Abstract
Purpose of Review
Although COVID-19 was originally characterized as a respiratory disease, recent findings have shown lingering side effects in those who have recovered, and much is still unknown about the long-term consequences of the illness. Thus, the potential of unearthing multi-system dysfunction is high, with current data revealing significant impacts on musculoskeletal health.
Recent Findings
Multiple animal models of COVID-19 infection have revealed significant post-infection bone loss at several different skeletal sites. While how this loss occurred is unknown, this current review discusses the primary bone loss studies, and examines the possible mechanisms of action including: direct infection of bone marrow macrophages or hematopoietic progenitors, a proinflammatory response as a result of the COVID-19 induced cytokine storm, and/or a result of hypoxia and oxidative stress. This review will further examine how therapeutics used to treat COVID-19 affect the skeletal system. Finally, this review will examine the possible consequence that delayed care and limited healthcare accessibility has on musculoskeletal-related patient outcomes.
Summary
It is important to investigate the potential impact COVID-19 infection has on musculoskeletal health.
Keywords: COVID-19, Cytokine storm, Osteoclast, Therapeutics, Skeletal system, Bone, Musculoskeletal, SARS-CoV-2
Introduction
Coronavirus disease 2019 (COVID-19), an infectious disease caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2), was classified as a global pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. Vaccines to prevent COVID-19 are perhaps the best hope for ending the pandemic. However, despite the nearly 7.5 billion vaccine doses administered globally, confirmed COVID-19 cases have surpassed 250 million globally, resulting in over 5 million deaths. Unfortunately, these numbers continue to increase each day [1]. While there is a general understanding of the symptoms and effects of COVID-19, the full scope of the effects the disease has on the human body is still largely unknown.
Disease severity for patients diagnosed with COVID-19 ranges from asymptomatic carriers to mild, moderate, severe, and critical disease which can result in death. Elderly patients and those with underlying medical conditions are at an increased risk of developing severe/critical symptoms [2]. Although several vaccines including mRNA vaccines, inactivated virus vaccines, adenovirus-vectored vaccines, and viral protein-based vaccines now exist, patients continue to contract COVID-19. Strikingly, those who survived COVID-19 infection may face lingering side effects of the disease. They likely have long-term health consequences and are currently known as “long haulers.” Some lingering symptoms include profound fatigue, cough, chest pain, heart palpitations, headache, joint pain, myalgia and weakness, insomnia, diarrhea, rashes, hair loss, impaired balance and gait, neurocognitive issues such as memory and concentration problems, and an overall reduction in quality of life [3].
As COVID-19 has existed for a short time, many longer-term impacts of this disease are just beginning to be understood or will be discovered with time. As an example, little is known about the effects of COVID-19 on the skeletal system [4, 5]. However, more is known about the skeletal health of patients infected with severe acute respiratory syndrome-associated coronavirus (SARS-CoV) that belongs to the same virus family and genus as SARS-CoV-2. The information of SARS-associated skeletal injury may be applicable to those with COVID-19. SARS patients had reduced bone mineral density (BMD), which was initially thought to primarily be dependent on treatment with corticosteroids [6, 7]. However, reduced BMD was observed in SARS patients with acute illness, suggesting bone loss is independent of treatment [8, 9]. Whether similar findings will be seen following SARS-CoV-2 infection remains elusive, but as detailed below, emerging reports suggest this is likely to be the case.
The current review will explore the unappreciated consequences of COVID-19 on the musculoskeletal system by examining the effect of SARS-CoV-2 infection on bone mass, the possible mechanisms resulting in increased osteoclasts (OCs), the effects therapeutics may have had on the skeletal system, as well as the indirect impacts of the pandemic on musculoskeletal health.
Impact of SARS-CoV-2 Infection on Bone Mass
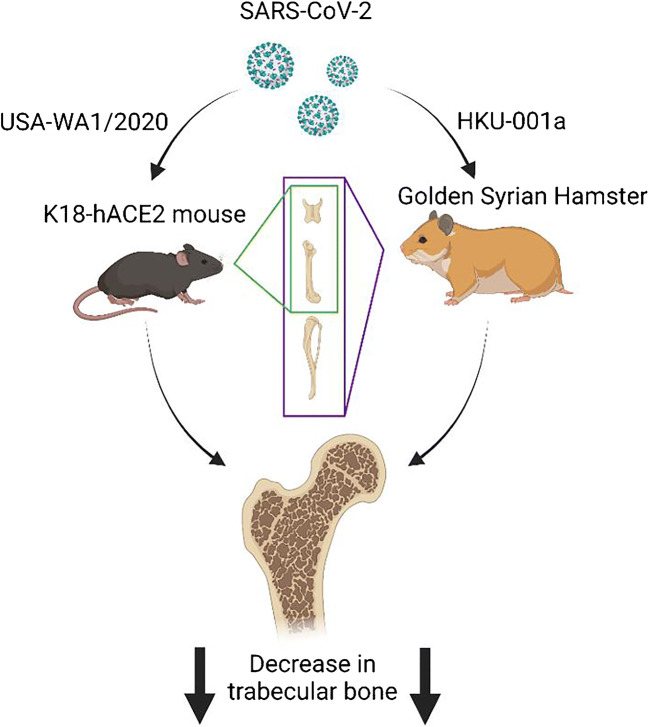
Recently, several groups have shown that both mouse and hamster models when infected with SARS-CoV-2 reveal a dramatic reduction of bone in a short period of time after infection (Fig. 1). Our group was the first to publish that infection of mice with SARS-CoV-2 (USA-WA1/2020) resulted in a dramatic loss in trabecular bone with a simultaneous increase in OC numbers [10••]. Briefly, the epithelial cell cytokeratin 18-human angiotensin I-converting enzyme 2 (K18-hACE2) transgenic mouse model was used. Importantly, with limited exceptions, successful COVID-19 mouse models have altered angiotensin-converting enzyme 2 (ACE2) expression in one manner or another, due to the fact that SARS-CoV-2 enters host cells by binding the receptor, ACE2, and the incompatibility of the murine ortholog [11, 12]. Thus, K18-hACE2 transgenic mice were used as no alterations of the human virus were required and hACE2 expression, driven by cytokeratin 18, results in hACE2 expression in epithelial cells of multiple organ systems allowing for successful inoculation and the development of severe disease [13, 14••]. In our recently reported studies [10••], 19-week-old young adult male K18-hACE2 mice were infected with 1 of 3 different viral doses which resulted in asymptomatic mice (1 × 103 plaque forming units or PFU), mice with moderate to severe disease where some mice recover and some die (1 × 104 PFU), and mice with severe disease where all mice died 6–7 days post-infection (dpi) (1 × 105 PFU). Mice that survived were humanely euthanized 12–14 dpi. Mock-infected K18-hACE2 and wild-type mice along with wild-type mice infected with virus served as controls. Femurs were examined for changes in bone parameters. Micro-computed tomography (μCT) analysis showed that infected mice exhibited a 24.4% reduction in trabecular bone volume fraction (BV/TV) and histomorphometric analysis showed a concomitant greater than 60% increase in OC number in infected mice as compared to non-infected controls. The bone loss and OC expansion observed in our model indicates bone marrow cell homeostasis is dysregulated. These observations are in agreement with clinical findings as one study revealed the presence of histiocytic hyperplasia with hemophagocytosis in the bone marrow of deceased COVID-19 patients and that dysregulated hematopoiesis could mark severe infection [15, 16]. Perhaps one of the most striking key findings was that even asymptomatic mice, exhibiting normal activity and behavior, exhibited these dramatic alterations in bone phenotype in just a 2-week period of time [10••]. This observation suggests that lack of mobility as seen with very sick mice could not be the cause of the dramatic bone loss observed in the asymptomatic mice. While not specifically tested in this study, it was hypothesized that an inflammatory response, such as the cytokine storm observed in human COVID-19 patients, was likely responsible for the dramatic increase in OC number. Whether asymptomatic mice exhibit a cytokine storm per se, or perhaps a lower grade elevation in cytokines remains to be tested. That said, investigators have shown that SARS-CoV-2 infection of K18-hACE2 mice results in a cytokine storm not unlike that observed in human COVID-19 patients [17–19].
