Abstract
The growth of marine bacteria under iron-limited conditions was investigated. Neither siderophore production nor bacterial growth was detected for Pelagiobacter sp. strain V0110 when Fe(III) was present in the culture medium at a concentration of <1.0 μM. However, the growth of V0110 was strongly stimulated by the presence of trace amounts of exogenous siderophore from an alpha proteobacterium, V0902, and 1 nM N-acyl-octanoylhomoserine lactone (C8-HSL), which is known as a quorum-sensing chemical signal. Even though the iron-binding functionality of a hydroxamate siderophore was undetected in the supernatant of V0902, a hydroxamate siderophore was detected in the supernatant of V0110 under the above conditions. These results indicated that hydroxamate siderophore biosynthesis by V0110 began in response to the exogenous siderophore from V0902 when in the presence of C8-HSL; however, C8-HSL production by V0110 and V0902 was not detected. Direct interaction between V0902 and V0110 through siderophore from V0902 was observed in the dialyzing culture. Similar stimulated growth by exogenous siderophore and HSL was also observed in other non-siderophore-producing bacteria isolated from marine sponges and seawater. The requirement of an exogenous siderophore and an HSL for heterologous siderophore production indicated the possibility that cell-cell communication between different species was occurring.
Iron is an essential element for all microorganisms (37, 43). To survive under iron-deficient conditions, terrestrial microorganisms produce siderophores, low-molecular-mass iron-chelating compounds which bind iron with high affinity (Kaff > 1030) (26). Due to the low iron concentration (<0.4 μM) in the ocean, marine bacteria are thought to be capable of producing siderophores (14, 41); however, few marine siderophores have been identified (22, 32). Recent geochemical investigations on the distribution of iron in seawater have demonstrated that more than 99.9% of the dissolved iron in the surface ocean is bound to organic compounds (13, 18, 33, 44). The chemical nature of these organic compounds is uncertain. Due to high binding activities with Fe(III), siderophores biosynthesized by marine bacteria are a likely candidate for the iron-binding organic compounds in the ocean.
It has been reported that siderophore biosynthesis in the pathogenic bacterium Pseudomonas aeruginosa is controlled by a quorum-sensing system (39). Quorum sensing is cell-density-dependent regulation of specific gene expression in response to extracellular chemical signals produced by the bacteria themselves (9). In a wide range of gram-negative bacteria, quorum sensing was identified to be based on one or more N-acyl homoserine lactones (HSLs) (11). All HSLs thus far reported are composed of an acyl chain with an even number of carbon atoms ranging from 4 to 14 in length, ligated to the homoserine lactone moiety (38). Although quorum-sensing-related siderophore biosynthesis has also been reported in the pathogenic bacterium Burkholderia cepacia (20), siderophores produced by this strain and P. aeruginosa were thought to be the virulence factors related to their pathogenesis. No quorum-sensing-controlled siderophore biosynthesis system has been found from the open ocean.
Recent investigations have reported that iron availability limits phytoplankton growth in large areas of the world's oceans and may influence biological carbon flow (4, 5, 21). The response of bacteria to iron enrichment has also been investigated, and marine bacteria were found to contain more iron per biomass than phytoplankton (40, 41). Iron uptake competition among phytoplankton through different siderophores has also been reported (16). It is suggested that siderophores produced by marine bacteria may be a very important factor which affects the “iron flow” in the ocean among bacteria or between bacteria and other microorganisms. To understand such bacterial communication related to iron uptake activity through siderophores in the ocean, we focused on the influence of siderophores and on quorum-sensing chemical signals during bacterial growth under iron-limited poor nutrient conditions similar to those of the natural ocean. In the present study, stimulated bacterial growth in the presence of siderophores and synthetic HSLs under iron-deficient conditions was reported.
MATERIALS AND METHODS
Strains and culture conditions.
Bacteria were isolated from 17 different marine sponges collected in Fiji and from seawater from four locations near Japan. The sponge tissue was squeezed, the solutions obtained were diluted from 10−1 to 10−4 times in sterilized seawater, and 100 μl of each solution was spread onto 1/10-diluted marine broth (Marine Broth 2216; Difco) agar plates. The plates were incubated at 30°C, and marine bacteria were identified by growth on the plates containing 3% NaCl in comparison with no growth on the agar plates containing 0.15% NaCl for the same strain.
The iron-deficient and low-nutrient seawater-based liquid medium containing 0.1 μM Fe(III) (IDSM medium) contained the following components (in grams/liter): NH4NO3, 1.0; NaCl, 30.0; MgSO4 · 7H2O, 0.5; KCl, 0.3; K2HPO4, 1.5; C8H18N2O4S (HEPES), 2.38; CaCl2, 0.2. It also contained 10% glucose (10 ml) and 0.1 ml of 1 mM FeCl3. The medium was adjusted to pH 7.2 and was treated by Chelex-100 (Sigma) (6) before the addition of FeCl3 solution. Liquid cultivation of bacteria was performed in 10 ml of IDSM medium with a Bio-Photorecorder TN-2612 (Advantec). The cultures were shaken at 50 rpm and 30°C for a week, and the optical density at 600 nm (OD600) was measured every 15 min. Glassware was acid washed in 6 N HCl for 24 h before use.
