Abstract
Gases released from anaerobic wastewater treatment facilities contain considerable amounts of volatile methyl and hydride derivatives of metals and metalloids, such as arsine (AsH3), monomethylarsine, dimethylarsine, trimethylarsine, trimethylbismuth (TMBi), elemental mercury (Hg0), trimethylstibine, dimethyltellurium, and tetramethyltin. Most of these compounds could be shown to be produced by pure cultures of microorganisms which are representatives of the anaerobic sewage sludge microflora, i.e., methanogenic archaea (Methanobacterium formicicum, Methanosarcina barkeri, Methanobacterium thermoautotrophicum), sulfate-reducing bacteria (Desulfovibrio vulgaris, D. gigas), and a peptolytic bacterium (Clostridium collagenovorans). Additionally, dimethylselenium and dimethyldiselenium could be detected in the headspace of most of the pure cultures. This is the first report of the production of TMBi, stibine, monomethylstibine, and dimethylstibine by a pure culture of M. formicicum.
Volatile methyl and hydride derivatives of metal(loid)s are found in gases released from natural environments such as sediments, wetlands (40), and hydrothermal springs (26), as well as from anthropogenic environments such as wastewater treatment plants and waste deposits (18). Some of the compounds are of anthropogenic origin, for example, alkylated tin and lead derivatives, whereas others are produced in environmental settings either due to chemical transalkylation or due to biologically mediated methylation and hydride formation (the addition of a formal hydride ion mediated by an organism) (11, 36). The production of volatile metal(loid) compounds is a significant part of the biogeochemical cycles of metals (e.g., Bi, Hg, and Sn) and metalloids (e.g., As, Sb, Se, and Te) because of the increased mobility of the resultant compounds. Additionally, most of the volatile derivatives exhibit higher toxicity than their inorganic counterparts, since organic derivatives are lipophilic and are thus more biologically active (35, 36). Exceptions are arsenic and selenium, in which cases the inorganic, nonmethylated forms are more toxic than the methylated derivatives (14).
Following the pioneering work of Gosio (23) and Challenger et al. (6, 7), the mechanism for the biomethylation of arsenic has been studied extensively with various fungi (10, 15), bacteria (3), archaea (3, 30), and mammals (1, 38, 41), including humans (13). As proposed by Challenger, the biomethylation of selenium and tellurium follows the same mechanism as arsenic but, in general, studies investigating these processes are rather rare (16, 22, 38). Mercury biomethylation, on the other hand, has been studied in more detail because of poisonings by methylmercury compounds (4, 8, 29, 33). Although stibine has been implicated as a cause in sudden infant death syndrome (31), up to now, only trimethylantimony was detected as a biovolatilization product of antimony in the headspace of soil samples (24) and of pure cultures of Scopulariopsis brevicaulis (12, 27). The biomethylation of inorganic tin has been reported (2, 25), although research on organotin compounds has focused mainly on the fate of organotin species, which are used as biocide additives. These compounds are leached out in aquatic environments and undergo dealkylation and methylation (9, 17). Trimethylbismuth (TMBi) has been found in gases released from municipal waste deposits and sewage gases (18, 20, 21), but the origin of this compound is unclear.
The present study is a further step toward an ecological evaluation of the process of biomethylation within a specific environmental scenario. The production of volatile metal(loid) compounds by the microflora adapted to the habitat of the anaerobic digestion of sewage sludge was investigated to estimate not only the ecological risk by the release of the mostly toxic compounds evolved during anaerobic sludge stabilization and other anaerobic processes established in waste management but also to provide a basis for the development of ecologically friendly practices. Therefore, we investigated the anaerobic bioconversion of metal(loid)s to their volatile derivatives in surplus sludge from a municipal wastewater treatment plant. Additionally, several microorganisms of different physiological groups, which are known representatives of the sewage sludge microflora (i.e., methanogenic archaea, including Methanobacterium formicicum, Methanosarcina barkeri, Methanobacterium thermoautotrophicum; sulfate-reducing bacteria, including Desulfovibrio vulgaris and D. gigas; and a peptolytic bacterium, Clostridium collagenovorans), were tested in pure cultures with respect to their ability to synthesize volatile metal(loid) compounds as a means to estimate their possible involvement in these processes during anaerobic sludge stabilization.
MATERIALS AND METHODS
Strains and culture media.
M. formicicum DSMZ 1535T, M. thermoautotrophicum DSMZ 1053T, and M. barkeri DSMZ 800 cultures were grown in medium DSMZ 120 (Deutsche Sammlung von Mikroorgnismen und Zellkulturen GmbH, Braunschweig, Germany), modified by the addition of 1 g of sodium acetate, 2 g of sodium formate, and 0.5 g each of valeric acid, isovaleric acid, 1-methylbutyric acid, and isobutyric acid per liter and lacking methanol and Na2S. The cultures were pressurized with CO2-H2 (200 kPa, 20:80% [vol/vol]).
