Abstract
Wheat (T1) and maize (T2) based sattu were formulated using chick pea, barley and other food adjuncts. Results revealed that, no significant difference was observed in the protein and ash content between control (chickpea basede sattu mix) and T1. However, significant difference was observed between the control and T2 sample between moisture, fat, insoluble and total fiber, with the exception of protein, soluble fiber and ash. In case of T1 and T2, significant difference was observed in fat and total fiber content. Coming on to the mineral composition, significant difference was observed between the mineral content in control and T1, with respect to calcium, potassium, sodium, magnesium, iron, manganese and copper. In case of control and T2, significant difference was observed with respect to calcium, potassium, sodium, magnesium, iron and copper, with the exception of manganese. Coming on to the mineral content of both the formulations, significant difference was observed with respect to all the minerals estimated. Significant difference was observed in the total amylose content between control and formulated samples on 0, 30 and 60 days. Microstructural studies of raw and roasted sattu and its ingredients by observation under scanning electron microscope revealed that substantial structural changes occurred during processing. The raw grains were tightly packed and contained no air spaces. However, a large number of air spaces are formed in the cotyledon of the roasted grain sample. It was observed that, T1and T2 had medium GI value (56 and 58% respectively), Whereas for control it was 60%. The formulated samples were found to be shelf stable for 60 days at RT, with an increase in moisture content of 4–6%. All the samples were sensorial acceptable and there was no perceptible off odor or off taste.
Keywords: Sattu, Proximate composition, Shelf life, Ready to eat, Convenience food
Introduction
Cereals are popular among the Indian population for their breakfast course, such as; rice flakes (poha) is common in western and central regions and bulgar wheat (dalia) in northern region of the country etc. Indian breakfast has always been a quintessential hot and cooked meal (naashta) and Indians find it quite difficult to shift to breakfast options like cereals with milk. However, with increase in purchasing power and need for convenience, lifestyles are gradually changing. These factors have stimulated Indians to opt for breakfast cereals, especially in urban areas. Convenience food is any food that can be prepared and packaged, ready to eat with minimum preparation. These are practical, easy solutions that have evolved enormously and ensure there is no need to compromise on taste or quality while consuming eatables. In the recent past, hot cereals and muesli have been the fastest growing breakfast categories. Oats with different varieties and flavours has gained high popularity and acceptance, among the hot cereals.
The breakfast cereal market already offers various product categories and they are introduced to meet the requirements of different age groups like Kellogg’s in kids category, Kellogg’s for women, for all family and for aging adults. The most popular breakfast cereal in India is corn flakes with a market share of over 50% in this category, followed by oats and muesli. Broadly breakfast cereals may be classified as Ready to eat (RTE) eg, cornflakes, multi grain flakes, puffed cereals, granola bars, popcorn etc., Ready to cook (RTC) eg. instant and quick cooking oats, oatmeal and muesli etc. Another category is of Precooked /Processed cereals where in the consumption level of these cereals is maximum among breakfast cereals. They can again be divided into RTE foods and RTC foods. RTE cereal foods here include cereal/malt based food, pulse and crisp snacks etc. RTC cereal foods include fermented / non fermented products, enriched products, such as idly, vada, dholka etc.
Sattu is a flour consisting of a mixture of roasted grains. The process of preparing sattu is known since ancient times and is popular in India, particularly Bihar. sattu is a wonder flour, nutritionally rich, with an excellent shelf life, that can be consumed uncooked. sattu is a wonder flour that can be consumed uncooked. The cooling property of sattu makes it a perfect summer drink. It is preferred for its high fiber content and medium to low Glycemic Index. High fiber foods normalize the bowel movement. The soluble fiber found in oats etc. may help lower total blood cholesterol level and insoluble fiber, promote the movement of material through digestive system and increase stool bulk. A variety of sattu is prepared by the people according to their choice and raw material prevalent in the area. Bengal gram sattu is commonly produced in Bihar and eastern Uttar Pradesh but in other parts of Uttar Pradesh, Barley based sattu is preferred by the population. In Punjab sattu is usually made with Barley and maize. It contains protein and provides high nutritional benefits for human population and is rich in minerals like Calcium, Magnesium, Iron, Sodium etc. This is a traditional age old product, which can be prepared in the homes. With this sattu mix we can derive many other food items like porridge, laddu, sherbet etc. It is easy to consume as a breakfast drink in different flavor with water. People can consume this early in the morning on an empty stomach. Major advantage is that the production cost is very less when compared with others breakfast cereals.
Though traditional Sattu mix is already available an attempt was taken to prepare instant sattu mix using multigrain and pulses, which has the goodness of chick pea, canadian pea, barley, wheat, maize, and oats. These grains enhance the nutritional quality which reflects increased health benefit as compared to the traditional sattu. More over the intermediate glycemic index of the developed sattu makes it suitable for obese and diabetic population. In the present study 2 types of instant sattu has been formulated wheat and maize based using chickpea and other ingredients and proximate, functional and shelf life studies have been carried out using market sattu as a control sample.
Methods and materials
Procurement of raw materials
The ingredients such as wheat, maize, canadian pea, chick pea, oats, barley were procured (as per the standards laid down by FSSAI) from APMC, Bandipalaya, Mysore, They were stored in an air tight container for protecting them from the pests and insects. Analytical grade (Sigma) chemicals were used for the study, unless stated otherwise. Standards of minerals were produced from Sigma, India.
Physical properties of the grains
All the dimensional parameters viz, grain length (L) breadth (B) and thickness (T) was measured with Vernier calipers. The bulk density was determined using the mass/volume relationship. Expansion ratio was estimated by measuring the volume of pre-weighed grains before and after puffing, using a graduated cylinder tapped 3-5 times to allow uniform compacting of the grain. Sphericity was done as per the method of Mohsenin (1986).
Steps for formulating sattu mix
Formulation of sattu mix
The grains like Canadian peas (4 hr), maize (2 hr) and chick pea (2 hr) were soaked in water for respective duration, after which water was drained off, and each grain was roasted separately at 100–120°C for approximately 45 minutes. After bringing down the temperature to room temperature, the grains were pulverized using hammer mill. wheat, oats and barley were pulverized after roasting. The powdered ingredients were passed through 60 mesh and mixed in appropriate proportion for making two formulations. The first formulation was T1 (wheat based sattu), wherein pulverized flours of chickpea, barley, oats, wheat and canadian peas, were mixed in the ratio of 3:3:1:2:1. The other formulation was maize based wherein pulverized flours of chickpea, barley, oats, maize and canadian peas were mixed in the ratio of 3:3:1:2:1. The flours were mixed and then packed in 100 gm pet packets.
Proximate analysis
The two formulations standardized were analyzed for proximate composition, keeping market sattu as control sample. Standard methods of analysis were used for proximate analysis of the samples. For the determination of moisture content, the sample was dried for two hours at 130 °C. Moisture was estimated by determining the loss in weight upon drying the sample in an oven maintained at 130 °C for 120 minutes. Initial weight of empty moisture cup was noted down (W1). Small amount of sample was taken in this pre-weighed moisture cup. Weight of the cup with sample is noted down (W2). Moisture cup with the sample is incubated for 120 minutes at 130 °C in hot air oven. A final weight of the moisture cup is taken after 120 minutes (W3). Fat was extracted using soxhlet apparatus, with petroleum ether and ash content was determined using the methods stated in AOAC (2000). The total nitrogen and crude protein (Nx5.95) was determined using micro-Kjeldahl method. For the estimation of dietary fiber, the method of Englyst and Hudson (1996) was used. Carbohydrate content was determined by difference. Carbohydrate (%) = 100 − (% moisture + fat + protein + ash). Whereas the energy content was calculated based on the formula, Energy (kJ/100g) = (Crude protein × 16.7) + (Crude fat × 37.7) + (Carbohydrate × 16.7). Mineral contents were determined using Atomic Absorption Spectrophotometry after digesting with concentrated hydrochloric acid using the standard method of analysis for Iron, Copper, Sodium, Magnesium and Potassium (AOAC 2000).
