Abstract
Conventional functional MRI (fMRI) with blood-oxygenation level dependent (BOLD) contrast is an important tool for mapping human brain activity non-invasively. Recent interest in quantitative fMRI has renewed the importance of oxidative neuroenergetics as reflected by cerebral metabolic rate of oxygen consumption (CMRO2) to support brain function. Dynamic CMRO2 mapping by calibrated fMRI require multi-modal measurements of BOLD signal along with cerebral blood flow (CBF) and/or volume (CBV). In human subjects this “calibration” is typically performed using a gas mixture containing small amounts of carbon dioxide and/or oxygen-enriched medical air, which are thought to produce changes in CBF (and CBV) and BOLD signal with minimal or no CMRO2 changes. However non-human studies have demonstrated that the “calibration” can also be achieved without gases, revealing good agreement between CMRO2 changes and underlying neuronal activity (e.g., multi-unit activity and local field potential). Given the simpler set-up of gas-free calibrated fMRI, there is evidence of recent clinical applications for this less intrusive direction. This up-to-date review emphasizes technological advances for such translational gas-free calibrated fMRI experiments, also covering historical progression of the calibrated fMRI field that is impacting neurological and neurodegenerative investigations of the human brain.
Keywords: Calibrated fMRI, calibrated BOLD, oxidative metabolism, glucose metabolism, gas calibration, hypercapnic calibration, hyperoxic calibration, dual calibration, gas-free calibration, vascular BOLD, neuronal BOLD, neuroenergetics, local-field potential, multi-unit activity, gradient-spin echo
Introduction
Prior to the discovery of blood-oxygenation level dependent (BOLD) functional magnetic resonance imaging (fMRI) of the human brain in 1992,1–5 positron emission tomography (PET) studies in the human brain measured changes in blood flow or volume (CBF, CBV) and/or in glucose (CMRglc) or oxygen (CMRO2) metabolism induced by external stimuli (for a historical perspective see 6 ) The BOLD contrast uses endogenous hemoglobin (i.e., oxyhemoglobin is diamagnetic and deoxyhemoglobin is paramagnetic) contained inside red blood cells as the intravascular contrast agent. As the fMRI method with BOLD contrast represents a complex combination of changes in CBV, CBF, and CMRO2 in the neuropil, 7 there were concerns in the late 1990’s about how well the BOLD signal reflected underlying changes in neuronal activity.8–10 Even in these earliest days of fMRI, it was clear that the CBV-CBF and CMRO2-CBF couplings will be critical to calibrating the BOLD contrast to obtain metabolic readouts, 11 as it directly related to neuronal activity.8–10 Regardless of this weakness of BOLD contrast, conventional fMRI rapidly became popular in the human neuroimaging community because the BOLD signal could be mapped repeatedly in the same session as no external tracer was needed (as in PET studies); the increased concentration of oxyhemoglobin (in relation to deoxyhemoglobin) during functional hyperemia provided the main contrast mechanism for a positive BOLD response.
Glucose is the main energy substrate in the normal brain and its oxidation efficiently produces energy to support function. 12 PET studies in the awake human brain during the late 1980’s revealed diminished glucose oxidation during sensory processing; while CMRglc and CBF changed significantly, but CMRO2 did not.13,14 These early PET results, along with Creutzfeld’s theoretical calculations, supported a model in which neuronal signaling had minimal oxidative energy costs, 15 contradicting the view of high energy costs for human brain function at rest.16,17
To test whether CMRO2 changed during functional brain activation, studies were performed in the animal somatosensory cortex and human visual cortex with a 1H MRS method called proton observe carbon edit (POCE)18–24 (for details on POCE see25–27) At Yale University, POCE was used in an anesthetized rat model to demonstrate a large increase in CMRO2 accompanying the BOLD response in the somatosensory cortex during forepaw stimulation.28,29 Similarly, human brain POCE studies conducted independently at the University of Minnesota and the University of Nottingham also showed a substantial increase in CMRO2 with BOLD signal increase in the primary visual cortex upon visual stimulation.30,31 Because the earliest interpretations of BOLD signal increase reflected minimal or no rise in CMRO2, 32 these POCE findings were considered controversial and a possible artefact at the time.
While these 1H MRS studies contributed to our understanding of the complexity of BOLD contrast, several groups focused on other non-BOLD methods for CBF and CBV imaging which proved to be important for calibrated fMRI studies.33–37 Labeling of arterial blood water with radio-frequency (RF) saturation or inversion (by arterial spin labelling, ASL) proved to be a sensitive perfusion tracer (similar to PET using injected 15O-water), obviating the need for exogenous contrast agents for CBF measurement. The CBV method, however, was based on similar BOLD-based intravascular contrast except that the agent was injected (superparamagnetic iron oxide (SPIO) nanoparticles) that overwhelmed the BOLD effect during functional hyperemia. Thus, a functional hyperemic change would increase BOLD contrast (no agent required), CBF contrast (no agent required), and decrease CBV contrast (with SPIO nanoparticles) – all indicating increases in blood oxygenation, flow, and volume.
The calibrated fMRI field emerged in response to a need to obtain the oxidative metabolic component (CMRO2) by using a biophysical model of BOLD contrast with independently measured hemodynamic changes (of CBF and/or CBV) that accompany functional hyperemia. The promise of calibrated fMRI was first demonstrated at Harvard University and McGill University in the human brain using gas mixture,38,39 and at Yale University in animal brain without gases.40,41 A gas mixture containing small amounts of carbon dioxide and/or oxygen-enriched medical air produce changes in CBF (and CBV) and BOLD signal with minimal or no CMRO2 changes. However essentially the same “calibration” can also be achieved without gases.
In recent years, the field of calibrated fMRI has undergone numerous cycles of development, testing and characterization. Despite this, clinical adoption of gas-based calibrated fMRI has remained relatively challenging due to additional hardware and subject cooperation needed. In this review, we briefly summarize the basic concepts and focus on the state-of-the-art of calibrated fMRI. We note that this field has benefitted from the momentum gained through multiple recent reviews.42–45 The distinguishing aspect of this review is that the focus on the potential for expanded clinical translation with innovations in gas-free calibrated fMRI. We first introduce the underlying biological processes and concepts that lead to the BOLD contrast. All referred terms are summarized in Table S1.
Vascular and metabolic origins of BOLD response
The brain makes up only 2% of the human body, but demands >20% of entire body’s energy needs.16,17 These are met through tightly regulated blood supply and metabolism, which in acts as the physiological basis for fMRI. However, the corresponding increase in CMRO2 during activation in the human brain, as measured by PET, was reported to be a small fraction of the increase in CBF or CMRglc.13,14 In other words, these functional PET studies demonstrated a tight CBF-CMRglc coupling, but very weak CBF-CMRO2 and CMRglc-CMRO2 couplings. Some level of increased aerobic glycolysis during brain activation has been suggested by 1H MRS studies that showed task-induced transient lactate generation and glucose depletion in human brain.46,47 However, these changes were quantitatively much smaller glycolytic change reported from the PET studies.13,14 Collectively these mismatches of CBF-CMRO2 (and CMRglc-CMRO2) changes during brain activation opened the door for alternative methods like calibrated fMRI.
While it has been proposed, through mathematical modelling, that this small CMRO2 increase results in functional hyperemia to regulate oxygen diffusion gradients on the abluminal side of the vascular wall, 48 accumulating experimental evidence suggests that neither tissue glucose nor oxygen availability regulates the CBF changes.49–54 Moreover, the mechanistic explanation for the CBF-CMRO2 mismatch continues to be challenged,55,56 and accounting for the dependence of oxygen diffusivity with CBF has revealed a CBF-CMRO2 coupling that does not vary much with CMRO2 change.50,51,57 In fact, oxygen dissociation from the red blood cell follows a pseudo-linear pattern along the length of the capillary such that changes in CBF-CMRO2 coupling are linear at physiological levels of stimulus driven functional hyperemia.49–51
It is also believed that astrocytes, which are ideally situated throughout the brain to modulate CBF, may also be actively involved in neurovascular coupling. 58 Astrocytic endfeet are found encompassing capillary walls throughout the brain, and are also part of the tripartite synapse where they can adjust synaptic activity and/or plasticity.10,59 Moreover, modulation of astrocytic activation causes corresponding changes in intracellular calcium (Ca2+), which can then lead to vasodilation or vasoconstriction.60–62 Neuronally-released glutamate has been shown to evoke these intracellular Ca2+ changes within astrocytes, thereby providing a link between neuronal activation and astrocytic modulation of CBF.63,64 However, in vivo studies assessing the effects of astrocytic Ca2+ on arteriolar blood flow have failed to substantiate these findings, and it has been suggested that perhaps it is only in certain parts of the microvasculature that astrocytes modulate functional hyperemia. 65
Another possible explanation for the functional disproportionality between CBF and CMRO2 is that energy needs may be met through transient increases in glycolytically generated lactate, specifically during brief moments of neuronal activity. 59 The predominant model is of lactate production in astrocytes, either from glycogen or glucose, 66 to meet energy needs for neurotransmitter clearance. 67 This lactate can then be shuttled into neurons for oxidative metabolism, though the bioenergetic significance of a shuttle mechanism and lactate oxidation is still unclear. 68 Moreover, this proposition from cell culture studies has been difficult to prove by in vivo or ex vivo experimentation. 69 In summary, neurovascular coupling is likely a multi-faceted phenomenon where a very neglected component is the commensurate neurometabolic coupling, 70 with complex interactions between the various cells in the brain. Due to the inherent complexity of neurovascular and neurometabolic couplings, functional imaging with conventional fMRI is a qualitative measure of neuronal activity and any quantitative interpretation of the BOLD signal is limited. Instead, calibrated fMRI can be used to quantitatively probe neurometabolic factors like CMRO2, which can then inform on the underlying complexities of neurovascular and neurometabolic states.
Neuronal origins of BOLD response
Neuronal firing is characterized by depolarization phases of 1–2 ms epochs that are followed by quiescent recharging periods lasting a few milliseconds. Neuronal depolarization, which follows the triggering of the neuronal action potential, depends on ionic distribution across the cell membrane. The resting neuron has an approximately 10:1 difference in ionic distribution between intracellular and extracellular compartments, and this membrane polarity switches during activation upon neuronal firing and the release of neurotransmitters to excite (or inhibit) other neurons in the neuropil. In contrast, synaptic activity is more graded with slowly varying membrane potential (lasting several milliseconds) and these changes are of lower amplitude than the peak membrane potential reached for an action potential. Since action and synaptic potential changes implicate altering ionic distributions across the cell membrane, reestablishment of the equilibrium ionic balances is achieved by ion pumping but at the cost of energy. 12
Finely tipped metal microelectrodes with high impedance placed in the extracellular spaces of neurons can record both action and synaptic potentials. If the electrical data are collected with high temporal resolution (<100 μs), the extracellular voltage can measure multi-unit activity (MUA) recordings to represent neuronal firing. But the extracellular signal is also sensitive to the slower waves representing the local field potential (LFP) that arise from graded synaptic events (subthreshold depolarizations that do not necessarily generate action potentials). Thus, appropriate bandwidths are needed to separate MUA (102–103 Hz) from LFP (<102 Hz), with MUA representing neuronal firing occurring in the electrode’s vicinity (spanning 10–100 μm) and LFP integrating non-firing signals over much larger distances (μm to mm). 71
Although the BOLD signal had been shown to correlate with LFP and MUA dynamics, 72 no specific mechanism for this linkage has been identified yet. The activities underlying MUA and LFP depend on ATP (adenosine triphosphate)-dependent ion pumps (e.g., sodium (Na+)/potassium (K+) ATPase) to help restore the ionic gradients, thereby allowing neurons ready to fire again. While advances in human brain calibrated fMRI studies in the last decade have contributed to improved understanding of metabolism in health and disease,73–75 preclinical calibrated fMRI studies have allowed the concurrent use of electrophysiological methods.76–81 These studies help answer questions about neurometabolic and neurovascular couplings across different brain regions, but also metabolic demands of neural-hemodynamic associated and dissociated areas within the brain. 82
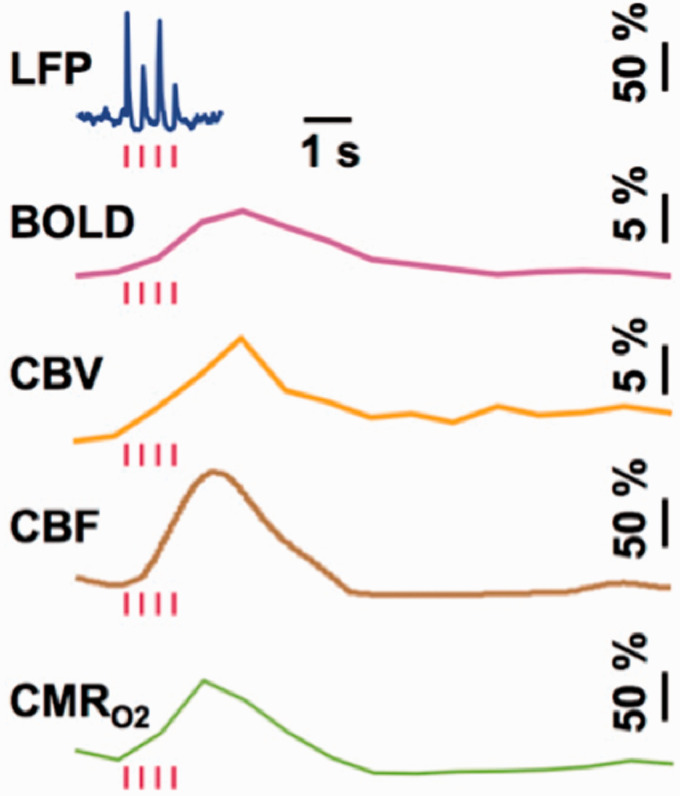
A comparison of LFP and MUA data with calibrated fMRI results, which are achievable only in animal models, shows tight neurovascular and neurometabolic couplings in block-design76,78 and event-related paradigms79,83 of the healthy brain, but also various types of epileptic seizure models.84–86 Although relationships between neuronal activity and stimulus features can range from linear to nonlinear, associations between hyperemic components (i.e., BOLD, CBF, CBV) and neuronal activity (i.e., LFP, MUA) are linear. The results collectively show that CMRO2 changes are correlated with LFP and MUA in the cerebral cortex, but the neural events occur in much faster time scales compared to the hyperemic factors (Figure 1).