C. collagenovorans DSMZ 3089T, D. gigas DSMZ 496, and D. vulgaris subsp. vulgaris DSMZ 644T were grown as recommended by the supplier. The various organisms were cultured under strictly anaerobic conditions in stoppered bottles, and the culture media (50 ml in 120-ml serum bottles) were reduced by either the addition of titanium citrate (ca. 1 to 2 mM, final concentration) (42) or the addition of l-cysteine (0.3 to 0.5 g liter−1). The cultures were grown in the dark to the early-exponential-growth phase in a rotary shaker (150 rpm) at 37°C for all of the cultures except M. thermoautotrophicum, which was grown at 60°C.
The sewage sludge samples (2% [wt/vol] dry matter, 0.8% [wt/vol] organic dry matter) originated from an anaerobic surplus stabilization tank of a municipal wastewater treatment plant from the Ruhrgebiet area in Germany, which is fed mainly with the domestic wastewater of approximately 70,000 inhabitants. The surplus stabilization tank is charged with primary sludge twice daily and is operated with a hydraulic retention time of 10 days. The sludge samples were used directly following collection.
Analytical methods.
The metal and metalloid content of the sewage sludge was determined by X-ray fluorescence (XRF) analysis with a Spectro XLAB 2000 as described elsewhere (34). Volatile metal(loid) compounds in the headspace of the samples were analyzed, as described previously (39), using a modified purge-and-trap gas chromatographic system coupled to an inductively coupled plasma mass spectrometer (ICP-MS; Fisons VG PlasmaQuad II) as an element specific detector. The volatile metal(loid) compounds were identified by boiling point retention time correlation (R = 0.998) and by comparison with the retention times of standards of trimethylarsines (TMAs) and TMBi, as well as those of arsine (AsH3), monomethylarsine (MMAs), and dimethylarsine (DMAs) produced by reaction of sodium borohydride with inorganic arsenic, monomethylarsenous acid, and cacodylic acid, respectively (40). The different volatile species were detected by mass/charge ratios of the respective metal(loid)s (m/z 75, As; m/z 78, Se; m/z 120, Sn; m/z 121, Sb; m/z 126, Te; m/z 202, Hg; m/z 209, Bi). Quantification was performed by external calibration to the signal from a 1-μg/liter Rh standard solution in 1% ultrapure nitric acid (Merck, Darmstadt, Germany) (19).
Assays for production of volatile derivatives of metal(loid)s.
If not otherwise indicated, pure cultures of methanogenic archaea, sulfate-reducing bacteria, and C. collagenovorans were spiked in the exponential growth phase with inorganic salts of the respective metal(loid)s and incubated 5 days, 2 days, or overnight, respectively, prior to analysis with an ICP-MS. Arsenic was spiked as KH2AsO4 (50 μM to 1 mM), Sb as SbCl3 (5 μM to 1 mM), Hg as HgCl2 (2 to 6 μM), Bi as Bi(NO3)3 (20 to 100 μM), Te as TeO2 (100 μM), and Se as SeO2 (100 μM). Antimony and tellurium salts were solubilized in ethanol. Control samples were prepared by using spiked sterile media of each organism without inoculation. Sludge samples (50 ml in 120-ml serum bottles) were pressurized with CO2-H2 (200 kPa, 20:80% [vol/vol]) and incubated anaerobically in stoppered bottles in a rotary shaker (150 rpm) at 37°C in the dark for 1 week prior to analysis of volatile derivatives of metal(loid)s. Control samples of the sewage sludge were autoclaved (30 min, 121°C) and incubated as described above.
Reagents.
The chemicals and solutions of the inorganic metals and metalloids used for ICP-MS analysis were of certified high-purity grade; water used for ICP-MS analysis was prepared with a PRO 90 CN (Seral, Ransbach-Baumbach, Germany). All other chemicals employed in the study were analytical reagent grade or better.
RESULTS
Production of volatile metal(loid)s in sewage sludge.
