Abstract
The phase II study of tirabrutinib monotherapy at a daily dose of 480 mg under fasting conditions for treatment‐naïve and relapsed/refractory Waldenström's macroglobulinemia (ONO‐4059‐05 study) demonstrated a promising efficacy and tolerable safety profile. We conducted an unplanned analysis with a median follow‐up of 24.8 months to update the efficacy and safety results and to report patient‐reported quality of life. Of 27 enrolled patients, 22 patients continued receiving the study drug. The major response assessed by an independent review committee was observed in 25 patients (93%), including one and five patients who newly achieved complete response and very good partial response, respectively, after the primary analysis. The progression‐free and overall survival rates at 24 months were 92.6% and 100%, respectively. Serum IgM levels in all patients except one declined and were maintained at low levels, although transient increases occurred after temporal interruption of the study drug. The disease‐related symptoms including recurrent fever and hyperviscosity mostly disappeared. Health‐related quality of life, assessed by cancer‐specific questionnaires, was mostly maintained. Grade 3–4 neutropenia, lymphopenia, and leukopenia were newly recognized in three, two, and one patient, respectively. Grade 3 treatment‐related hypertriglyceridemia was also recognized. Nine patients experienced grade 1–2 bleeding events (33%), one patient experienced grade 2 treatment‐related atrial fibrillation, and one patient experienced grade 1 treatment‐related hypertension. Treatment‐related skin adverse events were observed in 14 patients (52%). Taken together, tirabrutinib has durable efficacy with an acceptable safety profile for treatment‐naïve and refractory/relapsed Waldenström's macroglobulinemia.
Keywords: Bruton's tyrosine kinase inhibitor, Japanese, phase II, two‐year follow‐up, Waldenström's macroglobulinemia
Here, we present an update of the efficacy and safety results with a median follow‐up of 24.8 months in a phase II study of tirabrutinib monotherapy for treatment‐naïve and relapsed/refractory Waldenström's macroglobulinemia. The major response was observed in 25 (93%) out of 27 enrolled patients, including one complete response and eight very good partial responses. Nine patients experienced grade 1–2 bleeding events, and one patient each experienced grade 2 treatment‐related atrial fibrillation and grade 1 treatment‐related hypertension.
Abbreviations
- AE
Adverse events
- BTK
Bruton's tyrosine kinase
- CI
Confidence intervals
- CR
Complete response
- EGFR
Epidermal Growth Factor Receptor
- EQ‐5D
EuroQol 5 Dimension
- IRC
Independent review committee
- MR
Minor response
- MRR
Major response rate
- NHL
Non‐Hodgkin lymphoma
- ORR
Overall response rate
- OS
Overall survival
- PD
Progressive disease
- PFS
Progression‐free survival
- PR
Partial response
- QOL
Quality of life
- SD
Stable disease
- SPD
Sum of the products of the greatest diameters
- TMP‐SMZ
Trimethoprim‐Sulfamethoxazole
- TRAE
Treatment‐related adverse events
- VGPR
Very good partial response
- WHIM
Warts, Hypogammaglobulinemia, Infection, and Myelokathexis syndrome
- WM
Waldenström's macroglobulinemia
1. INTRODUCTION
Waldenström's macroglobulinemia (WM) is a rare, indolent B‐cell lymphoma that is characterized by an infiltration of the bone marrow and an accumulation of IgM monoclonal antibody in the serum. 1 In the 1980s, its 5‐year survival rate was 47.8%; however, it has increased to as high as 70% in the 2010s after approval of the anti‐CD20 antibody rituximab. 2
Bruton's tyrosine kinase (BTK) inhibitors have recently shown favorable efficacy for B‐cell malignancies including WM. 3 Ibrutinib is the first‐in‐class irreversible BTK inhibitor that dramatically reduced bone marrow involvement of neoplastic cells and serum IgM levels in both previously treated and treatment‐naïve patients with WM. 4 , 5 Due to its potential inhibition of off‐target kinases, 6 however, ibrutinib treatment poses a major concern of AEs including atrial fibrillation, bleeding events, and hypertension, which often lead to treatment discontinuation. 5 , 7 , 8 , 9 Tirabrutinib is a second‐generation, irreversible BTK inhibitor with high selectivity and fewer off‐target effects. 6 , 10 A phase II study (ONO‐4059‐05 study, JapicCTI‐184057) evaluated tirabrutinib monotherapy in patients with treatment‐naïve or relapsed/refractory WM, and demonstrated a promising efficacy with a MRR of 88.9% and a tolerable safety profile. 11 On the basis of these results, tirabrutinib has been approved in Japan for WM since August, 2020.
At the primary analysis of the ONO‐4059‐05 study, the median follow‐up period was relatively short at 6.5 months for treatment‐naïve patients and 8.3 months for relapsed/refractory patients, which limited the evaluation of some efficacy endpoints, such as PFS and OS, in addition to a lack of sufficient toxicity assessments. 11 Here, we analyzed the data of the ONO‐4059–05 study with a median follow‐up of 24.8 months, which was not a prespecified analysis in the study protocol. The outcome of WM‐related symptoms, such as hyperviscosity and peripheral neuropathy, and health‐related QOL of each patient as well as efficacy and safety were evaluated.
2. MATERIALS AND METHODS
2.1. Study design
The ONO‐4059‐05 study is a multicenter, open‐label, single‐arm, phase II study conducted in Japan to evaluate tirabrutinib monotherapy for WM. The study design has been described in detail previously. 11 In total, 27 patients were enrolled between 8 November 2018 and 22 February 2019; 18 patients who had not been treated previously were enrolled in Cohort A, and nine relapsed or refractory patients who received one or more systemic treatment for WM were in Cohort B. The data cutoff date for this analysis was 1 February 2021.
2.2. Patients and treatment
In brief, eligible patients had histologically confirmed WM that was treatment‐naïve (Cohort A) or relapsed/refractory (Cohort B) and had a monoclonal gammopathy with serum IgM levels of >500 mg/dl (both cohorts).