Figure 1.
Single trial responses from rat cortex to sensory stimuli (red bars) showing LFP, BOLD, CBV, CBF, and CMRO2 responses. From Herman et al (2009) J Neurochem 83 with permission.
Interpretation of neural origins of the BOLD response is provisional on whether LFP and MUA, across brain regions, are disassociated or associated. 82 Logothetis and colleagues found that the sensory-evoked BOLD response in the primate cerebral cortex is better correlated to LFP than to MUA. 72 But this also implies that MUA is disassociated from LFP, and furthermore this type of neural-hemodynamic disassociation has been reported by others (see citations in 82 ). Both vascular-based and circuitry-based differences can cause regional dissociation between neural (MUA or LFP) and hemodynamic (BOLD or CBF) measurements. But do metabolic demands of neural-hemodynamic associated and disassociated areas in brain differ? To answer this question, results from two studies in rat brain examining sensory-evoked responses of BOLD, CBF, CBV, and CMRO2 from rat’s ventral posterolateral thalamic nucleus (VPL) and cortical laminae of the somatosensory forelimb area (S1FL) in relation to both MUA and LFP recordings80,81 can be compared.
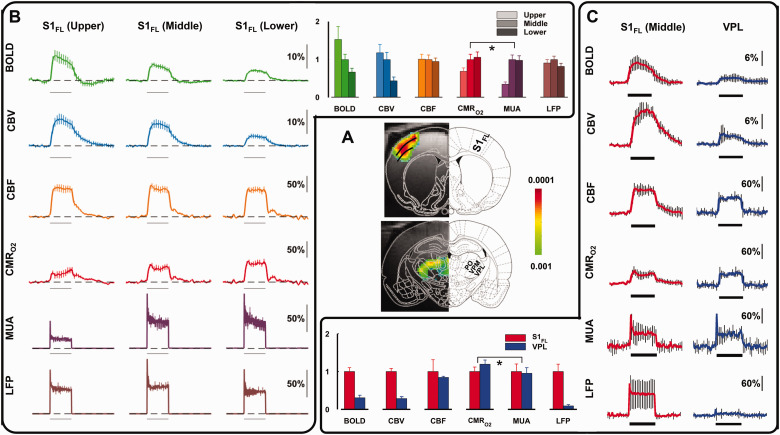
The rat’s VPL and S1FL are two model brain regions where LFP and MUA can be associated or disassociated from the respective BOLD responses (Figure 2(a)). Since the capillary density in the thalamus and most cortical structures are quite similar, 87 we expect the hemodynamic component to be comparable. However, the cellular morphology in these regions are dissimilar. The VPL consists of star-shaped thalamic cells such that the synaptic fields generated have potential to cancel out. 88 In contrast, the pyramidal cells in S1FL are oriented parallel along the cortex such that synaptic currents integrate and summate. 88 In other words, generally the LFP should be much lower in VPL than in S1FL. However, the upper, middle, and lower S1FL segments are also quite different: the upper layer comprises of neurons projecting to other adjacent cortical areas, the middle layer includes inputs from the thalamus and projections to the spinal cord, and the lower layer has reciprocal connections to and from deeper brain regions.
Figure 2.
Calibrated fMRI derived metabolic changes in rat brain, comparison between activations in forelimb cortex (S1FL) and thalamus separated into ventral posterolateral thalamic nucleus (VPL), ventral posteromedial nucleus (VPM), and posterior thalamic nuclear complex (PO) obtained during right forepaw stimulation (2 mA, 0.3 ms, 3 Hz) for 30 s (horizontal bar). (a) BOLD activation maps of S1FL and VPL. From Sanganahalli BG et al (2016) J Cereb Blood Flow Metab. 81 (b) Multimodal responses of BOLD, CBV, CBF, CMRo2, MUA, and LFP across cortical S1FL laminae (upper, middle, and lower segments, each ∼600 μm thick). Horizontal bar represents 30 s of forepaw stimulation. (c) Multimodal responses of BOLD, CBV, CBF, CMRo2, MUA, and LFP across cortical S1FL middle laminae and VPL thalamic region. A and C are from Sanganahalli BG et al. (2016) JCBFM, 81 where B is from Herman P et al. (2013) Proc Natl Acad Sci USA. 80
Layer-specific calibrated fMRI (BOLD, CBF, CBV, and CMRO2) and electrophysiology (LFP, MUA) data in S1FL during forepaw stimulation in the rat (Figure 2(b)) showed that BOLD and CBV responses were highest in superficial layers and lowest in the deepest layers, and these laminar trends did not correlate well with LFP or MUA responses.80,81 In contrast, however, CBF and LFP responses were quite stable across all layers. Changes in CMRO2 and MUA were varied across layers and were well correlated, lowest in the superficial layer and higher in the middle and deep layers. These animal results are similar to human calibrated fMRI findings of varied CBF-CMRO2 coupling across brain regions (see review 89 ) Comparison of calibrated fMRI and electrophysiology data in S1FL (middle layer) and VPL during the forepaw stimulation (Figure 2(c)) showed that BOLD and CBV responses were greater in S1FL than in VPL, and these hemodynamic changes were similar to LFP regional differences. However, CBF and CMRO2 responses were both much larger and were comparable in S1FL and VPL. Interestingly, despite different levels of CBF-CMRO2 coupling and LFP-MUA coupling in both regions, the CMRO2 was well matched with MUA in VPL and S1FL.80,81 These results suggest that neural-hemodynamic associated and dissociated areas can have similar metabolic demands, but this can only be revealed by comparing calibrated fMRI results.
The underpinnings for understanding the neuronal processes underlying BOLD contrast fMRI have largely been derived from animal studies, as such experiments are impossible to conduct in human subjects. However, most human calibrated fMRI studies are conducted in the awake state, while with animal models are executed under different types of anesthetics to achieve different levels of global brain activity.90,91 The implications of these will be outlined in subsequent sections. Table 1 summarizes the biophysical relationship amongst all key variables for the BOLD contrast mechanism, irrespective of the calibration approach.
Table 1.
The calibrated fMRI model based on BOLD contrast.
| Model building blocks | Equation |
|---|---|
| (1) | |
| (2) | |
| (3a) | |
| (3b) | |
| (3c) | |
| (4) | |
| (5) | |
| (6) |
A: field- and tissue-dependent proportionality constant; : model constant relating CBF and CBV; : model constant relating the BOLD signal to blood magnetic susceptibility; [dHb]: deoxyhemoglobin concentration; For definition of all acronyms see Table S1.
Calibrated fMRI using gas
The idea for calibrated fMRI rests on the assumption that it is possible to determine the maximum achievable BOLD signal intensity in tissue, which occurs when all deoxyhemoglobin (dHb) is eliminated from the vasculature (i.e., saturated by diamagnetic oxyhemoglobin). As it is impossible in reality to eliminate all dHb from the vasculature, calibrated fMRI with gas aims to manipulate the BOLD signal towards different conditions, thereby extrapolating the maximum BOLD signal change. Calibrated fMRI using inhaled gases has been thoroughly reviewed recently,43,45,92 and will be reviewed here only briefly.
Since its introduction in 1998, 38 the central focus of gas-calibrated fMRI has been to estimate the so-called “M-value” (see equations (1) to (6); Table 1). M represents the maximum achievable positive BOLD signal change, and it is field dependent. Given an accurate M, the CMRO2 response amplitude (ΔCMRO2) can be estimated from the CBF-CMRO2 coupling ratio (n = ΔCMRO2%/ΔCBF%) because the hemodynamic component, at least CBF (using ASL), is independently measured. Such a process also requires measurements of ΔCBF and ΔCBV. Given that CBV measurements have not been as accessible as those of CBF, it is customary in human studies to relate the CBF and CBV responses through a power-law relationship governed by the constant α (see equation (6); Table 1). Another important constant is β, which characterizes the relationship between deoxygenation-related field inhomogeneities in the reversible transverse relaxation rate (R2′; Table 1).
Hypercapnia, hyperoxia, dual and generalized calibrated fMRI
The gas perturbations are assumed to be physiologically benign. Table 2 (equations (7) to (16)) summarizes the systems of equations used in various calibrated fMRI to derive the “M-value”. It is evident from equation (5) that there are at least two unknowns, M and ΔCMRO2. In the seminal work by Davis et al., 38 hypercapnia was used as an iso-metabolic stimulus to simplify the calibrated fMRI equation (Table 1). As carbon dioxide presumably elicits CBF rise without altering CMRO2, hypercapnic manipulation (within 5% CO2) introduces a second BOLD (and CBF) measurement (in addition to that associated with neuronal activation) that allowed the two unknowns to be solved. In contrast, hyperoxic calibration 93 relies on BOLD manipulations driven by intravascular dHb concentration ([dHb]). The goal remains the same – using the gas manipulation to generate two measurements to solve two unknowns. The key assumption, however, is that hyperoxia (ranging from 25% to 100% O2) does not alter CBF. Both hypercapnic and hyperoxic calibration has been reviewed extensively in the literature,43,45,74,75,90,92,94 so the details will not be repeated here.
Table 2.
Summary of M-value estimation by different calibrated fMRI methods.
| Method | Equation | Comments |
|---|---|---|
| Hypercapnic calibration 101 | (7) | Advantages: Well understood, robust CO2 response, can provide time-resolved CMRO2 response measurementCaveats: Lengthy set up, requirement for special equipment, unverifiable assumptions, potentially uncomfortable, contraindicated for chronic obstructive pulmonary disease |
| Hyperoxic calibration 184 | (8)(9)(10) | Advantages: More comfortable, can provide time-resolved CMRO2 response measurementCaveats: Lengthy set up, requirement for special equipment, unverifiable assumptions : oxygen carrying capacity of hemoglobin : change in blood oxygenation : change in arterial oxygen tension : oxygen saturation of arterial hemoglobin : solubility coefficient of oxygen in blood |
| Dual calibration 96 | (11) (12) | Advantages: Builds upon well-understood signal models, can provide time-resolved CMRO2 response measurement, can also provide baseline CMRO2Caveats: Lengthy set up, requirement for special equipment, unverifiable assumptions, CO2-related caveats : arterial oxygen concentration |
| Gas-free: T2-based calibration 112 | (13) | Advantages: Methods is simple, with relatively high SNR. Caveats: T2 is only measured in a large vein, not accounting for spatial heterogeneity. Yv,0: baseline venous oxygenation |
| Gas-free: R2′-based calibration 40 | (14) (15) (16) | Advantages: No need for any gas or breathing challengeCaveats: Relies on accurate estimation of R2′ and β. |
Dual-gas calibrated fMRI methods were introduced to quantitatively measure the baseline oxygen extraction fraction (OEF).95–97 This more complicated setup relies on two types of respiratory challenges (hypercapnia and hyperoxia) to produce changes in the BOLD signal, along with similar measurements as described above for single gas (either CO2 or O2) calibrated fMRI. The generalized calibrated fMRI model was later presented by Gauthier and Hoge. 98 Using this model, it is not necessary for hyperoxia to be iso-capnic (or for that matter, for hypercapnia to be iso-oxic). Hyperoxic data is used to produce the functional relationship between M and the resting OEF0, while the hypercapnic data provides another relationship between M and OEF0. The use of these two relationships produces solutions for the two unknowns M and OEF0. The model is valid for arbitrary combinations of CBF and oxygenation changes. A more recent specific variant of the generalized calibrated fMRI model, known as QUantitative O2 (QUO2) MRI,99,100 involves the use of three gases to produce three conditions, namely hypercapnia, hyperoxia and hypercapnic-hyperoxia. The mean CBF and BOLD percent changes as well as the measured end-tidal partial pressure of oxygen (PetO2) are fed to the generalized calibration model, and a constant hyperoxia-induced CBF reduction is assumed.
It should be noted that the calibrated BOLD signal model proposed by Davis et al. 38 (and adapted by Hoge et al. 101 ) is fundamentally empirical. That is, the relationships between CBF and CMRO2, between CBF and CBV, and between BOLD and the M variable are derived from ad hoc fits to experimental data. While the functional form of the BOLD model may be able to fit many types of BOLD data, it remains difficult to substantially enhance the precision of calibrated fMRI parameters. This is a major ongoing challenge for the field.