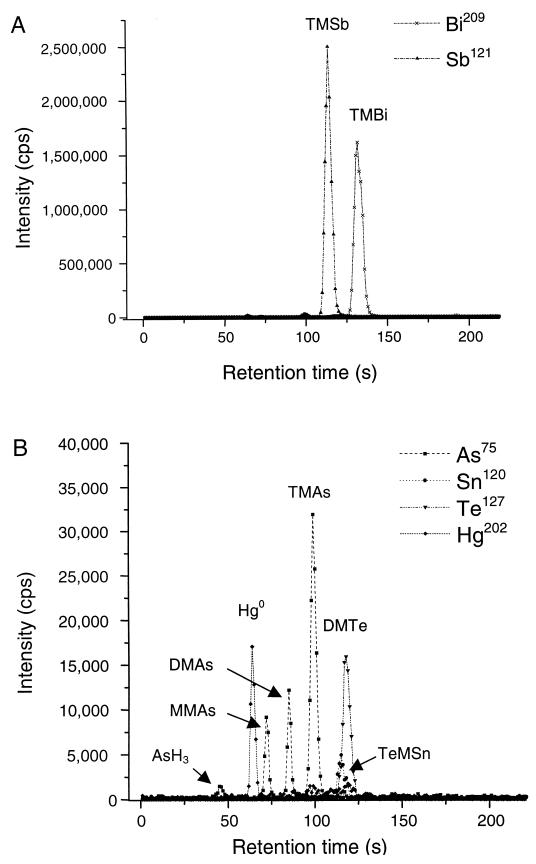
The sewage sludge samples contained high levels of metal(loid)s which are susceptible to biomethylation (Table 1). Following anaerobic incubation of the sewage sludge, several volatile organometal(loid) compounds, namely, tetramethyltin (TrMSn), elemental mercury, AsH3, MMAs, DMAs, TMAs, dimethyltellurium (DMTe), TMBi, and trimethylstibine (TMSb), could be detected in the headspace gas of the samples by the coupling of chromatographic separation and element-specific detection by ICP-MS analysis (Fig. 1). TMBi and TMSb were found as the dominant volatile metal(loid) species, with concentrations of up to 80 ng per liter, although the respective elements were present in the sewage sludge only at rather low concentrations (Table 1). No volatile metal(loid)s were detectable in autoclaved control samples which were purged extensively with CO2-H2 (20:80% [vol/vol]) to remove dissolved volatile products from the samples prior to incubation, suggesting that the production of the volatile metal(loid)s in the nonautoclaved samples was biologically mediated.
TABLE 1.
Metal(loid) content of sewage sludge determined by XRF analysis (only elements which are of interest for this study are listed) and volatile metal(loid)s detected in the headspace of the incubated sewage sludge samples after 1 week at 37°C
| Metal(loid) | Content (mg/kg [dry wt])a | Volatile species | Content (ng/liter)b |
|---|---|---|---|
| Te | 0.2 ± 0.1 | DMTe | 1.9 ± 0.15 |
| Bi | 1.4 ± 0.3 | TMBi | 46.2 ± 6.9 |
| Se | 1.5 ± 0.5 | ||
| Sb | 1.7 ± 0.3 | TMSb | 77.5 ± 4.6 |
| Hg | 3.0 ± 1.2 | Hg0 | 0.29 ± 0.16 |
| As | 15.2 ± 1.3 | AsH3 | 0.76 ± 0.21 |
| MMAs | 0.68 ± 0.45 | ||
| DMAs | 0.51 ± 0.36 | ||
| TMAs | 3.3 ± 1.3 | ||
| Sn | 25.4 ± 0.3 | TrMSn | 0.29 ± 0.18 |
Mean values ± absolute errors are listed.
Mean values of three independent measurements ± standard deviations are listed.
FIG. 1.
Chromatogram of the purge-and-trap gas chromatographic separation of the volatile metal(loid) compounds in the headspace of incubated sewage sludge samples after 1 week at 37°C. The compounds were identified by boiling-point retention time correlation and by the mass charge ratio (m/z) of the respective metal(loid) determined by ICP-MS analysis. (A) Recording for Sb and Bi. (B) Chromatogram of the same sample but at a 50-fold-higher sensitivity recording for As, Se, Sn, Te, and Hg. The recordings of Sb and Bi were omitted for clarity. cps, counts per second.
Production of volatile metal(loid)s by pure cultures.
In order to establish the biogenic origin of the volatile metal(loid) compounds observed in the headspace gas above incubated sewage sludge samples, the production of volatile derivatives of arsenic, antimony, bismuth, mercury, selenium, tellurium, and tin was studied with pure cultures of anaerobic microorganisms which are representatives of the sewage microflora.
The variety and the amounts of volatile metal(loid) compounds of arsenic, antimony, bismuth, selenium, and tellurium produced by the different pure cultures are shown in Tables 2 and 3 as mean values of at least three replicate experiments. None of the strains tested were able to produce volatile tin or methylated mercury species. No volatile metal(loid) compounds were detected in sterile control samples. Only the reduction of mercury(II) to elemental mercury was found in all cultures and also in all sterile controls, suggesting that mercury(II) is reduced chemically by the anaerobic media (not shown).
TABLE 2.
