Abstract
It is not clear whether pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB) should be selected for patients with advanced non–small‐cell lung cancer (NSCLC) exhibiting high PD‐L1 expression (tumor proportion score ≥ 50%). We performed a retrospective, multicenter study of 300 patients with NSCLC exhibiting high PD‐L1 expression who received MONO or COMB as first‐line treatment between December 2018 and January 2020. We reviewed the medical records of all consecutive patients with no driver mutations, and assessed the patient characteristics, therapeutic regimens, treatment periods, and adverse events. In total, 166 (55%; median age: 74 years) and 134 (45%; median age: 68 years) patients received MONO and COMB, respectively. Patients were younger and had better performance status (0–1) in the COMB group (p < 0.01). With a median follow‐up time of 10.6 (range: 0.1–20.6) months, the median progression‐free survival was 7.1 months with MONO and 13.1 months with COMB. The objective response rate was 42.2% with MONO and 67.9% with COMB. With respect to treatment discontinuation, 36 out of 166 (21.7%) and 28 out of 134 (20.1%) patients discontinued MONO and COMB, respectively. In conclusion, COMB may be a promising option for first‐line treatment for NSCLC with high PD‐L1 expression and good performance status.
Keywords: combination chemotherapy, non–small‐cell lung cancer, PD‐L1 inhibitor, pembrolizumab, retrospective study
Progression‐free survival was improved in the combination therapy group over the monotherapy group. We believe that our study makes a significant contribution to the literature because our findings suggest that combination therapy may be a promising option for first‐line treatment for NSCLC with high PD‐L1 expression.
![]()
Abbreviations
- AE
adverse event
- CI
confidence interval
- COMB
pembrolizumab plus platinum‐based chemotherapy
- CR
complete response
- CTCAE
common terminology criteria for AEs
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HR
hazard ratio
- MONO
pembrolizumab monotherapy
- NJLCG
North Japan Lung Cancer Study Group
- NSCLC
non–small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PD‐1
programmed cell death protein‐1
- PD‐L1
programmed cell death‐ligand‐1
- PFS
progression‐free survival
- PR
partial response
- PSM
propensity score matching
- TPS
tumor proportion score
1. INTRODUCTION
Recently, PD‐1/PD‐L1 inhibitors have shown efficacy against advanced NSCLC; therefore, they have emerged as standard therapies for patients with advanced‐stage disease.
Pembrolizumab monotherapy (MONO) for advanced NSCLC with high PD‐L1 expression (PD‐L1 tumor proportion score [TPS] ≥ 50%) was approved as a first‐line therapy based on the results of previous studies. 1 , 2 In the phase III randomized KEYNOTE‐024 trial, MONO was shown to prolong PFS and OS compared with platinum‐based chemotherapy alone. 1 Furthermore, in the subgroup population expressing high levels of PD‐L1 in the phase III randomized KEYNOTE‐042 trial, MONO was shown to prolong PFS and improve OS compared with platinum‐based chemotherapy alone, as noted in the KEYNOTE‐024 report. 2 Thereafter, in two other phase III trials (KEYNOTE‐189 and KEYNOTE‐407), pembrolizumab plus platinum‐based chemotherapy (COMB) was established as a first‐line treatment for patients with advanced NSCLC, regardless of PD‐L1 expression level, compared with platinum‐based chemotherapy alone. In particular, COMB was more effective for both PFS and OS in patients with high PD‐L1 expression compared with platinum‐based chemotherapy. 3 , 4 These clinical trials and some meta‐analyses support the notion that MONO and COMB are beneficial as first‐line treatments for advanced NSCLC with high PD‐L1 expression. 5 , 6 , 7
In terms of AEs, these studies suggest that there may be a lower incidence of treatment‐related AEs of both all grades and grades 3–5 in patients who received MONO compared with COMB. It is recognized that MONO is better tolerated than COMB. 1 , 2 , 3 , 4
Based on limited data, the selection of MONO or COMB for patients with high PD‐L1 expression is a major issue in clinical practice. Therefore, we retrospectively analyzed the data of patients with NSCLC with high PD‐L1 expression who received MONO or COMB in clinical practice. We aimed to understand the current status of treatment with pembrolizumab in clinical practice, and to conduct statistical analysis to help in selecting appropriate treatment for patients with high PD‐L1 expression.
