Abstract
Exosomes are produced by the majority of eukaryotic cells and are capable of transporting a variety of substances, including non-coding RNAs, between cells. Metastasis is a significant cause of death from cancer. Numerous studies have established an important role for exosomal non-coding RNAs in tumor metastasis. Exosomal non-coding RNAs from a variety of cells have been shown to affect tumor metastasis via several mechanisms. Exosomes transmit non-coding RNAs between tumor cells, fibroblasts, endothelial cells, and immune cells within the tumor microenvironment. Exosomal non-coding RNAs also have an effect on epithelial-mesenchymal transition, angiogenesis, and lymphangiogenesis. Exosomes derived from tumor cells have the ability to transport non-coding RNAs to distant organs, thereby facilitating the formation of the metastatic niche. Due to their role in tumor metastasis, exosomal non-coding RNAs have the potential to serve as diagnostic or prognostic markers as well as therapeutic targets for tumors. The purpose of this paper is to review and discuss the mechanisms of exosomal non-coding RNAs, their role in tumor metastasis, and their clinical utility, aiming to establish new directions for tumor metastasis, diagnosis, and treatment research.
Keywords: MT: Non-coding RNAs, exosome, tumor metastasis, non-coding RNA, microenvironment, immunity, EMT, angiogenesis, lymphangiogenesis, premetastatic niche
Graphical abstract
Exosomal non-coding RNAs have been found to play an important role in tumor metastasis. This paper reviews the important functions of exosome non-coding RNAs in tumor microenvironment, tumor immunity, EMT, angiogenesis, lymphangiogenesis, and distant metastasis formation. Exosomal non-coding RNAs may be new tumor biomarkers and therapeutic targets.
Introduction
Vesicles are produced and released frequently in eukaryotic cells. Extracellular vesicles (EVs) are capable of transporting a variety of substances, including proteins, nucleic acids, and lipids, between adjacent or distant cells, serving as a vital mode of information and material exchange between cells.1 EVs are mixtures of different subtypes of vesicles, such as ectosomes and exosomes. Ectosomes, which include microvesicles, microparticles, and large vesicles, are formed and released directly from the plasma membrane via outward budding. These EVs are formed from endosomes and range in diameter from 50 nm to 1 mm. Endosomes form early-sorting endosomes (ESEs), late-sorting endosomes (LSEs), and multivesicular bodies (MVBs) in the cytoplasm, and exosomes are formed when intraluminal vesicles (ILVs) in MVBs are released from the cell. Exosomes are about 40 nm–160 nm in diameter.2 Certain proteins are present in varying concentrations in different subtypes of EVs. CD9, CD63, and CD81 are abundant in exosomes, but they are also present in other vesicles. Syntenin-1, TSG101, ADAM10, and EHD4 were all found to be highly expressed in small EVs. Syntenin-1 and TSG101 are specifically distributed in exosomes and thus serve as exosome markers. GP96, actinin-4, and mitofilin are abundant in large vesicles and thus can be used as markers for them.3 At present, exosomes have been purified from nearly all mammalian cell types. Exosomes derived from antigen-presenting cells were discovered in 2002 to promote the activation of CD4+ and CD8+ T cells and the induction of a specific immune response.4 Since then, an increasing number of studies have established that exosomes play a role in a variety of physiological processes. In addition, exosomes have also been implicated in the progression of diseases. Exosomes, as components of the tumor microenvironment, also play a critical role in tumors. Tumor cells can exchange bioactive substances with other cells via exosomes, thereby affecting tumor progression.5 Exosomes, as intercellular messengers, play a critical role in tumor progression.
Tumor metastasis is a characteristic of cancer, and over 90% of cancer deaths are due to metastasis, not the primary site of the tumor.6,7 It is a clinical challenge to treat patients with tumor metastasis and improve their survival time and quality of life. At the moment, the mechanisms underlying tumor metastasis are unclear. It is a very complex process that involves the tumor microenvironment, angiogenesis, immunity, and drug resistance, among other factors. Exosomes, as a ubiquitous mode of intercellular communication, play a significant role in the interactions of tumor cells with other cells and the stroma as well as in the process of tumor metastasis. Exosomes derived from tumor cells, for example, contain a number of immunosuppressive proteins that aid in tumor immune escape.8 However, research on the role and mechanism of exosomes in tumor metastasis remains in its early stages.
Non-coding RNA is a type of nucleic acid that can be encased in exosomes and transported between tumor cells and other cells. MicroRNA (miRNA), long non-coding RNA (lncRNA), and circular RNA (circRNA) are the most common types of non-coding RNAs. They are involved in the regulation of various cellular activities, despite the fact that they do not encode proteins.9 miR-27b can prevent tumor cells from metastasizing in gastric cancer.10 lnc-PTAR regulates miR-101 and ZEB1 in ovarian cancer, promoting metastasis.11 circ-0008305 has been shown to inhibit transforming growth factor β (TGF-β)-induced epithelial-mesenchymal transition (EMT) and metastasis in non-small cell lung cancer.12 Exosomes can transport a variety of non-coding RNAs to recipient cells, affecting various aspects of tumor progression, including the microenvironment, immunity, angiogenesis, lymph angiogenesis, and the formation of a pre-metastatic niche. In this paper, we will discuss the role of exosomal non-coding RNAs in tumor metastasis.
Non-coding RNAs are packaged into exosomes during double invagination of the cell membrane
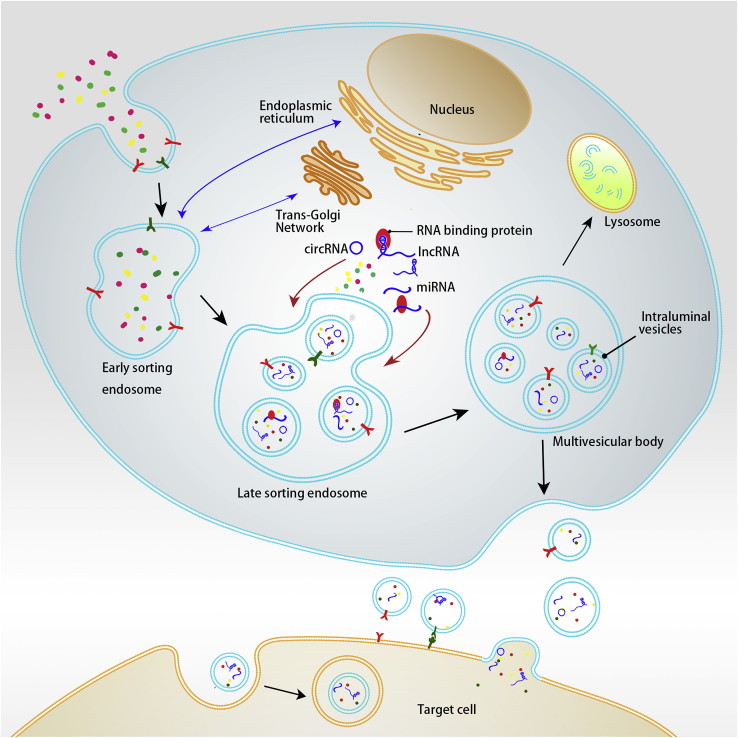
Exosome production requires a double invagination of the cell membrane (Figure 1). For the first time, the membrane invaginates, forming a cup-shaped structure that eventually forms ESEs composed of cell membrane surface and extracellular components. The newly formed ESEs can fuse with the cell’s already existing ESEs. ESEs mature and become LSEs. The second invagination occurs in LSEs, which results in the formation of MVBs that contain ILVs. Double invagination results in the entry of intracellular components into ILVs. MVBs can fuse with and be degraded by lysosomes. If MVBs are not degraded, they can be transported to and fuse with the cell membrane. Then, ILVs are released from the cell’s surface in the form of exosomes.2,13
Figure 1.
Biogenesis of exosomes
Early-sorting endosomes (ESEs) are formed when extracellular substances, such as lipids, proteins, ions, and cell-surface proteins, enter the cell via membrane invagination. The ESEs’ inner surface is formed by the outer side of the cell membrane. ESEs can fuse with the endoplasmic reticulum, the trans-Golgi network, or their derived vesicles or other existing ESEs. The membrane of ESEs invaginates a second time to form LSEs, and during this second invagination, ILVs are formed, with the ILVs’ outer side being formed by the outer side of the cell membrane. Non-coding RNAs are incorporated into ILVs along with other cytoplasmic constituents. In this process, RBP can bind miRNA or lncRNA and is associated with the selective sorting of non-coding RNAs. LSEs generate MVBs with a defined collection of ILVs. MVBs can fuse with lysosomes, which are eventually degraded. Undegraded MVBs migrate to the inner side of the cell membrane and fuse with it, while ILVs contained in MVBs are released as exosomes outside the cell. Exosomes eventually enter recipient cells via a variety of mechanisms, and exosomal components, such as non-coding RNAs, are also transported into the recipient cell.
Both extracellular and intracellular components, including non-coding RNA in the cytoplasm, can enter exosomes via the double membrane invagination process associated with exosome formation. The exact mechanism by which non-coding RNA enters exosomes is unknown. Apart from passively wrapping themselves around other substances in the cell, some studies have suggested that RNA-binding proteins (RBPs) are involved in the process by which exosomes sort non-coding RNAs, such as HnRNPA2B1, selectively (Figure 1). RBPs can recognize specific sequences on target RNAs and aid in cytoplasmic RNA transport, and RNA can enter exosomes selectively when bound to RBPs. HnRNPA2B1 is capable of recognizing and binding to the specific miR-198 sequence and thereby mediating miR-198 exosome packaging.14 Due to its specific binding to HnRNPA2B1, lnc-NMAT2 can be sorted into exosomes.15 YBX1 is another type of RBP involved in the packaging of miRNAs into exosomes. Both YBX1 and miR-223 possess binding sites, and YBX1 is homologous to the exosome surface molecule CD63. With the aid of YBX1, miR-223 can be selectively sorted into exosomes.16 However, the exact mechanism by which RBPs incorporate non-coding RNA into exosomes during exosome generation is unknown. In addition, miRNA was found to be packaged into exosomes via post-transcriptional uridylation of the 3′-terminal strand.17 This suggests that post-transcriptional modification of miRNA may be involved in exosome sorting. To summarize, the mechanism by which exosomes encapsulate RNA is unknown, and more research is required.
The mechanism of exosomal miRNAs in tumor metastasis
miRNAs are a class of non-coding RNAs with an approximate length of 21 nt that are derived from introns of protein-coding genes or RNA-polymerase-II-specific independent gene transcripts.18 The process of miRNA synthesis begins with the synthesis of the miRNA precursor with a hairpin structure, which is then exported to the cytoplasm for trimming into miRNA/miRNA double strands, one of which functions as a mature miRNA. Mature miRNAs and other proteins combine to form the miRNA-induced silencing complex (miRISC). In miRISC, miRNAs have a seed region containing nucleotides 2–8 at their 5′ ends that bind specifically to the target mRNA. The Watson-Crick base pairing between the miRNA seed region and the target mRNA’s 3′ untranslated region (3′ UTR) is required for miRNA target recognition and driving function. The seed region directs mature miRNAs to specifically target mRNA for post-transcriptional repression, which includes mRNA translational repression and mRNA degradation. Complementarity between miRNA and mRNA is critical in determining the type of post-transcriptional inhibition. Complete pairing of miRNA and mRNA target sites facilitates mRNA degradation, whereas incomplete pairing promotes mRNA translation repression. Translational suppression is widely accepted as the default and most prevalent mechanism by which miRNA suppresses mRNA. Both mechanisms can exist when miRNA and mRNA are completely paired.19 This is a relatively specific mechanism by which miRNAs regulate target gene expression.
Exosomes transport miRNA from the cytoplasm of tumor cells to other cells, where they regulate target genes via the mechanism described above. miR-105 can be transported to endothelial cells via exosomes derived from breast cancer cells. miR-105 contains a sequence that can bind to the 3ʹ UTR of the ZO-1 mRNA. After treatment with exosomal miR-105, the levels of ZO-1 in human microvascular endothelial cells (HMVECs) decreased, indicating that miR-105 can enter and reduce the level of ZO-1 in HMVECs. By inhibiting the expression of ZO-1 and destroying the adhesion between endothelial cells, exosomal miR-105 increases the permeability of the vascular wall.20 Similarly, exosomes from breast cancer cells contain a high level of miR-122. miR-122 can bind to the 3′ UTR of pyruvate kinase mRNA, thereby reducing pyruvate kinase’s mRNA and protein levels and impairing the glucose metabolism of receptor cells.21 In addition, miR-122 was found to be highly expressed in the serum exosomes of hepatocellular carcinoma (HCC) patients, according to the sequencing data.22 Numerous exosomal miRNAs have been implicated in the process of tumor metastasis (Table 1; Figure 2).