Patients received tirabrutinib orally at a daily dose of 480 mg under fasting conditions in a 28‐day cycle until disease progression or exhibiting unacceptable toxicity. Tirabrutinib administration could be interrupted due to AEs, and resumed with a reduced daily dose of 320 mg or 160 mg.
2.3. Assessments
Patient baseline characteristics were recorded upon enrollment. The L265P mutation in MYD88 was identified using real‐time polymerase chain reaction and an allele‐specific oligonucleotide, 12 and the warts, hypogammaglobulinemia, infection, and myelokathexis syndrome (WHIM)‐like mutations in CXCR4 13 , 14 were identified by a next generation sequencing system. 15
Responses were assessed according to the VIth International Workshop for Waldenström's Macroglobulinemia, 16 and classified into CR, VGPR, PR, MR, SD, and PD. The primary endpoint was the MRR assessed by the IRC; MRR refers to the proportion of patients with a best response of CR, VGPR, or PR and ORR refers to the proportion of patients with a best response of CR, VGPR, PR, or MR. Other secondary endpoints included ORR, time to response, duration of response, PFS, OS, change of serum IgM levels, and change of the sum of the products of the greatest diameters (SPD). To assess QOL, patients were periodically asked to answer the cancer‐specific QOL questionnaire (QLQ‐C30) developed by the European Organization for Research and Treatment of Cancer and using the EuroQol 5 Dimension (EQ‐5D) system.
Adverse events that emerged by 28 days after the last dose of the study drug and before the subsequent therapy were identified and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3.
2.4. Statistics
The safety was assessed in all patients who had received tirabrutinib at least once. The efficacy was assessed in patients who had the baseline serum IgM value and had one or more response scores evaluated by the IRC. The Clopper–Pearson method estimated 95% CIs for MRR and ORR. The duration of response, PFS, and OS, and their corresponding 95% CIs were analyzed using the Kaplan–Meier method. A transient increase of the serum IgM level was defined as a ≥25% and ≥500 mg/dl increase from the last measurement. 8 , 17
3. RESULTS
3.1. Patient characteristics
Patient baseline characteristics were summarized in the primary article 11 ; key baseline characteristics are shown in Table 1. The median age was 71 years, and 81% patients were male. The median serum IgM levels at baseline were 3787.5 mg/dl in Cohort A and 2105 mg/dl in Cohort B. A majority (76%) in Cohort A and all patients in Cohort B had the L265P‐mutated MYD88 and wild type CXCR4 genotype; the genotype in one patient in Cohort A was missing.
TABLE 1.
Key baseline characteristics
|
Cohort A Treatment naïve (N = 18) |
Cohort B Relapsed/refractory (N = 9) |
All (N = 27) | |
|---|---|---|---|
| Age | |||
| Median (years) | 70.5 (50–82) | 71 (60–83) | 71 (50–83) |
| <65 years | 6 (33) | 3 (33) | 9 (33) |
| 65–74 years | 5 (28) | 4 (44) | 9 (33) |
| ≥75 years | 7 (39) | 2 (22) | 9 (33) |
| Serum IgM | |||
| Median (mg/dl) | 3787.5 (1392–6340) | 2105 (730–6930) | 3600 (730–6930) |
| >4000 mg/dl | 7 (39) | 2 (22) | 9 (33) |
| Hemoglobin | |||
| Median (g/dl) | 10.45 (8.0–15.3) | 12.2 (9.1–13.9) | 10.6 (8.0–15.3) |
| ≤10 g/dl | 7 (39) | 4 (44) | 11 (41) |
| Symptoms observed in ≥10% patients | |||
| Recurrent fever, night sweats, weight loss, or fatigue | 5 (28) | 1 (11) | 6 (22) |
| Hyperviscosity | 6 (33) | 1 (11) | 7 (26) |
| Peripheral neuropathy due to WM | 2 (11) | 1 (11) | 3 (11) |
| Genotype a | |||
| MYD88 wildtype/CXCR4 WHIM | 1 (6) | 0 | 1 (4) |
| MYD88 L265P/CXCR4 wildtype | 13 (76) | 9 (100) | 22 (85) |
| MYD88 L265P/CXCR4 WHIM | 3 (18) | 0 | 3 (12) |
Data are numbers of patients (%) or median (range).
Gene mutation data were missing in one patient in Cohort A.
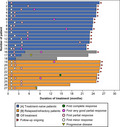
The median follow‐up period of all patients, Cohort A, and Cohort B was 24.8 months, 23.8 (range, 22.1–25.8) months, and 25.4 (range, 15.0–26.0) months, respectively. At the time of data cutoff, 15 (83%) and 7 (78%) patients in Cohorts A and B, respectively, were continued on the study drug (Figure 1). Reasons for discontinuation were AE (atypical mycobacterial infection; patient #18 in Figure 1), the physician's discretion (#17), and withdrawal of consent (#8) in Cohort A, and PD (#27) and withdrawal of consent (#26) in Cohort B. The tirabrutinib administration had been interrupted in one patient (#16) in Cohort A after 4.8‐month treatment because suppression of normal IgG and IgA level was observed and the physician considered it to be a risk of infection; this patient achieved VGPR during the interruption and was without progression.
FIGURE 1.
Duration of treatment and responses. A swimmer plot shows the duration of treatment, the first timings of responses, and the timing of progressive disease for each patient
3.2. Response and survival
The IRC‐assessed MRR was 92.6% in all patients; 94.4% in Cohort A and 88.9% in Cohort B (Table 2). In total, the number of patients who achieved a best response of CR and VGPR increased from 0 to 1 and from 3 to 8, respectively, after the time of data cutoff for the primary analysis; three patients in Cohort A newly achieved VGPR, and one and two patients in Cohort B newly achieved CR and VGPR, respectively. One patient with MR at the primary analysis in Cohort A achieved PR afterward. The patient who achieved CR had the MYD88L265P /CXCR4wildtype genotype. Major response rate (MRR) in three patients harboring the MYD88L265P /CXCR4WHIM genotype increased to 100% from 66.7% at the primary analysis. The median time to IRC‐assessed major response (CR, VGPR, and PR) was unchanged from the primary analysis: 1.9 (range, 1.0–20.3) months in Cohort A and 2.1 (range, 1.0–3.7) months in Cohort B.
