Abstract
Huge amount of mango peels is generated by the mango processing industries with rich composition of phenolic compounds having high antioxidant activity. In the present investigation, ultrasound assisted extraction was found to be an efficient extraction technique for recovery of phenolic compounds. The highest phenolic content was obtained using aqueous solution of ethanol (50%) over other extraction solvents. The highest phenolic content of 35.5 mg GAE/g was obtained using ultrasound assisted extraction method with solid to liquid ratio of 1:30 at 45 °C temperature, ultrasound amplitude 30% after 10 min of treatment time. The highest antioxidant activity of 92% was observed in the mango peels. The significant impact of ultrasonication treatment on the mango peels cell wall is evident from the scanning electron microscopy. The FTIR results indicated the rich composition of polyphenolic compounds present in the mango peels. Ultrasound assisted extraction can prove to be a sustainable green technique with high potential of bioactive compounds recovery like polyphenols for the valorization of mango by-products.
Keywords: Mango peels, Ultrasound assisted extraction, Maceration, Antioxidant activity, Total phenolic content
Introduction
Mango (Mangifera indica) fruit of the family Anacardiaceae is among the major variety of tropical fruit with worldwide production and consumer acceptability (FAO 2005). The valorization of agro-food chain of mango by the mango processing industries is associated with its conversion into different products like juice, jams, jellies, etc. (Wall-Medrano et al. 2020). Mango peels which constitute nearly 15–20% of the total fruit weight, is a major by-product left after mango processing. It is considered a potential source of bioactive substances like polyphenols, carotenoids, dietary fiber (Banerjee et al. 2018). The rich composition of such bioactive substances present in the mango peels is responsible for its high antioxidant activity.
A comparatively larger number of phenolic compounds are present in mango peels than the mango pulp (Ajila et al. 2007). These phenolic compounds due to their natural antioxidant capacity holds significant potential in replacement of synthetic food additives and as cancer-preventing agents (Peschel et al. 2006; Okonogi et al. 2007). A number of phenolic compounds have been reported in the mango peels including quercetin, mangiferin, rhamnetin, kaempferol, ellagic acid, and their conjugates (Masibo and He 2008).
The extraction of bioactive compounds from plant sources has gained considerable attention due to its immense health benefits. This is due to their potential antioxidant activities contributing to the prevention of oxidative stress related diseases (Guandalini et al. 2019). Phenolic compounds often present in several fruit by-products constitute one of the most important class of bioactive substances. The wastes generated from various fruit processing industries are prone to microbial spoilage leading to objectionable odors and environmental hazards. This urges the need for their proper disposal which is a great deal for processing industries considering economic feasibility of the process. The valorization of the fruit by-products by recovery of high-value compounds can be a sustainable means to solve the problem of the large quantity of biowaste generated and its disposal.
Conventional methods of extraction, like soxhlet (Castro-Vargas et al. 2019) and maceration (Rojas et al. 2020) have been extensively applied for obtaining polyphenols from mango peels. However, these techniques are accompanied with high temperature, more solvent consumption and longer extraction time. Since the conventional techniques are not efficient in terms of process yield and selectivity toward target compounds, non-conventional techniques can be of considerable interest. One of the green techniques, ultrasound-assisted extraction (UAE), has been considered a sustainable technique (Chemat et al. 2020) to recover phenolic compounds. It involves lower temperature, shorter time with simplified manipulation and high reproducibility, resulting in higher quality of the final product. With regard to environmental impacts, it require only a fraction of energy than that required in conventional extraction methods. Ultrasonication has been widely recognized as an effective and eco-friendly method for the extraction of bioactive compounds in a number of previous studies (Mercado-mercado et al. 2018; Martinez-Ramos et al. 2020; Torres-Leon et al. 2021). The rupture of cell walls of the treated plant material enables release of high quantities of bioactive substances thus establishing the efficacy of the process (Chemat et al. 2017). Thus, the present study was carried out to apply UAE technique for phenolic compounds extraction from the mango peels and assess its antioxidant activity by optimization of different extraction parameters (solid to liquid ratio, temperature, amplitude, and time).