Amounts of volatile arsenic and antimony compounds produced by pure cultures of various strains after 1 week of incubation at 37°C in the dark
| Metal(loid) | Concn (mM) | Volatile speciesb | Amt (ng)a produced by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| M. formicicum | M. barkeri | M. thermoautotrophicum | C. collagenovorans | D. vulgaris | D. gigas | |||
| As | 0 | AsH3 | 0.06 ± 0.04 | ND | ND | ND | ND | ND |
| MMAs | 0.06 ± 0.05 | ND | ND | ND | ND | ND | ||
| DMAs | 0.02 ± 0.001 | ND | ND | ND | ND | ND | ||
| TMAs | 0.002 ± 0.001 | ND | ND | ND | 0.002 ± 0.001 | 0.004 ± 0.002 | ||
| X | ND | ND | ND | ND | ND | ND | ||
| 0.05 | AsH3 | 1.13 ± 0.8 | 1.0 ± 0.63 | ND | ND | ND | 0.009 ± 0.002 | |
| MMAs | 8.2 ± 4.3 | ND | ND | ND | ND | ND | ||
| DMAs | 1.5 ± 0.7 | ND | ND | ND | ND | ND | ||
| TMAs | 0.5 ± 0.2 | ND | ND | 0.03 ± 0.01 | 0.01 ± 0.008 | 0.005 ± 0.003 | ||
| X | 6.4 ± 4.3 | 0.13 ± 0.08 | ND | ND | ND | ND | ||
| 0.1 | AsH3 | 6.3 ± 3.5 | 0.2 ± 0.06 | 0.073 ± 0.05 | ND | ND | ND | |
| MMAs | 14.3 ± 6.2 | ND | ND | ND | ND | ND | ||
| DMAs | 4.4 ± 3.2 | ND | ND | ND | ND | ND | ||
| TMAs | 7.2 ± 5.3 | ND | ND | 2.7 ± 1.7 | 0.004 ± 0.003 | 0.003 ± 0.002 | ||
| X | 9.4 ± 8.3 | 0.4 ± 0.3 | ND | ND | ND | ND | ||
| 0.3 | AsH3 | 9.6 ± 6.5 | 0.12 ± 0.09 | 10.4 ± 1.3 | ND | ND | ND | |
| MMAs | 9.8 ± 6.9 | ND | ND | ND | ND | ND | ||
| DMAs | 6.9 ± 4.8 | ND | ND | ND | ND | ND | ||
| TMAs | 6.9 ± 4.5 | ND | ND | 0.08 ± 0.05 | 0.001 ± 0.0006 | 0.002 ± 0.001 | ||
| X | 9.7 ± 7.4 | ND | ND | ND | ND | ND | ||
| 0.5 | AsH3 | 1.4 ± 0.6 | ND | 12.6 ± 3 | ND | ND | ND | |
| MMAs | ND | ND | ND | ND | ND | ND | ||
| DMAs | ND | ND | ND | ND | ND | ND | ||
| TMAs | ND | ND | ND | 0.04 ± 0.02 | ND | ND | ||
| X | ND | ND | ND | ND | ND | ND | ||
| Sb | 0.005 | SbH3 | 17.3 ± 10.6 | ND | ND | ND | ND | ND |
| MMSb | 1.7 ± 0.3 | ND | ND | ND | ND | ND | ||
| DMSb | 1.9 ± 0.5 | ND | ND | ND | ND | ND | ||
| TMSb | 16.1 ± 9.2 | ND | ND | ND | 0.06 ± 0.04 | ND | ||
| 0.05 | SbH3 | ND | ND | ND | ND | ND | ND | |
| MMSb | ND | ND | ND | ND | ND | ND | ||
| DMSb | ND | ND | ND | ND | ND | ND | ||
| TMSb | 8.4 ± 6.8 | ND | ND | 0.18 ± 0.07 | 0.22 ± 0.09 | ND | ||
| 0.1 | SbH3 | ND | ND | ND | ND | ND | ND | |
| MMSb | ND | ND | ND | ND | ND | ND | ||
| DMSb | ND | ND | ND | ND | ND | ND | ||
| TMSb | 7.2 ± 6.3 | ND | 0.06 ± 0.04 | 0.2 ± 0.07 | 0.08 ± 0.05 | ND | ||
| 0.3 | SbH3 | ND | ND | ND | ND | ND | ND | |
| MMSb | ND | ND | ND | ND | ND | ND | ||
| DMSb | ND | ND | ND | ND | ND | ND | ||
| TMSb | 9.5 ± 6.4 | 0.002 ± 0.001 | 0.2 ± 0.1 | 0.16 ± 0.08 | 0.07 ± 0.05 | ND | ||
| 0.5 | SbH3 | ND | ND | ND | ND | ND | ND | |
| MMSb | ND | ND | ND | ND | ND | ND | ||
| DMSb | ND | ND | ND | ND | ND | ND | ||
| TMSb | 8.9 ± 7.3 | 0.002 ± 0.001 | 0.05 ± 0.03 | ND | ND | ND | ||
The data shown are the mean values ± the standard deviations from at least three replicates. The amounts of the volatile species in the gas phase (70 ml) are given as absolute mass values of the respective metal(loid) in nanograms. ND, not detected.
