Abstract
Chronic pancreatitis (CP) is a chronic inflammatory and fibrotic disease of the pancreas. The incidence of CP is increasing worldwide but the effective therapies are lacking. Hence, it is necessary to identify economical and effective agents for the treatment of CP patients. Vitamin D (VD) and its analogues have been confirmed as pleiotropic regulators of cell proliferation, apoptosis, differentiation and autophagy. Clinical studies show that VD deficiency is prevalent in CP patients. However, the correlation between VD level and the risk of CP remains controversial. VD and its analogues have been demonstrated to inhibit pancreatic fibrosis by suppressing the activation of pancreatic stellate cells and the production of extracellular matrix. Limited clinical trials have shown that the supplement of VD can improve VD deficiency in patients with CP, suggesting a potential therapeutic value of VD in CP. However, the mechanisms by which VD and its analogues inhibit pancreatic fibrosis have not been fully elucidated. We are reviewing the current literature concerning the risk factors for developing CP, prevalence of VD deficiency in CP, mechanisms of VD action in PSC-mediated fibrogenesis during the development of CP and potential therapeutic applications of VD and its analogues in the treatment of CP.
Keywords: vitamin D, vitamin D receptor, chronic pancreatitis, fibrosis, inflammation
1 Introduction
Chronic pancreatitis (CP) is a multifactorial fibroinflammatory disease in which repeated episodes of pancreatic inflammation leads to extensive deposition of fibrotic tissue. The main clinical manifestations of CP are chronic pain, exocrine and endocrine pancreatic insufficiency, thereby declining life quality and shortening life expectancy. The pathophysiological processes of CP involve cellular injury, inflammation and fibrosis (Singh et al., 2019). The patients with CP in 5 years after diagnosis had a nearly eight-times increased risk for pancreatic cancer with a dismal prognosis (Kirkegård et al., 2017). The incidence and prevalence of CP are on the rise and extensive investigation on the treatment of CP has been done. However, there is still no effective treatment other than active care (Beyer et al., 2020). To explore agents that can be used for prevention or treatment of CP is needed urgently.
Vitamin D (VD) is a steroid hormone that has an important role in regulating body levels of calcium and phosphorus. It was initially widely used in skeletal system disorders because of its anti-rickets effect. Over the last several years, VD has been demonstrated to have pleiotropic effects including the regulation of cell proliferation, differentiation, apoptosis and autophagy as well as antagonizing inflammatory, fibrosis and cancer (Pike and Christakos, 2017; Golpour et al., 2019). Therefore, it has been also considered to be a promising therapeutic agent for non-skeletal system diseases such as cardiovascular disease, diabetes, cancer, infection, and autoimmune diseases (Jeon and Shin, 2018; Grant et al., 2020; Harrison et al., 2020; de la Guía-Galipienso et al., 2021). These exciting results inspire people to explore the correlation between VD and CP, and the potential therapeutic effects of VD in CP.
Previous epidemiological studies and clinical observations have found that VD deficiency is prevalent in patients with CP (Martínez-Moneo et al., 2016), but the correlation between VD level and the risk of CP remains controversial (Klapdor et al., 2012; Hoogenboom et al., 2016; Martínez-Moneo et al., 2016; Olesen et al., 2017). Several experimental studies have assessed the potential therapeutic benefits of VD in pancreatitis despite the therapeutic mechanism is not fully elucidated (Sherman et al., 2014; Bläuer et al., 2015; Kang et al., 2018; Wallbaum et al., 2018; Kang et al., 2021). VD analogue has been shown to suppress pancreatitis and the tumor stroma of pancreatic ductal adenocarcinoma via inhibiting pancreatic stellate cells (PSCs) activation (Sherman et al., 2014; Kang et al., 2021). Numerous studies are underway to elucidate the molecular mechanisms of VD/VD receptor (VDR) actions which involve in pancreatic and extra-pancreatic diseases. Some signaling pathways of VD/VDR in CP have been described, but their exact mechanisms need to be further clarified. Here we provide an up-to-date overview on these specific aspects, to better understand the potential therapeutic value of VD in CP. To our knowledge, this is the first review in this field.
2 Chronic Pancreatitis—Risk Factors and Pathogenesis
2.1 Risk Factors for Developing Chronic Pancreatitis
Excessive alcohol abuse is the most common cause of CP, affecting 42%–77% of patients with CP (Singh et al., 2019; Beyer et al., 2020). It has been also reported that the risk of developing CP in people with a long history of alcohol consumption was significantly higher than those not drinking (Singhvi and Yadav, 2018). Regular tobacco use is also a high risk factor of developing CP and there is a high prevalence (approximately 60%) of tobacco smoking among patients with CP (Beyer et al., 2020). Furthermore, the high risk of CP caused by smoking exhibits in a dose-dependent manner or in a combination with other risk factors, such as alcohol consumption (Rebours et al., 2012). Quitting smoking or alcohol or both can substantially reduce the risk of CP progression (Nikkola et al., 2013).
Additionally, several variants in genes including trypsin dependent and independent variants are also associated with CP, especially with idiopathic CP. These mutated genes include human cationic trypsinogen (PRSS1), pancreatic secretory trypsin inhibitor (SPINK1), chymotrypsin C (CTRC), cystic fibrosis transmembrane conductance regulator (CFTR), carboxypeptidase A1(CPA1) and claudin 2 (CLDN2) genes (Beyer et al., 2020). Other etiological risk factors include pancreatic duct obstruction, hypertriglyceridemia, hypercalcemia, IgG4-related disease, and chronic kidney disease (Singh et al., 2019; Beyer et al., 2020). CP is a multifactorial fibroinflammatory disease and its occurrence and progression can be usually promoted by multiple risk factors (HM et al., 2021).
2.2 Pathophysiology of Chronic Pancreatitis
The pathological features of CP are inflammatory cell infiltration, acinar atrophy, and pancreatic fibrosis. Pancreatic fibrosis is a pathological process characterized by the initial events of cellular damage and inflammatory cell infiltration, the involvement of multiple cytokines and inflammatory mediators, and the mediation of complex signal pathways, which in turn leads to PSC activation and extracellular matrix (ECM) production. Therefore, PSC plays a critical role in pancreatic fibrosis during the development of CP.
2.2.1 Cellular Injury
In normal pancreas, acinar cells play an important role in the synthesis and secretion of digestive enzymes. Ethanol damages acinar cells through oxidative metabolite acetaldehyde and non-oxidative metabolite fatty acid ethyl ester, both of which can also damage pancreatic duct cells and PSCs. Damaged acinar cells can induce the activation of transcriptional activator nuclear factor—kappa B (NF-κB) and the expression of pro-inflammatory cytokines resulting in the activation of inflammatory cascade and necro-inflammatory response (Clemens et al., 2016). Smoking causes acinar cell damage due to the toxic metabolites of nicotine. Additionally, the premature or increased intrapancreatic activation of trypsinogen due to variants in the PRSS1, SPINK1, and CTRC genes is the initial step of CP, which damages acinar cells through several mechanisms, such as endoplasmic reticulum stress, oxidative stress and impaired autophagy. The trypsin independent variants in the CFTR, CPA1, and CLDN2 genes also cause cell damage through different mechanisms (Witt et al., 2013; Giri et al., 2016).
2.2.2 Inflammation
Inflammation is mediated by cytokines, chemokines, and adhesion molecules. In the early stage of CP, injured acinar cells activate the key inflammatory cells such as macrophages, granulocytes and lymphocytes. All these cells then release a large number of proinflammatory cytokines, such as IL-1, IL-6, IL-8, tumor necrosis factor-alpha, transforming growth factor-beta 1 (TGF-β1), and platelet derived growth factor (PDGF). These proinflammatory cytokines can activate PSCs via paracrine stimuli. Meanwhile, the activated PSCs can also secrete cytokines for sustained activation of PSCs via autocrine stimuli. The sustained activation of PSCs leads to greater synthesis of ECM than degradation, eventually resulting in pancreatic fibrosis (Jin et al., 2020; Kandikattu et al., 2020; Zheng et al., 2021) (Figure 1). Additionally, NF-κB and activator protein 1 (AP-1) are important transcriptional factors that are involved in inflammatory responses. These two factors play an important role in initiating the inflammatory cascade in CP (Kandikattu et al., 2020).
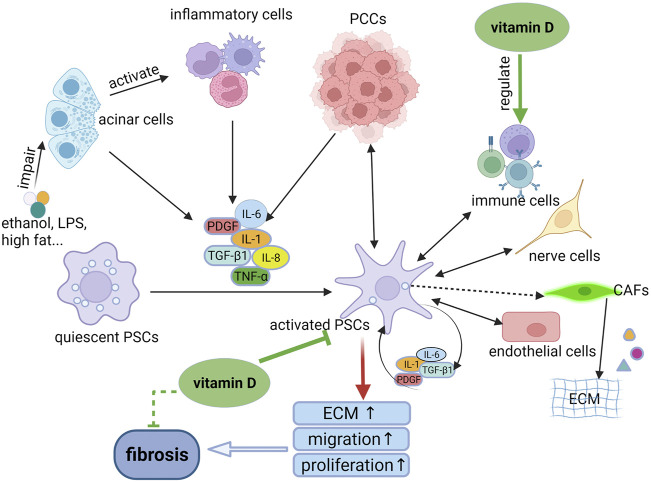
FIGURE 1.
The mechanism of PSC activation and the role of vitamin D in the process. When the pancreas is injured by ethanol, LPS or other factors, the damaged acinar cells can activate inflammatory cells to release pro-inflammatory cytokines which in turn activates quiescent PSCs to become activated phenotypes through paracrine stimuli. The activated PSCs can secrete cytokines to activate PSCs continuously through autocrine stimuli, resulting in pancreatic fibrosis. In addition, the activated PSCs can interact with other cell types, such as PCCs and immune cells, mediating the persistent inflammatory environment. Whereas, vitamin D can inhibit the activation and proliferation of PSCs, thereby reducing the synthesis of ECM. In addition, vitamin D play an anti-inflammatory and anti-fibrosis role via regulation of immune cells. LPS, lipopolysaccharide; PSCs, pancreatic stellate cells; ECM, extracellular matrix; PCCs, pancreatic cancer cells; CAFs, cancer-associated fibroblasts.
Previous in vivo studies have demonstrated that T cells and macrophages are the predominant immune cell types in the pancreas of CP (Sun et al., 2018; Kandikattu et al., 2020; Zheng et al., 2021). Pancreases from mice CP models and patients were infiltrated by M2 macrophages instead of M1 macrophages. The M2 macrophages can effectively activate PSCs via a “feedforward” process, suggesting that macrophages play a key role in the fibrogenesis of pancreas (Xue et al., 2015). Increased lymphocytes have been observed in pancreatic tissue samples from patients with CP, thereinto, CD8+ T cells that reside between the pancreatic parenchyma and the fibrotic region are considered as key contributors to disease severity, CD8+ T cell- or NKT cell-mediated cytotoxicity may play an important role in the pathogenesis of CP (Bhatia et al., 2020). Moreover, mast cells, dendritic cells, eosinophils, monocytes, and B cells are also involved in inflammation of CP (Kandikattu et al., 2020).
2.2.3 Fibrosis
PSCs are unique resident cells in the pancreas and play important roles in both the healthy and diseased pancreas. The activation of PSCs is a central link in pancreatic fibrogenesis (Bynigeri et al., 2017; Beyer et al., 2020; Li et al., 2022). PSCs can be activated by multiple triggers, such as ethanol and its metabolites, hyperglycemia, oxidative stress, cytokines, chemokines and stress, and then secrete excessive ECM, which causes interlobular and intralobular fibrosis. Advanced fibrosis can cause pancreatic exocrine and endocrine insufficiency. Among the cytokines, TGF-β1 is the most important driver of pancreatic fibrogenesis by promoting the activation of PSC and the production of ECM (Xu et al., 2017; Li et al., 2018b; Sun et al., 2018; Radoslavova et al., 2021; Zheng et al., 2021). Therefore, PSC is a potential target for antifibrotic therapy during the development of CP.
3 Vitamin D—Metabolism, Analogues, and Functions
3.1 Vitamin D Metabolism
VD is a fat-soluble steroid hormone which was first known by its use in treating rickets in the 1920s. It can be obtained from the diet and by the action of sunlight on the skin. VD exists in two forms: VD3 and VD2. VD3 is endogenously produced in the skin and is the most utilized source of VD in animals. Exposure of the skin to ultraviolet B (wavelength 290–315 nm) rays results in the conversion of 7-dehydrocholesterol (7-DHC) to pre-VD3, which is followed by thermal isomerization to VD3. VD2 is produced by ultraviolet irradiation of ergosterol in fungi or yeast (Figure 2).