Colour measurement
The colour values of the sattu samples after polishing was also determined using hunter lab scan XE model (M/S Hunter Associate Laboratory Inc., Reston –V.A., USA) with a view angle of 2˚. Hunter L* [black (0)/white (100)], a* [red (+)/green (−)] and b* [yellow (+)/blue (−)] colour scale was selected for all measurements. Sattu samples were kept on the specimen port adjusting at various degrees. Five measurements were made on each sample, after shaking the sample gently, and the average values of L, a and b were noted. The amount of variation, if any in the sample, was taken into account by shaking the samples every time the measurement was done, so that the effect of void space and orientation of the sattu powder was nullified.
Amylose estimation
Amylose content was estimated using the method of (Bhattacharya et al. 1971) from the respective defatted sattu flours. The total, soluble and insoluble amylose content equivalents were derived keeping potato amylose as standard.
Functional properties
2.7.1 Swelling and solubility characteristic
Swelling and solubility characteristic were measured at temperatures like 30, 60, 90 °C according to the method of Singh et al. 2000. About 500 mg (dry wt. basis) of sample was cooked in 20 ml of water at various temperature ranging from 30°C, 60°C, and 90°C for 30 minutes. They were weighed and made equivalent to 25.5 ml by adding distilled water. It was centrifuged at 3000 rpm for 15 minutes. Supernatant was collected and the residue was weighed for the determination of swelling power. 10 ml of the supernatant was taken in a pre-weighed petridish and kept on the water bath for evaporation. The dishes were dried at 105°C for 3 hrs. Cooled and weighed.
Water absorption and soluble index
Water absorption capacity (wai) of sattu mix and water solubility Index (wsi) was determined from the amount of dried solids recovered by evaporating the supernatant from the sattu mix according to the method of Anderson et al. 1969. In particular, the wai and wsi can be used to estimate the functional characteristics of foods and predict how the materials may behave if further processed. Moreover, these indices give information of the physicochemical changes of the biopolymers as a result of extrusion processing. 2.5 g of sample was suspended in 30 ml of water in a 50-ml pre-weighed centrifuge tube, stirred intermittently for 30 min at 30°C and centrifuged at 3000 rpm for 10 to15 min. The supernatant liquid was poured carefully into a pre-weighed evaporating dish. The remaining pellet was weighed and the wai calculated from its weight. As an index of water solubility, the amount of dried solids recovered by evaporating the supernatant from the water absorption test was expressed as percentage of dry solids in the 2.5 g sample.
Particle size analysis
Samples sieved with 60 mesh were used for particle size analysis by Microtrac Blue Wave Particle size analyzer.
Scanning electron microscopy
SPI conductive carbon paint was used to glue the samples to aluminum stubs. S150P Edwards sputter coater was used to coat the mounted specimens with gold/palladium film of 15nm thickness. Scanning electron Microscope was used to examine the specimens, at an accelerating potential of 20 kV. Images were captured and digitized. The final images were photographed in 20 µm scale and it have good resolution at 1000X.
Health promoting components
Estimated glycemic index
The Glycemic Index (eGI) of the samples was determined according to the methodology described by Goni et al. 1997, with some modifications. The Hydrolysis Index (HI) for each sample was calculated as the ratio between the AHC of each sample and the AHC of white bread, used as reference, and expressed in percentage. 50 mg of defatted sample was weighed and treated with 1 g of weighed pepsin enzyme which was dissolved in 0.2 ml of HCl-KCl buffer. 9.8 ml of HCl-KCL buffer, was added to this. It was incubated at 40 °C for 1 hr, in a shaking water bath. Subsequently, 25 ml of Tris-Malate buffer containing 100 µl of α-amylase was added. It was incubated in a shaking water bath at 37 °C. From this 1 ml aliquot was taken for every 30 minutes from 30 to 180 minutes. These aliquots were placed in a test tube at 100 °C and were energetically shaken for 15 minutes and refrigerated. After all the aliquots were collected till 3 hrs, 3 ml of 0.4 M of sodium acetate buffer followed by 60 ml of amyloglucosidase, then kept at 60 °C in shaking water bath for 45 minutes. This was made up to a known volume of 100 ml. 0.1 ml was taken and glucose released was measured using POD-GOD kit and the color reaction was measured in a UV/VIS spectrophotometer, 505 nm. Estimated GI was thereby estimated using the model Eq. 1.
| 1 |
In-vitro protein digestibility (IVPD)
IVPD was determined by pepsin followed by pancreatin digestion according to Akenson & Statman 1964. The digested protein relative to the total protein was expressed as percent digestibility. Protein digestibility was calculated by the formula (Eq. 2):
| 2 |
Self life analysis
Moisture
The sample was dried for two hours at 130 °C to determine the moisture content.
Free fatty analysis
Fat was extracted using petroleum ether (60–80°C) for 5hours/overnight at room temperature. Using Whattman filter paper no. 1, the extract was filtered and the filtrate was equally divided. In a pre-weighed Petri dish, half of the filtrate was evaporated on water bath and was dried for1 hour at 105 °C. To the other part of filtrate, equal volume of warm neutral alcohol was added and then titrated against known concentration of alkali using phenolphthalein as an indicator. FFA was expressed as Oleic Acid (%) and for determination, the following formula (Eq. 3) was used:
| 3 |
Sensory evaluation of sattu
Quantitative Descriptive Analysis (QDA) is used for profiling the samples (Lawless and Klein 1991). A suitable score card with selective sensory attributes such as, appearance, taste, mouthfeel after taste and overall quality were looked into. 9- points’ hedonic scale was used to judge the sensory quality of the product by 10 semi trained panellists. After the panel judgement, the mean value was calculated for each attribute.
Statistical analysis
The experiments were done with three duplicate samples and the average values were reported. The results are expressed as mean ± standard deviation. Paired t test was carried out between the proximate, minerals and colour profile for control, T1 and T2 samples.