Material and methods
Mango (Mangifera indica) peels of Chausa variety in the commercial ripening stage (based on the color of skin and firmness) were collected from the fruit processors in the local market of Longowal, Punjab, India. The sample was dried using sun drying method under open air and sunny conditions for 7 days until constant weight was achieved (Anwar et al. 2013). The dried peels (with moisture content of 11.1%) were grounded to fine powder (200 µm) using domestic grinder mill and stored (4 ± 1° C) till further analysis. The chemicals of analytical grade procured from SD fine chem ltd, Puja science house, Patiala) were used in the study.
Ultrasound assisted extraction
The phenolic compounds extraction from the mango peel powder was carried out by using UAE technique (Guandalini et al. 2019) with ultrasonic probe (Snaptech NexTgen Lab 500). The powdered sample (1 g) was extracted by using ethanol (50%, 30 mL) as solvent. After the extraction process, the extracts were centrifuged, collected in amber glass bottles and refrigerated. Solid to liquid ratio (1:10–1:40), temperature (25–55 °C), ultrasonic amplitude (20–50%) and treatment time (5–15 min) was varied to obtain the optimal conditions for the extraction of phenolic compounds.
Experimental design
The design matrix of the present investigation consisted of single factor experiments for the determination of optimal conditions of phenolic compounds extraction from mango peels and study of antioxidant activity. A total of four parameters namely solid to liquid ratio (1:10–1:40), extraction temperature (25–55 °C), ultrasonic amplitude (20–50%) and treatment time (5–15 min) were studied. At a time, single parameter was varied while the other parameters were kept constant. The optimal extraction conditions were determined on the basis of total phenolic content.
Determination of total phenolic content
Folin-ciocalteu method was used for the determination of total phenolic content in mango peels (Castro-Vargas et al. 2019). The liquid extract (100 µL) was mixed with folin-ciocalteu reagent (10% w/w, 750 µL) and after a gap of 5 min, sodium carbonate solution (6% w/w, 750 µL) was added. The mixture was stirred and left in dark for 90 min followed by measurement of absorbance (at 765 nm). Tests were run in triplicate and the final results were obtained as mg of gallic acid equivalents per g of raw material (mg GAE/g).
Determination of antioxidant activity
The DPPH radical scavenging assay was used for evaluation of antioxidant activity of mango peels extract (Ajila et al. 2007). Mango peels extract (200 µL) was mixed with DPPH solution prepared in ethanol (100 mM, 1 mL). The mixture was shaken, left in dark (20 min) followed by measurement of absorbance (As) at 517 nm. The solution containing all the reagents except the extract was taken as control. The calculation of DPPH radical scavenging activity was performed using the equation:
| 1 |
where As is the absorbance in presence of peel extract and A0 is the absorbance of the control at 517 nm.
Scanning electron microscopy
The scanning electron microscopy (SEM) was conducted to study the morphological characteristics of mango peel powder sample before and after ultrasound treatment. Scanning electron microscope (JSM- 7610 F plus, JEOL, Japan) was used for the analysis. Samples were sputter-coated using a thin layer of gold at room temperature before imaging (Altemimi et al. 2016).
Fourier-transform infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR) of the dried mango peel extract was studied with FTIR spectrophotometer (Perken Elmer Spectrum, RX-I, USA) at an absorbance range of 4000–600 cm−1 (Kannan et al. 2011).
Statistical analysis
Data obtained for the total phenolic content and antioxidant activity of mango peels at different conditions of time, temperature, solid to liquid ratio and amplitude using ultrasonication technique were analyzed by using SPSS 16.0 software. The results have been presented as means ± SD of three replicates. Statistically significant difference between the means in each group were tested using a 2-way ANOVA with significance level of 5% (P < 0.05) for all the calculations.
Results and discussion
The total phenolic content of mango peels obtained using conventional method of extraction (maceration) using 50% ethanol was found to be 20.5 mg GAE/g. The effect of various extraction parameters (solid to liquid ratio, temperature, ultrasonic amplitude and time) on total phenolic content and antioxidant activity of mango peels obtained using ultrasonication were studied and results have been discussed below.