2. PATIENTS AND METHODS
2.1. Study population and design
This study (HOT/NJLCG2001) was performed with a retrospective, multicenter, observational design to evaluate the efficacy and toxicity of MONO or COMB as first‐line treatments between December 2018 and January 2020. We reviewed the medical records of all consecutive patients with advanced or recurrent NSCLC with high PD‐L1 expression (TPS ≥ 50%) and no documented EGFR, ALK, or ROS1 aberrations who were treated with MONO or COMB in 34 institutions that belong to Hokkaido Lung Cancer Clinical Study Group HOT, North Japan Lung Cancer Study Group (NJLCG) in Japan. The data cutoff was August 31, 2020. This study was approved by the institutional review boards of all institutions, which also waived the need to obtain informed consent because the data were analyzed anonymously.
2.2. Data collection
We assessed the characteristics of the patients, therapeutic regimens, treatment period, and AEs. We recorded the patients’ age, sex, smoking status, histology, cancer stage, PD‐L1 status, and the Eastern Cooperative Oncology Group performance status (ECOG PS) at the start of initial treatment. We recorded therapeutic regimens (MONO or COMB) and the kind of COMB (carboplatin/cisplatin + pemetrexed, carboplatin + (nab‐)paclitaxel). We extracted AE types of grade ≥3 and AE types leading to treatment discontinuation using common terminology criteria for AEs (CTCAE v.5.0). 8 Tumor response was measured using RECIST version 1.1. 9 Because of the retrospective study design, CR and PR did not need confirmation. Assessments were performed in each participating institution.
2.3. Statistical analysis
A calculation of sample size and power was not performed because of the descriptive nature of this retrospective study. The chi‐squared test was used for comparing proportions of patients based on patient characteristics (age, sex, PS, and smoking), tumor factors (clinical stage, pathological diagnosis, and PD‐L1 status), and pembrolizumab treatment (MONO or COMB). PFS was defined as the time interval between initial treatment administration and disease progression or death. Patients without documented clinical or radiographic disease progression or who were still alive were censored on the date of the last follow‐up. PFS was evaluated with the Kaplan–Meier method and compared using a two‐sided log‐rank test according to age (<75 years and ≥75 years), PS (0–1 and ≥2), and PD‐L1 expression (TPS: 50%–89%, and ≥90%). To reduce selection bias and obtain similar comparison groups, we used PSM using age and PS as an adjustment factor to analyze patients treated with MONO or COMB with a caliper width equal to 0.2 standard deviation. Of the 166 patients in the MONO group and 134 patients in the COMB group, 84 pairs were matched. Univariate and multivariate analyses using Cox proportional hazards modeling were performed to determine the correlations between PFS and the following factors: patient characteristics (age, sex, PS, and smoking), tumor factors (clinical stage, pathological diagnosis, and PD‐L1 status) and pembrolizumab treatment (MONO or COMB). The HR and 95% CI were estimated using Cox proportional hazards regression model. All p‐values were two‐sided and the threshold for statistical significance was set at p < 0.05. All statistical analyses were computed with EZR v.1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan). 10
3. RESULTS
3.1. Patient characteristics
In total, 300 patients were enrolled. Out of these, 166 patients (55%) received MONO and 134 patients (45%) received COMB (Figure S1). The baseline patient characteristics are summarized in Table 1. The median age of the patients was 74 (range: 52–89) and 68 (range: 45–84) years in the MONO and COMB groups, respectively. Patients were younger and had better PS (0–1) in the COMB group (p < 0.01) (Table 1).
TABLE 1.