Results and discussion
Physical properties of the grains
The bulk density of the grains varies from 0.771 to 0.796 kg/m3 . Higher bulk density was observed in barley (0.796 kg/m3) followed by maize (0.792 kg/m3). Minimum bulk density was observed in Canadian peas (0.721 kg/m3). Significant difference was observed between the bulk density between Canadian peas and barley (Table 1). Chick pea and wheat had moderate bulk density value, i.e. 0.771 kg/m3 and 0.772 kg/m3 respectively. The bulk density of product thus increased with increasing fiber content. The grain length ranged from 5.406 mm (barley) for small ones to 10.43 mm (maize) for big ones, the width of the grain varied from 3.298 mm to 8.72 mm and the thickness of grain was between 2.251 mm and 6.879 mm. Principal dimensions of grains are useful in selecting sieve separators, which can aid in grain grading and uniformity. They can also be used to calculate volume of kernels, which are important during modelling of grain drying, aeration, heating and cooling (Sahay and Singh 1996). Significant difference was observed with respect to grain length between barley and chickpea, Canadian peas and maize. Similarly, significant difference was observed between the width of barley and chickpea, Canadian peas and maize. In case of thickness, significant difference was observed between barley and all the other grains. Expansion ratio is the ratio between the volume of raw grains and puffed grains. Maximum expansion ratio was observed for maize 2.679 %. Expansion volume correlated positively with sphericity. The sphericity values were high for canadian peas (93.91%), followed by chick pea (79.87%), maize (69.55%),wheat (67.28%) and barley (63.33%). A higher sphercity and higher expansion volume was seen in smaller, shorter and broader kernels. Maize required 2–3 minutes for puffing at 200 °C. In wheat and barley there was 10-fold increase in volume after puffing. Total expansion ratio of wheat and barley was 1.385 and 1.577 respectively. Canadian pea (1.23 %) and chick pea (1.12 %) showed least expansion ratio compared with others and it required 3 and 5 minutes respectively for popping at 200°C. Significant difference was observed in the expansion ratios between chickpea and barley, wheat and maize.
Table 1.
Physical, functional and shelf life studies of sattu mix
| S.No | Samples | Chickpea | Canadian Pea | Barley | Wheat | Maize |
|---|---|---|---|---|---|---|
| Physical Parameters | Bulk density(Kg/m3) | 0.771 ± 0.02a | 0.721 ± 0.04a | 0.796 ± 0.07b | 0.772 ± 0.02a | 0.792 ± 0.08a |
| Length (mm) | 8.886 ± 0.05c | 7.785 ± 0.06b | 5.406 ± 0.09a | 5.63 ± 0.04a |
10.430 ± .15d |
|
| Width (mm) | 6.483 ± 0.06b | 7.299 ± 0.10c | 3.298 ± 0.09a | 3.378 ± 0.10a |
8.72 ± 0.05d |
|
| Thickness(mm) | 6.206 ± 0.03b | 6.879 ± 0. 05c | 2.251 ± 0.02a | 2.858 ± 0.03d | 4.198 ± 0.04e | |
| Expansion Ratio (%) | 1.125 ± 0.03a | 1.236 ± 0.05a | 1.577 ± 0.03b | 1.385 ± 0.02c |
2.679 ± 0.06d |
|
| Spherisity(%) | 79.871 ± 0.047c | 93.919 ± 0.22d | 63.332 ± 0.067a | 67.282 ± 0.056a |
69.555 ± 0.08b |
|
| Amylose Content (%) | Days | Samples | Insoluble Amylose (%) | Soluble Amylose (%) | Total Amylose (%) | |
| 0 | Control Sample | 18.61 ± 0.15a | 6.53 ± 0.11a | 25.14 ± 0.05a | ||
| 0 | Formulation1 (T1) | 19.21 ± 0.12a | 11.58 ± 0.09b | 30.80 ± 0.04b | ||
| 0 | Formulation 2 (T2) | 17.80 ± 0.10a | 11.68 ± 0.10b | 29.48 ± 0.10b | ||
| 30 | Control Sample | 18.87 ± 0.09a | 6.28 ± 0.06a | 25.16 ± 0.11a | ||
| 30 | Formulation1 (T1) | 19.23 ± 0.10a | 11.61 ± 0.05b | 30.84 ± 0.09b | ||
| 30 | Formulation 2 (T2) | 17.87 ± 0.05a | 11.75 ± 0.06b | 29.62 ± 0.08b | ||
| 60 | Control Sample | 18.70 ± 0.10a | 6.45 ± 0.07a | 25.15 ± 0.02a | ||
| 60 | Formulation1 (T1) | 19.23 ± 0.11a | 11.68 ± 0.03b | 30.91 ± 0.06b | ||
| 60 | Formulation 2 (T2) | 17.86 ± 0.12a | 11.88 ± 0.12b | 29.74 ± 0.02b | ||
|
Functional Properties Swelling Power (%) |
Temperature | 30 °C | 50 °C | 70 °C | 90 °C | |
| Control sample | 36.89 ± 0.06a | 41.51 ± 0.08a | 41.51 ± 0.11a | 44.26 ± 0.11a | ||
| Formulation1 (T1) | 16.72 ± 0.06b | 24.76 ± 0.07b | 20.53 ± 0.06b | 27.89 ± 0.05b | ||
| Formulation 2 (T2) | 16.57 ± 0.09b | 22.65 ± 0.11b | 22.22 ± 0.02b | 27.480.06b | ||
| Solubility (%) | Temperature | 30 °C | 50 °C | 70 °C | 90 °C | |
| Control sample | 18.64 ± 0.06a | 20.73 ± 0.08a | 20.02 ± 0.06a | 23.00 ± 0.05a | ||
| Formulation1 (T1) | 9.43 ± 0.08b | 13.35 ± 0.08b | 12.17 ± 0.04b | 16.14 ± 0.11b | ||
| Formulation 2 (T2) | 9.16 ± 0.02b | 12.41 ± 0.06b | 12.87 ± 0.11b | 15.90 ± 0.10b | ||
| WAI ( g/g) | Days | 0 Day | 30 Day | 60 Day | ||
| Control sample | 3.19 ± 0.05a | 3.34 ± 0.06a | 3.47 ± 0.07a | |||
| Formulation1 (T1) | 2.69 ± 0.06b | 3.17 ± 0.08a | 3.37 ± 0.09a | |||
| Formulation 2 (T2) | 3.04 ± 0.08b | 3.18 ± 0.06a | 3.33 ± 0.08a | |||
| WSI (%) | Days | 0 Day | 30 Day | 60 Day | ||
| Control sample | 16.85 ± 0.03a | 13.30 ± 0.06a | 11.70 ± 0.03a | |||
| Formulation1 (T1) | 16.05 ± 0.06b | 10.06 ± 0.08b | 9.53 ± 0.02b | |||
| Formulation 2 (T2) | 14.50 ± 0.08b | 5.57 ± 0.07a | 8.75 ± 0.07b | |||
| Particle size | Diameter (µm) | Volume(%) | Width | |||
| Control sample | 48.37 ± 0.12 | 33.33 ± 0.10 | 26.93 ± 0.11 | |||
| Formulation1 (T1) | 76.79 ± 0.11 | 33.33 ± 0.12 | 56.84 ± 0.13 | |||
| Formulation 2 (T2) | 47.91 ± 0.15 | 50 ± 0.10 | 56.56 ± 0.15 | |||
| Shelf life studies | 0 Day | 15 Day | 30 Day | 45 Day | 60 Day | |
| Moisture (%) | Control sample | 8.3 ± 0.15a | 9.3 ± 0.10a | 9.3 ± 0.12a | 9.3 ± 0.11a | 9.5 ± 0.03a |
| Formulation1 (T1) | 6.6 ± 0.12b | 6.7 ± 0.12b | 6.8 ± 0.10b | 6.8 ± 0.09b | 7.0 ± 0.06b | |
| Formulation 2 (T2) | 6.6 ± 0.09b | 6.7 ± 0.06b | 6.8 ± 0.11b | 6.8 ± 0.08b | 6.9 ± 0.10b | |
| Free fatty acid (%) | Control sample | 0.18 ± 0.05a | 0.28 ± 0.06 | 0.36 ± 0.12 | 0.36 ± 0.15 | 0.38 ± 0.12 |
| Formulation1 (T1) | 0.21 ± 0.06b | 0.29 ± 0.05 | 0.35 ± 0.10 | 0.36 ± 0.12 | 0.39 ± 0.14 | |
