Abstract
The cry1Ac7 gene of Bacillus thuringiensis strain 234, showing activity against the sugarcane borer Eldana saccharina, was cloned under the control of the tac promoter. The fusion was introduced into the broad-host-range plasmid pKT240 and the integration vector pJFF350 and without the tac promoter into the broad-host-range plasmids pML122 and pKmM0. These plasmids were introduced into a Pseudomonas fluorescens strain isolated from the phylloplane of sugarcane and the endophytic bacterium Herbaspirillum seropedicae found in sugarcane. The ptac-cry1Ac7 construct was introduced into the chromosome of P. fluorescens using the integration vector pJFF350 carrying the artificial interposon Omegon-Km. Western blot analysis showed that the expression levels of the integrated cry1Ac7 gene were much higher under the control of the tac promoter than under the control of its endogenous promoter. It was also determined that multicopy expression in P. fluorescens and H. seropedicae of ptac-cry1Ac7 carried on pKT240 caused plasmid instability with no detectable protein expression. In H. seropedicae, more Cry1Ac7 toxin was produced when the gene was cloned under the control of the Nmr promoter on pML122 than in the opposite orientation and bioassays showed that the former resulted in higher mortality of E. saccharina larvae than the latter. P. fluorescens 14::ptac-tox resulted in higher mortality of larvae than did P. fluorescens 14::tox. An increased toxic effect was observed when P. fluorescens 14::ptac-tox was combined with P. fluorescens carrying the Serratia marcescens chitinase gene chiA, under the control of the tac promoter, integrated into the chromosome.
The gram-positive, aerobic, spore-forming bacterium Bacillus thuringiensis has been used as a safe alternative and supplement to chemical pesticides for over 2 decades. It is a pathogen of insect larvae which produces highly specific crystal inclusions during sporulation. These parasporal crystals consist predominantly of protoxin molecules known as δ-endotoxins, Cry toxins, or Cry proteins. The crystal inclusions dissolve in the larval midgut, where one or more protoxins are released and proteolytically converted into smaller toxic polypeptides. The activated toxins are highly specific to the insect and very specific in their activity (14). Despite the success of conventional B. thuringiensis-based products, they have several disadvantages as bioinsecticides. In the case of the sugarcane borer Eldana saccharina Walker (Lepidoptera: Pyralidae), a widespread sugarcane pest which causes considerable crop loss in the cane-growing areas of South Africa and Swaziland, these include instability in the environment and on the surface of sugarcane, as well as difficulty in reaching the internal regions where the larvae feed. The use of recombinant DNA technology has provided solutions to the problems through the development of two approaches, namely, genetically modified microorganisms and transgenic plants (18, 21, 22, 25, 26).
As part of an integrated pest management approach to the control of E. saccharina in South Africa, the cry1Ac7 gene from B. thuringiensis strain 234 was previously introduced into P. fluorescens isolate 14 (13, 33). This organism was isolated from the surface of sugarcane leaves, stems, and borings and shown to be a good colonizer of the phylloplane of sugarcane. Toxicity bioassays indicated that P. fluorescens 14 clones that expressed the gene were toxic to E. saccharina larvae, and greenhouse trials showed that sugarcane plants inoculated with the strain carrying the integrated gene were more resistant to E. saccharina damage than were untreated controls.
Although these results were encouraging, it was felt that there was room for further improvement in the use of recombinant bacteria for the control of this sugarcane pest. The aim of the work presented in this paper was to increase δ-endotoxin expression by cloning the cry1Ac7 gene under the control of the tac promoter with subsequent integration of the cassette into the chromosome of P. fluorescens 14. In addition, since recombinant P. fluorescens 14 populations are not stably maintained on sugarcane over long periods (33), the potential of endophytic bacteria present in the interior regions of healthy sugarcane plants that express the gene as a biocontrol agent was investigated. Of particular interest is the gram-negative, obligately endophytic, nitrogen-fixing bacterium Herbaspirillum seropedicae, which has been isolated only from monocotyledonous plants such as sugarcane, rice, sorghum, maize, 13 different graminaceous weeds, and the roots of a pigeonpea plant (3, 6, 7). The use of an endophytic bacterium was also seen as a possible solution to the problem of inaccessibility of conventional B. thuringiensis-based products to the interior regions of the plant. The advantages of using these recombinant endophytes is their high stability in sugarcane and the ability to be transferred to subsequent generations via sugarcane setts (4, 6, 7).