Effect of solid to liquid ratio
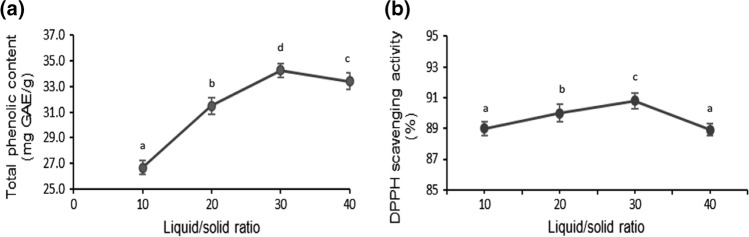
For the determination of phenolic content in mango peels, 50% ethanol was used as the extraction solvent. The effect of solid to liquid ratio on total phenolic content and antioxidant activity of mango peels using ultrasound assisted extraction was investigated. The different proportion of solvent significantly (p < 0.05) affected the extraction of phenolic compounds and antioxidant activity of mango peel (Fig. 1a, b). It was found that the highest total phenolic content (34.3 mg GAE/g) as well as the antioxidant activity (90.8%) was observed with 1:30 solid to liquid ratio, after which a decline in the value was observed.
Fig. 1.
Effect of solid to liquid ratio on total phenolic content a DPPH radical scavenging activity b of mango peel extract obtained using ultrasonication. The lower-case letters (a–d) indicate significant difference (p < 0.05)
The increase in total phenolic content can be due to a larger volume of solvent which accelerates the diffusion process. The non-significant change witnessed by the total phenolic content with increase in solid to liquid ratio after 1:30 might be due to saturation of the liquid in the extraction system (Medina-Torres et al. 2017). The obtained results were compatible with the mass transfer principle, where the driving force for the mass transfer was the increase in concentration gradient between the solid material and the liquid (Tao and Sun 2015). The reduction in total phenolic content at ratio greater than 1:30 might be due to extraction of other biomolecules like proteins and polysaccharides, which can dissolve in the medium and in turn, affecting the dissolution of phenolic compounds (Yang et al. 2010). The results correlated with a previous study on mango peels where 1:30 was taken as the optimum solid to liquid ratio for extraction of phenolic compounds using ultrasound assisted extraction (Morales et al. 2020). In another study on mango seed, solid to liquid ratio of 1:30 was taken as the optimum ratio for the solvent extraction of bioactive compounds responsible for antioxidant activity (Dorta et al. 2013). In case of variation of antioxidant activity with increase in solid to liquid ratio, no significant difference was observed in the values which indicated that the antioxidant activity was not much influenced by the variation in solid to liquid ratio. This was in agreement with the previous study on antioxidant activity of Clinacanthus nutans leaves where solid to liquid ratio had no significant impact on extraction yield (Sulaiman et al. 2017).
Effect of temperature
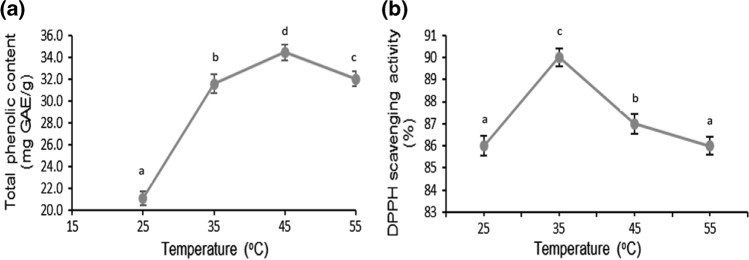
Significant (p < 0.05) effect of temperature was observed on the extraction of phenolic compounds as well as antioxidant activity of mango peels (Fig. 2a, 2b). Highest total phenolic content (34.4 mg GAE/g) was observed at 45 °C after which its value decreased. Whereas, in case of antioxidant activity, maximum values were observed at 35 °C, after which it was found to decrease.
Fig. 2.
Effect of temperature on total phenolic content a DPPH radical scavenging activity b of mango peel extract obtained using ultrasonication. The lower-case letters (a–d) indicate significant difference (p < 0.05)
Due to sensitivity of polyphenolic compounds to heat, their antioxidant activity might be lost when temperature was further raised to 55 °C. It implies thermal degradation of phenolic compounds and fall in the extraction yield (Osorio-Tobon 2020). The antioxidant activity of mango peels was found to increase up to 35 °C. However, further increase in temperature led to a decline in antioxidant activity. This behavior might be due to the principle of equilibrium, in which high temperature leads to increase in extraction rate and allow maximum recovery of phenolic compounds thus resulting in higher antioxidant activity. Increased temperature probably led to an increased phenolic solubility, better mass transfer, faster diffusion rate and therefore, increased extraction rate (Richter et al. 1996). However, increase in temperature above particular value may promote degradation of previously mobilized phenolic compounds as well as the residual phenolics (Mokrani and Madani 2016). Similar effect was observed in antioxidant activity of pomegranate peels, where it was found to increase when extraction temperature during ultrasonication was increased from 25 to 35 °C (Pan et al. 2012).