Nearly full-length 16S ribosomal DNA (rDNA) of strains V0902 and V0110 was amplified by using two oligonucleotide primers, fD (5′-AGAGTTTGATCCTGGCTCAG-3′) and rD (5′-AAGGAGGTGATCCAGCC-3′) (42), and was sequenced by using a 373 DNA sequencer (PE Biosystems).
Pelagiomicin production.
Pelagiomicin production by strain V0110 was determined by the following methods. After culturing of strain V0110 in marine broth liquid medium at 30°C for 24 h, the supernatant was collected by centrifugation and was extracted with CHCl3. The organic extract was concentrated to dryness. The residue was dissolved in 10 mM phosphate buffer (pH 7.0) and was analyzed by high-pressure liquid chromatography (HPLC) (Shiseido Capcell-CN column; 15 by 25 mm) at 265 nm. The elution gradient consisted of CH3CN in phosphate buffer (pH 7.0). HPLC active fraction was confirmed to be pelagiomicin A by analysis of nuclear magnetic resonance (NMR) spectra (17).
Siderophore detection.
The chrome azurol S (CAS) assay (35) was used to detect siderophores. On CAS agar plates, siderophore-producing (Sid+) bacteria form colonies with an orange halo. This occurs because iron is removed from the original blue CAS-Fe(III) complex during siderophore production. Formation of siderophore halos was evaluated following 5 days of colony incubation at 30°C. The CAS solution assay (14, 35) was used to quantitate siderophore activity in culture supernatant extract by measuring the decrease in the absorbance of blue color at 630 nm. Standard curves relating CAS reactivity to the iron-binding ligands were determined using the fungal siderophore desferrioxamine (Desferal; CIBA-GEIGY). The quantity of siderophores produced by the bacteria was reported in terms of iron-binding equivalents, expressed as moles per gram (dry weight) of bacteria (14). Hydroxamate and catechol functionality of 10-fold-concentrated siderophore extracts of V0902 and V0110 were examined by the Csaky test (12) and the Arnow reaction (1), respectively. In these assays, hydroxylamine and 2,3-dihydroxybenzoic acid, respectively, were used as the standards. Strains which neither grew nor formed a halo on the CAS agar plates containing different Fe(III) concentrations (10−4 to 10 μM) were defined as non-siderophore-producing (Sid−) bacteria.
Isolation of siderophores.
Bacterial cultures grown in 200 ml of IDSM medium for 2 days at 30°C were harvested by centrifugation at 8,000 rpm for 30 min. Iron-free siderophores were obtained by the following method. The supernatant was filtered through a 0.2-μm (pore-size) membrane filter to completely remove the cells and was acidified to pH 3 with concentrated HCl. Supernatants were extracted three times with equal volumes of ethyl acetate for catechols and benzyl alcohol for hydroxamates (27). The concentrated organic extracts were dissolved in 1 ml of a buffer (0.01 M phosphate buffer [pH 7.0] or 0.01 M acetate buffer [pH 4.0]). Partial purification of the siderophores was achieved by the fractionation of the organic extracts on a Sephadex LH-20 (Pharmacia) column in the respective buffers. The eluting solutions were purified with Chelex-100 to remove the iron. The CAS assay-reactive fractions were pooled and concentrated 10-fold by lyophilization.
Chemical synthesis of HSLs.
N-(3-Oxohexanoyl)-l-HSL was prepared according to the procedure of Chhabra et al. (3). N-(3-Oxooctanoyl)-, N-(3-oxodecanoyl)-, and N-(3-oxododecanoyl)-l-HSLs were synthesized as described previously (34, 46). The final products were purified by silica gel column chromatography (Merck Kieselgel 60, CHCl3-MeOH) followed by ODS open column chromatography (Cosmosil 5C18-AR; MeOH-H2O). Synthesis of N-[(S)-3-hydroxybutyryl]-l-HSL was as previously described (2). Unsubstituted acyl HSLs were synthesized as follows. Triethylamine (1.1 eq) and pyridine (1.5 eq) were successively added to a suspension of l-HSL hydrochloride (2.5 mmol) in anhydrous CH2Cl2 (10 ml) at 0°C. To this mixture, the corresponding acyl chloride (1.1 eq) was added dropwise. The mixture was stirred at room temperature overnight and poured into 1 M HCl. The aqueous layer was extracted with CH2Cl2, and the combined organic layer was dried over Na2SO4, filtrated, and concentrated to give an unsubstituted acyl HSL as a colorless powder. The purity of the synthetic HSL was checked by thin-layer chromatography (TLC), HPLC, and NMR.