| Formulation 2 (T2) | 0.21 ± 0.08b | 0.29 ± 0.10 | 0.33 ± 0.09 | 0.35 ± 0.10 | 0.36 ± 0.11 |
Values are mean ± Standard deviation of three determinations (n = 3). Values with the different superscript, within columns (I) within rows (II, III &IV) are significantly different at p < 0.05
Proximate analysis of sattu
Proximate composition of the samples is presented in Table 2 which indicates the protein, fat, fiber and ash of the samples formulated and control. Significant difference was observed in the moisture, fat, fiber soluble, insoluble and total fiber content, between control and T1, however no significant difference was observed in the protein and ash content between control and T1. Similarly, significant difference was observed between the control and formulation 2 (T2) between moisture, fat, insoluble and total fiber, with the exception of protein, soluble fiber and ash. In case of T1 and T2, significant difference was observed in fat, as reflected from t value, t (2) =8.55, p= 0.01 and total fiber content, t (2) =6.52, p=0.02. Barros et al. 2010 reported that, the use of whole wheat flour instead of refined flour significantly improved the nutritional profile of flour tortillas. Similarly, wheat flour used for formulating sattu had a positive impact on the nutritional composition of the sattu mix. There are different functional foods developed in different countries like USA, Japan, EU from whole grains considering its nutritional potential (Curic et al. 2006). Indrani et al. 2011, reported that the, effect of replacement of whole wheat flour with multigrain blend, increased the protein, fat, dietary fibre and mineral contents of north Indian parotta. Multi-whole grain mix also contributes significantly to RDA of protein (22 %), fibre (51 %) and calorie (18 %). This indicates that multigrain composition is nutritionally superior to refined grains. Fardet et al.2007, reported that, whole grain and refined wheat flours showed distinct metabolic profiles in rats due to difference in the content of health beneficial components. Jones 2007 reported that, whole grains and dietary fibre continue to win honors in preventing various diseases. Wholegrain foods like sattu and its products are healthy and contain high fiber which is good for gut bacteria. Coming on to the mineral composition, significant difference was observed between the mineral content in control and T1, with respect to calcium, potassium, sodium, magnesium, iron, manganese and copper. In case of control and T2, significant difference was observed with respect to calcium, potassium, sodium, magnesium, iron and copper, with the exception of manganese (Table 2). Coming on to the mineral content of both the formulations, significant difference was observed with respect to all the minerals estimated.
Table 2.
Paired t test table for proximate composition, minerals and colour for Sattu mix
| SAMPLES proximate data, minerals and colour (Paired t test between C v/s T1) |
Mean | Std. deviation | T value | df | Sig |
|---|---|---|---|---|---|
| Moisture (C) | 8.40 | 0.13 | 10.70 | 2 | 0.009 |
| Moisture (T1) | 6.66 | 0.15 | |||
| Protein(C) | 11.19 | 0.36 | 2.29 | 2 | 0.149 |
| Protein(T1) | 12.31 | 0.50 | |||
| Fat (C) | 6.90 | 0.23 | 42.57 | 2 | 0.001 |
| Fat (T1) | 3.34 | 0.09 | |||
| Fiber Insoluble (C) | 9.00 | 0.05 | 57.15 | 2 | 0.000 |
| Fiber Insoluble(T1) | 10.65 | 0.10 | |||
| Fiber soluble(C) | 3.44 | 0.11 | 22.25 | 2 | 0.002 |
| Fiber soluble(T1) | 4.33 | 0.05 | |||
| Total Fiber (C) | 12.58 | 0.07 | 12.42 | 2 | 0.006 |
| Total Fiber(T1) | 15.30 | 0.30 | |||
| Ash (C) | 2.55 | 0.05 | 1.000 | 2 | 0.423 |
| Ash(T1) | 2.60 | 0.10 | |||
| Carbohydrate(C) | 70.77 | 0.67 | 8.68 | 2 | 0.013 |
| Carbohydrate(T1) | 75.66 | 0.50 | |||
| Energy (C) | 1634 | 2.82 | 179.92 | 2 | 0.000 |
| Energy (T1) | 1383 | 0.93 | |||
| Minerals | |||||
| Calcium (C) | 43 | 1.0 | 20.00 | 2 | 0.002 |
| Calcium (T1) | 49.66 | 1.52 | |||
| Potassium(C) | 268.0 | 2.64 | 119.00 | 2 | 0.000 |
| Potassium (T1) | 347.33 | 2.51 | |||
| Sodium (C) | 46.66 | 1.52 | 16.83 | 2 | 0.028 |
| Sodium (T1) | 37.00 | 2.00 | |||
| Magnesium (C) | 51.72 | 1.42 | 33.78 | 2 | 0.002 |
| Magnesium (T1) | 93.33 | 3.05 | |||
| Iron (C) | 4.13 | 0.20 | 0.84 | 2 | 0.018 |
| Iron(T1) | 6.20 | 0.03 | |||
| Manganese (C) | 1.48 | 0.03 | 0.25 | 2 | 0.009 |
| Manganese (T1) | 1.91 | 0.03 | |||
| Copper (C) | 0.65 | 0.02 | 0.14 | 2 | 0.006 |
| Copper (T1) | 0.86 | 0.03 | |||
| Colour | |||||
| Lightness (C) | 80.49 | 0.13 | 3.02 | 2 | 0.094 |
| Lightness (T1) | 81.27 | 0.33 | |||
| Redness (C) | 3.22 | 0.20 | 0.032 | 2 | 0.977 |
| Redness (T1) | 3.21 | 0.03 | |||
| Yellowness (C) | 25.86 | 0.20 | 45.64 | 2 | 0.000 |
| Yellowness (T1) | 19.76 | 0.25 | |||
| Darkness (C) | 30.73 | 0.24 | 60.84 | 2 | 0.000 |
| Darkness (T1) | 25.46 | 0.30 | |||
| Paired t test between C v/s T2 | |||||
| Moisture (C) | 8.40 | 0.13 | 13.48 | 2 | 0.005 |
| Moisture (T2) | 6.53 | 0.08 | |||
| Protein(C) | 11.19 | 0.36 | 3.32 | 2 | 0.080 |
| Protein(T2) | 12.26 | 0.29 | |||
| Fat (C) | 6.90 | 0.23 | 12.17 | 2 | 0.007 |
| Fat (T2) | 4.47 | 0.13 | |||
| Fiber Insoluble (C) | 9.00 | 0.05 | 24.78 | 2 | 0.002 |
| Fiber Insoluble(T2) | 10.50 | 0.05 | |||
| Fiber soluble(C) | 3.44 | 0.11 | 0.776 | 2 | 0.519 |
| Fiber soluble(T2) | 3.60 | 0.43 | |||
| Total Fiber (C) | 12.58 | 0.07 | 22.91 | 2 | 0.002 |
| Total Fiber(T2) | 14.33 | 0.07 | |||
| Ash (C) | 2.55 | 0.05 | 1.73 | 2 | 0.225 |
| Ash(T2) | 2.70 | 0.13 | |||
| Carbohydrate(C) | 70.77 | 0.67 | 7.4 | 2 | 0.018 |
| Carbohydrate(T2) | 74.65 | 0.53 | |||
| Energy (C) | 1634 | 2.82 | 24.09 | 2 | 0.002 |
| Energy (T2) | 1610 | 1.09 | |||
| Minerals | |||||
| Calcium (C) | 43 | 1.00 | 9.60 | 2 | 0.011 |
| Calcium (T2) | 63 | 3.00 | |||
| Potassium(C) | 268 | 2.64 | 29.00 | 2 | 0.001 |
| Potassium (T2) | 287 | 2.51 | |||
| Sodium (C) | 46.66 | 1.52 | 17.32 | 2 | 0.003 |
| Sodium (T2) | 66.66 | 1.52 | |||
| Magnesium (C) | 52.72 | 1.42 | 6.39 | 2 | 0.024 |