Variability in the M value
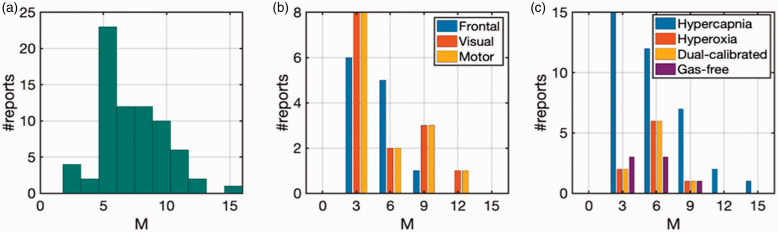
Gas calibration is an empirical approach as opposed to a first-principles-driven approach, and the estimation of the maximum achievable BOLD signal relies on our ability to accurately estimate the asymptotic BOLD value using two data points in the linear range. As recently summarized, 102 a very wide range of M-values has been reported in animal and human calibrated fMRI experiments. In this work, we focus on human studies, and the large variability of M estimates due to various factors amongst human studies is summarized in Figure 3, with the details provided in Table S2. Related biases in gas calibrated fMRI have also been simulated in detail previously,94,103 and will not be repeated here.
Figure 3.
Summary of calibrated BOLD results from human 3 T experiments from 2009 to 2020.45,98,161,184,222 Reported M values from (a) all studies, (b) specific to frontal, visual, and motor cortices, and (c) derived from different gas preparations. See Table S2 for details.
A fundamental limitation in gas-based calibrated fMRI is the over-reliance of accurate M-value estimation on a single gas-driven step. It should be noted that the experimental data obtained for BOLD and CBF changes during the gas exposures represent a very small portion of the pseudo-linear relation of a highly non-linear CBF-BOLD relationship. The measured CBF increases during gas exposure are less than 10–20%,38,96 whereas the exponential fitting that defines the M-value from the asymptotic limit for BOLD signal change is only reached when CBF increases are a fold or two higher,98,101 and it is difficult to reach such large CBF changes safely in humans.104–109 Thus, small experimental errors in BOLD or CBF measurements can substantially bias the M-value derived from asymptotic fitting. 102 This point is demonstrated by the fact that the M estimate depends on the optimal TE, which is field-strength dependent. 110
Moreover, as shown in Table 2, the high variability of M-value estimation could also be caused by differences in calibration approaches and their associated assumptions (see equation (7) vs. equations (8) to (10) vs. (11) and (12) vs. (13) vs. (14) to (16)). Blockley et al. measured M-values by two different methods in the human cerebral cortex: hypercapnia challenge and the R2′ method (which will be discussed later). They found small differences in M-values between visual cortex and the whole grey matter with the R2′ method, but regional variations in M-values were significantly higher with the hypercapnia challenge. Their study, in agreement with the observations by Shu et al. in the rat brain, 102 suggests that degree of regional M variations depends on the calibration method. The high variability in M-value has also been attributed to BOLD and CBF responses being calculated in separate steps. 111 Gas-free calibration, in contrast to gas-based calibration, does not rely on physiological assumptions, and may be an increasingly feasible approach for the eventual consolidation of calibrated fMRI findings.
Gas-free calibration
In gas-free conditions, OEF, hence [dHb] in the resting state, is estimated through intravascular transverse relaxation time (T2) relaxometry, 112 T2-oxygenation modeling 113 or venous susceptometry. 113 Specifically, these methods provide an estimate of global OEF, which has been demonstrated as uniform across the cortex. 115 However, in this review, we will focus on voxel-wise calibration methods for determining [dHb] to allow spatial mapping of OEF.
R2’-based calibration
It has long been known that the reversible transverse relaxation rate, R2′, is sensitive to venous cerebral blood volume (CBVv) and the concentration of deoxyhemoglobin ([dHb]). 116 These are the same entities that define the M-value, for what is “M” but the product of echo time (TE) used in the calibrated fMRI experiment and R2′ (see Table 1). Thus, the M- value can be determined from the baseline R2′ under well shimmed conditions. The use of R2′ mapping for estimating CMRO2 has been demonstrated in rat, mouse, and human brain calibrated fMRI studies.41,117–120
More recently, following the proposal of the quantitative BOLD (qBOLD) method 121 the feasibility of gas-free T2’ calibration has been revisited.94,122 The Yablonskiy-Haacke model of T2′ (inverse of R2′) dependence on vascular physiology offers the potential to simultaneously yet non-invasively quantify blood oxygenation saturation (SO2) and CBV.116,121,123 A summary of the various approaches to R2′ estimation is provided in Table 3.
Table 3.
Methods to map R2’.
| Method | Comments |
|---|---|
| GE-SE | Advantages: Simple to implement and analyze; low power deposition. Caveats: GE and SE acquired separately, effects such as ΔB0 and slice positioning may change between scans; isolation of R2 from R2* effects difficult at longer TEs. |
| ASE | Advantages: Low power deposition; low time consumption; matched ΔB0 and slice positioning between all echoes. Caveats: Limited number of samples along decay curve; isolation of R2 from R2* effects difficult at longer echo shifts. |
| GESFIDE | Advantages: Low power deposition; availability of numerous samples along the dephasing and rephasing curves. Caveats: Higher time consumption; sensitive to refocusing efficiency. |
| GESSE | Advantages: Low power deposition; availability of numerous samples along decay curve; insensitive to refocusing efficiencyCaveats: Higher time consumption; lower SNR than GESFIDE. |
| GESEPI | Advantages: Based on EPI, so low time consumption; low power deposition. Caveats: Image distortion due to EPI readout. |
| EPTI | Advantages: Highest temporal and spatial resolution of all available methods; availability of extremely high sampling of the dephasing and rephasing curves. Caveats: Sensitive to movement, due to simultaneous multi-slice reconstruction of down-sampled k-space; inter-echo spacing tied to spatial resolution, difficult to customize. |
| MRF | Advantages: Highest versatility in acquisition parameters of all available methods; high spatial resolution. Caveats: Randomization of sampling locations along the dephasing curve, difficult to customize for R2′ sensitivity to vasculature. |
Separate gradient- and spin-echo based methods
The easiest way to map R2′ is from independent R2 and R2* maps measured using gradient-echo (GE) and spin-echo (SE) EPI acquisitions, respectively. The principle of using an SE experiment to remove extravascular BOLD effects relies on the assumption that the majority of the BOLD effect emanates from the extravascular compartment. Several studies have used separate GE and SE acquisitions to obtain R2′ maps,102,120,124,125 in which M can be expressed as 94
| (17) |
where SSE and SGE are the SE and GE signals, respectively. A key consideration in such an approach is co-registration of the GE and SE images. Dynamic processes including head-motion, respiration and even heart beats can lead to mismatches between the GE and SE signals for a given voxel. In addition, stimulated echoes due to the use of multiple refocusing pulses can lead to R2 underestimation. Stimulated echoes most commonly arise from imperfect matching between excitation and refocusing pulse profiles in 2D image acquisitions or static magnetic field inhomogeneities (ΔB0).126,127 To enable whole-brain acquisitions in a reasonable amount of time, 3D imaging using non-selective RF pulses has been proposed. 128 To combat stimulated echoes, more elaborate pulse cycling schemes 129 can be employed, but at the cost of increased acquisition time. Nonetheless, the efficacy of phase cycling in regions of large ΔB0 remains low. 130 In addition to separately measuring R2* and R2 to find R2′, other methods have been developed in the past to measure R2′ in a single scan.131–134 However, the implementation of these R2′ mapping methods for calibrated fMRI scans remains unexplored.
Combined gradient- and spin-echo based methods
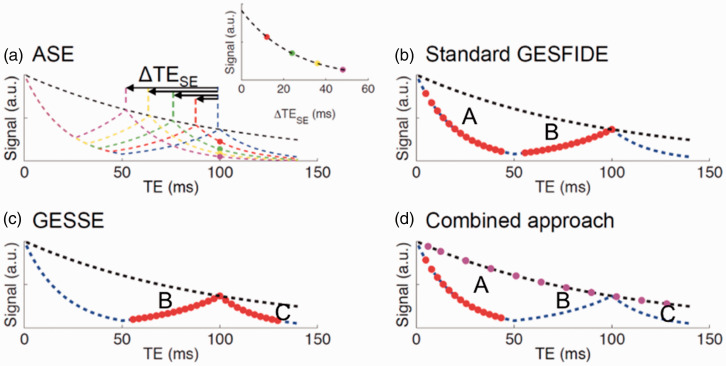
Combined GE-SE methods have become dominant in quantitative BOLD research. These methods include gradient-echo sampling of spin-echo (GESSE), gradient-echo sampling of free induction decay and echo (GESFIDE) and asymmetric spin echo (ASE)135,136 which all use a single spin-echo (instead of multiple spin-echoes as in the previous section) to quantify T2 (see Figure 4).
Figure 4.
TE dependence of asymmetric spin echo (ASE), gradient-echo sampling of free-induction decay and echo (GESFIDE), gradient-echo sampling of the spin echo (GESSE), and combined approach shown in a-d respectively. Figure is modified from 135 with permission.
ASE with EPI is the most intuitive way to measure R2′. 133 In the ASE acquisition (Figure 4(a)), the refocusing pulse is shifted by τ/2, and the signal (SASE) is acquired at TE, whereas the spin echo forms at TE-τ.
| (18) |
where F is the attenuation factor due to the shift τ from the spin-echo time. Using a series of different τ values, R2′ can be directly obtained from the ASE signal. Since ASE is most commonly based on conventional SE-EPI, it is unable to perfectly refocus the spin-echo due to susceptibility effects. Thus, ASE underestimates R2′, and hence M in a manner that depends on TE, ASE offset and vessel size. Specifically, imperfect SE refocusing from diffusion of water near microvasculature. 133 Another cause for M overestimation is inhomogeneities near air-tissue boundaries leading to loss in EPI quality.
The GESFIDE technique 132 (Figure 4(b)) involves sampling of the period starting immediately after the excitation pulse, spanning the free-induction decay (FID, t < 0.5TE) and rephrasing (t > 0.5TE), and ending at the centre of the spin-echo. R2′ is estimated from the FID and refocusing pulse as follows.
| (19a) |
| (19b) |
where τ represents the duration between the given sampling time and the spin-echo time. GESFIDE’s application in R2 and R2′ quantification was compared to that of a Carr-Purcel Meiboon-Gill multi-echo spin-echo technique. 119
The GESSE technique 134 derives from GESFIDE (Figure 4(c)), which shifts the sampling window to the rephasing stage, the spin-echo, and the dephasing stage. The rationale is that the signal segments flanking the spin-echo would be equally affected RF pulse profile imperfections in the refocusing pulse, resulting in less R2′ bias. However, the cost is signal-to-noise ratio (SNR), as the signal intensity would be lower than that of GESFIDE.
| (20a) |
| (20b) |
A natural extension, proposed by Ni et al., 135 is to combine GESFIDE and GESSE and compute R2′ from the FID, rephrasing and second-dephasing segments (Figure 4(d)). That is,
| (21a) |
| (21b) |
| (21c) |
A comparison of ASE, GESFIDE and GESSE for R2′ mapping has been reported in detail. 135 All of these methods suffer from underestimation of R2 (and thus overestimation of R2′) due to intravoxel through-plane dephasing. The attenuation can be described as
| (22) |
where Δω is the frequency difference across the voxel. This is a function of the slice-select gradient and the slice thickness. In regions with pronounced susceptibility gradients (e.g. air-tissue interfaces), the effect is magnified. Thus, Blockley et al. introduced the use of thin-slice EPI acquisition to minimize R2* effects in R2 measurements. 137 Simon et al. used two pairs of GESSE image series, one an early SE series (e.g. TE1 = 48 ms) and one a late SE series (e.g. TE2 = 98 ms), 138 each sampled at 0.63 ms spacing to produce 2 series of 64 samples. From these (S(t)), R2′ is estimated as
| (23) |
It is expected within these two spin-echoes, that R2′ and ΔR2* are similarly sensitive to variation in the baseline hematocrit, venous blood oxygenation and venous CBV but yet they are differentially sensitive to variation BOLD-related parameters. 138
Analogous to GESFIDE is gradient-spin-echo (GE-SE) echo-planar time-resolved imaging (EPTI), 139 in that it samples the refocusing and spin-echo portions of the T2 decay curve. It uses a hybrid k-time space in conjunction with slice-acceleration and multiple shots to sample a 3D k-space, and assumes small variations in RF coil-sensitivity profiles to inform the reconstruction kernel. It is able to achieve an inter-echo interval of 1 ms and a spatial resolution of 1.1 × 1.1 × 3 mm within 30 sec. Moreover, GE-SE EPTI has demonstrated robustness against ΔB0, as B0 effects are intrinsically corrected in the reconstruction process. However, there is residual R2* effect in the spin-echoes due to the interpolative nature of EPTI. This is nonetheless limited to a kernel full-width-at-half-minimum of ∼7 ms. 140 The high SNR and sampling rate of EPTI may make it an attractive alternative for R2′ mapping.
