Highlights
-
•
Low-dose interleukin-2 treatment increases the levels of cytokine IL-10 and TGFβ1.
-
•
Peripheral IL-10 and TGFβ1 signaling mediate the therapeutic effects of interleukin-2.
-
•
IL-10 and TGFβ1 directly reverses chronic migraine-related peripheral sensitization.
Keywords: Chronic migraine, Sensitization, Low-dose interleukin-2, Transforming growth factor beta-1, Interleukin-10, Trigeminal ganglion neuron
Abstract
Low-dose interleukin-2 (LD-IL-2) treatment has been shown to effectively reverse chronic migraine-related behaviors and the sensitization of trigeminal ganglion (TG) neurons through expansion and activation of peripheral regulatory T cells (Tregs) in mice. In this study, we investigated the molecular mechanisms underlying the effects of LD-IL-2 and Treg cells. LD-IL-2 treatment increases the production of cytokines interleukin-10 (IL-10) and transforming growth factor beta-1 (TGFβ1) in T cells, especially Treg cells, suggesting that they may mediate the therapeutic effect of LD-IL-2. Indeed, neutralizing antibodies against either IL-10 or TGFβ completely blocked the effects of LD-IL-2 on the facial mechanical hypersensitivity as well as the sensitization of TG neurons resulting from repeated nitroglycerin (NTG, a reliable trigger of migraine in patients) administration in mice, indicating that LD-IL-2 and Treg cells engage both peripheral IL-10 and TGFβ signaling pathways to reverse chronic-migraine related sensitizations. In an in vitro assay, incubation of TG culture with exogenous IL-10 or TGFβ1 fully reversed NTG-induced sensitization of TG neurons, suggesting that the IL-10 and TGFβ1 signaling in TG neurons contribute to LD-IL-2′s therapeutic effects. Collectively, these results not only elucidate the molecular mechanisms through which LD-IL-2 and Treg cells reverse chronic-migraine related sensitizations, but also suggest that the IL-10 and TGFβ1 signaling pathways in TG neurons are potential targets for chronic migraine therapy.
Introduction
With at least 15 headache days per month for at least 3 months, chronic migraine is highly debilitating and with limited treatment options (May and Schulte, 2016). Many patients are either unresponsive to current therapies or are intolerant to their side effects. There is an urgent need to develop better treatments and to understand their mechanisms of action.
A large body of clinical and animal studies support low-dose interleukin-2 (LD-IL-2) treatment as a safe and effective therapy for multiple autoimmune and neurological diseases, including chronic pain (Alves et al., 2017, Davoli-Ferreira et al., 2020, Gao et al., 2017, Hu et al., 2021, Klatzmann and Abbas, 2015, Zhang et al., 2018). In a mouse model of chronic migraine, we have shown that LD-IL-2 treatment effectively reverses chronic migraine-related behaviors and prevents the sensitization of trigeminal ganglion (TG) neurons through expansion and activation of the endogenous regulatory T cells (Tregs) at peripheral tissues (Guo et al., 2021, Zhang et al., 2020).
Tregs are a subpopulation of CD4+ T cells that selectively express the transcription factor Foxp3 in the nucleus and high-affinity IL-2 receptors on the plasma membrane to outcompete other immune cells for IL-2 (Schmidt et al., 2012). They are known to employ multiple mechanisms to suppress diverse types of immune cells (Schmidt et al., 2012), many of which have been implicated in migraine pathophysiology, including meningeal mast cells, dendritic cells, macrophages as well as microglia in the cervical/medullary dorsal horn (He et al., 2019, Levy et al., 2007, McIlvried et al., 2017, Reuter et al., 2001, Schain et al., 2018, Wieseler et al., 2017). To further validate LD-IL-2 and other Treg-targeted therapy [e.g. via activation of tumor necrosis factor receptor 2 on Treg cells (Fischer et al., 2019)] for chronic pain, we investigated the molecular mechanisms through which LD-IL-2 reverses chronic migraine-related behavioral and cellular sensitizations in the present study.
One of the main mechanisms that Treg cells use for immunosuppression is through the secretion of transforming growth factor beta-1 (TGFβ1), interleukin-10 (IL-10) and interleukin-35 (Sharabi et al., 2018). All of these cytokines, especially IL-10 and TGFβ1, have been shown to exhibit anti-nociceptive effects in various chronic pain models including neuropathic pain, back pain and pain related to experimental autoimmune encephalomyelitis (Chen et al., 2015, Duffy et al., 2019, Krukowski et al., 2016, Kwilasz et al., 2015, Laumet et al., 2020, Milligan et al., 2006, Zhang et al., 2017). In an attempt to determine the mechanism by which LD-IL-2 exerts in anti-migraine effects, we first examined whether LD-IL-2 treatment increases the production of IL-10 and TGFβ1 in T cells, especially Treg cells. Next, we modeled chronic migraine with repeated administration of nitroglycerin (NTG, a reliable migraine trigger in migraineurs) in mice and used neutralizing antibodies to investigate whether peripheral IL-10 and/or TGFβ signaling pathways mediate the beneficial effects of LD-IL-2 on chronic migraine-related behaviors and the sensitization of TG neurons. Since both calcitonin gene-related peptide (CGRP) and pituitary adenylate cyclase-activating polypeptide (PACAP) are strongly implicated in migraine pathophysiology (Messlinger and Russo, 2019), we used ratiometric Ca2+-imaging to identify TG neurons that respond to CGRP [i.e. CGRP-responsive (CGRP-R) neurons] and/or respond to PACAP [i.e. PACAP-responsive [(PACAP-R) neurons], respectively. This allowed us to investigate how IL-10 and TGFβ signaling modulate the sensitization of TG neurons. Finally, because both IL-10 and TGFβ1 have been shown to activate their cognate receptors in primary afferent neurons to regulate ion channel expression and neuronal excitability (Chen et al., 2015, Laumet et al., 2020, Shen et al., 2013), we used in vitro assays to test whether exogenous TGFβ1 and/or IL-10 can directly block the sensitization of TG neurons resulting from repeated NTG administration.
Materials and methods
Mice
All experiments were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at Washington University in St. Louis. All efforts were made to minimize mouse suffering, to reduce the number of mice used, and to utilize alternatives to in vivo techniques, if available. To avoid social isolation stress, all mice were group housed (2–5 per cage, same sex) in the animal facility of Washington University in St. Louis on a 12-hour light–dark cycle with constant temperature (23–24 °C), humidity (45–50%), and food and water ad libitum. All experiments were performed during the light phase (9 am to 4 pm). Female outbred Swiss Webster mice (8–15 weeks old, purchased from Charles River) were used in the majority of experiments. Female C57BL/6 mice (8–15 weeks old, generated from our mouse colony) were used in the behavioral assays (Fig. 2) when shipment of mice was not feasible in 2020. Our previous work indicates that both strains exhibit similar behavioral and neuronal responses to repeated NTG and LD-IL-2 treatment (Guo et al., 2021, Zhang et al., 2020).
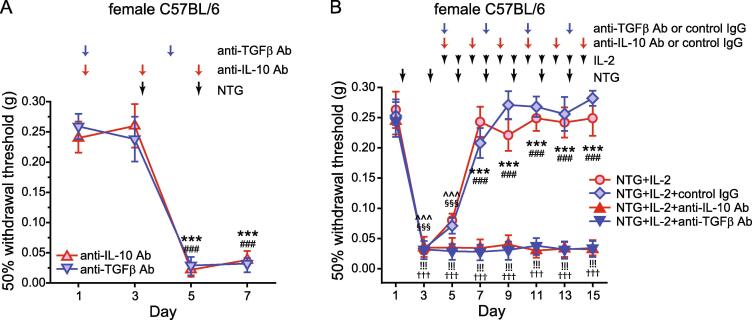
Fig. 2.