Table 1.
The role of exosomal non-coding RNA in tumor metastasis
| Exosomal non-coding RNA | Donor cell | Recipient cell | Role in tumor metastasis |
|---|---|---|---|
| miR-21, miR-10b23 | HCC cell | HCC cell | promoting HCC cells migration, invasion, and EMT |
| miR-15524 | gastric cancer cell | human umbilical vein endothelial cell (HUVEC) | promoting angiogenesis of HUVECs |
| miR-196a-125 | gastric cancer cell | gastric cancer cell | enhancing invasion ability of gastric cancer cells |
| miR-499a-5p26 | lung adenocarcinoma cell | lung adenocarcinoma cell | promoting cancer cell migration and epithelial-mesenchymal transition (EMT) |
| miR-122927 | colorectal cancer (CRC) cell | HUVEC | promoting tubulogenesis of HUVECs |
| miR-25-3p28 | CRC cell | HUVEC | promoting vascular permeability and angiogenesis to induce vascular leakiness |
| miR-25-3p, miR-92a-3p29 | liposarcoma cell | macrophage | inducing IL-6 release from macrophages |
| miR-106a30 | gastric cancer cell | peritoneal mesothelial cell | inducing peritoneal mesothelial cell damage, apoptosis, migration, and mesothelial-to-mesenchymal transition |
| miR-125b-5p31 | melanoma cell | macrophage | inducing the tumor-promoting phenotype of tumor-associated macrophages |
| miR-1910-3p32 | breast cancer cell | breast cancer cell | promoting migration of breast cancer cells |
| miR-933 | nasopharyngeal carcinoma cell | HUVEC | inhibiting HUVECs tube formation and migration |
| lnc-FMR1-AS134 | esophageal CSC | non-CSC | promoting stemness phenotypes of non-CSCs |
| lnc-UCA135 | CRC cell | CRC cell | promoting proliferation and migration of CRC cells |
| lnc-MALAT136 | CRC cell | CRC cell | promoting invasion and migration |
| lnc-RUNX2-AS137 | myeloma cell | mesenchymal stem cell | inhibiting the osteogenesis of mesenchymal stem cells |
| lnc-PTENP138 | normal cell | bladder cancer cell | suppressing bladder cancer cell invasion and migration |
| circ-MMP239 | HCC cell | normal liver cell | promoting the malignant phenotype formation of normal liver cells |
| circ-000028440 | cholangiocarcinoma cell | surrounding normal cell | promoting the migration and proliferation of surrounding normal cells and suppressing their apoptosis |
| circ-SATB241 | non-small cell lung cancer cell | lung cancer cell and bronchial epithelial cell | promoting the migration and invasion of lung cancer cells and inducing abnormal proliferation of bronchial epithelial cell |
Figure 2.
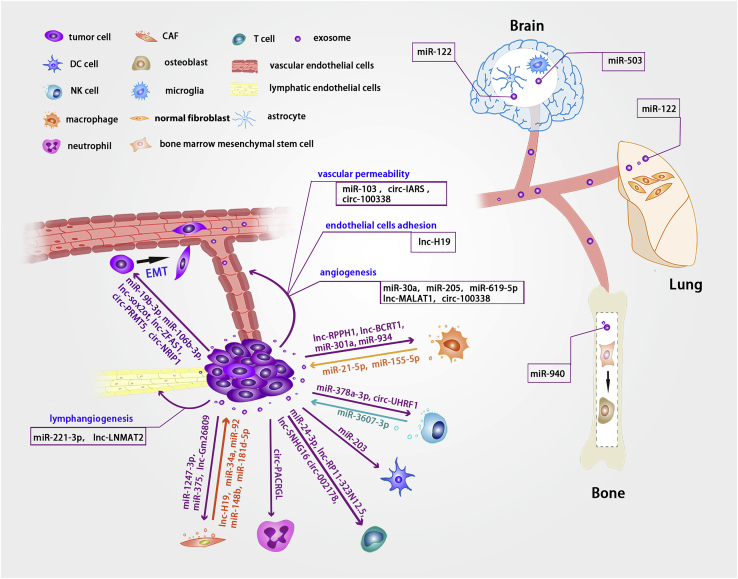
Illustration of important non-coding RNAs transported via exosomes between different cells in tumor metastasis
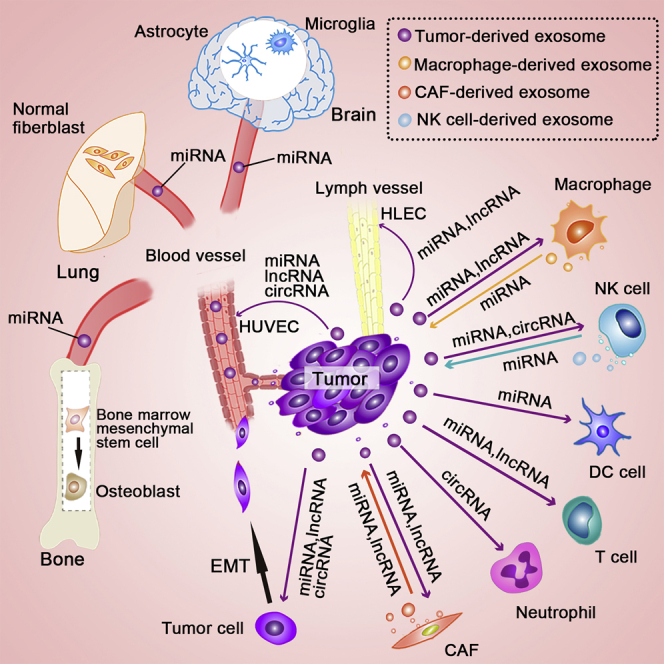
Exosomes transport non-coding RNAs between tumor cells and other types of cells within the tumor microenvironment, including immune and non-immune cells. CAF-derived exosomes transfer lnc-H19, miR-34a, miR-92, miR-148b, and miR-181d-5p to tumor cells, whereas tumor-cell-derived exosomes transfer miR-1247-3p, miR-375, and lnc-Gm26809 to fibroblasts to promote fibroblast activation. Tumor-cell-derived exosomes deliver lnc-H19, miR-103, circ-IARS, and circ-100338 to endothelial cells, where they regulate endothelial cell adhesion and vascular permeability. Tumor-cell-derived exosomes deliver non-coding RNAs to neutrophils, T cells, DC cells, NK cells, and TAMs. NK cells and macrophages can also deliver exosomal non-coding RNAs into tumor cells via exosomes. miRNAs, lncRNAs, and circRNAs can be transported to recipient tumor cells via tumor-cell-derived exosomes to promote EMT. Exosomal non-coding RNAs derived from tumors can be internalized by HUVECs and HLECs to promote angiogenesis and lymphangiogenesis. Tumor-cell-derived exosomes deliver non-coding RNAs to distant organs, such as the lung, bone, and brain, where they affect the cells and promote the formation of the pre-metastatic niche.
The mechanism of exosomal lncRNAs in tumor metastasis
lncRNAs are a class of non-coding RNAs that are approximately 200 nt in length and lack an open reading frame.42 While lncRNAs cannot be translated into proteins, they can be transmitted between cells via exosomes and may affect tumor metastasis (Table 1; Figure 2). Exosomal lncRNAs have been shown to play a critical role in a variety of tumor types. Exosomal lncRNAs function in a manner distinct from miRNAs. Exosomal lncRNAs function in a variety of ways, including miRNA sponging, mRNA stability reduction, interaction with RBPs, promoter combing, and epigenetic regulation in the nucleus.15,43,44
lncRNAs serve as competitive endogenous RNAs (ceRNAs) and have an important regulatory function. ceRNAs can bind specifically to miRNAs, and the specific sites on miRNAs where ceRNAs bind are known as miRNA response elements (MREs),45 which are also present in mRNAs. lncRNAs and mRNAs bind to miRNAs competitively, reducing miRNA-mediated inhibition of downstream mRNAs and forming an lncRNA-miRNA-mRNA regulatory network. In osteosarcoma, exosomes derived from bone marrow mesenchymal stem cells transported and increased the expression of lnc-PVT1 in osteosarcoma cells. lnc-PVT1 acts as a ceRNA, sponging miR-183-5p and reducing miR-183-5p’s inhibitory effect on its target, ERG, thereby promoting ERG expression, which in turn promotes osteosarcoma cell proliferation and migration.43 Certain lncRNAs can combine directly with mRNAs without involving miRNAs. lnc-APC1 can bind to specific sites on Rab5b mRNA in colorectal cancer (CRC). In this study, silencing lnc-APC1 prolonged the half-life of Rab5b mRNA, indicating that lnc-APC1 can regulate the stability of Rab5b mRNA, thereby reducing exosome production from CRC cells and thereby inhibiting tumor metastasis and angiogenesis.44
Certain exosomal lncRNAs are internalized by target cells and translocated into the nucleus to perform their functions. Exosomal lncRNAs have the ability to interact directly with DNA and regulate transcription. In bladder cancer, tumor cells produce exosomal lnc-LNMAT2, which is internalized by human lymphatic endothelial cells (HLECs) and interacts physiologically with the PROX1 promoter’s P3 region in the nucleus. The lnc-LNMAT2 and PROX1 promoters form a triple-stranded RNA-DNA structure and enhance PROX1 transcription.15 PROX1 is a lymphatic vascular marker that is required for the differentiation of endothelial cells into HLECs. PROX1 inhibition results in the absence of lymphatic vessels and abnormal development or function of lymphatic vessels.46 Exosomal lnc-LNMAT2 activates PROX1 in endothelial cells, promoting differentiation of endothelial cells into lymphatic endothelial cells, as well as lymphatic formation and extension. Increased lymphatic vessel formation in tumors promotes lymph node metastasis.15 In the nucleus, lncRNAs can collaborate with other molecules to regulate histone modification, which is required for epigenetic transcriptional regulation to occur. HnRNPA2B1 has the ability to bind to target DNA and participate in epigenetic regulation of H3K4 trimethylation.47 Exosomal lnc-LNMAT2 binds to the PROX1 promoter region in the nucleus and recruits hnRNPA2B1, which promotes H3K4 trimethylation on the PROX1 promoter and activates PROX1 expression.15
In addition, exosomal lncRNAs can interact directly with proteins. Exosomal lnc-91H may directly interact with HNRNPK in CRC, promoting tumor cell migration and invasion.48 HNRNPK has been linked to tumor metastasis.49 In CRC cells, the lnc-RPPH1 and TUBB3 proteins interact directly. The TUBB3 protein contains a unique domain that interacts with specific regions of lnc-RPPH1, promoting CRC cell migration and invasion. In addition, lnc-RPPH1 can inhibit the ubiquitination of TUBB3 and increase its stability.50 Thus, while some exosomal lncRNAs may not affect protein expression at the transcription or translation levels, they can affect protein stability via regulating ubiquitination and degradation.
The mechanism of exosomal circRNAs in tumor metastasis
circRNAs are non-coding circular RNAs that lack a PolyA tail and a 5′ end and are generated by backward splicing or exon skipping of the pre-mRNA.51 Certain circRNAs have been identified as being transmitted and functioning between cells via exosomes during tumor metastasis (Table 1; Figure 2). circRNAs can act as sponges for miRNAs or RBPs, and some can be translated into peptides.52
circRNAs derived from exosomes act primarily as ceRNAs in tumors by sponging miRNAs. circRNAs contain miRNA-binding sites that regulate gene expression by interacting with and affecting miRNAs. circ-PDE8A in exosomes was found to be negatively correlated with the prognosis of patients with pancreatic tumors and to promote tumor cell proliferation, migration, and invasion. Due to the presence of miR-338-binding sites on circ-PDE8A, circ-PDE8A has been identified as a miR-338 sponge. MACC1 is one of miR-338’s targets,53 has been implicated in tumor metastasis, and is a key regulator of MET signaling. MET, a tyrosine kinase receptor, is regarded as an oncogene and is a transcriptional target of MACC1.54 MET signaling is primarily mediated by the RAS-mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)-AKT pathways and has a direct effect on cell proliferation, migration, adhesion, and cytoskeleton organization.55 MACC1 and MET levels are decreased when miR-338 levels are increased. circ-PDE8A overexpression increases MACC1 and MET levels, implying that circ-PDE8A regulates the miR-338/MACC1/MET axis.53 Similarly, exosomal circ-RanGAP1 regulates miR-877-3p/VEGFA expression in gastric cancer by acting as a miRNA sponge.56 circ-WHSC1 and circ-PUM1 can be transported via tumor-derived exosomes and act as miRNA sponges, promoting tumor progression in ovarian cancer.57,58
Several studies have reported that exosomal circRNAs can interact directly with proteins, promoting tumor metastasis. circ-100338 was highly expressed in tumor-derived exosomes and was found to promote angiogenesis and metastasis in HCC. circ-100338 has the ability to bind to a wide variety of proteins, including RBPs, transcription factors, and enzymes. NOVA2 is one of the specific RBPs and is linked to angiogenesis; its interaction with circ-100338 may account for its angiogenesis-promoting effect.59 Exosomal circ-ABCC1 has been shown to interact directly with β-catenin, mediating β-catenin’s entry into the nucleus and activation of the Wnt pathway, thereby promoting CRC metastasis.60 Although some circRNAs can be translated into peptides, the mechanism by which exosomal circRNAs promote tumor metastasis via peptide translation remains unknown. In conclusion, exosomal circRNAs promote tumor metastasis mainly via interaction with miRNAs.
