Abstract
Aflatoxins (AFs) are genotoxic carcinogens and are a growing concern in peanuts and peanut products. This study aims to impact of different extraction processes on the transition of AFs from peanuts to oils. Peanuts were collected from nine different factories in Osmaniye, Turkey, during the period of November 2017–May 2018. While no aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) were detected in peanuts, aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) were determined in all peanut samples at levels varying from 26.7 to 234.7 µg kg−1 and from 4.44 to 44.0 µg kg−1, respectively. No AFs were quantified in oils obtained by the industrial application method. The ratios of AFB1 transitions to oils obtained by solvent extraction, cold pressing of roasted peanuts and cold pressing methods were 9.0–79.8%, 11.3–75.3% and 9.3–77.6%, respectively. The concentrations of AFB2 in oils obtained by solvent extraction, cold pressing of roasted peanuts and cold pressing methods were 0.46–17.2 µg kg−1, 0.84–33.0 µg kg−1 and 1.02–36.3 µg kg−1, respectively. This is the first demonstration of the impact of different extraction processes on the transition of AFs from peanuts to oils.
Keywords: Aflatoxins, Food safety, Extraction, Peanut, Peanut oil
Introduction
The peanut (Arachis hypogaea L.) is a very popular product throughout the world due to its high nutritional value and taste. China is the world’s largest producer of in-shell peanut, with 16.7 million metric tonnes produced in 2016, accounting for 37.9% of global peanut production, followed by India (15.6%), Nigeria (6.9%), the United States (5.9%) and Sudan (4.2%) (FAOSTAT 2018). Turkey is the leading producer of peanuts in Europe and associated countries, with almost 165 000 metric tonnes produced in 2017. In essence, more than 80% of Turkey peanuts are produced in Adana and Osmaniye provinces (TUIK 2018). The peanut is used not only as raw, roasted and salted but also for a variety of applications including peanut oil, peanut butter, peanut flour as well as many other uses. Peanuts that are damaged during harvesting, transportation and storage, are processed into peanut oil in Turkey. Peanut is one of the sources that have higher oil content (more than 40%) in grain (Abuagela et al. 2018). It has been expected to increase the production of peanut oil in Turkey with the decreasing of manufacturing costs and the transition to mechanization in agriculture.
Peanuts are susceptible to AFs contamination in the field conditions, during post-harvest drying and curing, and in storage and transportation. At these stages, as a result of fungal contamination of peanuts, aflatoxins (AFs) can be formed and can reach levels that may threaten the health of the consumer (Hepsag et al. 2014). AFs are toxic metabolites produced by fungi such as Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius. AFs are the most important mycotoxin group due to their toxic and carcinogenic potential. Of the 18 known AFs, only the four, aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) are naturally found in foodstuffs (EFSA 2004). In 1993, the International Agency for Research on Cancer (IARC) noted that AFB1 and naturally occurring mixtures of AFs are Group-1 agents (human carcinogen) (IARC 1993). A positive correlation has been found between the consumption of food contaminated with AFB1 and the risk of developing liver cancer in Asia and Africa (Probst et al. 2007).
AFs can be found in various agricultural products, including peanuts, tree nuts (pistachio, hazelnut, almond), Capsicums, maize and dried figs (EFSA 2007). Due to AFs are chemically stable, little or no reduction in toxin levels occurs as a result of thermal food processing (Kabak 2009). Therefore, controlling and monitoring agricultural and processed products for the presence of AFs are of great importance in terms of food safety and security. The European Commission has set the maximum levels (MLs) of 2 µg kg−1 for AFB1 and 4 µg kg−1 for sum of AFs (AFB1 + AFB2 + AFG1 + AFG2) in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs (European Commission 2006b).
The number of the Rapid Alert System for Food and Feed (RASFF) notifications on AFs in the category “nut, nut products and seeds” in 2020 was 220. The main notifications were concerned with peanuts (59.1%, 130 notifications), followed by pistachios (26.4%, 58 notifications) and hazelnuts (6.4%, 14 notifications) (RASFF 2021). While there have been many studies worldwide about AFs contamination in peanuts (Kaaya et al. 2006; Nakai et al. 2008; Blesa et al. 2003; Liao et al. 2009; Hepsag et al. 2014; Oulkar et al. 2018; Zhang and Banerjee 2020; Dhanshetty et al. 2021), limited data on the effects of extraction processes on AFs transitions to peanut oil. Thus, this study aims to impact different extraction processes on AFs transitions to peanut oil.
Materials and methods
Samples
Unshelled peanuts were provided from nine different peanut factories (A1–A9) located in Osmaniye, Turkey. The peanut kernels were collected four times in every two months during the years 2017–2018. Approximately 3 kg of peanut samples were randomly collected from peanuts bags at various depths in each shelling plant. The peanuts were stored in plastic bags at −18 °C to prevent any further mould development and AFs production until analysis.
Chemicals, reagents and standards
Methanol and acetonitrile were HPLC grade and ordered from Sigma–Aldrich (St. Louis, MO, USA). Sodium chloride, nitric acid (65%) and potassium bromide (KBr) were supplied by Merck (Darmstadt, Germany). Ultrapure water produced by Millipore Direct-Q3 purification system (Millipore, Molsheim, France) was used. The glass microfiber filter units were purchased from VWR international (Leuven, Belgium). The AflaTest® immunoaffinity columns were obtained from Vicam® (Watertown, MA, USA).