Baseline and treatment characteristics
| Characteristics | No. (%) | p | ||
|---|---|---|---|---|
| All (n = 300) | MONO (n = 166) | COMB (n = 134) | ||
| Age in years/Median (range) | 71 (45–89) | 74 (52–89) | 68 (45–84) | <0.01 |
| <75 y.o. | 200 (66.7) | 84 (50.6) | 116 (86.6) | |
| ≥75 y.o. | 100 (33.3) | 82 (49.4) | 18 (13.4) | |
| Sex | 0.89 | |||
| Male | 238 (79.0) | 131 (78.9) | 107 (79.9) | |
| Female | 62 (21.0) | 35 (21.1) | 27 (20.1) | |
| Performance status | <0.01 | |||
| 0–1 | 242 (80.7) | 118 (71.1) | 124 (92.5) | |
| ≥2 | 58 (19.3) | 48 (28.9) | 10 (7.5) | |
| Smoking status | 0.07 | |||
| Current/Former smoker | 259 (86.3) | 141 (84.9) | 118 (88.1) | |
| Never smoker | 41 (13.7) | 25 (15.1) | 16 (11.9) | |
| Stage | 0.03 | |||
| III/IV | 261 (87.0) | 140 (84.4) | 121 (90.3) | |
| Recurrence | 39 (13.0) | 26 (15.6) | 13 (9.7) | |
| Histology | 0.51 | |||
| Non‐squamous cell carcinoma | 216 (72.0) | 119 (71.7) | 97 (72.4) | |
| Adenocarcinoma | 171 (57.0) | 92 (55.4) | 79 (59.0) | |
| Non–small‐cell carcinoma | 27 (9.0) | 14 (8.4) | 13 (9.7) | |
| Others | 18 (6.0) | 13 (7.9) | 5 (3.7) | |
| Squamous cell carcinoma | 84 (28.0) | 47 (28.3) | 37 (27.6) | |
| PD‐L1 status | 0.01 a | |||
| 50–89% | 179 (59.7) | 101 (60.8) | 78 (58.3) | 0.69 b |
| ≥90% | 99 (33.0) | 60 (36.1) | 39 (29.1) | |
| ≥50% (details are unknown) | 22 (7.3) | 5 (3.1) | 17 (12.6) | |
| Regimens | ||||
| CDDP/CBDCA + PEM + Pembrolizumab | – | – | 83 (61.9) | |
| CBDCA + (nab‐)PTX + Pembrolizumab | – | – | 51 (38.1) | |
Abbreviations: CBDCA, carboplatin; CDDP, cisplatin; nab‐PTX, nab‐paclitaxel; PEM, pemetrexed; PTX, paclitaxel; y.o., years old.
Cases whose PD‐L1 status is unknown.
Excluding cases whose PD‐L1 status is unknown.
3.2. Efficacy
The data cutoff date was August 31, 2020 and the median follow‐up was for 10.6 (range: 0.1–20.6) months. The median PFS duration was 7.1 months (95% CI: 5.4–11.1) in the MONO group and 13.1 months (95% CI: 10.2–not reached [NR]) in the COMB group (HR 0.65; 95% CI: 0.47–0.89) (Figure 1A). In total 81, patients were alive in the MONO group and 97 patients were alive in the COMB group at the time of data cutoff. The median survival time was 16.6 months (95% CI: 13.2–NR) in the MONO group and NR (95% CI: 17.3–NR) in the COMB group (HR 0.46; 95% CI: 0.3–0.72), respectively (Figure 1B).
FIGURE 1.
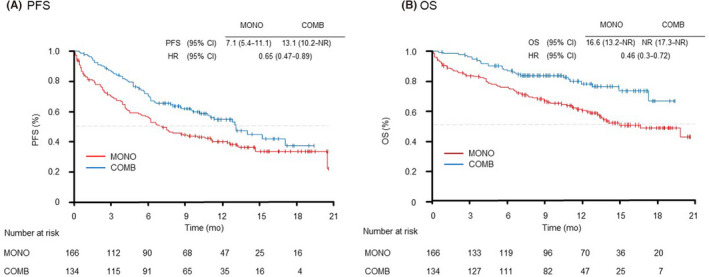
Kaplan–Meier curves of (A) progression‐free survival and (B) overall survival of all patients receiving pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB). mo, month; PFS, progression‐free survival; OS, overall survival
We evaluated the PFS in the selected subgroups of patients according to age (<75 years and ≥75 years), PS (0–1 and ≥2) and PD‐L1 expression (50%–89% and ≥90%). In the patients grouped by age, the younger group (<75 years) showed longer PFS with COMB than with MONO (14.0 vs. 6.2 months [HR 0.59; 95% CI: 0.4–0.87]) (Figure 2). In the PS 0–1 subgroup, although there were no significant differences in PFS between the MONO and COMB treatments (12.4 vs. 14.0 months [HR 0.8; 95% CI: 0.56–1.16]); the Kaplan–Meier curve for PFS showed that the COMB group was superior to the MONO group (Figure 3A). In the PS ≥2 subgroup, there were no significant differences in PFS between the MONO and COMB treatments (2.4 vs. 2.7 months [HR 0.95; 95% CI: 0.44–2.02]) (Figure 3B). In the subgroups of PS ≥2, the PFS of patients treated with MONO at PS3 and COMB at PS2 tended to be shorter than those of patients treated with MONO at PS2 (Figure S2). In both PD‐L1 subgroups (50%–89% and ≥90%), PFS tended to be better in the COMB group than in the MONO group. In particular, the COMB group exhibited a significantly longer PFS than MONO in the population with PD‐L1 expression ≥90% (NR vs. 11.2 months [HR 0.44; 95% CI: 0.21–0.89]) (Figure 4).