LD-IL-2 blocks NTG-induced behavioral sensitization through both peripheral IL-10 and TGFβ signaling pathways. (A) Female C57BL/6 mice received i.p. injections of neutralizing antibodies against IL-10 or TGFβ (anti-IL-10 Ab or anti-TGFβ Ab, 200 μg/mouse) before and during repeated NTG administration (n = 4 mice/group). Arrows indicate the injections of antibodies or NTG. On the days that the mouse behaviors were tested, NTG and/or the antibodies were always injected after completing the behavioral tests. Friedman test: p < 0.05 within the anti-TGFβ Ab group (F[3, 12] = 57.24); post hoc Student-Newman-Keuls test: ***p < 0.001, compared with the day 1 and 3 thresholds. Friedman test: p < 0.05 within the anti-IL-10 Ab group (F[3, 12] = 81.60); ###p < 0.001, compared with the day 1 and 3 thresholds. (B) The effect of LD-IL-2 on NTG-induced facial skin hypersensitivity was abolished by anti-IL-10 Ab and anti-TGFβ Ab. Arrows indicate the injection of NTG, LD-IL-2, anti-TGFβ Ab, anti-IL-10 Ab or control IgGs. On the days that the mouse behaviors were tested, drugs were always injected after completing the behavioral tests (n = 6 female C57BL/6 mice/group). In the NTG+IL-2+control IgG group, 3 mice received control IgG for anti-TGFβ Ab and the other 3 received the control IgG for anti-IL-10 Ab. Kruskal-Wallis ANOVA on ranks: p < 0.05 for group (F[3, 160] = 147.7), p < 0.001 for time (F[7, 160] = 68.3), and p < 0.001 for group × time interaction (F[21, 160] = 14.6); post hoc Student-Newman-Keuls test: ***p < 0.001, NTG+IL-2+anti-TGFβ Ab versus the corresponding NTG+IL-2 and NTG+IL-2+control IgG groups; ###p < 0.001, NTG+IL-2+anti-IL-10 Ab versus the corresponding NTG+IL-2 and NTG+IL-2+control IgG groups; §§§p < 0.001, compared with the baseline threshold (day 1) in the NTG+IL-2 group; ^^^p < 0.001, compared with the baseline threshold in the NTG+IL-2+control IgG group;!!!p < 0.001, compared with the baseline threshold in the NTG+IL-2+anti-TGFβ Ab group; †††p < 0.001, compared with the baseline threshold in the NTG+IL-2+anti-IL-10 Ab group.
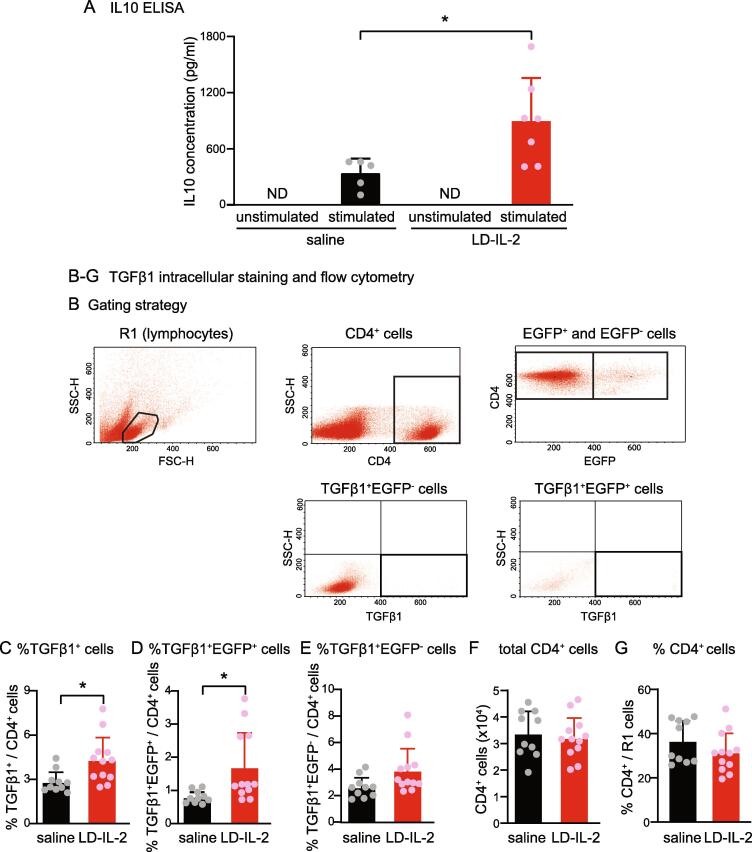
To differentiate between Foxp3+ Treg cells and CD4+ T helper cells, we used female DEREG (depletion of regulatory T cell) mice on C57BL/6 background for the intracellular staining of TGFβ1 and flow cytometry experiment (Fig. 1B-E). DEREG mice contain a transgenic allele that expresses the diphtheria toxin receptor-enhanced green fluorescent protein (DTR-EGFP) fusion protein under the control of the genomic sequences that regulate the expression of transcription factor foxp3 (Lahl et al., 2007). DEREG breeders (32050, Jackson Laboratory, Bar Harbor, ME) were crossed with C57BL/6 mice to generate heterozygotes as a reporter line for Foxp3+ Treg cells.
Fig. 1.
LD-IL-2 treatment increases the production of IL-10 and TGFβ1 in splenocytes. (A) The amount of IL-10 in culture supernatant in response to anti-CD3 and anti-CD28 stimulation measured by ELISA (n = 5 and 7 in saline and LD-IL-2 groups, respectively). *p < 0.05, two-tailed t test. (B) Gating strategy for individual cell populations. (C-E) The frequencies of TGFβ1+ cells (C), TGFβ1+EGFP+ cells (D), and TGFβ1+EGFP- cells (E) among CD4+ splenocytes from saline- and ld-IL-2-treated DEREG mice (n = 10 and 12 in saline and LD-IL-2 groups, respectively). *p < 0.05, two-tailed t test. (F-G) The number (F) and the frequency (G) of CD4+ cells in total splenocytes in R1 (gated for lymphocytes) from saline- and LD-IL-2-treated DEREG mice (same as in B-D).
IL-10 ELISA (enzyme-linked immunosorbent assay)
Female Swiss Webster mice received daily intraperitoneal (i.p.) injections of saline or LD-IL-2 for 5 consecutive days. Splenocytes were dissociated in HBSS buffer (with Ca2+ and Mg2+) containing 1% glutamine, 1% non-essential amino acids (NEAA), 2% fetal bovine serum (FBS) and 25 U/µl DNase. Cells were filtered through a sterile 70-µm cell strainer. After lysis of red blood cells (Biolegend, 420301, San Diego, CA), cells were pelleted and resuspended in RPMI1640 media (Invitrogen, Waltham, MA) for ELISA. Live cells were counted by the Vi-CELL Automated Cell Viability Analyzer (Beckman Coulter, Brea, CA).
Splenocytes were plated in 24-well plates (5 × 106 live cells per well) and stimulated overnight at 37 °C with antibodies against CD3 (1 µg/ml, Biolegend, 100340, clone 145-2C11) and CD28 (5 µg/ml, Biolegend, 102116, clone 37.51) in 1 ml complete RPMI1640 media containing 10% FBS, 1% glutamine, 1% NEAA and penicillin/streptomycin. IL-10 in the supernatants (50 μl per sample) were quantified by ELISA according to manufacturer’s instructions (Biolegend, 431411).
Intracellular staining of TGFβ1 and flow cytometry
Splenocytes were prepared from female DEREG mice after daily i.p. injections of saline or LD-IL-2 for 5 consecutive days and were plated in 24-well plates (5 × 106 live cells per well) in 1 ml complete RPMI1640 media containing 10% FBS, 1% glutamine, 1% NEAA, penicillin/streptomycin and 5 µg/ml brefeldin A (Biolegend, 420601) to block the secretion and to increase the intracellular accumulation of TGFβ1. After 4 h incubation at 37 °C, cells were stained with antibodies that recognize cell surface marker CD4 (Biolegend, clone GK1.5) for 30 min. Subsequently, cells were incubated in fixation buffer (Biolegend, 420801) for 20 min, blocked with anti-CD16/32 (Biolegend, 101310, clone 93) for 10 min, and were stained with the anti-mouse TGFβ1 antibody (Biolegend, clone TW7-16B4) in the intracellular staining permeabilization wash buffer (Biolegend, 421002) for 20 min. The frequency of individual cell subpopulations was determined via flow cytometric analysis. Data were collected with FACScan (Becton Dickinson Dickinson, Franklin Lakes, NJ) and analyzed with the CellQuest Pro (Becton Dickinson) and Rainbow X Alias (Cytek, Freemont, CA) software.
Mouse model of chronic migraine and drug treatments
To model chronic migraine, mice received repetitive i.p. injections of NTG (10 mg/kg and 10 ml/kg in saline with 1% propylene glycol) once every 2 days for 5 or more times as described previously (Guo et al., 2021, Zhang et al., 2020). The control mice received vehicle (saline with 1% propylene glycol, 10 ml/kg) injections every 2 days. NTG (SDM27, Copperhead Chemical, Tamaqua, PA) was freshly diluted from the stock (10% in propylene glycol, aliquoted in airtight glass vials, stored at room temperature and kept in darkness) with saline for every injection.
To reverse NTG-induced sensitization, mice received i.p. injections of 1 µg IL-2 in 100 µl saline every day for 11 days, starting 1 day after the 2nd NTG injection (Zhang et al., 2020). The control mice received daily i.p. injections of 100 µl saline. Recombinant mouse IL-2 (carrier-free; Biolegend) was freshly diluted from the stock (0.5–1 mg/ml aliquots at −80 °C) every day.