TABLE 2.
IRC‐assessed responses
| Genotype | Cohort A | Cohort B | All | |||
|---|---|---|---|---|---|---|
| All (N = 18) | All (N = 9) | All (N = 27) | MYD88WT /CXCR4WHIM (N = 1) | MYD88L265P /CXCR4WT (N = 22) | MYD88L265P /CXCR4WHIM (N = 3) | |
| MRR % (95% CI) | 94.4 (72.7–99.9) | 88.9 (51.8–99.7) | 92.6 (75.7–99.1) | 100 (2.5–100) | 90.9 (70.8–98.9) | 100 (29.2–100) |
| ORR % (95% CI) | 94.4 (72.7–99.9) | 100 (66.4–100) | 96.3 (81.0–99.9) | 100 (2.5–100) | 95.5 (77.2–99.9) | 100 (29.2–100) |
| Best overall response, n [n at the primary analysis] | ||||||
| CR | 0 [0] | 1 [0] | 1 [0] | 0 [0] | 1 [0] | 0 [0] |
| VGPR | 6 [3] | 2 [0] | 8 [3] | 0 [0] | 8 [3] | 0 [0] |
| PR | 11 [13] | 5 [8] | 16 [21] | 1[1] | 11 [17] | 3 [2] |
| MR | 0 [1] | 1 [1] | 1 [2] | 0 [0] | 1 [1] | 0 [1] |
| SD | 1 [1] | 0 [0] | 1 [1] | 0 [0] | 1 [1] | 0 [0] |
| PD | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
| NE | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
| Time to PR or better months, median (range) | 1.9 (1.0–20.3) | 2.1 (1.0–3.7) | 2.1 (1.0–20.3) | 5.6 | 1.9 (1.0–5.6) | 3.9 (1.9–20.3) |
| Duration of response months, median (range) | NR (0.0+–24.8+) | NR (6.0–24.0+) | NR (0.0+–24.8+) | NR (16.6+) | NR (4.6–24.8+) | NR (0.0+–20.3+) |
Abbreviations: +, censored; CI, confidence interval; CR, complete response; IRC, independent review committee; MR, minor response; MRR, major response rate; NE, not evaluated; NR, not reached; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response; WT, wild type.
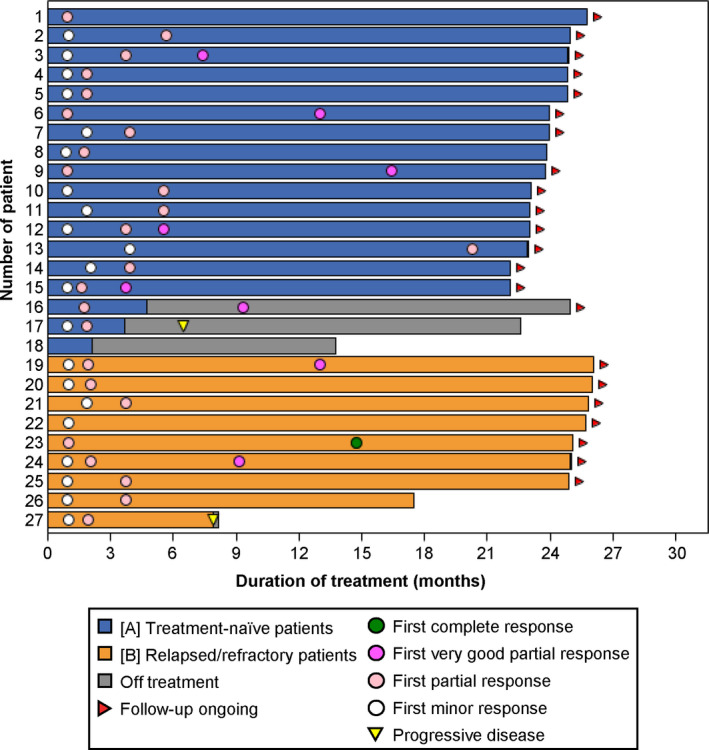
By the time of data cutoff, all patients were alive, and one patient in each cohort had PD after achieving PR (Figure 2). The two PD occurred in patients with the MYD88L265P /CXCR4wildtype genotype. The PFS rate at 24 months was 92.6% (95% CI, 73.5%–98.11%) in all patients, 94.4% (95% CI, 66.6%–99.2%) in Cohort A, and 88.9% (95% CI, 43.3%–98.4%) in Cohort B although multiple censors appeared at 22 months.
FIGURE 2.
Progression‐free (A) and overall (B) survival. Overall survival in all patients is shown
All patients, except one patient in Cohort A, achieved more than a 50% reduction in the serum IgM levels (Figure S1A); after the primary analysis, the serum IgM levels of most patients further reduced and were maintained at low levels (Figure S1B). One patient in Cohort A retained a serum IgM level comparable with that at enrollment. The serum IgM level increased upon discontinuation of the study drug in three patients. In total, six patients, four in Cohort A and two in Cohort B, experienced a transient increase (≥25% and ≥500 mg/dl increase) of the serum IgM level that occurred after temporal interruption of the study drug, predominantly due to AEs but not at any specific timing of the treatment course; the earliest one was observed at cycle 5 and the latest at cycle 25. The best response of these six patients was PR or VGPR, and no other withdrawal symptoms such as recurrent fever, night sweats, and fatigue (B‐symptoms) were concurrently recorded except for temporal reduction in hemoglobin level in one patient in Cohort B.