Agrobacterium tumefaciens A136 reporter strain assay.
The A. tumefaciens A136/(pCF218)(pCF372) reporter strain was kindly supplied by Clay Fuqua (University of Indiana) (10). This strain has been reported to detect a wide range of HSL compounds, including compounds with acyl chains of greater than four carbons and compounds with 3-oxo- or 3-hydroxyl substituents, including compounds that were unsubstituted at this position. After cultivation of the strain in 100 ml of IDSM medium or Marine Broth 2216 to stationary phase, the supernatant was collected by centrifugation at 8,000 rpm for 10 min. The supernatant was extracted with an equal volume of ethyl acetate three times. The combined organic fraction was dried over Na2SO4, concentrated, and dissolved in 100 μl of methanol and applied to a reversed-phase TLC system (Merck HPTLC plate RP-18 WF254S, 10 by 9 cm; 60% MeOH in water). HSL autoinducers were detected by TLC overlay assay using the reporter strain immobilized in the agar that contained the chromogenic X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (36, 47). HSL autoinducers cause the formation of blue spots on the TLC plates. The synthetic HSLs were used as standards.
With this assay, the limits of detection for synthetic C6-, 3OC6-, C8-, 3OC8-, and C10-HSLs were found to be 1, 10−3, 10−3, 10−4, and 1 nM, respectively.
Cross-feeding assay for Sid− bacteria with exogenous siderophores and synthetic HSLs.
Sid− strains were inoculated on four different CAS plates: (i) without any addition, (ii) with the addition of an exogenous siderophore, (iii) with the addition of an HSL, and (iv) with the addition of an exogenous siderophore plus an HSL. The exogenous siderophore was added to the plates by spreading 500 μl of filtered siderophore extract (0.155 nmol of ligands/g [dry weight]) buffer-dissolved solution from Sid+ strain V0902. Each HSL was added by spreading 100 μl of different concentrations (10−3 to 101 μM) of synthetic HSL solution. Plates and colonies were observed after incubation at 30°C for 7 days.
Dialyzing cultures of Sid− strain V0110 and Sid+ strain V0902.
A dialyzing culture of V0902 and V0110 was designed in Centriprep centrifugal filters (Amicon). The Centriprep centrifugal filter consists of two parts: a sample container and a filtrate collector with a low adsorptive regenerated cellulose dialyzing membrane. Centriprep-10 (nominal molecular weight limit, 10,000) was used because it has a wide range for compounds to pass through the membrane easily. The integrity of the Centriprep membrane after heat sterilization was checked by cultivating the bacteria in the filtrate collector or the sample container at 30°C for 14 days and spreading the cultures onto marine broth agar plates. No bacteria were found to pass through the membrane. The Sid+ strain V0902 was inoculated into the sample container with 5 ml of IDSM medium. The Sid− strain V0110 was inoculated into the filtrate collector with 5 ml of IDSM medium plus 1 nM synthetic C8-HSL. The filtrate collector was positioned with the air-seal cap to give a 0.7-cm space between the membrane support base and the bottom of the sample container. The Centriprep-10 was shaken at 100 rpm at room temperature for 7 days. Then, 100 μl of each culture was collected every 24 h, and the OD600 was measured using a Beckman spectrophotometer DU640 to estimate the bacterial growth.
Nucleotide sequence accession number.
The DDBJ GenBank accession number of the sequence for V0902 is AB012864.
RESULTS
Siderophore and HSL production of marine bacterial strains V0110 and V0902.
The marine bacterial strains V0110 and V0902 were isolated from the marine sponges Jaspis joinstoni and Plakortis lita de Laubenfels, respectively. They were identified as marine species by their halophilic growth (NaCl > 3.0%). The 16S rDNA sequence of V0110 is 98% identical to that of the marine bacterium Pelagiobacter variabilis, which is a halophilic gram-negative bacterium isolated from a macroalga (17). Pelagiomicin antibiotic production has only been reported in this genus (17). Production of pelagiomicin A (3.6 mg/liter) by strain V0110 also indicated that this bacterium is a Pelagiobacter sp. The 16S rDNA sequence of V0902 is 97% identical to that of an alpha proteobacterium (AB012864 [DDBJ]).
The bacterial growth of V0110 and V0902 under iron-deficient conditions was investigated with 0.1 μM Fe(III)-containing marine broth agar plates. V0902 was observed to form colonies for generations on the iron-deficient agar plate, while no colonies were observed for V0110. Siderophore production by V0902 and V0110 was investigated using the CAS agar plates. Strain V0110 was shown to be a Sid− strain by screening on CAS agar plates which contained 10−4 to 10 μM Fe(III). Strain V0902 showed siderophore production on CAS agar plates and was categorized to be a Sid+ strain. The siderophore component from V0902 was partially purified by extraction with ethyl acetate or benzyl alcohol (27) and was evaluated by the CAS solution assay (14, 35). The CAS assay result indicated that strain V0902 produced ca. 0.31 μmol of iron ligands per g (cell dry weight) after cultivation in 200 ml of IDSM medium (Table 1).