Magnetic resonance fingerprinting
Magnetic resonance fingerprinting (MRF) has been represented as a novel and versatile relaxometry technique, although most MRF studies have been concerned with estimating longitudinal (T1) and transverse (T2) relaxation times. More recently some effort has been put into estimating T2* (i.e., inverse of R2*) along with T1 and T2. The three published studies in this regard, which are all based on spiral readouts, have relied on a variable TE, 141 a combination of steady-state free precession (SSFP) with GE acquisition 142 or balanced SSFP (bSSFP) with RF phase variations. 143 The latter allows the separation between intravoxel dephasing and B0 offsets (two aspects of T2′), and can, in theory, be useful for quantitative fMRI. However, despite the dominance of spiral-based MRF methods, it should be recognized that spiral MRI is very sensitive to errors in gradient fields, leading to the need for more complex off-line reconstruction; this presents a nontrivial implementation challenge across different sites. Recently, EPI-based MRF methods 144 have been proposed that can quantify R2*. Such methods encapsulate the TE-dependence of R2* into a single R2* estimate, but are still sensitive to B0-dephasing effects, as is expected. As we are interested in the intravoxel frequency distribution underlying R2′ more than we are interested in the R2′ itself, MRF R2′ mapping methods need to minimize the macroscopic ΔB0 contribution. Moreover, it is important to remember that R2* and R2′ in fMRI are technique-specific, and it is still unclear how the R2* and R2 values estimated by MRF can be used to derive brain physiology.
Variability in estimates
Irrespective of the measurement technique, at lower fields there is a significant relaxation component from the intravascular compartment, which could induce some multi-exponential behaviours that are amplified at lower spatial resolution. These behaviours, in theory, bias R2′ estimates irrespective of the method used. At 9.4 T, Shu et al. benefited from high magnetic field strength with its inherent higher spatial resolution and reduced intravascular weighting to achieve mono-exponential R2′ decay.102,125 Under these experimental conditions, R2′ (and β) should in theory not vary significantly between R2′ methods, but further measurements are needed to confirm this observation.
Moreover, while it may seem technically straightforward to measure a value of R2′, the interpretation of R2′ in calibrated fMRI depends on a large number of modeling parameters that describe the architecture of the tissue vasculature. R2′ calibration schemes are sensitive to unmeasured model parameters describing CBV-CBF coupling, vascular size and orientation, as well as blood-tissue iso-susceptibility oxygenation, baseline hematocrit, oxygenation and volume of the deoxygenated compartment. 145 Furthermore, different R2/R2* methods, as detailed in the following, will result in different R2′ estimates, as described earlier. A main contributor to this variability is residual macroscopic ΔB0, which could result from poor shimming and incomplete refocusing.41,102 The variability in estimates can be gleaned from Table S3, and we also discuss these contributing factors in more detail in the following subsections. We also note that currently, calibrated fMRI is only performed in the grey matter. The white matter fMRI signal is low due to the limited blood supply, and can also be influenced by alterations in myelin susceptibility and iron content.
Applications in disease
Pathological conditions can challenge many of the long-standing assumptions in calibrated fMRI, and in broadening the applications of gas-based calibrated fMRI, it is important to design experiments that are robust against these uncertainties. Gas-free calibration harkens to a more quantitative approach to calibrated fMRI, by appropriately integrating a more complex biophysical model of the vasculature based on past modeling works.121,138,146,147 At the time of this review, we believe examples of calibrated fMRI, specifically with gas-free approaches, are on the verge of being applied more widely. Some cases of applications are given below.
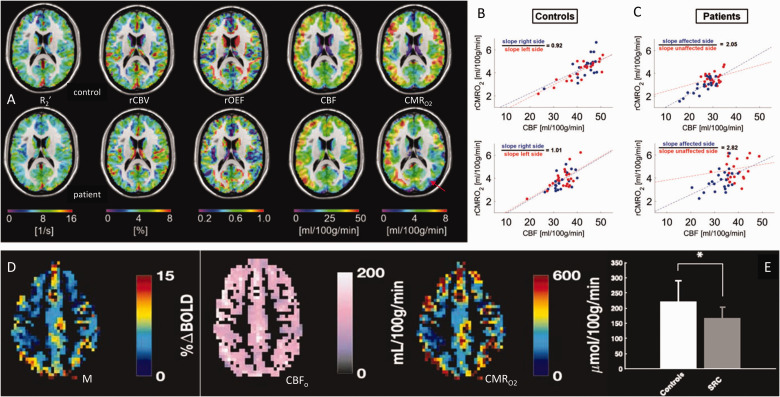
So far the largest group study that uses gas-calibrated fMRI among patients is by Lajoie et al. from the Hoge group in Montreal, Canada, 148 amongst 37 mild-moderate Alzheimer’s patients. CBF was measured using pseudo-continuous ASL, whereas OEF and CMRO2 are estimated using the comprehensive calibrated BOLD technique. CMRO2 deficits are found to be more widespread than those in CBF and OEF, reduced in the parietotemporal and precuneus regions, consistent with nuclear-medicine findings. 149 More recently, to assess if there are alterations in CBF-CMRO2 uncoupling in one side of the brain, patients with asymptomatic unilateral carotid artery stenosis were studied juxtaposed with normal age-matched controls by the Preibisch group in Munich, Germany. 150 They used gas-free calibrated fMRI, but to raise the precision they included an independent CBV measurement using contrast agent injection. Figure 5(a) shows that while the R2′, CBV, OEF or CBF maps look similar between control and patients, the CMRO2 maps can reveal subtle differences attributed to the mild pathology. Moreover, the CBF-CMRO2 couplings for each hemisphere within control (Figure 5(b)) and stenosis (Figure 5(c)) subjects clearly reveal CBF-CMRO2 uncoupling on the side with stenosis. Similarly, to assess if there are variations in metabolism with concussion, the Cook group from Queens University, Canada used gas-calibrated fMRI. 151 They studied normal college students and athletes who had suffered concussion. Using standard procedures for M-value estimation using hypercapnia challenges, their subject-specific data of M, CBF, and CMRO2 (Figure 5(d)) showed that the CMRO2 was significantly reduced in concussion patients (Figure 5(e)) in a manner similar to the CBF reduction in their brains. In other words, the CBF-CMRO2 couplings in students with and without concussion were not different, but their basal rates of flow and metabolism were dramatically altered.
Figure 5.
Calibrated fMRI in clinical scenarios at 3.0 T. (a) Multi-parametric maps of R2′, CBV, OEF, CBF and CMRO2 from a control subject (top row) and a patient with asymptomatic unilateral carotid artery stenosis (bottom row). The red arrow on the CMRO2 map of the patient shows the subtle change in metabolism in the left side of the brain, which is not clearly reflected in the other maps. (b,c) Interhemispheric flow-metabolism coupling is shown across gray matter areas in the left (red dots) and right (blue dots) hemispheres in (b) two control subjects and (c) two patients with asymptomatic unilateral carotid artery stenosis. The left side of the brain in patients clearly have an abnormal flow-metabolism coupling. (d) Demonstration of M, CBF, and CMRO2 maps of control subjects using gas-challenge calibrated fMRI. (e) CMRO2 was significantly reduced in sport-related concussion (SRC) group compared to controls. This drop in CMRO2 was similar to the CBF drop in the SRC group. (a–c) are from Figures 2 and 5 in 150 with permission. (d,e) are from Figures 2 and 5 in 151 with permission.
Remaining issues and potential solutions
Field inhomogeneity effects
As mentioned earlier, accounting for ΔB0 effects remain a challenge for R2′-weighted experiments. Early methods for correction of B0 effects in larger thickness than in-plane dimensions assumed that the B0 variation across a voxel is approximately linear. 152 One recent solution has been to decrease the slice thickness in 2D acquisitions. Specifically, the GESEPI (gradient-echo slice excitation profile imaging) technique 137 uses thin-sliced ASE EPI to circumvent the B0-related T2* effects. Liu et al. 153 used thin-sliced GESSE to the same end, in addition to using FLAIR to minimize cerebrospinal fluid (CSF)-related B0 inhomogeneity. Alternatively, He and Yablonskiy assumed that B0 effects have low spatial frequency, and regressed low-frequency harmonics out of the GESFIDE images. 121 However, neither of these approaches are likely to withstand severe B0 inhomogneities, such as found in the medial temporal/orbitofrontal lobes or at ultra-high field. Acquisition of a separate B0 map for B0 correction seems the most viable option in such cases.
Partial-volume effects in R2′
R2′ estimates are highly sensitive to MRI signal contamination by CSF, irrespective of the method used. On the one hand, the long T2 of CSF is likely not accurately captured by the range of TEs used in the methods described herein. Perhaps a less obvious source of bias is signal dephasing resulting from a small amount of CSF mixed with parenchyma. Dickson et al. estimated the off-resonance frequency resulting from dephasing due to partial-volumed CSF with 4% grey matter to be approximately 7 Hz. 154 Such dephasing would contribute to R2′. This is likened to a chemical-shift effect, and tends to cause M overestimation. These issues are relevant to GE-SE and MRF methods alike. To address this, fluid attenuation has been proposed, 155 with the caveat that fast flowing CSF cannot be suppressed. Simon et al. proposed to incorporate a CSF compartment when computing R2′, as well as use fluid-attenuated (FLAIR)-GESSE to minimize the contribution of CSF-related B0 inhomogeneities.138,153 On the other hand, it is desirable to achieve higher spatial resolution in order to avoid partial-volume effects. In that regard, EPTI and MRF-based methods, with their smaller voxels, represent promising options.
TE dependence of R2′
The R2′ value depends on the method of measurement, as discussed earlier. Moreover, the grey matter contains multiple T2 species (i.e. blood vessels, iron, etc) and additional diffusion processes that could lead to multiexponential T2 decay. It is hence little surprise that R2′ is found to increase with decreasing TESE, at least in the GESSE sequence. 135 In response, the use of two GESSE imaging sequences with different spin-echo times has been proposed, 138 as described earlier.
Vascular dependence of R2′
The calibrated fMRI models, whether gas-based or gas-free, all make certain assumptions about the composition of the voxels of interest. A summary of the cerebrovascular factors and assumptions affecting R2′ are shown in Figure 6.
Figure 6.
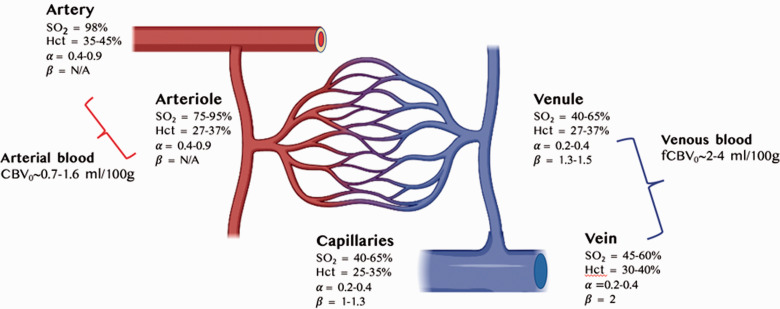
R2′ impacted by cerebrovascular factors and assumptions across arteries, veins and capillaries in the human grey matter. Blood volume and α are taken from a recent review, 214 hematocrit (Hct) values are taken from, 223 blood oxygenation (SO2) values are taken from,224,225 and β taken from125,166 as well as from our own simulations (results not shown) assuming 3.0 T.
Orientation dependence of R2′
It is well known that the blood susceptibility effects underlying BOLD have an orientation dependence,11,147 arising from dominant vascular orientations as well as the presence of neurites.156,157 In fact, diffusion anisotropy along white matter fibres has been demonstrated to account for 37% in OEF estimates, despite claims of orientation insensitivity in white-matter OEF estimates. 158 Conventionally, R2′ mapping for calibrated BOLD addresses only the grey matter, whereby the limited spatial resolution of conventional fMRI has allowed us to neglect this fibre orientation dependence. However, with increasing spatial resolution and the popularization of laminar imaging, R2′ orientation dependence may cease to be negligible. Recent work by Viessmann et al. 159 demonstrates that the BOLD signal amplitude can vary up to 60% due to orientation differences. This trade-off between higher spatial resolution for calibrated fMRI and inclusion of effects from diffusion anisotropy along white matter fibres will be matter for future research.
Vascular size dependence of R2′
As mentioned earlier, one of the factors contributing to multiexponential R2 (and hence R2′) in the grey matter is the presence of diffusion across different microenvironments (i.e. blood vessels, iron, etc), which cannot be refocused by the typical spin-echo pulse. The effect of diffusion depends on the size of vessels around which the diffusion occurs. The influence of vessel size on the GESSE-based R2′ estimate was demonstrated by Dickson et al. 160 Berman et al. recently showed that ASE underestimates M due precisely to this effect. 161 Thus, the sampling of the SE at multiple TEs is proposed, 161 and accounting for the TE-dependence of R2′ due to vascular size/diffusion effects lead to reduced M underestimation.