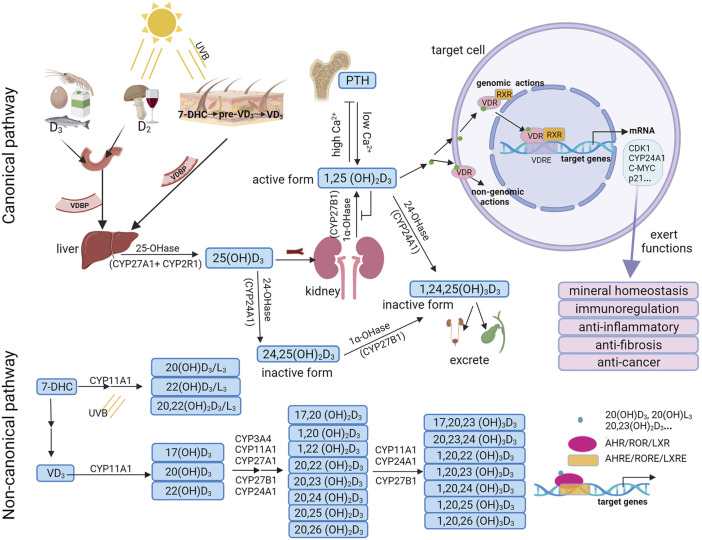
FIGURE 2.
Vitamin D3 metabolism and biological functions. In the canonical pathway, vitamin D3 can be hydroxylated by CYP27A1/CYP2R1 and CYP27B1 to form 25(OH)D3, and is then further hydroxylated to the active form 1,25(OH)2D3. 1,25(OH)2D3 can bind with VDR/RXR and translocate to the cell nucleus, where it binds to VDRE to regulate the transcription of target genes. Besides, 1,25(OH)2D3 may bind with a membrane-associated receptor to mediate non-genomic actions. In the non-canonical pathway, 7-DHC and vitamin D3 are first hydroxylated by CYP11A1 and further hydroxylated by various cytochrome enzymes including CYP24A1, CYP27A1, CYP27B1, CYP2R1, CYP3A4, and CYP11A1 to form dihydroxy or trihydroxy metabolites. These bioactive metabolites selectively act on not only VDR, but also on alternative nuclear receptors such as AHR, RORs or LXRs and binds to AHREs, ROREs or LXREs to regulate the transcription of target genes. UVB, ultraviolet B; 7DHC, 7-dehydrocholesterol; VDBP, vitamin D-binding protein; PTH, parathyroid hormone; VDR, vitamin D receptor; RXR, retinoid X receptor; VDREs, VDR response elements; CDK1, cyclin dependent kinase 1; AHR, aryl hydrocarbon receptor; RORs, retinoic acid orphan receptors; LXRs, liver X receptors; AHREs, AHR response elements; ROREs, ROR response elements; LXREs, LXR response elements.
In the canonical pathway, VD (D2 or D3) is carried by VD-binding protein (VDBP) from the blood to the liver, where it is hydroxylated by a vitamin D-25-hydroxylase enzyme (25-OHase), such as CYP27A1 in the mitochondria or CYP2R1 in the microsome, to produce 25(OH)D3. 25(OH)D3 is the highest concentration of VD metabolite in the blood, with a half-life of approximately 15 days. Therefore, 25(OH)D3 is an effective indicator for the evaluation of the VD status in the human body (Hollis, 2005). In the kidney, 25(OH)D3 is further hydroxylated by 1α -hydroxylase enzyme (1α-OHase) (known as CYP27B1), to form 1α, 25-dihydroxyvitamin D3 (1,25(OH)2D3). 1,25(OH)2D3 is the most bioactive VD metabolite. 25(OH)D3 and 1,25(OH)2D3 can be catalyzed by CYP24A1 into inactive forms, 24,25(OH)2D3 and 1,24,25(OH)3D3, both of which are excreted through bile and urine (Wei et al., 2021) (Figure 2).
In the non-canonical pathway, VD3 can be activated by CYP11A1 to form primary hydroxylation products, such as 17(OH)D3, 20(OH)D3, and 22(OH)D3 (Slominski et al., 2015b). CYP11A1 is expressed not only in classical steroidogenic tissues such as the placenta, adrenal glands, and epidermal keratinocytes, but also in other organs and tissues such as the brain, gastrointestinal tract, thymus, and immune cells [reviewed in (Slominski et al., 2014a)]. Serum 20(OH)D3 and 22(OH)D3 levels were 30 and 15 times lower than 25(OH)D3 levels, respectively (Slominski et al., 2015b). These products can be further selectively hydroxylated by various cytochrome enzymes including CYP24A1, CYP27A1, CYP27B1, CYP2R1, CYP3A4, and CYP11A1 to form dihydroxy or trihydroxy metabolites (Slominski et al., 2012c; Slominski et al., 2015b; Slominski et al., 2015c; Jenkinson, 2019; Slominski et al., 2021b). The major CYP11A1-derived VD3 products are 20(OH)D3 and 20,23(OH)2D3 (Slominski et al., 2005; Tuckey et al., 2008; Slominski et al., 2012b; Slominski et al., 2016; Bocheva et al., 2021) (Figure 2). Additionally, VD2 and 7-DHC can also be hydroxylated by CYP11A1 to produce various metabolites, such as 20(OH)D2, 17,20,24(OH)3D2, 20(OH)D3/L3, 22(OH)D3/L3 and 20,22(OH)2D3/L3 (Jenkinson, 2019; Bocheva et al., 2021).
3.2 Analogues of Vitamin D3
1,25(OH)2D3 is the most bioactive form of VD and is also a potent agonist of the transcription factor VDR. VDR is a nuclear hormone that directly affects chromatin structure and gene regulation. The physiological function of VD is to control calcium homeostasis for maintaining bone mineralization. Moreover, VD can modulate innate and adaptive immunity, induce cell differentiation, apoptosis, and autophagy; inhibit cell proliferation, angiogenesis and metastasis; and regulate other cellular signaling processes (El-Sharkawy and Malki, 2020; Adelani et al., 2021; Murdaca et al., 2021; Pi et al., 2021; Poursoltani et al., 2021; Zhao et al., 2021; Bhutia, 2022; Zhou et al., 2022). Since VD levels obtained from diet are often insufficient and VD deficiency is associated with a variety of diseases, a daily supplement of at least 25 μg (1,000 IU) of VD is recommended to prevent VD deficiency (Holick et al., 2011). The variety and sales of VD supplementation are increasing in recent years.
Although VD is of great benefit to human health, overdosing with natural VD metabolites, such as 1,25(OH)2D3 and 25(OH)D3 may result in an increased hypercalcemia risk. Numerous VD analogues have been designed as potent VDR agonists with higher VDR binding affinity, but with lower hypercalcemia risk. So far, a few analogues have entered the market, such as, cholecalciferol, calcidiol [25(OH)D3], calcitriol [1,25(OH)2D3], and calcipotriol [22-ene-26,27-dehydro-1,25(OH)2D3], the latter of which is the most potential.
The majority of synthetic VDR agonists are derived from modifications of the 1,25(OH)2D3 at its side-chain, A-ring, C-ring, or triene system. There is also an increasing number of nonsteroidal mimics in recent years. These VD analogues have high binding affinity with VDR and maintain a good metabolic stability. Calcipotriol has been shown to have anti-inflammatory and anti-cancer effects in pancreatitis and pancreatic cancer via VDR pathway (Sherman et al., 2014). Currently, researches on VD analogues are conducted almost exclusively in academia, and many interesting methods for optimizing VDR ligands have not yet explored their limits.
In contrast to 1,25(OH)2D3 and 25(OH)D3, the CYP11A1-derived secosteroids, 20(OH)D3, and 20,23(OH)2D3 have no risk of causing hypercalcemia at pharmacological doses (Slominski et al., 2010; Wang et al., 2012a; Chen et al., 2014). In addition, 20(OH)D3 and 20,23(OH)2D3 have anti-fibrosis, anti-rheumatoid arthritis, and anti-cancer activities without hypercalcemia in vivo and in vitro (Slominski et al., 2012a; Slominski et al., 2013; Tang et al., 2013; Slominski et al., 2015c). This provides an alternative approach to investigate the therapeutic role of VD analogues.
3.3 Functions of Vitamin D3
The classical, hormonally-active dihydroxy form of VD3, 1,25(OH)2D3, plays multiple roles by regulating target genes through VDR pathway. VDR is an endocrine receptor and is a member of the superfamily of nuclear receptors (Carlberg, 2018). VDR is a novel protein that is able to bind 1,25(OH)2D3 and its analogues at sub-nanomolar concentrations in the human genome (Haussler et al., 1997). VDR is not only located in the skeletal system but also widely distributed in other tissues such as the small intestine (Battistini et al., 2020), kidney (Chokhandre et al., 2015), heart (Lin et al., 2019), lung (Wang and Jiang, 2021), pancreas (Wallbaum et al., 2018), liver (Triantos et al., 2021a), and immune cells (Wang et al., 2012b) as well as other cell types (Wang et al., 2012b). VDR is located in the cytosol of VD-target cells (Udomsinprasert and Jittikoon, 2019; Triantos et al., 2021b). Upon activation by 1,25(OH)2D3, the VD/VDR complex forms a heterodimer with the retinoid X receptor (RXR). The heterodimer then translocates into the cell nucleus and binds to specific DNA sequences known as VD response element (VDRE) which triggers the transcription of downstream genes (Christakos et al., 2016). VDRE is mostly located in the upstream of the transcription start site where VDR/RXR binds. The binding of VDR/RXR to VDRE promotes the recruitment of co-regulators that are necessary for chromatin remodeling and for the regulation of VDR/RXR-induced transcription of target genes (Christakos et al., 2016). Intriguingly, the VD degrading enzyme CYP24A1, as a target gene of VDR, can regulate VD homeostasis and thus can be used as a marker of VDR activation. 1,25(OH)2D3 has exhibited a wide range of biological functions mainly via VDR pathway, including the regulation of bone and calcium homeostasis, inflammatory response, immune response, cell proliferation, cell differentiation, and apoptosis (Christakos et al., 2016) (Figure 2).
In addition to the classical pathway of VD/VDR/RXR exerting biological effects, CYP11A1-derived products of VD3 such as 20(OH)D3, 1,20(OH)2D3, 20,23(OH)2D3, 20(OH)L3, and 20,22(OH)2L3 can also act on alternative nuclear receptors including aryl hydrocarbon receptor (AHR) (Slominski et al., 2018b), retinoic acid orphan receptors (RORs) (Slominski et al., 2014b; Slominski et al., 2017) or liver X receptors (LXRs) (Slominski et al., 2021a), thereby exerting pleiotropic effects including anti-fibrosis, anti-rheumatoid arthritis, anti-tumor, immunomodulatory, and photoprotection through regulation of target genes (Slominski et al., 2012a; Slominski et al., 2013; Tang et al., 2013; Slominski et al., 2014a; Slominski et al., 2015a; Slominski et al., 2015c; Tongkao-On et al., 2015; Slominski et al., 2017; Slominski et al., 2018a). AHR is the major receptor for 20,23(OH)2D3 and can also be activated by other CYP11A1-derived products of VD3 like 20(OH)D3 (Slominski et al., 2018b). Intriguingly, the expression of VDR and AHR are mutually exclusive in ovarian endometriosis. This may be explained by a divergence between a more pro-differentiation fate mediated by VDR versus a more pro-proliferation fate induced by AHR (De Pascali et al., 2021). 20(OH)D3 and 20,23(OH)2D3 can function as antagonists or inverse agonists of RORα and RORγ, providing new possibilities for skin and systemic regulation (Slominski et al., 2014b; Slominski et al., 2017). LXRs have been demonstrated to be the nuclear receptors for several VD3 and lumisterol (L3) derivatives, including 1,25(OH)2D3, 1,20(OH)2D3, 25(OH)D3, 20(OH)D3, 20(OH)L3, and 20,22(OH)2L3 (Slominski et al., 2021a) (Figure 2).
Except for genomic actions, some non-genomic actions of VD have been reported, which are mediated by cell surface receptors, but this still remains controversial (Hii and Ferrante, 2016; Bhattarai et al., 2017; Cui et al., 2017; Bollen and Atherton, 2021). Numerous studies have indicated that the non-genomic functions may not be important for VD-mediated transcription of target genes. The enzyme, protein disulphide isomerase family A member 3 (PDIA3) has been reported as a potential membrane-associated receptor for VD (Hu et al., 2019; Gisbert-Ferrándiz et al., 2020) and VD can stimulate the nuclear translocation of PDIA3-STAT3 (Hu et al., 2019). However, the significance of PDIA3 is still not elucidated because no binding site for 1,25(OH)2D3 has been confirmed. More researches are required to confirm whether there is VD-induced genomic actions or non-genomic actions via membrane receptors.
4 Vitamin D and Chronic Pancreatitis
4.1 Prevalence of Vitamin D Deficiency in Chronic Pancreatitis
The definition of VD deficiency of US Endocrine Society guidelines was the serum concentration of 25(OH)D3 less than 20 ng/ml (50 nmol/L), VD insufficiency was 21–29 ng/ml (50–74 nmol/L), and the satisfactory status of VD was 30–100 ng/ml (75–250 nmol/L) (Holick et al., 2011).