Fig. 1.
Three different groups have shown significant post-infection bone loss in multiple animal species at several different skeletal sites. Two of the groups utilized the K18-hACE2 mouse model and trabecular bone loss was assessed in the vertebral body in one group and in the femur of the other group. A third group utilized the Golden Syrian hamster and observed bone loss was assessed in the femurs, tibias, and vertebrae. Created with BioRender.com
This finding is not in isolation; indeed, another group also used the same K18-hACE2 mouse model, but instead infected male and female juvenile mice which were 6 weeks old at the time of infection [20]. In this study, mice were infected with either 1 × 103 or 1 × 104 PFU and the L5 vertebral body was assessed. All mice died approximately 1-week post-infection and exhibited an approximate 20% reduction in weight. Compared to mock-infected control mice, infected mice exhibited a 10% decrease in BV/TV. Importantly, in these studies, virus was detected within different compartments of the musculoskeletal tissue including the bone marrow, femoral growth plate, and synovium [20].
More recently, a third group found similar findings in a differnt animal species, the golden Syrian hamster [21••]. This study utilized 6–10-week-old male hamsters that were inoculated with 1 × 105 PFU of the SARS-CoV-2 strain, HKU-001a. Hamsters were euthanized at 4, 30, and 60 dpi and femurs, tibias, and vertebrae were collected and analyzed at each time point. While even 4 dpi, significant losses in trabecular bone parameters were observed, perhaps the most striking finding was the up to 50% reduction in BV/TV observed at both 30 and 60 dpi. In this study, the authors demonstrated that infected hamsters, like humans, experience a cytokine storm which they speculate may be responsible for the striking loss of bone observed. The authors suggested that interleukin 1 beta (IL-1β), which was upregulated in the infected hamsters, could be the cytokine responsible for the increased osteoclastogenesis and bone loss. However, they did not specifically deplete IL-1β from infect animals to demonstrate the importance of SARS-CoV-2-induced increase in IL-1β on osteoclastogenesis and bone loss.
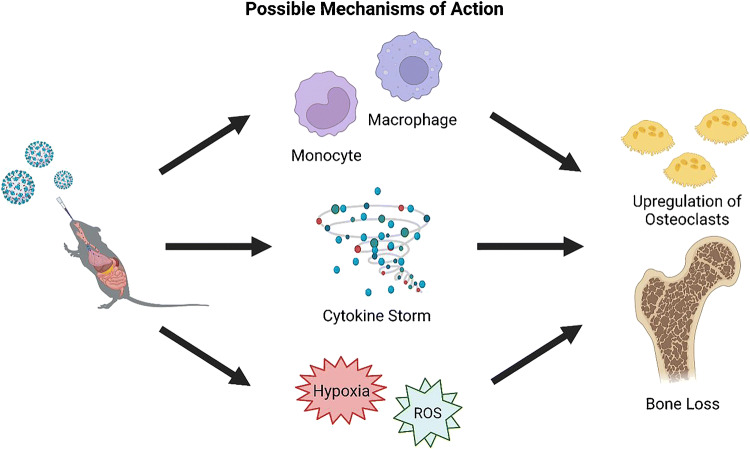
While further studies are required to understand the long-term consequences of SARS-CoV-2 infection on bone properties over time, these data suggest that bone loss occurs at multiple skeletal sites, in multiple species of animal, sexes, and ages [10••, 20, 21••]. The study with hamsters suggests that bone loss may continue/persist after the acute infection is resolved [21••]. Whether the bone loss can then be recovered remains to be tested, but likely may take time. Indeed, it is known that bone loss associated with disuse from spaceflight takes approximately 4 times the duration of the loss to recover (e.g., a 6-month spaceflight would take a human approximately 2 years post-flight to recover the bone) [22, 23]. Additionally, although clinical studies are required, as two animal species had similar bone losses, it is more likely that these data may in fact translate into humans. Finally, while the mechanism for how this bone loss occurs remains unknown, at least three possible explanations exist. First, the virus could directly infect macrophages or their progenitors; second, the systemic proinflammatory responses due to the infection-related elevation in cytokines could be responsible; or third, hypoxia and reactive oxygen species (ROS) could favor an osteoclastogenic environment (Fig. 2).
Fig. 2.
Possible mechanisms of action for the observed increase in osteoclastogenesis and bone loss in COVID-19 animal models. The three proposed mechanisms include: (1) alterations to osteoclast precursors; (2) cytokine storm-mediated impacts; and (3) impacts of hypoxia and reactive oxygen species (ROS). Created with BioRender.com
Upregulation of Osteoclasts
The monocyte/macrophage lineage are the progenitor cells for OCs. Therefore, it is possible that infection of hematopoietic lineage cells could cause skewing of progeny toward different cell types. SARS-CoV-2 can directly infect erythroid progenitors, but not other types of cells such as hemopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs), in human bone marrow [24]. Specifically, a decrease in lymphocytes was observed when HSCs treated with the SARS-CoV-2 S protein resulted in a significant reduction of multipotent lymphoid progenitor cells (MPCs) [25••]. Additionally, incubation with the S protein resulted in an initial increase in monocytes even being noted as CD14hi [25••]. An increase in the latter could contribute to the marked increase in osteoclastogenesis observed in the animal studies detailed above [10••, 21••].
Additionally, the studies by Christiansen et al. detected SARS-CoV-2 virus in multiple musculoskeletal tissues [20]. While their studies detected virus within the bone marrow, whether it was in bone marrow macrophages is not clear. Importantly, they also used immunohistochemical staining to show expression of ACE2 in musculoskeletal tissue. Consistent with these findings, another group recently showed that SARS-CoV-2 can enter bone marrow macrophages through neuropilin-1 (NRP1) which is constitutively expressed in aged and neonatal mice [26]. This article speculates that direct infection of bone marrow macrophages through NRP1 could impact OC differentiation. Therefore, whether SARS-CoV-2 can directly infect bone marrow macrophages or OCs, requires further investigation.
Cytokine Storm on Osteoclasts
As noted above, the systemic increase in proinflammatory cytokines observed following SARS-CoV-2 infection could be responsible for the profound increase in OC number and bone loss observed in animals. Indeed, one of the most well-described conditions associated with COVID-19 infection is what is known as the “cytokine storm.” Upon infection, an excessive proinflammatory response is triggered which leads to the uncontrolled release of cytokines [27••, 28]. Many of the proinflammatory cytokines, chemokines, and growth factors upregulated in those with COVID-19 have an established role in osteoclastogenesis and/or low BMD including IL-1β, IL-6, IL-17, C-X-C motif chemokine ligand 10 (CXCL10), tissue necrosis factor alpha (TNF-α), and vascular endothelial growth factor A (VEGF-A) (Table 1) [29–35].
Table 1.
