Abstract
Many mycotoxigenic fungi infect the food crops and affect the quality of the produce due to production of mycotoxins. Kodo millet is one of the important minor millets cultivated in India, mostly confined to marginal lands and tribal regions but has high yield potential under good management. The grains are nutritious and have anti-oxidant properties besides having many medicinal properties. However, the consumption is often hindered by the condition called ‘kodo poisoning’ resulting from fungal contamination producing cyclopiazonic acid, a toxic fungal secondary metabolite. An attempt has been made here to review the limited information available on kodo poisoning, its causes and effects, and proposed management practices by which the contamination can be checked. Further research efforts are essential for identifying sources of natural resistance to fungal metabolite, induction of host resistance through antimicrobial compounds or microbial antagonism to the pathogens to achieve cleaner grains from this crop even under high humid and rainy conditions. By effective adoption of both pre- and post-harvest management the kodo millet grains can be made safe for human consumption and can be popularized as a nutritious grain.
Keywords: Cyclopiazonic acid (CPA), Kodo millet, Malona, Mycotoxin, Paspalum
Introduction
Many small grained annual grasses of the rainfed ecosystem, commonly referred as ‘small millets’ are ancient grains, which fed the human population before wide spread adoption of fine cereals. Looking at the nutritional benefit of such grains along with other millets they are collectively called ‘Nutricereals’. Kodo millet (Paspalum scrobiculatum L.) is one such nutricereal grown on marginal and degraded lands producing high grain yields even with limited water. In India, it is grown largely in tribal regions and under poor environments, and finds place in the diet of poor people either as whole grains or in the preparation of traditional food products like idli, dosa, chapathi, pongal, soup, etc. Apart from food uses, several medicinal properties of grains as well as leaves are also known.
Despite all the beneficial properties, the kodo millet cultivation or consumption is limited by an enigma called ‘kodo poisoning’. Some early reports suggested that grains of kodo millet were poisonous and not fit for consumption based on a few poisoning incidences that had taken place in some of the kodo millet cultivating regions of North India, especially Uttar Pradesh and Madhya Pradesh. Limited research studies on this aspect have concluded that it was solely due to the contamination of the seeds with mycotoxin producing fungi. No study has so far shown kodo millet grains producing any inherent toxin naturally inside grain leading to poisoning. The fear of kodo poisoning has emerged as a serious economic constraint for kodo millet cultivation, consumption and popularization. Elaborate and in-depth understanding of the issue is lacking. Looking at the significance with respect to human and animal health, and very limited studies we have attempted to review the information available on kodo poisoning, the reasons, causal organisms and toxin, toxicological effects, methods for detection of toxin and suggested management measures to avoid the fungi responsible for poisoning. This review would help in understanding about kodo millet poisoning and its management thereby enabling its prevention, and enhancing the consumption and popularization.
Kodo millet—an ancient grain
Origin and distribution
Kodo millet is one among the genus Paspalum (Panicoidae) that includes nearly 400 species growing across the warmer region of the old-World tropics and subtropics (de Wet et al. 1983) and it is indigenous to India. The crop is grown in India, Pakistan, Philippines, Indonesia, Vietnam, Thailand and West Africa (Galinato et al. 1999). It is known by different names in different parts of the world viz., kodohirse, African Bastard millet, creeping paspalum, ditch millet, Indian paspalum, koda grass, scrobic, water couch, mijo koda, rice grass and herbe à épée (Heuzé et al. 2015; Knees and Gupta 2013; Hariprasanna 2017). Within India, it is called kodo (Hindi and Bengali), Varagu (Tamil and Malayalam), Arika (Telugu), Harka (Kannada), Kodra (Gujarati, Marathi & Punjabi) or Kodua (Oriya) in different regions depending on local language (Ayyangar and Panduranga Rao 1934; Deshpande et al. 2015; Hariprasanna 2015). It was widely grown in India since ancient times. During the excavations at the Neolithic sites of Maharashtra (Nevasa), Karnataka (Hallur) and Uttar Pradesh (Nagra), kodo millet was one of the ancient grains identified in the archeological deposits of India (Kajale 1974). It was cultivated as an annual cereal in Southern Rajasthan and some parts of Maharashtra in India from at least 3000 years ago (de Wet et al. 1983).
Cultivation
Kodo millet is one of the hardiest crops among the small millets. It has considerable production potential in marginal, low fertility soils and chronic moisture deficit areas of the country. It is a long duration crop (110–130 days) compared to other small millets and it grows well on shallow as well as deep soils. It is mostly grown on marginal or poor soils, in the sloppy or undulated terrains in tribal belts (Yadava and Jain 2006). India is the only contributing country to the world’s kodo millet production with about 0.08 million t of grains from an area of 0.2 million ha (Bhat et al. 2018). In India, it is grown in the states of Madhya Pradesh, Uttar Pradesh, Maharashtra, Chattishgarh, Tamilnadu and Karnataka. The crop possesses a number of agronomically valuable characteristics such as more herbage, branched ear, large number of seeds per raceme, high fertility and unique storage ability. The kodo millet seeds have an excellent storage life and can be stored even up to 100 years (Hegde and Gowda 1989).
Nutritional importance
Nutrient composition of kodo millet in terms of carbohydrate, protein and energy are as good as the popular cereals like rice and wheat. The nutritional composition of kodo millet verses milled raw rice and whole wheat are given in Table 1. When compared with rice and wheat, kodo millet has high fat and mineral content. It is rich in total folates (Longvah et al. 2017) which is used to treat anemia and given as a supplement during pregnancy to women to lessen the risk of neural disorders in the baby (Bibbins-Domingo et al. 2017). Total dietary fiber content (6.39 g) is higher in kodo millet than rice (2.81 g) and its fat has higher poly unsaturated fatty acid (PUFA) content (Longvah et al. 2017). It is very easy to digest and contains high amount of lecithin and is excellent for strengthening the nervous system (Deshpande et al. 2015; Malathi et al. 2012). Kodo millet grains are rich in B vitamins, especially Niacin (B3), Pantothenic Acid (B5), Biotin (B7) and total folates (B9), and minerals such as sodium, calcium, iron, potassium, magnesium and zinc (Longvah et al. 2017). Among the small millets studied, kodo millet had high phenols (368 mg catechol equivalents/100 g of dry flour), and antioxidant activity (free radical quenching capacity of 70% in comparison to 15–53% in other millet extracts) (Hegde and Chandra 2005). Kodo millet also has high phytate (452 mg/100 g) content compared to other small millets (Longvah et al. 2017). Glycemic index (GI) of whole kodo millet starch (47.81) is lower than rice flour (53.25) (Annor et al. 2013). The regular consumption of kodo millet is highly beneficial for post-menopausal women suffering from signs of cardiovascular disease, high blood pressure and high cholesterol levels (Malathi et al. 2012).
