroscience. 2019;405:92–102. doi: 10.1016/j.neuroscience.2017.10.034. [DOI] [PubMed] [Google Scholar]
- Panagiotakopoulou V, Ivanyuk D, De Cicco S, Haq W, Arsić A, Yu C, Deleidi M. Interferon-γ signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat Commun. 2020;11:5163. doi: 10.1038/s41467-020-18755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Morganti-Kossmann C, Lifshitz J, Ziebell JM. Rod microglia: a morphological definition. PLoS ONE. 2014;9:e97096. doi: 10.1371/journal.pone.0097096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73:327–348. doi: 10.1007/s00018-015-2067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ge H, Liu W, Zhu H, Chen Y, Zhang X, Lin J. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARγ signaling pathway. Oncotarget. 2017;8:19855–19865. doi: 10.18632/oncotarget.15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, He H, Qiao Y, Zhou T, He H, Yi S, You Z. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia. 2020;68:2674–2692. doi: 10.1002/glia.23878. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yao L, Yang J, Wang Z, Du G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review) Mol Med Rep. 2018;18:3547–3554. doi: 10.3892/mmr.2018.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol. 2013;8(4):888–899. doi: 10.1007/s11481-013-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article and its supplementary information files.