Cytokine storm factors known to regulate osteoclasts and bone loss
| Cytokines/chemokines/growth factors known to be upregulated in COVID-19+ patients | Impact on osteoclast function/activity | Impact on osteoclast number | Impact on bone loss |
|---|---|---|---|
| IL-1ß [36] | ↑ [37, 38] | ↑ [38] | ↑ [37] |
| IL-6 [36] | ↑ [39] | ↑ [35, 40] | ↑ [40–42] |
| IL-17 [36] | ↑ [43] | ↑ [29, 31] | ↑ [43, 44] |
| IP-10/ CXCL10 [36] | ↑ [45] | ↑ [45] | ↑ [32] |
| TNF-α [36] | ↑ [46, 47] | ↑ [46, 48] | ↑ [46–48] |
| VEGF-A [36] | ↑ [49, 50] | ↑ [51, 52] | ↑ [51] |
Here, we detail the known role that each of these cytokines/chemokines/growth factors play in osteoclastogenesis and bone loss independent of SARS-CoV-2 infection. IL-1 plays an important role in bone metabolism by regulation of both bone formation and resorption through the involvement of OC development in various steps. Specifically, IL-1β knockout (KO) mice had a significant ~7% increase in femur BV/TV, increased whole femur BMD, and significantly fewer number of OCs when compared to controls [53]. IL-6 is known to modulate osteoblast and OC differentiation. For example, IL-6 was shown to stimulate osteoclastic activity through the production of receptor activator of nuclear factor kappa B ligand (RANKL) in osteoblasts [41]. IL-17, while initially thought to only affect immune cells, has been shown to stimulate osteoclastogenesis in patients with rheumatoid arthritis (RA) by inducing OC-like multinucleated cell formation through prostaglandin E2 and expression of OC differentiation factor (ODF) [31, 54]. CXCL10 increases osteoclastogenic cytokines via toll-like receptor 4 (TLR4) and C-X-C chemokine receptor 3 (CXCR3) [55]. TNF-α induces osteoclastogenesis through a mechanism independent of ODF [48]. VEGF-A, key regulator of physiological angiogenesis and hematopoiesis, has been shown to increase bone resorption through the spontaneous recruitment of OCs [51, 52]. Thus, it appears that many of the cytokines/chemokines/growth factors upregulated in the COVID-19 induced cytokine storm are known to upregulate osteoclastogenesis, and could be responsible for the observed bone loss. However, initial findings of cytokine storm in COVID-19 have been challenged since most patients do not have remarkably high levels of inflammatory cytokines and only 4% of critically ill COVID-19 patients develop cytokine storm symptom (CSS) [56–61]. A study which examined the inflammatory profiles in the peripheral blood of COVID-19 patients did not find cytokine storm [62••]. Ultimately, further investigations into the cause and effects rather than the correlative data are required to determine the specific mechanisms of action.
Hypoxia and Oxidative Stress
Hypoxemia, a life-threatening healthy condition, is a hallmark of severe COVID-19 [63–65]. Systemic hypoxia can elicit excessive production of ROS relative to antioxidant defense and thereby alter redox balance [66–68]. Indeed, an imbalance between the production and scavenging of ROS occurs in COVID-19 patients [69–71], which is associated with the severity of COVID-19 [69–71]. ROS primarily act as second messengers whose signaling drives inflammasome activation, DNA damage, cell cycle arrest, and apoptosis [72–75]. While oxidative stressed has been linked to many diseases the most significant is osteoporosis. ROS has been found to induce the apoptosis of osteocytes and osteoblasts through the regulation of RANKL and osteoprotegerin (OPG) expression, thus favoring osteoclastogenesis and resulting in bone loss [76••, 77].
Recent reports have revealed that plasma levels of VEGF-A are highly elevated in COVID-19 patients and are associated with the severity of COVID-19 [62••]. VEGF-A, expression is mainly regulated by hypoxia, acts as a potent first messenger binding to its receptors (VEGFR-1 and VEGFR-2) to stimulate ROS production [78, 79]. VEGFR-2, a major receptor of VEGF-A [80], is highly expressed in vascular endothelial cells as well as activated T cells, allowing a direct effect of VEGF-A on T cell function [81, 82]. In advanced ovarian cancer, VEGF-A directly suppresses T cell proliferation and cytotoxic activity via VEGFR-2 [83, 84]. Strikingly, mice exposed to recombinant VEGF-A at the concentrations relevant to those observed in patients with advanced cancer develop a thymic atrophy with a reduced number of CD4/CD8 thymocytes [85], indicating VEGF-A directly interferes with the thymic development of T cells from hematopoietic stem/progenitor cells (HSPCs) [81]. Therefore, it is crucial to examine the effects hypoxia and excessive VEGF-A has on bone marrow hematopoiesis and the function and fate of lymphoid lineage cells in the peripheral blood in COVID-19.
Therapeutic Findings of COVID-19 Treatments on Bone
Antiviral Therapy
Remdesivir
As of November 10, 2021, the FDA has approved of only one drug, Remdesivir, to treat SARS-CoV-2. This treatment is approved for adults and children age 12 years older weighing at least 40 kg (88 lb) in patient cases requiring hospitalization. It has been reported that remdesivir decreases TNF-α, IL-β, IL-6, and IL-18 [86, 87]. As several of these factors increase osteoclastogenesis [30, 35, 38, 88], reductions in their expression would likely decrease osteoclastogenesis and may therefore help ameliorate COVID-related bone loss.
Immunomodulators
Corticosteroids
Corticosteroids are a class of drugs that work to reduce inflammation and suppress overactive immune system responses. In the context of COVID-19 treatment, corticosteroids are used to suppress the pro-inflammatory nature of the disease which can lead to lung injury and the development of multiorgan dysfunction [89]. Like with treatment with Remdesivir, the suppression of pro-inflammatory cytokines should reduce osteoclastogenesis. However, it is understood that in general, glucocorticoids (GCs) alter bone metabolism by acutely stimulating osteoclast-mediated bone resorption and reducing osteoblasts resulting in a net increase in bone resorption [90••, 91, 92]. GCs can affect osteoblasts through several pathways. One of which is through upregulation of peroxisome proliferator-activated receptor γ (PPARγ), decreasing osteoblast numbers by skewing precursors into differentiating into adipocytes [93, 94]. Several studies have shown that GCs have a dose-dependent effect on osteoblast autophagy, maintaining cell viability and function at low-dose or physiological levels but then accelerating apoptosis at high-doses [95–97]. Taken together, GCs have been shown to acutely stimulate osteoclast resorption and impair osteoblast biology, by decreasing the inflammation related OCs they have a mixed effect on resorption in this inflammatory setting.
IL-1, IL-6, and JAK Inhibitors
The use of IL-1, IL-6, and Janus kinase (JAK) inhibitors have been of great interest in hopes of decreasing the hyperinflammatory response the immune system has in response to SARS-CoV-2 infection. In SARS-CoV-2 infection, IL-1β is released from monocytes which are recruited by inflammatory cells further increasing IL-1β activity and innate immune cell activation. As detailed above, IL-1β is also known to increase OC number and function which can lead to an increase in bone loss. Therefore, IL-1 inhibitors could be useful in counteracting the autoinflammatory loop and potentially decreasing osteoclastogenesis associated with SARS-CoV-2 infection. Anakinra is a recombinant human IL-1 receptor antagonist and is typically used to treat rheumatoid arthritis [98]. A study that administered anakinra or a placebo to 594 hospitalized COVID-19 patients at risk of developing respiratory failure resulted in full recovery in just over 50% of the patients receiving the drug versus in only the 26.5% of patients given the placebo [99]. Additionally, anakinra reduced the risk of severe disease, progression to severe respiratory failure, and death. However, a different study that examined the effectiveness of anakinra in critically ill COVID-19 patients found that it was ineffective in reducing the in-hospital mortality and days required for organ support [100]. Related to bone measures, the use of anakinra was also shown to reduce epiphyseal growth plate thinning, epiphyseal bone volume loss, and osteoclastogenesis in the tibia of mice infected with two different kinds of arthritogenic alphaviruses [101].