Exosomal non-coding RNA and the non-immune tumor microenvironment
Rather than existing independently, tumor cells interact with their microenvironment to form a whole, analogous to “seeds” and “soil.” The tumor microenvironment contains a diverse array of cells and non-cellular elements. The tumor microenvironment can be classified as immune dominated by immune cells or non-immune dominated by fibroblasts and endothelial cells. Tumor cells and neighboring cells can exchange non-coding RNAs via exosomes within the tumor microenvironment. We begin this section by discussing the communication between tumor cells and the non-immune microenvironment via exosomes (Figure 3); the immune microenvironment will be discussed later in the section on exosomal non-coding RNAs and tumor immunity.
Figure 3.
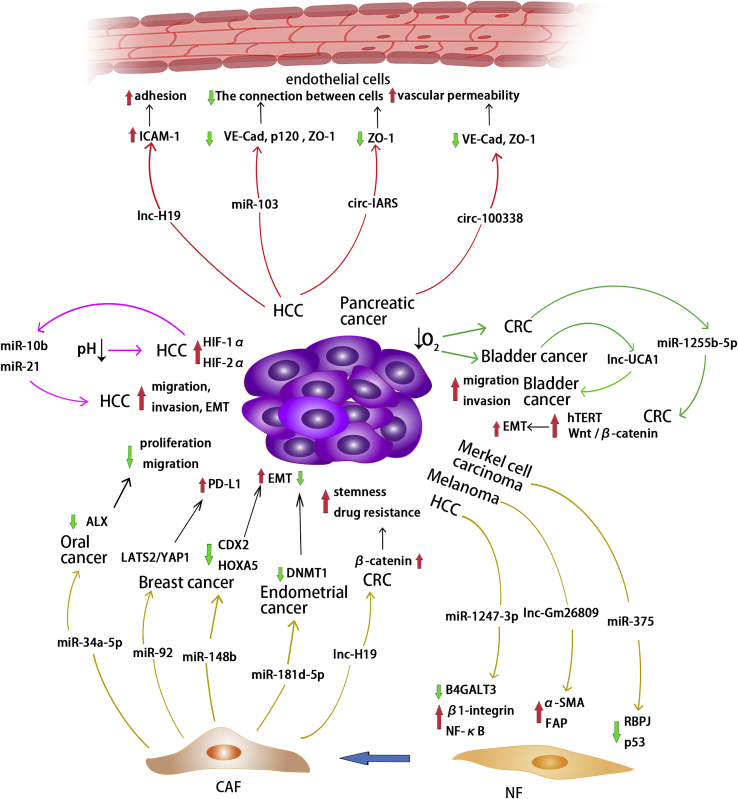
Exosomal non-coding RNAs and the non-immune tumor microenvironment
Exosomes derived from HCC, melanoma, and Merkel cell carcinoma deliver non-coding RNAs to regulatory targets and signaling pathways in fibroblasts, thereby promoting the transformation of NFs into CAFs. Exosomal non-coding RNAs derived from CAFs also regulate corresponding molecules, thereby affecting the progression of tumor cells in oral cancer, breast cancer, endometrial cancer, and CRC. Exosomes derived from HCC contain lnc-H19, which regulates ICAM-1 and promotes endothelial cell adhesion. Exosomes derived from HCC and pancreatic cancer contain non-coding RNAs that inhibit intercellular adhesion molecules and improve vascular permeability. In HCC, tumor cells in an acidic environment secrete exosomal miR-10b and miR-21 to promote tumor cell migration, invasion, and EMT. In addition, exosomal non-coding RNAs derived from CRC and bladder cancer cells in hypoxic environments promote tumor EMT, migration, and invasion.
Endothelial cells mediate angiogenesis in the tumor microenvironment. Adhesion between tumor cells and endothelial cells is a critical step in the progression of tumor cells into blood vessels. Exosomes can transport lncRNAs between cells, enhancing endothelial cell monolayer adhesion. In HCC, exosomal lnc-H19 derived from CD90+ cancer stem cells (CSCs) increases ICAM-1 expression in endothelial cells, thereby enhancing the adhesion ability of endothelial cells. This is critical for CSCs’ entry into vessels.61 After adhering to the endothelial cells, tumor cells must pass through the vessel wall to spread into the bloodstream and distant organs, as well as penetrate the vessel walls when implanted in distant organs. The connection between endothelial cells has a direct effect on vascular permeability. Exosomes secreted by HCC cells express a high level of miR-103, which downregulates VE-cadherin, p120, and ZO-1, all of which are important adhesion molecules within and between endothelial cells. As a result, exosomal miR-103 increases vascular permeability and promotes tumor metastasis.62 Pancreatic-cancer-secreted exosomal circ-IARS can inhibit ZO-1 expression in endothelial cells, increasing vascular permeability and promoting tumor metastasis.63 HCC exosomes carrying circ-100338 can be internalized by human umbilical vein endothelial cells (HUVECs) and downregulate VE-cadherin and ZO-1 expression in HUVECs, thereby enhancing vascular permeability.59 These findings suggest that non-coding RNA contained in exosomes secreted by tumor cells can promote metastasis by regulating endothelial cell surface adhesion molecules, thereby destroying endothelial cell-to-cell connections and increasing vascular permeability.
Cancer-associated fibroblasts (CAFs) can secrete a variety of cell factors that affect tumor progression. When activated by tumors, normal fibroblasts differentiate into CAFs, which can alter the tumor microenvironment and interact with tumor cells. Exosomal non-coding RNAs secreted by tumor cells contribute to the transformation of fibroblasts into CAFs.64,65 Exosomal miR-1247-3p derived from cancer cells specifically targets B4GALT3 in fibroblasts and regulates the β1-integrin/nuclear factor κB (NF-κB) axis, activating fibroblasts to transform into CAFs in HCC.64 Similarly, exosomal miR-375 can downregulate its targets, RBPJ and p53, promoting fibroblast polarization to CAFs in Merkel cell carcinoma.66 RBPJ, also known as CSL, is a Notch signaling molecule that inhibits CAF activation.67,68 Exosomes secreted by melanoma cells and their contents of lnc-Gm26809 upregulate the specific CAF markers, α-SMA and FAP, in fibroblasts.65 CAFs, on the other hand, can secrete exosomes and interact with tumor cells. Primary CAF and NF cells from CRC and normal colorectal tissues were cultured, and exosomes of CAFs and NFs were collected. RNA sequencing revealed significant differences in the expression profiles of lncRNA and short non-coding RNA (including miRNA) in CAF and NF exosomes. Furthermore, it is suggested that these non-coding RNAs play a role in the interaction of fibroblasts and cancer cells.69 Through exosomes, CAFs deliver lnc-H19 to CRC cells, which sponges miR-141 and activates the β-catenin pathway, enhancing the stemness and resistance of CRC cells to oxaliplatin.70 CAF-derived exosomal miR-181d-5p can regulate CDX2 and HOXA5 in breast cancer cells, thereby promoting EMT of cancer cells.71 On the other hand, CAF-derived exosomal non-coding RNAs can act as tumor suppressors. CAFs transport miR-34a-5p to adjacent tumor cells via exosomes and regulate ALX expression in oral cancer. ALX deficiency can increase intercellular adhesion and inhibit tumor cell growth, metastasis, invasion, and EMT.72,73 miR-34a-5p inhibits ALX expression and the proliferation, motility, and EMT of oral cancer cells. The miR-34a-5p/ALX axis has an effect on tumor cell EMT via the AKT/GSK-3β/β-catenin/Snail signaling pathway.74 Similarly, exosomal miR-34 derived from CAFs has been reported to inhibit tumor invasion in gastric cancer.75 In addition, RNA sequencing of exosomes extracted from the urine of prostate cancer patients and normal controls revealed a decreased level of miR-34a expression in patients’ urine exosomes.76 miR-148b in CAF-derived exosomes was significantly reduced in endometrial cancer and can be transferred to tumor cells via exosomes and inhibit DNMT1, which promotes EMT of endometrial cancer. As a result, CAF-derived exosomal miR-148b suppresses tumor cell invasion.77 CAF-derived exosomal non-coding RNAs can promote tumor immune escape. In breast cancer cells treated with CAF-derived exosomes, miR-92 and PD-L1 expression levels have increased. miR-92 targets LATS2, which interacts with YAP1, and YAP1 can regulate the transcription of PD-L1, implying that CAF-derived exosomes inhibit T cell function by regulating PD-L1 levels in breast cancer cells via the miR-92/LATS2/YAP1 pathway.78
The tumor microenvironment, in addition to cells, contains a variety of non-cellular components that affect tumor progression, such as oxygen and pH. Under normal conditions, the aerobic respiration of cells provides the majority of the energy required for cell activity. In solid tumors, the absence of blood vessels results in a hypoxic environment, which is widely regarded as one of the most fundamental tumor microenvironmental pressures. It does not promote the tumor’s rapid growth.79 circ-133 is abundant in anoxic tumor cells in CRC. Exosomal circ-133 derived from hypoxic tumor cells has the ability to enter normoxic tumor cells and promote their migration via the miR-133a/GEF-H1/RhoA axis.80 Exosomal lnc-UCA1 derived from hypoxic bladder cancer cells promotes bladder cancer cell migration and invasion.81 Under hypoxia in CRC, the content of miR-1255b-5p in exosomes derived from cancer cells decreased, resulting in an increase in the expression of human telomerase reverse transcriptase, which activates Wnt/β-catenin and promotes EMT of tumor cells.82
The increased glycolysis in tumor cells, hypoxia within the solid tumor, and abnormal blood flow in the tumor microenvironment all contribute to an imbalance in the pH of the tumor microenvironment, making the extracellular environment acidic. It is advantageous for tumor metastasis. Acidic conditions have been shown to increase miR-21 and miR-10b levels in tumor-cell-derived exosomes in HCC by increasing HIF-1α and/or HIF-2α. Under acidic conditions, high miR-21 and miR-10b levels in exosomes promote the migration, invasion, and EMT of tumor cells.23 miR-21 has been demonstrated to promote tumor metastasis in CRC, breast cancer, and lung cancer.83, 84, 85 In gastric cancer, exosome miR-21 has been found to promote peritoneal metastasis via mesothelial-to-mesenchymal transition of gastric cancer cells.86 Furthermore, miR-10b was found to be highly expressed in the plasma exosomes of patients with lung squamous cell carcinoma, pancreatic ductal carcinoma, and gastric cancer.87, 88, 89 As a result, both hypoxic and acidic tumor microenvironments promote tumor metastasis.
Exosomal non-coding RNA and tumor immunity
Tumor cells can affect a variety of immune cells via exosomal non-coding RNAs (Figure 4).
Figure 4.