X, unidentified arsenic-containing compound.
TABLE 3.
Amounts of volatile bismuth, selenium, and tellurium compounds produced by pure cultures of various strains after 1 week of incubation at 37°C in the dark
| Metal(loid) | Concn (mM) | Volatile species | Amt (ng)a produced by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| M. formicicum | M. barkeri | M. thermoautotrophicum | C. collagenovorans | D. vulgaris | D. gigas | |||
| Bi | 0.02 | TMBi | 7.6 ± 3.2 | ND | ND | 0.02 ± 0.01 | ND | ND |
| 0.1 | TMBi | 2.7 ± 1.5 | ND | ND | ND | ND | ND | |
| Se | 0.1 | DMSe | 4.1 ± 3.4 | 19.1 ± 6.7 | ND | 1.8 ± 0.6 | 0.026 ± 0.02 | 1.1 ± 0.8 |
| DMDSe | 22.6 ± 7.2 | 10 ± 4.3 | ND | 11.4 ± 6.2 | 2.6 ± 1.5 | 10.3 ± 4.7 | ||
| Te | 0.1 | DMTe | 0.23 ± 0.1 | ND | ND | 0.68 ± 0.37 | ND | 0.1 ± 0.04 |
The data shown are the mean values from at least three replicates. The amounts of the volatile species in the gas phase (70 ml) are given as absolute mass values of the respective metal(loid) in nanograms. ND, not detected.
Production of volatile arsenic compounds.
In general, the highest amounts of volatilized arsenic derivatives were detected at spiked inorganic arsenic concentrations between 0.1 and 0.3 mM (Table 2), whereas no volatile arsenic compounds could be detected in any of the cultures at 1.0 mM spiked arsenate (not shown). The greatest variety and the highest amounts of volatile arsenic derivatives were detected in the headspace cultures of M. formicicum cultures (Table 2). The production of AsH3, MMAs, DMAs, and TMAs by M. formicicum was already apparent from trace levels of arsenic in the medium, prior to spiking, suggesting that the process is facile even at low concentration levels. Cultures of M. formicicum spiked with 0.05 to 0.3 mM arsenate also produced an unidentified volatile arsenic-containing compound; only arsine was detected when the culture was spiked with 0.5 mM arsenate. Compared to M. formicicum, all other organisms produced fewer volatile arsenic species and typically in smaller amounts: M. barkeri formed arsine and small amounts of the unidentified arsenic-containing compound, whereas M. thermoautotrophicum produced only arsine. The sulfate-reducing bacteria and C. collagenovorans formed only TMAs, and small amounts of arsine were detected in cultures of D. gigas.
Production of volatile antimony compounds.
The highest production of the volatile antimony compounds was found at antimony chloride concentrations between 0.005 and 0.1 mM for M. formicicum, C. collagenovorans, and D. vulgaris, whereas the highest amounts of TMSb produced by M. barkeri and M. thermoautotrophicum were detected at concentrations of antimony chloride between 0.3 and 0.5 mM. No volatile antimony compounds were detected in any of the cultures at 1 mM antimony chloride (not shown). In most cultures of the organisms tested, TMSb was detected as the only volatile antimony compound, and no volatilization product of antimony was detected in cultures of D. gigas (Table 2). In three of five cultures of M. formicicum spiked with 5 μM antimony chloride, stibine, mono-, di-, and trimethylstibine were detected in amounts up to 18, 0.2, 0.4, and 16 ng, respectively. However, the volatile antimony hydrides were observed only in cultures of M. formicicum which were spiked in the stationary phase and incubated for a shorter time period (15 h); after incubation for 5 days, TMSb was the only volatile antimony compound detected. In comparison with the other organisms, M. formicicum, produced as much as 3 orders of magnitude more TMSb.
Production of volatile bismuth compounds.
The biogenic production of TMBi by pure cultures was demonstrated for the first time in this study. Of the cultures tested, TMBi was synthesized only by M. formicicum, which produced up to 8 ng of TMBi, and C. collagenovorans, which produced TMBi at lower levels (0.01 ng) from an initial solution concentration of 20 μM Bi (Table 3). Following spiking with 100 μM Bi, the production of TMBi was detected only in cultures of M. formicicum but in decreased amounts (2.7 ng). No other volatile bismuth compounds were detected in any other culture.
Production of volatile selenium compounds.
Of the elements investigated in this study, the organisms produced the highest amounts of volatile compounds from selenium. In all cultures, with the exception of M. thermoautotrophicum, DMSe and DMDSe were found as volatilization products from inorganic selenium in amounts as high as 35 ng of DMSe and 40 ng of DMDSe from solution concentrations of 0.1 mM Se (Table 3).