Table 1.
Nutrient composition of kodo millet compared to raw milled rice and whole wheat (Values expressed per 100 g edible portion)
| Nutrient | Kodo millet | Rice (raw, milled) | Wheat (whole) |
|---|---|---|---|
| Moisture (g) | 14.23 | 9.93 | 10.58 |
| Protein (g) | 8.92 | 7.94 | 10.59 |
| Fat (g) | 2.55 | 0.52 | 1.47 |
| Total dietery fiber (g) | 6.39 | 2.81 | 11.23 |
| Carbohydrates (g) | 66.19 | 78.24 | 64.72 |
| Energy (kcal) | 331.7 | 356.3 | 321.9 |
| Mineral matter (g) | 1.72 | 0.56 | 1.42 |
| Vitamins | |||
| Thiamine (mg) | 0.29 | 0.05 | 0.46 |
| Riboflavin (mg) | 0.20 | 0.05 | 0.15 |
| Niacin (mg) | 1.49 | 1.69 | 2.68 |
| Pantothenic Acid (mg) | 0.63 | 0.57 | 1.08 |
| Biotin (μg) | 1.49 | 0.60 | 1.03 |
| Folates (μg) | 39.49 | 9.32 | 30.09 |
| Minerals and trace elements | |||
| Calcium (mg) | 15.27 | 7.49 | 39.36 |
| Iron (mg) | 2.34 | 0.65 | 3.97 |
| Zinc (mg) | 1.65 | 1.21 | 2.85 |
| Magnesium (mg) | 122 | 19.30 | 125 |
| Phosphorus (mg) | 101 | 96 | 315 |
| Sodium (mg) | 3.35 | 2.34 | 2.50 |
| Amino acids (per 100 g protein) | |||
| Methionine (g) | 2.69 | 2.60 | 1.75 |
| Lysine (g) | 1.42 | 3.70 | 3.13 |
| Tryptophan (g) | 1.32 | 1.27 | 1.40 |
| Total starch (g) | 64.96 | 75.70 | 57.53 |
| Polyunsaturated fatty acid (mg) | 597 | 253 | 141 |
| Phytochemicals | |||
| Phytate (mg) | 452 | 266 | 638 |
| Total polyphenols (mg) | 0.53 | 3.14 | 14.33 |
| Total Phenols (aqueous extracts) (μmol FAE*/g cooked grain, dry weight) | 4.33 | – | – |
| Total flavonoids (μmol CE**/g cooked grain, dry weight) | 1.28 | – | – |
| Antioxidant activity (μmol TE***/g cooked grain) | 2.55 | – | – |
Kodo Poisoning
History of events
In the manual of Medical Jurisprudence for India, under poisonous grains and legumes, consumption of kodo grains has been indicated to result in some adverse health conditions. This had happened due to the intake of kodo grains whose maturing and harvesting had coincided with rainfall, resulting in fungal infection of the grains leading to ‘poisoned kodo’ locally known as ‘Matawna Kodoo’ or ‘Matona Kodo’ in northern India (Chevers 1870). This is the reason why in some places where kodo is cultivated, farmers believe that kodo millet is poisonous after rains. In Indian medical gazette 1922, there was a report on acute ‘kodon’ poisoning that was witnessed by an assistant surgeon from district Shahjehanpur, Uttar Pradesh, India that described four unconscious cases of acute poisoning caused by the consumption of bread made from flour of kodo millet where the victims suffered from vomiting, giddiness and unconsciousness after taking their meal (Swarup 1922). Assistant surgeon stated that kodo is one of the cheapest grains and largely eaten by poor people in Uttar Pradesh and it had developed poisonous properties under certain unknown conditions. Nervous and cardio-vascular systems are mainly affected by poison and the chief symptoms include vomiting, giddiness, unconsciousness, small and rapid pulse, cold extremities, shaking of limbs, tremors and resistance to outside interference. However, no cases of death were observed. Poisoned individuals could be recovered by washing out the stomach, by giving stimulants, hot tea or milk and warmth to the extremities (Swarup 1922). The symptoms of kodo poisoning lasted for one to three days followed by recovery (Bhide 1962). In some instances, it produces derilium with violent tremors of voluntary muscles and detrimental effects that even lead to death in very extreme cases. Frequently, the husk and leaves acquire poisonous character which was popularly attributed to heavy rainfalls (Bhide 1962; Bhide and Aimen 1959). The poisoning was locally also known as Malona in Madhya Pradesh and Chattisgarh states (Pall et al. 1980; Ansari and Shrivastava 1991).
Reasons of poisoning
The association of the mycotoxin, cyclopiazonic acid (CPA), with kodo millet seeds causing ‘kodua poisoning’ was first identified during mid-eighties (Rao and Husain 1985). CPA was isolated and identified from a sample of kodo millet seed that caused symptoms of ‘kodua poisoning’ in man. The extract of the toxic grain when injected into mice produced symptoms of depression and complete loss of mobility. The seed was infected by Aspergillus flavus and A. tamarii and both fungi produced cyclopiazonic acid. This is the first report of the association of a mycotoxin with kodua poisoning (Rao and Husain 1985). The finding suggested that consumption or feeding of contaminated kodo millet is a serious health hazard to humans and animals because of the risk of exposure to CPA produced by the toxigenic fungi in kodo millet. Fungal growth in fodder also causes a decrease in nutritional value and results in health hazards. CPA is named after the strain, Penicillium cyclopium Westling from which it was isolated (Holzapfel 1968) and later it was identified that P. griseofulvum Dierckx was the CPA-producing strain originally (Frisvad 1989; Chang et al. 2009). Species of Penicillium including P. griseofulvum, P. camemberti, P. urticae and P. commune are also reported to consistently produce CPA (Burdock and Flamm 2000). Apart from Aspergillus flavus and A. tamarii other species of Aspergillus viz., A. oryzae, A. fumigatus, A. versicolor are also known to produce CPA (Chang et al. 2009). Few isolates of A. flavus were found to produce both mycotoxins cyclopiazonic acid and aflatoxin (Gallagher et al. 1978). List of fungi reported to produce CPA are given in Table 2.