TABLE 1.
Concentration of iron ligands in V0902 and V0110 in supernatant extracts of 200 ml of IDSM medium containing 0.1 μM Fe(III)
| Strain | Mean siderophore content ± SDa as detected by:
|
||
|---|---|---|---|
| CAS assay | Csaky test | Arnow reaction | |
| V0902 | 0.31 ± 0.15 | ND | ND |
| V0110 (induced) | 0.26 ± 0.11 | 0.16 ± 0.09 | |
Values are given as micromoles of ligand per gram (dry weight) of cells and represent the mean of three experiments. ND, not detected (i.e., the concentration was below the detection limit of the assay).
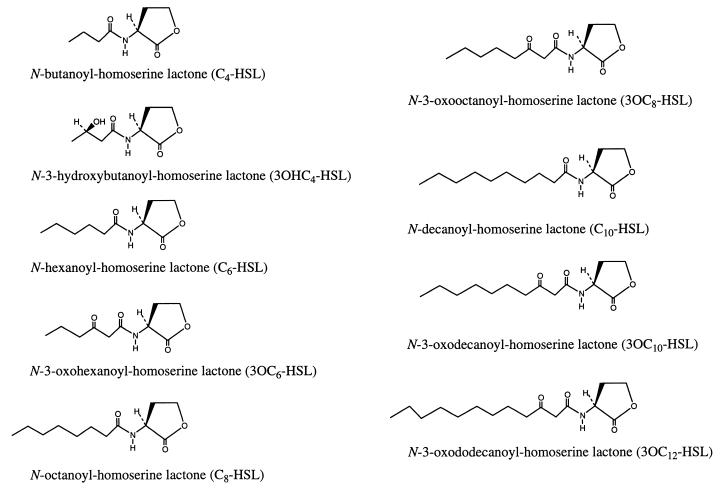
The chemical structures of synthetic HSL autoinducers are shown in Fig. 1. Production of HSL autoinducers by V0110 and V0902 was investigated with the A. tumefaciens A136 reporter strain assay (47) using the synthetic compounds as standards. C6-, 3OC6-, C8-, 3OC8-, and C10-HSLs were not detected in supernatant extracts of V0110 and V0902 by this reporter strain assay.
FIG. 1.
Chemical structures of nine synthetic N-acyl HSL autoinducers which have been characterized in various bacteria (2, 7, 8, 19, 23, 25, 28, 29, 47).
Stimulated growth of V0110 with the addition of exogenous siderophore and C8-HSL.
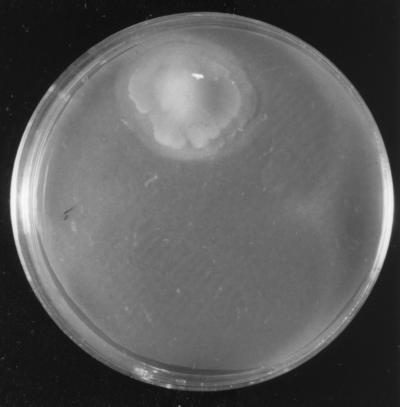
A cross-feeding assay for V0110 was performed on CAS agar plates with the addition of each siderophore extract (0.155 nmol/g [dry weight]) from V0902 or of one of the HSLs at 1 nM or with the addition of a siderophore extract from V0902 plus one of the HSLs at 1 nM. V0110 was observed to grow only on the agar plate that contained both the siderophore extract from V0902 and 1 nM C8-HSL (Fig. 2 and Table 2). Figure 2 shows the colony formation of V0110 on the CAS plate with the above-described additions. The siderophore component from V0902 added to the CAS agar plate was 0.155 nmol of iron ligands/g (dry weight), and this amount was 200 times less than that used for the CAS assay. This amount did not induce any color change on the CAS agar plates. The colony halo indicated the possible siderophore production by V0110.
FIG. 2.
Pelagiobacter sp. strain V0110 following incubation with exogenous siderophore extract (0.155 nmol/g [dry weight]) from alpha proteobacterium V0902 and 1 nM C8-HSL on a CAS agar plate at 30°C for 7 days.
TABLE 2.