Production of volatile tellurium compounds.
Tellurium was converted to DMTe in cultures of M. formicicum, C. collagenovorans, and D. gigas (Table 3) but only in amounts below the range of 0.1 to 0.7 ng.
In general, the amounts of volatile metal(loid) derivatives detected in the gas phase above the cultures ranged from 0.001 to 50 ng as the absolute mass of the respective metal(loid), depending on the organism tested and on the concentration of metal(loid) added.
DISCUSSION
The diversity of the volatile organometal(loid) compounds produced by microorganisms under anaerobic conditions and the variation in the production of such compounds among organisms, as observed in this study, has implications for the risks associated with the release of toxic gases from anaerobic digestion of sewage sludge.
The headspace of anaerobic incubated sewage sludge samples contained volatile methyl and hydride derivatives of antimony, arsenic, bismuth, tellurium, and tin which are very similar in composition to the gas released from the sewage sludge treatment plant, indicating that the experimental conditions correspond largely to those found in practice. Surprisingly, the content of volatile metal(loid) derivatives in the headspace of the incubated sludge samples does not correlate with the concentrations of the respective metal(loid)s in the sewage sludge. In the cases of antimony and bismuth, the respective volatile compounds TMSb and TMBi are found in high concentrations in the sewage gas, whereas the content of the corresponding elements in the sewage sludge is rather low compared to the content of other elements such as tin and arsenic. A possible explanation is that these metal(loid)s are more susceptible to volatilization or that their uptake by microbial cells is more facile.
With the exception of TrMSn, all of the volatile metal(loid) derivatives found in sewage gas could be detected in the headspace of pure cultures of strains which typically occur in the habitat of sewage sludge that is being anaerobically digested, suggesting that these organisms are among those responsible for the production of volatile metal(loid) derivatives in the more complex habitat. On the other hand, some variations are apparent which suggest differences between the compounds involved in the metabolism of the pure cultures and those present in the sewage sludge: the diversity of the volatile metal(loid) compounds in pure cultures is higher than in the sludge treatment process, and most of the pure cultures produced the volatile compounds dimethylselenium (DMSe) and dimethyldiselenium (DMDSe), which were absent in sewage gas. Additionally, the volatile stibines (stibine [SbH3], monomethylstibine [MMSb], and dimethylstibine [DMSb]) synthesized by pure cultures of M. formicicum and an unidentified volatile compound produced by M. formicicum and M. barkeri, which was established in this study as a volatile arsenic-containing species, were not detectable in sewage gas. The lack of these compounds in the sewage gas may be due to unfavorable reaction conditions in the sample matrix of the sewage sludge, as well as to possible interfering trans- and demethylation reactions in this heterogeneous milieu.
The biosynthetic capacity of the organisms tested here varies significantly with respect to their ability to synthesize volatile metal(loid) species. Even among closely related strains, considerable variations could be observed: M. formicicum, which proved to be the most effective organism in producing volatile metal(loid) derivatives, differs significantly from the other methanogens with regard to the quantity and diversity of the volatile metal(loid) derivatives produced. In addition to several methylated and hydride derivatives of other metal(loid)s, this organism synthesizes TMBi and volatile stibines, which have not been previously reported as resulting from biological activity. Also, the two closely related Desulfovibrio strains included in this study, D. gigas and D. vulgaris, differ in their abilities to produce volatile metal(loid) species with respect to the quantities and diversity of the compounds produced, and although both species are close relatives of D. desulfuricans, they are unable to produce DMHg, which, however, is reported to be synthesized by the latter (5). These observations indicate that related organisms do not necessarily exhibit similar conversion patterns.
At the moment, knowledge regarding the parameters governing the production of volatile metal(loid) derivatives is scarce. As shown in this study, the capability of biomethylation and biohydride formation not only depends on external conditions [e.g., the concentration of the respective metal(loid)] but also depends on the physiological state of the organism. For example, production of SbH3, MMSb, and DMSb by M. formicicum could only be observed in the stationary phase. For biomethylation, methylcobalamine and S-adenosylmethionine have been shown to be the methyl donors (32); however, it is clear that the presence of these cofactors does not represent the only prerequisite for methylation of metal(loid)s, since the methylation capacity of M. formicicum is significantly higher than that of M. thermoautotrophicum and M. barkeri, although the intracellular methylcobalamine concentration of M. formicicum is virtually the same as that found in M. thermoautotrophicum and four times lower than that found in M. barkeri (28). Finally, to our knowledge, nothing is known about the biochemical reactions responsible for the formation of volatile metal(loid) hydrides and the cofactors involved. Thus, despite the great ecological importance of these bioconversions, the molecular processes responsible for the volatilization of metal(loid)s are largely unknown and thus additional intensive research activity in this field is needed.