Importantly, as detailed before, IL-6 is known to increase OC number and/or activity, resulting in bone loss. Tocilizumab is a recombinant humanized anti-IL-6 receptor monoclonal antibody which has classically been used to treat rheumatic disorders. In one study, patients with rheumatoid arthritis given tocilizumab had a significant increase in the BMD in the lumbar spine and the femoral neck, while patients with normal BMD maintained their BMD when given tocilizumab [102]. Although the concept of using tocilizumab to reduce IL-6 signaling is attractive to both reduce disease severity in COVID-19 patients and reduce osteoclastogenesis and associated bone loss, in two separate clinical trials tocilizumab was found to have no effect in decreasing mortality in hospitalized COVID-19 patients with moderate to severe illness [103, 104].
Among the cytokines upregulated during the cytokine storm, several of them are in the JAK signaling pathway. IL-6 is just one example, whereby IL-6 activates the JAK-signal transducer and activator of transcription (JAK-STAT) pathway, which then regulates numerous biological functions such as lymphocyte growth and differentiation, immune regulation, and oxidative stress [28, 105]. Baricitinib is a selective inhibitor of JAK1/2 and is known to inhibit the signaling pathways of the cytokines associated with the cytokine storm and also inhibits SARS-CoV-2 entry by impairing the viral association of adaptor protein complex 2 (AP2)-associated protein kinase 1 [106]. Additionally, baricitinib was found to inhibit osteoclastogenesis in vitro by inhibiting RANKL expression in osteoblast, but did not directly impact the ability of bone marrow macrophages to differentiated into OCs [107]. In the FDA issued EUA announcement, baricitinib was not authorized or approved as a stand-alone treatment for COVID-19, but was only approved for use in combination with remdesivir for hospitalized COVID-19 patients requiring high-flow oxygen and noninvasive ventilation. The ACCT-2 clinical trial reported that patients given baricitinib plus remdesivir had a lower median recovery time, less frequent adverse events, and a decreased 28-day mortality when compared to the control group [106]. Thus, it may be that not only do JAK inhibitors help reduce disease severity and improve recovery in COVID-19 patients, but they may also help reduce osteoclastogenesis and bone loss associated with SARS-CoV-2 infection. Thus, such investigations would be important to examine in animal models and humans.
Supplementation
Vitamin D and COVID-19
Vitamin D has been shown to play an important role in maintaining bone mass and calcium homeostasis as well as a role in modulating inflammation [108•, 109]. Indeed, vitamin D regulates calcium homeostasis in part through its regulation of RANKL expression in osteoblasts which results in the upregulation of OC formation and bone resorption, but perhaps its most important role is calcium absorption in the small intestine [108•, 110]. Vitamin D also modulates both the adaptive and innate immune systems by regulating certain cell signaling pathways and cytokines. However, the vitamin D receptor is also present on the surface of B and T cells with vitamin D deficiency being associated with increased autoimmunity and infection. As such it has been shown to inhibit pro-inflammatory cytokines through the suppression of T cell proliferation resulting in a Th1 to Th2 shift [111, 112]. This shift in T helper (Th) cell phenotype decreases INF-γ, TNF-α, IL-2, and IL-12 and increases IL-4, IL-5, and IL-10 [108•, 113]. Additionally, vitamin D was reported to suppress the differentiation of Th17 cells with a shift toward T regulatory cells resulting in a decrease in IL-17 and IL-23 and an increase in anti-inflammatory cytokine IL-10 [108•, 114].
With the known role of vitamin D in regulating inflammation, there has been increasing interest in whether there is an association between vitamin D deficiency and COVID-19 disease severity and mortality. A cross-sectional study conducted at Masih Daneshvari Hospital in Iran revealed that there was a negative inverse relationship between serum vitamin D levels and COVID-19 disease severity [115]. Similar findings were reported in a study in India, in which significantly lower levels of vitamin D were observed in severely ill COVID-19 patients as compared to asymptomatic patients [116•]. Of note, the severely ill COVID-19 patients also had significantly higher IL-6 levels as compared to those seen in asymptomatic patients [116•]. Contrary to expectation, in a study that observed the prevalence of high and low COVID-19 cases and vitamin D status across several European countries, sunny countries were reported to having lower vitamin D levels, higher vitamin D deficiency, and the higher rates of infections than countries that received less UVB sunlight [109]. However, association studies are incapable of demonstrating causation. For example, it could be that people who have generally compromised health and comorbidities also have tendency to low vitamin D intake and low sun exposure, hence predisposing them to both vitamin D deficiency and to severe illness if they get COVID. Thus, vitamin D deficiency may not be to blame at all.
However, reports on whether administering vitamin D to COVID-19 patients decreases mortality or disease severity appear to depend on when administration begins and possibly on whether they are deficient in vitamin D at that time. Specifically, an observational study found that administering high-dose parenteral vitamin D3 to ICU patients with severe COVID-19 and vitamin D deficiency did not decrease the need for endotracheal intubation, time spent in the hospital, or mortality [117•]. Similar findings were also reported in another study where vitamin D was administered to patients with moderate to severe COVID-19 resulting in no significant differences in hospital stay, mechanical intubation, and mortality between the vitamin D and placebo groups [118•]. On the other hand, a clinical trial that administered repeated high dose calcifediol to hospitalized patients who had yet to develop severe ARDS showed it significantly reduced the ICU admission, eliminated mortalities, and all patients were discharged without complications [119]. Consistent with the idea that early treatment is beneficial, another study examining the impacts of vitamin D supplementation on hospitalized frail elderly patients reported that regular vitamin D3 supplementation prior to COVID-19 diagnosis was associated with less severe outcomes and higher survival rates than with patients given a single oral supplement after diagnosis [120•]. Taken together, it appears that in general vitamin D levels inversely correlate with COVID-19 disease severity but it remains unclear whether vitamin D interventions are relevant to COVID-19 outcomes. However, since vitamin D has a well-known role in skeletal homeostasis, treatment of COVID-19 patients to achieve or maintain vitamin D sufficiency may also improve overall skeletal health.
Sequential Effects of Restricted Medical Accessibility and Delayed Care
A major consequence of the COVID-19 outbreak was that hospitals and medical clinics alike were forced to restrict access to the public. This was to contain the spread of the outbreak and ease the stress on resources, one of which was health care provider’s time that have been limited in terms of the ability to effectively take care of the surges of patients [121, 122]. Consequently, this change in deferred care has resulted in unfavorable musculoskeletal-related patient outcomes. As an example, there has been an increase in preventable amputations especially in diabetic patients. At a level one trauma center in Ohio, the probability of undergoing any amputation was 10.8 times higher during the pandemic than during prepandemic times, and the risk of major amputations had a 12.5 times increase in odds ratio [123]. Similar findings have been reported in southern India, whereby researchers also reported a significant increase major amputations when compared to the prepandemic period [124]. Likewise, another study found that there was an increase in patients with extensive ischemic damage during lockdown and reported a 42% increase in the number of major amputations in 2020 when compared to previous years [125].