Patients with CP are often complicated with pancreatic exocrine insufficiency (PEI) of which steatorrhea, diarrhea, bloating, and weight loss are common symptoms. The main consequences of PEI are malnutrition and poor life quality. Deficiencies of fat-soluble vitamin, transferrin, and some kinds of micronutrient such as magnesium and zinc are common in PEI patients. Numerous studies have reported that the prevalence of VD deficiency in patients with CP ranging from 22% to 86.5% (Klapdor et al., 2012; Jøker-Jensen et al., 2020) (Table 1). Recently, our research found that 25(OH)D3 levels were significantly low in patients with alcoholic chronic pancreatitis as compared with healthy population.
TABLE 1.
Overview of reports on vitamin D (VD) deficiency/insufficiency in CP.
| Country | Participants | Testing indicators | Mean value | Criteria for deficiency/insufficiency | Prevalence of deficiency/insufficiency | References |
|---|---|---|---|---|---|---|
| Denmark | 115 cases | VD (nmol/L) | 57.8 ± 36.9 (10.0–175.0) | <25 | 22% (25/115) | Jøker-Jensen et al. (2020) |
| Germany | 37 cases; 108 controls | 25(OH)D3 (ng/ml) | CP: 15.6 ± 13.6 control: 17.5 ± 9.7 | <30 | CP: 94.2% control: 87% (p > 0.05) | Klapdor et al. (2012) |
| Germany | 211 cases | 25(OH)D3 (ng/ml) | 20.2 ± 12 | <20 | 56.39% (119/211) | Stigliano et al. (2018) |
| Ireland | 62 cases; 66 matched controls | 25(OH)D3 (nmol/L) | Not available | <50 | CP: 58% control: 61.7% (p = 0.894) | Duggan et al. (2014) |
| United Kingdom | 91 cases | VD | Not available | Not available | 62.5% (55/88) | Min et al. (2018) |
| United States | 100 pediatric cases | VD (ng/ml) | Not available | <20 | 5% (5/99) | McEachron et al. (2021) |
| India | 72 TCP; 100 controls | 25(OH)D3 (nmol/L) | CP: 24.0 (17.3–42.0) control: 27.5 (20.5–37.5) (p = 0.88) | <50 | CP: 86% control: 85% (85/100) (p = 0.19) | Joshi et al. (2011) |
| Ireland | 29 cases; 29 controls | 25(OH)D3 (nmol/L) | CP: 31 control: 42 (p = 0.0126) | <50 | CP: 69% (20/29) control: 62% (18/29) (p = 0.401) | Duggan et al. (2015) |
VD, vitamin D; TCP, tropical calcific pancreatitis; CP, chronic pancreatitis.
A study from Denmark enrolled 115 consecutive CP outpatients and showed that micronutrient deficiencies in CP outpatients were varied and that VD deficiency (22%) was the most common micronutrient deficiency (Jøker-Jensen et al., 2020). A prospective multicenter study from Europe that enrolled 211 CP patients indicated 56% of VD deficiency (Stigliano et al., 2018). A study from United States, 62.5% (55/88) of patients had VD deficiency, and the rate of VD deficiency was higher in women as compared with men (67.3% vs. 54.5%, respectively) and was also higher in smokers versus nonsmokers (Min et al., 2018). Another study from United States showed that VD deficiency is also common in children. The total rate of VD deficiency and VD insufficiency is 27% in children with CP, and it is even higher (30%) after total pancreatectomy with islet auto-transplantation (McEachron et al., 2021). An earlier study from Germany reported that the prevalence of VD deficiency and insufficiency was 86.5% in patients with CP and 87% in normal controls, showing no difference between the two groups (Klapdor et al., 2012). A case-matched study from Ireland found no significant difference in serum 25(OH)D3 deficiency rates between CP patients and controls. Subgroup analysis demonstrated that VD levels were significantly lower in CP patients with osteoporosis than in CP patients without osteoporosis (Duggan et al., 2015). Taken together, VD deficiency is common in patients with CP, however, it is still unclear whether VD deficiency is a potential risk factor for the development of CP. Large-scale, high-quality prospective clinical studies are needed to elucidate the exact relationship between VD deficiency and the risk of CP.
4.2 Therapeutic Implications of Vitamin D
4.2.1 In Vivo and In Vitro Studies
The activation of PSCs is a key step in the initiation and development of CP. Current in vitro studies mainly focus on the effect of VD on PSCs (Table 2). Primary PSCs from healthy mice were isolated and cultured. The activated cells were treated with VD2, VD3, and calcipotriol. The results showed that VD could increase lipid droplet storage, inhibit PSC activation, and decrease the expression of α-SMA and interleukin 6. However, VD didn’t have significant effects on type 1 collagen (Col1) and TGF-β1 production (Wallbaum et al., 2018).
TABLE 2.
Summary on the role of vitamin D in CP from in vivo and in vitro studies.
| Function | Biological effects | References |
|---|---|---|
| Inhibition of activation of PSCs | ↑Lipid droplet ↑VDR expression | Sherman et al. (2014), Wallbaum et al. (2018) |
| Anti-inflammatory | ↓Pro-inflammatory cytokines | Wallbaum et al. (2018) |
| Anti-fibrosis | ↓ECM | Sherman et al. (2014), Bläuer et al. (2015), Kang et al. (2018), Wallbaum et al. (2018), Kang et al. (2021) |
| Anti-proliferation | ↓PSCs activation ↓PSC number ↑cyclin-dependent kinase inhibitors p21/p27 ↑cell cycle arrest at the G (1)/S checkpoint | Sherman et al. (2014), Bläuer et al. (2015), Kang et al. (2018), Wallbaum et al. (2018), Kang et al. (2021) |
| Induction of differentiation | ↑VDR binding ↓SMAD3 binding ↓p-STAT3 | Sherman et al. (2014) |
VDR, vitamin D receptor; ECM, extracellular matrix; PSCs, pancreatic stellate cells.
In 2015, Finnish researchers investigated the anti-proliferation and anti-fibrosis effects of 1,25(OH)2D3 in PSCs. The activated PSCs were exposed to different physiological concentrations of 1,25(OH)2D3. The results showed that 1,25(OH)2D3 could inhibit the expression of fibronectin and Col1 and the proliferation of PSCs, with a positive correlation between anti-proliferation ability and 1,25(OH)2D3 concentrations. 1,25(OH)2D3 could also promote the expression of VDR in PSCs (Bläuer et al., 2015). A compound named as 9c has been recognized as one of the novel series of non-secosteriodal VD analogues to inhibit the expression of fibrotic genes and ECM deposition in vitro and in vivo (Kang et al., 2018) (Table 2).
4.2.2 Clinical Studies
In addition to the above in vitro and in vivo studies, several observational studies and randomized controlled trials (RCTs) have also been conducted to investigate the therapeutic potential of VD in patients with CP. Due to the differences in population and the methods of biochemical analysis among these studies, the results of VD deficiencies in patients with CP versus controls are highly different (Table 3). Therefore, the existing studies are not enough to say whether VD deficiency is related to the risk of CP (Olesen et al., 2017).
TABLE 3.
Summary on the roles of vitamin D (VD) in CP from clinical studies.
| Country | Research type | Number of patients | Aim of the study | RR/HR/OR (95%CI, p) | Conclusion | References |
|---|---|---|---|---|---|---|
| Spain | Meta | 548 | To determine the prevalence of fat-soluble vitamin deficiency in CP patients | 1.17 (0.77–1.78, p = 0.46) I2 = 0% | Fat-soluble vitamins deficiency is frequent in CP patients, but no significant increased risk of VD deficiency | Martínez-Moneo et al. (2016) |
| Netherlands | Meta | 465 | To determine the prevalence of VD insufficiency and deficiency in CP patients | 1.14 (0.70–1.85, p > 0.05) I2 = 0% | High prevalence of VD insufficiency and deficiency in CP patients, but no significant difference between patients and healthy controls | Hoogenboom et al. (2016) |
| Germany | Meta | 220 | To analyze the results from RCTs of dietary interventions for CP patients and make further dietary recommendations | Not available | VD can improve VD deficiency in CP, while other nutritional support therapies have no evidence of effectiveness | Wiese et al. (2021) |
| Denmark | RCT | 30 | To assess intestinal absorption of cholecalciferol in patients with CP and fat malabsorption | p < 0.001 | Daily VD supplementation increased 25(OH)D3 in CP patients compared to placebo, but this was not the case with weekly tanning bed sessions | Bang et al. (2011) |
| Denmark | RCT | 30 | To investigate the effect of changes in 25(OH)D3 and 1,25(OH)2D3 on Tregs in patients with CP with fat malabsorption | p < 0.05 | Changes in VD significantly correlate with maturation of CD4+ and CD8+ Tregs | Bang et al. (2012) |
| India | RCT | 40 | To assess the relative efficacy of two different doses of VD in patients with CP with VD deficiency | p < 0.001 | The 600,000 IU dose was more effective in achieving VD sufficiency over 6 months compared to 300,000 IU, but no longer after 9 months | Reddy et al. (2013) |
CP, chronic pancreatitis; RCT, randomized controlled trial; VD, vitamin D; Tregs, regulatory T cells; RR, relative risk; HR, hazard ratio; OR, odds ratio; CI, confidence interval.
A latest systematic review and meta-analysis about nutritional management of CP enrolled five RCTs suggest that the supplementation of VD is a potential therapy for CP (Bang et al., 2011; Bang et al., 2012; Reddy et al., 2013) and that oral or intravenous VD can improve VD deficiency in patients with CP (Bang et al., 2011; Wiese et al., 2021). However, another RCT showed that 600,000 IU was more effective in achieving VD sufficiency over six months compared to 300,000 IU, but no longer after nine months (Reddy et al., 2013). Several other related RCTs are underway or completed but the results have not yet been published (Table 4).
TABLE 4.
Clinical trials (http://clinicaltrials.gov/).
| Clinical Trials.gov number | Conditions/diseases | Drugs | Intervention/treatment | Enrollment | Phase |
|---|---|---|---|---|---|
| *Unregistered Bang et al. (2011), Bang et al. (2012) | CP and fat malabsorption | Cholecalciferol | Cholecalciferol 1520 IU daily and calcium 800 mg weekly for 10 weeks, PO | 30 | Not applicable |
| *NCT00956839 Reddy et al. (2013) | TCP | Cholecalciferol | 3,00,000/6,00,000 Units single dose, IM | 40 | VI |
| NCT02965898 | CP | VD | 100/10 μg daily for at least 7 years, PO | 260 | Not applicable |
| NCT01141998 | CP with malabsorption syndromes | Calcium | 400 mg two times daily week 0–10 and week 14–52, PO | 27 | Not applicable |
| NCT02108509 | CP with osteopenia/osteoporosis | Not applicable | Not applicable | 55 | Not applicable |
CP, chronic pancreatitis; TCP, tropical calcific pancreatitis; PO, oral intake; IM, intramuscular injection; VD, vitamin D.
5 Mechanisms of Vitamin D Action in Chronic Pancreatitis
5.1 Anti-Inflammatory and Anti-Fibrotic Effects
PSCs have similar physiological properties and functions to those of hepatic stellate cells (HSCs). So far, many in vitro studies by culturing PSCs or HSCs have confirmed the therapeutic potential of VD in pancreatic or liver diseases. Since HSCs were discovered earlier than PSCs, there are more studies on the mechanism of VD in anti-hepatic fibrosis as compared with anti-pancreatic fibrosis, thereby providing ideas and research methods for reference in the study of VD in anti-pancreatic fibrosis. For instance, an early study has shown that VD analogue calcipotriol antagonizes TGF-β -mediated pre-fibrotic gene expression in human HSCs through VDR/SMAD interaction (Ding et al., 2013). Based on some relevant researches on liver diseases, five signaling pathways of VD in anti-inflammatory and anti-fibrosis have been summarized as follows: 1) VD inhibit cyclin D1 expression, which is a key marker of the cell cycle, resulting in anti-proliferation of HSCs; 2) VD reduces SMAD3 occupancy at co-regulating genes, revealing an intersecting VDR/SMAD genomic circut that regulate hepatic fibrogenesis, thereby inhibiting TGF-β/SMAD-mediated pro-fibrotic effects; 3) VD inhibits the transcription of pro-fibrotic genes and activity of HSC by interacting with HSC-specific p62 and VDR; 4) VD activates VDR to bind with IKKβ by which the NF-κB transcriptional activity is impaired, thus reducing inflammatory response; 5) VD/VDR signaling attenuates TLR4-mediated inflammatory response by enhancing negative feedback regulation (Triantos et al., 2021b).