Vascular size, orientation and β
In 1993, Ogawa et al modeled the dependencies of R2* in the presence of diffusion roughly to show that large vessels affect R2* linearly, while small vessels affect R2* quadratically. 11 This dependence of R2* on the susceptibility (Δχ) produced by blood within vessels was termed “β” by Davis et al. 38 In essence, ΔR2*∝ Δχβ, where β can be determined empirically by examining the relationship between R2′ and susceptibility levels of blood. In the work by Shu et al. 125 β was measured in vivo across multiple regions in the white and grey matter of the rat brain by capitalizing on intravascular susceptibility variations. R2′ was measured at different concentrations of SPIO nanoparticles contrast agent.
| (24) |
where Cm and Cm,0 are concentrations of contrast agent at two levels to modulate R2′. At 9.4 T, β was found to be ∼0.8 across multiple cortical regions in the rat brain and moreover it was shown to be stable across different metabolic states.
In humans, however, the value of β is more difficult to determine empirically. Not only does β vary with vascular size, β is maximal if the blood vessels’ axes are perpendicular to B0. But if the blood vessels are positioned parallel to the magnetic field, β is equal to zero. 116 In a blood vessel network, geometry of individual vessels is of less concern, but diffusion and vessel size effects still dominate, and the fact that β > 1 is due to extravascular diffusion. At one extreme, diffusion is very fast and β is 2. In the slow and intermediate diffusion regimes, however, β is between 1 and 2, depending on the choice of SE or GE sequence for measuring R2. 162 Thus, in large vessels, such as arterioles and venules, where diffusion is viewed as slow relative to the size of the vessels, diffusion effects are outweighed by static dephasing effects and do not play any remarkable role in the BOLD contrast. For this reason also, most high field (e.g., ≥7.0 T) animal studies have usually assumed a β value of 1, 41 as signal from large veins is suppressed by the short intravascular T2*. β may in fact drop below 1 at higher fields. However it is in the smaller capillaries, and at low fields (at 2.0 T and lower), where field inhomogeneities are much reduced, that diffusion effect on relaxation rates become significant. 116 For these conditions, β of 2 has been reported, as diffusion effects prevail.11,116,163
Practically, β is often assumed based on Monte Carlo simulations at 2.0 T146,162 which have produced a value of 1.5 for gradient-echo BOLD at 1.5 T. To date, this β value is assumed in most human calibrated fMRI studies. However, concerns have been raised regarding potential biases introduced into CMRO2 calculation due to the assumed β.164,165 Moreover, the interpretation of β extends beyond the dHb, embodying effects of diffusion, vascular size, CBV, tissue orientation and hematocrit among other factors (Figure 6). Very recently, a mouse cortical vascular model was inserted into the Monte Carlo framework for estimating β 166 through simulations. It was found that the β value decreases with B0. At lower field strengths, β depends on the details of the vasculature and can vary across subjects and regions for a uniform distribution of Δχ.
At high field, ΔR2 is generally very small when compared to ΔR2*. Additionally, ΔR2 is uniform throughout the brain, whereas ΔR2* (owing to ΔR2′) is higher in superficial layers of the cortex, due to their proximity to larger veins and CSF. Previous imaging studies showed that cortical microvessels are thinner in deeper layers,167,168 but despite the gradients observed in cortical ΔR2′ due to vessel size or blood volume fraction differences, β values among all neocortical regions appeared homogeneous, in contrast to the implications from former simulations and analyses.11,116,146 The smoothness of β values in the cortex suggests relatively homogeneous coupling between metabolism and hemodynamics. 125 However, further studies with vascular staining are needed to confirm β’s physiological dependence on vessel size across regions.
Assumptions of arterial CBV
It is commonly assumed in the calibrated fMRI literature that arterial blood does not contribute to the BOLD effect. This assumption is a concern for both gas-based and gas-free calibrated fMRI. As was mentioned earlier, since oxy-Hb is diamagnetic, the iso-susceptibility oxygenation of arterial blood can be as low as 89%. 147 Thus, as arterial blood is generally known to be more oxygenated than this, the susceptibility difference between arterial blood and brain parenchyma also leads to intravoxel signal dephasing and BOLD signal loss. However, the definition of “arterial” can influence the accuracy of this assumption. Early measurements in rodents have uncovered substantial precapillary O2 loss,169,170 which, coupled with the high elasticity of arterioles, could fundamentally alter the interpretation of BOLD signal localization. Previous studies in animals have recorded arterial O2 decreases in the range of 10-40% with increasing degrees of branching. 171 If we assume the average arterial O2 saturation is only 70% rather than 100%, the arterial-venous difference in blood oxygenation that the OEF model is based on becomes more complicated to solve for calibrated fMRI. Furthermore, the high arterial flow can introduce potential T1-based signal perturbations to the steady state BOLD signal (i.e., inflow effects). While this is deemed a negligible effect for typical fMRI acquisition protocols, 172 the increasing adoption of shorter repetition times, such as permitted by slice-acceleration schemes.173,174 This effect is expected to be magnified at higher field strengths. The implications of arterial-CBV participation include: (1) higher CBF-CBV coupling (exponent α in Table 1); (2) reduced BOLD signal change due to tasks; (3) the occurrence of negative BOLD response to an increase in neural activity. The latter could occur due to the small volume fraction of arterial vessels and their high capacity for expansion during activation, leading to increased intra-voxel mesoscopic B0 inhomogeneity.
The extravascular assumption
The empirically derived calibrated fMRI model38,101 assumes that the intravascular BOLD signal contribution is negligible, as the susceptibility effects that underlie the β parameter are dominated by extravascular mesoscopic B0 inhomogeneities. Moreover, only blood vessels oriented perpendicular to the main magnetic field B0 contribute to these B0 inhomogeneities. Furthermore, this extravascular contribution depends on voxel size; in larger vessels, it is more likely that the B0-offset effects form a complete set of dipoles that cancel each other, a scenario less likely to happen in smaller voxels. The intravascular BOLD contribution, on the other hand, has been found to range from 60%-70% at 1.5 T to 20% at 7.0 T. 175 Moreover, the intravascular BOLD contribution varies with TE, being maximized when TE equals blood T2*, with shorter TEs introducing more intravascular weighting.176,177 It is unclear if intravascular BOLD follows the same empirical BOLD model, therefore there is a potentially sizeable portion of the BOLD signal that cannot be explained by the existing calibration process.
Assumptions specific to gas-calibrated fMRI
For children, the elderly and clinical populations, gas calibration, especially via hypercapnia, remains challenging. Safety concerns during acute hypercapnia would most likely contraindicate participants with respiratory diseases (such as asthma), pulmonary occlusions and kidney disease. Compliance and attrition issues would also in general be more likely in the above populations. 178 Furthermore, there are gas-calibration specific assumptions about respiratory physiology.
Hypercapnia-related assumptions
Notwithstanding the nervous response initiation by the macrovascular CO2 receptors, the homeostatic blood pH is actively regulated. Thus, hypercapnic challenges, in which the increased arterial CO2 content induces large CBF increases without a significant concomitant increase in CMRO2,114,179 have been widely used to study cerebrovascular coupling. While the broad validity of this assumption has recently been confirmed, 138 it is important to realize that such an assumption is only likely to hold at mild levels of hypercapnia. 179 Sustained increases in arterial CO2 is known to suppress neuronal activity,180–182 as measured using electroencephalography 183 and magnetoencephalography.180,181
As typical calibrated fMRI studies are not equipped to enforce strict control on CO2 levels, it is more feasible to account for the effect of CMRO2. Driver et al. proposed a graded-hypercapnia under the assumption that increased CO2 will have a concordant increase in CMRO2. This can be used to calculate M without any knowledge of baseline CMRO2. 104
| (25) |
where κ relates end-tidal partial pressure of CO2 (PetCO2) during hypercapnia (HC) to CMRO2. This method produced M values that were on average lower, potentially as it accounts for CMRO2 decreases incurred during hypercapnia, thus reducing the baseline-CMRO2 underestimation. An alternative is to adopt a CO2-free calibration method, such as hyperoxic calibration, or gas-free calibration.
Hyperoxia-related assumptions
The iso-hemodynamic assumption refers to the assumption that CBF remains unaltered during hyperoxic calibration. However, in practice, CBF has been known to decrease at high inhaled O2 levels. 93 Thus, the use of a CBF correction factor with equation (10) (Table 2) is advisable,93,184
| (26) |
where r is the correction factor for O2-induced CBF reduction. Moreover, the generalized calibration model makes no assumptions about CBF. 98 Beyond CBF, dissolved O2 in the plasma has also been raised as a potential source of BOLD signal bias, as the extra O2 delivered in hyperoxia is carried by the plasma. However, this was demonstrated not to be a notable factor.118,185–187
The Hct and baseline OEF are assumed as constants in the oxygen saturation equation, but potential variations in Hct and their consequences are described in subsections to follow.
The end-tidal assumptions
Arterial partial pressure of CO2 (PaCO2) and O2 (PaO2) are in turn inferred from the PetO2, taking into account the alveolar-arterial gas gradient and assuming that arterial blood is well equilibrated with gases in the alveoli. 94 This latter assumption relies upon healthy lung function for validity; for instance, it would not hold in patients with pneumonia have a physical barrier within the alveoli that limits the diffusion of oxygen into the capillaries. 188 In addition, the alveolar gas equation is valid only in the setting of steady-state conditions.
The assumption of the end-tidal gas levels reflecting intravascular gas levels is less critical for hypercapnic than for hyperoxic calibration. Hypercapnic calibration relies on a known relationship between PaCO2 and CBF. Conversely, in hyperoxic calibration, [dHb]/[dHb]0 is estimated by modeling oxygen carrying capacity of the blood as a function of the PaO2.
Assumptions of hematocrit
The hematocrit refers to the proportion, by volume, of the blood that consists of red blood cells. The role of the hematocrit in shaping the range of hemodynamic and metabolic estimates in fMRI has become increasingly investigated.103,189–191 In hyperoxic, dual calibration and gas-free calibration, it is necessary to assume a baseline [Hb], which is proportional to Hct. Specifically, blood R2 dependence on both SO2 and Hct.192–198 Moreover, accounting for the role of hematocrit in between-subject variations in MRI-derived baseline cerebral hemodynamic parameters. 190 In a rat model, laser doppler flowmetry combined with MRI (contrast-enhanced using a plasma-borne contrast agent like SPIO nanoparticles) has been used to estimate Hct in vivo. 189 In humans, the dependence of blood T1 on Hct199,200 has been capitalized, resulting in rapid intravascular (arterial and venous) T1 assessment using a flow-compensated Look-Locker technique. Contrast-enhanced methods have also been proposed. 201 Hematocrit abnormalities are observed in a number of clinical conditions. For instance, sickle cell anaemia is associated with reduced Hct, and calibrated fMRI would require blood sampling. 202
Assumptions of α
Despite the widespread use of Grubb’s relationship, 203 its adoption by Grubb et al. was based on empirical rather than theoretical discovery. Since the BOLD signal is dependent primarily upon deoxygenated, instead of total, blood volume change, the relevant ΔCBV in the context of BOLD modeling may be overestimated when using Grubb’s coefficient of 0.38, leading to the potential underestimation of ΔCMRO2 for a given ΔCBF and ΔBOLD. Simulations show that an overestimation of α leads to an underestimation of ΔCMRO2 with a mirroring overestimation in the neurovascular coupling ratio n.94,204
The current consensus is that the power-law α coefficient is much lower when it concerns the BOLD-specific CBV changes, 204 capillary volume, 205 and total CBV. 203 Moreover, different parts of the brain are associated with different α values,206–208 as are different cortical layers. 209 To further complicate the issue, veins and capillaries may be associated with different CBF-CBV coupling,205,210,211 fuelling the continued debate into the differential participation of capillaries and veins in hyperemia.205,210,212,213 MRI-based CBV mapping methods is the topic of a recent review, 214 and will not be reiterated here. Finally, in terms of the potential for dynamic calibration, 145 the CBV-CBF relationship is far from being clear through the phase of the functional hyperemic response. 215 In pathological conditions, α may not be accurately represented by the published values, which pertain mostly to healthy young adults. It should also be mentioned that the vasoactive mechanisms of functional and CO2-related hyperemia are not identical. While microvascular dilation is mainly linked to the presence of Ca2+, the CO2 response is believed to be mainly explained by the effect of bicarbonate ions and pH. 216 While nitric oxide (NO) has been suggested as a mediator of CO2-related vasodilation, the source of the NO release, whether it is the neurons or the endothelium, is still debated. This is even more relevant in pathological conditions in which either the neurons or the endothelium could be impaired, then the equivalence of the functional and vascular response may break down.
Pathologies such as small-vessel disease (SVD) have been linked to systemic inflammation, and have been associated with microbleeds as well as increased stroke risk. SVD may trigger perivascular space dysfunction leading to white-matter hyperintensities. 217 It has been hypothesized that enlarged periventricular spaces involve inflammation and increased CMRO2.218,219 It has also been suggested, based on animal studies, that reduced cerebrovascular reactivity may precede the development of visible white-matter hyperintensities. 220 Moreover, SVD may be related to hypoperfusion despite the hypermetabolism, suggesting an altered neurovascular (vs. neurometabolic) coupling as well as a potential alteration in the α coefficient in SVD patients and those exhibiting perivascular enlargement.