Influence of V0902 siderophore and HSL autoinducers on bacterial growth of Sid− marine bacteria under iron-deficient conditions
| Sid− strain | Origin | Bacterial growtha with siderophore from V0902 and 1 nM:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No HSL | C4-HSL | 3OHC4-HSL | C6-HSL | 3OC6-HSL | C8-HSL | 3OC8-HSL | C10-HSL | 3OC10-HSL | 3OC12-HSL | ||
| V0110 | Sponge | − | − | − | − | − | + | − | + | − | − |
| V0101 | Sponge | − | − | − | − | − | − | + | − | − | − |
| V0211 | Sponge | − | − | − | − | − | − | − | + | + | − |
| V0328 | Sponge | − | − | − | + | + | + | − | − | − | − |
| V0701 | Sponge | − | − | − | − | − | − | − | − | + | + |
| V0801 | Sponge | − | − | − | − | − | − | + | − | + | − |
| V0803 | Sponge | − | − | − | − | − | − | + | + | − | − |
| V1104 | Sponge | − | − | − | − | − | + | + | − | − | − |
| GMO-22 | Seawater | − | − | − | + | − | − | − | − | − | − |
| GMO-25 | Seawater | − | − | − | + | − | − | − | − | − | − |
| GMO-31 | Seawater | − | − | − | − | − | + | − | − | − | − |
| GMO-54 | Seawater | − | − | − | − | − | − | − | − | − | + |
+, the same phenomenon was observed with V0110 (Fig. 2) in this study.
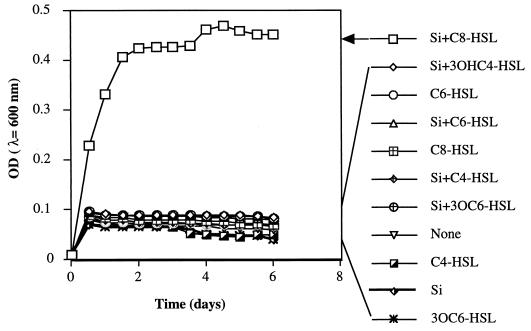
To confirm the cooperative influences of the exogenous siderophore and HSL on the growth of V0110 in the liquid medium, V0110 was grown in IDSM medium containing 0.1 μM Fe(III) with the addition of the same amount of siderophore extract from V0902 and each of the HSLs. As shown in Fig. 3, there was no obvious bacterial growth with exogenous siderophore only; with 1 nM C4-HSL, 3OHC4-HSL, C6-HSL, 3OC6-HSL, and C8-HSL alone; or with exogenous siderophore plus four of the HSLs except for C8-HSL. However, when 1 nM C8-HSL and the siderophore extract of V0902 were added together, growth began on the first day and increased from 0.0097 to 0.4673 (λ = 600 nm) over 4 days. These results coincided with observations on the CAS agar plate shown in Fig. 2.
FIG. 3.
Growth of Pelagiobacter sp. strain V0110 at 30°C in IDSM medium with or without siderophore extract from V0902 (0.155 nmol/g [dry weight]) plus 1 nM synthesized C4-, 3OHC4-, C6-, 3OC6-, and C8-HSLs or with siderophore extraction of V0902 and synthesized HSL. Si, siderophore of V0902. Each point represents the mean coaggregation value from three separate experiments.
The Csaky test and Arnow reaction were used to estimate the typical functional groups bound to iron in the siderophore extracts of V0902 and V0110 (Table 1). These two assays are well known for the detection of hydroxamate or catechol groups, respectively (1, 12). The CAS-detectable iron-chelating component produced by V0902 did not have positive reactions in either assay. Negative results for V0902 shown in Table 1 indicated two possibilities: (i) the produced siderophore did not have either of two functional groups, hydroxamate or catecholate, and (ii) the two assays were not sensitive enough to detect a low production of siderophore from V0902. The siderophore extract of V0110 gave a positive result in the Csaky test, while it gave a negative result in the Arnow test. This revealed that a hydroxamate moiety was present in the siderophore component of V0110.
Dialyzing culture of V0902 and V0110.
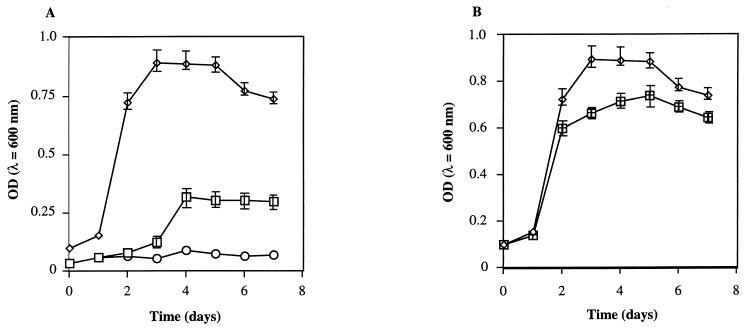
To analyze direct interactions between the Sid− strain V0110 and the Sid+ strain V0902, simultaneous cultivation of the two strains in Centriprep centrifugal filters was designed. The monitored growth of both strains in the dialyzing culture or independent culture is shown in Fig. 4. In the presence of 1 nM C8-HSL, the bacterial growth of the Sid− strain V0110 was not detected during the first 2 days and started after strain V0902 reached stationary phase. The same stimulated growth of V0110 in the presence of 1 nM C8-HSL was observed when 0.155 nmol/g (dry weight) of siderophore extract from V0902 was added to the sample container instead of the inoculation of strain V0902 (data not shown). The growth of V0110 in an independent culture with the direct addition of siderophore extract was observed to be faster than that seen in dialyzing culture, but the maximum cell density was almost the same (data not shown). No growth was observed without C8-HSL under any conditions of dialyzing cultivation (Fig. 4A). The growth of Sid+ strain V0902 under dialyzing cultivation with Sid− strain V0110 showed a higher density than that seen under independent cultivation with an equal starting cell number.