Many studies in rheumatoid arthritis, chronic obstructive pulmonary disease and cardiovascular disease have demonstrated that VD regulates the inflammatory microenvironment of the diseases through enhancement of p38 MAPK pathway, inhibition of NF-κB signaling and regulation of prostaglandin pathway (Moreno et al., 2005; Yang et al., 2015; Ishizawa et al., 2017; Gil et al., 2018; Wen et al., 2018; Derakhshanian et al., 2019; Qian et al., 2019; Yao et al., 2019; Zhou et al., 2019; Cimmino et al., 2020). 1,25(OH)2D3 can restrain macrophage-mediated inflammation processes by suppressing the AKT/NF-κB/COX-2 pathway in a carrageen-induced paw edema mouse model and it can also reduce the proliferation of fibroblast-like synoviocytes and the production of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, and PGE2) in a rheumatoid arthritis rat model. (Wang et al., 2014; Fan et al., 2017).
5.2 Immunomodulatory Effect
The inflammatory cell storm plays an important role in the progress of CP in which many cell types including monocytes, macrophages, mast cells, and T cells are implicated (Kandikattu et al., 2020). Activated macrophages have been demonstrated as a critical regulator of inflammation and fibrosis that promote the production of collagen and fibronectin in PSCs via paracrine-cytokine signaling (Schmid-Kotsas et al., 1999). During the development of CP, local imbalances of T-cell subsets in inflammatory have also been observed (Schmitz-Winnenthal et al., 2010). The numbers of central memory T-cell subsets (CCR7+/CD45RA) were increased in blood samples from CP patients. Moreover, the increased CCR7+ memory T cells were not changed between unresected CP patients and subjects who had undergone pancreatic resection due to CP, suggesting that a persistent increase of central memory T lymphocytes may be important for maintaining the inflammatory process in CP (Grundsten et al., 2005). Therefore, targeting T cells may be a potential therapy to reverse the process of CP.
Various immune cells including macrophages, dendritic cells and lymphocytes express VDR constitutively or inductively, thus increasing immune response to antigens (von Essen et al., 2010; Scolletta et al., 2013). VD/VDR complex has been confirmed to play a role in T cell antigen receptor signaling and T cell activation as well as in the regulation of immune responses (von Essen et al., 2010; Di Rosa et al., 2011; Bang et al., 2012; Sarkar et al., 2016; Cantorna et al., 2019). Moreover, VDR agonists have significant inhibitory effects on macrophage- and monocyte-mediated inflammatory processes through controlling the expression and activities of VDR and CYP27B1 (Morán-Auth et al., 2013; Dionne et al., 2017; Martens et al., 2020; Wherry et al., 2021). 1,25(OH)2D3 or its analogue treated dendritic cells can modulate human autoreactive T cells via the selective induction of apoptosis (van Halteren et al., 2004; Gil et al., 2018; Vanherwegen et al., 2019). VDR agonists exert a significant suppression of inflammatory processes by switching the immune response from T helper 1 (Th1) to T helper 2 (Th2) dominance and by counteracting the self-enhancing inflammatory loop between immune cells and resident cells (Scolletta et al., 2013). VD suppresses the expression of IL-17 and IL-2 in CD4+ T cells and reduces CD8+ T cell-mediated cytotoxicity, which leads to an overall effect of blocking Th1-mediated responses (Meehan et al., 1992). Moreover, VD stimulates the development and differentiation of regulatory T cells (Tregs) and enhances their suppressive function (Treiber et al., 2015; Bogdanou et al., 2017; Di Liberto et al., 2019; Fisher et al., 2019). Likewise, B cell proliferation, plasmacyte differentiation, and immunoglobulin secretion are also influenced by VDR ligands perhaps via their effects on antigen-presenting cells or T cells (Chen et al., 2007; Vanherwegen et al., 2017b; Vanherwegen et al., 2017a).
5.3 Regulation of Proliferation
VDR agonists can inhibit the cell cycle of a variety cells, especially cancer cells. 1,25(OH)2D3 upregulates the expression of cyclin-dependent kinase inhibitors p21(Waf1/Cip1) and p27(Kip1), which plays a key role in G0/G1 phase cell cycle arrest and anti-proliferation (Wu et al., 2007; Irazoqui et al., 2014; Spath et al., 2017; Trump, 2018; Li et al., 2019; Gesmundo et al., 2020). A cross-talk between 1,25(OH)2D3/VDR non-genomic and genomic signaling at the level of MAPK activation has been demonstrated to reduce the proliferation of human osteosarcoma cells (Wu et al., 2007). The human p21(waf1/cip1) gene has been recognized as a primary 1,25(OH)2D3-responding gene with at least three VDR binding promoter regions, in two of which are also co-localized with p53, therefore it is a primary anti-proliferative target for the VDR in the presence of 1,25(OH)2D3 (Saramäki et al., 2006; Li et al., 2017a). VDR is involved in the induction of p27(Kip1) by VD3 and may interact with Sp1 to modulate the expression of target genes in LNCaP cancer cells (Huang et al., 2004). In addition, 1,25(OH)2D3 induces the expression of other cyclin-dependent kinase inhibitors, such as p15(Ink4b) and p16 (Ink4a) (Chiang and Chen, 2013; Chen et al., 2019).
5.4 Induction of Differentiation
WNT/β-catenin signaling is activated in colon cancer cells which is associated with tumor cell malignancy and dedifferentiation (González-Sancho et al., 2020). 1,25(OH)2D3 can induce the transcription of genes involved in differentiation of bone, skin and brain cells by repressing WNT/β-catenin signaling (González-Sancho et al., 2020). VDR agonist can also reduce the amount of β-catenin binding to transcription factor T cell factor (TCF) by inducing the interaction between β-catenin and VDR (Larriba et al., 2013). E-cadherin is a transmembrane glycoprotein that connects epithelial cells together at adherens junctions. In normal cells, E-cadherin exerts its tumor suppressing role mainly by sequestering β-catenin from its binding to lymphoid enhancer factor (LEF)/TCF. 1,25(OH)2D3 induces high expression of E-cadherin and WNT inhibitor (DKK-1) leading to β-catenin nuclear export and relocation to the adherens junctions at the plasma membrane, thereby suppressing colonic carcinogenesis (Pendás-Franco et al., 2008; Larriba et al., 2013; Xin et al., 2017).
5.5 Induction of Apoptosis
VD has been confirmed to promote apoptosis in various cell types through different signaling pathways. 1,25(OH)2D3 induces apoptosis in adipocytes via activation of Ca2+-dependent calpain and Ca2+/calpain-dependent caspase-12 (Sergeev, 2009; 2020), providing a potential therapy for obesity. VD analogue paricalcitol reduced fibroid tumor size of nude mice through upregulation of apoptosis (Halder et al., 2014). 1,25(OH)2D3 induces apoptosis through inhibiting anti-apoptotic proteins BCL-2 and BCL-XL and inducing pro-apoptotic proteins such as BAX, BAK, and BAD in cancer cells (Díaz et al., 2000; Halder et al., 2012; Giammanco et al., 2015; Aslam et al., 2021), while VD shows an anti-apoptotic effect in peripheral blood mononuclear cells in systemic lupus erythematosus via increasing the expression of BCL-2 and decreasing the expression of BAX (Tabasi et al., 2015). VD also induces apoptosis of ovarian cancer cells through downregulating the activity of telomerase and the level of telomerase reverse transcriptase (Jiang et al., 2004). Furthermore, 1,25(OH)2D3 can also enhance the pro-apoptotic effects of gemcitabine, paclitaxel and cisplatin in squamous cell carcinoma through different pathways (Hershberger et al., 2001; Hershberger et al., 2002). 1,25(OH)2D3 enhances cisplatin-mediated cell apoptosis by decreasing the expression of ERK and AKT and increasing the expression of BAX, p21, and p27 in gastric cancer cells (Bao et al., 2014).
5.6 Induction of Autophagy
Autophagy is a cellular process in degrading of long-lived proteins and organelles and misfolded proteins in the cytosol for maintaining cellular homeostasis, which has been linked to many states of human health and disease (Xu et al., 2018; Zhang et al., 2021). Recently, VD has been demonstrated to alleviate ethanol-induced hepatotoxicity by enhancing autophagy (Yuan et al., 2021). 1,25(OH)2D3 has also been confirmed to improve hepatic steatosis by upregulating autophagy induced by ATG16L1 (Li et al., 2017b). Moreover, 1,25(OH)2D3 can increase cell viability and insulin secretion of rat insulinoma cells and protects cells from oxidative damage induced by streptozotocin via autophagy activation (He et al., 2019). PRSS1-related hereditary pancreatitis is characterized by episodes of acute pancreatitis and recurrent acute pancreatitis with frequent progression to CP, which damages acinar cells through several mechanisms including oxidative stress and impaired autophagy (Witt et al., 2013; Giri et al., 2016).
There are several studies showed that the autophagy is required for activation of PSC (Endo et al., 2017). Saikosaponin A inhibits the activation of PSCs by suppressing autophagy and the NLRP3 (nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3) inflammasome (Cui et al., 2020). Additionally, inhibiting autophagy can also suppress pancreatic fibrosis through promoting ECM degradation by decreasing the expression of TGF-β1 and increasing MMPs/TIMPs ratio (Li et al., 2018a). Retinoblastoma coiled coil protein 1-induced autophagy can facilitate PSC activation and pancreatic fibrosis in CP (Li et al., 2018c; Zhang et al., 2021). Contrarily, PDGF inhibits autophagy in HSC and increases the release of extracellular vesicle (EV) (Gao et al., 2020), while the release of EV can promote the interaction between cells and fibrosis (Xu et al., 2018), suggesting that autophagy in HSC alleviated liver fibrosis by reducing the release of HSC-derived EV (Gao et al., 2020). The role of autophagy is different in various cell types linked to liver diseases. Targeting autophagy has been considered as a potential strategy to treat acute liver injury and non-alcoholic fatty liver disease (Allaire et al., 2019). However, the role of autophagy on PSC activation and pancreatic fibrosis and the therapeutic value of VD-induced autophagy need to be further clarified.