The mixed standard of AFs (1 μg AFB1, 0.3 μg AFB2, 1 μg AFG1 and 0.3 μg AFG2) in one ml of methanol was supplied by Supelco® (Bellefonte, PA, USA). A stock solution of multi-standard (0.1 μg ml−1 for AFB1 and AFG1; 0.03 μg ml−1 for AFB2 and AFG2) was prepared in methanol and kept in the dark brown vials at −18 °C. From the stock standard, working calibration standard solutions were prepared.
Apparatus
The screw press (Koçmaksan Industrial Machinery Manufacturing and Service, Turkey) was used for the cold pressing of oils. A semi-automatic soxhlet device (Gerhardt, Germany) was used for solvent extraction of oils. A reversed-phase high-performance liquid chromatography coupled with a fluorescence detector (HPLC-FLD) (Agilent 1100, USA) was used for the determination of AFs.
Oil extraction processes from peanuts
After peanut kernels were screened for the presence of AFs using the HPLC-FLD method, they were processed into peanut oil by four different methods.
Oils obtained by solvent extraction method
Peanut samples were first ground in a blender to make it homogenous, and water was removed until it reached constant weight in the oven at 100 ± 2 °C for 4–5 h. A 200 g of grounded and dried sample was loaded in two separate cartridges, and the cartridges were placed in a semi-automatic glass soxhlet device. A total of 800 ml of petroleum ether was added to the soxhlet apparatus, and then they were placed in the device. The apparatus was left to run for 4 h at 50 °C. At the end of this period, the micella (oil + solvent) was taken to the flask, and the solvent (petroleum ether) was removed in a rotary evaporator connected to the cooling water unit (+ 3 °C) and vacuum pump at 37 °C for 20 min to obtain oil (Anyasor et al. 2009).
Oils obtained by cold pressing method
An amount of 200 g of peanuts was weighed and finely pulverized to a maximum size of 0.1 mm in miller. Peanut powders were transferred to the cartridge and squeezed in a pressing machine to obtain oil (Chu and Hsu 1999). The amount of oil remaining in the press was approximately 10–15%.
Oils obtained by cold pressing of roasted peanuts
A portion of 200 g of peanuts was roasted in an oven at 200 °C for 15 min and cooled to room temperature. Roasted peanuts were finely powdered in a miller with a size of less than 0.1 mm and transferred to a press cartridge. Then, peanuts were passed through a pressing machine to obtain peanut oil (Megahed 2001). The amount of oil remaining in the press was approximately 10–15%.
Extraction using hot pressing and refining process
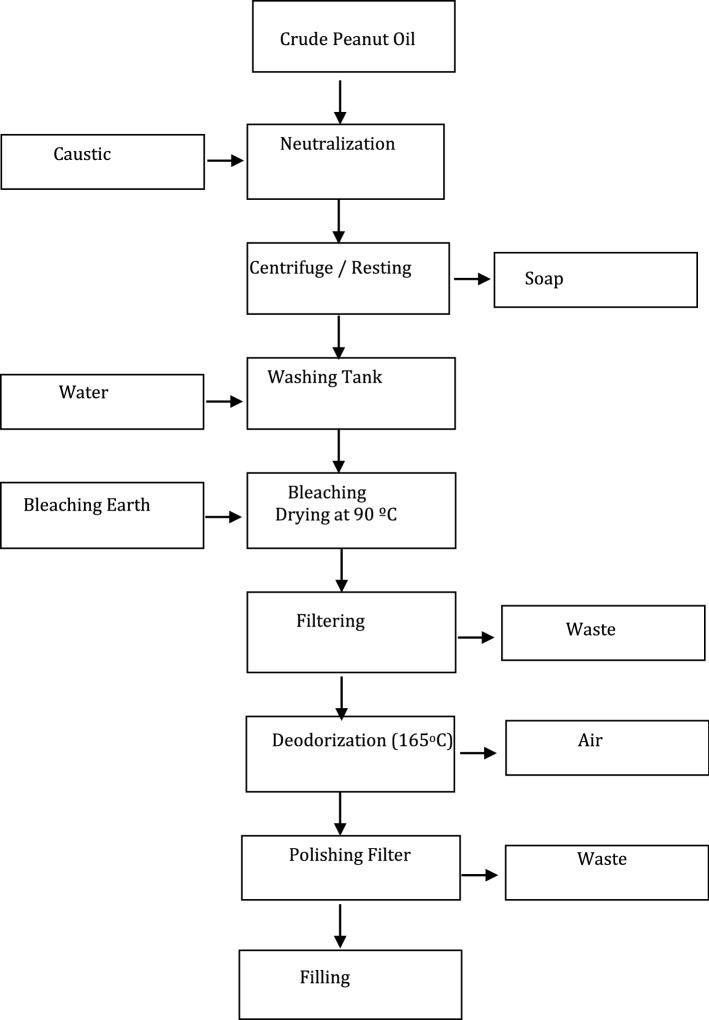
Peanut oil was produced in a private oil company operating in Osmaniye, Turkey. The peanuts (approx. 3 kg) were ground and finely powdered to a size below 0.1 mm. The finely ground samples were roasted at 90 °C for 30 min, during which time the proteins were coagulated, oil droplets joined together, and a suitable medium was prepared for the flow of oil from the peanuts. Then, the humidity was reduced to 4–4.5% at 110–115 °C and transferred to the pressing device. It was heated to 90 °C and squeezed in a hot steam pressing system to obtain crude oil. Hydraulic press (Ekmekçi Machine Ltd. Co., Hatay, Turkey) was used in the pressing process. The amount of oil remaining in the press cake was around 8–10%. After pressing, the collected oil was filtered and subjected to a refining process. The refining process of peanut oil was illustrated in Fig. 1.