ACKNOWLEDGMENTS
We thank K. Imhoff and V. Neitzel (Ruhrverband, Essen, Germany) for rendering the sampling of sewage sludge from the waste water treatment plant, and we thank J. Koppen and J. Mertens (Department of Geology, University of Essen, Essen, Germany) for the XRF analysis of the sewage sludge samples.
This research was supported by the Graduiertenkolleg GRK 153/2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aposhian H V. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol. 1997;37:397–419. doi: 10.1146/annurev.pharmtox.37.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Ashby J R, Craig P J. Environmental methylation of tin: an assessment. Sci Tot Environ. 1988;73:127–133. doi: 10.1016/0048-9697(88)90193-3. [DOI] [PubMed] [Google Scholar]
- 3.Bachofen R, Birch L, Buchs U, Ferloni P, Flynn I, Jud G, Tahedel H, Chasteen T G. Volatilization of arsenic compounds by microorganisms. In: Hinchee R E, editor. Bioremediation of inorganics. Columbus, Ohio: Batelle Press; 1995. pp. 103–108. [Google Scholar]
- 4.Bakir F, Damluji S F, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi N Y, Tikriti S, Dhahir H I, Clarkson T W, Smith J C, Doherty R A. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 5.Baldi F, Parati F, Filippelli M. Dimethylmercury and dimethylmercury-sulfide of microbial origin in the biogeochemical cycle of Hg. Water Air Soil Pollut. 1995;80:805–815. [Google Scholar]
- 6.Challenger F. Biological methylation. Chem Rev. 1945;36:315–318. [Google Scholar]
- 7.Challenger F, Higginbottom C, Ellis L. The formation of organo-metalloidal compounds by microorganisms. J Chem Soc. 1933;5:95–101. [Google Scholar]
- 8.Choi S C, Chase T, Jr, Bartha R. Enzymatic catalysis of mercury methylation by Desulfovibrio desulfuricans LS. Appl Environ Microbiol. 1994;60:1342–1346. doi: 10.1128/aem.60.4.1342-1346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark E A, Sterritt R M, Lester J N. The fate of tributyltin in the aquatic environment. Environ Sci Technol. 1988;22:600–604. [Google Scholar]
- 10.Cox D P, Alexander M. Production of trimethylarsine gas from various arsenic compounds by three sewage fungi. Bull Environ Contam Toxicol. 1973;9:84–88. doi: 10.1007/BF01684760. [DOI] [PubMed] [Google Scholar]
- 11.Craig P J, editor. Organometallic compounds in the environment: principles and reactions. New York, N.Y: John Wiley & Sons, Inc.; 1986. [Google Scholar]
- 12.Craig P J, Jenkins R O, Dewick R, Miller D P. Trimethylantimony generation by Scopulariopsis brevicaulis during aerobic growth. Sci Tot Environ. 1999;229:83–88. [Google Scholar]
- 13.Crecelius E A. Changes in the chemical speciation of arsenic following ingestion by man. Environ Health Perspect. 1977;19:147–150. doi: 10.1289/ehp.7719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen W R, Reimer K J. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- 15.Cullen W R, Li H, Pergantis S A, Eigendorf G K, Mosi A A. Arsenic biomethylation by the microorganism Apiotrichum humicola in the presence of l-methionine-methyl-d3. Appl Organomet Chem. 1995;9:507–515. [Google Scholar]
- 16.Doran J W. Microorganisms and the biological cycling of selenium. Adv Microbiol Ecol. 1982;6:1–32. [Google Scholar]
- 17.Errécalde O, Astruc M, Maury G, Pinel R. Biotransformation of butyltin compounds using pure strains of microorganisms. Appl Organometal Chem. 1995;9:23–28. [Google Scholar]
- 18.Feldmann J, Grümping R, Hirner A V. Determination of volatile metal and metalloid compounds in gases from domestic waste deposits with GC/ICP-MS. Fresenius J Anal Chem. 1994;350:228–234. [Google Scholar]
- 19.Feldmann J. A calibration method for the analysis of volatile metal(loid) species in environmental gas samples by using GC-ICP-MS. J Anal Atomic Spectrom. 1997;12:1069–1076. [Google Scholar]
- 20.Feldmann J, Hirner A V. Occurrence of volatile metal and metalloid species in landfill and sewage gases. Int J Environ Anal Chem. 1995;60:339–359. [Google Scholar]
- 21.Feldmann J, Krupp E M, Glindemann D, Hirner A V, Cullen W R. Methylated bismuth in the environment. Appl Organometal Chem. 1999;13:1–10. [Google Scholar]