6 Conclusion and Perspectives
VD deficiency is prevalent in patients with CP which is associated with the risk and the prognosis of CP. VD supplementation is expected to reduce the risk and improve the prognosis of CP. VD plays a variety of biological functions in the body and has been widely used in the study of inflammatory diseases. VD and its analogues have been confirmed to inhibit PSC activation and reduce ECM deposition, thereby alleviating pancreatic fibrosis. These evidences suggest that VD may be a potential anti-fibrotic therapeutic agent for CP. However, some meta-analyses and clinical studies have found that the relationship between VD deficiency and CP is unclear. At present, large-scale and high-quality prospective studies are needed to confirm the exact role of VD on anti-fibrosis in CP. In the future, more clinical trials of VD and its analogues for the treatment of CP should be carried out, especially RCT studies. There is still much effort to be done to translate clinical trials into clinical practice. These efforts will contribute to the development of an economical and effective agent for the treatment of CP.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
MZ conceptualized the manuscript. RG edited and made significant revisions to the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (81770629).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Adelani I. B., Rotimi O. A., Maduagwu E. N., Rotimi S. O. (2021). Vitamin D: Possible Therapeutic Roles in Hepatocellular Carcinoma. Front. Oncol. 11, 642653. 10.3389/fonc.2021.642653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire M., Rautou P. E., Codogno P., Lotersztajn S. (2019). Autophagy in Liver Diseases: Time for Translation? J. Hepatol. 70 (5), 985–998. 10.1016/j.jhep.2019.01.026 [DOI] [PubMed] [Google Scholar]
- Aslam A., Ahmad J., Baghdadi M. A., Idris S., Almaimani R., Alsaegh A., et al. (2021). Chemopreventive Effects of Vitamin D3 and its Analogue, Paricalcitol, in Combination with 5-fluorouracil against Colorectal Cancer: The Role of Calcium Signalling Molecules. Biochim. Biophys. Acta Mol. Basis Dis. 1867 (3), 166040. 10.1016/j.bbadis.2020.166040 [DOI] [PubMed] [Google Scholar]
- Bang U. C., Brandt L., Benfield T., Jensen J. E. (2012). Changes in 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D Are Associated with Maturation of Regulatory T Lymphocytes in Patients with Chronic Pancreatitis: a Randomized Controlled Trial. Pancreas 41 (8), 1213–1218. 10.1097/MPA.0b013e31824da377 [DOI] [PubMed] [Google Scholar]
- Bang U. C., Matzen P., Benfield T., Beck Jensen J. E. (2011). Oral Cholecalciferol versus Ultraviolet Radiation B: Effect on Vitamin D Metabolites in Patients with Chronic Pancreatitis and Fat Malabsorption - a Randomized Clinical Trial. Pancreatology 11 (4), 376–382. 10.1159/000330224 [DOI] [PubMed] [Google Scholar]
- Bao A., Li Y., Tong Y., Zheng H., Wu W., Wei C. (2014). 1,25-Dihydroxyvitamin D₃ and Cisplatin Synergistically Induce Apoptosis and Cell Cycle Arrest in Gastric Cancer Cells. Int. J. Mol. Med. 33 (5), 1177–1184. 10.3892/ijmm.2014.1664 [DOI] [PubMed] [Google Scholar]
- Battistini C., Ballan R., Herkenhoff M. E., Saad S. M. I., Sun J. (2020). Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 22 (1), 362. 10.3390/ijms22010362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer G., Habtezion A., Werner J., Lerch M. M., Mayerle J. (2020). Chronic Pancreatitis. Lancet 396 (10249), 499–512. 10.1016/s0140-6736(20)31318-0 [DOI] [PubMed] [Google Scholar]
- Bhatia R., Thompson C., Ganguly K., Singh S., Batra S. K., Kumar S. (2020). Alcohol and Smoking Mediated Modulations in Adaptive Immunity in Pancreatitis. Cells 9 (8), 1880. 10.3390/cells9081880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai P., Bhattarai J. P., Kim M. S., Han S. K. (2017). Non-genomic Action of Vitamin D3 on N-Methyl-D-Aspartate and Kainate Receptor-Mediated Actions in Juvenile Gonadotrophin-Releasing Hormone Neurons. Reprod. Fertil. Dev. 29 (6), 1231–1238. 10.1071/rd15357 [DOI] [PubMed] [Google Scholar]
- Bhutia S. K. (2022). Vitamin D in Autophagy Signaling for Health and Diseases: Insights on Potential Mechanisms and Future Perspectives. J. Nutr. Biochem. 99, 108841. 10.1016/j.jnutbio.2021.108841 [DOI] [PubMed] [Google Scholar]
- Bläuer M., Sand J., Laukkarinen J. (2015). Physiological and Clinically Attainable Concentrations of 1,25-dihydroxyvitamin D3 Suppress Proliferation and Extracellular Matrix Protein Expression in Mouse Pancreatic Stellate Cells. Pancreatology 15 (4), 366–371. 10.1016/j.pan.2015.05.044 [DOI] [PubMed] [Google Scholar]
- Bocheva G., Slominski R. M., Slominski A. T. (2021). The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 22 (16), 9097. 10.3390/ijms22169097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanou D., Penna-Martinez M., Filmann N., Chung T. L., Moran-Auth Y., Wehrle J., et al. (2017). T-lymphocyte and Glycemic Status after Vitamin D Treatment in Type 1 Diabetes: A Randomized Controlled Trial with Sequential Crossover. Diabetes Metab. Res. Rev. 33 (3), 2865. 10.1002/dmrr.2865 [DOI] [PubMed] [Google Scholar]
- Bollen S. E., Atherton P. J. (2021). Myogenic, Genomic and Non-genomic Influences of the Vitamin D axis in Skeletal Muscle. Cell. Biochem. Funct. 39 (1), 48–59. 10.1002/cbf.3595 [DOI] [PubMed] [Google Scholar]
- Bynigeri R. R., Jakkampudi A., Jangala R., Subramanyam C., Sasikala M., Rao G. V., et al. (2017). Pancreatic Stellate Cell: Pandora's Box for Pancreatic Disease Biology. World J. Gastroenterol. 23 (3), 382–405. 10.3748/wjg.v23.i3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T., Rogers C. J., Arora J. (2019). Aligning the Paradoxical Role of Vitamin D in Gastrointestinal Immunity. Trends Endocrinol. Metab. 30 (7), 459–466. 10.1016/j.tem.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C. (2018). Vitamin D Genomics: From In Vitro to In Vivo . Front. Endocrinol. (Lausanne) 9, 250. 10.3389/fendo.2018.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang J., Kim T. K., Tieu E. W., Tang E. K., Lin Z., et al. (2014). Novel Vitamin D Analogs as Potential Therapeutics: Metabolism, Toxicity Profiling, and Antiproliferative Activity. Anticancer Res. 34 (5), 2153 [PMC free article] [PubMed] [Google Scholar]
- Chen L., Yang R., Qiao W., Zhang W., Chen J., Mao L., et al. (2019). 1,25-Dihydroxyvitamin D Exerts an Antiaging Role by Activation of Nrf2-Antioxidant Signaling and Inactivation of P16/p53-Senescence Signaling. Aging Cell. 18 (3), e12951. 10.1111/acel.12951 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen S., Sims G. P., Chen X. X., Gu Y. Y., Chen S., Lipsky P. E. (2007). Modulatory Effects of 1,25-dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 179 (3), 1634–1647. 10.4049/jimmunol.179.3.1634 [DOI] [PubMed] [Google Scholar]
- Chiang K. C., Chen T. C. (2013). The Anti-cancer Actions of Vitamin D. Anticancer Agents Med. Chem. 13 (1), 126–139. 10.2174/187152013804487443 [DOI] [PubMed] [Google Scholar]
- Chokhandre M. K., Mahmoud M. I., Hakami T., Jafer M., Inamdar A. S. (2015). Vitamin D and its Analogues in Type 2 Diabetic Nephropathy: a Systematic Review. J. Diabetes Metab. Disord. 14, 58. 10.1186/s40200-015-0186-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. (2016). Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 96 (1), 365–408. 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino G., Morello A., Conte S., Pellegrino G., Marra L., Golino P., et al. (2020). Vitamin D Inhibits Tissue Factor and CAMs Expression in Oxidized Low-Density Lipoproteins-Treated Human Endothelial Cells by Modulating NF-Κb Pathway. Eur. J. Pharmacol. 885, 173422. 10.1016/j.ejphar.2020.173422 [DOI] [PubMed] [Google Scholar]
- Clemens D. L., Schneider K. J., Arkfeld C. K., Grode J. R., Wells M. A., Singh S. (2016). Alcoholic Pancreatitis: New Insights into the Pathogenesis and Treatment. World J. Gastrointest. Pathophysiol. 7 (1), 48–58. 10.4291/wjgp.v7.i1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Li C., Zhuo Y., Yang L., Cui N., Li Y., et al. (2020). Saikosaponin A Inhibits the Activation of Pancreatic Stellate Cells by Suppressing Autophagy and the NLRP3 Inflammasome via the AMPK/mTOR Pathway. Biomed. Pharmacother. 128, 110216. 10.1016/j.biopha.2020.110216 [DOI] [PubMed] [Google Scholar]
- Cui X., Gooch H., Petty A., McGrath J. J., Eyles D. (2017). Vitamin D and the Brain: Genomic and Non-genomic Actions. Mol. Cell. Endocrinol. 453, 131–143. 10.1016/j.mce.2017.05.035 [DOI] [PubMed] [Google Scholar]
- de la Guía-Galipienso F., Martínez-Ferran M., Vallecillo N., Lavie C. J., Sanchis-Gomar F., Pareja-Galeano H. (2021). Vitamin D and Cardiovascular Health. Clin. Nutr. 40 (5), 2946–2957. 10.1016/j.clnu.2020.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascali F., Casarini L., Kuhn C., Simoni M., Mahner S., Jeschke U., et al. (2021). Nuclear Expression of VDR and AHR Is Mutually Exclusive in Glandular Cells in Endometriosis. Histochem Cell. Biol. 156 (4), 391–399. 10.1007/s00418-021-02005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshanian H., Djazayery A., Javanbakht M. H., Eshraghian M. R., Mirshafiey A., Jahanabadi S., et al. (2019). Vitamin D Downregulates Key Genes of Diabetes Complications in Cardiomyocyte. J. Cell. Physiol. 234 (11), 21352–21358. 10.1002/jcp.28743 [DOI] [PubMed] [Google Scholar]
- Di Liberto D., Scazzone C., La Rocca G., Cipriani P., Lo Pizzo M., Ruscitti P., et al. (2019). Vitamin D Increases the Production of IL-10 by Regulatory T Cells in Patients with Systemic Sclerosis. Clin. Exp. Rheumatol. 37 Suppl 119 (Suppl. 1194), 76 [PubMed] [Google Scholar]
- Di Rosa M., Malaguarnera M., Nicoletti F., Malaguarnera L. (2011). Vitamin D3: a Helpful Immuno-Modulator. Immunology 134 (2), 123–139. 10.1111/j.1365-2567.2011.03482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz G. D., Paraskeva C., Thomas M. G., Binderup L., Hague A. (2000). Apoptosis Is Induced by the Active Metabolite of Vitamin D3 and its Analogue EB1089 in Colorectal Adenoma and Carcinoma Cells: Possible Implications for Prevention and Therapy. Cancer Res. 60 (8), 2304 [PubMed] [Google Scholar]
- Ding N., Yu R. T., Subramaniam N., Sherman M. H., Wilson C., Rao R., et al. (2013). A Vitamin D Receptor/SMAD Genomic Circuit Gates Hepatic Fibrotic Response. Cell. 153 (3), 601–613. 10.1016/j.cell.2013.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne S., Duchatelier C. F., Seidman E. G. (2017). The Influence of Vitamin D on M1 and M2 Macrophages in Patients with Crohn's Disease. Innate Immun. 23 (6), 557–565. 10.1177/1753425917721965 [DOI] [PubMed] [Google Scholar]
- Duggan S. N., Purcell C., Kilbane M., O'Keane M., McKenna M., Gaffney P., et al. (2015). An Association between Abnormal Bone Turnover, Systemic Inflammation, and Osteoporosis in Patients with Chronic Pancreatitis: a Case-Matched Study. Am. J. Gastroenterol. 110 (2), 336–345. 10.1038/ajg.2014.430 [DOI] [PubMed] [Google Scholar]
- Duggan S. N., Smyth N. D., O'Sullivan M., Feehan S., Ridgway P. F., Conlon K. C. (2014). The Prevalence of Malnutrition and Fat-Soluble Vitamin Deficiencies in Chronic Pancreatitis. Nutr. Clin. Pract. 29 (3), 348–354. 10.1177/0884533614528361 [DOI] [PubMed] [Google Scholar]
- El-Sharkawy A., Malki A. (2020). Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 25 (14),219. 10.3390/molecules25143219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S., Nakata K., Ohuchida K., Takesue S., Nakayama H., Abe T., et al. (2017). Autophagy Is Required for Activation of Pancreatic Stellate Cells, Associated with Pancreatic Cancer Progression and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 152 (6), 1492–e24. 10.1053/j.gastro.2017.01.010 [DOI] [PubMed] [Google Scholar]