| Magnesium (T2) | 65.33 | 2.51 | |||
| Iron (C) | 4.13 | 0.32 | 9.50 | 2 | 0.011 |
| Iron(T2) | 5.40 | 0.10 | |||
| Manganese (C) | 1.48 | 0.03 | 3.33 | 2 | 0.079 |
| Manganese (T2) | 1.58 | 0.02 | |||
| Copper (C) | 0.65 | 0.02 | 43.13 | 2 | 0.001 |
| Copper (T2) | 1.27 | 0.04 | |||
| Colour | |||||
| Lightness (C) | 80.49 | 0.13 | 33.53 | 2 | 0.001 |
| Lightness (T2) | 77.30 | 0.28 | |||
| Redness (C) | 3.22 | 0.20 | 1.49 | 2 | 0.006 |
| Redness (T2) | 4.33 | 0.06 | |||
| Yellowness (C) | 25.86 | 0.20 | 1.94 | 2 | 0.009 |
| Yellowness (T2) | 22.52 | 0.48 | |||
| Darkness (C) | 30.73 | 0.24 | 0.65 | 2 | 0.543 |
| Darkness (T2) | 30.60 | 0.20 | |||
| Paired t test between T1 v/s T2 | |||||
| Moisture (T1) | 6.66 | 0.15 | 1.00 | 2 | 0.423 |
| Moisture (T2) | 6.53 | 0.15 | |||
| Protein(T1) | 12.31 | 0.50 | 1.62 | 2 | 0.246 |
| Protein(T2) | 11.82 | 0.13 | |||
| Fat (T1) | 3.34 | 0.09 | 8.55 | 2 | 0.013 |
| Fat (T2) | 4.47 | 0.13 | |||
| Fiber Insoluble (T1) | 10.65 | 0.10 | 1.63 | 2 | 0.243 |
| Fiber Insoluble(T2) | 10.50 | 0.05 | |||
| Fiber soluble(T1) | 4.33 | 0.05 | 3.05 | 2 | 0.093 |
| Fiber soluble(T2) | 3.60 | 0.43 | |||
| Total Fiber (T1) | 15.30 | 0.30 | 6.52 | 2 | 0.023 |
| Total Fiber(T2) | 14.33 | 0.07 | |||
| Ash (T1) | 2.60 | 0.10 | 2.00 | 2 | 0.184 |
| Ash(T2) | 2.70 | 0.13 | |||
| Carbohydrate(T1) | 75.66 | 0.28 | 12.44 | 2 | 0.006 |
| Carbohydrate(T2) | 74.65 | 0.31 | |||
| Energy (T1) | 1383 | 0.54 | 411.84 | 2 | 0.000 |
| Energy (T2) | 1610 | 0.63 | |||
| Minerals | |||||
| Calcium (T1) | 49.66 | 1.52 | 6.10 | 2 | 0.026 |
| Calcium (T2) | 63.00 | 3.00 | |||
| Potassium(T1) | 347.33 | 2.51 | 2 | ||
| Potassium (T2) | 287.33 | 2.51 | |||
| Sodium (T1) | 37.00 | 2.00 | 14.63 | 2 | 0.005 |
| Sodium (T2) | 66.66 | 1.52 | |||
| Magnesium (T1) | 93.33 | 3.05 | 18.33 | 2 | 0.003 |
| Magnesium (T2) | 65.33 | 2.51 | |||
| Iron (T1) | 6.20 | 0.20 | 5.23 | 2 | 0.035 |
| Iron(T2) | 5.40 | 0.10 | |||
| Manganese (T1) | 1.91 | 0.03 | 0.33 | 2 | 0.001 |
| Manganese (T2) | 1.58 | 0.02 | |||
| Copper (T1) | 0.86 | 0.03 | 13. 95 | 2 | 0.005 |
| Copper (T2) | 1.27 | 0.04 | |||
| Colour | |||||
| Lightness (T1) | 81.27 | 0.33 | 11.23 | 2 | 0.008 |
| Lightness (T2) | 77.30 | 0.28 | |||
| Redness (T1) | 3.21 | 0.03 | 36.66 | 2 | 0.001 |
| Redness (T2) | 4.33 | 0.06 | |||
| Yellowness (T1) | 19.76 | 0.25 | 6.60 | 2 | 0.022 |
| Yellowness (T2) | 22.52 | 0.48 | |||
| Darkness (T1) | 25.46 | 0.30 | 20.09 | 2 | 0.002 |
| Darkness (T2) | 30.60 | 0.20 | |||
Color
Food color and appearance are almost the important parameter that can influence a consumer need towards the product whether aid of colorimeter, food color can be measured as much the same as human eye. Paired t test revealed, a significant difference in the yellowness and darkness values between control and T1, t (2) =45.64, P=0.000 for yellowness and for darkness, t (2) = 60.84, p=0.000, respectively (Table 2). Paired t test between control and T2 samples revealed, significant difference in the lightness, t (2) =33.53, p=0.001, redness t (2) =12.63, p=0.006, yellowness t (2) =10.30, p=0.009, respectively. Paired t test betweenT1 and T2 samples revealed, significant difference in the lightness, t (2) =11.23, p=0.008, redness t (2) =36.661, p=0.001, yellowness t (2) =6.601, p=0.022 and darkness, t (2) =20.094, p=0.002, respectively.
Amylose estimation
The Total amylose content of the control and formulated samples varied from 25 to 30.80 %, the highest amylose content was observed for TI (30.803 %) followed by T2 (29.48 %) and in control (25.148 %). Significant difference was observed in the total amylose content between control and formulated samples on 0, 30 and 60 days (Table 1). No significant difference in the insoluble amylose content was observed in all the three samples, over a period of 60 days. However, in the case of soluble amylose, significant difference was observed in the control and formulated samples T1 and T2 on the zero day as well as 30 and 60 days.
Functional properties of sattu
Swelling power and solubility
On increasing the temperature from 30 to 90°C it was observed that the swelling power of the sattu mix was increasing (Table 1). Swelling power of sattu mix at 30 °C was found to be 16.72 (T1) and 16.57 (T2) which indicates its water holding capacity (Lee and Osman 2003) and was dependent on the amylose content. In swelling power, the crystalline structure of starch was disrupted when it was heated in excess water. An increase in granule swelling and solubility is caused when the water molecule gets linked, by hydrogen bonding, to the exposed hydroxyl group of amylose and amylopectin of the starch. (Singh et al. 2003). Significant difference in swelling power between control and both the formulated samples (T1 and T2) was observed at 30, 50, 70 and 90°C. Significant difference was also observed for solubility between control and formulated samples (T1 and T2) at 30, 50, 70 and 90 °C respectively. The swelling power ranged from 36 to 44 % for control whereas 16–27% for sample 1 and 2. The solubility for control ranged from 18 to 23 % whereas for sample 1 and 2 ranged from 9 to 16%.The high swelling power and solubility of control sample indicates the higher susceptibility of its starch granules to disintegrate and the linear molecules get leached out. A shorter average amylopectin chain length could be one of the reasons for the excessive leaching of starch in the control sample (Miazukami et al. 1999). The low swelling power and solubility of T1 and T2 proposes the presence of more amylose-lipid complex and a stronger bonding force within the interior of the starch granules (Tester and Morrison 1990). Ong and Blanshard (1995), concluded that long chain of amylopectin interacts with amylose and form double helix structure and on cooking, it lowers the swelling and leaching of materials. This may also be the reason for low solubility and swelling power in the samples T1 and T2.