Stroke, on the other hand, can lead to extreme alterations in the basal vascular physiology, creating a scenario in which calibrated fMRI can be particularly useful for identifying salvageable tissue. However, the type of stroke, whether haemorrhagic or ischemic, also determines the applicability of gas calibration. Moreover, one might observe a gas-calibration BOLD response while the neuronal hyperemic response is diminished or absent, violating the assumption surrounding the gas-based M value estimation. 221
Summary and future outlook
Calibrated fMRI studies in animals show that neural (LFP, MUA) and hyperemic (BOLD, CBV, CBF) components are well coupled for steady-state and dynamic stimuli, lending confidence to application of the same biophysical models of calibrated fMRI for a wide range of stimulus conditions. Importantly, the CMRO2 increases during stimuli of different epochs indicate relevance of oxidative metabolism for supporting neural events separated by less than 200 ms. While gas-free mapping of M-values used for these CMRO2 calculations are an enticing advantage for clinical translation of calibrated fMRI, some considerations must be met as to how R2′ values are mapped across cortical grey matter, and these same caveats may apply to the translation of calibrated fMRI to clinical populations. Given the rapid rise of calibrated fMRI studies in recent years it is expected that clinical translation with gas-free setups will be important for understanding the variations of flow-metabolism coupling in neurological and neurodegenerative patients, and for realizing the potential of calibrated fMRI.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221077338 for Mapping oxidative metabolism in the human brain with calibrated fMRI in health and disease by J Jean Chen, Biranavan Uthayakumar and Fahmeed Hyder in Journal of Cerebral Blood Flow & Metabolism
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JJC was supported by the Canadian Institutes of Health Research (FDN 148398, PJT 169688) and the Natural Sciences and Engineering Research Council of Canada (FGPIN 418443). FH was supported by the National Institutes of Health (R01 NS-100106, R01 MH-067528, R01 MH-111424).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Bandettini PA, Wong EC, Hinks RS, et al. Time course EPI of human brain function during task activation. Magn Reson Med 1992; 25: 390–397. [DOI] [PubMed] [Google Scholar]
- 2.Blamire AM, Ogawa S, Ugurbil K, et al. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A 1992; 89: 11069–11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frahm J, Bruhn H, Merboldt KD, et al. Dynamic MR imaging of human brain oxygenation during rest and photic stimulation. J Magn Reson Imaging 1992; 2: 501–505. [DOI] [PubMed] [Google Scholar]
- 4.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 1992; 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 1992; 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME. A brief history of human brain mapping. Trends Neurosci 2009; 32: 118–126. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990; 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barinaga M. What makes brain neurons run? Science 1997; 276: 196–198. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick SM, Rothman D. New approaches to functional neuroenergetics. J Cogn Neurosci 1999; 11: 467–471. [DOI] [PubMed] [Google Scholar]
- 10.Pellerin L, Sibson NR, Hadjikhani N, et al. What you see is what you think – or is it? Trends Neurosci 2001; 24: 71–72. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J 1993; 64: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siesjo BK. Brain energy metabolism. New York, USA: Wiley and Sons, Ltd, 1978. [Google Scholar]
- 13.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 1986; 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox PT, Raichle ME, Mintun MA, et al. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988; 241: 462–464. [DOI] [PubMed] [Google Scholar]
- 15.Creutzfeldt OD. Neurophysiological correlates of different functional states of the brain. In Ingvar DH, Lassen NA. (eds) Brain work. The coupling of function, metabolism and blood flow in the brain. Alfred benzon symposium VIII. New York: Academic Press, 1975, pp.21–46. [Google Scholar]
- 16.Sokoloff L. Relationship between functional activity and energy metabolism in the nervous system: whether, where and why? In: Lassen NA, Ingvar DH, Raichle ME, et al. (eds) Brain work and mental activity. Copenhagen: Munksgaard, 1991, pp.52–64. [Google Scholar]
- 17.Aiello LC, Wheeler P. The expensive-tissue hypothesis – the brain and the digestive-system in human and primate evolution. Curr Anthropol 1995; 36: 199–221. [Google Scholar]
- 18.Rothman DL, Behar KL, Hetherington HP, et al. 1H-observe/13C-decouple spectroscopic measurements of lactate and glutamate in the rat brain in vivo. Proc Natl Acad Sci U S A 1985; 82: 1633–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behar KL, Petroff OA, Prichard JW, et al. Detection of metabolites in rabbit brain by 13C NMR spectroscopy following administration of [1-13C]glucose. Magn Reson Med 1986; 3: 911–920. [DOI] [PubMed] [Google Scholar]
- 20.Petroff OA, Prichard JW, Ogino T, et al. Combined 1H and 31P nuclear magnetic resonance spectroscopic studies of bicuculline-induced seizures in vivo. Ann Neurol 1986; 20: 185–193. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick SM, Hetherington HP, Behar KL, et al. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab 1990; 10: 170–179. [DOI] [PubMed] [Google Scholar]
- 22.Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem 1990; 265: 12916–12926. [PubMed] [Google Scholar]
- 23.Rothman DL, Novotny EJ, Shulman GI, et al. 1H-[13C] NMR measurements of [4-13C]glutamate turnover in human brain. Proc Natl Acad Sci 1992; 89: 9603–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruetter R, Rothman DL, Novotny EJ, et al. Localized 13C NMR spectroscopy of myo-inositol in the human brain in vivo. Magn Reson Med 1992; 25: 204–210. [DOI] [PubMed] [Google Scholar]
- 25.Shulman RG, Rothman DL, Behar KL, et al. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci 2004; 27: 489–495. [DOI] [PubMed] [Google Scholar]
- 26.Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage 2012; 62: 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyder F, Rothman DL. Advances in imaging brain metabolism. Annu Rev Biomed Eng 2017; 19: 485–515. [DOI] [PubMed] [Google Scholar]
- 28.Hyder F, Chase JR, Behar KL, et al. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci 1996; 93: 7612–7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyder F, Rothman DL, Mason GF, et al. Oxidative glucose metabolism in rat brain during single forepaw stimulation: a spatially localized 1H[13C] nuclear magnetic resonance study. J Cereb Blood Flow Metab 1997; 17: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhu XH, Gruetter R, et al. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using (1)H-[(13)C] MRS and fMRI. Magn Reson Med 2001; 45: 349–355. [DOI] [PubMed] [Google Scholar]
- 31.Chhina N, Kuestermann E, Halliday J, et al. Measurement of human tricarboxylic acid cycle rates during visual activation by (13)C magnetic resonance spectroscopy. J Neurosci Res 2001; 66: 737–746. [DOI] [PubMed] [Google Scholar]
- 32.Kim SG, Uğurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn Reson Med 1997; 38: 59–65. [DOI] [PubMed] [Google Scholar]
- 33.Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 1992; 89: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med 1992; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 35.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med 1995; 34: 293–301. [DOI] [PubMed] [Google Scholar]
- 36.Kennan RP, Scanley BE, Innis RB, et al. Physiological basis for BOLD MR signal changes due to neuronal stimulation: separation of blood volume and magnetic susceptibility effects. Magn Reson Med 1998; 40: 840–846. [DOI] [PubMed] [Google Scholar]
- 37.Mandeville JB, Marota JJ, Kosofsky BE, et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 1998; 39: 615–624. [DOI] [PubMed] [Google Scholar]
- 38.Davis TL, Kwong KK, Weisskoff RM, et al. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A 1998; 95: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoge RD, Atkinson J, Gill B, et al. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A 1999; 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kida I, Hyder F, Kennan RP, et al. Toward absolute quantitation of bold functional MRI. Adv Exp Med Biol 1999; 471: 681–689. [DOI] [PubMed] [Google Scholar]
- 41.Kida I, Kennan RP, Rothman DL, et al. High-resolution CMRO2 mapping in rat cortex: a multiparametric approach to calibration of BOLD image contrast at 7 tesla. J Cereb Blood Flow Metab 2000; 20: 847–860. [DOI] [PubMed] [Google Scholar]
- 42.Blockley NP, Griffeth VE, Simon AB, et al. Calibrating the BOLD response without administering gases: comparison of hypercapnia calibration with calibration using an asymmetric spin echo. Neuroimage 2015; 104: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bright MG, Croal PL, Blockley NP, et al. Multiparametric measurement of cerebral physiology using calibrated fMRI. Neuroimage 2019; 187: 128–144. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier CJ, Fan AP. BOLD signal physiology: models and applications. Neuroimage 2019; 187: 116–127. [DOI] [PubMed] [Google Scholar]
- 45.Germuska M, Wise RG. Calibrated fMRI for mapping absolute CMRO2: practicalities and prospects. Neuroimage 2019; 187: 145–153. [DOI] [PubMed] [Google Scholar]
- 46.Prichard J, Rothman D, Novotny E, et al. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A 1991; 88: 5829–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Novotny EJ, Zhu XH, et al. Localized 1H NMR measurement of glucose consumption in the human brain during visual stimulation. Proc Natl Acad Sci U S A 1993; 90: 9896–9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 1997; 17: 64–72. [DOI] [PubMed] [Google Scholar]
- 49.Hudetz AG. Mathematical model of oxygen transport in the cerebral cortex. Brain Res 1999; 817: 75–83. [DOI] [PubMed] [Google Scholar]
- 50.Hyder F, Shulman RG, Rothman DL. A model for the regulation of cerebral oxygen delivery. J Appl Physiol (1985) 1998; 85: 554–564. [DOI] [PubMed] [Google Scholar]
- 51.Mintun MA, Lundstrom BN, Snyder AZ, et al. Blood flow and oxygen delivery to human brain during functional activity: Theoretical modeling and experimental data. Proc Natl Acad Sci U S A 2001; 98: 6859–6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers WJ, Hirsch IB, Cryer PE. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am J Physiol 1996; 270: H554–H559. [DOI] [PubMed] [Google Scholar]
- 53.Lin A-L, Fox PT, Hardies J, et al. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA 2010; 107: 8446–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanovic B, Warnking JM, Rylander KM, et al. The effect of global cerebral vasodilation on focal activation hemodynamics. Neuroimage 2006; 30: 726–734. [DOI] [PubMed] [Google Scholar]
- 55.Ress D, Thompson JK, Rokers B, et al. A model for transient oxygen delivery in cerebral cortex. Front. Neuroenerget 2009; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng Y, Martindale J, Johnston D, et al. A model of the hemodynamic response and oxygen delivery to brain. Neuroimage 2002; 16: 617–637. [DOI] [PubMed] [Google Scholar]
- 57.Vafaee MS, Gjedde A. Model of blood–brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab 2000; 20: 747–754. [DOI] [PubMed] [Google Scholar]
- 58.Cauli B, Hamel E. Brain perfusion and astrocytes. Trends Neurosci 2018; 41: 409–413. [DOI] [PubMed] [Google Scholar]
- 59.Magistretti PJ, Pellerin L, Rothman DL, et al. Energy on demand. Science 1999; 283: 496–497. [DOI] [PubMed] [Google Scholar]
- 60.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 2004; 95 [DOI] [PubMed] [Google Scholar]
- 61.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 2003; 6: 43–50. [DOI] [PubMed] [Google Scholar]
- 62.Guerra-Gomes S, Sousa N, Pinto L, et al. Functional roles of astrocyte calcium elevations: from synapses to behavior. Front Cell Neurosci 2018; 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 2009; 32: 160–169. [DOI] [PubMed] [Google Scholar]
- 64.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron 2011; 71: 782–797. [DOI] [PubMed] [Google Scholar]
- 65.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 2017; 96: 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A 2001; 98: 6417–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 1994; 91: 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothman DL, Dienel GA, Behar KL, et al. Glucose sparing by glycogenolysis determines the relationship between brain metabolism and neurotransmission. J Cereb Blood Flow Metab 2022; 0271678X2110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel AB, Lai JC, Chowdhury GM, et al. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci U S A 2014; 111: 5385–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyder F, Sanganahalli BG, Herman P, et al. Neurovascular and neurometabolic couplings in dynamic calibrated fMRI: transient oxidative neuroenergetics for block-design and event-related paradigms. Front Neuroenergetics 2010; 2: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyder F. Dynamic imaging of brain function. Methods Mol Biol 2009; 489: 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logothetis NK, Pauls J, Augath M, et al. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001; 412: 150–157. [DOI] [PubMed] [Google Scholar]