Fig. 1.
Refining process of peanut oil
Sample extraction and IAC clean-up
An amount of 25 g ground peanuts was extracted with 125 mL of methanol–water (87.5:37.5, v/v) extraction solution and 5 g of NaCl with a Waring blender for 2 min at high speed. The extract was passed through a filter paper. A 15 ml filtrate was diluted with 30 ml of ultra-pure water, and the mixture was passed through a glass microfiber filter. In the next step, the IACs containing specific antibodies against AFs were placed in a vacuum manifold, and 15 ml of the diluted filtrate was passed through the IAC at a rate of 1–2 drops per second. After the sample extract completely passed through the column, the IACs were washed with 20 ml of ultra-pure water and dried with air. AFs were eluted from the column with 1 ml of methanol (HPLC purity) in a clean vial. In the next stage, 2–3 ml of air was passed to ensure that no methanol was left in the column, and 1 ml of ultrapure water was passed through the column. The mixture (representing 2 ml = 1 g of sample) was stirred in a vortex mixer and kept in a refrigerator (4–8 °C) until chromatographic analysis (Stroka et al. 2000).
For peanut oils, 10 g of sample was taken into 250 mL of a separatory funnel and shaken by adding 50 ml hexane and 40 ml deionized water. The lower phase was collected in 100 ml of the flask. The supernatant was washed twice with 20 ml of phosphate buffer (PBS) solution, and the lower phase was again collected in the same flask. The volume was completed to 100 ml with PBS. A 10 ml of this mixture was passed through the IAC at two drops per second. The column was then washed with 20 ml distilled water and dried. Finally, 1.5 ml of methanol (0.75 ml × 2) and 1.5 ml of water were passed through the column and collected in the vial. The eluate was kept in a refrigerator (4–8 °C) until HPLC-FLD analysis (AOAC 1990).
HPLC-FLD analysis
The instrument and chromatographic conditions were as follows: column: ACE 5 C18 (250 × 4.6 mm, 5 μm), detector: fluorescence detector (excitation wavelength: 360 nm, emission wavelength: 430 nm), column temperature: 25 °C, mobile phase: water–methanol-acetonitrile (6:3:2, v/v/v), mobile phase flow rate: 1 ml per min, injection volume: 100 μl. In addition, the post-column derivatization unit (Cobra cell) was included in the HPLC system. A 132 mg of potassium bromide (KBr) and 385 μl of 4 M nitric acid (HNO3) were added to the mobile phase (1100 ml) to provide ionization in the derivatization.
The linearity of the method was determined using calibration standards at seven concentrations ranging from 0.25 to 12 μg l−1 for AFB1 and AFG1 and from 0.075 to 3.6 μg l−1 for AFB2 and AFG2. The coefficients of determination (R2) values of > 0.99 were acceptable. For the recovery, the blank materials of peanuts and peanut oil were fortified with AFs standards (3 μg kg−1 for AFB1 and AFG1, and 1 μg kg−1 for AFB2 and AFG2). The fortified samples were analysed according to sample preparation and chromatographic analysis procedures as described previously, and the recovery was calculated as follows (Eq. 1):
| 1 |
Statistical analysis
The results were evaluated according to the analysis of variance, and the differences between the significant groups were determined by Duncan multiple comparison test at a 5% significance level. For this purpose, SPSS 18.0 statistical package program was used.
Results and discussion
Validation data
The linearity was maintained over the levels of 0.25–12 µg l−1 for AFB1 and AFG1, and 0.075–3.6 μg l−1 for AFB2 and AFG2, with the coefficient of determination (R2) value ≥ 0.999. The limit of quantification (LOQ) values of AFB1, AFB2, AFG1 and AFG2 were 0.50, 0.23, 0.73 and 0.20 μg kg−1, respectively. The recoveries of AFs were in the range of 104–109% in peanut and 90–104% in peanut oil matrices. The repeatabilities (RSD%) were in the range of 4.20–11.43%. These recovery values fulfilled the requirements of the Commission Regulation Guideline that recommend a recovery rate of 70–110% for mass fraction in the range 1–10 µg kg−1 (European Commission 2006a).
The transition of AFs from peanuts to peanut oils
The levels of AFs in peanuts collected from nine different companies and transitions of AFs to peanut oils obtained by different extraction methods are shown in Table 1. While AFB1 and AB2 were detected in all peanut samples collected from nine peanut factories, AFG1 and AFG2 could not be determined in any sample. This can be explained by the fact that the raw peanuts might be contaminated with only B-group AFs-producing A. flavus. The concentrations of AFB1 and AFB2 in peanuts ranged between 26.7 and 234.7 µg kg−1 and between 4.44 and 44.0 µg kg−1, respectively. These differences in AFB1 and AFB2 levels may be explained by the peanuts coming to the peanut companies to be physically and chemically different and, most importantly, the waiting time of the waste peanuts in inappropriate conditions. These levels are higher than that reported by Hepsag et al. (2014), who found AFs in 19.2% of peanut samples (29/151) at levels of 0.16–60.9 µg kg−1. The researchers also reported that ten peanut samples contained AFs above the ML of 10 µg kg−1 set by Turkish Food Codex (TGK 2011).