Table 2.
List of fungal species reported to produce CPA
| Fungal species | Product | CPA (ppm) | References |
|---|---|---|---|
| Penicillium spp. | |||
| P. cyclopium | Barley | – | Holzapfel (1968), Burdock and Flamm (2000) |
| P. patulum | Peanuts | – | Holzapfel (1968), Burdock and Flamm (2000) |
| P. viridicatum, | Ham | – | Holzapfel (1968), Burdock and Flamm (2000) |
| P. camemberti | Meat, cheese | – | Bars (1979), Burdock and Flamm (2000) |
| P. griseofulvum | Peanut leaf and stem | – | Hermansen et al. (1984), Burdock and Flamm (2000) |
| P. urticae | Navy beans | 6–8 | Burdock and Flamm (2000) |
| P. chrysogenum | Chicken | – | El-Banna et al. (1987) |
| P. crustosum | Raw meat | – | |
| P. viridicatum | Ham meat | – | |
| P. commune | Artificial media | – | |
| P. nalgiovense | Artificial media | – | |
| P. hirsutum | Artificial media | – | |
| Aspergillus spp. | |||
| A. flavus | Kodo millet | – | Luk et al. (1977), Dorner et al. (1983) |
| Chicken (artificial inoculation) | 100 | ||
| A. tamarii | Kodo millet | – | Dorner et al. (1983); Rao and Husain (1985), Chang et al. (2009) |
| A. oryzae | Artificial media | – | Burdock and Flamm (2000); Vinokurova et al. (2007) |
| A. fumigatus | Artificial media | – | Burdock and Flamm (2000) |
| A. versicolor | Artificial media | – | Burdock and Flamm (2000) |
| A. phoenicis | Artificial media | – | Vinokurova et al. (2007) |
Symptoms and effects on human and animal health
Since CPA is the principal components of kodo poisoning the symptoms of kodo poisoning are similar to CPA toxicity. CPA toxicity has been studied in many animals. CPA toxin causes degeneration and necrosis of the liver, lesions of the myocardium, and neurotoxic effects through alteration of calcium homeostasis and cellular transduction processes in many animal species (Yu and Chu 1998) and also alters the ion transport across cell membranes (Bennett and Klich 2003). Symptoms of CPA toxicity has been reported in various organisms as loss of mobility, depression and reluctance to move in mice (Rao and Husain 1985); mortality, decreased weight gain, and poor feed conversion in chickens (Dorner et al. 1983); acute hepatotoxicity in rats (Antony et al. 2003); and weakness, inactivity, anorexia, rough hair coats, reduced body weight, gastric ulcers, mucosal hyperemia, and hemorrhage in pigs (Lomax et al. 1984). The poisoning in cattle led to nervousness, lack of muscular co-ordination, staggering gait, tremors, clonic convulsions, coma, depression and spasms (Bhide 1962; Rao and Husain 1985) and even death in some cases (Harrison 1971; Burdock and Flamm 2000). Sleepiness, tremors, giddiness and nausea have been described in humans after accidental consumption of kodo millet seeds contaminated with Aspergillus flavus and A. tamari (Swarup 1922; Rao and Husain 1985). CPA was one of the responsible mycotoxins in turkey ‘X’ disease outbreak in 1960 (Bradburn et al. 1994).
CPA contamination in other agricultural products
CPA is a mycotoxin produced by numerous species of Aspergillus and Penicillium growing on stored grains as a secondary metabolite (Hayashi and Yoshizawa 2005). Therefore, many agricultural products are contaminated by CPA. Other than kodo millet, CPA has been found as a natural contaminant on agricultural products like cheese (Bars 1979), wheat (Gallagher et al. 1978), rice (Gallagher et al. 1978; Gonçalves et al. 2019), corn, peanuts (Lansden and Davidson 1983; Cole and Dorner 1999; Dorner 2002) and dried figs (Heperkan et al. 2012) which makes the commodities unfit for consumption.
Chemical nature of CPA
CPA (α-cyclopiazonic acid) is made of indole tetramic acid. It is a secondary metabolite produced by few strains of fungi like Aspergillus and Penicillium. Fungal spores present in the air land on grain. In event of high humidity (caused by rain) the spores germinate and enter inside the grain through its surface. During this process the fungus releases specific enzymes that facilitate degradation of grain components and release of energy for its growth. Research to explain the mechanism of CPA biosynthesis was carried in the 1970s. The origin of the carbon skeleton of CPA was established by feeding studies using radiolabeled substrates (Holzapfel and Wilkins 1971). Chemical degradation analysis indicated that CPA is derived from tryptophan, a C5-unit formed from mevalonic acid and two molecules of acetic acid (McGrath et al. 1976). For detail information on biosynthetic pathway recent reviews on the subject may be referred (Chang et al. 2009; Liu and Walsh 2009). CPA possesses a metal-chelating ability and is an optically active, colourless, odourless, crystalline metabolite (Vinokurova et al. 2007) (Fig. 1).
Fig. 1.