Effect of amplitude
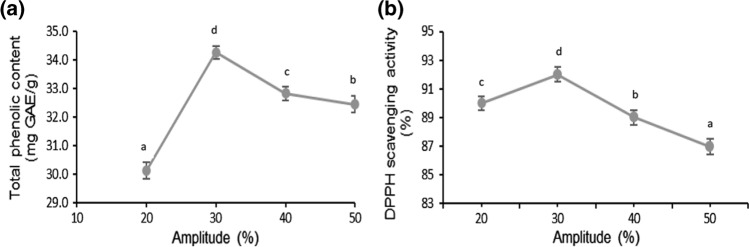
Ultrasonic amplitude had a significant (p < 0.05) effect on phenolic compounds extraction and antioxidant activity of mango peels (Fig. 3a, b). Highest phenolic content (32.8 mg GAE/g) was observed at ultrasonic amplitude of 40% after which a decrease was noted. In relation to antioxidant activity, its highest value (92%) was observed at 30% ultrasonic amplitude after which it decreased.
Fig. 3.
Effect of amplitude on total phenolic content a DPPH radical scavenging activity b of mango peel extract obtained using ultrasonication. The lower-case letters (a–d) indicate significant difference (p < 0.05)
With increase in ultrasonic amplitude, major alterations occur in plant matrix caused by cavitation. This enhanced solvent penetration allowing maximum recovery of phenolic compounds. However, further increase in amplitude might cause degradation of phenolic compounds (Osorio-Tobon 2020). Similar effect of amplitude was observed on the antioxidant activity of mango peel extract except that in this case, amplitude did not have much effect on the antioxidant activity. Similar findings were observed in a study on extraction of carotenoids from mango by-products (Mercado-mercado et al. 2018) where ultrasonic amplitude of 30% was selected as an optimum extraction parameter.
Effect of time
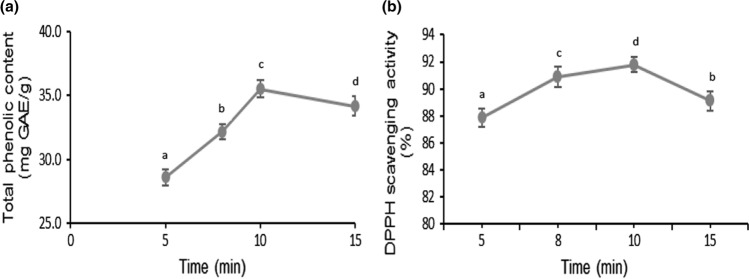
The treatment time also significantly (p < 0.05) affected the phenolic compounds extraction and antioxidant activity of the mango peels (Fig. 4a, b). The highest total phenolic content (35.5 mg GAE/g) and antioxidant activity (92%) was observed after 10 min of treatment time. Further increase in treatment time caused no significant change in the phenolic content as well as the antioxidant activity.
Fig. 4.
Effect of time on total phenolic content a DPPH radical scavenging activity (b) of mango peel extract obtained using ultrasonication. The lower-case letters (a–d) indicate significant difference (p < 0.05)
This could be explained by Fick’s second law of diffusion, according to which, a state of equilibrium is attained between the bulk solution and solute concentration in the solid matrix after certain period of time. Therefore, additional increase in time may not be required for further extraction of phenolics (Silva et al. 2007). The progressive release of the metabolites and the efficiency of extraction process is significantly influenced by the time of contact of the extraction solvent and plant matrix. The results correlated with the previous studies on mango seed (Morales et al. 2020) and pomegranate peels (Pan et al. 2012; Kaderides et al. 2015). Prolonging the extraction time did not cause any significant change in the antioxidant activity of mango peels with highest observed antioxidant activity of 92% after 10 min treatment time. The results were similar to a previous study on citrus peels (Xu et al. 2008) and grape pomace extract (Pinelo et al. 2005), where no change in antioxidant activity was observed with increase in the extraction time.