FIG. 4.
Dialyzing culture of Sid+ strain V0902 and Sid− strain V0110. (A) Growth of V0902 and V0110 each in 5 ml of IDSM medium separated in a Centriprep-10 centrifugal filter at room temperature for 7 days. Symbols: ◊, V0902; □, V0110 with 1 nM C8-HSL; ○, without C8-HSL. (B) Comparison of dialyzing culture (◊) and independent culture (⊞) of V0902 in 5 ml of IDSM medium in a Centriprep-10 centrifugal filter at room temperature for 7 days. The data represent the averages of three measurements.
Influence of exogenous siderophores and autoinducers on Sid− marine bacteria from sponges and seawater.
To estimate whether the effect of the exogenous siderophore and the HSL autoinducer is a specific activity of V0110 or is universal for marine bacteria, we investigated another 20 Sid− bacteria which did not respond by growth to exogenous siderophore from V0902. Table 2 shows the positive results of bacterial colony and siderophore halo formation with the addition of exogenous siderophore from V0902 and one of nine HSLs on CAS agar plates. All strains shown were Sid− and non-HSL-producing bacteria as determined by CAS plate and A. tumefaciens A136 reporter strain assays. The stimulated growth of such Sid− strains under iron-limited conditions was similar to that observed with strain V0110 when incubated in the presence of the V0902 siderophore and C8-HSL. It is interesting that more than one kind of autoinducer showed a positive result in the same strain such as in C10, where the bacterial growth of V0110 was stimulated in response to the same siderophore. However, the HSLs with butanoyl chains did not affect any tested Sid− strains here.
DISCUSSION
Some terrestrial Sid− bacteria have been reported to survive under iron stress in the presence of exogenous siderophores (30, 31). However, it is not clear whether those bacteria might be stimulated to synthesize their own siderophores. Only the Csaky assay gave a positive result for siderophore extract from V0110 cultured with a trace amount of siderophore extract from V0902 and C8-HSL, while siderophore extract from V0902 gave a negative result in both of the assays. This finding indicates that a hydroxamate siderophore was synthesized by V0110 only in the presence of trace amounts of exogenous siderophore from V0902 and C8-HSL autoinducer. This phenomenon has not been reported before. The dialyzing culture of V0110 and V0902 also indicates direct bacterial interaction through siderophores in the presence of HSL. The growth of V0110 in the dialyzing culture with C8-HSL suggests that the siderophore produced by V0902 in the sample container diffused to the filtrate collector through the membrane and enabled the growth of V0110 to commence after 2 days. It is well known that siderophore production reaches a peak level in the stationary phase (27). The independent culture of V0110 with the direct addition of siderophore extract and C8-HSL was more rapid in the growth than in the dialyzing culture. The 2-day delay in the commencement of V0110 growth in the dialyzing culture might be due to the delay in reaching the threshold concentration of V0902 siderophore in the filtrate collector. Almost the same maximum cell density was observed for V0110 in both the dialyzing and the independent cultures. This result indicates that the growth rate of V0110 does not depend on the amount of siderophore from V0902 and thus suggests that the siderophore from V0902 is a signal for V0110 to commence synthesis of its own hydroxamate siderophore. Direct interaction of V0110 and V0902 through the siderophores suggests that siderophores might be a signal for interspecies bacterial communication.