The neutralizing antibody against IL-10 [200 µg/mouse, BE0049, BioXcell, Labanon, NH (Li et al., 2012)] or control immunoglobulin G (IgG, BioXcell, BE0088) was i.p. injected every 2 days. The neutralizing antibody against all TGFβ isoforms [200 µg/mouse, BioXcell, BE0057, (Littwitz-Salomon et al., 2018)] or control IgG (BioXcell, BE0083) was i.p. injected every 3 days.
Behavioral test – withdrawal responses to facial mechanical stimuli
Mice were extensively handled by the experimenters for 2 weeks and were well-habituated to the test room and the test apparatus before each experiment. The experimenters were blinded to the treatments mice received during data collection and analysis.
The hair on the mouse forehead (above and between the eyes) was shaved the day before testing. On the test day, the experimenter gently held the mouse on the palm with minimal restraint and applied the calibrated von Frey filament (Bioseb, Pinellas Park FL) perpendicularly to the shaved skin, causing the filament to bend for 5 s. A positive response was determined by the following criteria as previously described: mouse vigorously stroked its face with the forepaw, head withdrawal from the stimulus, or head shaking (Zhang et al., 2020). The up-down paradigm was used to determine the 50% withdrawal threshold (Chaplan et al., 1994). The withdrawal thresholds to facial von Frey stimuli were measured at baseline and two days after each NTG injection.
Primary culture of mouse TG neurons and drug treatments
TG tissues were collected and were treated with 2.5 mg/ml collagenase IV in HBSS solution without serum (Invitrogen) for 10 min, followed by 2.5 mg/ml trypsin in HBSS solution without serum at 37 °C for 15 min. Cells were dissociated by triturating with fire-polished glass pipettes, resuspended in MEM-based culture medium (Invitrogen) containing 5% FBS, 25 ng/ml nerve growth factor (R&D, Minneapolis, MN),10 ng/ml glial cell-derived neurotrophic factor (R&D), and seeded on Matrigel-coated coverslips. The media did not contain penicillin/streptomycin. Ratiometric Ca2+ imaging was performed at 2 days in vitro.
In three experiments, recombinant mouse TGFβ1 (R&D, 7666-MB, 2, 10 or 50 ng/ml) and/or recombinant mouse IL-10 (R&D, 417-ML, 0.2, 1 or 50 ng/ml) were added to the culture media at 1 day in vitro. After overnight incubation, neurons were used for Ca2+ imaging.
Ratiometric Ca2+ imaging
TG cultures were incubated with HBSS/HEPES solution containing 2.5 µM Ca2+ indicator Fura-2 AM and 10% Pluronic F-68 (both from Molecular Probes, Eugene, OR) at 37 °C for 45 min. De-esterification of the dye was carried out by washing the coverslips 3 times with HBSS/HEPES solution and incubating the coverslips in HBSS/HEPES solution in the dark for an additional 15 min at 37 °C. Neurons were used for Ca2+ imaging experiments within 1 h after Fura-2 loading as in our previous study (Guo et al., 2021).
Coverslips were placed in a flow chamber mounted on a Nikon TE2000S inverted epifluorescent microscope and were perfused with room temperature Tyrode’s solution (1 ml/min) containing (in mM): 130 NaCl, 2 KCl, 2 CaCl2, 2 MgCl2, 25 Hepes, 30 glucose, pH 7.3–7.4 with NaOH, and 310 mosmol/kgH2O. Healthy neurons were chosen based on their differential interference contrast images (Guo et al., 2021). Coverslips were alternately excited by 340 and 380 nm light (Sutter Lambda LS, Sutter Instrument, Navato, CA) and the emission was detected at 510 ± 20 nm by a UV-transmitting 20x objective (N.A. 0.75) and a Prime BSI Express SCMOS camera (Photometrics, Tuscon AZ). The frame capture period was 50 ms at 1.5 s interval. MetaMorph software (Molecular Devices, San Jose, CA) was used for controlling and synchronizing the devices as well as image acquisition and analysis.
Regions of interest (ROIs) encompassing individual neurons were defined a priori. The ratio of fluorescence excited by 340 nm divided by fluorescence excited by 380 nm (R340/380) was determined on a pixel-by-pixel basis and was averaged for each ROI. An additional background area was recorded in each field for off-line subtraction of background fluorescence. Peak responses (ΔF) were determined by calculating the relative increase in R340/380 above baseline (F0, the average R340/380 during the 2–3 min baseline measurement). A ΔF/F0 > 20% was set as the threshold for a response.
After a 2–3 min baseline measurement in Tyrode’s solution, neurons were perfused with 50 nM PACAP1-38 (Tocris, Minneapolis, MN, 1136) for 1 min followed by washing with Tyrode’s for 4 min. Subsequently, the coverslip was incubated with 3 µM human αCGRP (Tocris, 3012) for 1 min followed by washing with Tyrode’s for 4 min. CGRP and PACAP were freshly diluted from the stock (aliquots at −80 °C) in Tyrode’s solution before each experiment.
Statistical analysis
For behavioral experiments, power analysis was conducted to estimate sample size with > 80% power to show an effect size of 0.8, alpha (two-sided) of 0.05, and a simple covariance structure for repeated measures. The experimenters were blinded to the treatments mice received. For Ca2+ imaging, all groups were tested in parallel in individual experiments. Each group contained neurons from at least 3 batches of culture.
All data were reported as mean ± standard error of the mean. Shapiro–Wilk test showed that all data were normally distributed. Statistical significance between or within experimental groups was assessed in Origin10 (OriginLab Corporation, Northampton, MA) or Statistica (StatSoft Inc, Tulsa, OK). Two-tailed t-test was used to analyze ELISA and flow cytometry data. The non-parametric Friedman test or Kruskal-Wallis ANOVA on ranks with multiple comparisons (Student-Newman-Keuls method) was used to analyze the differences in the withdrawal threshold to mechanical stimuli. The χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction were used to compare the distribution of TG subpopulations between various treatment groups. Differences with p < 0.05 were considered statistically significant. The statistical analysis for individual experiments was described in figure legends.
Results
LD-IL-2 treatment increases the production of IL-10 and TGFβ1 in splenocytes
In a previous study, we found that treating naïve mice with LD-IL-2 doubles the number of Treg cells in the spleen without altering the total number of CD4+ T helper cells or CD3+ lymphocytes (Zhang et al., 2020). In this study, we examined whether LD-IL-2 treatment alters the production of IL-10 and TGFβ1 in mice, as both cytokines are secreted by Treg cells and have been shown to reverse chronic pain-related behaviors in rodent models (Chen et al., 2015, Krukowski et al., 2016, Kwilasz et al., 2015, Laumet et al., 2020, Milligan et al., 2006, Zhang et al., 2017). We used ELISA to compare the amount of IL-10 secreted by splenocytes from mice that received daily injections of saline or LD-IL-2 for 5 consecutive days. IL-10 was not detectable in supernatants of unstimulated splenocytes (Fig. 1A). After overnight stimulation with anti-CD3 and anti-CD28 antibodies, splenocytes from saline-treated mice produced 326 ± 79 pg/ml IL-10 in the supernatant, likely secreted from stimulated Treg and other T cells (Fig. 1A). Stimulated splenocytes from LD-IL-2-treated mice produced 799 ± 207 pg/ml IL-10 in the supernatant (Fig. 1A). On average, LD-IL-2 treatment led to a 2.5-fold increase in IL-10 secretion. This likely results from the higher number of Treg cells in splenocytes after LD-IL-2 treatment.
Among all 3 TGFβ isoforms, TGFβ1 predominantly mediates Treg-induced suppression (Moreau et al., 2022). However, many other types of cells in the spleen also produce TGFβ1 in addition to Tregs. Since supernatants of unstimulated splenocytes from naïve mice already contained high level of TGFβ1 (2298 ± 148 pg/ml, n = 5 female Swiss Webster mice), the effect of LD-IL-2 on TGFβ1 production may be masked in the ELISA test. We therefore used intracellular staining and flow cytometry to measure the number of Treg cells and other CD4+ T helper cells that express TGFβ1. Female DEREG mice received daily injections of saline or LD-IL-2 for 5 consecutive days. Splenocytes were collected to stain for intracellular TGFβ1. T helper cells were identified by the anti-CD4 antibody. Foxp3+ Treg cells were identified by the EGFP signal (Lahl et al., 2007, Zhang et al., 2020). LD-IL-2 treatment significantly increased the frequency of TGFβ1+ cells among CD4+ cells (Fig. 1B). In particular, the percentage of EGFP+ Treg cells that express TGFβ1 was increased with treatment (Fig. 1C). Conversely, LD-IL-2 did not alter the percentage of EGFP-negative T helper cells that express TGFβ1 (Fig. 1D) or the total number of CD4+ cells in the spleen (Fig. 1E). Together, these results indicate that LD-IL-2 treatment increases the production of IL-10 and TGFβ1 in splenocytes. In a previous study, we have shown that 2 weeks of ld-IL-2 treatment leads to a 4–9 fold increase in the number of Treg cells in dura and TG (Zhang et al., 2020), it is highly likely that ld-IL-2 upregulates the production of IL-10 and TGFβ1 in these tissues.