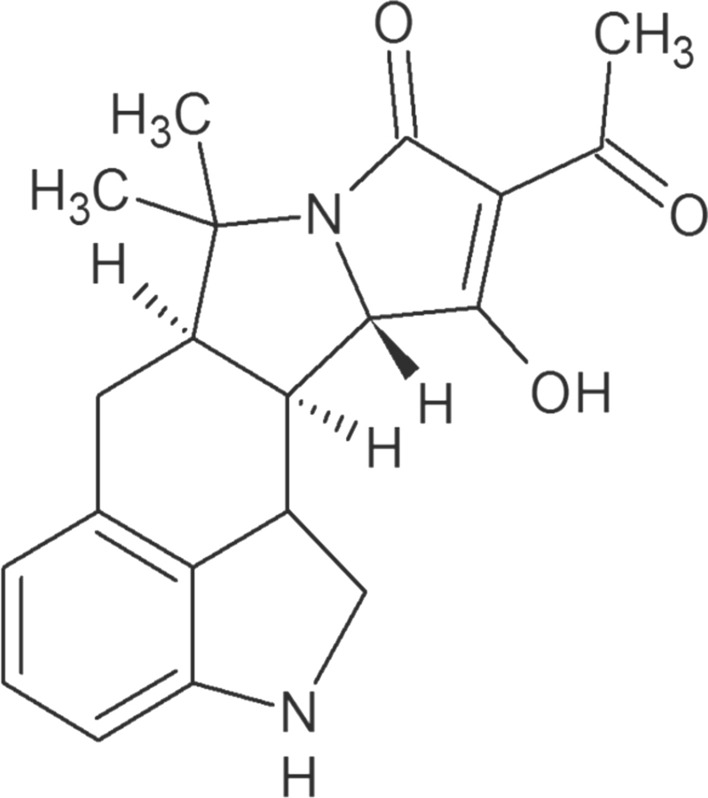
Chemical structure of Cyclopiazonic acid (
Source: Ostry et al. 2018)
Detection of CPA contamination in kodo millet
Various analytical methods including chromatographic, mass spectrometric and immunochemical techniques have been developed to detect CPA (Table 3). Thin layer chromatography (TLC) is widely used for qualitative estimation of mycotoxins. In nuts and grains TLC has been successfully used to determine CPA (Lansden 1986). Rao and Husain (1985) purified the toxin from infected kodo grains using preparatory TLC on oxalic acid impregnated plates. The plates were developed in chloroform:methyl ethyl ketone reagent and when the plate was sprayed with Ehlrich reagent, the region containing CPA developed violet coloured spots. The coloured area was scrapped off, eluted with chloroform and dried in vacuum. The purity of the toxin obtained was tested on TLC and identity of the compound was confirmed using mass spectrometry. Currently High Performance Liquid Chromatography (HPLC) coupled with Mass Spectrometry is the most employed method for quantification of CPA (Hayashi and Yoshizawa 2005; Moldes-Anaya et al. 2009; Zorzete et al. 2013; Ansari and Haeubl 2016). Immunoassays also have been developed for CPA detection (Hahnau and Weiler 1983; Maragos et al. 2017).
Table 3.
Commonly used methods for CPA detection
| Method of detection | Technique | LOD | LOQ | References |
|---|---|---|---|---|
| Capillary electrophoresis | Capillary electrophoresis | 20 ppb | – | Prasongsidh et al. (1998) |
| Spectrophotometry | Visible spectrophotometry | 0.08 mg/kg | – | Chang-Yen and Bidasee (1990) |
| Chromatography | Thin layer chromatography | 0.3 ng | – | Matsudo and Sasaki (1995) |
| Liquid chromatography | 4 ng | – | Lansden (1984) | |
| LC/UV (PDA) | 0.01 µg/ml | 0.03 µg/ml | Saito et al. (2015) | |
| LC–MS/MS | 2.17 µg/kg | 7.15 µg/kg | Vulić et al. (2021) | |
| Solid phase micro-extraction coupled to HPLC | 3–12 ng/ml | 7-29 ng/ml | Aresta et al. (2003) | |
| HPLC–MS/MS | 0.2 µg/kg | 0.5 µg/kg | Ansari and Haeubl (2016) | |
| Immunochemical techniques | Competitive enzyme-linked immunosorbent assay (CI-ELISA) | 2 µg | – | Maragos et al. (2017) |
| Immunoassay utilizing Imaging Surface plasmon resistance | 6–17 µg/kg | – | Hossain et al. (2019) |
LOD Limit of detection; LOQ Limit of Quantification
Possibility of inherent plant toxins
Inherent plant toxins are naturally occurring components in plants that are toxic and/or have negative effects on the bioavailability of nutrients. Toxins in the latter category are often referred to as anti-nutritional factors or anti-nutrients. Some plant toxins exhibit chronic effects, others acute effects, or both (van Egmond 2004). For example, hydrocyanic acid (HCN) in forage sorghum (Hunt and Taylor 1976; Pandey et al. 2011; Karthika and Kalpana 2017; Pushpa et al. 2019), the glucosinolates, a group of sulphur‐containing glucosides in brassica family (Heaney and Fenwick 1995) and trypsin inhibitors (anti-nutritional factors) in soybean (Gilani et al. 2005) are inherent plant toxin or anti-nutritional factors. So far there is no report of presence of such plant toxins or anti-nutritional factors in kodo millets. In case of kodo millet poisoning also, till date there are no reports or data proving that the poisoning is due to inherent plant toxin. Available reports mostly substantiate that the poisoning is due to contamination of kodo millet with fungal metabolites (mycotoxins).
Management
In integrated management system, mycotoxins contamination should be minimized in every phase of production, harvesting, processing and distribution. Prevention through pre-harvest management is the best method for controlling mycotoxins contamination. Although, if contamination prevails, the risk associated with the toxins must be managed through post-harvest methods.
Pre-harvest management
Pre-harvest mitigating strategies include breeding for resistant cultivars. There have been several efforts to breed resistant cultivars for reduced mycotoxin contamination in several crops. In case of sorghum for management of grain mold disease complex, using host plant resistance was the major focus (Das et al. 2012) for development of less susceptible varieties and hybrids. In maize aflatoxin resistant genotypes were identified in West and Central Africa and these sources of resistance are being used in breeding programmes to develop aflatoxin-resistant, high-yielding cultivars adapted to tropical Africa (Hell et al. 2008). In peanut also same strategy is being followed (Leslie et al. 2008). In case of kodo millet screening for mycotoxins (CPA) resistance need to be initiated to manage the CPA contamination before harvest like in other crops. The common pre-harvest management practices for mycotoxins in sorghum or maize or peanut are good crop management practices, such as crop rotation, timely planting and harvesting, adjusting the planting date to avoid end-of-season rains coinciding with the harvest time, maintaining the optimal plant population in the field, taking necessary precautions to control the pest and diseases of the crop by adopting pest management practices, harvesting the crop at right maturity (18–20% moisture in the grains) and avoiding over maturity of the crop (Das et al. 2012; Hell et al. 2008; Rose et al. 2018). These cultural practices can very well be used in kodo millet for reducing or avoiding the contamination by CPA in the field.
Post-harvest management
Kodo poisoning can be minimized by employing proper post-harvest grain management practices. In the post-harvest control measures, drying, storage and processing are the significant aspects where contamination can be prevented in grains (Choudhary and Kumari 2010). Drying is an important step in ensuring good quality grain that is free of fungi and microorganisms (Villers 2014). The harvested field crops should be dried as quickly as possible to recommended moisture levels of 10–13% for cereals (Hell et al. 2008). During storage, the moisture, temperature and relative humidity of the grains are the three main factors to be controlled to keep the growth of fungi in control. A relative humidity of 70% or less (Kabak 2009; Neme and Mohammed 2017) and grain moisture content of 12% or less will reduce the growth of storage molds and their toxins. Water activity of less than 0.7 will not favour any fungal growth/mycotoxin production in the grains (Manna and Kim 2017). Proper drying after harvest and use of air tight containers for safe storage of grains reduces or inhibits the mold growth. Development of the fungus was found to stop in corn when moisture content was below 12–13% (Villers 2014). Proper drying of harvested kodo millet immediately and avoiding further moisture contact by storing in dry, air tight conditions can prevent the fungal contamination to a great extent.