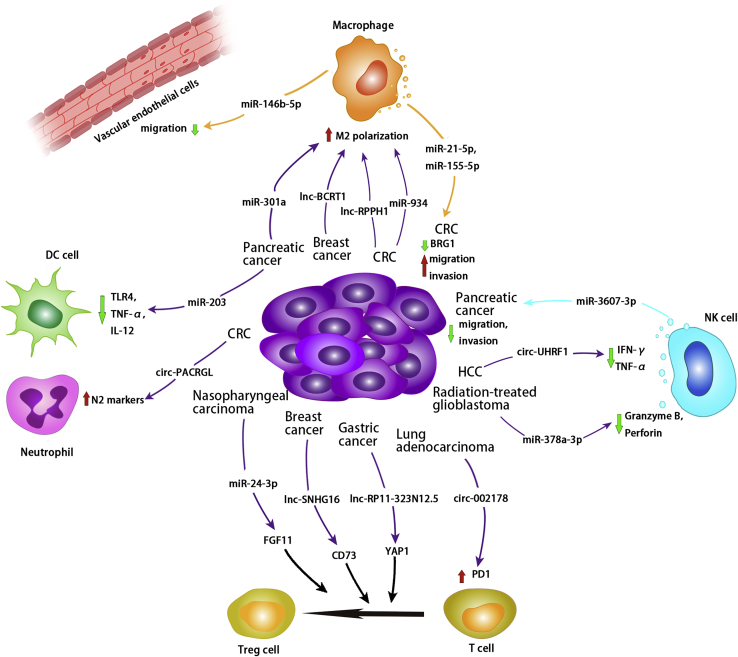
Exosomal non-coding RNAs and the immune tumor microenvironment
Exosomal non-coding RNAs from tumor cells can be internalized by macrophages and regulate their targets in macrophages, promoting M2 polarization in CRC, breast cancer, and pancreatic cancer cells. TAM-derived exosomal miR-21-5p and miR-155-5p downregulate BRG1, thereby promoting tumor migration and invasion in CRC. Macrophage-derived exosomal miR-146b-5p can inhibit vascular endothelial cell migration. Tumor-cell-derived exosomal non-coding RNAs regulate T cell differentiation into Treg cells in nasopharyngeal carcinoma, breast cancer, and gastric cancer. In addition, exosomal circ-002178 derived from lung adenocarcinoma has been shown to increase PD1 expression on T cells. Radiation-treated-glioblastoma-derived exosomal miR-378a-3p suppresses granzyme B and perforin expression in NK cells, whereas HCC-derived exosomal circ-UHRF1 suppresses IFN-γ and TNF-α expression in NK cells. However, exosomal miR-3607-3p derived from NK cells inhibits pancreatic tumor growth. Exosomal miR-203 derived from pancreatic cancer cells inhibits DC cell function by suppressing TLR4, TNF-α, and IL-12 expression. Exosomal circ-PACRGL derived from CRC cells increases N2 markers in neutrophils.
Macrophages comprise the majority of immune cells in the tumor microenvironment.90 Macrophages are classified into two subtypes: classically activated M1 and transformed M2. M1 macrophages are capable of secreting pro-inflammatory factors and exerting anti-tumor activity. M2 macrophages have the ability to promote tumor metastasis, angiogenesis, and growth. Exosomes secreted by tumor cells promote macrophage transformation from M1 to M2. After macrophages internalized exosomes derived from CRC cells, the levels of M2 macrophage markers increased significantly without any significant changes in the levels of M1 macrophage markers, indicating that exosomes secreted by CRC cells promote macrophage M2 polarization. The level of lnc-RPPH1 in the exosomes of CRC cells is responsible for this effect. lnc-RPPH1 was found to be overexpressed in CRC tissues in seven CRC patients with liver metastasis via next-generation sequencing, and lnc-RPPH1 was found to be abundant in serum exosomes in these patients. High lnc-RPPH1 levels in exosomes can upregulate the expression of M2 markers in macrophages.50 Breast-cancer-cell-derived exosomal lnc-BCRT1 has the ability to promote macrophage M2 polarization, enhancing macrophage migration and chemotaxis and promoting TGF-β secretion.91 Cancer cells with activated CXCL12/CXCR4 can deliver upregulated miRNAs to macrophages via exosomes, activating the PI3K/AKT signaling pathway and regulating PTEN expression to promote M2 polarization in CRC.92 CRC-derived exosomal miR-934 induces M2 macrophage polarization by downregulating PTEN and activating the PI3K/AKT signaling pathway. In addition, M2 macrophages can increase miR-934 expression in CRC cells by activating the CXCL13/CXCR5/NF-κB/p65/miR-934 feedback loop, which promotes the formation of CRC liver metastases.93 Hypoxia can enhance exosome secretion and miR-301a expression in pancreatic cancer. Exosomes containing a high level of miR-301a induced M2 macrophage polarization by downregulating PTEN and activating PI3Kg.94 Tumor-associated macrophages (TAMs) can also affect tumor cells by secreting exosomes. Exosomes derived from M2 macrophages contain significant levels of miR-21-5p and miR-155-5p. These two miRNAs are delivered to colon cancer cells via exosomes and inhibit BRG1 expression, hence promoting tumor cell migration and invasion.95 TAMs can deliver miR-95 to prostate cancer cells via exosomes. The complementary pairing of mir-95 and the 3′ UTR of JunB mRNA inhibits JunB mRNA translation, and miR-95 promotes EMT in prostate cancer by inhibiting JunB expression.96 In addition, TAMs can interact with other cells within the tumor microenvironment. In ovarian cancer, exosomal miR-146b-5p produced by TAMs has been shown to inhibit endothelial cell migration by modulating TRAF6/NF-κB/MMP2 expression in endothelial cells. The inhibitory effect of TAM-derived exosomes on endothelial cell migration was reversed when exosomes from ovarian epithelial cancer cells were added. The authors speculated that lncRNA contained in tumor exosomes restored endothelial cells’ migration ability.97 This study indicates that cell communication in the tumor microenvironment is a complex network, with various cells exerting inconsistent effects via exosome communication.
Natural killer (NK) cells are essential components of the innate immune system because they are capable of secreting cytokines. NK cells act as immunomodulators and directly kill cells via cytotoxicity. They are important immune cells that act as inhibitors of tumor growth in the tumor microenvironment. Exosomes derived from NK cells are rich in miR-3607-3p in pancreatic cancer, and these exosomes can inhibit pancreatic cancer cell migration and invasion, as miR-3607-3p in exosomes can directly regulate interleukin-26 (IL-26) in cancer cells.98 On the other hand, exosomal miR-378a-3p from radiation-treated glioblastoma cells can enter NK cells, resulting in a decrease in the expression of granzyme B/perforin, the primary cytotoxic effector molecule of NK cells. This indicates that tumor-derived exosomes inhibit the immune killing ability of NK cells.99 Cancer-derived exosomal circ-UHRF1 inhibited interferon (IFN)-γ and tumor necrosis factor alpha (TNF-α) secretion by NK cells via miR-449c/TIM-3 in HCC. circ-UHRF1/miR-449c/TIM-3 is specific to NK cells and has no effect on CD8+ T cells. On NK cells, inhibiting tumor exosomal circ-UHRF1 enhanced resistance to anti-PD1 therapy.100 These findings suggest tumor cells and NK cells can fight each other via exosomes during tumor progression, potentially affecting tumor development.
Exosomal non-coding RNA can also affect T cells. Exosomes derived from tumors not only inhibit proliferation but also affect T cell subtype differentiation. miR-24-3p is abundant in exosomes derived from nasopharyngeal carcinoma. miR-24-3p inhibited ERK and STAT phosphorylation by inhibiting FGF11 in T cells following exosome treatment, resulting in decreased T cell proliferation, inhibition of Th1 and Th17 differentiation, and induction of regulatory T (Treg) cell differentiation.101 Th1 and Th17 cells stimulate the immune response, whereas Treg cells suppress the immune response. In breast cancer, V δ 1T cells are the predominant Treg cell type, and exosomes from breast cancer cells transfer lnc-SNHG16 to V δ 1T cells. lnc-SNHG16 can upregulate CD73 expression via the miR-16-5p/Smad5 axis, and CD73+V δ 1T cells inhibit the immune response of breast cancer.102 In gastric cancer, lnc-RP11-323N12.5 can be transferred to T cells via exosomes and combined with c-myc to increase YAP1 levels in T cells, promoting Treg cell differentiation and immunosuppression.103 Exosomal non-coding RNAs can also impair T cell function by upregulating the expression of immunosuppressive molecules on their surface. Lung adenocarcinoma-derived exosomal circ-002178 sponged miR-28-5p and increased PD-1 on the surface of T cells. On the other hand, circ-002178 expression was significantly higher in lung adenocarcinoma cells than in normal tissues, promoting PD-L1 expression in tumor cells.104 The high level of PD-1/PD-L1 inhibited the immune killing effect of CD8+ T cells.
In addition, exosomal miR-203 derived from pancreatic cancer can inhibit dendritic cell function by downregulating TLR4.105 In CRC, tumor-derived exosomal circ-PACRGL can promote tumor cell migration and invasion. After neutrophils were treated with CRC-derived exosomes, circ-PACRGL increased the expression of N2 markers via the miR-1423p/miR-506-3p-TGF-β1 axis and regulated N1-N2 neutrophil differentiation.106 N2 neutrophils have been shown to promote tumor metastasis. Taken together, tumor-derived exosomes have the potential to inhibit the immune response to tumor cells by transporting non-coding RNA to various immune cells, which may be one of the mechanisms by which tumors evade the immune system.
Exosomal non-coding RNA and EMT
EMT is critical for tumor invasion. During the initial stages of tumor metastasis, epithelial tumor cells undergo transformation into cells with interstitial characteristics. Tumor cells lose their polarity and intercellular connections, which facilitate migration and invasion. Numerous EMT drivers, including SNAIL1 and SNAIL2, have been shown to be significantly associated with disease recurrence and survival in many tumor types, indicating that EMT is associated with the prognosis of patients with malignancies.107 EMT is characterized by a decrease in E-cadherin and an increase in vimentin and N-cadherin expression. Exosomal non-coding RNAs can affect tumor metastasis by regulating EMT-related pathways and transcription factors (Figure 5).
Figure 5.
Exosomal non-coding RNAs have an effect on EMT
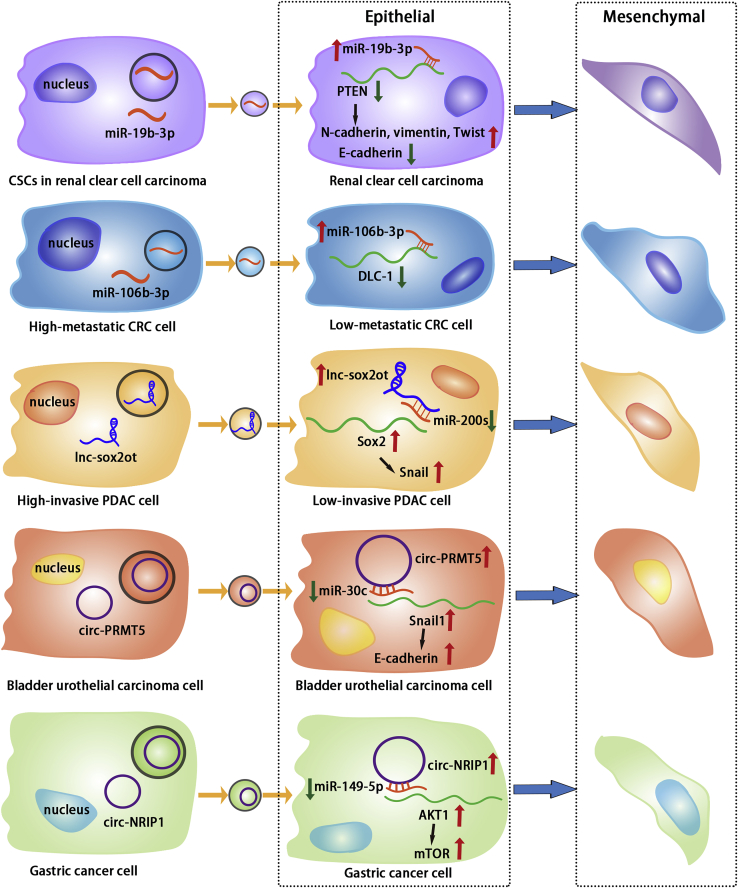
Tumor-cell-derived exosomal non-coding RNAs regulate target molecules and signaling pathways to promote tumor cell EMT. In renal clear cell carcinoma, CSCs-derived exosomal miR-19b-3p suppresses PTEN expression in cancer cells, promoting EMT. Exosomes from highly metastatic CRC cells transfer miR-106b-3p to low-metastatic CRC cells and inhibit DLC-1, thereby promoting EMT. Exosomal lnc-sox2ot secreted by highly invasive PDAC cells promotes low-invasive PDAC cell EMT via miR-200s/Sox2/snail. In bladder urothelial carcinoma, cancer cells secrete exosomal circ-PRMT5 and promote EMT in recipient cancer cells via miR-30c/snail1/E-cadherin. Exosomal circ-NRIP1 from gastric cancer cells promotes EMT via miR-149-5p/AKT1/mTOR.