- 22.Gao S, Tanji K K. Model for biomethylation and volatilization of selenium from agricultural evaporation ponds. J Environ Qual. 1995;24:191–197. [Google Scholar]
- 23.Gosio B. Zur Frage, wodurch die Giftigkeit arsenhaltiger Tapeten bedingt wird. Ber Deutsch Chem Gesamte. 1897;30:1024–1026. [Google Scholar]
- 24.Gürleyük H, Van Fleet-Stalder V, Chasteen T G. Confirmation of the biomethylation of antimony compounds. Appl Organometal Chem. 1997;11:471–483. [Google Scholar]
- 25.Hallas E, Means J C, Cooney J J. Methylation of tin by estuarine microorganisms. Science. 1982;215:1505. doi: 10.1126/science.215.4539.1505. [DOI] [PubMed] [Google Scholar]
- 26.Hirner A V, Feldmann J, Krupp E, Grümping R, Goguel R, Cullen W R. Metal(loid)organic compounds in geothermal gases and waters. Organic Geochem. 1998;29:1765–1778. [Google Scholar]
- 27.Jenkins R O, Craig P J, Goessler W, Miller D, Ostah N, Irgolic K J. Biomethylation of inorganic antimony compounds by an aerobic fungus: Scopulariopsis brevicaulis. Environ Sci Technol. 1998;32:882–885. [Google Scholar]
- 28.Krzycki J, Zeikus J G. Quantification of corrinoids in methanogenic bacteria. Curr Microbiol. 1980;3:243–245. doi: 10.1007/BF02602456. [DOI] [PubMed] [Google Scholar]
- 29.Luke G T. The Minamata disease. Am J For Med Pat. 1982;3:335–338. doi: 10.1097/00000433-198212000-00010. [DOI] [PubMed] [Google Scholar]
- 30.McBride B C, Wolfe R S. Biosynthesis of dimethylarsine by Methanobacterium. Biochemistry. 1971;10:4312–4317. doi: 10.1021/bi00799a024. [DOI] [PubMed] [Google Scholar]
- 31.Richardson B A. Sudden Infant Death Syndrome: a possible primary cause. J Forensic Sci Soc. 1994;34:199–204. doi: 10.1016/s0015-7368(94)72915-7. [DOI] [PubMed] [Google Scholar]
- 32.Ridley W P, Dizikes L J, Wood J M. Biomethylation of toxic elements in the environment. Science. 1977;197:329–332. doi: 10.1126/science.877556. [DOI] [PubMed] [Google Scholar]
- 33.Robinson J B, Tuovinen O H. Mechanisms of microbial resistance and detoxification of mercury and organomercury compounds: physiological, biochemical, and genetic analyses. Microbiol Rev. 1984;48:95–124. doi: 10.1128/mr.48.2.95-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreiber U, Anders D, Koppen J. Mixing and chemical interdiffusion of trachytic and latitic magma in a subvolcanic complex of the tertiary Westerwald (Germany) Lithos. 1999;46:695–714. [Google Scholar]
- 35.Thayer J S. Organometallic compounds and living organisms. Orlando, Fla: Academic Press, Inc.; 1984. [Google Scholar]
- 36.Thayer J S. Environmental chemistry of the heavy elements: hydrido and organo compounds. Weinheim, Germany: VCH Publishers, Inc.; 1995. [Google Scholar]
- 37.Thompson D J. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–114. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- 38.Van Fleet-Stalder V, Chasteen T G. Using fluorine-induced chemiluminescence to detect organo-metalloids in the headspace of phototrophic bacterial cultures amended with selenium and tellurium. J Photochem Photobiol B Biol. 1998;43:193–203. [Google Scholar]
- 39.Wickenheiser E B, Michalke K, Drescher C, Hirner A V, Hensel R. Development and application of liquid and gas-chromatographic speciation techniques with element specific (ICP-MS) detection to the study of anaerobic arsenic metabolism. Fresenius J Anal Chem. 1998;362:498–501. [Google Scholar]
- 40.Wickenheiser E B, Michalke K, Hensel R, Drescher C, Hirner A V, Brutishauser B, Bachofen R. Volatile compounds in gases emitted from the wetland bogs near Lake Cadagno. In: Peduzzi R, Bachofen R, Tonolla M, editors. Lake Cadagno: a meromictic lake. Documenta dell'Instituto Italiano di Idrobiologia, no. 63. Verbania Pallanza, Italy: Instituto Italiano di Idrobiologia; 1998. pp. 137–140. [Google Scholar]
- 41.Zakharyan R, Wu Y, Bogdan G M, Aposhian H V. Enzymatic methylation of arsenic compounds: assay, partial purification, and properties of arsenite methyltransferase and monomethylarsonic acid methyltransferase of rabbit liver. Chem Res Toxicol. 1995;8:1029–1038. doi: 10.1021/tx00050a006. [DOI] [PubMed] [Google Scholar]
- 42.Zehnder A J, Wuhrmann K. Titanium(III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976;194:1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]