Temperature is another key factor that prevents the growth of molds in the stored grains. Ideally, grain should be cooled after drying and maintained at 1 to 4 °C for the duration of storage (Munkvold 2003). At low or cold temperature, fungal contaminants are not killed, but their growth and metabolism are minimal (Neme and Mohammed 2017). Levels of mycotoxins in contaminated commodities prior to consumption can be reduced by food processing methods such as wet and dry milling, grain cleaning, autoclaving, roasting, baking, frying, extrusion cooking, etc. There are diverse traditional food processing methods that significantly reduce the amount of mycotoxin (aflatoxin) in food prepared from corn and peanuts in different parts of Africa (Hell et al. 2008). It has been reported that in case of rainfall affected kodo millet it was never eaten as flour but as rice and was well washed before being cooked and this produced no bad effects (Chevers 1870). Washing followed by cooking at high temperature not only kills harboring fungi and their spores but also deactivate mycotoxins thus alleviating bad effects.
To prevent poisoning, grains are carefully removed from the glumes, lemma and palea or in other words milled before cooking. When different rice cooking methods used by Indians were compared, it was found that pressure cooking at 15 psi for 5 min gave maximum destruction (72%) of aflatoxin in comparison to the method of ordinary cooking (50%) and cooking with excess water (50%) (Choudhary and Kumari 2010; Fasiha et al. 1979). Pressure cooking of rice not only destroyed the maximum amount of aflatoxins but also preserved nutrients in rice. These management practices can be effectively used for mitigating mycotoxin in different food grains. As CPA is also a secondary metabolite of Aspergillus and Penicillium, like aflatoxins, it may be presumed that the similar practices can be employed for reducing the CPA contamination in kodo millet.
Bio-management
Different microorganisms have been tested to limit fungal development and mycotoxin production as a possible alternative method. These microorganisms may control plant diseases through one or more mechanisms like induction of host resistance to the disease by the production of antimicrobial compounds or direct antagonism to the pathogens and competition with pathogens for space and nutrients (Gonçalves et al. 2019; Compant et al. 2005). Biocontrol agents have been tested successfully as control agents for cereal diseases caused by Fusarium species (Gonçalves et al. 2019; Khan et al. 2006; Khan and Doohan 2009). Trichoderma was found efficient in inhibition of mycotoxin production in rice by Fusarium culmorum and F. graminearum (Matarese et al. 2012). Inhibition of six major rice fungal pathogens has been achieved by the use of Streptomyces corchorusii strain UCR3-16 (Tamreihao et al. 2016). In peanuts aflatoxin and cyclopiazonic acid contamination caused by Aspergillus flavus and A. parasiticus is a serious economic, and potential human and animal health problem. A biological control strategy involving inoculation of fields for several years with competitive, non-toxigenic strains of the same fungi resulted in reductions in aflatoxin contamination (by 30 to 90%) and cyclopiazonic acid (by 85.7%) (Cole and Dorner 1999). In managing the grain mold pathogens in sorghum, Trichoderma viride, T. harzianum and Pseudomonas spp. showed promising results both at laboratory and field levels (Das et al. 2012). As kodo poisoning is caused by different strains of Aspergillus the above practices may be useful and can be practiced to reduce or minimize the contamination of mycotoxin, CPA.
Conclusion
Kodo millet is a nutricereal, and staple food for many tribal and economically weaker sections in India. It is one of the hardiest crops, drought tolerant with high yield potential and excellent storage properties. But production and consumption are on the decline due to various factors. Kodo poisoning is one of the major factors impeding its consumption and the problem needs an immediate attention for effective management. So far studies have concentrated only on detecting the causal organism and its toxin, CPA. Though many other agricultural crops suffer from CPA contamination, major adverse effects have been recorded only in kodo millet because of lack of scientific management. What makes this crop more vulnerable than others for CPA contamination is not yet understood. Agro-climatic requirements of the crop, micro climates surrounding seed, chemical composition of grain, husk and leaves are some of the aspects that should get immediate research attention to unveil the mystery of kodo poisoning. In addition to CPA, Aspergillus species also produces aflatoxin. Is there any synergistic effect of co-presence of CPA and aflatoxins on kodo millet needs further investigation. Efforts should also be made to examine all parts of the plant including green leaves, grain with husk, dehulled grain and fodder for finding out if traces of CPA occur naturally in the crop. To reduce the risk of CPA contamination, integrated management system needs to be adopted for kodo millet and utmost care should be taken at every stage of the crop (pre-harvest to processing) to curb poisoning. Pre-harvest management of mycotoxin contamination is vital to harvest clean grains and to maintain contamination levels near to zero. Breeding for cultivars or traits showing resistance to fungal infection and use of antifungal crop protection chemical agents that are non-toxic to human beings are important measures that can be thought of to nullify kodo millet poisoning and popularizing the crop.
Acknowledgements
The authors gratefully acknowledge the support of Indian Council of Agricultural Research (ICAR), New Delhi, and ICAR-Indian Institute of Millets Research (ICAR-IIMR), Hyderabad.
Author contributions
DC performed literature search and prepared draft. HK reviewed the first draft and critically revised the final version. IKD, JJ and RCV provided inputs in their respective areas. VAT conceived the idea. Rest of the authors commented on the draft.
Funding
The article has not been supported by any special grant.
Declarations
Conflict of interest
No conflict of interest with any agency/researcher.