- Fan P., He L., Hu N., Luo J., Zhang J., Mo L. F., et al. (2017). Effect of 1,25-(OH)2D3 on Proliferation of Fibroblast-like Synoviocytes and Expressions of Pro-inflammatory Cytokines through Regulating MicroRNA-22 in a Rat Model of Rheumatoid Arthritis. Cell. Physiol. Biochem. 42 (1), 145–155. 10.1159/000477123 [DOI] [PubMed] [Google Scholar]
- Fisher S. A., Rahimzadeh M., Brierley C., Gration B., Doree C., Kimber C. E., et al. (2019). The Role of Vitamin D in Increasing Circulating T Regulatory Cell Numbers and Modulating T Regulatory Cell Phenotypes in Patients with Inflammatory Disease or in Healthy Volunteers: A Systematic Review. PLoS One 14 (9), e0222313. 10.1371/journal.pone.0222313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Wei B., de Assuncao T. M., Liu Z., Hu X., Ibrahim S., et al. (2020). Hepatic Stellate Cell Autophagy Inhibits Extracellular Vesicle Release to Attenuate Liver Fibrosis. J. Hepatol. 73 (5), 1144–1154. 10.1016/j.jhep.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesmundo I., Silvagno F., Banfi D., Monica V., Fanciulli A., Gamba G., et al. (2020). Calcitriol Inhibits Viability and Proliferation in Human Malignant Pleural Mesothelioma Cells. Front. Endocrinol. (Lausanne) 11, 559586. 10.3389/fendo.2020.559586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giammanco M., Di Majo D., La Guardia M., Aiello S., Crescimannno M., Flandina C., et al. (2015). Vitamin D in Cancer Chemoprevention. Pharm. Biol. 53 (10), 1399–1434. 10.3109/13880209.2014.988274 [DOI] [PubMed] [Google Scholar]
- Gil Á., Plaza-Diaz J., Mesa M. D. (2018). Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 72 (2), 87–95. 10.1159/000486536 [DOI] [PubMed] [Google Scholar]
- Giri A. K., Midha S., Banerjee P., Agrawal A., Mehdi S. J., Dhingra R., et al. (2016). Common Variants in CLDN2 and MORC4 Genes Confer Disease Susceptibility in Patients with Chronic Pancreatitis. PLoS One 11 (1), e0147345. 10.1371/journal.pone.0147345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert-Ferrándiz L., Cosin-Roger J., Hernández C., Macias-Ceja D. C., Ortiz-Masiá D., Salvador P., et al. (2020). The Vitamin D Receptor Taq I Polymorphism Is Associated with Reduced VDR and Increased PDIA3 Protein Levels in Human Intestinal Fibroblasts. J. Steroid Biochem. Mol. Biol. 202, 105720. 10.1016/j.jsbmb.2020.105720 [DOI] [PubMed] [Google Scholar]
- Golpour A., Bereswill S., Heimesaat M. M. (2019). Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-independent Approaches to Tackle Bacterial Infections - Lessons Learnt from a Literature Survey. Eur. J. Microbiol. Immunol. (Bp) 9 (3), 80–87. 10.1556/1886.2019.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sancho J. M., Larriba M. J., Muñoz A. (2020). Wnt and Vitamin D at the Crossroads in Solid Cancer. Cancers 12 (11), 3434. 10.3390/cancers12113434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. B., Lahore H., McDonnell S. L., Baggerly C. A., French C. B., Aliano J. L., et al. (2020). Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 12 (4), 988. 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundsten M., Liu G. Z., Permert J., Hjelmstrom P., Tsai J. A. (2005). Increased Central Memory T Cells in Patients with Chronic Pancreatitis. Pancreatology 5 (2-3), 177–182. 10.1159/000085269 [DOI] [PubMed] [Google Scholar]
- Halder S. K., Sharan C., Al-Hendy A. (2012). 1,25-dihydroxyvitamin D3 Treatment Shrinks Uterine Leiomyoma Tumors in the Eker Rat Model. Biol. Reprod. 86 (4), 116. 10.1095/biolreprod.111.098145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder S. K., Sharan C., Al-Hendy O., Al-Hendy A. (2014). Paricalcitol, a Vitamin D Receptor Activator, Inhibits Tumor Formation in a Murine Model of Uterine Fibroids. Reprod. Sci. 21 (9), 1108–1119. 10.1177/1933719114537721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. R., Li D., Jeffery L. E., Raza K., Hewison M. (2020). Vitamin D, Autoimmune Disease and Rheumatoid Arthritis. Calcif. Tissue Int. 106 (1), 58–75. 10.1007/s00223-019-00577-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., Haussler C. A., Jurutka P. W., Thompson P. D., Hsieh J. C., Remus L. S., et al. (1997). The Vitamin D Hormone and its Nuclear Receptor: Molecular Actions and Disease States. J. Endocrinol. 154 Suppl (Suppl. l), S57 [PubMed] [Google Scholar]
- He D., Wang Y., Liu R., He A., Li S., Fu X., et al. (2019). 1,25(OH)2D3 Activates Autophagy to Protect against Oxidative Damage of INS-1 Pancreatic Beta Cells. Biol. Pharm. Bull. 42 (4), 561–567. 10.1248/bpb.b18-00395 [DOI] [PubMed] [Google Scholar]
- Hershberger P. A., McGuire T. F., Yu W. D., Zuhowski E. G., Schellens J. H., Egorin M. J., et al. (2002). Cisplatin Potentiates 1,25-dihydroxyvitamin D3-Induced Apoptosis in Association with Increased Mitogen-Activated Protein Kinase Kinase Kinase 1 (MEKK-1) Expression. Mol. Cancer Ther. 1 (10), 821 [PubMed] [Google Scholar]
- Hershberger P. A., Yu W. D., Modzelewski R. A., Rueger R. M., Johnson C. S., Trump D. L. (2001). Calcitriol (1,25-dihydroxycholecalciferol) Enhances Paclitaxel Antitumor Activity In Vitro and In Vivo and Accelerates Paclitaxel-Induced Apoptosis. Clin. Cancer Res. 7 (4), 1043 [PubMed] [Google Scholar]
- Hii C. S., Ferrante A. (2016). The Non-genomic Actions of Vitamin D. Nutrients 8 (3), 135. 10.3390/nu8030135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., Gordon C. M., Hanley D. A., Heaney R. P., et al. (2011). Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 96 (7), 1911–1930. 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- Hollis B. W. (2005). Circulating 25-hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D. J. Nutr. 135 (2), 317–322. 10.1093/jn/135.2.317 [DOI] [PubMed] [Google Scholar]
- Hoogenboom S. A., Lekkerkerker S. J., Fockens P., Boermeester M. A., van Hooft J. E. (2016). Systematic Review and Meta-Analysis on the Prevalence of Vitamin D Deficiency in Patients with Chronic Pancreatitis. Pancreatology 16 (5), 800–806. 10.1016/j.pan.2016.07.010 [DOI] [PubMed] [Google Scholar]
- Hu W., Zhang L., Li M. X., Shen J., Liu X. D., Xiao Z. G., et al. (2019). Vitamin D3 Activates the Autolysosomal Degradation Function against Helicobacter pylori through the PDIA3 Receptor in Gastric Epithelial Cells. Autophagy 15 (4), 707–725. 10.1080/15548627.2018.1557835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. C., Chen J. Y., Hung W. C. (2004). Vitamin D3 receptor/Sp1 Complex Is Required for the Induction of p27Kip1 Expression by Vitamin D3. Oncogene 23 (28), 4856–4861. 10.1038/sj.onc.1207621 [DOI] [PubMed] [Google Scholar]
- Irazoqui A. P., Boland R. L., Buitrago C. G. (2014). Actions of 1,25(OH)2-vitamin D3 on the Cellular Cycle Depend on VDR and P38 MAPK in Skeletal Muscle Cells. J. Mol. Endocrinol. 53 (3), 331–343. 10.1530/jme-14-0102 [DOI] [PubMed] [Google Scholar]
- Ishizawa M., Akagi D., Yamamoto J., Makishima M. (2017). 1α,25-Dihydroxyvitamin D3 Enhances TRPV6 Transcription through P38 MAPK Activation and GADD45 Expression. J. Steroid Biochem. Mol. Biol. 172, 55–61. 10.1016/j.jsbmb.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Jenkinson C. (2019). The Vitamin D Metabolome: An Update on Analysis and Function. Cell. Biochem. Funct. 37 (6), 408–423. 10.1002/cbf.3421 [DOI] [PubMed] [Google Scholar]
- Jeon S. M., Shin E. A. (2018). Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 50 (4), 20–14. 10.1038/s12276-018-0038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Bao J., Li P., Nicosia S. V., Bai W. (2004). Induction of Ovarian Cancer Cell Apoptosis by 1,25-dihydroxyvitamin D3 through the Down-Regulation of Telomerase. J. Biol. Chem. 279 (51), 53213–53221. 10.1074/jbc.M410395200 [DOI] [PubMed] [Google Scholar]
- Jin G., Hong W., Guo Y., Bai Y., Chen B. (2020). Molecular Mechanism of Pancreatic Stellate Cells Activation in Chronic Pancreatitis and Pancreatic Cancer. J. Cancer 11 (6), 1505–1515. 10.7150/jca.38616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jøker-Jensen H., Mathiasen A. S., Køhler M., Rasmussen H. H., Drewes A. M., Olesen S. S. (2020). Micronutrient Deficits in Patients with Chronic Pancreatitis: Prevalence, Risk Factors and Pitfalls. Eur. J. Gastroenterol. Hepatol. 32 (10), 1328–1334. 10.1097/meg.0000000000001866 [DOI] [PubMed] [Google Scholar]
- Joshi A., Reddy S. V., Bhatia V., Choudhuri G., Singh R. K., Singh N., et al. (2011). High Prevalence of Low Bone Mineral Density in Patients with Tropical Calcific Pancreatitis. Pancreas 40 (5), 762–767. 10.1097/MPA.0b013e31821396b2 [DOI] [PubMed] [Google Scholar]
- Kandikattu H. K., Venkateshaiah S. U., Mishra A. (2020). Chronic Pancreatitis and the Development of Pancreatic Cancer. Endocr. Metab. Immune Disord. Drug Targets 20 (8), 1182–1210. 10.2174/1871530320666200423095700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z., Wang C., Tong Y., Li Y., Gao Y., Hou S., et al. (2021). Novel Nonsecosteroidal Vitamin D Receptor Modulator Combined with Gemcitabine Enhances Pancreatic Cancer Therapy through Remodeling of the Tumor Microenvironment. J. Med. Chem. 64 (1), 629–643. 10.1021/acs.jmedchem.0c01197 [DOI] [PubMed] [Google Scholar]
- Kang Z. S., Wang C., Han X. L., Du J. J., Li Y. Y., Zhang C. (2018). Design, Synthesis and Biological Evaluation of Non-secosteriodal Vitamin D Receptor Ligand Bearing Double Side Chain for the Treatment of Chronic Pancreatitis. Eur. J. Med. Chem. 146, 541–553. 10.1016/j.ejmech.2018.01.073 [DOI] [PubMed] [Google Scholar]
- Kirkegård J., Mortensen F. V., Cronin-Fenton D. (2017). Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 112 (9), 1366–1372. 10.1038/ajg.2017.218 [DOI] [PubMed] [Google Scholar]
- Klapdor S., Richter E., Klapdor R. (2012). Vitamin D Status and Per-Oral Vitamin D Supplementation in Patients Suffering from Chronic Pancreatitis and Pancreatic Cancer Disease. Anticancer Res. 32 (5), 1991 [PubMed] [Google Scholar]
- Larriba M. J., González-Sancho J. M., Barbáchano A., Niell N., Ferrer-Mayorga G., Muñoz A. (2013). Vitamin D Is a Multilevel Repressor of Wnt/b-Catenin Signaling in Cancer Cells. Cancers (Basel) 5 (4), 1242–1260. 10.3390/cancers5041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. X., Cui L. H., Zhuo Y. Z., Hu J. G., Cui N. Q., Zhang S. K. (2018a). Inhibiting Autophagy Promotes Collagen Degradation by Regulating Matrix Metalloproteinases in Pancreatic Stellate Cells. Life Sci. 208, 276–283. 10.1016/j.lfs.2018.07.049 [DOI] [PubMed] [Google Scholar]
- Li H., Wen W., Luo J. (2022). Targeting Endoplasmic Reticulum Stress as an Effective Treatment for Alcoholic Pancreatitis. Biomedicines 10 (1), 108. 10.3390/biomedicines10010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xiu M., Wang S., Brigstock D. R., Sun L., Qu L., et al. (2018b). Role of Gut-Derived Endotoxin on Type I Collagen Production in the Rat Pancreas after Chronic Alcohol Exposure. Alcohol Clin. Exp. Res. 42 (2), 306–314. 10.1111/acer.13550 [DOI] [PubMed] [Google Scholar]
- Li L., Shang F., Zhu Y., Sun Y., Sudi R. S. (2019). Modulation of VDR and Cell Cycle-Related Proteins by Vitamin D in Normal Pancreatic Cells and Poorly Differentiated Metastatic Pancreatic Cancer Cells. Nutr. Cancer 71 (5), 818–824. 10.1080/01635581.2018.1521445 [DOI] [PubMed] [Google Scholar]
- Li L., Wang G., Hu J. S., Zhang G. Q., Chen H. Z., Yuan Y., et al. (2018c). RB1CC1-enhanced Autophagy Facilitates PSCs Activation and Pancreatic Fibrogenesis in Chronic Pancreatitis. Cell. Death Dis. 9 (10), 952. 10.1038/s41419-018-0980-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Li L., Zhang L., Hu W., Shen J., Xiao Z., et al. (2017a). 1,25-Dihydroxyvitamin D3 Suppresses Gastric Cancer Cell Growth through VDR- and Mutant P53-Mediated Induction of P21. Life Sci. 179, 88–97. 10.1016/j.lfs.2017.04.021 [DOI] [PubMed] [Google Scholar]
- Li R., Guo E., Yang J., Li A., Yang Y., Liu S., et al. (2017b). 1,25(OH)2 D3 Attenuates Hepatic Steatosis by Inducing Autophagy in Mice. Obes. (Silver Spring) 25 (3), 561–571. 10.1002/oby.21757 [DOI] [PubMed] [Google Scholar]