Delayed care for osteoporotic patients requiring parenteral treatments has also had deleterious effects [126, 127]. Indeed, a Steering Committee comprised of medical specialists provided a guide for care for such osteoporotic patients which was proactively designed to prevent such deleterious effects [128••]. The report stated that delays in intravenous bisphosphonate treatment are unlikely to be harmful even for several months primarily due to their sustained actions on BMD [128••]. However, for those being treated with denosumab, the Steering Committee strongly recommended the temporary transition to an oral bisphosphonate due to the high bone turnover observed in patients discontinuing denosumab treatment. [128••, 129–132]. Discontinuation of romosozumab also causes rapid bone loss if no other osteoporosis treatment is prescribed [133]. Therefore, the Steering Committee suggested a delay in romosozumab treatment can be allowed if less than 2–3 months; however, if greater than 2–3 months, transition to an oral bisphosphonate is recommended [128••]. If a patient has been on romosozumab for longer than 6 months, a more permanent transition to oral bisphosphonates is suggested [128••]. Finally, for those patients treated with teriparatide or abaloparatide, due to the lack of rebound fracture risk after discontinuation of treatment, the Steering Committee recommended a 2–3-month delay in treatment would be feasible, but if longer delays are required a temporary transition to an oral bisphosphonate was recommended [128••, 134].
Importantly, COVID-19 did result in some positive changes in musculoskeletal health. One area of musculoskeletal health that saw improvements was telehealth, specifically in relation to physical therapy. In a report from the American Physical Therapy Association, the number of physical therapists (PTs) providing video consultations significantly increased with 13% of PTs reporting they treated more than 10 patients per week via video consultation by July 2020. Increases in telehealth can have long-term beneficial impacts on musculoskeletal patient outcomes, especially for those that live far away from health providers or for other reasons cannot easily or safely access healthcare providers in person [135, 136••].
Conclusion
In conclusion, the last 2 years have seen significant changes due to the COVID-19 pandemic. With every day that passes, more knowledge is revealed about the short- and long-term consequences of infection. While initially, the pulmonary complications were the main focus of COVID-19 disease, other physiologic systems, such as the musculoskeletal system have been affected either directly or indirectly as consequences of COVID-19, and also need to be fully further investigated in humans.
Acknowledgements
This project was supported by the Cooperative Center of Excellence in Hematology (CCEH) Award, funded in part by NIH U54 DK106846 (MAK, UCD). This work was also supported by NIH T32 HL007910 (ODA), NIH T32 AR065971 (UCD), NIH T32 DK007519 (UCD), NIH P30 AR072581 (EAI), and the Indiana Clinical and Translational Sciences Institute, funded in part by NIH UL1TR002529 (MAK, EAI). Additional funding for this project was received from the ASBMR Fund for Research and Education (UCD). The results of this work were also supported with resources and the use of facilities at the Richard L. Roudebush VA Medical Center, Indianapolis, IN: VA Merit #BX003751 (MAK). Finally, the views expressed in this article are solely those of the authors and do not necessarily represent the official position or policy of any of the aforementioned agencies.
Author Contribution
Melissa A. Kacena conceived of the idea, Olatundun D. Awosanya and Melissa A. Kacena performed the literature search and analyses, and Olatundun D. Awosanya wrote the first manuscript draft. All authors wrote sections of the manuscript, critically revised the manuscript, and approved the final manuscript.
Declarations
Conflict of Interest
Melissa A. Kacena serves as the Editor-in-Chief of Current Osteoporosis Reports. The other authors declare that they have no competing interests to declare that are relevant to the content of this article.
Human and Animal Right and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Muscle and Bone
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyaolu A, et al. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine, 2020: p. 1-8. 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed]
- 3.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773. doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamopoulos IE. Inflammation in bone physiology and pathology. Curr Opin Rheumatol. 2018;30(1):59–64. doi: 10.1097/BOR.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 6.Griffith JF. Musculoskeletal complications of severe acute respiratory syndrome. Semin Musculoskelet Radiol. 2011;15(05):554–560. doi: 10.1055/s-0031-1293500. [DOI] [PubMed] [Google Scholar]
- 7.Lau EMC, Chan FWK, Hui DSC, Wu AKL, Leung PC. Reduced bone mineral density in male severe acute respiratory syndrome (SARS) patients in Hong Kong. Bone. 2005;37(3):420–424. doi: 10.1016/j.bone.2005.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orford NR, Pasco JA, Kotowicz MA. Osteoporosis and the critically ill patient. Crit Care Clin. 2019;35(2):301–313. doi: 10.1016/j.ccc.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 9.van Niekerk G, Engelbrecht A-M. Inflammation-induced metabolic derangements or adaptation: an immunometabolic perspective. Cytokine Growth Factor Rev. 2018;43:47–53. doi: 10.1016/j.cytogfr.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 10.••.Awosanya OD, et al. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone. 2021:116227. 10.1016/j.bone.2021.116227This study was one of the first to report bone loss and post COVID-19 infection bolstered by a dramatic loss trabecular bone and a significant increase in osteoclasts numbers in a K18-hACE2 mouse model. Of importance, revealed that even asymptomatic mice exhibited the dramatic bone loss no contributed to a decrease in activity after infection. [DOI] [PMC free article] [PubMed]
- 11.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11(8):875–9. 10.1038/nm1267. [DOI] [PMC free article] [PubMed]
- 13.Johansen MD, Irving A, Montagutelli X, Tate MD, Rudloff I, Nold MF, Hansbro NG, Kim RY, Donovan C, Liu G, Faiz A, Short KR, Lyons JG, McCaughan GW, Gorrell MD, Cole A, Moreno C, Couteur D, Hesselson D, et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13(6):877–91. 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed]
- 14.••.McCray Paul B, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prieto-Pérez L, Fortes J, Soto C, Vidal-González Á, Alonso-Riaño M, Lafarga M, Cortti MJ, Lazaro-Garcia A, Pérez-Tanoira R, Trascasa Á, Antonio A, Córdoba R, Rodríguez-Pinilla SM, Cedeño O, Peces-Barba G, Fernández-Ormaechea I, Díez Medrano MJ, López de Las Heras M, Cabello A, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33(11):2139–46. 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed]
- 16.Wang X, Wen Y, Xie X, Liu Y, Tan X, Cai Q, Zhang Y, Cheng L, Xu G, Zhang S, Wang H, Wei L, Tang X, Qi F, Zhao J, Yuan J, Liu L, Zhu P, Ginhoux F, et al. Dysregulated hematopoiesis in bone marrow marks severe COVID-19. Cell Discov. 2021;7(1):60. 10.1038/s41421-021-00296-9. [DOI] [PMC free article] [PubMed]
- 17.Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, Fox JM, Chen RE, Earnest JT, Keeler SP, Ritter JH, Kang LI, Dort S, Robichaud A, Head R, Holtzman MJ, Diamond MS. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327–1335. doi: 10.1038/s41590-020-0778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oladunni FS, Park JG, Pino PA, Gonzalez O, Akhter A, Allué-Guardia A, Olmo-Fontánez A, Gautam S, Garcia-Vilanova A, Ye C, Chiem K, Headley C, Dwivedi V, Parodi LM, Alfson KJ, Staples HM, Schami A, Garcia JI, Whigham A, et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat Commun. 2020;11(1):6122. 10.1038/s41467-020-19891-7. [DOI] [PMC free article] [PubMed]
- 19.Yinda CK, Port JR, Bushmaker T, Offei Owusu I, Purushotham JN, Avanzato VA, Fischer RJ, Schulz JE, Holbrook MG, Hebner MJ, Rosenke R, Thomas T, Marzi A, Best SM, de Wit E, Shaia C, van Doremalen N, Munster VJ. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021;17(1):e1009195. doi: 10.1371/journal.ppat.1009195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christiansen B, Ball E, Haudenschild A, Yik J, Coffey L, Haudenschild D. Bone loss following SARS-CoV-2 infection in mice. J Bone Miner Res. 2021;36(Suppl 1). https://www.asbmr.org/education/AbstractDetail?aid=8b6290cc-b73b-45db-8d42-b747751c0ab1 [DOI] [PMC free article] [PubMed]
- 21.••.Qiao W, et al. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat Commun. 2022;13:3139. 10.1038/s41467-022-30952-xUtilized golden Syrian hamsters inoculated them with SARS-CoV-2 and evaluated changes in bone parameters changes after euthanasia 4-, 30-, and 60-days post infection. Demonstrated a decrease in trabecular bone volume fraction, and suggested IL-1β induced bone loss.