FIGURE 2.
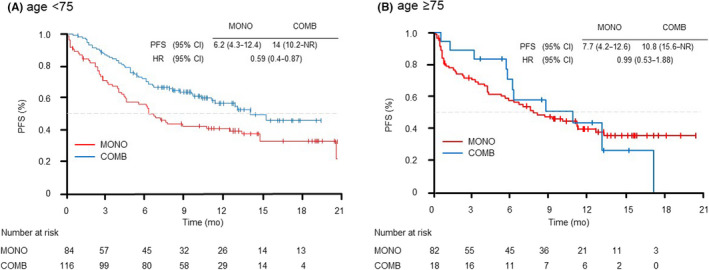
Kaplan–Meier curves of progression‐free survival of patients receiving pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB) according to age: (A) <75 years, (B) ≥75 years. mo, month; PFS, progression‐free survival
FIGURE 3.
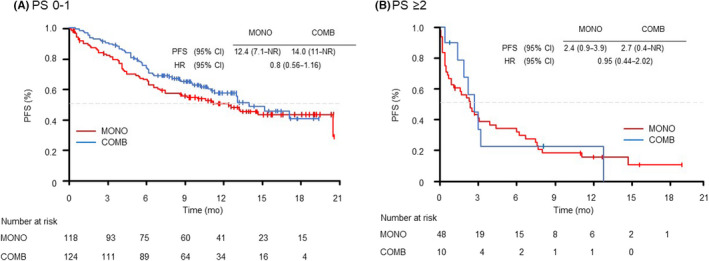
Kaplan–Meier curves of progression‐free survival of patients receiving pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB) according to ECOG performance status: (A) PS 0–1, (B) PS ≥2. ECOG, Eastern Cooperative Oncology Group; mo, month; PFS, progression‐free survival; PS, performance status
FIGURE 4.
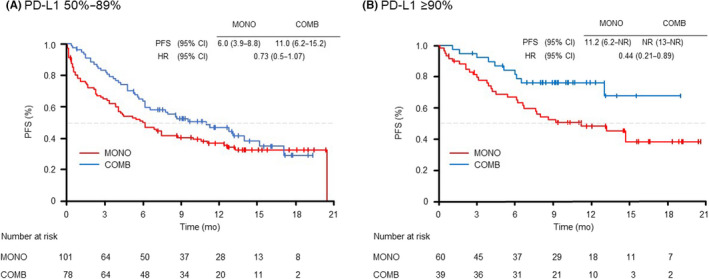
Kaplan–Meier curves of progression‐free survival of patients receiving pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB) according to a PD‐L1 tumor proportion score of (A) 50%–89%, (B) ≥90%. mo, month; PFS, progression‐free survival
The objective response rates (ORRs) were 42.2% (70/166) with MONO and 67.9% (91/134) with COMB. In addition, the progressive disease rate was 27.7% (46/166) with MONO and 11.1% (15/134) with COMB (Table 2).
TABLE 2.