LD-IL-2 blocks NTG-induced behavioral sensitization through both peripheral TGFβ and IL-10 signaling pathways
To test whether endogenous IL-10 and/or TGFβ1 mediate the therapeutic effects of LD-IL-2, we treated female C57BL/6 mice with neutralizing antibodies against IL-10 or against all TGFβ isoforms to inhibit peripheral IL-10 or TGFβ signaling. Baseline mechanical thresholds were not altered by the neutralizing antibodies (Fig. 2A, day 1 versus day 3). Repeated NTG administration (10 mg/kg, i.p., every 2 days) still produced robust and long-lasting facial mechanical hypersensitivity in the presence of neutralizing antibodies (Fig. 2A, day 5–7 versus day 1–3), indicating that the peripheral TGFβ or IL-10 signaling pathway is not involved in basal mechanical nociception or the development of NTG-induced behavioral sensitization.
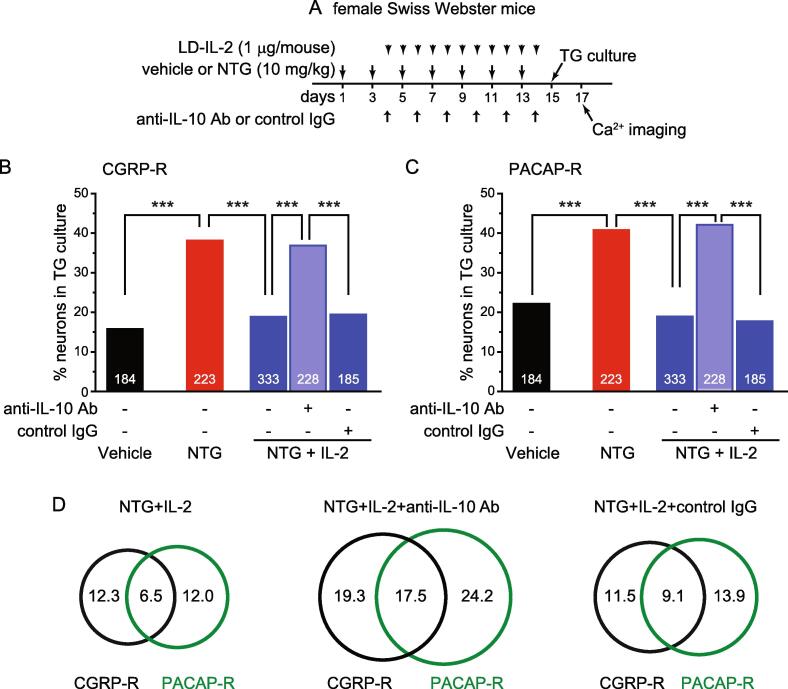
Next, we investigated how the neutralizing antibodies affect LD-IL-2′s effect on NTG-induced behavioral sensitization. First, we confirmed that NTG-induced persistent facial mechanical hypersensitivity in female C57BL/6 mice could be completely reversed after 3 daily LD-IL-2 injections (Fig. 2B, NTG+IL-2 group, day 3 to 7), consistent with previous reports (Guo et al., 2021, Zhang et al., 2020). Moreover, subsequent NTG injections failed to establish persistent mechanical hypersensitivity on facial skin as long as the LD-IL-2 treatment was continued (Fig. 2B, NTG+IL-2 group, day 7 to 15), indicating that LD-IL-2 prevents the development of chronic migraine-related behaviors as well as reversed the established sensitization. Co-administration of control IgGs during LD-IL-2 treatment did not alter the effects of LD-IL-2 (Fig. 2B, NTG+IL-2+control IgG group). In contrast, the effect of LD-IL-2 was completely abolished in mice that received neutralizing antibody against IL-10 (Fig. 2B, NTG+IL-2+anti-IL-10 Ab group), and NTG-induced facial mechanical hypersensitivity persisted throughout the experiment. Thus, although peripheral IL-10 signaling is not involved in the development of NTG-induced sensitization (Fig. 2A), it is required for the reversal of repeated NTG-induced hypersensitivity by LD-IL-2 (Fig. 2B).
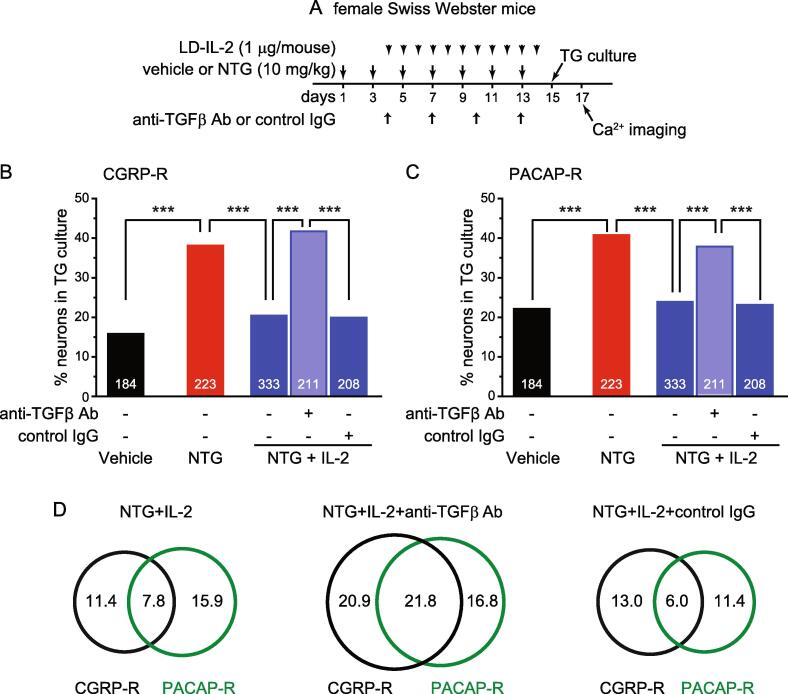
Likewise, the neutralizing antibody against all TGFβ isoforms also completely blocked the effects of LD-IL-2 (Fig. 2B, NTG+IL-2+anti-TGFβ Ab group), indicating that peripheral TGFβ signaling does not contribute to NTG-induced hypersensitivity (Fig. 2A), but mediates the anti-migraine effects of LD-IL-2 (Fig. 2B). Collectively, these results support that LD-IL-2 needs to engage both peripheral IL-10 and TGFβ signaling pathways to reverse NTG-induced sensitization.
LD-IL-2 treatment blocks NTG-induced cellular sensitization through both peripheral IL-10 and TGFβ signaling pathways
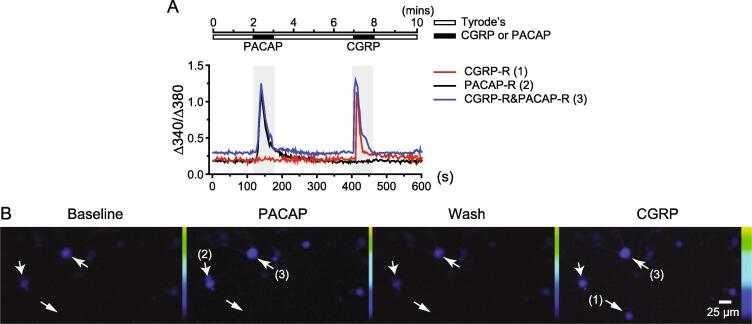
In our previous study, LD-IL-2 not only reverses NTG-induced behavioral sensitization, but also prevents repeated NTG-induced increase in the number of TG neurons that respond to neuropeptides CGRP and PACAP (Guo et al., 2021, Zhang et al., 2020). Here, we asked whether peripheral IL-10 and TGFβ signaling pathways mediate the effects of LD-IL-2 on NTG-induced sensitization of TG neurons. As in our previous study, bath application of 50 nM PACAP1-38 and 3 µM αCGRP evoked significant intracellular Ca2+ increase in cultured TG neurons (Fig. 3A-B). This allowed us to define CGRP-R and PACAP-R neurons as well as neurons that respond to both CGRP and PACAP (CGRP-R&PACAP-R) in TG cultures.
Fig. 3.