Table 1.
The effect of extraction methods and sampling period on AFB1 and AFB2 in peanut oils (a: A1 Company, b: A2 Company, c: A3 Company, d: A4 Company, e: A5 Company, f: A6 Company, g: A7 Company, h: A8 Company, i: A9 Company) (Mean value ± standard deviation, μg kg−1)
| Period | Applied method | |||||
|---|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | Industrial method | ||
| a | ||||||
| AFB1 | 1 | 187.74Aaxy ± 4.7 | 31.89 Da ± 1.0 | 61.63Ca ± 0.6 | 89.52Ba ± 1.7 | nd |
| 2 | 68.93Ad ± 1.2 | 17.95Dd ± 0.6 | 45.63Bc ± 0.7 | 33.82Cd ± 0.5 | nd | |
| 3 | 117.77Ac ± 0.9 | 25.64Db ± 0.7 | 47.21Cbc ± 0.2 | 61.41Bb ± 1.2 | nd | |
| 4 | 138.65Ab ± 1.3 | 20.11Dc ± 0.6 | 48.98Bb ± 1.3 | 44.69Cc ± 1.0 | nd | |
| AFB2 | 1 | 29.72Aa ± 2.4 | 6.01 Da ± 0.2 | 20.46Ca ± 0.8 | 24.22Ba ± 0.5 | nd |
| 2 | 11.27Ad ± 1.2 | 2.49Dc ± 0.6 | 9.71Bc ± 0.2 | 7.44Cc ± 0.1 | nd | |
| 3 | 18.76Ab ± 0.9 | 3.49Db ± 0.2 | 8.88Cd ± 0.5 | 10.46Bb ± 0.1 | nd | |
| 4 | 17.40Ac ± 0.8 | 3.58Db ± 0.1 | 14.31Bb ± 0.5 | 10.86Cb ± 0.2 | nd | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| b | |||||
| AFB1 | 1 | 188.54Aaxy ± 2.4 | 17.05Cc ± 2.7 | 73.27Ba ± 1.6 | 71.35Bab ± 2.2 |
| 2 | 134.24Ac ± 3.2 | 21.43Dbc ± 0.9 | 46.91Cc ± 1.0 | 72.67Ba ± 1.0 | |
| 3 | 181.89Aa ± 6.1 | 25.06Dab ± 1.2 | 40.11Cd ± 1.14 | 61.47Bbc ± 4.8 | |
| 4 | 158.55Ab ± 1.8 | 28.58Ca ± 0.8 | 52.43Bb ± 1.14 | 54.67Bc ± 5.5 | |
| AFB2 | 1 | 16.32Ac ± 0.9 | 1.76Dc ± 0.5 | 13.05Ba ± 0.4 | 10.73Cb ± 0.9 |
| 2 | 15.76Ad ± 1.2 | 2.99Db ± 0.4 | 8.92Cb ± 0.6 | 12.92Ba ± 0.8 | |
| 3 | 21.05Aa ± 0.9 | 3.33Db ± 0.8 | 7.42Cc ± 0.6 | 10.49bB ± 0.9 | |
| 4 | 17.45Ab ± 0.8 | 3.98Ca ± 0.6 | 9.48Bb ± 0.8 | 9.95Bb ± 1.0 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| c | |||||
| AFB1 | 1 | 41.26cAxy ± 0.8 | 8.58bB ± 0.5 | 6.65Cb ± 0.1 | 7.47BCc ± 0.1 |
| 2 | 52.62aA ± 0.9 | 8.88aC ± 0.4 | 8.74aC ± 0.1 | 15.70aB ± 1.4 | |
| 3 | 26.68dA ± 1.1 | 2.51dC ± 0.3 | 4.33cB ± 0.7 | 5.98 dB ± 0.2 | |
| 4 | 45.79bA ± 1.4 | 4.35cD ± 0.2 | 6.81bC ± 0.5 | 12.87bB ± 0.6 | |
| AFB2 | 1 | 4.44cA ± 0.2 | 1.49aB ± 0.5 | 1.01bC ± 0.2 | 1.02dC ± 0.0 |
| 2 | 9.27aA ± 0.3 | 1.39aD ± 0.2 | 1.72aC ± 0.1 | 2.75aB ± 0.0 | |
| 3 | 4.56cA ± 0.3 | 0.46cC ± 0.0 | 0.84cB ± 0.2 | 1.16cB ± 0.1 | |
| 4 | 5.24bA ± 0.8 | 0.64bD ± 0.2 | 0.98bC ± 0.1 | 1.73bB ± 0.0 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| d | |||||
| AFB1 | 1 | 218.49bcAxy ± 0.8 | 48.58bD ± 0.4 | 57.92abC ± 1 | 72.49bB ± 0.3 |
| 2 | 214.13cA ± 0.6 | 36.14dD ± 0.4 | 56.50abC ± 1.2 | 73.25bB ± 0.4 | |
| 3 | 219.88bA ± 2.4 | 43.46cD ± 0.1 | 65.45aC ± 0.4 | 79.96aB ± 1.9 | |
| 4 | 234.68aA ± 2.5 | 96.97aB ± 1.1 | 53.38bD ± 6.4 | 75.15bC ± 1.4 | |
| AFB2 | 1 | 32.75cA ± 0.1 | 5.73bC ± 3.3 | 26.52bB ± 0.1 | 28.69bAB ± 0.1 |
| 2 | 43.77aA ± 0.3 | 6.68bC ± 0.1 | 32.27aB ± 1.22 | 33.63aB ± 0.9 | |
| 3 | 44.00aA ± 0.2 | 8.42bD ± 0.2 | 25.86bC ± 2.2 | 30.10bB ± 1.3 | |