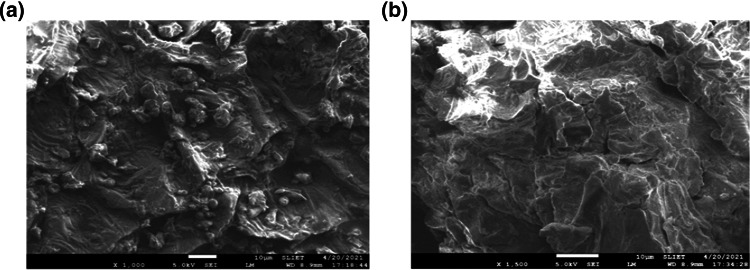
SEM analysis
The SEM images for mango peel powder sample were taken before and after ultrasonication treatment (Fig. 5). The non-treated sample and the ultrasound treated material sample showed significant difference in the structural morphology. From the results it can be clearly stated that ultrasonic processing led to an increased cell damage thus leading to an increase in the rate of mass transfer of bioactive compounds across the cell wall (Altemimi et al. 2016). Similar effect of ultrasound was observed in the previous studies, where ultrasonic cavitation energy caused significant changes on the surface structure of prickly pear peel. The numerous cracks and pores produced on the surface of peel indicated effective bioactive components extraction using ultrasound technique (Karunanithi and Venkatachalam 2019).
Fig. 5.
Scanning electron microscopy of mango peel powder. a Non-treated sample b Ultrasound treated sample
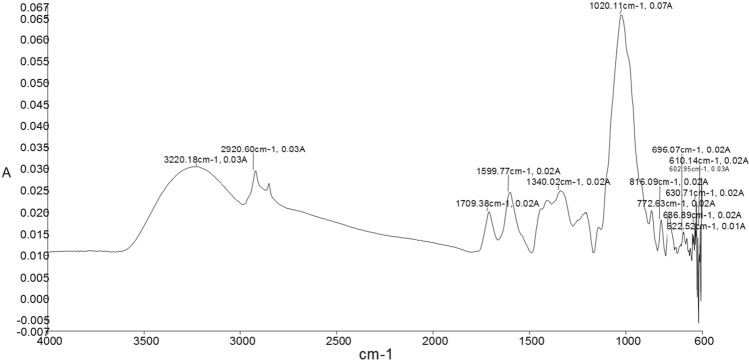
FTIR analysis
The FTIR spectral analysis for phenolic extract of mango peels was carried out in the mid-infrared region (4000–600 cm−1) (Fig. 6). The prominent band at 3220 cm−1 represented the O–H of carboxylic acid indicating strong intermolecular hydrogen bonding of gallic acid (Patle et al. 2020). The absorption bands around 2920 cm−1 indicate the C–H stretching of the CH2 groups. These bands indicate the presence of aliphatic CH groups in the present compounds (Kannan et al. 2011). The sharp absorption peaks near 1709–1600 cm−1 reflect the C=O stretching vibrations in carbonyl compounds. This may be attributed to the presence of high flavonoids content in the mango peels. The low intensity absorption bands near 1450 cm−1 represents the C–C–O stretching vibration whereas absorption bands near 1300–1100 cm−1 indicate O–H bending vibration which arise due to presence of compounds such as rutin, gallic acid, quercetin, and tannic acid (Patle et al. 2020). A sharp absorption band at 1020 cm−1 correspond to the C–O group of molecules. The absorption bands from 815 to 630 cm−1 can be assigned to the phenolic compounds like quercetin, tannic acid, gallic acid and vanillin respectively. The bands near 607 cm−1 correspond to the stretching vibration of phenols (Valchos et al. 2006).
Fig. 6.
FTIR spectra of mango peel extract obtained using ultrasound assisted extraction
Conclusions
The present study revealed the potential of ultrasonication technique for the extraction of phenolic compounds. Maximum extraction of phenolic compounds (35.5 mg GAE/g) was observed with solid to liquid ratio 1:30 at 45 °C temperature, 30% ultrasonic amplitude, after 10 min treatment time. Ultrasound assisted extraction is an advantageous green technique with wider applications in food industries as compared to conventional technique thus making the overall process economical. Mango peels possess considerable amounts of polyphenols and antioxidants which make it a potential food supplement in various food preparations such as bakery products, dairy products, beverages, etc. Extensive research can be conducted for total utilization of mango by-products to adopt the concept of zero waste generation thus establishing sustainability of the process.