Representative traces and images of PACAP-R and CGRP-R TG neurons. (A) The protocol to measure PACAP- and CGRP-evoked intracellular Ca2+ increase and the representative traces of PACAP- and CGRP-induced Ca2+ transients in TG neurons. (B) Representative images of Fura-2-loaded TG neurons before, during and after applications of 50 nM PACAP and 3 μM CGRP, respectively. The arrows indicate the same CGRP-R, PACAP-R and CGRP-R&PACAP-R neurons shown in A, respectively.
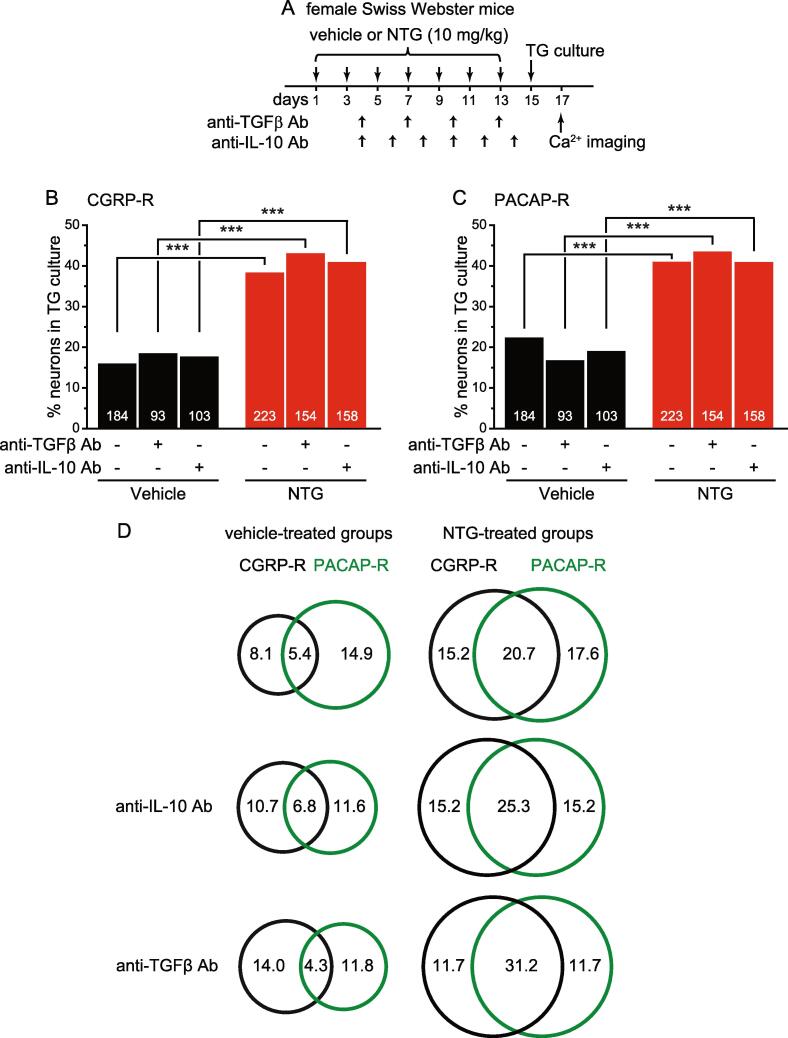
Female Swiss Webster mice received vehicle or 10 mg/kg NTG injections every 2 days for 7 times. Two days after the last injection, we cultured TG neurons and measured CGRP- and PACAP-induced intracellular Ca2+ increase in individual neurons (Fig. 4A). The number of CGRP-R and PACAP-R neurons was significantly higher in TG cultures from NTG-treated mice than those from vehicle controls (Fig. 4B-C), indicating sensitization of TG neurons as in our previous work (Guo et al., 2021). In parallel experiments, we treated another four groups of mice with anti-IL-10 or anti-TGFβ antibody during repeated vehicle- or NTG-injections (Fig. 4A). This did not change the number of CGRP-R or PACAP-R neurons in TG cultures from either vehicle- or NTG-treated mice (Fig. 4B-C). We also plotted the CGRP-R and PACAP-R TG subpopulations in Venn diagrams to indicate CGRP-R&PACAP-R neurons (Fig. 4D). Repeated NTG administration resulted in 3.8-fold increase in CGRP-R&PACAP-R neurons in TG culture (from 5.4% to 20.7% of total TG neurons, p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction). This was not altered by anti-IL-10 or anti-TGFβ antibodies either (Fig. 4D). These data suggest that peripheral IL-10 and TGFβ signaling pathways are not involved in the regulation of basal TG neuron activity or the establishment of NTG-induced sensitization of TG neurons.
Fig. 4.
Peripheral IL-10 or TGFβ signaling pathway does not regulate the number of CGRP-R and PACAP-R TG neurons at basal level or after repeated NTG administration. (A) Time line of the experiment in B-D. Data from individual experimental groups in Fig. 4, Fig. 5, Fig. 6 were collected in parallel but plotted separately for clarity. (B) The percentages of CGRP-R neurons in TG cultures from female Swiss Webster mice after repeated injections of vehicle, NTG as well as neutralizing antibodies against IL-10 or TGFβ (n = 3–6 mice/group). The number of neurons in each group is indicated in the figure. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (C) The percentages of PACAP-R neurons in TG cultures from vehicle- or NTG-treated mice (same neurons as in B). ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (D) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in each group (same neurons as in B).
As shown in our previous work (Guo et al., 2021), NTG-induced increase in CGRP-R and PACAP-R TG neurons was blocked by LD-IL-2 treatment (Fig. 5A-C, NTG versus NTG+IL-2 groups), supporting that LD-IL-2 prevents the sensitization of TG neurons. In cultures from mice that received the anti-IL-10 antibody along with LD-IL-2 treatment, the percentages of CGRP-R and PACAP-R TG neurons were comparable to those of NTG-treated mice (Fig. 5B-D, NTG+IL-2 versus NTG+IL-2+anti-IL-10 Ab groups). The percentages of CGRP-R and PACAP-R TG neurons in cultures from mice that received the anti-TGFβ antibody along with LD-IL-2 treatment were also similar to those of NTG-treated mice (Fig. 6A-D). In addition, LD-IL-2 suppressed NTG-induced increase in CGRP-R&PACAP-R neurons, and this effect was also negated by the anti-IL-10 antibody (Fig. 5D, p < 0.01, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction) and the anti-TGFβ antibody (Fig. 6D, p < 0.01, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction). Taken together, both neutralizing antibodies abolish the effects of LD-IL-2 but not the effects of NTG on TG neurons, suggesting that neither peripheral IL-10 nor TGFβ signaling contributes to NTG-induced sensitization of TG neurons (Fig. 4), but both pathways are required for LD-IL-2 to reverse NTG-induced peripheral sensitization (Fig. 5, Fig. 6).
Fig. 5.
The IL-10 neutralizing antibody abolishes the effects of LD-IL-2 on NTG-induced sensitization of TG neurons. (A) Time line of the experiment in B-D. (B) The percentages of CGRP-R neurons in TG cultures from female Swiss Webster mice that received repeated NTG, LD-IL-2 as well as anti-IL-10 antibody or control IgG (n = 5–8 mice/group). The number of neurons in each group is indicated in the figure. The vehicle and NTG groups are the same as in Fig. 4B. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (C) The percentages of PACAP-R neurons in TG cultures from female Swiss Webster mice that received repeated NTG, LD-IL-2 as well as anti-IL-10 antibody or control IgG (same neurons as in B). The vehicle and NTG groups are the same as in Fig. 4C. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (D) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in the NTG+IL-2 groups (same neurons as in B).
Fig. 6.
The TGFβ neutralizing antibody abolishes the effects of LD-IL-2 on NTG-induced sensitization of TG neurons. (A) Time line of the experiment in B-D. (B) The percentages of CGRP-R neurons in TG cultures from female Swiss Webster mice that received repeated NTG, LD-IL-2 as well as anti-TGFβ antibody or control IgG (n = 5–8 mice/group). The number of neurons in each group is indicated in the figure. The vehicle, NTG and NTG+IL-2 groups are the same as in Fig. 5B. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (C) The percentages of PACAP-R neurons in TG cultures from female Swiss Webster mice that received repeated NTG, LD-IL-2 as well as anti-TGFβ antibody or control IgG (same neurons as in B). The vehicle, NTG, and NTG+IL-2 groups are the same as in Fig. 5C. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (D) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in the NTG+IL-2 groups (same neurons as in B).
Activation of IL-10 and/or TGFβ1 signaling pathways in TG neurons reverses repeated NTG-induced neuronal sensitization.