- 73.Buxton RB. Dynamic models of BOLD contrast. Neuroimage 2012; 62: 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoge RD. Calibrated fMRI. Neuroimage 2012; 62: 930–937. [DOI] [PubMed] [Google Scholar]
- 75.Pike GB. Quantitative functional MRI: concepts, issues and future challenges. Neuroimage 2012; 62: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 76.Smith AJ, Blumenfeld H, Behar KL, et al. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A 2002; 99: 10765–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kida I, Smith AJ, Blumenfeld H, et al. Lamotrigine suppresses neurophysiological responses to somatosensory stimulation in the rodent. Neuroimage 2006; 29: 216–224. [DOI] [PubMed] [Google Scholar]
- 78.Maandag NJ, Coman D, Sanganahalli BG, et al. Energetics of neuronal signaling and fMRI activity. Proc Natl Acad Sci U S A 2007; 104: 20546–20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanganahalli BG, Herman P, Blumenfeld H, et al. Oxidative neuroenergetics in event-related paradigms. J Neurosci 2009; 29: 1707–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herman P, Sanganahalli BG, Blumenfeld H, et al. Quantitative basis for neuroimaging of cortical laminae with calibrated functional MRI. Proc Natl Acad Sci U S A 2013; 110: 15115–15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanganahalli BG, Herman P, Rothman DL, et al. Metabolic demands of neural-hemodynamic associated and disassociated areas in brain. J Cereb Blood Flow Metab 2016; 36: 1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ekstrom A. How and when the fMRI BOLD signal relates to underlying neural activity: the danger in dissociation. Brain Res Rev 2010; 62: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herman P, Sanganahalli BG, Blumenfeld H, et al. Cerebral oxygen demand for short-lived and steady-state events. J Neurochem 2009; 109 Suppl 1: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Englot DJ, Mishra AM, Mansuripur PK, et al. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci 2008; 28: 9066–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mishra AM, Ellens DJ, Schridde U, et al. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/rij rat. J Neurosci 2011; 31: 15053–15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schridde U, Khubchandani M, Motelow JE, et al. Negative BOLD with large increases in neuronal activity. Cereb Cortex 2008; 18: 1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol 1989; 288: 401–413. [DOI] [PubMed] [Google Scholar]
- 88.Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 1985; 65: 37–100. [DOI] [PubMed] [Google Scholar]
- 89.Mark CI, Mazerolle EL, Chen JJ. Metabolic and vascular origins of the BOLD effect: implications for imaging pathology and resting-state brain function. J Magn Reson Imaging 2015; 42: 231–246. [DOI] [PubMed] [Google Scholar]
- 90.Hyder F, Rothman DL, Bennett MR. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci U S A 2013; 110: 3549–3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu Y, Herman P, Rothman DL, et al. Evaluating the gray and white matter energy budgets of human brain function. J Cereb Blood Flow Metab 2018; 38: 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blockley NP, Griffeth VEM, Simon AB, et al. A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed 2013; 26: 987–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bulte DP, Chiarelli PA, Wise RG, et al. Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab 2007; 27: 69–75. [DOI] [PubMed] [Google Scholar]
- 94.Blockley NP, Griffeth VE, Buxton RB. A general analysis of calibrated BOLD methodology for measuring CMRO2 responses: comparison of a new approach with existing methods. Neuroimage 2012; 60: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bulte DP, Kelly M, Germuska M, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage 2012; 60: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gauthier CJ, Hoge RD. Magnetic resonance imaging of resting OEF and CMRO2 using a generalized calibration model for hypercapnia and hyperoxia. Neuroimage 2012; 60: 1212–1225. [DOI] [PubMed] [Google Scholar]
- 97.Wise RG, Harris AD, Stone AJ, et al. Measurement of OEF and absolute CMRO2: MRI-based methods using interleaved and combined hypercapnia and hyperoxia. Neuroimage 2013; 83: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gauthier CJ, Hoge RD. A generalized procedure for calibrated MRI incorporating hyperoxia and hypercapnia. Hum Brain Mapp 2013; 34: 1053–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan AP, Schäfer A, Huber L, et al. Baseline oxygenation in the brain: Correlation between respiratory-calibration and susceptibility methods. NeuroImage 2016; 125: 920–931. [DOI] [PubMed] [Google Scholar]
- 100.Gauthier CJ, Desjardins-Crepeau L, Madjar C, et al. Absolute quantification of resting oxygen metabolism and metabolic reactivity during functional activation using QUO2 MRI. Neuroimage 2012; 63: 1353–1363. [DOI] [PubMed] [Google Scholar]
- 101.Hoge RD, Atkinson J, Gill B, et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 1999; 42: 849–863. [DOI] [PubMed] [Google Scholar]
- 102.Shu CY, Herman P, Coman D, et al. Brain region and activity-dependent properties of M for calibrated fMRI. Neuroimage 2016; 125: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage 2007; 35: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Driver ID, Wise RG, Murphy K. Graded Hypercapnia-calibrated BOLD: beyond the iso-metabolic hypercapnic assumption. Front Neurosci 2017; 11: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lu M, He Q, Li Y, et al. Influence of carbon dioxide plasma treatment on the dry adhesion of vertical aligned carbon nanotube arrays. Nanotechnology 2020; 31: 345701. [DOI] [PubMed] [Google Scholar]
- 106.Rodgers ZB, Jain V, Englund EK, et al. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J Cereb Blood Flow Metab 2013; 33: 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 2005; 25: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thesen T, Leontiev O, Song T, et al. Depression of cortical activity in humans by mild hypercapnia. Hum Brain Mapp 2012; 33: 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zappe AC, Uludağ K, Oeltermann A, et al. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex 2008; 18: 2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hare HV, Blockley NP, Gardener AG, et al. Investigating the field-dependence of the Davis model: calibrated fMRI at 1.5, 3 and 7T. Neuroimage 2015; 112: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Germuska M, Merola A, Murphy K, et al. A forward modelling approach for the estimation of oxygen extraction fraction by calibrated fMRI. Neuroimage 2016; 139: 313–323. [DOI] [PubMed] [Google Scholar]
- 112.Lu H, Zhao C, Ge Y, et al. Baseline blood oxygenation modulates response amplitude: physiologic basis for intersubject variations in functional MRI signals. Magn Reson Med 2008; 60: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Driver ID, Hall EL, Wharton SJ, et al. Calibrated BOLD using direct measurement of changes in venous oxygenation. Neuroimage 2012; 63: 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Englund EK, Fernández-Seara MA, Rodríguez-Soto AE, et al. Calibrated fMRI for dynamic mapping of CMRO2 responses using MR-based measurements of whole-brain venous oxygen saturation. J Cereb Blood Flow Metab 2020; 40: 1501–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hyder F, Herman P, Bailey CJ, et al. Uniform distributions of glucose oxidation and oxygen extraction in gray matter of normal human brain: no evidence of regional differences of aerobic glycolysis. J Cereb Blood Flow Metab 2016; 36: 903–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yablonskiy DA, Haacke EM. Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 1994; 32: 749–763. [DOI] [PubMed] [Google Scholar]
- 117.Hyder F, Kida I, Behar KL, et al. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed 2001; 14: 413–431. [DOI] [PubMed] [Google Scholar]
- 118.Fujita N, Matsumoto K, Tanaka H, et al. Quantitative study of changes in oxidative metabolism during visual stimulation using absolute relaxation rates. NMR Biomed 2006; 19: 60–68. [DOI] [PubMed] [Google Scholar]
- 119.Fujita N, Shinohara M, Tanaka H, et al. Quantitative mapping of cerebral deoxyhemoglobin content using MR imaging. Neuroimage 2003; 20: 2071–2083. [DOI] [PubMed] [Google Scholar]
- 120.Xu M, Bo B, Pei M, Chen Y, Shu CY, Qin Q, et al. High-resolution relaxometry-based calibrated fMRI in murine brain: metabolic differences between awake and anesthetized states. J Cereb Blood Flow Metab 2021; 271678X211062279. doi: 10.1177/0271678X211062279. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He X, Yablonskiy DA. Quantitative BOLD: mapping of human cerebral deoxygenated blood volume and oxygen extraction fraction: default state. Magn Reson Med 2007; 57: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stone AJ, Blockley NP. A streamlined acquisition for mapping baseline brainoxygenation using quantitative BOLD. Neuroimage 2017; 147: 79–88. [DOI] [PubMed] [Google Scholar]
- 123.An H, Lin W. Impact of intravascular signal on quantitative measures of cerebral oxygen extraction and blood volume under normo- and hypercapnic conditions using an asymmetric spin echo approach. Magn Reson Med 2003; 50: 708–716. [DOI] [PubMed] [Google Scholar]
- 124.Sedlacik J, Boelmans K, Löbel U, et al. Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. Neuroimage 2014; 84: 1032–1041. [DOI] [PubMed] [Google Scholar]
- 125.Shu CY, Sanganahalli BG, Coman D, et al. Quantitative β mapping for calibrated fMRI. Neuroimage 2016; 126: 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hennig J. Multiecho imaging sequences with low refocusing flip angles. J Magn Reson (1969) 1988; 78: 397–407. [Google Scholar]
- 127.Uddin MN, Marc Lebel R, Wilman AH. Transverse relaxometry with reduced echo train lengths via stimulated echo compensation. Magn Reson Med 2013; 70: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 128.Kaczmarz S, Hyder F, Preibisch C. Oxygen extraction fraction mapping with multi-parametric quantitative BOLD MRI: reduced transverse relaxation bias using 3D-GraSE imaging. NeuroImage 2020; 220: 117095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev 1954; 94: 630–638. [Google Scholar]
- 130.Jarenwattananon NN, Bouchard L-S. Breakdown of Carr-Purcell Meiboom-Gill spin echoes in inhomogeneous fields. J Chem Phys 2018; 149: 084304. [DOI] [PubMed] [Google Scholar]
- 131.Hyder F, Renken R, Kennan RP, et al. Quantitative multi-modal functional MRI with blood oxygenation level dependent exponential decays adjusted for flow attenuated inversion recoveries (BOLDED AFFAIR). Magn Reson Imaging 2000; 18: 227–235. [DOI] [PubMed] [Google Scholar]
- 132.Ma J, Wehrli FW. Method for image-based measurement of the reversible and irreversible contribution to the transverse-relaxation rate. J Magn Reson B 1996; 111: 61–69. [DOI] [PubMed] [Google Scholar]
- 133.Stables LA, Kennan RP, Gore JC. Asymmetric spin-echo imaging of magnetically inhomogeneous systems: theory, experiment, and numerical studies. Magn Reson Med 1998; 40: 432–442. [DOI] [PubMed] [Google Scholar]
- 134.Yablonskiy DA, Haacke EM. An MRI method for measuring T2 in the presence of static and RF magnetic field inhomogeneities. Magn Reson Med 1997; 37: 872–876. [DOI] [PubMed] [Google Scholar]
- 135.Ni W, Christen T, Zun Z, et al. Comparison of R2′ measurement methods in the normal brain at 3 tesla: comparison of R2′ measurement methods in brain at 3T. Magn Reson Med 2015; 73: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yablonskiy DA, Sukstanskii AL, He X. Blood oxygenation level-dependent (BOLD)-based techniques for the quantification of brain hemodynamic and metabolic properties – theoretical models and experimental approaches. NMR Biomed 2013; 26: 963–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blockley NP, Stone AJ. Improving the specificity of R2′ to the deoxyhaemoglobin content of brain tissue: prospective correction of macroscopic magnetic field gradients. Neuroimage 2016; 135: 253–260. [DOI] [PubMed] [Google Scholar]
- 138.Simon AB, Dubowitz DJ, Blockley NP, et al. A novel bayesian approach to accounting for uncertainty in fMRI-derived estimates of cerebral oxygen metabolism fluctuations. Neuroimage 2016; 129: 198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang F, Dong Z, Reese TG, et al. Echo planar time-resolved imaging (EPTI). Magn Reson Med 2019; 81: 3599–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang F, Dong Z, Tian Q, et al. Cortical-depth dependence of pure T2-weighted BOLD fMRI with minimal T2? contamination using Echo-planar time-resolved imaging. Proceedings of the ISMRM 2020; 1229.