Best tumor response to first‐line pembrolizumab monotherapy or pembrolizumab plus platinum‐based chemotherapy
| No. (%) | ||
|---|---|---|
| MONO | COMB | |
| (n = 166) | (n = 134) | |
| Tumor response | ||
| ORR | 70 (42.2) | 91 (67.9) |
| DCR | 109 (65.7) | 117 (87.3) |
| Best overall response | ||
| CR | 5 (3.0) | 3 (2.2) |
| PR | 65 (39.2) | 88 (65.7) |
| SD | 39 (23.5) | 26 (19.4) |
| PD | 46 (27.7) | 15 (11.1) |
| NE | 11 (6.6) | 2 (1.5) |
Abbreviations: COMB, pembrolizumab plus platinum‐based chemotherapy; CR, complete response; DCR, disease control rate; MONO, pembrolizumab monotherapy; NE, not evaluated; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
3.3. Safety and toxicity
Treatment discontinuation rates at data cutoff were 78% (129/166) in the MONO group and 63% (84/134) in the COMB group. The most frequent reason for treatment discontinuation was disease progression. Of 166 patients, 36 (21.6%) in the MONO group and 28 of 134 (20.9%) in the COMB group discontinued treatment due to AEs. The most frequently reported AE was pneumonitis, which was reported in 15 of 166 patients (9.0%) receiving MONO and 14 of 134 patients (10.4%) receiving COMB (Table 3). There were no associations between pneumonitis and poor PS. Only one patient in each of the MONO and COMB groups developed pneumonitis at PS2; the rest had PS 0–1. No treatment‐related deaths were observed in the two groups.
TABLE 3.
Treatment‐related adverse events grade ≥3 and those leading to the discontinuation of all treatment
| No. (%) | ||||
|---|---|---|---|---|
| MONO (n = 166) | COMB (n = 134) | |||
| AE | ≥Grade 3 | DISCON | ≥Grade 3 | DISCON |
| Total | 36 (21.6) | 36 (21.6) | 33 (24.6) | 28 (20.9) |
| Pneumonitis | 8 (4.9) | 15 (9.0) | 6 (4.5) | 14(10.4) |
| Rash | 3 (1.8) | 2 (1.2) | 4 (3.0) | 5 (3.7) |
| Hepatic dysfunction | 3 (1.8) | 2 (1.2) | 3 (2.2) | 3 (2.2) |
| Thromboembolic event | 3 (1.8) | 2 (1.2) | – | – |
| Adrenal insufficiency | 2 (1.2) | 1 (0.6) | 1 (0.7) | 1 (0.7) |
| Cholangitis | 2 (1.2) | 1 (0.6) | – | – |
| Enterocolitis | 2 (1.2) | – | – | – |
| Decreased neutrophil count | 1 (0.6) | 1 (0.6) | 6 (4.5) | – |
| Bronchiolitis | 1 (0.6) | 1 (0.6) | – | – |
| Fever | 1 (0.6) | 1 (0.6) | – | – |
| Kidney infection | 1 (0.6) | 1 (0.6) | – | – |
| Myelodysplastic syndrome | 1 (0.6) | 1 (0.6) | – | – |
| Nervous system disorder | 1 (0.6) | 1 (0.6) | – | – |
| Pericarditis | 1 (0.6) | 1 (0.6) | – | – |
| Pharyngeal ulcer | 1 (0.6) | 1 (0.6) | – | – |
| Uveitis | 1 (0.6) | 1 (0.6) | – | – |
| Hyponatremia | 1 (0.6) | – | 1 (0.7) | – |
| Biliary obstruction | 1 (0.6) | – | – | – |
| Polymyalgia rheumatica | 1 (0.6) | – | – | – |
| Vasculitis | 1 (0.6) | – | – | – |
| Diarrhea | – | 2 (1.2) | – | – |
| Eyelid function disorder | – | 1 (0.6) | – | – |
| Lung infection | – | 1 (0.6) | – | – |
| Intestinal perforation | – | – | 3 (2.3) | – |
| Renal dysfunction | – | – | 2 (1.5) | 3 (2.2) |
| Anemia | – | – | 2 (1.5) | – |
| Heart failure | – | – | 1 (0.7) | 1 (0.7) |
| Anorexia | – | – | 1 (0.7) | – |
| Peritonitis | – | – | 1 (0.7) | – |
| Decreased platelet count | – | – | 1 (0.7) | – |
| Thyroid gland malfunction | – | – | 1 (0.7) | – |
| Edema | – | – | – | 1 (0.7) |
Abbreviations: AE, adverse event; COMB, pembrolizumab plus platinum‐based chemotherapy; DISCON, discontinuation; MONO, pembrolizumab monotherapy.