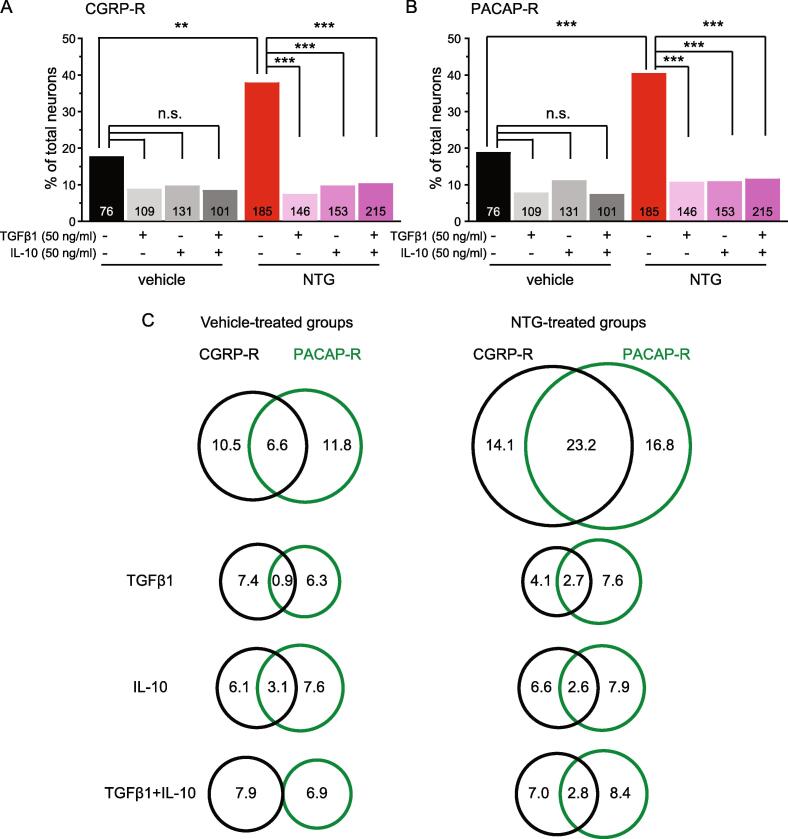
Receptors for TGFβ1 and IL-10 are expressed in primary afferent neurons (Chen et al., 2015, Laumet et al., 2020, Shen et al., 2013). To investigate whether TGFβ1 and/or IL-10 can directly regulate NTG-induced cellular sensitization through activation of their cognate receptors on primary afferent neurons, we cultured mouse TG neurons after 5 repeated vehicle or NTG administrations and incubated neurons in media containing recombinant TGFβ1 [50 ng/ml, (Liu et al., 2006)] and/or IL-10 [50 ng/ml, (Komai et al., 2018)] overnight. Either TGFβ1 or IL-10 at high concentration significantly reduced the number of CGRP-R and PACAP-R neurons in TG cultures from NTG-treated mice (Fig. 7A-B, NTG groups). The reduction of CGRP-R and PACAP-R TG neurons in cultures from vehicle-treated mice did not reach statistical significance (Fig. 7A-B, vehicle groups). Cultures treated with both TGFβ1 and IL-10 did not show further decrease in CGRP-R and PACAP-R neurons (Fig. 7, TGFβ1 + IL-10 groups). In cultures from vehicle-treated mice, the percentage of CGRP-R&PACAP-R TG neurons was not reduced by IL-10 but was significantly reduced by TGFβ1 and TGFβ1 + IL-10 (Fig. 7C, left column, p < 0.01, compared with the untreated group, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction). In contrast, the number of CGRP-R&PACAP-R TG neurons in cultures from NTG-treated mice was drastically decreased by TGFβ1 and IL-10, either alone or in combination (Fig. 7C, right column, p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction).
Fig. 7.
The effect of high concentrations of TGFβ1 and/or IL-10 on NTG-induced neuronal sensitization. (A) The percentages of CGRP-R neurons in TG cultures from female Swiss Webster mice that received repeated vehicle or NTG (n = 4–7 mice/group). The number of neurons in each group and the overnight treatment conditions are indicated in the figure. **p < 0.01. ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (B) The percentages of PACAP-R neurons in TG cultures from female Swiss Webster mice that received repeated vehicle or NTG (same neurons as in A). ***p < 0.001, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (C) Venn diagrams of the percentages of CGRP-R and PACAP-R neurons in each group (same neurons as in A and B).
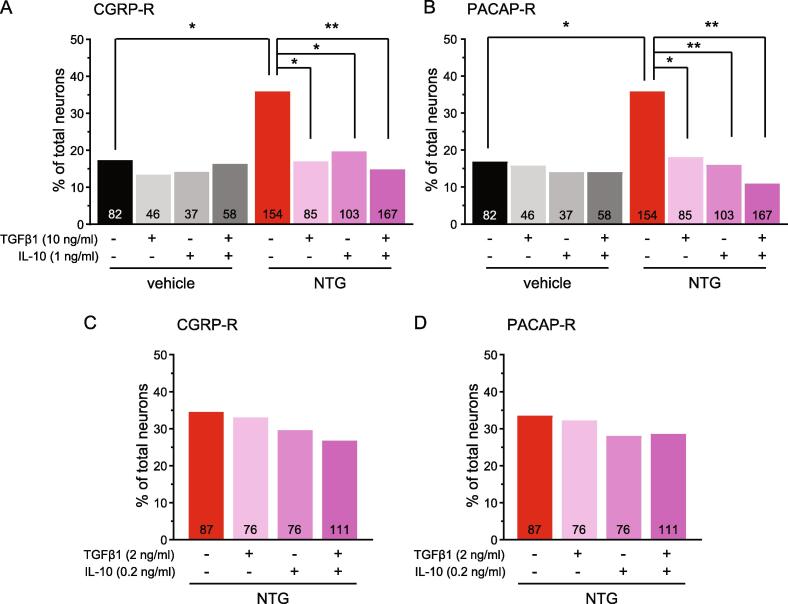
In the next experiment, we treated TG cultures with lower concentrations of TGFβ1 [10 ng/ml, (Chen et al., 2015, Zhang et al., 2017)] and/or IL-10 [1 ng/ml, (Krukowski et al., 2016, Laumet et al., 2020, Shen et al., 2013)]. This did not alter the percentage of CGRP-R and PACAP-R TG neurons from vehicle-treated mice (Fig. 8A-B, vehicle groups), but significantly reduced the numbers of CGRP-R and PACAP-R neurons in TG cultures from NTG-treated mice to the control level (Fig. 8A-B, NTG groups). Incubation of TG cultures from NTG-treated mice with 2 ng/ml TGFβ1 and 0.2 ng/ml IL-10 did not reverse NTG-induced cellular sensitization (Fig. 8C-D). Together, these data suggest that LD-IL-2 engages both TGFβ1 and IL-10 signaling pathways in TG neurons to reverse NTG-induced increase in the numbers of functional CGRP and PACAP receptors.
Fig. 8.
The effect of low concentrations of TGFβ1 and/or IL-10 on NTG-induced neuronal sensitization. (A-B) The percentages of CGRP-R neurons (A) and PACAP-R neurons (B) in TG cultures from female Swiss Webster mice that received repeated vehicle or NTG (n = 3–5 mice/group). Cultures were treated with 10 ng/ml TGFβ1 and/or 1 ng/ml IL-10 overnight. The number of neurons in each group and the overnight treatment conditions are indicated in the figure. *p < 0.05. **p < 0.01, χ2 test followed by post hoc Fisher’s exact test with Bonferroni correction. (C-D) The percentages of CGRP-R neurons (C) and PACAP-R neurons (D) in TG cultures from female Swiss Webster mice that received repeated NTG (n = 3 mice/group). Cultures were treated with 2 ng/ml TGFβ1 and/or 0.2 ng/ml IL-10 overnight.
Discussion
Our recent studies show that peripheral Treg cells mediate the therapeutic effects of LD-IL-2 in mouse models of chronic headache disorders (Guo et al., 2021, Zhang et al., 2020). In the present work, we progressed to elucidate the molecular mechanisms through which LD-IL-2 and Treg cells reverse chronic migraine-related behavioral and cellular sensitizations in mice. First, we confirmed that LD-IL-2 treatment increases the production of IL-10 and TGFβ1 in T cells, especially Treg cells, from the spleen. Since LD-IL-2 results in a higher magnitude of Treg increase in dura and TG (4–9 fold) than in the spleen [2-fold, (Zhang et al., 2020)], it is highly likely that the secretion of both IL-10 and TGFβ1 are upregulated by LD-IL-2 treatment in TG and dura. IL-2-activated Treg cells may also induce the secretion of IL-10 and TGFβ1 from macrophages and other cells (Schmidt et al., 2012, Sharabi et al., 2018). More work is needed to determine the levels of IL-10 and TGFβ1 in serum, TG and dura after LD-IL-2 treatment; and to investigate whether Treg cells are the main source of IL-10 and TGFβ1 in TG and dura.