WAI and WSI
The water absorption index (wai) can be used as an index of gelatinization and it measures the amount of water absorbed by starch. As an index of water solubility (wsi), the amount of dried solids recovered by evaporating the supernatant form the water absorption index. Higher wai was observed in control (3.19 g/g), as compared to formulated samples T1 and T2. Significant difference in wai with respect to control and formulated samples was observed at 0 day, however on 30 and 60 day no significant difference was observed. It was also observed that wai increased during storage period. Increased wai indicates the high amylose content. Decreased wai indicates high water binding capacity and thus reduced water availability for gelatinization of the starch granule (Table 1).
In case of wsi, significant difference was observed with respect to control and formulated samples at 0, 30 (control and T1) and 60 days, respectively. WSI decreased during storage period as compared to wai. The reduced viscosity and the increased protein made the product less soluble and affected the reconstitution ability. Because of this, raw material which, consists of starch molecule can expand and absorb water well (Ding et al. 2005) Hence, the samples which have low wsi were highly soluble in water but had less protein (Table 1). The previous studies reported that, water is absorbed and bound to the starch molecule with a resulting change in the starch granules and the degree of gelatinization decreased with increasing moisture (Ding et al. 2006).
Particle size analysis
The particle size distribution values of formulated samples and control revealed, that T1 had larger diameter followed by control and then T1 (Table 1). Generally, the viscosity of solutions of the products is significantly affected by differences in particle size distribution amongst products. Dispersion containing coursed fractions exhibited more viscous behavior than dispersions made up of “medium fine” or “very fine” particles (Ihekoronye and Oladunjoue 1988). Width of the T1 was observed very less followed by control and T2 respectively.
Scanning electron microscope (SEM)
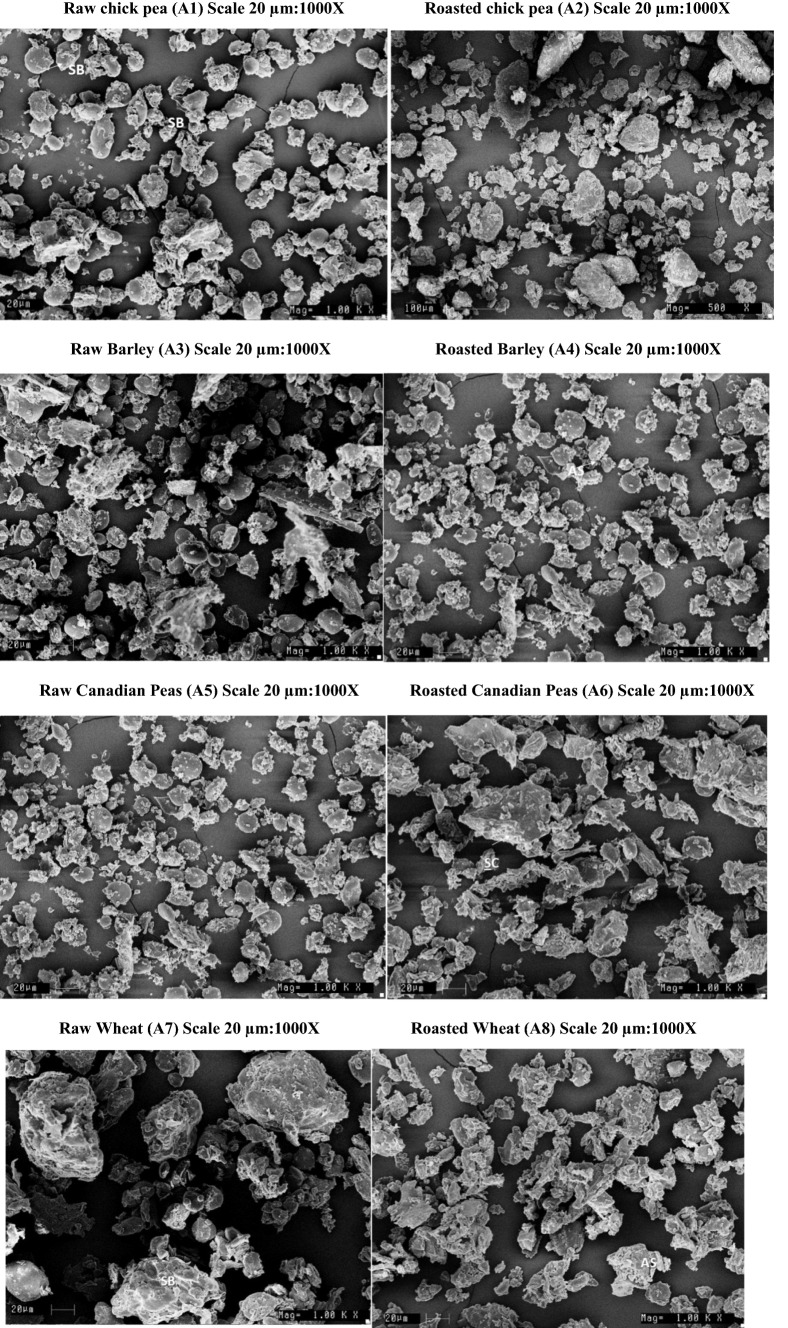
Microstructural studies of raw and roasted sattu and its ingredients by observation under scanning electron microscope revealed that during processing, substantial structural changes were occurred. The raw grains were tightly packed and contained no air spaces. However, in the cotyledon the roasted grain sample a large number of air spaces are formed. The chemical and physical changes during processing could also have incorporated air into the structure. During roasting, the water changes from liquid to vapor inside the grains. The compact structure of chickpeas, wheat, Canadian pea, barley, oats, maize may cause an increase in the vapor pressure of water so that the steam that is generated causes the grain to expand during roasting (Fig. 1). (Cenkowski and Sosulski 1989) in their study of infrared heat treatment (roasting) of lentils concluded that during the processing temperature is attained as high as 125 °C in the lentils, so that the superheated steam has sufficient pressure to create voids in the cellular matrix of grains. A porous like structure is caused due to the exit of steam and dehydration of starchy matrix, at the later stages of roasting. T1 was more tightly packed as compared to T2.
Fig. 1.
Scanning Electron Microscopy Images of different ingredients used for sattu formulation at 1000 X magnifications, Scale 20 µm, (A1) Raw chick pea, (A2) Roasted chick pea, (A3) Raw Barley, (A4) Roasted Barley, (A5) Raw Canadian Peas, (A6) Roasted Canadian Peas, (A7) Raw Wheat, (A8) Roasted Wheat, (A9) Raw Oats, (A10) Roasted Oats, (A11) Raw Maize, (A12) Roasted Maize, (T1) Wheat based sattu, (T2) Maize based sattu (SB-Starch Bundles, SC-Starch cemented, AS-Air spaces)
Health promoting factors
Analysis of glycemic index
Glycemic index (GI) is a measure of various carbohydrates on blood glucose level. Carbohydrates that are digested rapidly and release glucose into blood have high GI where as those that digested slowly have low GI. A low GI food release glucose into blood slowly and steadily as compares to high GI food that increase blood glucose level rapidly. The use of fibers, which aids in the control of postprandial insulin release, can help in obtaining a reduced glycemic index (eGI) of starch based foods. (Roberts 2000).