3.4. Propensity score matching analysis
To control the unbalanced conditions at baseline between the groups, we used PSM with age and PS as adjustment factors and the 1:1 matching yielded match pairs of 84 patients in the two groups, resulting in no differences in any of the characteristics (Figure S1 and Table S1). The median PFS duration was 9.2 months (95% CI: 6.1–NR) with MONO and 13.0 months (95% CI: 9.6–17.1) with COMB (HR 0.84; 95% CI: 0.55–1.3) (Figure 5A). The median survival duration was 19.8 months (95% CI: 13.5–NR) in the MONO group and NR (95% CI: 17.3–NR) in the COMB group (HR 0.73; 95% CI: 0.42–1.3), respectively (Figure 5B).
FIGURE 5.
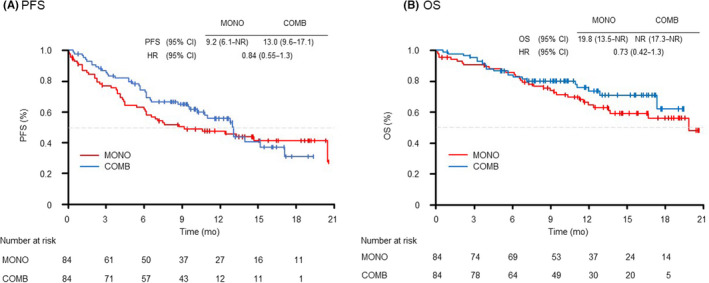
Kaplan–Meier curves of (A) progression‐free survival and (B) overall survival of patients receiving pembrolizumab monotherapy (MONO) or pembrolizumab plus platinum‐based chemotherapy (COMB) after propensity score matching. mo, month; OS, overall survival; PFS, progression‐free survival
3.5. Analyses of PFS according to various factors
Univariate and multivariate analyses using Cox proportional hazards modeling were performed to measure the correlations between various factors and PFS of all patients and selected patients after PSM. The evaluated factors were patient characteristics (age, sex, PS, smoking), tumor factors (clinical stage, pathological diagnosis, PD‐L1 status) and pembrolizumab treatment (MONO or COMB). In multivariate regression analyses, two independent factors were identified as good PFS factors: PS 0–1 and PD‐L1 ≥90% (Table 4).
TABLE 4.
Univariate and multivariate analysis of progression‐free survival
| Variable | All patients (n = 300) | Patients after PSM (n = 168) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| Age (y.o.) | <75 vs. ≥75 | 0.77 | (0.56–1.05) | 0.1 | 0.94 | (0.64–1.37) | 0.75 | 0.89 | (0.54–1.49) | 0.11 | 0.83 | (046–1.47) | 0.51 |
| Sex | Male vs. Female | 1.16 | (0.78–1.71) | 0.47 | 1.12 | (0.7–1.8) | 0.63 | 1.66 | (0.9–3.07) | 0.1 | 1.81 | (0.84–3.9) | 0.13 |
| PS | 0–1 vs. ≥2 | 0.28 | (0.2–0.4) | <0.001 | 0.26 | (0.18–0.38) | <0.001 | 0.22 | (0.13–0.38) | <0.001 | 0.22 | (0.12–0.4) | <0.001 |
| Smoking | yes vs. no | 0.88 | (0.58–1.35) | 0.56 | 0.7 | (0.41–1.2) | 0.19 | 0.72 | (0.41–1.28) | 0.27 | 0.48 | (0.22–1.03) | 0.06 |
| Stage | III/IV vs. Rec | 1.26 | (0.78–2.04) | 0.34 | 1.09 | (0.66–1.79) | 0.75 | 1.09 | (0.62–1.94) | 0.76 | 0.98 | (0.54–1.77) | 0.94 |
| Pathology | non‐SQ vs. SQ | 0.74 | (0.53–1.02) | 0.07 | 0.76 | (0.53–1.08) | 0.13 | 0.69 | (0.44–1.09) | 0.67 | 0.69 | (0.42–1.13) | 0.14 |
| PD‐L1 status | ≥90% vs 50–89% | 0.6 | (0.42–0.86) | 0.005 | 0.5 | (0.35–0.72) | <0.001 | 0.55 | (0.34–0.91) | 0.02 | 0.52 | (0.31–0.87) | 0.01 |
| Treatment | COMB vs. MONO | 0.65 | (0.47–0.9) | 0.008 | 0.83 | (0.57–1.23) | 0.35 | 0.84 | (0.55–1.29) | 0.42 | 0.72 | (0.45–1.15) | 0.17 |
Abbreviations: COMB, combination therapy; HR, hazard ratio; I, confidence interval; MONO, monotherapy; PS, performance status; PSM, propensity score matching; Rec, recurrence; S, squamous cell carcinoma; y.o., years old.