- 22.Williams D, Kuipers A, Mukai C, Thirsk R. Acclimation during space flight: effects on human physiology. CMAJ : Canadian Medical Association Journal. 2009;180(13):1317–1323. doi: 10.1503/cmaj.090628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang T, et al. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res. 2006;21:1224–30. 10.1359/jbmr.060509. [DOI] [PubMed]
- 24.Huerga Encabo H, Grey W, Garcia-Albornoz M, Wood H, Ulferts R, Aramburu IV, Kulasekararaj AG, Mufti G, Papayannopoulos V, Beale R, Bonnet D. Human erythroid progenitors are directly infected by SARS-CoV-2: implications for emerging erythropoiesis in severe COVID-19 patients. Stem Cell Rep. 2021;16(3):428–436. doi: 10.1016/j.stemcr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••.Ropa J, et al. Human hematopoietic stem, progenitor, and immune cells respond ex vivo to SARS-CoV-2 spike protein. Stem Cell Rev Rep. 2021;17(1):253–265. doi: 10.1007/s12015-020-10056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, et al. Neuropilin-1 mediates SARS-CoV-2 infection in bone marrow-derived macrophages: Adv Biol. 2022;6(5):e2200007. 10.1002/adbi.202200007. [DOI] [PMC free article] [PubMed]
- 27.••.Ragab D, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battina HL, Alentado VJ, Srour EF, Moliterno AR, Kacena MA. Interaction of the inflammatory response and megakaryocytes in COVID-19 infection. Exp Hematol. 2021;104:32–39. doi: 10.1016/j.exphem.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Disser NP, de Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal consequences of COVID-19. J Bone Joint Surg. 2020;102(14):1197–1204. doi: 10.2106/jbjs.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-α. Endocrinology. 2000;141(11):3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 31.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Investig. 1999;103(9):1345–1352. doi: 10.1172/jci5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu P, Lee S, Knoll J, Rauch A, Ostermay S, Luther J, Malkusch N, Lerner UH, Zaiss MM, Neven M, Wittig R, Rauner M, David JP, Bertolino P, Zhang CX, Tuckermann JP. Loss of menin in osteoblast lineage affects osteocyte–osteoclast crosstalk causing osteoporosis. Cell Death Differ. 2017;24(4):672–682. doi: 10.1038/cdd.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao H, Ge G, Li W, Liang X, Wang H, Li N, Sun H, Zhang W, Geng D. Dysimmunity and inflammatory storm: watch out for bone lesions in COVID-19 infection. Med Hypotheses. 2020;145:110332. doi: 10.1016/j.mehy.2020.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Feng X, He Y, Gao Y, Yang S, Shao Z, Yang C, Wang H, Ye Z. IL-4 administration exerts preventive effects via suppression of underlying inflammation and TNF-α-induced apoptosis in steroid-induced osteonecrosis. Osteoporos Int. 2016;27(5):1827–1837. doi: 10.1007/s00198-015-3474-6. [DOI] [PubMed] [Google Scholar]
- 35.Wu Q, Zhou X, Huang D, JI Y, Kang F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Physiol Biochem. 2017;41(4):1360–1369. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- 36.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2020;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, di Benedetto P, Berardicurti O, Alesse E, Giacomelli R. The Role of IL-1<i>β</i> in the bone loss during rheumatic diseases. Mediat Inflamm. 2015;2015:782382. doi: 10.1155/2015/782382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiratori T, Kyumoto-Nakamura Y, Kukita A, Uehara N, Zhang J, Koda K, Kamiya M, Badawy T, Tomoda E, Xu X, Yamaza T, Urano Y, Koyano K, Kukita T. IL-1β induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of Kindlin-3 and Talin-1. J Immunol. 2018;200(1):218–228. doi: 10.4049/jimmunol.1602035. [DOI] [PubMed] [Google Scholar]
- 39.Adebanjo OA, Moonga BS, Yamate T, Sun L, Minkin C, Abe E, Zaidi M. Mode of action of interleukin-6 on mature osteoclasts. Novel interactions with extracellular Ca2+ sensing in the regulation of osteoclastic bone resorption. J Cell Biol. 1998;142(5):1347–1356. doi: 10.1083/jcb.142.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32(1):1–7. doi: 10.1016/S8756-3282(02)00915-8. [DOI] [PubMed] [Google Scholar]
- 41.Kwan Tat S, et al. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Harmer D, Falank C, Reagan MR. Interleukin-6 interweaves the bone marrow microenvironment, bone loss, and multiple myeloma. Front Endocrinol. 2019;9:788–8. 10.3389/fendo.2018.00788. [DOI] [PMC free article] [PubMed]
- 43.Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJJ, Kolls JK, Joosten LAB, van den Berg WB. IL-17 Promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-κB ligand/osteoprotegerin balance. J Immunol. 2003;170(5):2655–2662. doi: 10.4049/jimmunol.170.5.2655. [DOI] [PubMed] [Google Scholar]
- 44.Pacifici R. The role of IL-17 and TH17 cells in the bone catabolic activity of PTH. Front Immunol. 2016;7:57. doi: 10.3389/fimmu.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee J-H, Kim HN, Kim KO, Jin WJ, Lee S, Kim HH, Ha H, Lee ZH. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Res. 2012;72(13):3175–3186. doi: 10.1158/0008-5472.can-12-0481. [DOI] [PubMed] [Google Scholar]
- 46.Boyce BF, et al. TNF-alpha and pathologic bone resorption. Keio J Med. 2005;54(3):127–131. doi: 10.2302/kjm.54.127. [DOI] [PubMed] [Google Scholar]
- 47.Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48–8. 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed]
- 48.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191(2):275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aldridge SE, Lennard TWJ, Williams JR, Birch MA. Vascular endothelial growth factor acts as an osteolytic factor in breast cancer metastases to bone. Br J Cancer. 2005;92(8):1531–1537. doi: 10.1038/sj.bjc.6602417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sipola A, Nelo K, Hautala T, Ilvesaro J, Tuukkanen J. Endostatin inhibits VEGF-A induced osteoclastic bone resorption in vitro. BMC Musculoskelet Disord. 2006;7(1):56. doi: 10.1186/1471-2474-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niida S, Kaku M, Amano H, Yoshida H, Kataoka H, Nishikawa S, Tanne K, Maeda N, Nishikawa SI, Kodama H. Vascular endothelial growth factor can substitute for macrophage colony-stimulating factor in the support of osteoclastic bone resorption. J Exp Med. 1999;190(2):293–298. doi: 10.1084/jem.190.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niida S, Kondo T, Hiratsuka S, Hayashi SI, Amizuka N, Noda T, Ikeda K, Shibuya M. VEGF receptor 1 signaling is essential for osteoclast development and bone marrow formation in colony-stimulating factor 1-deficient mice. Proc Natl Acad Sci U S A. 2005;102(39):14016–14021. doi: 10.1073/pnas.0503544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y-M, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010;22(10):805–816. doi: 10.1093/intimm/dxq431. [DOI] [PubMed] [Google Scholar]
- 54.Lee Y. The role of interleukin-17 in bone metabolism and inflammatory skeletal diseases. BMB Rep. 2013;46(10):479–483. doi: 10.5483/bmbrep.2013.46.10.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J-H, Kim B, Jin WJ, Kim HH, Ha H, Lee ZH. Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: relevance for arthritis. Arthritis Res Ther. 2017;19(1):163–3. 10.1186/s13075-017-1353-6. [DOI] [PMC free article] [PubMed]