| 4 | 43.15bA ± 0.3 | 17.22aD ± 0.19 | 20.13cC ± 1.2 | 27.78bB ± 0.7 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| e | |||||
| AFB1 | 1 | 202.52aAxy ± 2.8 | 23.84dD ± 0.6 | 34.58cC ± 0.6 | 64.89bB ± 1.8 |
| 2 | 178.13cA ± 1.8 | 77.19aB ± 1.7 | 20.10dD ± 0.2 | 28.44cC ± 0.5 | |
| 3 | 192.83bA ± 0.2 | 67.93bC ± 0.7 | 59.26bD ± 1.5 | 75.28aB ± 0.3 | |
| 4 | 194.78bA ± 2.5 | 56.13cC ± 1 | 85.62aB ± 1.3 | 18.14dD ± 0.5 | |
| AFB2 | 1 | 14.99cA ± 0.1 | 5.05cD ± 0.1 | 9.45bC ± 0.1 | 12.76bB ± 0.16 |
| 2 | 13.15dA ± 0.1 | 10.95aB ± 1 | 3.97cD ± 0.1 | 6.88cC ± 0.15 | |
| 3 | 32.80aA ± 0.2 | 11.17aD ± 0.1 | 19.19aC ± 0.7 | 25.85aB ± 0.2 | |
| 4 | 29.41bA ± 0.4 | 9.39bC ± 0.2 | 19.33aB ± 0.3 | 3.10dD ± 0.1 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| f | |||||
| AFB1 | 1 | 195.26aAxy ± 1.2 | 70.83bC ± 0.4 | 64.94bD ± 1.8 | 78.62cB ± 1.4 |
| 2 | 157.45bA ± 0.4 | 55.65cD ± 1.4 | 64.53bC ± 0.9 | 99.21bB ± 1.8 | |
| 3 | 200.71aA ± 4.1 | 34.27dD ± 0.7 | 90.14aC ± 1.8 | 105.71aB ± 1.2 | |
| 4 | 206.89aA ± 9 | 78.79aB ± 1.2 | 67.83bB ± 2.6 | 71.17 dB ± 0.9 | |
| AFB2 | 1 | 21.55dA ± 0.8 | 17.07aB ± 0.7 | 14.88dC ± 1.2 | 10.21dD ± 0.8 |
| 2 | 33.48cA ± 0.6 | 11.76bC ± 0.9 | 27.46bB ± 1.6 | 27.52cB ± 0.6 | |
| 3 | 43.06aA ± 0.9 | 6.68cD ± 0.5 | 32.96aB ± 1.2 | 30.66bC ± 1.4 | |
| 4 | 39.48bA ± 1.2 | 16.92aD ± 1.2 | 23.22cC ± 0.9 | 36.33aB ± 1.2 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| g | |||||
| AFB1 | 1 | 190.77aAxy ± 2.4 | 43.68cD ± 1.2 | 89.56bB ± 1.5 | 81.20bC ± 1.4 |
| 2 | 163.43bA ± 6.4 | 56.79bC ± 2.3 | 77.50cB ± 1.9 | 68.08cB ± 2.2 | |
| 3 | 178.38abA ± 1.6 | 54.59bD ± 2 | 128.68aB ± 5.2 | 81.0bC ± 1.7 | |
| 4 | 183.83abA ± 15 | 72.56aC ± 1.3 | 44.63dD ± 1.5 | 97.25aB ± 1.8 | |
| AFB2 | 1 | 39.18aA ± 1.8 | 9.65cD ± 1.2 | 24.89bC ± 1.0 | 27.02bB ± 2.2 |
| 2 | 37.26bA ± 1.4 | 11.61bD ± 1.4 | 28.54aC ± 1.3 | 31.41aB ± 0.9 | |
| 3 | 33.19dA ± 0.9 | 10.32cD ± 1.8 | 20.92cC ± 1.1 | 27.41bB ± 1.8 | |
| 4 | 34.43cA ± 1.6 | 16.52aC ± 1.2 | 8.65dD ± 1.4 | 27.36bB ± 1.6 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| h | |||||
| AFB1 | 1 | 73.45bAxy ± 1.6 | 41.76bC ± 1.5 | 55.31aB ± 1.3 | 57.00aB ± 2.8 |
| 2 | 66.96cA ± 1.6 | 53.46aB ± 3.1 | 44.62bC ± 1.8 | 45.55bBC ± 5.0 | |
| 3 | 79.46aA ± 2.5 | 37.17bcC ± 1.9 | 17.55cD ± 1.6 | 57.53aB ± 1.9 | |
| 4 | 61.11dA ± 3.2 | 34.88cC ± 1.8 | 43.91bB ± 1.4 | 44.81bB ± 2.8 | |
| AFB2 | 1 | 15.51bA ± 1.4 | 9.19bC ± 0.9 | 12.07bB ± 1.3 | 12.42aB ± 1.6 |
| 2 | 17.46aA ± 1.3 | 10.98aC ± 1.6 | 14.95aB ± 1.9 | 10.99aC ± 1.4 | |
| 3 | 17.54aA ± 1.7 | 8.71bC ± 1.2 | 5.54dD ± 1.8 | 12.09aB ± 1.8 | |
| 4 | 13.44cA ± 1.2 | 7.05cC ± 1.7 | 9.83cB ± 0.8 | 9.47bB ± 1.2 | |
| Period | Applied method | ||||
|---|---|---|---|---|---|
| Peanut | Solvent extraction | Cold pressing of roasted peanuts | Cold pressing | ||
| i | |||||
| AFB1 | 1 | 49.76bAxy ± 1.8 | 27.96cB ± 1.1 | 10.53dC ± 0.5 | 8.94dC ± 1.2 |