Consent for publication
All authors have agreed for its publication in JFST.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Annor GA, Marcone M, Bertoft E, Seetharaman K. In-vitro starch digestibility and expected glycemic index of Kodo millet (Paspalum scrobiculatum) as affected by starch-protein-lipid Interactions. Cereal Chem. 2013;90(3):211–217. [Google Scholar]
- Ansari AA, Shrivastava AK. Susceptibility of minor millets to Aspergillus flavus for aflatoxin production. Indian Phytopath. 1991;44:533–534. [Google Scholar]
- Ansari P, Haeubl G. Determination of cyclopiazonic acid in white mould cheese by liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) using a novel internal standard. Food Chem. 2016;211:978–982. doi: 10.1016/j.foodchem.2016.05.063. [DOI] [PubMed] [Google Scholar]
- Antony M, Shukla Y, Janardhanan KK. Potential risk of acute hepatotoxicity of kodo poisoning due to exposure to cyclopiazonic acid. J Ethnopharmacol. 2003;87(2–3):211–214. doi: 10.1016/s0378-8741(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Aresta A, Cioffi N, Palmisano F, Zambonin CG. Simultaneous determination of ochratoxin A and cyclopiazonic, mycophenolic, and tenuazonic acids in cornflakes by solid phase microextraction coupled to high-performance liquid chromatography. J Agric Food Chem. 2003;51:5232–5237. doi: 10.1021/jf034385r. [DOI] [PubMed] [Google Scholar]
- Ayyangar GNR, Panduranga Rao V. Studies in Paspalum scrobiculatum L.—the kodra millet. Madras Agric J. 1934;22:419–425. [Google Scholar]
- Bars JLE. Cyclopiazonic acid production by Penicillium camemberti Thom. and natural occurrence of this mycotoxin in cheese. Appl Environ Microb. 1979;38(6):1052–1055. doi: 10.1128/aem.38.6.1052-1055.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat BV, Dayakar Rao B, Tonapi VA (eds) (2018) The story of millets, Karnataka State Department of Agriculture, Benguluru, India & ICAR-Indian Institute of Millets Research, Hyderabad, India. 58
- Bhide NK. Pharmacological study and fractionation of Paspalum scrobiculatum extract. Br J Pharmacol Chemother. 1962;18(1):7–18. doi: 10.1111/j.1476-5381.1962.tb01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide NK, Aimen RA. Pharmacology of a tranquillizing principle in Paspalum scrobiculatum grain. Nature. 1959;183(4677):1735–1736. doi: 10.1038/1831735b0. [DOI] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, García FA, Kemper AR, Krist AH, Kurth AE, Landefeld CS, et al. Folic acid supplementation for the prevention of neural tube defects: US preventive services task force recommendation statement. J Am Med Assoc. 2017;317(2):183–189. doi: 10.1001/jama.2016.19438. [DOI] [PubMed] [Google Scholar]
- Bradburn N, Coker RD, Blunden G. The aetiology of turkey ‘X’ disease. Phytochemistry. 1994;35(3):817. [Google Scholar]
- Burdock GA, Flamm WG. Safety assessment of the mycotoxin cyclopiazonic acid. Int J Toxicol. 2000;19:195–218. [Google Scholar]
- Chandrasekara A, Shahidi F. Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Funct Food. 2012;4(1):226–237. [Google Scholar]
- Chang PK, Ehrlich KC, Fujii I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins (basel) 2009;1:74–99. doi: 10.3390/toxins1020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Yen I, Bidasee K. Improved spectrophotometric determination of cyclopiazonic acid in poultry feed and corn. J Assoc off Ana Chem. 1990;73(2):257–259. [PubMed] [Google Scholar]
- Chevers N. A manual of medical jurisprudence for India, including the outline of a history of crime against the person in India. Calcutta, India: Thacker, Spink & Co; 1870. p. 861. [Google Scholar]
- Choudhary AK, Kumari P. Management of mycotoxin contamination in preharvest and post harvest crops: present status and future prospects. J Phytology. 2010;46(6):1435–1437. [Google Scholar]
- Cole RJ, Dorner JW. Biological control of aflatoxin and cyclopiazonic acid contamination of peanuts. JSM Mycotoxins Suppl. 1999;2:70–73. [Google Scholar]
- Compant S, Duffy B, Nowak J, Clement C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microb. 2005;71(9):4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das IK, Audilakshmi S, Patil JV. Fusarium grain mold: the major component of grain mold disease complex in sorghum (Sorghum bicolor L. Moench) Eur J Plant Sci Biotech. 2012;6:45–55. [Google Scholar]
- de Wet JMJ, Prasada Rao KE, Mengesha MH, Brink DE. Diversity in kodo millet, Paspalum scrobiculatum. Econ Bot. 1983;37:159–163. [Google Scholar]
- Deshpande SS, Mohapatra D, Tripathi MK, Sadvatha RH. Kodo millet: nutritional value and utilization in Indian foods. J Grain Process Storage. 2015;2(2):16–23. [Google Scholar]
- Dorner JW. Mycotoxins and food safety. Boston, MA: Springer; 2002. Recent advances in analytical methodology for cyclopiazonic acid; pp. 107–116. [DOI] [PubMed] [Google Scholar]
- Dorner JW, Cole RJ, Lomax LG, Gosser HS, Diener UL. Cyclopiazonic acid production by Aspergillus flavus and its effects on broiler chickens. Appl Environ Microb. 1983;46:1435–1437. doi: 10.1128/aem.46.3.698-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Banna AA, Pitt JI, Leistner L. Production of mycotoxins by Penicillium species. Syst Appl Microbiol. 1987;1:42–46. [Google Scholar]
- Fasiha R, Basappa SC, Murthy VS. Destruction of aflatoxin in rice by different cooking methods. J Food Sci Tech. 1979;16(3):111–112. [Google Scholar]
- Frisvad JC. The connection between the Penicillia and Aspergilli and mycotoxins with special emphasis on misidentified isolates. Arch Environ Contam Toxicol. 1989;18:452–467. doi: 10.1007/BF01062373. [DOI] [PubMed] [Google Scholar]
- Galinato MI, Moody K, Piggin CM. Upland rice weeds of South and Southeast Asia. Manila, Philippines: International Rice Research Institute; 1999. p. 154. [Google Scholar]