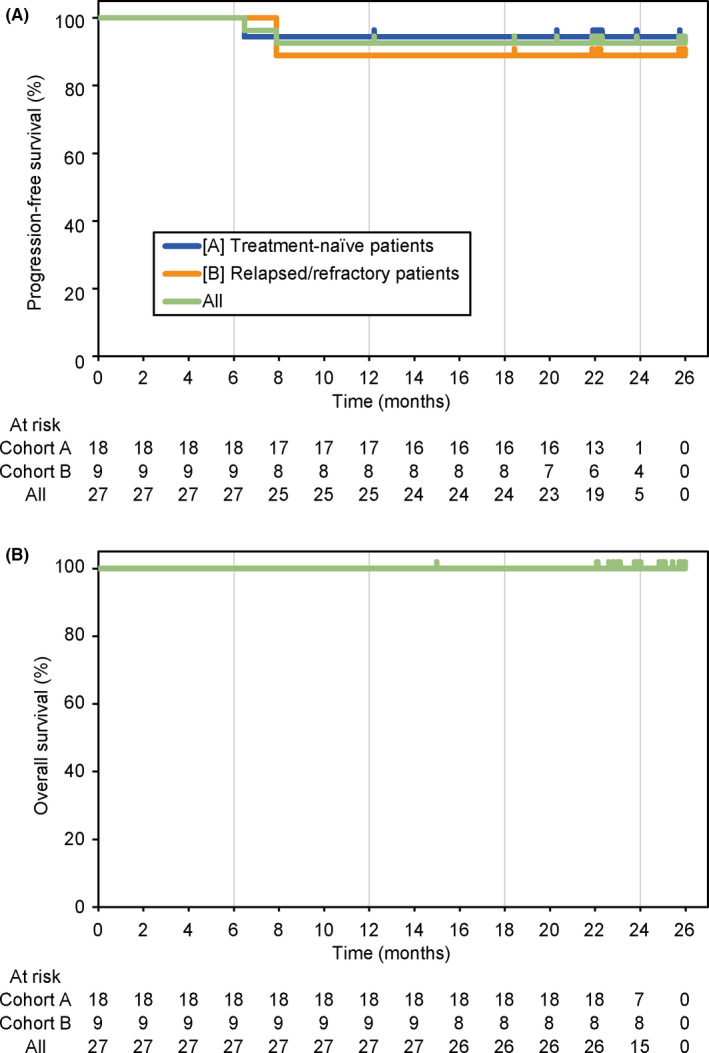
All patients who had measurable lesions achieved more than a 50% reduction in the SPD, and mostly maintained the shrunken size (Figure S2). Median hemoglobin levels were retained at the favorable levels during the study drug administration (Figure S3). Waldenström's macroglobulinemia‐related symptoms observed in ≥3 (10%) patients were B‐symptoms or fatigue; hyperviscosity; peripheral neuropathy due to WM; and anemia, hemoglobin ≤10 g/dl, which mostly resolved over time (Figure 3 and Figure S4; each symptoms).
FIGURE 3.
Patients with major symptoms. Major symptoms that were observed in ≥3 patients (recurrent fever, night sweats, weight loss, or fatigue; hyperviscosity; peripheral neuropathy due to WM; hemoglobin, ≤10 g/dl) were monitored overtime. The number of patients with any of these symptoms was set to 100%. The number of patients who had symptoms at baseline and who continued to be followed up at day 1 in each 28‐day cycle is shown
3.3. QOL
Figure S5 shows the score change in QLQ‐C30. The mean baseline score (standard deviation) was 69.1 (24.0) in the global health status, 92.6 (10.4) in the physical functioning, 90.7 (14.1) in the role functioning, 84.9 (13.3) in the emotional functioning, 82.7 (19.9) in the cognitive functioning, 93.8 (9.4) in the social functioning, 23.9 (17.4) in fatigue, 1.2 (4.4) in nausea and vomiting, 11.1 (15.3) in pain, 14.8 (23.3) in dyspnea, 9.9 (20.3) in insomnia, 9.9 (22.3) appetite loss, 12.3 (18.8) in constipation, 12.3 (22.9) in diarrhea, and 6.2 (20.7) in the financial difficulties. Overall, these scores were maintained during the study drug administration. The EQ‐5D index score and the visual analog scale (EQ‐VAS) score at baseline were 0.858 (standard deviation, 0.158) and 77.3 (standard deviation, 16.1), respectively, and were also maintained during the study drug administration (Figure S6). Figure S7 shows QOL in patient subgroups classified by the response, CR/VGPR or PR/MR/SD.
3.4. Safety
All patients exhibited any grade AEs, and 39% and 56% of the patients in Cohort A and B, respectively, exhibited grade 3–4 AEs (Table 3). The major grade 3–4 AEs were neutropenia (22%), lymphopenia (19%), and leukopenia (11%); after the time of data cutoff for the primary analysis, three, two, and one patients newly experienced grade 3–4 neutropenia, lymphopenia, and leukopenia, respectively. A grade 3 hypertriglyceridemia that was considered to be related to the study drug was newly observed in Cohort A. A grade 3 anaphylactic reaction and a grade 3 increased lipase were also newly observed, both of which were not considered to be related to the study drug. A grade 1 hypertension considered to be related with the study drug was observed in one patient in Cohort B. No grade 5 AEs were observed.
TABLE 3.