Exosomes derived from CSCs were found to transport miR-19b-3p into renal clear cell carcinoma. miR-19b-3p can regulate PTEN to significantly upregulate the expression of N-cadherin, vimentin, and Twist and downregulate the expression of E-cadherin in renal clear cell carcinoma cells, thereby promoting EMT.108 Patients with CRC metastasis had significantly increased serum exosomal miR-106b-3p levels. Exosomal miR-106b-3p can promote EMT of CRC cells by suppressing DLC-1, a factor that promotes CRC cell invasion and lung metastasis in vivo.109
Not only exosomal miRNAs but also some exosomal lncRNAs can influence the EMT process in tumors. Tumor-derived exosomes were found to promote tumor invasion and stem cell characteristics in PDAC and showed a significant increase in lnc-Sox2ot. When the tumor cells were treated with exosomes from the tumor, the lnc-Sox2ot expression increased in the receptor cells. lnc-Sox2ot regulated Sox2 expression in tumor cells by sponging miR-200 family members and altering the expression of E-cadherin, N-cadherin, and vimentin.110 Similarly, lnc-ZFAS1 expression is high in exosomes derived from gastric cancer. Exosomes can be internalized into gastric cancer cells, and lnc-ZFAS1 in exosomes regulates Slug, Snail, Twist, and ZEB1 in tumor cells, thereby promoting tumor cell EMT and metastasis.111
The role of exosomal circRNA in EMT has also garnered attention. circ-PRMT5 has been shown to be highly expressed in bladder urothelial carcinoma and to promote tumor cell invasion and EMT. circ-PRMT5 was highly expressed in patient serum and urine exosomes, with a positive correlation between its level and lymph node metastasis.112 RNA sequencing detected the high expression of 33 circRNAs in gastric tumorous tissues, with circ-NRIP1 being the most abundant. Serum exosomal circ-NRIP1 from patients was also highly expressed. Exosomes from gastric cancer cells expressing a high level of circ-NRIP1 can promote the expression of EMT markers in tumor cells.113 These findings suggest that tumor cells may release exosomal circRNA to neighboring tumor cells, thereby promoting EMT and enhancing tumor invasiveness and metastasis.
Exosomal non-coding RNAs affect angiogenesis and lymphangiogenesis
Angiogenesis is an important characteristic of tumors.6 Angiogenesis enables malignant tumor cells to migrate away from their primary sites to the circulatory system or distant organs. Exosomes play a role in tumor angiogenesis. Exosomes from lnc-APC1-silenced CRC cells, for example, promote angiogenesis in endothelial cells by activating the MAPK pathway.44
Exosomal non-coding RNA can stimulate endothelial cells to form blood vessels (Figure 6). In gastric cancer, miR-130a was found to be transported from cancer cells to HUVECs via exosomes. miR-130a directly binds and inhibits c-MYB in HUVECs, whereas c-MYB inhibits the proliferation, migration, and tube formation of HUVECs. As a result, gastric cancer cells have the ability to secrete exosomes in order to promote angiogenesis.114 Exosomes from ovarian cancer cells contain miR-205 and can be internalized by HUVECs via a lipid-raft-related pathway. In endothelial cells, exosomal miR-205 regulated the PTEN-AKT pathway. Treatment with tumor-derived exosomal miR-205 significantly enhanced the proliferation, migration, and angiogenesis of HUVECs.115 miR-619-5p expression was increased in exosomes derived from non-small cell lung cancer cells, and exosomal miR-619-5p was found to regulate the level of RCAN1.4 in HUVECs, thereby promoting angiogenesis.116 In addition, ovarian-cancer-derived exosomes can transport lnc-MALAT1 to HUVECs, resulting in a significant increase in lnc-MALAT1 expression in HUVECs. lnc-MALAT1 may contribute to angiogenesis in part by modulating the expression of VEGF-A, VEGF-D, bFGF, PlGF, angiopoietin, ENA-78, leptin, and IL-8. Certain circRNAs contained in exosomes have been shown to promote angiogenesis as well.117 circRNA-100338 was found to be highly expressed in HCC-cell-derived exosomes. The treatment of HUVECs with tumor-cell-derived exosomes enhanced their proliferation and tubule formation, and the serum exosomal circ-100338 level in patients with HCC may be a predictor of lung metastasis.59
Figure 6.
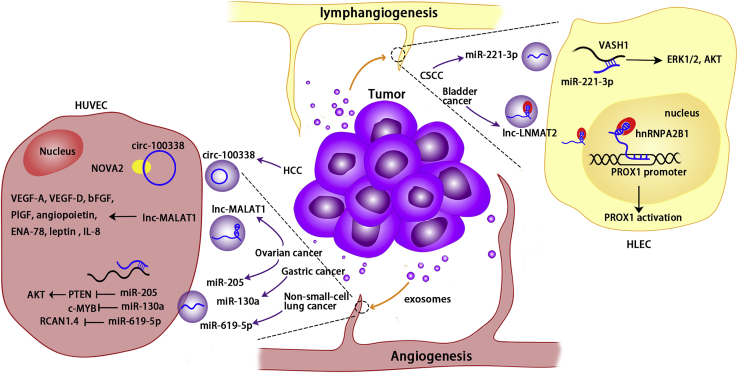
Exosomal non-coding RNAs have an effect on angiogenesis and lymphangiogenesis
Cancer-cell-derived exosomal non-coding RNAs regulate targets and pathways in vascular and lymphatic endothelial cells, thereby promoting angiogenesis and lymphangiogenesis. Exosomal circ-100338 from HCC cells can be internalized by HUVECs and promote angiogenesis via regulating NOVA2. Cancer-derived exosomal lnc-MALAT1 and miR-205 derived from ovarian cancer cells can enter HUVECs and regulate their targets, promoting angiogenesis. Exosomal miR-130a from gastric cancer cells promotes angiogenesis via c-MYB in HUVECs. Exosomal miR-619-5p from non-small cell lung cancer cells can enter HUVECs and regulate RCAN1.4 to promote angiogenesis. Exosomal lnc-LNMAT2 derived from bladder cancer cells can enter HLECs’ nuclei and activate PROX1 via hnRNPA2B1, thereby promoting lymphangiogenesis. In CSCC, miR-221-3p from cancer cells can be internalized and inhibit VASH1, thereby promoting lymphangiogenesis via ERK1/2 and AKT.
Along with vascular metastasis, lymphatic metastasis is a significant mode of tumor dissemination. The lymphatic vessels surrounding the tumor serve as conduits for tumor cells to spread from their primary sites to regional lymph nodes. Exosomal non-coding RNAs have been shown to affect lymphangiogenesis (Figure 6). lnc-LNMAT2 was found to be significantly overexpressed in muscle-invasive and lymph-node-positive bladder cancer, as determined by next-generation sequencing. Exosomal lnc-LNMAT2 derived from bladder cancer cells can directly interact with the PROX1 promoter sequence and increase PROX1 transcription in HLECs’ nucleus. In addition, lnc-LNMAT2 and RBP can promote H3K4 trimethylation of the PROX1 promoter.15 PROX1 is involved in the formation of lymphatic vessels, regulates endothelial cell differentiation, and induces lymphatic endothelial cell germination and extension.118 Exosomal lnc-LNMAT2 promotes lymphangiogenesis by upregulating PROX1 in lymphatic endothelial cells.15 Lymphatic metastasis is the main mechanism of cervical cancer metastasis. In cervical squamous cell carcinoma (CSCC), miR-221-3p was found to be transferred via exosomes from cancer cells to HLECs, promoting HLEC migration and tubule formation. Exosomal miR-221-3p inhibits vasohibin-1, a negative regulator of lymphangiogenesis, and increases ERK1/2 and AKT phosphorylation levels in CSCC cells, thereby promoting lymphangiogenesis and lymphatic metastasis.119 In addition, cancer-derived exosomes can transport miR-221-3 from CSCC cells to vascular endothelial cells, promoting angiogenesis.120 In summary, exosomal non-coding RNAs are involved in both vascular and lymphatic metastasis.
Exosomal non-coding RNA and distant metastasis
Tumor cells can spread in different ways to distant organs. To colonize and grow in new organs, tumor cells need an optimal microenvironment. It has long been observed that tumors do not appear to randomly select organs to metastasize to but rather exhibit a tendency to travel to and grow in specific organs based on the original tumor type.121 Tumor-derived exosomes play a role in organ-specific metastasis.122 Tumor cells release exosomes during this process to prepare recipient organs and establish a microenvironment conducive to metastasis. Tumor-derived exosomes remained in organs where cancer cells metastasize, promoting the formation of a pre-metastatic niche.122
Exosomal non-coding RNA affects the formation of the metastatic niche in metastasis-prone organs (Figure 7). Exosomes derived from cancer cells promote the migration and proliferation of lung fibroblasts in breast cancer. Sequencing results revealed that treatment with cancer-derived exosomes increased the content of certain lncRNAs in lung fibroblasts, implying that cancer-secreted exosomes may deliver lncRNA to lung fibroblasts to participate in pre-metastatic niche formation.123 Prostate cancer frequently metastasizes to the bone, and osteogenic lesions are common in prostatic bone metastases. miR-940 promotes the differentiation of human mesenchymal stem cells (HMCs) into osteoblasts. HMCs can internalize exosomal miR-940 derived from prostate cancer cells, promoting osteogenic differentiation via regulating ARHGAP1 and FAM134A. In addition, exosomes derived from cancer cells enhanced the osteogenic ability of host cells in xenograft models. miR-940 expression was higher in prostate cancer patients with bone metastasis than in normal prostate epithelial cells or bone tissues.124 This study suggests that exosomal miR-940 derived from prostate cancer promotes bone metastasis. miR-122 was detected to be highly expressed in exosomes derived from breast cancer cells. Exosomal miR-122 inhibits glucose uptake in lung fibroblasts and brain astrocytes by downregulating PKM2 and GLUT1 while increasing the glucose supply to cancer cells. In vivo, tumor-derived exosomal miR-122 reduced glucose utilization in niche tissues and promoted tumor cell metastasis.21 This indicated that exosomes from tumor cells reduced glucose uptake by niche cells, resulting in glucose redistribution in the pre-metastatic niche and promoting tumor cell growth in the metastatic organs. In breast cancer, the level of lnc-XIST was found to be negatively correlated with brain metastasis. lnc-XIST silencing increases exosomal miR-503 secretion, which induces M1-M2 polarization in microglia. This conversion upregulates PD-L1 expression in microglia, thereby inhibiting T cell proliferation. Microglia reprogramming by exosomal miR-503 promotes the brain metastasis of breast cancer.125 These findings imply that exosomal non-coding RNA derived from tumor cells can alter the microenvironment in distant organs, thereby facilitating tumor metastasis.
Figure 7.
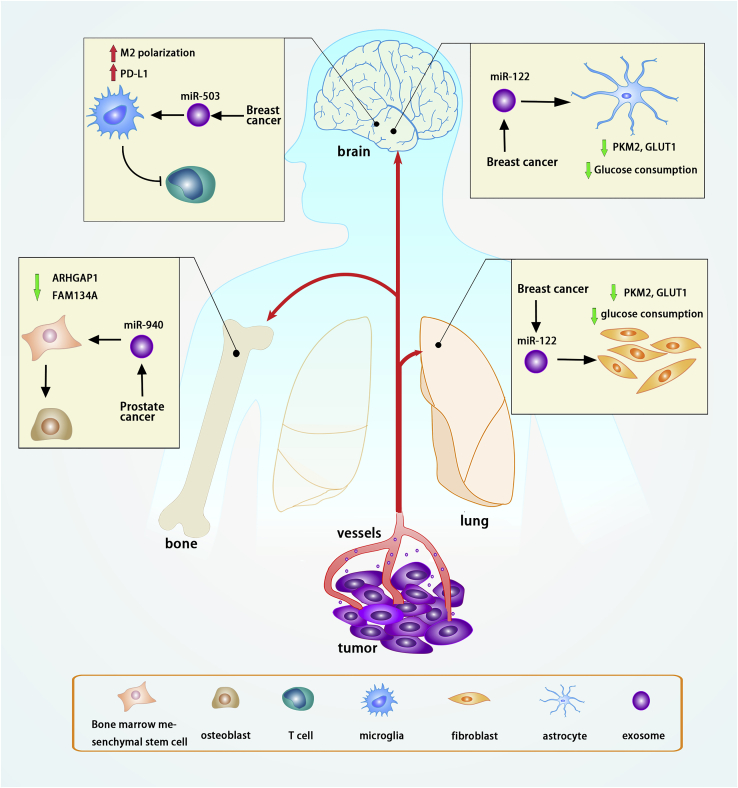
Exosomal non-coding RNAs and distant metastasis
Exosomal non-coding RNAs derived from tumor cells can be transported to distant organs and promote the formation of the pre-metastatic niche. In breast cancer, miR-122 can be transported to the lung and brain via cancer-cell-derived exosomes and inhibits PKM2 and GLUT1 expression, thereby decreasing glucose consumption in lung fibroblasts and brain astrocytes. Exosomal miR-503 derived from breast cancer promotes M2 polarization of microglia and increases PD-L1 expression on the microglial surface, inhibiting T cells. Exosomal miR-940 from prostate cancer regulates ARHGAP1 and FAM134A expression in bone marrow mesenchymal stem cells, promoting their differentiation into osteoblasts.