Previous studies indicate that activation of either IL-10 or TGFβ1 signaling pathway is sufficient to exert anti-hyperalgesic effects in animal models of chronic somatic pain (Chen et al., 2015, Krukowski et al., 2016, Kwilasz et al., 2015, Laumet et al., 2020, Milligan et al., 2006, Zhang et al., 2017). Our work shows that IL-10 and TGFβ signaling pathways do not contribute to NTG-induced sensitizations. However, treating mice with either neutralizing antibodies against IL-10 or TGFβ completely blocked the effects of LD-IL-2 on chronic migraine-related behaviors and the sensitization of TG neurons. Thus, LD-IL-2 no longer exhibits anti-migraine effects when IL-10 signaling is inhibited and TGFβ signaling is unperturbed, or vice versa. This led us to conclude that both IL-10 and TGFβ signaling pathways are required for LD-IL-2 to reverse NTG-induced behavioral and cellular sensitizations. Collectively, results from the present study and previous work suggest that distinct cytokine signaling pathways mediate the natural and treatment-facilitated resolution of individual types of chronic pain. Antibodies do not normally cross the blood brain barrier (BBB), and infusion of NTG in migraine patients triggers migraine-like episodes without compromising the integrity of BBB (Schankin et al., 2016). Although the repeated administration of high-dose NTG in the mouse model may increase BBB permeability (Chen et al., 2022), LD-IL-2 expands Treg cells in peripheral tissues but not in the brain of mice that have received repeated NTG administration (Zhang et al., 2020). Collectively, these results support that the therapeutic effects of LD-IL-2 is predominantly mediated through peripheral TGFβ and IL-10 signaling pathways. In addition to chronic migraine, LD-IL-2 reverses headache-related behaviors in mouse models of post-traumatic headache and medication overuse (Zhang et al., 2020). It is likely that the peripheral TGFβ and IL-10 signaling pathways also mediate the effects of LD-IL-2 on these chronic headache disorders.
TGFβ1 first binds to the high-affinity TGFβ receptor II subunit, and then recruit TGFβ receptor I subunit to the complex to initiate signaling events (Heldin and Moustakas, 2016). The IL-10 receptor is composed of two α and two β subunits, both are required for IL-10 signaling (Moore et al., 2001). Primary afferent neurons express functional TGFβ1 and IL-10 receptors (Chen et al., 2015, Krukowski et al., 2016, Laumet et al., 2020, Shen et al., 2013). Exogenous TGFβ1 rapidly suppresses nerve injury-evoked spinal synaptic plasticity and the hyperexcitability of dorsal root ganglion (DRG) neurons via TGF-β receptor 1-mediated noncanonical signaling (Chen et al., 2015). IL-10 normalizes chemotherapy-induced hyperexcitability of DRG neurons by lowering the resting membrane potential and increasing action potential thresholds (Krukowski et al., 2016, Laumet et al., 2020). This likely results from the down-regulation of mRNA and protein levels of multiple voltage-gated Na+ channels (Shen et al., 2013). In this study, overnight incubation of TG culture with either 10 ng/ml TGFβ1 or 1 ng/ml IL-10 completely reversed NTG-induced increase in CGRP-R, PACAP-R as well as CGRP-R&PACAP-R neurons, indicating that many TG neurons co-express functional TGFβ1 and IL-10 receptors. This likely occurs in human primary afferent neurons as well, given that the expression of IL-10 receptor α subunit in human DRG neurons is broader than that in mice (Tavares-Ferreira et al., 2022). One of the limitations of this study is that we did not generate full dose–response curves of TGFβ1- and IL-10-regulation of CGRP-R and PACAP-R TG neurons, making it difficult to assess the interactions between these two signaling pathways. Future in-depth studies are warranted to investigate whether TGFβ1 and IL-10 signaling synergistically regulate the level of functional CGRP and PACAP receptors in TG neurons under chronic migraine-related state, as in the case of TGFβ1- and IL-10-mediated modulation of metabolic signals in humoral immunity (Komai et al., 2018). Another caveat is that, although the selectivity of neutralizing antibodies are well documented, it remains to be tested whether inhibition of TGFβ1 signaling indirectly affects the strength of IL-10 signaling, or vice versa.
Female inbred and outbred mice were used in the present study as migraine is far more common in women than in men, and female sex increases the risk of chronic migraine (Ashina et al., 2021, May and Schulte, 2016). LD-IL-2 reverses chronic migraine-related sensitizations in both male and female, inbred and outbred mice through peripheral Treg cells (Zhang et al., 2020). It is likely that the effects of LD-IL-2 in male mice are also mediated through peripheral TGFβ1 and IL-10 signaling pathways but this requires further assessment.
Repeated NTG administration is widely used to establish a chronic migraine-related state in rodents (Guo et al., 2021, Pradhan et al., 2014, Sufka et al., 2016, Tipton et al., 2016, Zhang et al., 2020). However, the dose of NTG required to establish persistent behavioral sensitization and hyperalgesic priming in naïve mice is much higher than that used to trigger migraine-like headache in migraine patients (Olesen, 2008), and it only models one of the many mechanisms underlying migraine pathophysiology. Future studies need to investigate whether peripheral IL-10 and TGFβ1 signaling mediate LD-IL-2′s effects on chronic migraine-related sensitizations generated through other mechanisms.
In addition to the release of IL-10 and TGFβ1, Treg cells employ many other mechanisms to mediate immunosuppression, for example, the release of interleukin-35, the metabolism of extracellular ATP to adenosine by CD39 and CD73, as well as the PD-1/PD-L1 co-inhibitory pathway (Schmidt et al., 2012, Sharabi et al., 2018). All of these mechanisms have been shown to contribute to the regulation of chronic pain (Chen et al., 2017, Duffy et al., 2019, Luongo et al., 2021). Whether LD-IL-2 and Treg cells engage these mechanisms to inhibit chronic migraine-related behavioral and cellular sensitizations merits further investigation.
Taken together, the present study reveals the molecular mechanisms through which LD-IL-2 and Treg cells reverse chronic-migraine related behavioral and cellular sensitizations, providing further support for future clinical assessment of LD-IL-2 as a treatment for chronic migraine. Moreover, our results suggest that the TGFβ1 and IL-10 signaling pathways in TG neurons can be targeted for chronic migraine therapy.
Data availability statement
Raw data is available upon request to the corresponding author.
Funding
This work was supported by the Department of Defense Investigator-Initiated Research Award W81XWH2110597 to YQC. The sponsor was not involved in any of the stages from study design to submission of the paper for publication.
CRediT authorship contribution statement
Zhaohua Guo: Conceptualization, Data curation, Formal analysis, Methodology, Visualization. Jintao Zhang: Data curation, Formal analysis, Methodology, Visualization. Xuemei Liu: Data curation, Formal analysis, Methodology, Visualization. Jacqueline Unsinger: Data curation, Formal analysis, Methodology, Visualization. Richard S Hotchkiss: Conceptualization, Supervision, Visualization. Yu-Qing Cao: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors thank Dr. Drew Walton for valuable comments on the manuscript.