- 141.Wyatt CR, Smith TB, Sammi MK, et al. Multi-parametric T2 * magnetic resonance fingerprinting using variable echo times. NMR Biomed 2018; 31: e3951. [DOI] [PubMed] [Google Scholar]
- 142.Hong T, Han D, Kim D-H. Simultaneous estimation of PD, T1, T2, T2*, and ΔB0 using magnetic resonance fingerprinting with background gradient compensation. Magn Reson Med 2019; 81: 2614–2623. [DOI] [PubMed] [Google Scholar]
- 143.Wang CY, Coppo S, Mehta BB, et al. Magnetic resonance fingerprinting with quadratic RF phase for measurement of T2* simultaneously with δf, T1, and T2. Magn Reson Med 2019; 81: 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rieger B, Zimmer F, Zapp J, et al. Magnetic resonance fingerprinting using echo-planar imaging: joint quantification of T1 and T2∗ relaxation times. Magn Reson Med 2017; 78: 1724–1733. [DOI] [PubMed] [Google Scholar]
- 145.Simon AB, Buxton RB. Understanding the dynamic relationship between cerebral blood flow and the BOLD signal: Implications for quantitative functional MRI. Neuroimage 2015; 116: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boxerman JL, Hamberg LM, Rosen BR, et al. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 1995; 34: 555–566. [DOI] [PubMed] [Google Scholar]
- 147.Uludağ K, Müller-Bierl B, Uğurbil K. An integrative model for neuronal activity-induced signal changes for gradient and spin echo functional imaging. Neuroimage 2009; 48: 150–165. [DOI] [PubMed] [Google Scholar]
- 148.Lajoie I, Nugent S, Debacker C, et al. Application of calibrated fMRI in Alzheimer’s disease. Neuroimage Clin 2017; 15: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ishii K, Mori E, Kitagaki H, et al. The clinical utility of visual evaluation of scintigraphic perfusion patterns for Alzheimer’s disease using I-123 IMP SPECT. Clin. Nucl. Med 1996; 21: 106–110. [DOI] [PubMed] [Google Scholar]
- 150.Göttler J, Kaczmarz S, Kallmayer M, et al. Flow-metabolism uncoupling in patients with asymptomatic unilateral carotid artery stenosis assessed by multi-modal magnetic resonance imaging. J Cereb Blood Flow Metab 2019; 39: 2132–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Champagne AA, Coverdale NS, Fernandez-Ruiz J, et al. Compromised resting cerebral metabolism after sport-related concussion: a calibrated MRI study. Brain Imaging Behav 2021; 15: 133–146. [DOI] [PubMed] [Google Scholar]
- 152.Fernández-Seara MA, Wehrli FW. Postprocessing technique to correct for background gradients in image-based R2* measurements. Magn Reson Med 2000; 44: 358–366. [DOI] [PubMed] [Google Scholar]
- 153.Liu EY, Guo J, Simon AB, et al. The potential for gas-free measurements of absolute oxygen metabolism during both baseline and activation states in the human brain. Neuroimage 2020; 207: 116342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dickson JD, Ash TWJ, Williams GB, et al. Quantitative BOLD: the effect of diffusion. J Magn Reson Imaging 2010; 32: 953–961. [DOI] [PubMed] [Google Scholar]
- 155.Christen T, Schmiedeskamp H, Straka M, et al. Measuring brain oxygenation in humans using a multiparametric quantitative blood oxygenation level dependent MRI approach. Magn Reson Med 2012; 68: 905–911. [DOI] [PubMed] [Google Scholar]
- 156.Kor D, Birkl C, Ropele S, et al. The role of iron and myelin in orientation dependent R2* of white matter. NMR Biomed 2019; 32: e4092. [DOI] [PubMed] [Google Scholar]
- 157.Rudko DA, Klassen LM, de Chickera SN, et al. Origins of R2* orientation dependence in gray and white matter. Proc Natl Acad Sci U S A 2014; 111: E159–E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaczmarz S, Göttler J, Zimmer C, et al. Characterizing white matter fiber orientation effects on multi-parametric quantitative BOLD assessment of oxygen extraction fraction. J Cereb Blood Flow Metab 2020; 40: 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Viessmann O, Scheffler K, Bianciardi M, et al. Dependence of resting-state fMRI fluctuation amplitudes on cerebral cortical orientation relative to the direction of B0 and anatomical axes. Neuroimage 2019; 196: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dickson JD, Ash TWJ, Williams GB, et al. Quantitative phenomenological model of the BOLD contrast mechanism. J Magn Reson 2011; 212: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berman AJL, Mazerolle EL, MacDonald ME, et al. Gas-free calibrated fMRI with a correction for vessel-size sensitivity. Neuroimage 2018; 169: 176–188. [DOI] [PubMed] [Google Scholar]
- 162.Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 1994; 31: 9–21. [DOI] [PubMed] [Google Scholar]
- 163.Kiselev VG and Posse S, Analytical model of susceptibility-induced MR signal dephasing: effect of diffusion in a microvascular network. Magnetic Resonance in Medicine 1999; 41: 499–509. [DOI] [PubMed]
- 164.Buxton RB, Uludağ K, Dubowitz DJ, et al. Modeling the hemodynamic response to brain activation. Neuroimage 2004; 23 Suppl 1: S220–33. [DOI] [PubMed] [Google Scholar]
- 165.Mohtasib RS, Lumley G, Goodwin JA, et al. Calibrated fMRI during a cognitive stroop task reveals reduced metabolic response with increasing age. Neuroimage 2012; 59: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 166.Cheng X, Berman AJL, Polimeni JR, et al. Dependence of the MR signal on the magnetic susceptibility of blood studied with models based on real microvascular networks. Magn Reson Med 2019; 81: 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lapi D, Marchiafava PL, Colantuoni A. Geometric characteristics of arterial network of rat pial microcirculation. J Vasc Res 2008; 45: 69–77. [DOI] [PubMed] [Google Scholar]
- 168.Zhao F, Wang P, Hendrich K, et al. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. NeuroImage 2006; 30: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 169.Duling BR, Berne RM. Longitudinal gradients inperiarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res 1970; 27: 669–678. [DOI] [PubMed] [Google Scholar]
- 170.Pittman RN, Duling BR. Measurement of percentoxyhemoglobin in the microvasculature. J Appl Physiol 1975; 38: 321–327. [DOI] [PubMed] [Google Scholar]
- 171.Gagnon L, Sakadžić S, Lesage F, et al. Quantifying the microvascular origin of BOLD-fMRI from first principles with two-photon microscopy and an oxygen-sensitive nanoprobe. J Neurosci 2015; 35: 3663–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gao J-H, Liu H-L. Inflow effects on functional MRI. Neuroimage 2012; 62: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 173.Cauley SF, Polimeni JR, Bhat H, et al. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med 2014; 72: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zahneisen B, Grotz T, Lee KJ, et al. Three-dimensional MR-encephalography: fast volumetric brain imaging using rosette trajectories. Magn Reson Med 2011; 65: 1260–1268. [DOI] [PubMed] [Google Scholar]
- 175.Kim S-G, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 2012; 32: 1188–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ragot DM, Chen JJ. Characterizing contrast origins and noise contribution in spin-echo EPI BOLD at 3 T. Magn. Reson. Imaging 2019; 57: 328–336. [DOI] [PubMed] [Google Scholar]
- 177.Triantafyllou C, Wald LL, Hoge RD. Echo-time and field strength dependence of BOLD reactivity in veins and parenchyma using flow-normalized hypercapnic manipulation. PLoS One 2011; 6: e24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Spano VR, Mandell DM, Poublanc J, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology 2013; 266: 592–598. [DOI] [PubMed] [Google Scholar]
- 179.Chen JJ, Pike GB. Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab 2010; 30: 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Driver ID, Whittaker JR, Bright MG, et al. Arterial CO2 fluctuations modulate neuronal rhythmicity: implications for MEG and fMRI studies of resting-state networks. J Neurosci 2016; 36: 8541–8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hall EL, Driver ID, Croal PL, et al. The effect of hypercapnia on resting and stimulus induced MEG signals. Neuroimage 2011; 58: 1034–1043. [DOI] [PubMed] [Google Scholar]
- 182.Uh J, Xu F, Yezhuvath U, et al. The effect of hypercapnia on resting state fMRI. NeuroImage 2009; 47: S185. [Google Scholar]
- 183.Xu F, Uh J, Brier MR, et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab 2011; 31: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chiarelli PA, Bulte DP, Wise R, et al. Calibration method for quantitative BOLD fMRI based on hyperoxia. Neuroimage 2007; 37: 808–820. [DOI] [PubMed] [Google Scholar]
- 185.Ma Y, Berman AJL, Pike GB. The effect of dissolved oxygen on the relaxation rates of blood plasma: implications for hyperoxia calibrated BOLD. Magn Reson Med 2016; 76: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 186.Schwarzbauer C, Deichmann R. Vascular component analysis of hyperoxic and hypercapnic BOLD contrast. Neuroimage 2012; 59: 2401–2412. [DOI] [PubMed] [Google Scholar]
- 187.Berman AJL, Ma Y, Hoge RD, et al. The effect of dissolved oxygen on the susceptibility of blood. Magn Reson Med 2016; 75: 363–371. [DOI] [PubMed] [Google Scholar]
- 188.Hantzidiamantis PJ, Amaro E. Physiology, alveolar to arterial oxygen gradient. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. [PubMed] [Google Scholar]
- 189.Herman P, Sanganahalli BG, Hyder F. Multimodal measurements of blood plasma and red blood cell volumes during functional brain activation. J Cereb Blood Flow Metab 2009; 29: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu F, Li W, Liu P, et al. Accounting for the role of hematocrit in between-subject variations of MRI-derived baseline cerebral hemodynamic parameters and functional BOLD responses. Hum Brain Mapp 2018; 39: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang Z, Craddock RC, Milham MP. Impact of hematocrit on measurements of the intrinsic brain. Front Neurosci 2015; 8: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen JJ, Pike GB. Human whole blood T2 relaxometry at 3 tesla. Magn Reson Med 2009; 61: 249–254. [DOI] [PubMed] [Google Scholar]
- 193.Liu P, Dimitrov I, Andrews T, et al. Multisite evaluations of a T2 -relaxation-under-spin-tagging (TRUST) MRI technique to measure brain oxygenation. Magn Reson Med 2016; 75: 680–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magn Reson Med 2003; 49: 568–571. [DOI] [PubMed] [Google Scholar]
- 195.Stefanovic B, Pike GB. Human whole-blood relaxometry at 1.5 T: assessment of diffusion and exchange models. Magn Reson Med 2004; 52: 716–723. [DOI] [PubMed] [Google Scholar]
- 196.Zhao JM, Clingman CS, Narvainen MJ, et al. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn Reson Med 2007; 58: 592–597. [DOI] [PubMed] [Google Scholar]
- 197.Liu P, Chalak LF, Krishnamurthy LC, et al. T1 and T2 values of human neonatal blood at 3 tesla: dependence on hematocrit, oxygenation, and temperature. Magn Reson Med 2016; 75: 1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Portnoy S, Seed M, Sled JG, et al. Non-invasive evaluation of blood oxygen saturation and hematocrit from T1 and T2 relaxation times: in-vitro validation in fetal blood. Magn Reson Med 2017; 78: 2352–2359. [DOI] [PubMed] [Google Scholar]
- 199.Li W, Liu P, Lu H, et al. Fast measurement of blood T1 in the human carotid artery at 3T: accuracy, precision, and reproducibility. Magn Reson Med 2017; 77: 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lu H, Clingman C, Golay X, et al. Determining the longitudinal relaxation time (T1) of blood at 3.0 tesla. Magn Reson Med 2004; 52: 679–682. [DOI] [PubMed] [Google Scholar]
- 201.Calamante F, Ahlgren A, van Osch MJP, et al. A novel approach to measure local cerebral haematocrit using MRI. J Cereb Blood Flow Metab 2016; 36: 768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li W, Xu X, Liu P, et al. Quantification of whole-brain oxygenation extraction fraction and cerebral metabolic rate of oxygen consumption in adults with sickle cell anemia using individual T2-based oxygenation calibrations. Magn Reson Med 2020; 83: 1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grubb RL, Raichle ME, Eichling JO, et al. Ter-Pogossian MM. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 1974; 5: 630–639. [DOI] [PubMed] [Google Scholar]
- 204.Chen JJ, Pike GB. BOLD-specific cerebral blood volume and blood flow changes during neuronal activation in humans. NMR Biomed 2009; 22: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 205.Stefanovic B, Hutchinson E, Yakovleva V, et al. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab 2008; 28: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ances BM, Leontiev O, Perthen JE, et al. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: implications for BOLD-fMRI. Neuroimage 2008; 39: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Griffeth VEM, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage 2011; 58: 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mark CI, Pike GB. Indication of BOLD-specific venous flow-volume changes from precisely controlled hyperoxic vs. hypercapnic calibration. J Cereb Blood Flow Metab 2012; 32: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tian P, Teng IC, May LD, et al. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci U S A 2010; 107: 15246–15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim T, Kim S-G. Temporal dynamics and spatial specificity of arterial and venous blood volume changes during visual stimulation: implication for BOLD quantification. J Cereb Blood Flow Metab 2011; 31: 1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wesolowski R, Blockley NP, Driver ID, et al. Coupling between cerebral blood flow and cerebral blood volume: contributions of different vascular compartments. NMR Biomed 2019; 32: e4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duan A, Bedggood PA, Bui BV, et al. Evidence of flicker-induced functional hyperaemia in the smallest vessels of the human retinal blood supply. PLoS One 2016; 11: e0162621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hillman EMC, Devor A, Bouchard MB, et al. Depth-resolved optical imaging and microscopy of vascular compartment dynamics during somatosensory stimulation. NeuroImage 2007; 35: 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hua J, Liu P, Kim T, et al. MRI techniques to measure arterial and venous cerebral blood volume. Neuroimage 2019; 187: 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: implications for dynamic functional magnetic resonance imaging calibration. J Cereb Blood Flow Metab 2007; 27: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kontos HA, Raper AJ, Patterson JL. Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke 1977; 8: 358–360. [DOI] [PubMed] [Google Scholar]
- 217.Aribisala BS, Wiseman S, Morris Z, et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014; 45: 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018; 114: 1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 220.Lacombe P, Oligo C, Domenga V, et al. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke 2005; 36: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 221.Shen Q, Ren H, Cheng H, et al. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab 2005; 25: 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Blockley NP, Griffeth VEM, Stone AJ, et al. Sources of systematic error in calibrated BOLD based mapping of baseline oxygen extraction fraction. Neuroimage 2015; 122: 105–113. [DOI] [PubMed] [Google Scholar]
- 223.McHedlishvili G, Varazashvili M. Hematocrit in cerebral capillaries and veins under control and ischemic conditions. J Cereb Blood Flow Metab 1987; 7: 739–744. [DOI] [PubMed] [Google Scholar]
- 224.Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res 1996; 32: 632–643. [PubMed] [Google Scholar]
- 225.Sakadžić S, Mandeville ET, Gagnon L, et al. Large arteriolar component of oxygen delivery implies a safe margin of oxygen supply to cerebral tissue. Nat Commun 2014; 5: 5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X221077338 for Mapping oxidative metabolism in the human brain with calibrated fMRI in health and disease by J Jean Chen, Biranavan Uthayakumar and Fahmeed Hyder in Journal of Cerebral Blood Flow & Metabolism