- Lin L., Zhang L., Li C., Gai Z., Li Y. (2019). Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 20 (10), 984–995. 10.2174/1389203720666190807130504 [DOI] [PubMed] [Google Scholar]
- Martens P. J., Gysemans C., Verstuyf A., Mathieu A. C. (2020). Vitamin D's Effect on Immune Function. Nutrients 12 (5), 1248. 10.3390/nu12051248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Moneo E., Stigliano S., Hedström A., Kaczka A., Malvik M., Waldthaler A., et al. (2016). Deficiency of Fat-Soluble Vitamins in Chronic Pancreatitis: A Systematic Review and Meta-Analysis. Pancreatology 16 (6), 988–994. 10.1016/j.pan.2016.09.008 [DOI] [PubMed] [Google Scholar]
- McEachron K. R., Downs E. M., Schwarzenberg S. J., Chinnakotla S., Bellin M. D. (2021). Fat-soluble Vitamin Deficiency Is Common in Children with Chronic Pancreatitis Undergoing Total Pancreatectomy with Islet Autotransplantation. J. Pediatr. Gastroenterol. Nutr. 72 (1), 123–126. 10.1097/mpg.0000000000002950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan M. A., Kerman R. H., Lemire J. M. (1992). 1,25-Dihydroxyvitamin D3 Enhances the Generation of Nonspecific Suppressor Cells while Inhibiting the Induction of Cytotoxic Cells in a Human MLR. Cell. Immunol. 140 (2), 400–409. 10.1016/0008-8749(92)90206-5 [DOI] [PubMed] [Google Scholar]
- Min M., Patel B., Han S., Bocelli L., Kheder J., Vaze A., et al. (2018). Exocrine Pancreatic Insufficiency and Malnutrition in Chronic Pancreatitis: Identification, Treatment, and Consequences. Pancreas 47 (8), 1015–1018. 10.1097/mpa.0000000000001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morán-Auth Y., Penna-Martinez M., Shoghi F., Ramos-Lopez E., Badenhoop K. (2013). Vitamin D Status and Gene Transcription in Immune Cells. J. Steroid Biochem. Mol. Biol. 136, 83–85. 10.1016/j.jsbmb.2013.02.005 [DOI] [PubMed] [Google Scholar]
- Moreno J., Krishnan A. V., Swami S., Nonn L., Peehl D. M., Feldman D. (2005). Regulation of Prostaglandin Metabolism by Calcitriol Attenuates Growth Stimulation in Prostate Cancer Cells. Cancer Res. 65 (17), 7917–7925. 10.1158/0008-5472.can-05-1435 [DOI] [PubMed] [Google Scholar]
- Murdaca G., Gerosa A., Paladin F., Petrocchi L., Banchero S., Gangemi S. (2021). Vitamin D and Microbiota: Is There a Link with Allergies? Ijms 22 (8), 4288. 10.3390/ijms22084288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ní Chonchubhair H. M., Duggan S. N., Egan S. M., Kenyon M., O'Toole D., McManus R., et al. (2021). A High Prevalence of Genetic Polymorphisms in Idiopathic and Alcohol-Associated Chronic Pancreatitis Patients in Ireland. HPB Oxf. 23 (2), 231–237. 10.1016/j.hpb.2020.06.002 [DOI] [PubMed] [Google Scholar]
- Nikkola J., Räty S., Laukkarinen J., Seppänen H., Lappalainen-Lehto R., Järvinen S., et al. (2013). Abstinence after First Acute Alcohol-Associated Pancreatitis Protects against Recurrent Pancreatitis and Minimizes the Risk of Pancreatic Dysfunction. Alcohol Alcohol 48 (4), 483–486. 10.1093/alcalc/agt019 [DOI] [PubMed] [Google Scholar]
- Olesen S. S., Poulsen J. L., Vestergaard P., Drewes A. M. (2017). Vitamin-D Deficiency in Patients with Chronic Pancreatitis - Prevalence and Pitfalls. Pancreatology 17 (1), 22–23. 10.1016/j.pan.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Pendás-Franco N., Aguilera O., Pereira F., González-Sancho J. M., Muñoz A. (2008). Vitamin D and Wnt/beta-Catenin Pathway in Colon Cancer: Role and Regulation of DICKKOPF Genes. Anticancer Res. 28 (5a), 2613 [PubMed] [Google Scholar]
- Pi Y., Tian X., Ma J., Zhang H., Huang X. (2021). Vitamin D Alleviates Hypoxia/reoxygenation-Induced Injury of Human Trophoblast HTR-8 Cells by Activating Autophagy. Placenta 111, 10–18. 10.1016/j.placenta.2021.05.008 [DOI] [PubMed] [Google Scholar]
- Pike J. W., Christakos S. (2017). Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. North Am. 46 (4), 815–843. 10.1016/j.ecl.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poursoltani F., Nejati V., Pazhang Y., Rezaie J. (2021). Sulindac and Vitamin D3 Synergically Inhibit Proliferation of MCF-7 Breast Cancer Cell through AMPK/Akt/β-catenin axis In Vitro . Cell. Biochem. Funct. 39 (8), 991–997. 10.1002/cbf.3668 [DOI] [PubMed] [Google Scholar]
- Qian X., Zhu M., Qian W., Song J. (2019). Vitamin D Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Inflammation via Suppressing the RhoA/ROCK/NF-ĸB Pathway. Biotechnol. Appl. Biochem. 66 (5), 850–857. 10.1002/bab.1797 [DOI] [PubMed] [Google Scholar]
- Radoslavova S., Folcher A., Lefebvre T., Kondratska K., Guénin S., Dhennin-Duthille I., et al. (2021). Orai1 Channel Regulates Human-Activated Pancreatic Stellate Cell Proliferation and TGFβ1 Secretion through the AKT Signaling Pathway. Cancers (Basel) 13 (10), 2395. 10.3390/cancers13102395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebours V., Vullierme M. P., Hentic O., Maire F., Hammel P., Ruszniewski P., et al. (2012). Smoking and the Course of Recurrent Acute and Chronic Alcoholic Pancreatitis: a Dose-dependent Relationship. Pancreas 41 (8), 1219–1224. 10.1097/MPA.0b013e31825de97d [DOI] [PubMed] [Google Scholar]
- Reddy S. V., Ramesh V., Bhatia E. (2013). Double Blind Randomized Control Study of Intramuscular Vitamin D3 Supplementation in Tropical Calcific Pancreatitis. Calcif. Tissue Int. 93 (1), 48–54. 10.1007/s00223-013-9726-6 [DOI] [PubMed] [Google Scholar]
- Saramäki A., Banwell C. M., Campbell M. J., Carlberg C. (2006). Regulation of the Human P21(waf1/cip1) Gene Promoter via Multiple Binding Sites for P53 and the Vitamin D3 Receptor. Nucleic Acids Res. 34 (2), 543–554. 10.1093/nar/gkj460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Hewison M., Studzinski G. P., Li Y. C., Kalia V. (2016). Role of Vitamin D in Cytotoxic T Lymphocyte Immunity to Pathogens and Cancer. Crit. Rev. Clin. Lab. Sci. 53 (2), 132–145. 10.3109/10408363.2015.1094443 [DOI] [PubMed] [Google Scholar]
- Schmid-Kotsas A., Gross H. J., Menke A., Weidenbach H., Adler G., Siech M., et al. (1999). Lipopolysaccharide-activated Macrophages Stimulate the Synthesis of Collagen Type I and C-Fibronectin in Cultured Pancreatic Stellate Cells. Am. J. Pathol. 155 (5), 1749–1758. 10.1016/s0002-9440(10)65490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Winnenthal H., Pietsch D. H., Schimmack S., Bonertz A., Udonta F., Ge Y., et al. (2010). Chronic Pancreatitis Is Associated with Disease-specific Regulatory T-Cell Responses. Gastroenterology 138 (3), 1178–1188. 10.1053/j.gastro.2009.11.011 [DOI] [PubMed] [Google Scholar]
- Scolletta S., Colletti M., Di Luigi L., Crescioli C. (2013). Vitamin D Receptor Agonists Target CXCL10: New Therapeutic Tools for Resolution of Inflammation. Mediat. Inflamm. 2013, 876319. 10.1155/2013/876319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev I. N. (2009). 1,25-Dihydroxyvitamin D3 Induces Ca2+-Mediated Apoptosis in Adipocytes via Activation of Calpain and Caspase-12. Biochem. Biophys. Res. Commun. 384 (1), 18–21. 10.1016/j.bbrc.2009.04.078 [DOI] [PubMed] [Google Scholar]
- Sergeev I. N. (2020). Vitamin D Status and Vitamin D-dependent Apoptosis in Obesity. Nutrients 12 (5), 1392. 10.3390/nu12051392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. H., Yu R. T., Engle D. D., Ding N., Atkins A. R., Tiriac H., et al. (2014). Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell. 159 (1), 80–93. 10.1016/j.cell.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K., Yadav D., Garg P. K. (2019). Diagnosis and Management of Chronic Pancreatitis: A Review. Jama 322 (24), 2422–2434. 10.1001/jama.2019.19411 [DOI] [PubMed] [Google Scholar]
- Singhvi A., Yadav D. (2018). Myths and Realities about Alcohol and Smoking in Chronic Pancreatitis. Curr. Opin. Gastroenterol. 34 (5), 355–361. 10.1097/mog.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A., Janjetovic Z., Tuckey R. C., Nguyen M. N., Bhattacharya K. G., Wang J., et al. (2013). 20S-hydroxyvitamin D3, Noncalcemic Product of CYP11A1 Action on Vitamin D3, Exhibits Potent Antifibrogenic Activity In Vivo . J. Clin. Endocrinol. Metab. 98 (2), E298–E303. 10.1210/jc.2012-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A., Semak I., Zjawiony J., Wortsman J., Li W., Szczesniewski A., et al. (2005). The Cytochrome P450scc System Opens an Alternate Pathway of Vitamin D3 Metabolism. Febs J. 272 (16), 4080–4090. 10.1111/j.1742-4658.2005.04819.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Brożyna A. A., Skobowiat C., Zmijewski M. A., Kim T. K., Janjetovic Z., et al. (2018a). On the Role of Classical and Novel Forms of Vitamin D in Melanoma Progression and Management. J. Steroid Biochem. Mol. Biol. 177, 159–170. 10.1016/j.jsbmb.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Janjetovic Z., Fuller B. E., Zmijewski M. A., Tuckey R. C., Nguyen M. N., et al. (2010). Products of Vitamin D3 or 7-dehydrocholesterol Metabolism by Cytochrome P450scc Show Anti-leukemia Effects, Having Low or Absent Calcemic Activity. PLoS One 5 (3), e9907. 10.1371/journal.pone.0009907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Janjetovic Z., Kim T. K., Wasilewski P., Rosas S., Hanna S., et al. (2015a). Novel Non-calcemic Secosteroids that Are Produced by Human Epidermal Keratinocytes Protect against Solar Radiation. J. Steroid Biochem. Mol. Biol. 148, 52–63. 10.1016/j.jsbmb.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Janjetovic Z., Kim T. K., Wright A. C., Grese L. N., Riney S. J., et al. (2012a). Novel Vitamin D Hydroxyderivatives Inhibit Melanoma Growth and Show Differential Effects on Normal Melanocytes. Anticancer Res. 32 (9), 3733 [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Chen J., Nguyen M. N., Li W., Yates C. R., et al. (2012b). Cytochrome P450scc-dependent Metabolism of 7-dehydrocholesterol in Placenta and Epidermal Keratinocytes. Int. J. Biochem. Cell. Biol. 44 (11), 2003–2018. 10.1016/j.biocel.2012.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Hobrath J. V., Oak A. S. W., Tang E. K. Y., Tieu E. W., et al. (2017). Endogenously Produced Nonclassical Vitamin D Hydroxy-Metabolites Act as "biased" Agonists on VDR and Inverse Agonists on RORα and RORγ. J. Steroid Biochem. Mol. Biol. 173, 42–56. 10.1016/j.jsbmb.2016.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Janjetovic Z., Brożyna A. A., Żmijewski M. A., Xu H., et al. (2018b). Differential and Overlapping Effects of 20,23(OH)₂D3 and 1,25(OH)₂D3 on Gene Expression in Human Epidermal Keratinocytes: Identification of AhR as an Alternative Receptor for 20,23(OH)₂D3. Int. J. Mol. Sci. 19 (10). 10.3390/ijms19103072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Li W., Postlethwaite A., Tieu E. W., Tang E. K. Y., et al. (2015b). Detection of Novel CYP11A1-Derived Secosteroids in the Human Epidermis and Serum and Pig Adrenal Gland. Sci. Rep. 5, 14875. 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Li W., Tuckey R. C. (2016). Classical and Non-classical Metabolic Transformation of Vitamin D in Dermal Fibroblasts. Exp. Dermatol 25 (3), 231–232. 10.1111/exd.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Li W., Yi A. K., Postlethwaite A., Tuckey R. C. (2014a). The Role of CYP11A1 in the Production of Vitamin D Metabolites and Their Role in the Regulation of Epidermal Functions. J. Steroid Biochem. Mol. Biol. 144 Pt A, 28–39. 10.1016/j.jsbmb.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Qayyum S., Song Y., Janjetovic Z., Oak A. S. W., et al. (2021a). Vitamin D and Lumisterol Derivatives Can Act on Liver X Receptors (LXRs). Sci. Rep. 11 (1), 8002. 10.1038/s41598-021-87061-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Shehabi H. Z., Semak I., Tang E. K., Nguyen M. N., et al. (2012c). In Vivo evidence for a Novel Pathway of Vitamin D₃ Metabolism Initiated by P450scc and Modified by CYP27B1. Faseb J. 26 (9), 3901–3915. 