References
- Alves S., Churlaud G., Audrain M., Michaelsen-Preusse K., Fol R., Souchet B., Braudeau J., Korte M., Klatzmann D., Cartier N. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain. 2017;140(3):826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- Ashina M., Katsarava Z., Do T.P., Buse D.C., Pozo-Rosich P., Ozge A., Krymchantowski A.V., Lebedeva E.R., Ravishankar K., Yu S., Sacco S., Ashina S., Younis S., Steiner T.J., Lipton R.B. Migraine: epidemiology and systems of care. Lancet. 2021;397(10283):1485–1495. doi: 10.1016/S0140-6736(20)32160-7. [DOI] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen G., Kim Y.H., Li H., Luo H., Liu D.L., Zhang Z.J., Lay M., Chang W., Zhang Y.Q., Ji R.R. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci. 2017;20(7):917–926. doi: 10.1038/nn.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Park C.K., Xie R.G., Ji R.R. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J. Clin. Investig. 2015;125(8):3226–3240. doi: 10.1172/JCI80883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Tang X., Li J., Hu B., Yang W., Zhan M., Ma T., Xu S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: a novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain. 2022;23(1):1. doi: 10.1186/s10194-021-01374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli-Ferreira M., de Lima K.A., Fonseca M.M., Guimaraes R.M., Gomes F.I., Cavallini M.C., Quadros A.U., Kusuda R., Cunha F.Q., Alves-Filho J.C., Cunha T.M. Regulatory T cells counteract neuropathic pain through inhibition of the Th1 response at the site of peripheral nerve injury. Pain. 2020;161(8):1730–1743. doi: 10.1097/j.pain.0000000000001879. [DOI] [PubMed] [Google Scholar]
- Duffy S.S., Keating B.A., Perera C.J., Lees J.G., Tonkin R.S., Makker P.G.S., Carrive P., Butovsky O., Moalem-Taylor G. Regulatory T cells and their derived cytokine, interleukin-35, reduce pain in experimental autoimmune encephalomyelitis. J. Neurosci. 2019;39(12):2326–2346. doi: 10.1523/JNEUROSCI.1815-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R., Sendetski M., Del Rivero T., Martinez G.F., Bracchi-Ricard V., Swanson K.A., Pruzinsky E.K., Delguercio N., Rosalino M.J., Padutsch T., Kontermann R.E., Pfizenmaier K., Bethea J.R. TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. PNAS. 2019;116(34):17045–17050. doi: 10.1073/pnas.1902091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Li F., Zhou Z., Xu X., Wu Y., Zhou S., Yin D., Sun D., Xiong J., Jiang R., Zhang J. IL-2/Anti-IL-2 complex attenuates inflammation and BBB disruption in mice subjected to traumatic brain injury. Front. Neurol. 2017;8:281. doi: 10.3389/fneur.2017.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Czerpaniak K., Zhang J., Cao Y.Q. Increase in trigeminal ganglion neurons that respond to both calcitonin gene-related peptide and pituitary adenylate cyclase-activating polypeptide in mouse models of chronic migraine and posttraumatic headache. Pain. 2021;162(5):1483–1499. doi: 10.1097/j.pain.0000000000002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Long T., Pan Q., Zhang S., Zhang Y., Zhang D., Qin G., Chen L., Zhou J. Microglial NLRP3 inflammasome activation mediates IL-1beta release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J. Neuroinflammation. 2019;16(1):78. doi: 10.1186/s12974-019-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C.H., Moustakas A. Signaling Receptors for TGF-beta Family Members. Cold Spring Harb. Perspect. Biol. 2016;8(8) doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Zhang J., Liu X., Huang D., Cao Y.Q. Low-dose interleukin-2 and regulatory T cell treatments attenuate punctate and dynamic mechanical allodynia in a mouse model of sciatic nerve injury. J Pain Res. 2021;14:893–906. doi: 10.2147/JPR.S301343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Abbas A.K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 2015;15(5):283–294. doi: 10.1038/nri3823. [DOI] [PubMed] [Google Scholar]
- Komai T., Inoue M., Okamura T., Morita K., Iwasaki Y., Sumitomo S., Shoda H., Yamamoto K., Fujio K. Transforming growth factor-beta and interleukin-10 synergistically regulate humoral immunity via modulating metabolic signals. Front. Immunol. 2018;9:1364. doi: 10.3389/fimmu.2018.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukowski K., Eijkelkamp N., Laumet G., Hack C.E., Li Y., Dougherty P.M., Heijnen C.J., Kavelaars A. CD8+ T cells and endogenous IL-10 are required for resolution of chemotherapy-induced neuropathic pain. J. Neurosci. 2016;36(43):11074–11083. doi: 10.1523/JNEUROSCI.3708-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwilasz A.J., Grace P.M., Serbedzija P., Maier S.F., Watkins L.R. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G., Bavencoffe A., Edralin J.D., Huo X.J., Walters E.T., Dantzer R., Heijnen C.J., Kavelaars A. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. 2020;161(10):2344–2352. doi: 10.1097/j.pain.0000000000001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Burstein R., Kainz V., Jakubowski M., Strassman A.M. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1–2):166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Huang L., Ye H., Song S.P., Bajwa A., Lee S.J., Moser E.K., Jaworska K., Kinsey G.R., Day Y.J., Linden J., Lobo P.I., Rosin D.L., Okusa M.D. Dendritic cells tolerized with adenosine A(2)AR agonist attenuate acute kidney injury. J. Clin. Investig. 2012;122(11):3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littwitz-Salomon E., Malyshkina A., Schimmer S., Dittmer U. The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory T cells during acute retroviral infection. Front. Immunol. 2018;9:1947. doi: 10.3389/fimmu.2018.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Teige I., Birnir B., Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006;12(5):518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- Luongo L., Guida F., Maione S., Jacobson K.A., Salvemini D. Adenosine metabotropic receptors in chronic pain management. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.651038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A., Schulte L.H. Chronic migraine: risk factors, mechanisms and treatment. Nature reviews. Neurology. 2016;12(8):455–464. doi: 10.1038/nrneurol.2016.93. [DOI] [PubMed] [Google Scholar]
- McIlvried L.A., Cruz J.A., Borghesi L.A., Gold M.S. Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura. Cephalalgia. 2017;37(1):36–48. doi: 10.1177/0333102416637832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K., Russo A.F. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia. 2019;39(13):1661–1674. doi: 10.1177/0333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan E.D., Sloane E.M., Langer S.J., Hughes T.S., Jekich B.M., Frank M.G., Mahoney J.H., Levkoff L.H., Maier S.F., Cruz P.E., Flotte T.R., Johnson K.W., Mahoney M.M., Chavez R.A., Leinwand L.A., Watkins L.R. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126(1–3):294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19(1):683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Moreau J.M., Velegraki M., Bolyard C., Rosenblum M.D., Li Z. Transforming growth factor-beta1 in regulatory T cell biology. Sci. Immunol. 2022;7(69):eabi4613. doi: 10.1126/sciimmunol.abi4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Pradhan A.A., Smith M.L., McGuire B., Tarash I., Evans C.J., Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155(2):269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter U., Bolay H., Jansen-Olesen I., Chiarugi A., Sanchez del Rio M., Letourneau R., Theoharides T.C., Waeber C., Moskowitz M.A. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124(Pt 12):2490–2502. doi: 10.1093/brain/124.12.2490. [DOI] [PubMed] [Google Scholar]
- Schain A.J., Melo-Carrillo A., Borsook D., Grutzendler J., Strassman A.M., Burstein R. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann. Neurol. 2018;83(3):508–521. doi: 10.1002/ana.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schankin C.J., Maniyar F.H., Seo Y., Kori S., Eller M., Chou D.E., Blecha J., Murphy S.T., Hawkins R.A., Sprenger T., VanBrocklin H.F., Goadsby P.J. Ictal lack of binding to brain parenchyma suggests integrity of the blood-brain barrier for 11C-dihydroergotamine during glyceryl trinitrate-induced migraine. Brain. 2016;139(Pt 7):1994–2001. doi: 10.1093/brain/aww096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Oberle N., Krammer P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi A., Tsokos M.G., Ding Y., Malek T.R., Klatzmann D., Tsokos G.C. Regulatory T cells in the treatment of disease. Nat. Rev. Drug Discovery. 2018;17(11):823–844. doi: 10.1038/nrd.2018.148. [DOI] [PubMed] [Google Scholar]
- Shen K.F., Zhu H.Q., Wei X.H., Wang J., Li Y.Y., Pang R.P., Liu X.G. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp. Neurol. 2013;247:466–475. doi: 10.1016/j.expneurol.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Sufka K.J., Staszko S.M., Johnson A.P., Davis M.E., Davis R.E., Smitherman T.A. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J Headache Pain. 2016;17:40. doi: 10.1186/s10194-016-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares-Ferreira D., Shiers S., Ray P.R., Wangzhou A., Jeevakumar V., Sankaranarayanan I., Cervantes A.M., Reese J.C., Chamessian A., Copits B.A., Dougherty P.M., Gereau R.W., Burton M.D., Dussor G., Price T.J. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 2022;14(632) doi: 10.1126/scitranslmed.abj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton A.F., Tarash I., McGuire B., Charles A., Pradhan A.A. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia. 2016;36(11):1048–1056. doi: 10.1177/0333102415623070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieseler J., Ellis A., McFadden A., Stone K., Brown K., Cady S., Bastos L.F., Sprunger D., Rezvani N., Johnson K., Rice K.C., Maier S.F., Watkins L.R. Supradural inflammatory soup in awake and freely moving rats induces facial allodynia that is blocked by putative immune modulators. Brain Res. 2017;1664:87–94. doi: 10.1016/j.brainres.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xia Y., Ye Q., Yu F., Zhu W., Li P., Wei Z., Yang Y., Shi Y., Thomson A.W., Chen J., Hu X. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J. Neurosci. 2018;38(47):10168–10179. doi: 10.1523/JNEUROSCI.3411-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Czerpaniak K., Huang L., Liu X., Cloud M.E., Unsinger J., Hotchkiss R.S., Li D., Cao Y.Q. Low-dose interleukin-2 reverses behavioral sensitization in multiple mouse models of headache disorders. Pain. 2020;161(6):1381–1398. doi: 10.1097/j.pain.0000000000001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Li Z., Chen F., Liu H., Wang H., Li X., Liu X., Wang J., Zheng Z. TGF-beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp. Mol. Med. 2017;49(9):e379. doi: 10.1038/emm.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data is available upon request to the corresponding author.