Quorum-sensing-controlled siderophore production has been shown to occur in P. aeruginosa (39) and B. cepacia (20). Quorum-sensing systems in gram-negative bacteria have been well studied (9), and HSLs have been known to be the membrane-permeable signaling molecule. So far, most of the quorum sensing has been found to occur in symbiotic or pathogenic interactions. It is not unexpected that V0110, which was isolated from a marine sponge, responds to HSL signaling because a marine sponge is a symbiotic organism with large amounts of bacteria and microalgae. The requirement of C8-HSL or C10-HSL for the stimulated biosynthesis of heterologous siderophore indicates that HSL also might be a signaling molecule for V0110. No production of C8-HSL and C10-HSL was detected in V0110 and V0902 by the A. tumefaciens A136 reporter strain assay. This suggests possible interspecies communication through C8-HSL or C10-HSL produced by other species. Communication between different species through HSLs has been reported in Vibrio harveyi for its bioluminescence (15) and during B. cepacia for its production of virulence factors (24). McKenney et al. (24) reported that siderophore production by B. cepacia was observed to increase sevenfold when supernatant from Sid+ P. aeruginosa PAO1 was added to Sid+ B. cepacia. P. aeruginosa PAO1 was known to produce C4 and 3OC12-HSLs (28, 29), while B. cepacia was observed to produce C4-, C6-, and 3OC6-HSLs (24). Siderophore production by B. cepacia with the addition of the supernatant of lasR deletion mutant P. aeruginosa PAO-RI was higher than that with the addition of the supernatant of B. cepacia. The P. aeruginosa PAO-RI mutant has been shown to produce 1,000- and 20-fold-less 3OC12-HSL and C4-HSL, respectively, than the wild-type strain P. aeruginosa PAO1 (29). Those suggested that the increased siderophore production by B. cepacia may be stimulated by other undetected HSLs in the P. aeruginosa PAO-RI mutant (24). Our study suggests that siderophores produced by P. aeruginosa PAO1 were possible corporate signals with HSLs which stimulated the siderophore production by B. cepacia.
Stimulated growth by exogenous siderophores and HSLs was also observed in marine planktonic Sid− bacteria. This suggests that such interspecies communication may occur in an open aquatic environment. The interactions through siderophores and HSLs between marine bacteria may be one of the factors which affects the uptake of iron by bacteria. These findings contribute useful information to our knowledge of “iron flow” between different microorganisms in the ocean.
Bacteria are abundant in the ocean; however, <0.1% of these bacteria are thought to have been isolated. The majority are believed to be nonculturable species since most marine bacteria cannot be cultivated by traditional microbiological protocols (45). The marine bacterium, V0110, studied here, for example, is unculturable under iron-limited stress, but this stress can be alleviated with an exogenous siderophore and a quorum-sensing chemical signal such as HSL. Thus, it may be possible to isolate marine species under natural, aqueous, nutrient-poor conditions with the addition of trace siderophores and autoinducers.
ACKNOWLEDGMENTS
This work was performed as a part of The Industrial Science and Technology Project, Technological Development of Biological Resources in Bioconsortia, supported by New Energy and Industrial Technology Development Organization.
We thank Clay Fuqua, University of Indiana, for kindly supplying us with the reporter strain. We also are grateful for the support of Y. Shizuri, Marine Biotechnology Institute, Shimizu Laboratories.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the components of 3,4-hydroxyphenylalanine-tyrosine mixtures. Annu Rev Biochem. 1937;50:715–731. [Google Scholar]
- 2.Cao J-G, Meighen E A. Biosynthesis and stereochemistry of the autoinducer controlling luminescence in Vibrio harveyi. J Bacteriol. 1993;175:3856–3862. doi: 10.1128/jb.175.12.3856-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhabra S R, Stead P, Bainton N J, Salmond G P C, Stewart G S A B, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J Antibiot. 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 4.Coale K H, Fitzwater S E, Gordon R M, Johnson K S, Barber R T. Control of community growth and export production by upwelled iron in the equatorial Pacific Ocean. Nature. 1996;379:621–624. [Google Scholar]
- 5.de Baar H J, de Jong W, Bakker J T M, Loscher B M, Veth C, Bathmann U, Smetacek V. Importance of iron for plankton blooms and carbon dioxide drawndown in the Southern Ocean. Nature. 1995;373:412–415. [Google Scholar]
- 6.Dominique P A G, Mottle B, Morck D W, Brown M R W, Costerton J W. A simplified rapid method for the removal of iron and other cations from complex media. J Microbiol Methods. 1990;12:13–22. [Google Scholar]
- 7.Eberhard A, Burlingame A L, Eberhard C, Kenyon G L, Nealson K H, Oppenheimer N J. Structural identification of autoinducer of Photobacterium fischeri. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 8.Eberhard A, Widrig C A, McBath P, Schineller J B. Analogs of the autoinducer of bioluminescence in Vibrio fischeri. Arch Microbiol. 1986;155:294–297. doi: 10.1007/BF00690155. [DOI] [PubMed] [Google Scholar]
- 9.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystem: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 10.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuqua C, Eberhard A. Signal generation in autoinduction systems: synthesis of acylated homoserine lactones by LuxI-type proteins. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 211–230. [Google Scholar]