Acknowledgements
Authors would like to acknowledge the financial support provided by ASEAN India Science & Technology Development Fund (AISTDF), by Science & Engineering Research Board, Department of Science & Technology (SERB-DST) under Grant No. CRD/2019/000141. Further, the infrastructural support provided by Sant Longowal Institute of Engineering and Technology (SLIET), for carrying out the research is also acknowledged.
Abbreviations
- UAE
Ultrasound assisted extraction
- SEM
Scanning electron microscopy
- FTIR
Fourier-transform infrared spectroscopy
Author contributions
BK: Investigation, Methodology, Writing—original draft, Formal analysis. PSP: Conceptualization, Writing—review & editing, Supervision, Project administration. AKA: Conceptualization, Supervision.
Funding
The research project was funded by ASEAN India Science & Technology Development Fund (AISTDF), Science & Engineering Research Board- Department of Science & Technology (SERB-DST) under Grant No. CRD/2019/000141.
Declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajila C, Bhat S, Rao UP. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007;102:1006–1011. doi: 10.1016/j.foodchem.2006.06.036. [DOI] [Google Scholar]
- Altemimi A, Watson DG, Choudhary R, et al. Ultrasound assisted extraction of phenolic compounds from peaches and pumpkins. PLoS One. 2016;11(2):e0148758. doi: 10.1371/journal.pone.0148758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar F, Kalsoom U, Sultana B, et al. Effect of drying method and extraction solvent on the total phenolics and antioxidant activity of cauliflower (Brassica oleracea L.) Extracts. Int Food Res J. 2013;20:653–659. [Google Scholar]
- Banerjee J, Singh R, Vijayaraghavan R, et al. A hydrocolloid based biorefinery approach to the valorisation of mango peel waste. Food Hydrocoll. 2018;77:142–151. doi: 10.1016/j.foodhyd.2017.09.029. [DOI] [Google Scholar]
- Castro-Vargas HI, Ballesteros Vivas D, Ortega Barbosa J, et al. Bioactive phenolic compounds from the agroindustrial waste of Colombian mango cultivars ‘sugar mango’ and ‘tommy atkins’-an alternative for their use and valorization. Antioxidants. 2019;8(2):41. doi: 10.3390/antiox8020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CheSulaiman IS, Basri M, FardMasoumi HR, et al. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem Cent J. 2017;11(1):54. doi: 10.1186/s13065-017-0285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemat F, AbertVian M, Fabiano-Tixier AS, et al. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020;22(8):2325–2353. doi: 10.1039/c9gc03878g. [DOI] [Google Scholar]
- Chemat F, Rombaut N, Meullemiestre A, et al. Review of green food processing techniques. Preservation, transformation, and extraction. Innov Food Sci Emerg Technol. 2017;41:357–377. doi: 10.1016/j.ifset.2017.04.016. [DOI] [Google Scholar]
- Dorta E, Lobo MG, González M. Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioprocess Technol. 2013;6:1067–1081. doi: 10.1007/s11947-011-0750-0. [DOI] [Google Scholar]
- Food and Agriculture Organization (2005) FAOSTAT database collections, agricultural data, food and agriculture organization of the United Nations
- Guandalini BBV, Rodrigues NP, Marczak LDF. Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res Int. 2019;119:455–461. doi: 10.1016/j.foodres.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Kaderides K, Goula AM, Adamopoulos KG. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov Food Sci Emerg Technol. 2015;31:204–215. doi: 10.1016/j.ifset.2015.08.006. [DOI] [Google Scholar]
- Kannan RRR, Arumugam R, Anantharaman P. Fourier transform infrared spectroscopy analysis of seagrass polyphenols. Curr Bioact Compd. 2011;7(2):118–125. doi: 10.2174/157340711796011142. [DOI] [Google Scholar]
- Karunanithi A, Venkatachalam S. Ultrasonic-assisted solvent extraction of phenolic compounds from Opuntia ficus-indica peel: phytochemical identification and comparison with soxhlet extraction. J Food Process Eng. 2019 doi: 10.1111/jfpe.13126. [DOI] [Google Scholar]