Any grade adverse events occurring in ≥2 patients and grade 3–4 adverse events
| Adverse events |
Cohort A Treatment‐naïve (N = 18) |
Cohort B Relapsed/refractory (N = 9) |
All (N = 27) | |||
|---|---|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Any | 18 (100) | 7 (39) | 9 (100) | 5 (56) | 27 (100) | 12 (44) |
| Rash | 11 (61) | 0 | 1 (11) | 0 | 12 (44) | 0 |
| Neutropenia | 3 (17) | 2 (11) | 5 (56) | 4 (44) | 8 (30) | 6 (22) |
| Nasopharyngitis | 3 (17) | 0 | 4 (44) | 0 | 7 (26) | 0 |
| Leukopenia | 2 (11) | 0 | 4 (44) | 3 (33) | 6 (22) | 3 (11) |
| Lymphopenia | 2 (11) | 2 (11) | 3 (33) | 3 (33) | 5 (19) | 5 (19) |
| Stomatitis | 3 (17) | 0 | 2 (22) | 0 | 5 (19) | 0 |
| Constipation | 3 (17) | 0 | 1 (11) | 0 | 4 (15) | 0 |
| Insomnia | 3 (17) | 0 | 1 (11) | 0 | 4 (15) | 0 |
| Pneumonia | 3 (17) | 0 | 1 (11) | 0 | 4 (15) | 0 |
| Hypertriglyceridemia | 2 (11) | 1 (6) | 1 (11) | 0 | 3 (11) | 1 (4) |
| Diarrhea | 3 (17) | 0 | 0 | 0 | 3 (11) | 0 |
| Pruritus | 3 (17) | 0 | 0 | 0 | 3 (11) | 0 |
| Rash maculopapular | 3 (17) | 0 | 0 | 0 | 3 (11) | 0 |
| Thrombocytopenia | 3 (17) | 0 | 0 | 0 | 3 (11) | 0 |
| Nausea | 2 (11) | 0 | 1 (11) | 0 | 3 (11) | 0 |
| Pyrexia | 2 (11) | 0 | 1 (11) | 0 | 3 (11) | 0 |
| Purpura | 1 (6) | 0 | 2 (22) | 0 | 3 (11) | 0 |
| Dry skin | 2 (11) | 0 | 0 | 0 | 2 (7) | 0 |
| Joint pain | 2 (11) | 0 | 0 | 0 | 2 (7) | 0 |
| Paronychia | 2 (11) | 0 | 0 | 0 | 2 (7) | 0 |
| Upper respiratory tract infection | 2 (11) | 0 | 0 | 0 | 2 (7) | 0 |
| Urinary tract infection | 2 (11) | 0 | 0 | 0 | 2 (7) | 0 |
| Atrial fibrillation | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Cataract | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Epistaxis | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Hyperkalemia | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Indigestion | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Pharyngitis | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Weight decreased | 1 (6) | 0 | 1 (11) | 0 | 2 (7) | 0 |
| Bronchitis | 0 | 0 | 2 (22) | 0 | 2 (7) | 0 |
| Contusion | 0 | 0 | 2 (22) | 0 | 2 (7) | 0 |
| Fall | 0 | 0 | 2 (22) | 0 | 2 (7) | 0 |
| Anaphylactic reaction | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Atypical mycobacterial infection | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Erythema multiforme | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Increased lipase | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Rash erythematous | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Rhegmatogenous retinal detachment | 1 (6) | 1 (6) | 0 | 0 | 1 (4) | 1 (4) |
| Type 2 diabetes | 0 | 0 | 1 (11) | 1 (11) | 1 (4) | 1 (4) |
Adverse events occurring in ≥2 patients and those with grade 3–4 are listed. Number (%) of patients is shown.
Including six patients who were reported at the primary analysis, a total of nine patients, five (28%) in Cohort A and four (44%) in Cohort B, experienced bleeding AEs, grades of which were 1–2; no grade 3 or higher bleeding events were observed (Table 4). After the time of data cutoff for the primary analysis, one patient in Cohort B who achieved PR reported grade 1 treatment‐related atrial fibrillation at day 1 of cycle 13. The administration of the study drug was interrupted in cycle 15, and resumed from cycle 16; grade 2 atrial fibrillation was observed during the study drug interruption. Another patient in Cohort B reported atrial fibrillation at the end of cycle 1 that was not considered to be related to the study drug, and continued the therapy with an achievement of VGPR. Treatment‐related AEs (TRAEs) of interest are shown in Table S1. Skin TRAEs including rash and erythema multiforme were observed in 13 (72%) and 1 (11%) patients in Cohorts A and B, respectively (Table S1). Fifteen patients in Cohort A and eight patients in Cohort B took sulfamethoxazole–trimethoprim (TMP–SMZ) as a prophylactic treatment for Pneumocystis jirovecii pneumonia, of whom 10 patients in Cohort A and one patient in Cohort B suffered skin TRAEs. Conversely, three patients in Cohort A who did not receive TMP–SMZ experienced skin TRAEs. One grade 3 erythema multiforme, and one rash erythematous were recognized in patients in Cohort A who received TMP–SMZ.
TABLE 4.
Bleeding adverse events
| Grade |
Cohort A Treatment‐naïve (N = 18) |
Cohort B Relapsed/refractory (N = 9) |
All (N = 27) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3–5 | 1 | 2 | 3–5 | 1 | 2 | 3–5 | |
| Any | 5 (28) | 0 | 0 | 3 (33) | 1 (11) | 0 | 8 (30) | 1 (4) | 0 |
| Purpura | 1 (6) | 0 | 0 | 2 (22) | 0 | 0 | 3 (11) | 0 | 0 |
| Epistaxis | 1 (6) | 0 | 0 | 1 (11) | 0 | 0 | 2 (7) | 0 | 0 |
| Contusion | 0 | 0 | 0 | 2 (22) | 0 | 0 | 2 (7) | 0 | 0 |
| Traumatic hematoma | 0 | 0 | 0 | 0 | 1 (11) | 0 | 0 | 1 (4) | 0 |
| Anal hemorrhage | 1 (6) | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 |
| Hematoma | 1 (6) | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 |
| Mouth hemorrhage | 1 (6) | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 |
| Petechiae | 1 (6) | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 | 0 |
| Conjunctival hemorrhage | 0 | 0 | 0 | 1 (11) | 0 | 0 | 1 (4) | 0 | 0 |
| Hemorrhage subcutaneous | 0 | 0 | 0 | 1 (11) | 0 | 0 | 1 (4) | 0 | 0 |
Number (%) of patients is shown.
The time of the first onset of TRAEs of interest is shown in Figure 4. Most skin disorders were developed within 1 month after the first dose, whereas the first onset of cytopenia and infection were observed throughout the observation period.
FIGURE 4.