- 12.Gillam A H, Lewis A G, Andersen R J. Quantitative determination of hydroxamic acids. Anal Chem. 1981;53:841–844. [Google Scholar]
- 13.Gledhill M, van der Berg C M G. Determination of complexation of iron (III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar Chem. 1994;47:41–54. [Google Scholar]
- 14.Granger J, Price N M. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol Oceanogr. 1999;44:541–555. [Google Scholar]
- 15.Greenberg E P, Hastings J W, Ulizur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. [Google Scholar]
- 16.Hutchins D A, Witter A E, Bulter A, Luther G W., III Competition among marine phytoplankton for different chelated iron species. Nature. 1999;400:858–861. [Google Scholar]
- 17.Imamura N, Nishijima M, Taketera T, Adachi K, Sakai M, Sano H. New anticancer antibiotics pelagiomicins, produced by a new marine bacterium Pelagiobacter variabilis. J Antibiot. 1997;50:8–12. doi: 10.7164/antibiotics.50.8. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K S, Gordon R M, Coale K H. What controls dissolved iron concentrations in the world ocean? Mar Chem. 1997;57:137–161. [Google Scholar]
- 19.Kuo A, Blough N V, Dunlap P V. Multiple N-acyl-l-homoserine lactone autoinducers of luminescence in marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1994;176:7558–7565. doi: 10.1128/jb.176.24.7558-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewenza S, Conway B, Greenberg E P, Sokol P A. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J Bacteriol. 1999;181:748–756. doi: 10.1128/jb.181.3.748-756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J H, Fitzwater S E. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Limnol Oceanogr. 1988;36:1793–1802. [Google Scholar]
- 22.Martinez J S, Zhang G P, Holt P D, Jung H-T, Carrano C J, Haygood M G, Bulter A. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- 23.McClean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation and violacein production and inhibition for the detection of N-acylhomoserine lactone. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 24.McKenney D, Brown K E, Allison D G. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J Bacteriol. 1995;177:6989–6992. doi: 10.1128/jb.177.23.6989-6992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milton D L, Hardman A, Camara M, Chhabra S R, Bycroft B W, Stewart G S A B, Williams P. Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-l-homoserine lactone. J Bacteriol. 1997;179:3004–3012. doi: 10.1128/jb.179.9.3004-3012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 27.Payne S M. Detection, isolation, and characterization of siderophores. Methods Enzymol. 1994;235:329–344. doi: 10.1016/0076-6879(94)35151-1. [DOI] [PubMed] [Google Scholar]
- 28.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence gene. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson J P, Passador K L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole K, Young L, Nesshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990;172:6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabsch W, Voigt W, Reissbrodt R, Tsolis R M, Baumler A J. Salmonella typhimurium TroN and FepA proteins mediated uptake of enterobactin but differ in their specificity for other siderophores. J Bacteriol. 1999;181:3610–3612. doi: 10.1128/jb.181.11.3610-3612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid R T, Live D H, Falkner D J, Bulter A. A siderophore from a marine bacterium with an exceptional ferric iron affinity constant. Nature. 1993;366:455–458. doi: 10.1038/366455a0. [DOI] [PubMed] [Google Scholar]
- 33.Rue E L, Bruland K W. Complexation of iron(III) by natural ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem. 1995;50:117–138. [Google Scholar]
- 34.Schaefer A L, Hanzelka B L, Eberhard A, Greenberg E P. Quorum sensing in Vibrio fischeri: probing autoinducer-LuxR interactions with autoinducer analogs. J Bacteriol. 1996;178:2897–2901. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 36.Shaw P D, Ping G, Daly S L, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1994;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigel A, Sigel H, editors. Metal ions in biological systems. New York, N.Y: Marcel Dekker, Inc.; 1998. pp. 1–29. [Google Scholar]
- 38.Simon S, Williams P, Stewart G S A B. N-Acylhomoserine lactones and quorum sensing in proteobacteria. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 291–313. [Google Scholar]
- 39.Stintzi A, Evans K, Meyer J M, Poole K. Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol Lett. 1998;15:341–345. doi: 10.1111/j.1574-6968.1998.tb13910.x. [DOI] [PubMed] [Google Scholar]
- 40.Tortell P L, Maldonado M T, Price N M. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature. 1996;383:330–332. [Google Scholar]
- 41.Tortell P D, Maldonado M T, Granger J, Price N M. Marine bacteria and biogeochemical cycling of iron in the oceans. FEMS Microbiol Ecol. 1999;29:1–11. [Google Scholar]
- 42.Weiburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VHC Press; 1987. [Google Scholar]
- 44.Wu J, Luther G W., III Complexation of Fe(III) by natural organic ligands in the northwest Atlantic Ocean by a competitive ligand equilibration method and a kinetic approach. Mar Chem. 1995;50:159–177. [Google Scholar]
- 45.Yoshinaga I, Katanozaka K, Ueno Y. VBNC (viable but nonculturable) marine bacteria on the molecular biological aspects. Microbes Environ. 1999;14:123–129. [Google Scholar]
- 46.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Beaber J W, More M I, Fuqua C, Eberhard A, Winans S C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J Bacteriol. 1998;180:5398–5405. doi: 10.1128/jb.180.20.5398-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]