- 56.Mudd PA, Crawford JC, Turner JS, Souquette A, Reynolds D, Bender D, Bosanquet JP, Anand NJ, Striker DA, Martin RS, Boon ACM, House SL, Remy KE, Hotchkiss RS, Presti RM, O'Halloran JA, Powderly WG, Thomas PG, Ellebedy AH. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv. 2020;6:6(50). doi: 10.1126/sciadv.abe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scherger S, Henao-Martínez A, Franco-Paredes C, Shapiro L. Rethinking interleukin-6 blockade for treatment of COVID-19. Med Hypotheses. 2020;144:110053. doi: 10.1016/j.mehy.2020.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324:1565. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Syed F, Li W, Relich RF, Russell PM, Zhang S, Zimmerman MK, Yu Q. Excessive matrix metalloproteinase-1 and hyperactivation of endothelial cells occurred in COVID-19 patients and were associated with the severity of COVID-19. J Infect Dis. 2021;224:60–69. doi: 10.1093/infdis/jiab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur Respir J. 2020;56:56(4). doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinha P, Matthay MA, Calfee CS. Is a "cytokine storm" relevant to COVID-19? JAMA Intern Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 62.••.Syed F, et al. Excessive matrix metalloproteinase-1 and hyperactivation of endothelial cells occurred in COVID-19 patients and were associated with the severity of COVID-19. J Infect Dis. 2021;224(1):60–69. doi: 10.1093/infdis/jiab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Vecchio L, Locatelli F. Hypoxia response and acute lung and kidney injury: possible implications for therapy of COVID-19. Clin Kidney J. 2020;13(4):494–499. doi: 10.1093/ckj/sfaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dogani B, Månsson F, Resman F, Hartman H, Tham J, Torisson G. The application of an oxygen mask, without supplemental oxygen, improved oxygenation in patients with severe COVID-19 already treated with high-flow nasal cannula. Crit Care. 2021;25(1):319. doi: 10.1186/s13054-021-03738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galvan-Pena S, et al. Profound Treg perturbations correlate with COVID-19 severity. Proc Natl Acad Sci U S A. 2021;118(37). 10.1073/pnas.2111315118. [DOI] [PMC free article] [PubMed]
- 66.Debevec T, Millet GP, Pialoux V. Hypoxia-induced oxidative stress modulation with physical activity. Front Physiol. 2017;8:84. doi: 10.3389/fphys.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakane M. Biological effects of the oxygen molecule in critically ill patients. J Intensive Care. 2020;8(1):95. doi: 10.1186/s40560-020-00505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and oxidative stress. Biochemistry (Mosc) 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doğan S, Bal T, Çabalak M, Dikmen N, Yaqoobi H, Ozcan O. Oxidative stress index can be a new marker related to disease severity in COVID-19. Turkish J Biochem. 2021;46(4):349–357. doi: 10.1515/tjb-2021-0013. [DOI] [Google Scholar]
- 72.Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perillo B, di Donato M, Pezone A, di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.••.Domazetovic V, et al. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14(2):209–16. 10.11138/ccmbm/2017.14.1.209A brief but in-depth review on how ROS affects bone metabolism. [DOI] [PMC free article] [PubMed]
- 77.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2007;9(6):731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 80.Shibuya M. Vascular endothelial growth factor (VEGF)-Receptor2: its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium. 2006;13(2):63–69. doi: 10.1080/10623320600697955. [DOI] [PubMed] [Google Scholar]
- 81.Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol. 2021;12:616837. doi: 10.3389/fimmu.2021.616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Terpos E, Antsaklis A, Dimopoulos MA, Bamias A. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107(11):1869–1875. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Antsaklis A, Dimopoulos MA, Bamias A. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130(4):857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 85.Ohm JE, Gabrilovich DI, Sempowski GD, Kisseleva E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 86.Heimfarth L, Serafini MR, Martins-Filho PR, Quintans JSS, Quintans-Júnior LJ. Drug repurposing and cytokine management in response to COVID-19: A review. Int Immunopharmacol. 2020;88:106947–7. 10.1016/j.intimp.2020.106947. [DOI] [PMC free article] [PubMed]
- 87.Tan YL, Tan KSW, Chu JJH, Chow VT. Combination treatment with remdesivir and ivermectin exerts highly synergistic and potent antiviral activity against murine coronavirus infection. Front Cell Infect Microbiol. 2021;11:700502–2. 10.3389/fcimb.2021.700502. [DOI] [PMC free article] [PubMed]
- 88.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1β and tumour necrosis factor α. Ann Rheum Dis. 2004;63(11):1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133(9):906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 90.••.Mitra R. Adverse effects of corticosteroids on bone metabolism: a review. PM&R. 2011;3(5):466–471. doi: 10.1016/j.pmrj.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Olney RC. Mechanisms of impaired growth: effect of steroids on bone and cartilage. Horm Res. 2009;72(Suppl 1):30–35. doi: 10.1159/000229761. [DOI] [PubMed] [Google Scholar]
- 92.Hartmann K, Koenen M, Schauer S, Wittig-Blaich S, Ahmad M, Baschant U, Tuckermann JP. Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol Rev. 2016;96(2):409–447. doi: 10.1152/physrev.00011.2015. [DOI] [PubMed] [Google Scholar]
- 93.Ito S, Suzuki N, Kato S, Takahashi T, Takagi M. Glucocorticoids induce the differentiation of a mesenchymal progenitor cell line, ROB-C26 into adipocytes and osteoblasts, but fail to induce terminal osteoblast differentiation. Bone. 2007;40(1):84–92. doi: 10.1016/j.bone.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 94.Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16(8):437–447. doi: 10.1038/s41574-020-0341-0. [DOI] [PubMed] [Google Scholar]
- 95.Han Y, Zhang L, Xing Y, Zhang L, Chen X, Tang P, Chen Z. Autophagy relieves the function inhibition and apoptosis-promoting effects on osteoblast induced by glucocorticoid. Int J Mol Med. 2018;41(2):800–808. doi: 10.3892/ijmm.2017.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang S, Liu Y, Liang Q. Low-dose dexamethasone affects osteoblast viability by inducing autophagy via intracellular ROS. Mol Med Rep. 2018;17(3):4307–4316. doi: 10.3892/mmr.2018.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Heckmann BL, Yang X, Long H. Osteoblast autophagy in glucocorticoid-induced osteoporosis. J Cell Physiol. 2019;234(4):3207–3215. doi: 10.1002/jcp.27335. [DOI] [PubMed] [Google Scholar]
- 98.Mertens M, Singh JA. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol. 2009;36(6):1118–1125. doi: 10.3899/jrheum.090074. [DOI] [PubMed] [Google Scholar]
- 99.Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, Fragkou A, Rapti A, Damoulari C, Fantoni M, Kalomenidis I, Chrysos G, Angheben A, Kainis I, Alexiou Z, Castelli F, Serino FS, Tsilika M, Bakakos P, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27(10):1752–60. 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed]
- 100.The, R.-C.A.P.I. and L.P.G. Derde. Effectiveness of Tocilizumab, Sarilumab, and Anakinra for critically ill patients with COVID-19 The REMAP-CAP COVID-19 Immune Modulation Therapy Domain Randomized Clinical Trial. medRxiv, 2021: p. 2021.06.18.21259133. 10.1101/2021.06.18.21259133.