It was observed that, T1and T2 had medium GI value (56 and 58 % respectively), Whereas for control it was 60%. It indicates its medium GI and slow digestion and slow release of glucose into blood. The reason for medium digestibility is partial gelatinization of the starches present in various grains which have been roasted. The crystalline structure of starch, which limits enzyme hydrolytic action and protects the glucoside bonds causes low digestibility in legumes (Ruiz et al. 2008). Once the gelatinization is completed, this crystalline structure is lost, leaving the molecules open for hydrolysis, which breaks the glucoside bonds, and therefore increases digestibility. The presence of fiber and polyphenols in whole grains, reduce the digestibility of starch may also cause low digestibility (Shobana et al. 2009, Yadav et al. 2010).
Protein digestibility
The nutritional quality of cereals also depends upon the digestibility of protein and the bioavailability of amino acids, not only on their protein, energy and amino acid composition. The digestion of protein meals by proteolytic enzymes such as pepsin and trypsin under in vitro condition has been used to evaluate the quality of protein (Sleisenger et al. 1977). In vitro digestibility was found to be maximum in T1 (88.01%), which is wheat variety followed by T2, that was maize variety. Jood et al. (1995) reported that in vitro protein digestibility of wheat was 72.92 per cent. The protein digestibility can be increased by cooking and heat treatment (Gupta 1994). Hence, the combination of the wheat and chick pea in T1 increases the In vitro protein digestibility while compared with T2 (Maize based). The increase in protein digestibility in formulated sample can be attributed to the reduction of ant nutrients and in activation of enzyme inhibitors during heat treatment (Nergiz and Gokgoz 2007).
Shelf life analysis of sattu
Significant difference in moisture content was observed over a storage of 60 days, between control and T1 and control and T2 samples. An 11% increase in moisture content was observed in control sample, whereas for T1 and T2 increase was 6% and 4% respectively (Fig. 2a). It is known that the rate of increase of FFA will be in commensuration with the moisture content (Vijayalakshmi et al. 2009). In case of Free fatty acid, a significant increase (40–52%) was observed over the storage period, in all the samples (Fig. 2b). Significant difference was observed in the FFA content on the 0 day between control and T1 and T2 samples (Table 1). However, prolong storage did not show any significant interaction for FFA within the samples.
Fig. 2.
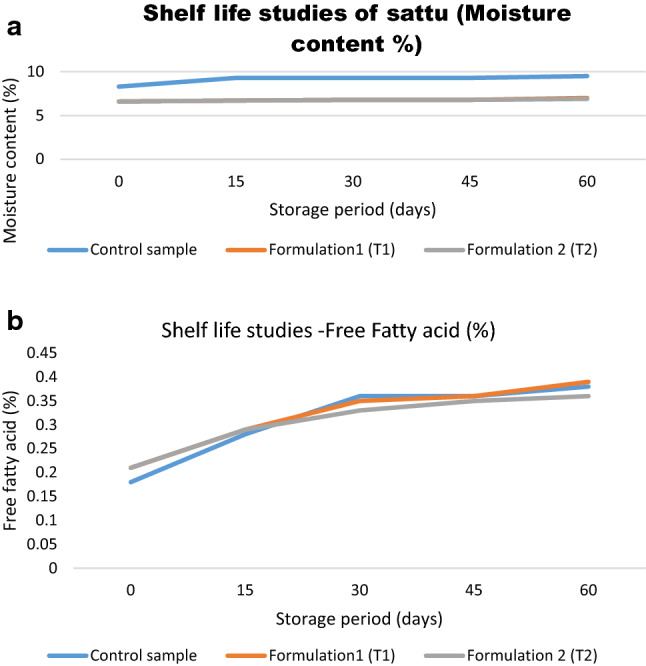
a Shelf life studies for sattu—(moisture content %) b Shelf life studies for sattu- (Free fatty acid content %)
Sensory analysis
There was no perceptible off odor or off taste in the samples which made them acceptable (Fig. 3). Acceptability of the maize based sattu was found to be more as compared to wheat based. Maximum score for flavor, appearance, taste and after taste were in the category of liked moderately for maize based sattu and comparable to control. As from Fig. 3 we can infer that, Maximum score for flavor, appearance, taste and after taste were in the category of liked moderately for maize based sattu and comparable to control. Overall both the formulated sattu mixes were acceptable and had no off taste or odour. However the control sample was also equally acceptable (Fig. 3).
Fig. 3.
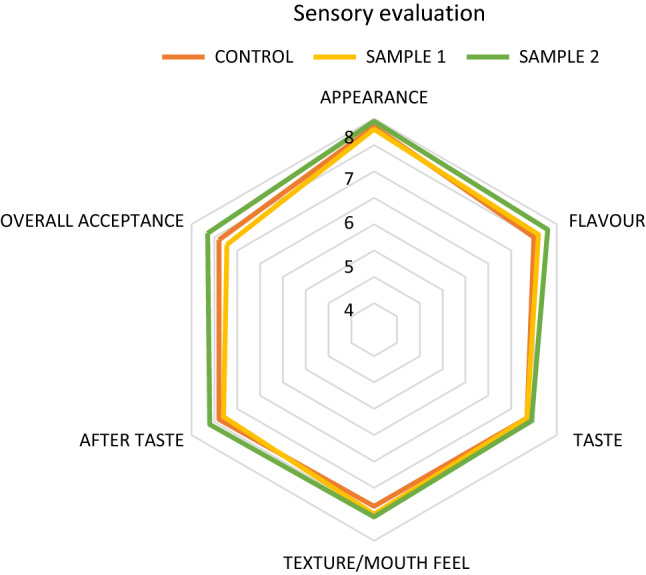
Graphical representation of sensory profile of Sattu mix
Overall both the formulated sattu mixes were acceptable and had no off taste or odour.
Conclusion
Sattu is a traditional Indian food which is consumed as an energy drink, usually in summers, that helps to keep our body fit and healthy. It has various health benefits for all age groups. It is healthy for our intestines considering its high fiber content and a perfect blend of balanced nutrients. The results obtained from this study have shown that it is a good and healthier combination of cereal with an appropriate proportion of pulses to create a value added food drink of acceptable organoleptic and nutritional properties. The product moisture content varied from 6 to 7 % and hence can be stored for 60 days at RT. Among the two types of formulations, wheat based had higher digestibility and lower GI as compared to maize based. However, both the formulations were better in terms of proximate and minerals composition, swelling solubility and glycemic index, as compared to control sample. This instant cereal breakfast beverage powder (Sattu) is convenient and can be easily prepared by dissolving in hot or cold water prior to consumption corresponding for urban lifestyle and health consciousness of the customer. The product was found to be sensorial acceptable. It has a “true nutritive potential” and can get global recognition outside India as well. There is a growing awareness about the health impact of carbonated drinks or packaged drinks. Hence, in this scenario, there is a resurgence of not only healthy, but also traditional foods as people are linking back to traditional lifestyles, and sattu is one such food which can be popularized.
Acknowledgements
The authors wish to thank Director, CSIR-CFTRI, Mysore, Karnataka, India, for his support and encouragement.