- Martinez-Ramos T, Benedito-Fort J, Watson NJ, et al. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.) Food Bioprod Process. 2020;122:41–54. doi: 10.1016/j.fbp.2020.03.011. [DOI] [Google Scholar]
- Masibo M, He Q. Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci Food Saf. 2008;7:309–319. doi: 10.1111/j.1541-4337.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- Medina-Torres N, Ayora-Talavera T, Espinosa-Andrews H, et al. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy. 2017;7(3):47. doi: 10.3390/agronomy7030047. [DOI] [Google Scholar]
- Mercado-mercado G, Gonzalez EM, Gonzalez-Aguilar GA, et al. Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci. 2018;21:215–131. doi: 10.1016/j.fbio.2017.12.012. [DOI] [Google Scholar]
- Mokrani A, Madani K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol. 2016;162:68–76. doi: 10.1016/j.seppur.2016.01.043. [DOI] [Google Scholar]
- Morales M, Zapata K, Sagaste CA, Angulo AA, Rojano B. Optimization of the ultrasound-assisted extraction of polyphenol mangiferin, and its antioxidant expression in mango peel (Mangifera indica) using response surface methodology. Acta Sci Pol Technol Aliment. 2020;19(1):5–14. doi: 10.17306/J.AFS.0733. [DOI] [PubMed] [Google Scholar]
- Okonogi S, Duangrat C, Anuchpreeda S, et al. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007;103:839–846. doi: 10.1016/j.foodchem.2006.09.034. [DOI] [Google Scholar]
- Osorio-Tobon JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol. 2020;57(12):4299–4315. doi: 10.1007/s13197-020-04433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Qu W, Ma H, et al. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason Sonochem. 2012;19(2):365–372. doi: 10.1016/j.ultsonch.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Patle TK, Shrivas K, Kurrey R, et al. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–Vis and FTIR spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc. 2020 doi: 10.1016/j.saa.2020.118717. [DOI] [PubMed] [Google Scholar]
- Peschel W, Sanchez-Rabaneda F, Diekmann W, et al. An industrial approach in the search of natural antioxidants from vegetables and fruit wastes. Food Chem. 2006;97:137–150. doi: 10.1016/j.foodchem.2005.03.033. [DOI] [Google Scholar]
- Pinelo M, Rubilar M, Jerez M, et al. Effect of Solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–2117. doi: 10.1021/jf0488110. [DOI] [PubMed] [Google Scholar]
- Richter BE, Jones BA, Ezzell JL, et al. Accelerated solvent extraction: a technique for sample preparation. Anal Chem. 1996;68(6):1033–1039. doi: 10.1021/ac9508199. [DOI] [Google Scholar]
- Rojas R, Alvarez-Prerez OB, Contreras-Esquivel JC, et al. Valorisation of mango peels: extraction of pectin and antioxidant and antifungal polyphenols. Waste Biomass Valoriz. 2020;11(1):89–98. doi: 10.1007/s12649-018-0433-4. [DOI] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55(3):381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Tao Y, Sun DW. Enhancement of food processes by ultrasound: a review. Crit Rev Food Sci Nutr. 2015;55(4):570–594. doi: 10.1080/10408398.2012.667849. [DOI] [PubMed] [Google Scholar]
- Torres-Leon C, Ramos BDA, Santos Correia MTD, et al. Antioxidant and anti-staphylococcal activity of polyphenolic-rich extracts from Ataulfo mango seed. LWT. 2021 doi: 10.1016/j.lwt.2021.111653. [DOI] [Google Scholar]
- Valchos N, Akopelitia Y, Psaroudaki M, et al. Applications of Fourier transform infrared (FTIR) spectroscopy to edible oils. Anal Chem Acta. 2006 doi: 10.1016/j.aca.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Wall-Medrano A, Olivas-Aguirre FJ, Ayala-Zavala JF, Domínguez-Avila JA, Gonzalez-Aguilar GA, Herrera-Cazares LA, Gaytan-Martinez M (2020) Health benefits of mango by-products. In: Campos-Vega R, Oomah BD, Vergara-Castañeda HA (eds) Food wastes and by-products. 10.1002/9781119534167.ch6
- Xu GH, Chen JC, Liu DH, Zhang YH, Jiang P, Ye XQ. Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J Food Sci. 2008;73:11–18. doi: 10.1111/j.1750-3841.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- Yang L, Cao YL, Jiang JG, et al. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J Sep Sci. 2010;33(9):1349–1355. doi: 10.1002/jssc.200900776. [DOI] [PubMed] [Google Scholar]