4. DISCUSSION
In this retrospective multicenter observational study for advanced NSCLC with high PD‐L1 expression, we found that COMB was associated with a longer PFS and higher ORR compared with MONO in clinical practice. The discontinuation rates due to AEs were also similar in both groups. Furthermore, using PSM, we found that the median PFS and OS of COMB showed a trend toward better than MONO, although they were not statistically significant. Based on our results, we suggest that COMB may be a promising option for first‐line treatment for advanced NSCLC with high PD‐L1 expression and good PS.
The median PFS was 7.1 months (95% CI: 5.4–11.1) in MONO and 13.1 months (95% CI: 10.2–NR) in COMB, and there was a significant difference in the PFS between the groups (HR 0.65; 95% CI: 0.47–0.89). In addition, the ORR was 41% in MONO and 67.4% in COMB. These results were comparable with those reported previously. 1 , 3 , 4 , 11 , 12 We evaluated PFS in subgroups such as age, PS, and PD‐L1 expression, which are relevant to treatment decisions in clinical practice. These previous reports mainly focused on patients with PS in the range 0–1 and aged <75 year, and, consequently, there were insufficient data to recommend treatment options for patients with PS ≥2 and aged ≥75 years. Therefore, one of our objectives in this study was to elucidate the treatment options in clinical practice for patients with PS ≥2 and aged ≥75 years. In the subgroups of PS ≥2, the PFS of patients treated with MONO at PS3 and COMB at PS2 tended to be shorter than the PFS of patients treated with MONO at PS2. Based on our results, even in patients with high PD‐L1 expression, MONO is recommended for patients with PS2 and immune checkpoint inhibitor treatment is not recommended for patients with PS3. In addition, the multivariate analysis showed that PS was the most important factor for PFS. In patients aged ≥75 years, there was no significant difference in PFS between COMB and MONO treatments. However, patients aged ≥75 years are rarely selected for COMB (n = 18) in clinical practice, and statistical validation was difficult in this study. By contrast, the number of patients selected for MONO was sufficient (n = 82), and the PFS results of MONO were recommendable for patients ≥75 years. Several studies have shown that higher PD‐L1 expression levels (<1%, 1%–49%, ≥50%) are associated with better survival when the first‐line immunotherapy was used alone and with immunotherapy plus chemotherapy. 2 , 3 , 4 Even in patients with PD‐L1 expression ≥50%, patients with an expression level of 90%–100% had a significantly higher ORR, longer PFS, and longer OS than patients with PD‐L1 expression of 50%–89%. 13 In our study, the PFS tended to be longer with COMB than with MONO in both subgroups (50%–89% and ≥90%). In particular, the COMB group exhibited significantly longer PFS than MONO in the population with PD‐L1 expression ≥90%. In the multivariate analysis, PD‐L1 expression ≥90% was identified as a good PFS factors.
In our study, the incidences of grade 3 or higher AEs, and AEs associated with treatment discontinuation were similar in the MONO and COMB groups. Compared with the findings of previous studies, the incidence of AEs associated with treatment discontinuation was higher in both COMB and MONO. 1 , 2 , 3 , 4 As patients with good PS are enrolled in clinical trials, our data may reflect the role of clinical practice in the rate of treatment discontinuation due to AEs. Of the patients included in our study, 19.3% had PS of 2–3, and were ≥80 years. These patients tended to receive MONO.
To control biases in patient characteristics, we used a PSM analysis for PFS and OS. Although there was no significant difference between MONO and COMB, the Kaplan–Meier curve for PFS and OS showed that the COMB group was superior to the MONO group. Although a longer observation period is required, the results of PFS and OS after PSM show that COMB is not inferior to MONO.
Our study had some limitations. First, this was a retrospective observational study. The bias in the selection of patients in the MONO and COMB groups by the physicians is a limitation of this study. However, we believe that our results are valuable as a survey of the current status of first‐line treatment with pembrolizumab in clinical practice to determine the treatment that should be selected according to the patient background. Second, we had a low number of patients in this cohort, especially for some subgroup analyses and the PSM evaluation. Third, it would have been helpful if all grades of AEs could have been included strictly according to CTCAE 5.0. Fourth, the observation period was short; therefore, it is necessary to extend the observation period and obtain in‐depth data on long‐term safety and OS. Therefore, we plan to have extend the next data cutoff of this study.