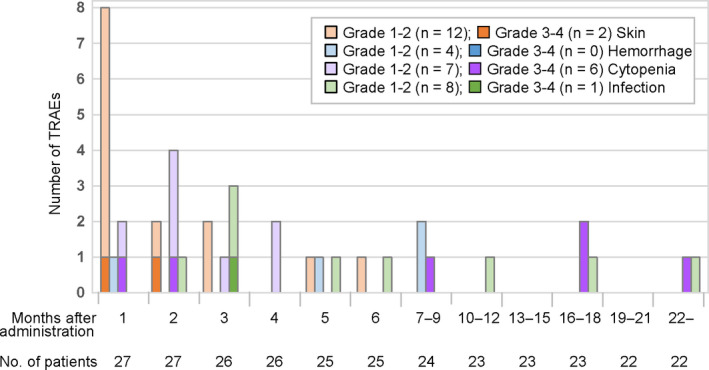
First onset of each category of treatment‐related adverse events (TRAEs). TRAEs in each category are listed in Table S1. The number of patients who continued to be followed at the first day of each month or period is shown. Note: Two patients initially exhibited grade 1–2 cytopenia and later developed grade 3–4 cytopenia
4. DISCUSSION
With a median follow‐up of 24.8 months, tirabrutinib in treatment‐naïve and refractory/relapsed WM demonstrated an improvement of response status; one, five, and one patients newly achieved CR, VGPR, and PR, respectively. Decreased serum IgM levels and shrunken lesion sizes were mostly maintained throughout the 24‐month treatment period, suggesting a durable response to tirabrutinib. Additionally, WM‐related symptoms including recurrent fever, fatigue, hyperviscosity, and peripheral neuropathy mostly resolved after the administration of tirabrutinib. Concerning the survival analysis, the 24‐month PFS and OS rate were 92.6% and 100%, respectively.
Several clinical trials of BTK inhibitors including ibrutinib, acalabrutinib, and zanubrutinib for symptomatic WM have been reported. 18 , 19 , 20 Although the direct comparison of different studies may be inappropriate due to different patient characteristics, the efficacy and safety in these studies with follow‐up periods comparable with those of the current study would be useful to interpret the difference in efficacy and safety of each BTK inhibitor in patients with WM. With a median follow‐up of 59 months, the MRR of ibrutinib monotherapy was 79.4%, and the 5‐year PFS rate was 54% in previously treated patients. 18 A second‐generation BTK inhibitor, acalabrutinib, achieved an MRR of 78.3%, and the 2‐year PFS rates in treatment‐naïve and previously treated WM were 90% and 82%, respectively. 19 Recently, the results of the phase III clinical trial comparing ibrutinib with a second‐generation BTK inhibitor, zanubrutinib, monotherapy for untreated and previously treated WM (ASPEN clinical trial) were reported. 20 The MRR of ibrutinib and zanubrutinib were not different, 78% and 77%, respectively, and the 18‐month PFS rates were 84% and 85%, respectively. Focusing efficacies in our study, the MRR was 92.6% and the 24‐month PFS rate was 92.6%, which are consistent with or even higher than those of other BTK inhibitor studies.
In the current study, six out of 27 patients experienced a transient increase of the serum IgM level due to temporal interruption of tirabrutinib. These events occurred not at any specific timing of the treatment because the timing ranged between cycles 5–25 and all of these patients were responders with PR or better. A retrospective review at the Dana‐Farber Cancer Institute reported that IgM rebounded following ibrutinib discontinuation occurred in 73% of patients with WM, mostly during the first 2 months after the discontinuation. 8 Furthermore, 114 of 189 patients with WM (60%) required temporal ibrutinib interruption during ibrutinib therapy for WM, and 22 patients (19%) developed withdrawal symptoms including transient IgM elevation, fever, body aches, and night sweats. 21 These symptoms, however, resolved rapidly following the reinsertion of ibrutinib. In the 3‐year follow‐up data of a phase I/II study of zanubrutinib, 36 of 77 patients with WM (47%) required dose holds, and 18 experienced an IgM rebound of ≥50%, most of which resolved after re‐administration of zanubrutinib. 22 The mechanisms of IgM rebound and withdrawal symptoms after transient BTK inhibitor interruption are interpreted as a hyperactive immune state similar to the cytokine‐release syndrome. The optimal strategy of interrupting BTK inhibitors should be further studied.
Compared with individuals without cancer, patients with NHL reported a greater decline in physical and mental health over 2 years. 23 Other studies similarly demonstrated that patients with NHL with active disease or receiving chemotherapy had a worse QOL. 24 , 25 Although current knowledge of QOL in patients with WM is still limited, in the ASPEN clinical trial, exploratory QOL results using EQ‐5D and QLQ–C30 scales demonstrated a trend toward improvement throughout zanubrutinib and ibrutinib treatments, especially among patients who obtained a VGPR. 20 In the present study, QOL was mostly maintained during the study drug administration. A longer observation is warranted to further assess the impact of tirabrutinib regarding QOL assessments. Recently, a global WM patient‐derived data registry capturing treatment and QOL outcomes, named the WhiMSICAL project, was launched. 26 In the preliminary results of the project using the QLQ‐C30 global scale, patients taking BTK inhibitor had higher QOL scores compared with those not receiving BTK inhibitor.
Ibrutinib is often accompanied by AEs including atrial fibrillation, bleeding events, and hypertension, leading to discontinuation of the treatment in 5%–10% of patients with WM. 5 , 7 , 8 , 9 Systematic reviews of ibrutinib studies using eight randomized controlled trials for B‐cell malignancies revealed a relative risk for atrial fibrillation and hypertension of 4.69 and 2.82, whereas patients suffering life‐threatening arrhythmias have also been reported. 27 , 28 , 29 In the ASPEN clinical trial, patients receiving zanubrutinib had a lower incidence of atrial fibrillation, bleeding AEs, and hypertension compared with those taking ibrutinib, leading to lower treatment discontinuation rate in patients on zanubrutinib treatment. 20 These results supported the idea that the second‐generation BTK inhibitor could minimize off‐target effects such as HER2 and TEC kinases and reduce toxicities related to BTK inhibitors. In the present analysis, two patients, including one reported at the primary analysis, experienced grade 1–2 atrial fibrillation. Focusing bleeding AEs and hypertension, nine patients experienced grade 1–2 bleeding AEs, and one patient had grade 1 hypertension. No new grade ≥3 TRAEs were observed except for hypertriglyceridemia. Throughout the study period, only one patient discontinued tirabrutinib administration due to an AE (atypical mycobacterial infection).