Authors contribution
MS carried out pilot plant operation and generated engineering data; DEK carried out lab experiments and generated data; SP conceived, carried out the experiments and supervised the work and wrote the MS.
Funding
The Institute has provided the funding for the work.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest related to this article.
Ethics approval
The Research has been conducted in an ethical and responsible manner.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akeson WR, Stahmann MA. A pepsin pancreatin digest Index of protein quality evaluation. J Nutr. 1964;83:257–261. doi: 10.1093/jn/83.3.257. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Conway VFP, Griffin EL. Gelatinization of corn grits by roll- and extrusion-cooking. Cereal Sci Today. 1969;14:4–7. [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 17. Gaitherburg, USA: AOAC International Inc.; 2000. [Google Scholar]
- Barros F, Alviola JN, Rooney LW. Comparison of quality of refined and whole wheat tortillas. J Cereal Sci. 2010;51(1):50–56. doi: 10.1016/j.jcs.2009.10.001. [DOI] [Google Scholar]
- Bhattacharya KR, Sowbhagya CM. Water uptake by rice during cooking. Cereal Sci Today. 1971;16:420–424. [Google Scholar]
- Cenkowski S, Sokhansanj S, Sosulski FW. Equilibrium moisture content of lentils. Can Agric Engng. 1989;31:159–162. [Google Scholar]
- Curic D, Galic K. Development of functional cereal based foodstuffs. In: Proceed the 3rd int congress flour - bread '05 and 5th croatian congress of cereal technologists. pp. 121–133
- Ding QB, Ainsworth P, Tucker G, Marson H. The effect of extrusion conditions on the physicochemical properties and sensory characteristics of rice based expanded snacks. J Food Eng. 2005;66:283–289. doi: 10.1016/j.jfoodeng.2004.03.019. [DOI] [Google Scholar]
- Ding QB, Ainsworth P, Plunkett A, Tucker G, Marson H. The effect of extrusion conditions on the functional and physical properties of wheat based expanded snacks. J Food Eng. 2006;73:142–148. doi: 10.1016/j.jfoodeng.2005.01.013. [DOI] [Google Scholar]
- Englyst HN, Hudson GJ. The classification and measurement of dietary carbohydrates. Food Chem. 1996;57:15–21. doi: 10.1016/0308-8146(96)00056-8. [DOI] [Google Scholar]
- Fardet A, Canlet C, Gottardi G, Lyan B, Llorach R, Remesy C, et al. Whole-grain and refined wheat flours show distinct metabolic profiles in rats as assessed by a H-1 NMR-based metabonomic approach. J Nutr. 2007;137(4):923–929. doi: 10.1093/jn/137.4.923. [DOI] [PubMed] [Google Scholar]
- Goni I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic Index. Nutr Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Gupta HO. Protein quality evaluation of sprouted maize. Plant Foods Hum Nutr. 1994;46:85–91. doi: 10.1007/BF01088465. [DOI] [PubMed] [Google Scholar]
- Ihekoronye AI, Oladunjoye MG. Formulation and physicochemical properties of high-protein food beverage powders based on protein concentrate from the Nigerian red skin groundnut. Trop Sci. 1988;23:62–72. [Google Scholar]
- Indrani D, Swetha P, Soumya C, Rajiv J, Rao GV. Effect of multigrains on rheological, microstructural and quality characteristics of north Indian parotta—an Indian flat bread. Lwt-Food Sci Technol. 2011;44(3):719–724. doi: 10.1016/j.lwt.2010.11.017. [DOI] [Google Scholar]
- Jones JM. Whole grains and dietary fiber continue to win honors in preventing various diseases. Cereal Foods World. 2007;52:286–288. [Google Scholar]
- Jood S, Kapoor AC, Singh R. Amino acid composition and chemical evaluation of protein quality of cereals as affected by insect infestation. Plant Foods Hum Nutr. 1995;48:159–167. doi: 10.1007/BF01088312. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Klein BP. Sensory science theory and applications in foods. Adv Sens Sci. 1991;14:25–30. [Google Scholar]
- Lee YE, Osman EM. Pasting and crystalline property differences of commercial and isolated rice starch with added amino acids. J Food Sci. 2003;68:832–838. doi: 10.1111/j.1365-2621.2003.tb08251.x. [DOI] [Google Scholar]
- Mizukami H, Takeda Y, Hizukari S. The structure of the hot water soluble components in the starch granules of new Japanese rice cultivars. Carbohyd Polym. 1999;38:329–335. doi: 10.1016/S0144-8617(98)00120-9. [DOI] [Google Scholar]
- Mohsenin NN. Physical properties of plant and animal materials: structure, physical characteristics, and mechanical properties. 2. New York, NY: Gordon and Breach Science Publishers; 1986. [Google Scholar]
- Nergiz C, Gokgoz E. Effects of traditional cooking methods on some anti-nutrients and in vitro protein digestibility of dry bean varieties grown in Turkey. Int J Food Sci Technol. 2007;42:868–873. doi: 10.1111/j.1365-2621.2006.01297.x. [DOI] [Google Scholar]
- Ong MH, Blanshard JMV. Texture determinants of cooked, parboiled rice. Physicochemical properties and leaching behavior of rice. J Cereal Sci. 1995;21:261–269. doi: 10.1006/jcrs.1995.0029. [DOI] [Google Scholar]
- Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev. 2000;58(6):163–169. doi: 10.1111/j.1753-4887.2000.tb01855.x. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz J, Martinez-Ayala A, Dargo S, Gonzalez R, Betancur-Ancona D, Chel-Guerrero L. Extrusion of hard to cook bean (Phaseolus vulgaris L.) and quality protein maize (Zea mays L.) flour blend. LWT. Food Sci Technol. 2008;41:1799–807. doi: 10.1016/j.lwt.2008.01.005. [DOI] [Google Scholar]
- Sahay KM, Singh KK (1996) Unit operations of agricultural processing. Vikas Publishing House, New Delhi, pp. 12
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of [alpha]-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- Singh V, Ali SZ. Acid degradation of starch. The effect of acid and starch type. Carbohyd Polym. 2000;41:191–195. doi: 10.1016/S0144-8617(99)00086-7. [DOI] [Google Scholar]
- Singh N, Sodhi NS, Kaur M. Physio-chemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels. Food Chem. 2003;82:433–439. doi: 10.1016/S0308-8146(03)00007-4. [DOI] [Google Scholar]
- Sleisenger MH, Pelling D, Burston D, Matthews DM. Amino acid concentrations in portal venous plasma during absorption from the small intestine of the guinea pig of an amino acid mixture simulating casein and a partial enzymic hydrolysate of casein. Clin Sci Molec Med. 1977;52:259–267. doi: 10.1042/cs0520259. [DOI] [PubMed] [Google Scholar]
- Tester RF, Morrison WR. Swelling and gelatinization of cereal starches. Cereal Chem. 1990;67:551–558. [Google Scholar]
- Vijayalakshmi NS, Indiramma AR, Rangarao GCP, Ramesh BS. Package design for chips based on their deteriorative characteristics, size and permeability of packages. J Food Sci Technol. 2009;46(5):450–454. [Google Scholar]
- Yadav BS, Sharma A, Yadav RB. Effect of storage on resistant starch content and in vitro starch digestibility of some pressure-cooked cereals and legumes commonly used in India. Int J Food Sci Technol. 2010;45:2449–2455. doi: 10.1111/j.1365-2621.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.