In conclusion, based on this real‐world cohort, we believe that COMB may be a promising option for first‐line treatment for NSCLC with high PD‐L1 expression and good PS. MONO may be used depending on a patient’s background, such as age and PS. Our results may be helpful in selecting an appropriate pembrolizumab treatment for patients with high PD‐L1 expression in clinical practice.
DISCLOSURE
The authors have no conflict of interest.
ETHICAL CONSIDERATIONS
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review boards of all participating institutions (clinical trial registration no. UMIN000040223).
Supporting information
Fig S1
Fig S2
Fig S1
ACKNOWLEDGMENTS
We thank all patients and their families, and Fumihiro Hommura (Sapporo City General Hospital), Noriaki Sukoh (National Hospital Organization Hokkaido Cancer Medical Center), Kenichiro Ito (KKR Sapporo Medical Center), Takashi Kikuchi (Iwate Prefecture Isawa Hospital), Toshihiko Agatsuma(Shinshu Ueda Medical Center), Toshiyuki Harada (JCHO Hokkaido Hospital), Yoshitsugu Narumi (Nayoro City General Hospital), Daisuke Jingu (Saka General Hospital), Kenichi Nishie (Iida Municipal Hospital), Ryota Ozawa (Japanese Red Cross Society Nagano Hospital), Kazuhiro Usui (NTT Medical Center Tokyo), Akane Kato (Ina Central Hospital), Makoto Kosaka (Nagano Prefectural Shinshu Medical Center), Taichi Takashina (Iwamizawa Municipal General Hospital), Takashige Miyahara (Nagano Matsushiro General Hospital), and Nobumitsu Kobayashi (Okaya City Hospital) for proofreading the manuscript.
Ikezawa Y, Mizugaki H, Morita R, et al. Current status of first‐line treatment with pembrolizumab for non–small‐cell lung cancer with high PD‐L1 expression. Cancer Sci. 2022;113:2109–2117. doi: 10.1111/cas.15361
REFERENCES
- 1. Reck M, Rodríguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 2. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 3. Gadgeel S, Rodríguez‐Abreu D, Speranza G, et al. Updated analysis from KEYNOTE‐189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small‐cell lung cancer. J Clin Oncol. 2020;38:1505‐1517. [DOI] [PubMed] [Google Scholar]
- 4. Paz‐Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo‐controlled trial of pembrolizumab plus chemotherapy in patient with metastatic squamous NSCLC: protocol‐specified final analysis of KEYNOTE‐407. J Thorac Oncol. 2020;15:1657‐1669. [DOI] [PubMed] [Google Scholar]
- 5. Wang C, Qiao W, Jiang Y, et al. The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non‐small‐cell lung cancer: a systematic review and meta‐analysis. J Cell Physiol. 2020;235:4913‐4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qu J, Wang L, Jiang M, et al. A review about pembrolizumab in first‐line treatment of advanced NSCLC: focus on KEYNOTE Studies. Cancer Manag Res. 2020;12:6493‐6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu J, Li C, Seery S, Yu J, Meng X. Identifying optimal first‐line interventions for advanced non‐small cell lung carcinoma according to PD‐L1 expression: a systematic review and network meta‐analysis. Oncoimmunology. 2020;9:1746112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freites‐Martinez A, Santana N, Arias‐Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE ‐ Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90‐92. [DOI] [PubMed] [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 10. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Velcheti V, Chandwani S, Chen X, Pietanza MC, Piperdi B, Burke T. Outcomes of first‐line pembrolizumab monotherapy for PD‐L1‐positive (TPS ≥50%) metastatic NSCLC at US oncology practices. Immunotherapy. 2019;11:1541‐1554. [DOI] [PubMed] [Google Scholar]
- 12. Amrane K, Geier M, Corre R, et al. First‐line pembrolizumab for non‐small cell lung cancer patients with PD‐L1 ≥ 50% in a multicenter real‐life cohort: the PEMBREIZH study. Cancer Med. 2020;9:2309‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first‐line pembrolizumab in patients with non‐small‐cell lung cancer and very high PD‐L1 expression. Ann Oncol. 2019;30:1653‐1659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S1