Clinical application of exosomal non-coding RNA in tumor
Interestingly, non-coding RNAs have the potential to serve as novel biomarkers for tumor diagnosis and prognosis.126, 127, 128 However, isolating non-coding RNA from body fluids for diagnostic purposes is challenging because RNA degrades rapidly outside tissue and cells. Exosomes provide a similar intracellular environment for non-coding RNAs, protecting RNAs and maintaining their stability. Extracting exosomal RNA from body fluids for diagnostic purposes represents a novel strategy for clinical diagnosis. For instance, exosomal miR-30d-5p and let-7d-3p were found to be expressed at low levels in the serum of cervical cancer patients but were differentially expressed in cervical tumors and adjacent normal tissues. Therefore, serum exosomal miR-30d-5p and let-7d-3p may be used as non-invasive screening biomarkers for cervical cancer.129 Exosomal lnc-HOTTIP is highly expressed in the plasma of patients with gastric cancer and is correlated with tumor node metastasis (TNM) stage, invasion depth, and overall survival. Therefore, plasma exosomal lnc-HOTTIP is suggested as a potential diagnostic and prognostic marker for gastric cancer.130 Furthermore, serum exosomal circRNAs have been shown to distinguish cancer patients from healthy individuals and can serve as biomarkers for tumor diagnosis.131 The researchers analyzed circ-PNN expression in the serum of 50 patients with CRC and 50 healthy subjects and revealed that circ-PNN is useful in diagnosing CRC and may be used as a potential biomarker for CRC detection.132
On the other hand, exosomal non-coding RNAs have the potential to be used as a novel tumor-treatment strategy. Non-coding RNAs play a critical role in tumor progression, implying that they may be therapeutic targets. For instance, clinical trials have been conducted to determine the efficacy of miR-34 mimics in tumors.133 Exosomes have a number of advantages as drug carriers and are currently being investigated for use in tumor therapy. Exosomes, when injected into the circulatory system of mice, are less clear and are more efficiently taken up by tumor cells than liposomes.134 Due to the fact that exosomes are derived from tissues or cells, they are largely nontoxic. Although nucleic acid drugs targeting non-coding RNAs represent a novel strategy for tumor therapy, they need carriers to avoid degradation. Exosomes as carriers for nucleic acid drugs regulating non-coding RNAs have garnered considerable attention in recent years. Intravenous injection of miR-126-loaded exosomes into lung cancer cells in mice effectively internalized them, and miR-126 inhibited lung cancer progression by disrupting the PTEN/PI3K/AKT signaling pathway in cancer cells.135 In gastric cancer, miR-214 is associated with chemoresistance to cisplatin, and intravenous injection of an antisense inhibitor of miR-214-loaded exosomes sensitizes the tumors’ response to cis-diamminedichloroplatinum in mice.136 Furthermore, exosomes can be modified artificially to enhance the targeting of nucleic acid drugs delivered via exosomes. In breast cancer cells, the transmembrane domain of the recombinant-tissue-derived growth factor receptor was fused to the GE11 peptide to enable exosomes to efficiently target EGFR+ breast cancer cells and deliver let-7a to inhibit cancer cells.137
Discussion
In this review, we explored the possible mechanisms by which exosomes package non-coding RNAs. In addition, we discussed the role of important exosomal non-coding RNAs discovered recently in the tumor microenvironment, tumor immunity, angiogenesis, lymphangiogenesis, and distant metastasis (Table 1; Figure 2).
Exosomes are small vesicles secreted by cells that contain a variety of substances and mediate intercellular communication.2,5 Exosomes, which act as a “bridge” for cell-to-cell communication, transport non-coding RNAs and are critical in the progression of tumors. As demonstrated in our review, this view is shared by a large number of researchers. Although non-coding RNAs play an important role in tumorigenesis and have the potential to be used as diagnostic markers and therapeutic targets, overcoming RNA’s extracellular instability is difficult in clinical application. Exosomes, which are vesicles with phospholipid bilayers secreted by cells, have been shown to protect non-coding RNA and enhance its extracellular stability. Exosomes can be extracted not only from blood but also from urine, saliva, and other bodily fluids.138, 139, 140 Numerous studies have indicated that exosomal non-coding RNAs may be useful for non-invasive or minimally invasive tumor diagnosis.129, 130, 131,141
Exosomes can evade circulatory clearance, accumulate at the tumor site, and are essentially nontoxic.134,142 Thus, exosomes as RNA vectors have a number of advantages in the treatment of cancer. Exosomes modified to contain a high level of tumor-suppressive or short hairpin RNAs (shRNAs) and small interfering RNAs (siRNAs) cancer-promoting non-coding RNAs can be used to inhibit tumor progression.136,143,144 In this way, exosomes can be used to treat cancer. However, challenges remain in the clinical application of exosomal non-coding RNA. There are currently no convenient and rapid methods for extracting a large number of high-purity exosomes, limiting the use of exosomal non-coding RNAs in clinical diagnosis and treatment. Existing methods for exosome extraction do not completely separate exosomes from other types of vesicles, which casts doubt on the clinical utility of exosomes. Additional research on the production and regulation of exosomes may be needed to overcome these obstacles.
Exosomal non-coding RNA is involved in tumor metastasis, and because exosomes have many advantages as RNA carriers, exosomal non-coding RNA may be useful for tumor diagnosis and treatment. With the advancement of exosome and non-coding RNA research and the development of exosome extraction technology, we believe that exosomal non-coding RNA will have a broad range of applications in the near future.
Acknowledgments
The present study was supported by the National Natural Science Foundation of China (32071349 and 81701820). This research was supported by the Zhejiang Provincial Natural Science Foundation of China (grant no. LY20H180014), the Department of Health of Zhejiang Province (grant no. 2018PY025), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2019A1515110433), and the Shenzhen Science and Technology Planning Project (grant no. JCYJ20190808123207674).
Author contributions
Conceptualization, J.X. and H.C.; funding acquisition, J.X. and H.C.; writing – original draft preparation, D.W. and W.Z.; writing – reviewing and editing, D.W. and W.Z.; visualization, C.Z. and L.W.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Heng Chen, Email: polyhengchen@szu.edu.cn.
Jianbin Xu, Email: xu9709426@zju.edu.cn.
References
- 1.Lee Y., El Andaloussi S., Wood M.J.A. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U S A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Indirect activation of naive CD4(+) T cells by dendritic cell-derived exosomes. Nat. Immun. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 5.Neviani P., Fabbri M. Exosomic microRNAs in the tumor microenvironment. Front. Med. 2015;2:47. doi: 10.3389/fmed.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Steeg P.S. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 8.Clayton A., Mason M.D. Exosomes in tumour immunity. Curr. Oncol. 2009;16:187–190. doi: 10.3747/co.v16i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita Y., Yoshioka Y., Ochiya T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016;107:385–390. doi: 10.1111/cas.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Q., Wu X., Li F., Ning B., Lu X., Zhang Y., Pan Y., Guan W. miR-27b inhibits gastric cancer metastasis by targeting NR2F2. Protein Cell. 2017;8:114–122. doi: 10.1007/s13238-016-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H., Yu T., Han Y., Jiang H., Wang C., You T., Zhao X., Shan H., Yang R., Yang L., et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer. 2018;17:119. doi: 10.1186/s12943-018-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Tong X., Zhou Z., Wang S., Lei Z., Zhang T., Liu Z., Zeng Y., Li C., Zhao J., et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-β-induced epithelial-mesenchymal transition and metastasis by controlling TIF1γ in non-small cell lung cancer. Mol. Cancer. 2018;17:140. doi: 10.1186/s12943-018-0889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Niel G., D'Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 14.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D.J., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C., Luo Y., He W., Zhao Y., Kong Y., Liu H., Zhong G., Li Y., Li J., Huang J., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Invest. 2020;130:404–421. doi: 10.1172/jci130892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shurtleff M.J., Temoche-Diaz M.M., Karfilis K.V., Ri S., Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/elife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koppers-Lalic D., Hackenberg M., Bijnsdorp I.V., van Eijndhoven M.A.J., Sadek P., Sie D., Zini N., Middeldorp J.M., Ylstra B., de Menezes R.X., et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 20.Zhou W., Fong M.Y., Min Y., Somlo G., Liu L., Palomares M.R., Yu Y., Chow A., O’Connor S., Chin A.R., et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O'Connor S.T.F., Li S., Chin A.R., et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mjelle R., Dima S.O., Bacalbasa N., Chawla K., Sorop A., Cucu D., Herlea V., Sætrom P., Popescu I. Comprehensive transcriptomic analyses of tissue, serum, and serum exosomes from hepatocellular carcinoma patients. BMC Cancer. 2019;19:1007. doi: 10.1186/s12885-019-6249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian X.P., Wang C.Y., Jin X.H., Li M., Wang F.W., Huang W.J., Yun J.P., Xu R.H., Cai Q.Q., Xie D. Acidic microenvironment up-regulates exosomal miR-21 and miR-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 2019;9:1965–1979. doi: 10.7150/thno.30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng T., Zhang H., Yang H., Wang H., Bai M., Sun W., Wang X., Si Y., Ning T., Zhang L., et al. RETRACTED: exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF Axis of endothelial cells. Mol. Ther. Nucleic Acids. 2020;19:1449–1459. doi: 10.1016/j.omtn.2020.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Feng C., She J., Chen X., Zhang Q., Zhang X., Wang Y., Ye J., Shi J., Tao J., Feng M., et al. Exosomal miR-196a-1 promotes gastric cancer cell invasion and metastasis by targeting SFRP1. Nanomedicine. 2019;14:2579–2593. doi: 10.2217/nnm-2019-0053. [DOI] [PubMed] [Google Scholar]
- 26.He S., Li Z., Yu Y., Zeng Q., Cheng Y., Ji W., Xia W., Lu S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019;379:203–213. doi: 10.1016/j.yexcr.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 27.Hu H.Y., Yu C.H., Zhang H.H., Zhang S.Z., Yu W.Y., Yang Y., Chen Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019;132:470–477. doi: 10.1016/j.ijbiomac.2019.03.221. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Z., Li Y., Pan Y., Lan X., Song F., Sun J., Zhou K., Liu X., Ren X., Wang F., et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadei L., Calore F., Creighton C.J., Guescini M., Batte K., Iwenofu O.H., Zewdu A., Braggio D.A., Bill K.L., Fadda P., et al. Exosome-derived miR-25-3p and miR-92a-3p stimulate liposarcoma progression. Cancer Res. 2017;77:3846–3856. doi: 10.1158/0008-5472.can-16-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu M., Zhang N., He S., Lu X. Exosomal miR-106a derived from gastric cancer promotes peritoneal metastasis via direct regulation of Smad7. Cell Cycle. 2020 doi: 10.1080/15384101.2020.1749467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerloff D., Lützkendorf J., Moritz R.K.C., Wersig T., Mäder K., Mäder K., Müller L.P., Müller L.P., Sunderkötter C., Sunderkötter C. Melanoma-derived exosomal miR-125b-5p educates tumor associated macrophages (TAMs) by targeting lysosomal acid lipase A (LIPA) Cancers. 2020;12:464. doi: 10.3390/cancers12020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B., Mao J.H., Wang B.Y., Wang L.X., Wen H.Y., Xu L.J., Fu J.X., Yang H. Exosomal miR-1910-3p promotes proliferation, metastasis, and autophagy of breast cancer cells by targeting MTMR3 and activating the NF-κB signaling pathway. Cancer Lett. 2020;489:87–99. doi: 10.1016/j.canlet.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Lu J., Liu Q.H., Wang F., Tan J.J., Deng Y.Q., Peng X.H., Liu X., Zhang B., Xu X., Li X.P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018;37:147. doi: 10.1186/s13046-018-0814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W., Zhang L., Guo B., Deng J., Wu S., Li F., Wang Y., Lu J., Zhou Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol. Cancer. 2019;18:22. doi: 10.1186/s12943-019-0949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luan Y., Li X., Luan Y., Zhao R., Li Y., Liu L., Hao Y., Oleg Vladimir B., Jia L. Circulating lncRNA UCA1 promotes malignancy of colorectal cancer via the miR-143/MYO6 Axis. Mol. Ther. Nucleic Acids. 2020;19:790–803. doi: 10.1016/j.omtn.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J., Xiao Y., Liu B., Pan S., Liu Q., Shan Y., Li S., Qi Y., Huang Y., Jia L. Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2020;39:54. doi: 10.1186/s13046-020-01562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B., Xu H., Han H., Song S., Zhang X., Ouyang L., Qian C.a., Hong Y., Qiu Y., Zhou W., et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37:5508–5519. doi: 10.1038/s41388-018-0359-0. [DOI] [PubMed] [Google Scholar]