- 101.Wolf S, Taylor A, Zaid A, Freitas J, Herrero LJ, Rao S, Suhrbier A, Forwood MR, Bucala R, Mahalingam S. Inhibition of interleukin-1β signaling by anakinra demonstrates a critical role of bone loss in experimental arthritogenic alphavirus infections. Arthritis Rheum. 2019;71(7):1185–1190. doi: 10.1002/art.40856. [DOI] [PubMed] [Google Scholar]
- 102.Kume K, Amano K, Yamada S, Kanazawa T, Ohta H, Hatta K, Amano K, Kuwaba N. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology (Oxford) 2014;53(5):900–903. doi: 10.1093/rheumatology/ket468. [DOI] [PubMed] [Google Scholar]
- 103.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, CORIMUNO-19 Collaborative Group, Bureau S, Dougados M, Tibi A, Azoulay E, Cadranel J, Emmerich J, Fartoukh M, Guidet B, Humbert M, Lacombe K, Mahevas M, Pene F, et al. Effect of Tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed]
- 104.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, et al. Efficacy of Tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–44. 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed]
- 105.Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol Sci. 2020;41(8):531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, el Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed]
- 107.Murakami K, Kobayashi Y, Uehara S, Suzuki T, Koide M, Yamashita T, Nakamura M, Takahashi N, Kato H, Udagawa N, Nakamura Y. A Jak1/2 inhibitor, baricitinib, inhibits osteoclastogenesis by suppressing RANKL expression in osteoblasts in vitro. PLoS ONE. 2017;12(7):e0181126. doi: 10.1371/journal.pone.0181126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.•.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi: 10.2310/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81. [PubMed] [Google Scholar]
- 110.Laird E, Ward M, McSorley E, Strain JJ, Wallace J. Vitamin D and bone health: potential mechanisms. Nutrients. 2010;2(7):693–724. doi: 10.3390/nu2070693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bhalla AK, et al. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133(4):1748–1754. [PubMed] [Google Scholar]
- 112.Pichler J, et al. 1α,25(OH)2D3 Inhibits not only Th1 but Also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52(1):12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 113.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HFJ, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 114.Zhang H, Shih DQ, Zhang X. Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur J Microbiol Immunol. 2013;3(4):237–240. doi: 10.1556/EuJMI.3.2013.4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vasheghani M, Jannati N, Baghaei P, Rezaei M, Aliyari R, Marjani M. The relationship between serum 25-hydroxyvitamin D levels and the severity of COVID-19 disease and its mortality. Sci Rep. 2021;11(1):17594. doi: 10.1038/s41598-021-97017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.•.Jain A, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.•.Güven M, Gültekin H. The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study. Eur J Clin Nutr. 2021;75(9):1383–1388. doi: 10.1038/s41430-021-00984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.•.Murai IH, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751–1. 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed]
- 120.•.Annweiler G, et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12(11):3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czeisler MÉ. Delay or avoidance of medical care because of COVID-19–related concerns—United States, June 2020. MMWR. Morbidity and Mortality Weekly Report, 2020. 69. 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed]
- 122.Fu SJ, George EL, Maggio PM, Hawn M, Nazerali R. The consequences of delaying elective surgery: surgical perspective. Ann Surg. 2020;272(2):e79–e80. doi: 10.1097/SLA.0000000000003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Casciato DJ, Yancovitz S, Thompson J, Anderson S, Bischoff A, Ayres S, Barron I. Diabetes-related major and minor amputation risk increased during the COVID-19 pandemic. J Am Podiatr Med Assoc. 2020. 10.7547/20-224. [DOI] [PubMed]
- 124.Viswanathan V, Nachimuthu S. Major lower-limb amputation during the COVID pandemic in South India. Int J Low Extrem Wounds. 2021:15347346211020985. 10.1177/15347346211020985. [DOI] [PubMed]
- 125.Schuivens PME, Buijs M, Boonman-de Winter L, Veen EJ, de Groot HGW, Buimer TG, Ho GH, van der Laan L. Impact of the COVID-19 lockdown strategy on vascular surgery practice: more major amputations than usual. Ann Vasc Surg. 2020;69:74–79. doi: 10.1016/j.avsg.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuggle NR, Singer A, Gill C, Patel A, Medeiros A, Mlotek AS, Pierroz DD, Halbout P, Harvey NC, Reginster JY, Cooper C, Greenspan SL. How has COVID-19 affected the treatment of osteoporosis? An IOF-NOF-ESCEO global survey. Osteoporos Int. 2021;32(4):611–617. doi: 10.1007/s00198-020-05793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kocijan R, Behanova M, Reichardt B, Haschka J, Kocijan A, Zwerina J. Poor adherence to parenteral osteoporosis therapies during COVID-19 pandemic. Arch Osteoporos. 2021;16(1):46–6. 10.1007/s11657-021-00904-x. [DOI] [PMC free article] [PubMed]
- 128.••.Yu EW, et al. Osteoporosis Management in the Era of COVID-19. J Bone Miner Res. 2020;35(6):1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miller PD, Wagman RB, Peacock M, Lewiecki EM, Bolognese MA, Weinstein RL, Ding B, Martin JS, McClung MR. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: six-year results of a Phase 2 clinical trial. J Clin Endocrinol Metab. 2011;96(2):394–402. doi: 10.1210/jc.2010-1805. [DOI] [PubMed] [Google Scholar]
- 130.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC. Effects of denosumab treatment and discontinuation on bone mineral Density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 131.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JEB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 132.Gonzalez-Rodriguez E, Aubry-Rozier B, Stoll D, Zaman K, Lamy O. Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res Treat. 2020;179(1):153–159. doi: 10.1007/s10549-019-05458-8. [DOI] [PubMed] [Google Scholar]
- 133.McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, Bolognese MA, Goemaere S, Bone HG, Zanchetta JR, Maddox J, Bray S, Grauer A. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: a randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33(8):1397–1406. doi: 10.1002/jbmr.3452. [DOI] [PubMed] [Google Scholar]
- 134.Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SAM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915–2921. doi: 10.1210/jc.2008-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hirko KA, Kerver JM, Ford S, Szafranski C, Beckett J, Kitchen C, Wendling AL. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020;27(11):1816–1818. doi: 10.1093/jamia/ocaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.••.Demeke HB, et al. Telehealth Practice Among Health Centers During the COVID-19 Pandemic - United States, July 11-17, 2020. MMWR. Morbidity and mortality weekly report, 2020. 69(50): p. 1902-1905. 10.15585/mmwr.mm6950a4. A report on the availability of telehealth practices before and during the pandemic and a discussion on the benefits of the expansion and their study limitations. [DOI] [PMC free article] [PubMed]