- Gallagher RT, Richard JL, Stahr HM, Cole RJ. Cyclopiazonic acid production by aflatoxigenic and non-aflatoxigenic strains of Aspergillus flavus. Mycopathologia. 1978;66(1–2):31–36. doi: 10.1007/BF00429590. [DOI] [PubMed] [Google Scholar]
- Gilani GS, Cockell KA, Sepehr E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J AOAC Int. 2005;88(3):967–987. [PubMed] [Google Scholar]
- Gonçalves A, Gkrillas A, Dorne JL, Dall'Asta C, Palumbo R, Lima N, Battilani P, Venâncio A, Giorni P. Pre-and postharvest strategies to minimize mycotoxin contamination in the rice food chain. Compr Rev Food Sci F. 2019;18(2):441–454. doi: 10.1111/1541-4337.12420. [DOI] [PubMed] [Google Scholar]
- Hahnau S, Weiler EW. Monoclonal antibodies for the enzyme immunoassay of the mycotoxin cyclopiazonic acid. J Agric Food Chem. 1983;41:1076–1080. [Google Scholar]
- Hariprasanna K. Genetic improvement in kodo millet. In: Tonapi VA, Patil JV, editors. Millets: ensuring climate resilience and nutritional security. New Delhi, India: Daya Publishing House; 2015. pp. 305–317. [Google Scholar]
- Hariprasanna K. Kodo millet, Paspalum scrobiculatum L. In: Patil JV, editor. Millets and sorghum: biology and genetic improvement. UK: John Wiley & Sons; 2017. pp. 199–225. [Google Scholar]
- Harrison J. Food moulds and their toxicity. Trop Sci. 1971;13:57–63. [Google Scholar]
- Hayashi Y, Yoshizawa T. Analysis of cyclopiazonic acid in corn and rice by a newly developed method. Food Chem. 2005;93(2):215–221. [Google Scholar]
- Heaney RK, Fenwick GR. Natural toxins and protective factors in Brassica species, including rapeseed. Nat Toxins. 1995;3(4):233–237. doi: 10.1002/nt.2620030412. [DOI] [PubMed] [Google Scholar]
- Hegde BR, Gowda BKL. Cropping systems and production technology for small millets in India. In: Seetharam A, Riley KW, Harinarayana G, editors. Small millets in global agriculture. New Delhi: Oxford & IBH Publishing Co.; 1989. pp. 209–236. [Google Scholar]
- Hegde PS, Chandra TS. ESR spectroscopic study reveals higher free radical quenching potential in kodo millet (Paspalum scrobiculatum) compared to other millets. Food Chem. 2005;92(1):177–182. [Google Scholar]
- Hell K, Fandohan P, Bandyopadhyay R, Kiewnick S, Sikora R, Cotty PJ. Pre- and postharvest management of aflatoxin in maize: an African perspective. In: Leslie JF, Bandyopadhyay R, Viscont A, editors. Mycotoxins: detection methods, management, public health and agricultural trade. Wallingford: CAB International; 2008. pp. 219–229. [Google Scholar]
- Heperkan D, Somuncuoglu S, Karbancioglu-Güler F, Mecik N. Natural contamination of cyclopiazonic acid in dried figs and co-occurrence of aflatoxin. Food Control. 2012;23(1):82–86. [Google Scholar]
- Hermansen K, Frisvad JC, Emborg C, Hansen J. Cyclopiazonic acid production by submerged cultures of Penicillium and Aspergillus strains. FEMS Microbiol Let. 1984;21(2):253–261. [Google Scholar]
- Heuzé V, Tran G, Giger-Reverdin S (2015) Scrobic (Paspalum scrobiculatum) forage and grain. In: Feedipedia, a programme by INRA, CIRAD, AFZ and FAO; https://feedipedia.org/node/401
- Holzapfel CW. The isolation and structure of cyclopiazonic acid, a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron. 1968;24(5):2101–2119. doi: 10.1016/0040-4020(68)88113-x. [DOI] [PubMed] [Google Scholar]
- Holzapfel CW, Wilkins DC. On the biosynthesis of cyclopiazonic acid. Phytochemistry. 1971;10:351–358. [Google Scholar]
- Hossain Z, Busman M, Maragos CM. Immunoassay utilizing imaging surface plasmon resonance for the detection of cyclopiazonic acid (CPA) in maize and cheese. Anal Bioanal Chem. 2019;411(16):3543–3552. doi: 10.1007/s00216-019-01835-w. [DOI] [PubMed] [Google Scholar]
- Hunt BJ, Taylor AO. Hydrogen cyanide production by field grown sorghums. New Zeal J Exp Agr. 1976;4(2):191–194. [Google Scholar]
- Kabak B. The fate of mycotoxins during thermal food processing. J Sci Food Agr. 2009;89(4):549–554. [Google Scholar]
- Kajale MD. Ancient grains from India. Bull Deccan College Post-Grad Res Inst. 1974;34(1/4):55–74. [Google Scholar]
- Karthika N, Kalpana R. HCN content and forage yield of multi-cut forage sorghum under different organic manures and nitrogen levels. Chem Sci Rev Lett. 2017;6:1659–1663. [Google Scholar]
- Khan M, Doohan FM. Bacterium-mediated control of Fusarium head blight disease of wheat and barley and associated mycotoxin contamination of grain. Biol Control. 2009;48(1):42–47. [Google Scholar]
- Khan M, Fischer S, Egan D, Doohan F. Biological control of Fusarium seedling blight disease of wheat and barley. Phytopathology. 2006;96(4):386–394. doi: 10.1094/PHYTO-96-0386. [DOI] [PubMed] [Google Scholar]
- Knees SG, Gupta AK (2013) Paspalum scrobiculatum, The IUCN Red List of Threatened Species 2013; e.T168983A1260955. 10.2305/IUCN.UK.2013-1.RLTS.T168983A1260955.en
- Lansden JA. Liquid chromatographic analysis system for cyclopiazonic acid in peanuts. J Assoc off Anal Chem. 1984;67(4):728–731. [PubMed] [Google Scholar]
- Lansden JA. Determination of cyclopiazonic acid in peanuts and corn by thin layer chromatography. J Assoc off Anal Chem. 1986;69(6):964–966. [PubMed] [Google Scholar]
- Lansden JA, Davidson JI. Occurrence of cyclopiazonic acid in peanuts. Appl Environ Microbiol. 1983;45(3):766–769. doi: 10.1128/aem.45.3.766-769.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JF, Bandyopadhyay R, Visconti A, editors. Mycotoxins: detection methods, management, public health and agricultural trade. Wallingford: CAB International; 2008. p. 480. [Google Scholar]