| 2 | 62.01aA ± 1.2 | 21.54 dB ± 1.3 | 12.93cC ± 0.2 | 11.05cC ± 1.9 | |
| 3 | 52.95bA ± 1.8 | 37.82aB ± 2.2 | 18.22bC ± 1.3 | 17.80bC ± 0.8 | |
| 4 | 44.97cA ± 1.6 | 31.96bB ± 1.7 | 25.41aC ± 0.8 | 32.67aB ± 1.4 | |
| AFB2 | 1 | 6.98cA ± 1.2 | 5.27cB ± 0.6 | 2.46dC ± 0.8 | 2.04dD ± 0.0 |
| 2 | 10.58aA ± 1.8 | 4.28 dB ± 1.2 | 4.55cB ± 1.0 | 3.84cC ± 0.0 | |
| 3 | 10.53aA ± 0.8 | 7.07aB ± 1.2 | 6.47bC ± 0.4 | 5.88bC ± 0.2 | |
| 4 | 9.69bA ± 1.0 | 6.34bC ± 1.2 | 8.105aB ± 0.6 | 8.42aB ± 0.2 | |
xThe difference between the values shown in different capital letters on the same line is statistically significant (p < 0.05).
yThe difference between the values shown in different lower case letters for each toxin in the same column is statistically significant (p < 0.05).
nd: not detected
The effect of the processing method and sampling period on the amount of AFB1 and AFB2 in oils was found to be significant (p < 0.05). Among the processing methods used for obtaining peanut oils, the most effective was the industrial application (extraction using hot pressing and refining process). No transition of both AFB1 and AFB2 to peanut oils was observed after the industrial application method. This could be explained by process steps during refining in the industrial oil-making process. It is thought that the use of sodium hydroxide at high temperatures in the neutralization process causes the elimination of AFs. This result is confirmed by a previous study by Idris et al. (2010), who did not detect AFs in refined peanut, sesame and cottonseed oils. However, Elzupir et al. (2010) determined AFB1 in 11 out of 21 peanut oils at levels from 2.9 to 36.1 µg kg−1, with a mean concentration of 16.3 µg kg−1.
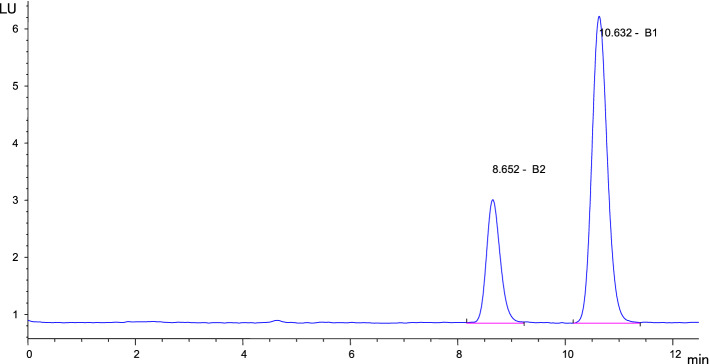
The solvent extraction method was the second most effective method for the reduction of transitions both of AFB1 and AFB2 to peanut oils. In the solvent extraction method, AFB1 present in peanuts was transferred to oils in the ranges of 9.0–79.8%, with a mean level of 31.7%, depending on initial toxin concentration and sampling period. The levels of AFB2 in oils obtained by solvent extraction method were ranged between 0.46 and 17.2 µg kg−1, corresponding to 10.1–83.3% of AFB2 present in peanuts. An HPLC-FLD chromatogram of AFB1 (43.5 μg kg−1) and AFB2 (8.4 μg kg−1) in peanut oils obtained by solvent extraction method is shown in Fig. 2. In the solvent extraction method, transferring of AFB1 from peanuts to oils in the samples taken from companies A1, A2, A6 and A7 were found to be significantly lower (p < 0.05) compared to cold pressing and cold pressing of roasted peanuts. Similarly, the transferring of AFB2 to oils obtained by solvent extraction method was significantly lower (p < 0.05) than other oils obtained by cold pressing and cold pressing of roasted peanuts except for oils from A3, A5, A8 and A9 factory.
Fig. 2.