10.1096/fj.12-208975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Kim T. K., Takeda Y., Janjetovic Z., Brozyna A. A., Skobowiat C., et al. (2014b). RORα and ROR γ Are Expressed in Human Skin and Serve as Receptors for Endogenously Produced Noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. Faseb J. 28 (7), 2775–2789. 10.1096/fj.13-242040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A. T., Li W., Kim T. K., Semak I., Wang J., Zjawiony J. K., et al. (2015c). Novel Activities of CYP11A1 and Their Potential Physiological Significance. J. Steroid Biochem. Mol. Biol. 151, 25–37. 10.1016/j.jsbmb.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski R. M., Raman C., Elmets C., Jetten A. M., Slominski A. T., Tuckey R. C. (2021b). The Significance of CYP11A1 Expression in Skin Physiology and Pathology. Mol. Cell. Endocrinol. 530, 111238. 10.1016/j.mce.2021.111238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath L., Ulivieri A., Lavra L., Fidanza L., Carlesimo M., Giubettini M., et al. (2017). Antiproliferative Effects of 1α-OH-vitD3 in Malignant Melanoma: Potential Therapeutic Implications. Sci. Rep. 7, 40370. 10.1038/srep40370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigliano S., Waldthaler A., Martinez-Moneo E., Lionetto L., Robinson S., Malvik M., et al. (2018). Vitamins D and K as Factors Associated with Osteopathy in Chronic Pancreatitis: A Prospective Multicentre Study (P-BONE Study). Clin. Transl. Gastroenterol. 9 (10), 197. 10.1038/s41424-018-0066-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xiu M., Wang S., Brigstock D. R., Li H., Qu L., et al. (2018). Lipopolysaccharide Enhances TGF-Β1 Signalling Pathway and Rat Pancreatic Fibrosis. J. Cell. Mol. Med. 22 (4), 2346–2356. 10.1111/jcmm.13526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabasi N., Rastin M., Mahmoudi M., Ghoryani M., Mirfeizi Z., Rabe S. Z., et al. (2015). Influence of Vitamin D on Cell Cycle, Apoptosis, and Some Apoptosis Related Molecules in Systemic Lupus Erythematosus. Iran. J. Basic Med. Sci. 18 (11), 1107. 10.22038/IJBMS.2015.6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang E. K., Chen J., Janjetovic Z., Tieu E. W., Slominski A. T., Li W., et al. (2013). Hydroxylation of CYP11A1-Derived Products of Vitamin D3 Metabolism by Human and Mouse CYP27B1. Drug Metab. Dispos. 41 (5), 1112–1124. 10.1124/dmd.113.050955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tongkao-On W., Carter S., Reeve V. E., Dixon K. M., Gordon-Thomson C., Halliday G. M., et al. (2015). CYP11A1 in Skin: an Alternative Route to Photoprotection by Vitamin D Compounds. J. Steroid Biochem. Mol. Biol. 148, 72–78. 10.1016/j.jsbmb.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Treiber G., Prietl B., Fröhlich-Reiterer E., Lechner E., Ribitsch A., Fritsch M., et al. (2015). Cholecalciferol Supplementation Improves Suppressive Capacity of Regulatory T-Cells in Young Patients with New-Onset Type 1 Diabetes Mellitus - A Randomized Clinical Trial. Clin. Immunol. 161 (2), 217–224. 10.1016/j.clim.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Triantos C., Aggeletopoulou I., Thomopoulos K., Mouzaki A. (2021a). Vitamin D-Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology 74 (2), 1065–1073. 10.1002/hep.31699 [DOI] [PubMed] [Google Scholar]
- Triantos C., Aggeletopoulou I., Thomopoulos K., Mouzaki A. (2021b). Vitamin D -Liver Disease Association: Biological Basis and Mechanisms of Action. Hepatology 74, 1065–1073. 10.1002/hep.31699 [DOI] [PubMed] [Google Scholar]
- Trump D. L. (2018). Calcitriol and Cancer Therapy: A Missed Opportunity. Bone Rep. 9, 110–119. 10.1016/j.bonr.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey R. C., Li W., Zjawiony J. K., Zmijewski M. A., Nguyen M. N., Sweatman T., et al. (2008). Pathways and Products for the Metabolism of Vitamin D3 by Cytochrome P450scc. Febs J. 275 (10), 2585–2596. 10.1111/j.1742-4658.2008.06406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomsinprasert W., Jittikoon J. (2019). Vitamin D and Liver Fibrosis: Molecular Mechanisms and Clinical Studies. Biomed. Pharmacother. 109, 1351–1360. 10.1016/j.biopha.2018.10.140 [DOI] [PubMed] [Google Scholar]
- van Halteren A. G., Tysma O. M., van Etten E., Mathieu C., Roep B. O. (2004). 1alpha,25-dihydroxyvitamin D3 or Analogue Treated Dendritic Cells Modulate Human Autoreactive T Cells via the Selective Induction of Apoptosis. J. Autoimmun. 23 (3), 233–239. 10.1016/j.jaut.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Vanherwegen A. S., Eelen G., Ferreira G. B., Ghesquière B., Cook D. P., Nikolic T., et al. (2019). Vitamin D Controls the Capacity of Human Dendritic Cells to Induce Functional Regulatory T Cells by Regulation of Glucose Metabolism. J. Steroid Biochem. Mol. Biol. 187, 134–145. 10.1016/j.jsbmb.2018.11.011 [DOI] [PubMed] [Google Scholar]
- Vanherwegen A. S., Gysemans C., Mathieu C. (2017a). Regulation of Immune Function by Vitamin D and its Use in Diseases of Immunity. Endocrinol. Metab. Clin. North Am. 46 (4), 1061–1094. 10.1016/j.ecl.2017.07.010 [DOI] [PubMed] [Google Scholar]
- Vanherwegen A. S., Gysemans C., Mathieu C. (2017b). Vitamin D Endocrinology on the Cross-Road between Immunity and Metabolism. Mol. Cell. Endocrinol. 453, 52–67. 10.1016/j.mce.2017.04.018 [DOI] [PubMed] [Google Scholar]
- von Essen M. R., Kongsbak M., Schjerling P., Olgaard K., Odum N., Geisler C. (2010). Vitamin D Controls T Cell Antigen Receptor Signaling and Activation of Human T Cells. Nat. Immunol. 11 (4), 344–349. 10.1038/ni.1851 [DOI] [PubMed] [Google Scholar]
- Wallbaum P., Rohde S., Ehlers L., Lange F., Hohn A., Bergner C., et al. (2018). Antifibrogenic Effects of Vitamin D Derivatives on Mouse Pancreatic Stellate Cells. World J. Gastroenterol. 24 (2), 170–178. 10.3748/wjg.v24.i2.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Slominski A., Tuckey R. C., Janjetovic Z., Kulkarni A., Chen J., et al. (2012a). 20-hydroxyvitamin D₃ Inhibits Proliferation of Cancer Cells with High Efficacy while Being Non-toxic. Anticancer Res. 32 (3), 739–746. [PMC free article] [PubMed] [Google Scholar]
- Wang Q., He Y., Shen Y., Zhang Q., Chen D., Zuo C., et al. (2014). Vitamin D Inhibits COX2 Expression and Inflammatory Response by Targeting Thioesterase Superfamily Member 4. J. Biol. Chem. 289 (17), 11681–11694. 10.1074/jbc.M113.517581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jiang L. (2021). Role of Vitamin D-Vitamin D Receptor Signaling on Hyperoxia-Induced Bronchopulmonary Dysplasia in Neonatal Rats. Pediatr. Pulmonol. 56 (7), 2335–2344. 10.1002/ppul.25418 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu J., DeLuca H. F. (2012b). Where Is the Vitamin D Receptor? Arch. Biochem. Biophys. 523 (1), 123–133. 10.1016/j.abb.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Wei D., Wang L., Zuo X., Bresalier R. S. (2021). Vitamin D: Promises on the Horizon and Challenges Ahead for Fighting Pancreatic Cancer. Cancers (Basel) 13 (11), 2716. 10.3390/cancers13112716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Liu Y., Li J., Wei D., Liu D., Zhao F. (2018). Inhibitory Effect and Mechanism of 1,25-dihydroxy Vitamin D3 on RANKL Expression in Fibroblast-like Synoviocytes and Osteoclast-like Cell Formation Induced by IL-22 in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 36 (5), 798 [PubMed] [Google Scholar]
- Wherry T. L. T., Dassanayake R. P., Casas E., Mooyottu S., Bannantine J. P., Stabel J. R. (2021). Exogenous Vitamin D3 Modulates Response of Bovine Macrophages to Mycobacterium avium Subsp. Paratuberculosis Infection and Is Dependent upon Stage of Johne's Disease. Front. Cell. Infect. Microbiol. 11, 773938. 10.3389/fcimb.2021.773938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M., Gärtner S., Doller J., Tran Q. T., Frost F., Bannert K., et al. (2021). Nutritional Management of Chronic Pancreatitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Gastroenterol. Hepatol. 36 (3), 588–600. 10.1111/jgh.15230 [DOI] [PubMed] [Google Scholar]
- Witt H., Beer S., Rosendahl J., Chen J. M., Chandak G. R., Masamune A., et al. (2013). Variants in CPA1 Are Strongly Associated with Early Onset Chronic Pancreatitis. Nat. Genet. 45 (10), 1216–1220. 10.1038/ng.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Zhang X., Zanello L. P. (2007). 1alpha,25-Dihydroxyvitamin D(3) Antiproliferative Actions Involve Vitamin D Receptor-Mediated Activation of MAPK Pathways and AP-1/p21(waf1) Upregulation in Human Osteosarcoma. Cancer Lett. 254 (1), 75–86. 10.1016/j.canlet.2007.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y., He L., Luan Z., Lv H., Yang H., Zhou Y., et al. (2017). E-cadherin Mediates the Preventive Effect of Vitamin D3 in Colitis-Associated Carcinogenesis. Inflamm. Bowel Dis. 23 (9), 1535–1543. 10.1097/mib.0000000000001209 [DOI] [PubMed] [Google Scholar]
- Xu J., Camfield R., Gorski S. M. (2018). The Interplay between Exosomes and Autophagy - Partners in Crime. J. Cell. Sci. 131 (15), 15210. 10.1242/jcs.215210 [DOI] [PubMed] [Google Scholar]
- Xu M., Wang G., Zhou H., Cai J., Li P., Zhou M., et al. (2017). TGF-β1-miR-200a-PTEN Induces Epithelial-Mesenchymal Transition and Fibrosis of Pancreatic Stellate Cells. Mol. Cell. Biochem. 431 (1-2), 161–168. 10.1007/s11010-017-2988-y [DOI] [PubMed] [Google Scholar]
- Xue J., Sharma V., Hsieh M. H., Chawla A., Murali R., Pandol S. J., et al. (2015). Alternatively Activated Macrophages Promote Pancreatic Fibrosis in Chronic Pancreatitis. Nat. Commun. 6, 7158. 10.1038/ncomms8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Long F., Zhang Y., Yu R., Zhang P., Li W., et al. (2015). 1α,25-Dihydroxyvitamin D3 Induces Neutrophil Apoptosis through the P38 MAPK Signaling Pathway in Chronic Obstructive Pulmonary Disease Patients. PLoS One 10 (4), e0120515. 10.1371/journal.pone.0120515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B., He J., Yin X., Shi Y., Wan J., Tian Z. (2019). The Protective Effect of Lithocholic Acid on the Intestinal Epithelial Barrier Is Mediated by the Vitamin D Receptor via a SIRT1/Nrf2 and NF-Κb Dependent Mechanism in CaCo2 Cells. Toxicol. Lett. 316, 109–118. 10.1016/j.toxlet.2019.08.024 [DOI] [PubMed] [Google Scholar]
- Yuan F., Xu Y., You K., Zhang J., Yang F., Li Y. X. (2021). Calcitriol Alleviates Ethanol-Induced Hepatotoxicity via AMPK/mTOR-mediated Autophagy. Arch. Biochem. Biophys. 697, 108694. 10.1016/j.abb.2020.108694 [DOI] [PubMed] [Google Scholar]
- Zhang T., Zhang G., Yang W., Chen H., Hu J., Zhao Z., et al. (2021). Lnc-PFAR Facilitates Autophagy and Exacerbates Pancreatic Fibrosis by Reducing Pre-miR-141 Maturation in Chronic Pancreatitis. Cell. Death Dis. 12 (11), 996. 10.1038/s41419-021-04236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Zhang W., Ma C., Zhao Y., Xiong R., Wang H., et al. (2021). Immunomodulatory Function of Vitamin D and its Role in Autoimmune Thyroid Disease. Front. Immunol. 12, 574967. 10.3389/fimmu.2021.574967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Li H., Sun L., Brigstock D. R., Gao R. (2021). Interleukin-6 Participates in Human Pancreatic Stellate Cell Activation and Collagen I Production via TGF-β1/Smad Pathway. Cytokine 143, 155536. 10.1016/j.cyto.2021.155536 [DOI] [PubMed] [Google Scholar]
- Zhou W., Wang W., Yuan X. J., Xiao C. C., Xing Y., Ye S. D., et al. (2022). The Effects of RBP4 and Vitamin D on the Proliferation and Migration of Vascular Smooth Muscle Cells via the JAK2/STAT3 Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 3046777. 10.1155/2022/3046777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Yuan G., Wang Q. (2019). Vitamin D Attenuates Lipopolysaccharide-Induced Inflammatory Response in Endothelial Cells through Inhibition of PI3K/Akt/NF-Κb Signaling Pathway. Pharmazie 74 (7), 412–417. 10.1691/ph.2019.9373 [DOI] [PubMed] [Google Scholar]