- 38.Zheng R., Du M., Wang X., Xu W., Liang J., Wang W., Lv Q., Qin C., Chu H., Wang M., et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer. 2018;17:143. doi: 10.1186/s12943-018-0880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D., Kang H., Gao M., Jin L., Zhang F., Chen D., Li M., Xiao L. Exosome-transmitted circ_MMP2 promotes hepatocellular carcinoma metastasis by upregulating MMP2. Mol. Oncol. 2020;14:1365–1380. doi: 10.1002/1878-0261.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S., Hu Y., Lv X., Li B., Gu D., Li Y., Sun Y., Su Y. Circ-0000284 arouses malignant phenotype of cholangiocarcinoma cells and regulates the biological functions of peripheral cells through cellular communication. Clin. Sci. 2019;133:1935–1953. doi: 10.1042/cs20190589. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N., Nan A., Chen L., Li X., Jia Y., Qiu M., Dai X., Zhou H., Zhu J., Zhang H., Jiang Y. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol. Cancer. 2020;19:101. doi: 10.1186/s12943-020-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tano K., Akimitsu N. Long non-coding RNAs in cancer progression. Front. Genet. 2012;3:219. doi: 10.3389/fgene.2012.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao W., Qin P., Zhang D., Cui X.C., Gao J., Yu Z.Z., Chai Y.T., Wang J.X., Li J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY) 2019;11:9581–9596. doi: 10.18632/aging.102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F.W., Cao C.H., Han K., Zhao Y.X., Cai M.Y., Xiang Z.C., Zhang J.X., Chen J.W., Zhong L.P., Huang Y., et al. APC-activated long noncoding RNA inhibits colorectal carcinoma pathogenesis through reduction of exosome production. J. Clin. Invest. 2019;129:727–743. doi: 10.1172/jci122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 47.Tchurikov N.A., Kretova O.V., Fedoseeva D.M., Sosin D.V., Grachev S.A., Serebraykova M.V., Romanenko S.A., Vorobieva N.V., Kravatsky Y.V. DNA double-strand breaks coupled with PARP1 and HNRNPA2B1 binding sites flank coordinately expressed domains in human chromosomes. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao T., Liu X., He B., Nie Z., Zhu C., Zhang P., Wang S. Exosomal lncRNA 91H is associated with poor development in colorectal cancer by modifying HNRNPK expression. Cancer Cell Int. 2018;18:11. doi: 10.1186/s12935-018-0506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue A., Sawata S.Y., Taira K., Wadhwa R. Loss-of-function screening by randomized intracellular antibodies: identification of hnRNP-K as a potential target for metastasis. Proc. Natl. Acad. Sci. U S A. 2007;104:8983–8988. doi: 10.1073/pnas.0607595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Z.X., Liu H.S., Wang F.W., Xiong L., Zhou C., Hu T., He X.W., Wu X.J., Xie D., Wu X.R., Lan P. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis. 2019;10:829. doi: 10.1038/s41419-019-2077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeck W.R., Sharpless N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng X., Lin X., Zhang Y., Li Q., Guo Y., Fang C., Wang H. Exosomal circular RNA sorting mechanisms and their function in promoting or inhibiting cancer. Oncol. Lett. 2020;19:3369–3380. doi: 10.3892/ol.2020.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z., Yanfang W., Li J., Jiang P., Peng T., Chen K., Zhao X., Zhang Y., Zhen P., Zhu J., Li X. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer Lett. 2018;432:237–250. doi: 10.1016/j.canlet.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 54.Stein U., Walther W., Arlt F., Schwabe H., Smith J., Fichtner I., Birchmeier W., Schlag P.M. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat. Med. 2009;15:59–67. doi: 10.1038/nm.1889. [DOI] [PubMed] [Google Scholar]
- 55.Gherardi E., Birchmeier W., Birchmeier C., Woude G.V. Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 56.Lu J., Wang Y.H., Yoon C., Huang X.Y., Xu Y., Xie J.W., Wang J.B., Lin J.X., Chen Q.Y., Cao L.L., et al. Circular RNA circ-RanGAP1 regulates VEGFA expression by targeting miR-877-3p to facilitate gastric cancer invasion and metastasis. Cancer Lett. 2020;471:38–48. doi: 10.1016/j.canlet.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 57.Zong Z.H., Du Y.P., Guan X., Chen S., Zhao Y. CircWHSC1 promotes ovarian cancer progression by regulating MUC1 and hTERT through sponging miR-145 and miR-1182. J. Exp. Clin. Cancer Res. 2019;38:437. doi: 10.1186/s13046-019-1437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan X., Zong Z.H., Liu Y., Chen S., Wang L.L., Zhao Y. circPUM1 promotes tumorigenesis and progression of ovarian cancer by sponging miR-615-5p and miR-6753-5p. Mol. Ther. Nucleic Acids. 2019;18:882–892. doi: 10.1016/j.omtn.2019.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X.-Y., Huang Z.-L., Huang J., Xu B., Huang X.-Y., Xu Y.-H., Zhou J., Tang Z.-Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020;39:20. doi: 10.1186/s13046-020-1529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao H., Chen S., Fu Q. Exosomes from CD133 + cells carrying circ-ABCC1 mediate cell stemness and metastasis in colorectal cancer. J. Cell. Biochem. 2020;121:3286–3297. doi: 10.1002/jcb.29600. [DOI] [PubMed] [Google Scholar]
- 61.Conigliaro A., Costa V., Lo Dico A., Saieva L., Buccheri S., Dieli F., Manno M., Raccosta S., Mancone C., Tripodi M., et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang J.H., Zhang Z.J., Shang L.R., Luo Y.W., Lin Y.F., Yuan Y., Zhuang S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 2018;68:1459–1475. doi: 10.1002/hep.29920. [DOI] [PubMed] [Google Scholar]
- 63.Li J., Li Z., Jiang P., Peng M., Zhang X., Chen K., Liu H., Bi H., Liu X., Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. J. Exp. Clin. Cancer Res. 2018;37:177. doi: 10.1186/s13046-018-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang T., Lv H., Lv G., Li T., Wang C., Han Q., Yu L., Su B., Guo L., Huang S., et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018;9:191. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu T., Hu J. Melanoma-derived exosomes induce reprogramming fibroblasts into cancer-associated fibroblasts via Gm26809 delivery. Cell Cycle. 2019;18:3085–3094. doi: 10.1080/15384101.2019.1669380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan K., Spassova I., Gravemeyer J., Ritter C., Horny K., Lange A., Gambichler T., Odum N., Schrama D., Schadendorf D., et al. Merkel cell carcinoma-derived exosome-shuttle miR-375 induces fibroblast polarization by inhibition of RBPJ and p53. Oncogene. 2020;40:980–996. doi: 10.1038/s41388-020-01576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Procopio M.-G., Laszlo C., Al Labban D., Kim D.E., Bordignon P., Jo S.-H., Goruppi S., Menietti E., Ostano P., Ala U., et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat. Cell Biol. 2015;17:1193–1204. doi: 10.1038/ncb3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goruppi S., Procopio M.-G., Jo S., Clocchiatti A., Neel V., Dotto G.P. The ULK3 kinase is critical for convergent control of cancer-associated fibroblast activation by CSL and GLI. Cell Rep. 2017;20:2468–2479. doi: 10.1016/j.celrep.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrera M., Llorens C., Rodríguez M., Herrera A., Ramos R., Gil B., Candia A., Larriba M.J., Garre P., Earl J., et al. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and Cancer-associated fibroblasts in colorectal cancer. Mol. Cancer. 2018;17:114. doi: 10.1186/s12943-018-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren J., Ding L., Zhang D., Shi G., Xu Q., Shen S., Wang Y., Wang T., Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang H., Wei H., Wang J., Li L., Chen A., Li Z. MicroRNA-181d-5p-Containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. Nucleic Acids. 2020;19:654–667. doi: 10.1016/j.omtn.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y., Ye X., Tan C., Hongo J.A., Zha J., Liu J., Kallop D., Ludlam M.J.C., Pei L. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 73.Cichoń M.A., SZentpetery Z., Caley M.P., Papadakis E.S., Mackenzie I.C., Brennan C.H., O'Toole E.A. The receptor tyrosine kinase Axl regulates cell-cell adhesion and stemness in cutaneous squamous cell carcinoma. Oncogene. 2014;33:4185–4192. doi: 10.1038/onc.2013.388. [DOI] [PubMed] [Google Scholar]
- 74.Li Y.Y., Tao Y.W., Gao S., Li P., Zheng J.M., Zhang S.E., Liang J., Zhang Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–220. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi L., Wang Z., Geng X., Zhang Y., Xue Z. Exosomal miRNA-34 from cancer-associated fibroblasts inhibits growth and invasion of gastric cancer cells in vitro and in vivo. Aging (Albany NY) 2020;12:8549–8564. doi: 10.18632/aging.103157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Rodríguez M., Bajo-Santos C., Hessvik N.P., Lorenz S., Fromm B., Berge V., Sandvig K., Linē A., Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer. 2017;16:156. doi: 10.1186/s12943-017-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li B.L., Lu W., Qu J.J., Ye L., Du G.Q., Wan X.P. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell. Physiol. 2019;234:2943–2953. doi: 10.1002/jcp.27111. [DOI] [PubMed] [Google Scholar]
- 78.Dou D., Ren X., Han M., Xu X., Ge X., Gu Y., Wang X. Cancer-associated fibroblasts-derived exosomes suppress immune cell function in breast cancer via the miR-92/PD-L1 pathway. Front. Immunol. 2020;11:2026. doi: 10.3389/fimmu.2020.02026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Gillies R.J., Verduzco D., Gatenby R.A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang H., Zhang H., Yang Y., Wang X., Deng T., Liu R., Ning T., Bai M., Li H., Zhu K., et al. Hypoxia induced exosomal circRNA promotes metastasis of Colorectal Cancer via targeting GEF-H1/RhoA axis. Theranostics. 2020;10:8211–8226. doi: 10.7150/thno.44419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue M., Chen W., Xiang A., Wang R., Chen H., Pan J., Pang H., An H., Wang X., Hou H., Li X. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol. Cancer. 2017;16:143. doi: 10.1186/s12943-017-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang X., Bai J., Yin H., Long L., Zheng Z., Wang Q., Chen F., Yu X., Zhou Y. Exosomal miR-1255b-5p targets human telomerase reverse transcriptase in colorectal cancer cells to suppress epithelial-to-mesenchymal transition. Mol. Oncol. 2020;14:2589–2608. doi: 10.1002/1878-0261.12765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Asangani I.A., Rasheed S.A.K., Nikolova D.A., Leupold J.H., Colburn N.H., Post S., Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 84.Li X., Wu X. MiR-21-5p promotes the progression of non-small-cell lung cancer by regulating the expression of SMAD7. Onco Targets Ther. 2018;11:8445–8454. doi: 10.2147/ott.s172393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang H., Tan Z., Hu H., Liu H., Wu T., Zheng C., Wang X., Luo Z., Wang J., Liu S., et al. microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer. 2019;19:738. doi: 10.1186/s12885-019-5951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Q., Li B., Li Q., Wei S., He Z., Huang X., Wang L., Xia Y., Xu Z., Li Z., et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9:854. doi: 10.1038/s41419-018-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y., Han T., Feng D., Li J., Wu M., Peng X., Wang B., Zhan X., Fu P. Screening of non-invasive miRNA biomarker candidates for metastasis of gastric cancer by small RNA sequencing of plasma exosomes. Carcinogenesis. 2020;41:582–590. doi: 10.1093/carcin/bgz186. [DOI] [PubMed] [Google Scholar]