An HPLC-FLD chromatogram of AFB1 (43.5 μg kg−1) and AFB2 (8.4 μg kg−1) in peanut oils obtained by solvent extraction method
The amounts of AFB1 and AFB2 in oils obtained by cold pressing of roasted peanuts ranged from 4.33 to 128.7 µg kg−1 and from 0.84 to 33.0 µg kg−1. This means that 11.3–75.3% of AFB1 and 18.4–86.2% of AFB2 present in peanuts were transferred to oils obtained by cold pressing of roasted peanuts. The least effective method in the reduction of transitions of AFB1 and AFB2 to oils was the cold pressing of peanuts. AFB1 levels in peanut oils obtained by cold pressing of peanuts were in the range 5.98–105.7 µg kg−1 (mean = 54.9 µg kg−1), which represented 9.3–77.6% of AFB1 present in peanuts. The ranges of AFB2 concentration in oils obtained by cold pressing of peanuts were 1.02–36.3 µg kg−1 (mean = 15.4 µg kg−1), which represented 10.5–92.0% of AFB2 present in peanuts. However, there was no significant difference (p > 0.05) in the transferring of AFB1 from peanuts to oils from A2, A3, A5, A7 and A9 factories between the processes of cold pressing and cold pressing of roasted peanuts. For AFB2, there was no significant difference (p > 0.05) between the oil samples obtained by cold pressing and cold pressing of roasted peanuts, except for oils from A7.
In order to make a general evaluation, the results were evaluated according to the three-way analysis of variance (Table 2). The differences in the transitions of AFB1 and AFB2 from peanuts to oils were found to be significant (p < 0.01) between process methods. The highest transitions of AFB1 and AFB2 to oils were obtained by cold pressing of peanuts (39.8% for AFB1, 68.7% for AFB2), followed by cold pressing of roasted peanuts (34.9% for AFB1, 61.7% for AFB2) and solvent extraction (28.5% for AFB1, 32.8% for AFB2) methods. The difference between peanut sampling periods was also found to be significant (p < 0.01). This is the first demonstration of the impact of different extraction processes, including industrial application, on the transition of AFs from peanuts to oils.
Table 2.
The effect of extraction method, company and sampling period on AFB1 and AFB2 in peanut oils
| Parameter | AFB1 (mean ± SDa, μg kg−1) | AFB2 (mean ± SD, μg kg−1) | |
|---|---|---|---|
| Company | A1 | 65.0d ± 46.23 | 12.44c ± 7.71 |
| A2 | 76.77c ± 56.12 | 10.53e ± 5.53 | |
| A3 | 16.21 g ± 15.98 | 2.42 g ± 1.32 | |
| A4 | 102.91a ± 71.35 | 26.67a ± 12.22 | |
| A5 | 86.23b ± 65.58 | 14.22b ± 8.79 | |
| A6 | 102.63a ± 54.73 | 24.58a ± 10.83 | |
| A7 | 100.75a ± 50.60 | 24.28a ± 10.02 | |
| A8 | 50.91e ± 15.29 | 11.70d ± 3.24 | |
| A9 | 29.13f ± 16.27 | 6.41f ± 2.59 | |
| Significance level | ** | ** | |
| Applied method | Peanut | 137.93a ± 66.75 | 22.47a ± 12.61 |
| Solvent extraction | 39.34d ± 22.59 | 7.38d ± 4.73 | |
| Cold pressing of roasted peanuts | 48.11c ± 27.94 | 13.87c ± 9.38 | |
| Cold Pressing | 54.90b ± 28.84 | 15.44b ± 8.55 | |
| Significance level | ** | ** | |
| Period | 1 | 73.54a ± 60.66 | 13.79ab ± 9.02 |
| 2 | 63.12b ± 49.55 | 14.58a ± 11.58 | |
| 3 | 72.11a ± 57.84 | 15.76a ± 11.10 | |
| 4 | 71.50a ± 56.55 | 15.01a ± 10.01 | |
| significance level | ** | ** |
aArithmetic mean ± standard deviation
The difference between the values indicated by different letters in the same column is statistically significant (p < 0.05), **: p < 0.01
Conclusion
AFB1 and AFB2 were detected in all peanut samples intended for oil production in Turkey obtained from nine peanuts factories, up to levels of 234.7 and 44.0 µg kg−1, respectively. Peanut samples showed neither AFG1 nor AFG2 contamination. The most effective method in the prevention of AFs transfer from peanuts to oils was the industrial method, followed by solvent extraction, cold pressing of roasted peanuts and cold pressing methods. AFB1 and AFB2 could not be detected in peanut oils produced by industrial method. Codes of practices should be applied that describes preventive measures established by Codex for the prevention and reduction of AFs formation in peanuts.
Acknowledgements
This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK project no. 116O068) and Scientific and Research Council of Osmaniye Korkut Ata University (project no. MUH19004.15.008).
Author contributions
Conceptualization: A. B. and B. K.; methodology: A. B. and B. K.; formal analysis: K. S., K.Y. and T.E.; investigation: K. S., K.Y. and T.E.; method validation: K. S. funding acquisition: A. B.; writing-review and editing: A. B. and B. K.
Funding
This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK project no. 116O068) and Scientific and Research Council of Osmaniye Korkut Ata University (project no. MUH19004.15.008).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
All authors have contributed to this manuscript.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Ethical approval
Ethical approval was not required for this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adnan Bozdogan, Email: bozdogan@osmaniye.edu.tr.
Bulent Kabak, Email: bulentkabak@hitit.edu.tr.