Other notable TRAEs were skin TRAEs including rash and erythema. A recent review reported that both acalabrutinib and zanubrutinib are associated with a range of dermatological AEs not different from those in ibrutinib treatment. 30 In contrast, the incidence of skin TRAEs in the present study, which appeared mostly within 1 month after the first dose, was relatively higher than those of other BTK inhibitor studies, although grade ≥3 of them were rare. Rash is usually considered to be an EGFR‐related toxicity using BTK inhibitors 31 , 32 and dermatologic AEs are relatively common in patients with EGFR inhibitors. 31 , 32 , 33 , 34 EGFR inhibition was also recognized in ibrutinib and zanubrutinib, but not in acalabrutinib and tirabrutinib according to the percentage of inhibition and IC50 values reported for BTK inhibitors. 30 In the present study, 10 of 15 patients in Cohort A and one of eight patients in Cohort B taking TMP–SMZ had skin TRAEs, whereas three patients in Cohort A without TMP–SMZ prophylaxis experienced skin TRAEs. One grade 3 erythema multiforme, and one rash erythematous were recognized in Cohort A with TMP–SMZ. In a phase I/II study of tirabrutinib for relapsed/refractory primary central nervous system lymphoma, 35 24 of 44 patients were complicated with skin AEs (54.5%). Thirty‐six patients (81.8%) received TMP–SMZ, and five of seven patients who developed grade ≥3 skin AEs received TMP–SMZ. Taken together, TMP–SMZ might affect the incidence and severity of skin TRAEs in this study. Additionally, the rate of skin AEs was obviously higher in Cohort A in the present study. Recently, Uchida and colleagues reported that non‐prior chemotherapy was a significantly high‐risk factor for skin toxicities in patients with NHL receiving bendamustine alone or with rituximab. 36 The underlying mechanism was not clarified; however, they speculated that T‐cell function was maintained in chemotherapy‐naïve patients compared with that in relapsed or refractory patients. Regarding skin AEs in tirabrutinib, further studies would be required.
Because this phase II study was conducted only in Japan with a small number of patients, our findings should be further verified in a larger patient population. Although the overall efficacy and safety of tirabrutinib for WM is apparently comparable with those of other BTK inhibitors, this single‐arm study could not make a direct comparison of BTK inhibitors. While most patients achieved favorable responses including CR, WM has generally indolent clinical progression and, therefore, a longer follow‐up observation may be required for appropriate assessments of the tirabrutinib treatment.
In summary, data presented in this 24‐month follow‐up analysis revealed that tirabrutinib has durable efficacy with an acceptable safety profile for treatment‐naïve and refractory/relapsed WM.
DISCLOSURE
N. Sekiguchi has received research funding from Ono. S. Rai has received honoraria from Chugai and Ono. W. Munakata has received research funding from Ono. H. Handa has received honoraria from Celgene, Janssen, Ono, and Takeda; and research funding from Celgene, Kyowa Kirin, and Takeda. H. Shibayama has received honoraria from AstraZeneca, Bristol‐Myers Squibb, Chugai, Eisai, Janssen, Kyowa Kirin, Mundi Pharma, Nippon Shinyaku, Novartis, Ono, Otsuka, Sanofi, SymBio, and Takeda; and research funding from AbbVie, AstraZeneca, Celgene, Chugai, Janssen, Novartis, Ono, and Sanofi. Y. Terui has received honoraria from AbbVie, Celgene, Chugai, Eisai, Janssen, MSD, Ono, and Takeda. N. Fukuhara has received honoraria from Chugai, HUYA Japan, and Kyowa Kirin; and research funding from Abbie, Bayer, Celgene, Chugai, Eisai, Gilead, Incyte, Ono, and Solasia. S. Iida is an editorial board member of Cancer Science and has received honoraria from Celgene, Janssen, Ono, Sanofi, and Takeda; and research funding from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Daiichi Sankyo, GlaxoSmithKline, Janssen, Ono, Pfizer, and Takeda; and scholarship endowments from Chugai, Ono, Sanofi, and Takeda. D. Iguchi is an employee of Ono Pharma USA, Inc. K. Izutsu has received honoraria from Eisai, Janssen, Kyowa Kirin, and Ono; and research funding from AbbVie, AstraZeneca, Bayer, Beigene, Celgene, Chugai, Daiichi Sankyo, Eisai, Genmab, Incyte, Janssen, Novartis, Ono, Pfizer, Symbio, and Yakult. The other authors have no relationships to disclose. This work was supported by Ono Pharmaceutical. The study sponsor was involved in developing the study design, providing the study drug, writing the report, and deciding submission of the article for publication. All authors had full access to all of the data in the study and had the final responsibility for the decision to submit for publication.
ETHICAL APPROVAL
The institutional review board of each site approved this trial. This study was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients who participated and their supportive families for making this study possible. We also thank the medical professionals in the study sites. We are grateful to Dr. Kazuo Tamura (General Medical Research Center, Fukuoka University, Fukuoka, Japan) and Dr. Hirokazu Nagai (Nagoya Medical Center, Nagoya, Japan) for reviewing clinical data as members of the Efficacy and Safety Monitoring Committee. A medical written support was provided by Masatoshi Esaki, PhD, of Ono Pharmaceutical, Co., Ltd., and this study was funded by Ono Pharmaceutical.