- 88.Lai X., Wang M., McElyea S.D., Sherman S., House M., Korc M. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett. 2017;393:86–93. doi: 10.1016/j.canlet.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin X., Chen Y., Chen H., Fei S., Chen D., Cai X., Liu L., Lin B., Su H., Zhao L., et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non-small cell lung cancer using next-generation sequencing. Clin. Cancer Res. 2017;23:5311–5319. doi: 10.1158/1078-0432.ccr-17-0577. [DOI] [PubMed] [Google Scholar]
- 90.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Liang Y., Song X., Li Y., Chen B., Zhao W., Wang L., Zhang H., Liu Y., Han D., Zhang N., et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol. Cancer. 2020;19:85. doi: 10.1186/s12943-020-01206-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Wang D., Wang X., Si M., Yang J., Sun S., Wu H., Cui S., Qu X., Yu X. Exosome-encapsulated miRNAs contribute to CXCL12/CXCR4-induced liver metastasis of colorectal cancer by enhancing M2 polarization of macrophages. Cancer Lett. 2020;474:36–52. doi: 10.1016/j.canlet.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Zhao S., Mi Y., Guan B., Zheng B., Wei P., Gu Y., Zhang Z., Cai S., Xu Y., Li X., et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J. Hematol. Oncol. 2020;13:156. doi: 10.1186/s13045-020-00991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Hypoxic tumor-derived exosomal miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to promote pancreatic cancer metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.can-17-3841. [DOI] [PubMed] [Google Scholar]
- 95.Lan J., Sun L., Xu F., Liu L., Hu F., Song D., Hou Z., Wu W., Luo X., Wang J., et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.can-18-0014. [DOI] [PubMed] [Google Scholar]
- 96.Guan H., Peng R., Fang F., Mao L., Chen Z., Yang S., Dai C., Wu H., Wang C., Feng N., et al. Tumor-associated macrophages promote prostate cancer progression via exosome-mediated miR-95 transfer. J. Cell. Physiol. 2020;235:9729–9742. doi: 10.1002/jcp.29784. [DOI] [PubMed] [Google Scholar]
- 97.Wu Q., Wu X., Ying X., Zhu Q., Wang X., Jiang L., Chen X., Wu Y., Wang X. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer Cell Int. 2017;17:62. doi: 10.1186/s12935-017-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun H., Shi K., Qi K., Kong H., Zhang J., Dai S., Ye W., Deng T., He Q., Zhou M. Natural killer cell-derived exosomal miR-3607-3p inhibits pancreatic cancer progression by targeting IL-26. Front. Immunol. 2019;10:2819. doi: 10.3389/fimmu.2019.02819. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Briand J., Garnier D., Nadaradjane A., Clément-Colmou K., Potiron V., Supiot S., Bougras-Cartron G., Frenel J.-S., Heymann D., Vallette F.M., Cartron P.F. Radiotherapy-induced overexpression of exosomal miRNA-378a-3p in cancer cells limits natural killer cells cytotoxicity. Epigenomics. 2020;12:397–408. doi: 10.2217/epi-2019-0193. [DOI] [PubMed] [Google Scholar]
- 100.Zhang P.-F., Gao C., Huang X.-Y., Lu J.-C., Guo X.-J., Shi G.-M., Cai J.-B., Ke A.-W. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol. Cancer. 2020;19:110. doi: 10.1186/s12943-020-01222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye S.B., Zhang H., Cai T.T., Liu Y.N., Ni J.J., He J., Peng J.Y., Chen Q.Y., Mo H.Y., Cui J., et al. Exosomal miR-24-3p impedes T-cell function by targeting FGF11 and serves as a potential prognostic biomarker for nasopharyngeal carcinoma. J. Pathol. 2016;240:329–340. doi: 10.1002/path.4781. [DOI] [PubMed] [Google Scholar]
- 102.Ni C., Fang Q.Q., Chen W.Z., Jiang J.X., Jiang Z., Ye J., Zhang T., Yang L., Meng F.B., Xia W.J., et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+γδ1 Treg cells. Signal Transduct. Target. Ther. 2020;5:41. doi: 10.1038/s41392-020-0129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang J., Huang F., Shi Y., Zhang Q., Xu S., Yao Y., Jiang R. RP11-323N12.5 promotes the malignancy and immunosuppression of human gastric cancer by increasing YAP1 transcription. Gastric Cancer. 2020 doi: 10.1007/s10120-020-01099-9. [DOI] [PubMed] [Google Scholar]
- 104.Wang J., Zhao X., Wang Y., Ren F., Sun D., Yan Y., Kong X., Bu J., Liu M., Xu S. circRNA-002178 act as a ceRNA to promote PDL1/PD1 expression in lung adenocarcinoma. Cell Death Dis. 2020;11:32. doi: 10.1038/s41419-020-2230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou M., Chen J.H., Zhou L.J., Chen W.C., Ding G.P., Cao L.P. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol. 2014;292:65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Shang A., Gu C., Wang W., Wang X., Sun J., Zeng B., Chen C., Chang W., Ping Y., Ji P., et al. Exosomal circPACRGL promotes progression of colorectal cancer via the miR-142-3p/miR-506-3p- TGF-β1 axis. Mol. Cancer. 2020;19:117. doi: 10.1186/s12943-020-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thiery J.P., Acloque H., Huang R.Y.J., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 108.Wang L., Yang G., Zhao D., Wang J., Bai Y., Peng Q., Wang H., Fang R., Chen G., Wang Z., et al. CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: role of remote MiR-19b-3p. Mol. Cancer. 2019;18:86. doi: 10.1186/s12943-019-0997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu H., Liu Y., Sun P., Leng K., Xu Y., Mei L., Han P., Zhang B., Yao K., Li C., et al. Colorectal cancer-derived exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1 expression. Clin. Sci. 2020;134:419–434. doi: 10.1042/cs20191087. [DOI] [PubMed] [Google Scholar]
- 110.Li Z., Jiang P., Li J., Peng M., Zhao X., Zhang X., Chen K., Zhang Y., Liu H., Gan L., et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene. 2018;37:3822–3838. doi: 10.1038/s41388-018-0237-9. [DOI] [PubMed] [Google Scholar]
- 111.Pan L., Liang W., Fu M., Huang Z.-H., Li X., Zhang W., Zhang P., Qian H., Jiang P.-C., Xu W.-R., Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2017;143:991–1004. doi: 10.1007/s00432-017-2361-2. [DOI] [PubMed] [Google Scholar]
- 112.Chen X., Chen R.X., Wei W.S., Li Y.H., Feng Z.H., Tan L., Chen J.W., Yuan G.J., Chen S.L., Guo S.J., et al. PRMT5 circular RNA promotes metastasis of urothelial carcinoma of the bladder through sponging miR-30c to induce epithelial-mesenchymal transition. Clin. Cancer Res. 2018;24:6319–6330. doi: 10.1158/1078-0432.ccr-18-1270. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X., Wang S., Wang H., Cao J., Huang X., Chen Z., Xu P., Sun G., Xu J., Lv J., Xu Z. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol. Cancer. 2019;18:20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang H., Zhang H., Ge S., Ning T., Bai M., Li J., Li S., Sun W., Deng T., Zhang L., et al. Exosome-derived mir-130a activates angiogenesis in gastric cancer by targeting c-myb in vascular endothelial cells. Mol. Ther. 2018;26:2466–2475. doi: 10.1016/j.ymthe.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115.He L., Zhu W., Chen Q., Yuan Y., Wang Y., Wang J., Wu X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9:8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim D.H., Park S., Kim H., Choi Y.J., Kim S.Y., Sung K.J., Sung Y.H., Choi C.-M., Yun M., Yi Y.-S., et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1. Cancer Lett. 2020;475:2–13. doi: 10.1016/j.canlet.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 117.Qiu J.J., Lin X.J., Tang X.Y., Zheng T.T., Lin Y.Y., Hua K.Q. Exosomal metastasis-associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 2018;14:1960–1973. doi: 10.7150/ijbs.28048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saharinen P., Tammela T., Karkkainen M.J., Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 119.Zhou C.F., Ma J., Huang L., Yi H.Y., Zhang Y.M., Wu X.G., Yan R.M., Liang L., Zhong M., Yu Y.H., et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene. 2019;38:1256–1268. doi: 10.1038/s41388-018-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu X.G., Zhou C.F., Zhang Y.M., Yan R.M., Wei W.F., Chen X.J., Yi H.Y., Liang L.J., Fan L.S., Liang L., et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis. 2019;22:397–410. doi: 10.1007/s10456-019-09665-1. [DOI] [PubMed] [Google Scholar]
- 121.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. doi: 10.1016/s0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 122.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng T., Zhang P., Sun Y., Wang Y., Tong J., Dai H., Hua Z. High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation. Gene. 2019;710:258–264. doi: 10.1016/j.gene.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 124.Hashimoto K., Ochi H., Sunamura S., Kosaka N., Mabuchi Y., Fukuda T., Yao K., Kanda H., Ae K., Okawa A., et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. U S A. 2018;115:2204–2209. doi: 10.1073/pnas.1717363115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xing F., Liu Y., Wu S.Y., Wu K., Sharma S., Mo Y.Y., Feng J., Sanders S., Jin G., Singh R., et al. Loss of XIST in breast cancer activates MSN-c-met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res. 2018;78:4316–4330. doi: 10.1158/0008-5472.can-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee Y.S., Dutta A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhan A., Soleimani M., Mandal S.S. Long noncoding RNA and cancer: a new paradigm. Cancer Res. 2017;77:3965–3981. doi: 10.1158/0008-5472.can-16-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Li P., Wu M. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol. Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng M., Hou L., Ma Y., Zhou L., Wang F., Cheng B., Wang W., Lu B., Liu P., Lu W., Lu Y. Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol. Cancer. 2019;18:76. doi: 10.1186/s12943-019-0999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao R., Zhang Y., Zhang X., Yang Y., Zheng X., Li X., Liu Y., Zhang Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer. 2018;17:68. doi: 10.1186/s12943-018-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie Y., Li J., Li P., Li N., Zhang Y., Binang H., Zhao Y., Duan W., Chen Y., Wang Y., et al. RNA-seq profiling of serum exosomal circular RNAs reveals circ-PNN as a potential biomarker for human colorectal cancer. Front. Oncol. 2020;10:982. doi: 10.3389/fonc.2020.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., Lee J.J., Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nie H., Xie X., Zhang D., Zhou Y., Li B., Li F., Li F., Cheng Y., Mei H., Meng H., Jia L. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12:877–887. doi: 10.1039/c9nr09011h. [DOI] [PubMed] [Google Scholar]
- 136.Wang X., Zhang H., Bai M., Ning T., Ge S., Deng T., Liu R., Zhang L., Ying G., Ba Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to Cisplatin in gastric cancer. Mol. Ther. 2018;26:774–783. doi: 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohno S.-i., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Motamedinia P., Scott A.N., Bate K.L., Sadeghi N., Salazar G., Shapiro E., Ahn J., Lipsky M., Lin J., Hruby G.W., et al. Urine exosomes for non-invasive assessment of gene expression and mutations of prostate cancer. PLoS One. 2016;11:e0154507. doi: 10.1371/journal.pone.0154507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang J., Wei F., Schafer C., Wong D.T.W. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS One. 2014;9:e110641. doi: 10.1371/journal.pone.0110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu Y., Qi C., Liu X., Zhang C., Gao J., Wu Y., Yang J., Zhao Q., Li J., Wang X., Shen L. Malignant ascites-derived exosomes promote peritoneal tumor cell dissemination and reveal a distinct miRNA signature in advanced gastric cancer. Cancer Lett. 2019;457:142–150. doi: 10.1016/j.canlet.2019.04.034. [DOI] [PubMed] [Google Scholar]
- 141.Lin L.Y., Yang L., Zeng Q., Wang L., Chen M.L., Zhao Z.H., Ye G.D., Luo Q.C., Lv P.Y., Guo Q.W., et al. Tumor-originated exosomal lncUEGC1 as a circulating biomarker for early-stage gastric cancer. Mol. Cancer. 2018;17:84. doi: 10.1186/s12943-018-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Meel R., Fens M.H.A.M., Vader P., van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J. Control. Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 143.Xie C., Du L.Y., Guo F., Li X., Cheng B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell. Biochem. 2019;458:11–26. doi: 10.1007/s11010-019-03526-7. [DOI] [PubMed] [Google Scholar]
- 144.Lou G., Song X., Yang F., Wu S., Wang J., Chen Z., Liu Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015;8:122. doi: 10.1186/s13045-015-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]