References
- Abuagela MO, Iqdiam BM, Baker GL, Macintosh AJ. Temperature-controlled pulsed light treatment: impact on aflatoxin level and quality parameters of peanut oil. Food Bioprocess Technol. 2018;11:1350–1358. doi: 10.1007/s11947-018-2105-6. [DOI] [Google Scholar]
- Anyasor GN, Ogunwenmo KO, Oyelana OA, Ajayi D, Dangana J. Chemical analyses of groundnut (Arachis hypogaea) oil. Pakistan J Nutr. 2009;8:269–272. doi: 10.3923/pjn.2009.269.272. [DOI] [Google Scholar]
- AOAC (1990) Methods of analysis of the association of official analytical chemists. 1990. 15th edition, Virginia, USA, pp:1184–1213
- Blesa J, Soriano JM, Molto JC, Marin R, Mañes J. Determination of aflatoxins in peanuts by matrix solid-phase dispersion and liquid chromatography. J Chromatogr A. 2003;1011:49–54. doi: 10.1016/S0021-9673(03)01102-6. [DOI] [PubMed] [Google Scholar]
- Chu Y-H, Hsu H-F. Effects of antioxidants on peanut oil stability. Food Chem. 1999;66:29–34. doi: 10.1016/S0308-8146(98)00082-X. [DOI] [Google Scholar]
- Dhanshetty M, Elliott CT, Banerjee K. Decontamination of aflatoxin B1 in peanuts using various cooking methods. J Food Sci Technol. 2021;58:2547–2554. doi: 10.1007/s13197-020-04761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (2004) Opinion on the scientific panel on contaminants in the food chain on a request from the commission related to aflatoxin B1 as undesirable substance in animal feed. The EFSA J 39 1–27
- EFSA (2007) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. The EFSA J 446 1–227
- Elzupir AO, Suliman MA, Ibrahim IA, Fadul MH, Elhussein AM. Aflatoxins levels in vegetable oils in Khartoum State, Sudan. Mycotoxin Res. 2010;26:69–73. doi: 10.1007/s12550-010-0041-z. [DOI] [PubMed] [Google Scholar]
- European Commission (2006a) Commission regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Official Journal of the European Union L70 12–34
- European Commission (2006b) Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union L364 5–23
- FAOSTAT (2018) FAO statistical databases and data sets. http://www.faostat.fao.org. Accessed 1 Feb 2020
- Hepsag F, Golge O, Kabak B. Quantitation of aflatoxins in pistachios and groundnuts using HPLC-FLD method. Food Control. 2014;38:75–81. doi: 10.1016/j.foodcont.2013.10.005. [DOI] [Google Scholar]
- IARC (1993) Some naturally occurring substances, food items and constituents, heterocyclic aromatic amines and mycotoxins, 56. Lyon France: World Health Organization 489–521
- Idris YMA, Mariod AA, Elnour IA, Mohamed AA. Determination of aflatoxin levels in Sudanese edible oils. Food Chem Toxicol. 2010;48:2539–2541. doi: 10.1016/j.fct.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Kaaya AN, Harris C, Eigel W. Peanut aflatoxin levels on farms and in markets of Uganda. Peanut Sci. 2006;33:68–75. doi: 10.3146/0095-3679(2006)33[68:PALOFA]2.0.CO;2. [DOI] [Google Scholar]
- Kabak B. The fate of mycotoxins during thermal food processing. J Sci Food Agric. 2009;89:549–554. doi: 10.1002/jsfa.3491. [DOI] [Google Scholar]
- Liao B, Zhuang W, Tang R, Zhang X, Shan S, Jiang H, Huang J. Peanut aflatoxin and genomics research in China: progress and perspectives. Peanut Sci. 2009;36:21–28. doi: 10.3146/AT07-004.1. [DOI] [Google Scholar]
- Megahed MG. Microwave roasting of peanuts: Effects on oil characteristics and composition. Nahrung. 2001;45:255–257. doi: 10.1002/1521-3803(20010801)45:4<255::AID-FOOD255>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Nakai VK, Rocha LO, Gonçalez E, Fonseca H, Ortega EMM, Corrêa B. Distribution of fungi and aflatoxins in a stored peanut variety. Food Chem. 2008;106:285–290. doi: 10.1016/j.foodchem.2007.05.087. [DOI] [Google Scholar]
- Oulkar D, Goon A, Dhanshetty M, Khan Z, Satav S, Banerjee K. High sensitivity direct analysis of aflatoxins in peanuts and cereal matrices by ultra-performance liquid chromatography with fluorescence detection involving a large volume flow cell. J Environ Sci Health Part B. 2018;53:255–260. doi: 10.1080/03601234.2017.1410416. [DOI] [PubMed] [Google Scholar]
- Probst C, Njapau H, Cotty PJ. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the casual agent. Appl Environ Microbiol. 2007;73:2762–2764. doi: 10.1128/AEM.02370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASFF (2021). https://webgate.ec.europa.eu/rasff-window/screen/list. Accessed 28 June 2021
- Stroka J, Anklam E, Jörissen U, Gilbert J. Immunoaffinity column cleanup with liquid chromatography using post-column bromination for determination of aflatoxins in peanut butter, pistachio paste, fig paste, and paprika powder: collaborative study. J AOAC Int. 2000;83:320–340. doi: 10.1093/jaoac/83.2.320. [DOI] [PubMed] [Google Scholar]
- TGK (2011) Resmi gazete, sayı: 28157. http://www.resmigazete.com.tr. Accessed 12 Feb 2020
- TUIK (2018) The summary of agricultural statistics. http://tuik.gov.tr. Accessed 16 Oct 2019
- Zhang K, Banerjee K. A review: sample preparation and chromatographic technologies for detection of aflatoxins in foods. Toxins. 2020;12:539. doi: 10.3390/toxins12090539. [DOI] [PMC free article] [PubMed] [Google Scholar]