Sekiguchi N, Rai S, Munakata W, et al. Two‐year outcomes of tirabrutinib monotherapy in Waldenström’s macroglobulinemia. Cancer Sci. 2022;113:2085–2096. doi: 10.1111/cas.15344
Name of trial register: Japan Pharmaceutical Information Center
Clinical trial registration number: JapicCTI‐184057
REFERENCES
- 1. Owen RG, Treon SP, Al‐Katib A, et al. Clinicopathological definition of Waldenstrom’s Macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110‐115. [DOI] [PubMed] [Google Scholar]
- 2. Yin X, Chen L, Fan F, et al. Trends in incidence and mortality of Waldenström Macroglobulinemia: a population‐based study. Front Oncol. 2020;10:1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s Macroglobulinemia. N Engl J Med. 2015;372:1430‐1440. [DOI] [PubMed] [Google Scholar]
- 5. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment‐naïve patients with Waldenström Macroglobulinemia. J Clin Oncol. 2018;36:2755‐2761. [DOI] [PubMed] [Google Scholar]
- 6. Liclican A, Serafini L, Xing W, et al. Biochemical characterization of tirabrutinib and other irreversible inhibitors of Bruton’s tyrosine kinase reveals differences in on ‐ and off ‐ target inhibition. Biochim Biophys Acta. 2020;1864:129531. [DOI] [PubMed] [Google Scholar]
- 7. Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström’s Macroglobulinemia. N Engl J Med. 2018;378:2399‐2410. [DOI] [PubMed] [Google Scholar]
- 8. Gustine JN, Meid K, Dubeau T, et al. Ibrutinib discontinuation in Waldenström macroglobulinemia: etiologies, outcomes, and IgM rebound. Am J Hematol. 2018;93:511‐517. [DOI] [PubMed] [Google Scholar]
- 9. Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26:e233‐e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozaki R, Vogler M, Walter H, et al. Responses to the selective Bruton’s Tyrosine Kinase (BTK) inhibitor tirabrutinib (ONO/GS‐4059) in diffuse large B‐cell lymphoma cell lines. Cancers. 2018;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sekiguchi N, Rai S, Munakata W, et al. A multicenter, open‐label, phase II study of tirabrutinib (ONO/GS‐4059) in patients with Waldenström’s macroglobulinemia. Cancer Sci. 2020;111:3327‐3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiménez C, del Chillón MC, Balanzategui A, et al. Detection of MYD88 L265P mutation by real‐time allele‐specific oligonucleotide polymerase chain reaction. Appl Immunohistochem Mol Morphol. 2014;22:768‐773. [DOI] [PubMed] [Google Scholar]
- 13. Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM‐like CXCR4 mutations, and small somatic deletions associated with B‐cell lymphomagenesis. Blood. 2014;123:1637‐1646. [DOI] [PubMed] [Google Scholar]
- 14. Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood. 2014;123:2791‐2796. [DOI] [PubMed] [Google Scholar]
- 15. Rothberg JM, Hinz W, Rearick TM, et al. An integrated semiconductor device enabling non‐optical genome sequencing. Nature. 2011;475:348‐352. [DOI] [PubMed] [Google Scholar]
- 16. Owen RG, Kyle RA, Stone MJ, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171‐176. [DOI] [PubMed] [Google Scholar]
- 17. Abeykoon JP, Zanwar S, Ansell SM, et al. Ibrutinib monotherapy outside of clinical trial setting in Waldenström macroglobulinaemia: practice patterns, toxicities and outcomes. Br J Haematol. 2020;188:394‐403. [DOI] [PubMed] [Google Scholar]
- 18. Treon SP, Meid K, Gustine J, et al. Long‐term follow‐up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenström Macroglobulinemia. J Clin Oncol. 2021;39(6):565‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Owen RG, McCarthy H, Rule S, et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: a single‐arm, multicentre, phase 2 study. Lancet Haematol. 2020;7:e112‐e121. [DOI] [PubMed] [Google Scholar]
- 20. Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: the ASPEN study. Blood. 2020;136:2038‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castillo JJ, Gustine JN, Meid K, Dubeau T, Severns P, Treon SP. Ibrutinib withdrawal symptoms in patients with Waldenström macroglobulinemia. Haematologica. 2018;103:e307‐e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trotman J, Opat S, Gottlieb D, et al. Zanubrutinib for the treatment of patients with Waldenström macroglobulinemia: 3 years of follow‐up. Blood. 2020;136:2027‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reeve BB, Potosky AL, Smith AW, et al. Impact of cancer on health‐related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SK, Zimmerman S, Williams CS, Zebrack BJ. Health status and quality of life among non‐hodgkin lymphoma survivors. Cancer. 2009;115:3312‐3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mols F, Aaronson NK, Vingerhoets AJJM, et al. Quality of life among long‐term non‐hodgkin lymphoma survivors: a population‐based study. Cancer. 2007;109(8):1659‐1667. [DOI] [PubMed] [Google Scholar]
- 26. Tohidi‐Esfahani I, Warden A, Malunis E, et al. WhiMSICAL: a global Waldenström’s Macroglobulinemia patient‐derived data registry capturing treatment and quality of life outcomes. Am J Hematol. 2021;96:E218‐E222. [DOI] [PubMed] [Google Scholar]
- 27. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta‐analysis. PLoS One. 2019;14:e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lampson BL, Yu L, Glynn RJ, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581‐2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ntanasis‐Stathopoulos I, Gavriatopoulou M, Fotiou D, Dimopoulos MA. Current and novel BTK inhibitors in Waldenström’s macroglobulinemia. Ther Adv Hematol. 2021;12:2040620721989586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Estupiñán HY, Berglöf A, Zain R, Smith CIE. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front Cell Dev Biol. 2021;9:630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lucchini E, Pilotto S, Spada E, Melisi D, Bria E, Tortora G. Targeting the epidermal growth factor receptor in solid tumors: focus on safety. Expert Opin Drug Saf. 2014;13:535‐549. [DOI] [PubMed] [Google Scholar]
- 32. Kozuki T. Skin problems and EGFR‐tyrosine kinase inhibitor. Jpn J Clin Oncol. 2016;46:291‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Ran YG, Wang KJ. Risk of severe rash in cancer patients treated with EGFR tyrosine kinase inhibitors: a systematic review and meta‐analysis. Future Oncol. 2016;12:2741‐2753. [DOI] [PubMed] [Google Scholar]
- 34. Hsu WH, Yang JCH, Mok TS, Loong HH. Overview of current systemic management of EGFR‐mutant NSCLC. Ann Oncol. 2018;29(Supplement 1):i3‐i9. [DOI] [PubMed] [Google Scholar]
- 35. Narita Y, Nagane M, Mishima K, et al. Phase I/II study of tirabrutinib, a second‐generation Bruton’s tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021;23:122‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchida M, Mori Y, Akiba K, et al. Risk factors for skin toxicities associated with bendamustine‐based chemotherapy in patients with non‐hodgkin lymphoma. Biol Pharm Bull. 2020;43:1577‐1582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