- Liu X, Walsh CT. Cyclopiazonic acid biosynthesis in Aspergillus sp.: characterization of a Reductase-like R Domain in Cyclopiazonate Synthetase that forms and releases cyclo-acetoacetyl-l-tryptophan. Biochemistry. 2009;48(36):8746–8757. doi: 10.1021/bi901123r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax LG, Cole RJ, Dorner JW. The toxicity of cyclopiazonic acid in weaned pigs. Vet Pathol. 1984;21(4):418–424. doi: 10.1177/030098588402100408. [DOI] [PubMed] [Google Scholar]
- Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K, editors. Indian food composition tables. Hyderabad, India: National Institute of Nutrition, Indian Council of Medical Research; 2017. p. 505. [Google Scholar]
- Luk KC, Kobbe B, Townsend JM. Production of cyclopiazonic acid by Aspergillus flavus Link. Appl Environ Microbiol. 1977;33(1):211–212. doi: 10.1128/aem.33.1.211-212.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi D, Thilagavathi T, Sindhumathi G. Traditional Recipes from Kodo millet. Coimbatore, India: Tamil Nadu Agricultural University; 2012. p. 14. [Google Scholar]
- Manna M, Kim KD. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobiology. 2017;45(4):240–254. doi: 10.5941/MYCO.2017.45.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos CM, Sieve KK, Bobell J. Detection of cyclopiazonic acid (CPA) in maize by immunoassay. Mycotoxin Res. 2017;33(2):157–165. doi: 10.1007/s12550-017-0275-0. [DOI] [PubMed] [Google Scholar]
- Matarese F, Sarrocco S, Gruber S, Seidl-Seiboth V, Vannacci G. Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology. 2012;158(1):98–106. doi: 10.1099/mic.0.052639-0. [DOI] [PubMed] [Google Scholar]
- Matsudo T, Sasaki M. Simple determination of cyclopiazonic acid. Biosci Biotech Bioch. 1995;59(3):355–357. [Google Scholar]
- McGrath RM, Steyn PS, Ferreira NP, Neethling DC. Biosynthesis of cyclopiazonic acids in Penicillium cyclopium: the isolation of dimethylallylpyrophosphate: cyclo-acetoacetyltryptophanyl dimethylallyltransferase. Bioorg Chem. 1976;4:11–23. [Google Scholar]
- Moldes-Anaya AS, Eriksen GS, Skaar I, Rundberget T. Determination of cyclopiazonic acid in food and feeds by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2009;1216:3812–3818. doi: 10.1016/j.chroma.2009.02.061. [DOI] [PubMed] [Google Scholar]
- Munkvold GP. Cultural and genetic approaches to managing mycotoxins in maize. Ann Rev Phytopathol. 2003;41(1):99–116. doi: 10.1146/annurev.phyto.41.052002.095510. [DOI] [PubMed] [Google Scholar]
- Neme K, Mohammed A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. Food Control. 2017;78:412–425. [Google Scholar]
- Ostry V, Toman J, Grosse Y, Malir F. Cyclopiazonic acid: 50th anniversary of its discovery. World Mycotoxin J. 2018;11(1):135–148. [Google Scholar]
- Pall BS, Jain AC, Singh SP. Diseases of lesser millets. Jabalpur, Madhya Pradesh, India: JNKVV; 1980. p. 66. [Google Scholar]
- Pandey RK, Kumar D, Jadhav KM. Assessment of determinants for reducing HCN content in Sorghum used for ruminant in Gujarat, India. Livest Res Rural Dev. 2011;23:66. [Google Scholar]
- Prasongsidh BC, Kailasapathy K, Skurray GR, Bryden WL. Analysis of cyclopiazonic acid in milk by capillary electrophoresis. Food Chem. 1998;61:515–519. [Google Scholar]
- Pushpa K, Madhu P, Venkatesh Bhat B. Estimation of HCN content in sorghum under irrigated and stressed conditions. J Pharmacogn Phytochem. 2019;8(3):2583–2585. [Google Scholar]
- Rao BL, Husain A. Presence of cyclopiazonic acid in kodo millet (Paspalum scrobiculatum) causing ‘kodua poisoning’ in man and its production by associated fungi. Mycopathologia. 1985;89(3):177–180. doi: 10.1007/BF00447028. [DOI] [PubMed] [Google Scholar]
- Rose LJ, Okoth S, Flett BC, van Rensburg BJ, Viljoen A. Pre-harvest management strategies and their impact on Mycotoxigenic fungi and associated Mycotoxins—Impact and Management Strategies. London: IntechOpen; 2018. [Google Scholar]
- Saito K, Baba N, Sasaki M, Watanabe M, Ito R, Kato M, Ishii R, Hosoe T. Development of analytical method for cyclopiazonic acid in liquid seasoning by LC/UV(PDA) and LC/TOF-MS, and its validation study. Jpn J Food Chem Safety. 2015;22(3):163–169. [Google Scholar]
- Swarup A. Acute “Kodon” Poisoning. Indian Med Gazette. 1922;57(7):257. [PMC free article] [PubMed] [Google Scholar]
- Tamreihao K, Ningthoujam DS, Nimaichand S, Singh ES, Reena P, Singh SH, Nongthomba U. Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol Res. 2016;192:260–270. doi: 10.1016/j.micres.2016.08.005. [DOI] [PubMed] [Google Scholar]
- van Egmond HP. Natural toxins: risks, regulations and the analytical situation in Europe. Anal Bioanal Chem. 2004;378(5):1152–1160. doi: 10.1007/s00216-003-2373-4. [DOI] [PubMed] [Google Scholar]
- Villers P. Aflatoxins and safe storage. Front Microbiol. 2014;5:158. doi: 10.3389/fmicb.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinokurova NG, Ivanushkina NE, Khmel’nitskaya II, Arinbasarov MU. Synthesis of α-cyclopiazonic acid by fungi of the genus Aspergillus. Appl Biochem Microbiol. 2007;43(4):435. [PubMed] [Google Scholar]
- Vulić A, Lešić T, Kudumija N, Zadravec M, Kiš M, Vahčić N, Pleadin J. The development of LC-MS/MS method of determination of cyclopiazonic acid in dry-fermented meat products. Food Control. 2021;123:107814. [Google Scholar]
- Yadava HS, Jain AK. Advances in Kodo millet research. New Delhi, India: DIPA, Indian Council of Agricultural Research; 2006. p. 95. [Google Scholar]
- Yu W, Chu FS. Improved direct competitive enzyme-linked immunosorbent assay for cyclopiazonic acid in corn, peanuts, and mixed feed. J Agric Food Chem. 1998;46:1012–1017. [PubMed] [Google Scholar]
- Zorzete P, Baquião AC, Atayde DD, Reis TA, Gonçalez E, Corrêa B. Mycobiota, aflatoxins and cyclopiazonic acid in stored peanut cultivars. Food Res Int. 2013;52:380–386. [Google Scholar]