A further strategy to improve the biocontrol of E. saccharina involved combining P. fluorescens strains producing the Cry1Ac7 protein and a Serratia marcescens chitinase, ChiA. Reports have shown that coapplication of B. thuringiensis δ-endotoxins and bacterial chitinases significantly increased the insecticidal effect of the former against insect larvae (28, 31). It is believed that the chitinase causes perforations in the chitin-containing peritrophic membrane of the larvae, thereby increasing the accessibility of the midgut membranes to the δ-endotoxin (28). The introduction of both Cry and ChiA into bacteria or plants offers great potential for increasing the insecticidal activity in transgenic systems where the Cry toxins are expressed at low levels and/or in a crystalline form (28).
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Rifampin-resistant P. fluorescens 14 was grown on Luria-Bertani medium (LB) or LB medium with agar supplemented with rifampin (100 μg/ml). The sugarcane endophyte H. seropedicae HRC54 was provided by J. Döbereiner of the Empresa Brasiliera de Pesquisa Agnopecuaria, Brasilia, Brazil. A spontaneous nalidixic acid-resistant mutant, H. seropedicae Nal1, was isolated. These strains were grown in JNFb medium, which contained, per liter, 5 g of malic acid, 0.6 ml of K2HPO4, 1.8 ml of KH2PO4, 0.2 g of MgSO4 · 7H2O, 0.1 g of NaCl, 0.2 g of CaCl2 · H2O, 0.066 g of FeEDTA, 2 ml of bromothymol blue, 2 ml of micronutrients, 0.02 g of yeast extract, and 4.5 g of KOH (pH 5.8) and was supplemented with the appropriate antibiotic. For solid JNFb, 17 g of agar was added per liter; for semisolid JNFb, 1.9 g of agar was added per liter with the yeast extract omitted; and for liquid JNFb medium, 1 g of NH4Cl was added per liter in addition to yeast extract. The micronutrients consisted of 0.2 g of Na2MoO4 · 2H2O, 0.235 g of MnSO4 · H2O, 0.28 g of H3BO3, 0.008 g of CuSO4 · 5H2O, and 0.024 g of ZnSO4 · 7H2O per 200 ml of H2O.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant genotype/phenotype | Reference or source |
|---|---|---|
| Strains | ||
| E. coli JM105 | F′ traD36 lacIq Δ(lacZ)M15 proAB thi rpsL (Strr) endA sbcB15 (sbcC) hsdR4 (rK− mK+) Δ(lac-proAB) | 38 |
| E. coli S17-1 | recA thi pro hsdR4 (rK− mK+) (RP4-2Tc-Mu-Km-Tn7) Tpr Smr | 30 |
| P. fluorescens Rif1 | Rifr | 8 |
| P. fluorescens 14 | Rifr Nalr | 17 |
| P. fluorescens 14::tox | Rifr Nalr Omegon-Km–cry1Ac7 | 13 |
| H. seropedicae HRC54 | Apr | 3 |
| H. seropedicae Nal1 | Nalr | This work |
| Plasmids | ||
| pKT240 | Kmr Apr | 2 |
| pKK223-3 | Apr ptac rrnB T1 T2 | Pharmacia Biotech |
| pML122 | Gmr Nm PNm | 19 |
| pJFF350 | Kmr | 9 |
| pGH37D-1 | Aprcry1Ac7 | 13 |
| pJTT | Kmr ptac-cry1Ac7 | This work |
| pKTT | Kmr Apr ptac-cry1Ac7 | This work |
| pMT7 | Gmr PNm-cry1Ac7ay | This work |
| pMT11 | Gmrcry1Ac7 | This work |
| pKmM0 | Kmr | 32 |
| pKmM0-tox | Kmrcry1Ac7 | This work |
Promoter of the neomycin resistance gene upstream of the cry1Ac7 gene.
All bacteria were grown at 30°C. P. fluorescens was maintained in 0.1 M MgSO4 at 4°C, and H. seropedicae strains were maintained in JNFb medium supplemented with 10% glycerol at −70°C.
Molecular techniques.
Molecular techniques were performed as described by Sambrook et al. (29).
Western blot analysis.
Determination of the expression of the cry1Ac7 gene in P. fluorescens 14 and H. seropedicae Nal1 was carried out by Western blot analysis. Cell extracts were prepared from 1 ml of stationary-phase cultures by resuspending cell pellets in 100 μl of denaturing loading buffer (20). Samples (20 μl) were loaded onto a denaturing gradient (10 to 5%) acrylamide gel, and the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method of Laemmli (20). For the quantitative analysis of Cry1Ac7 production, Escherichia coli cultures were grown overnight at 30°C in LB medium supplemented with the appropriate antibiotic, diluted 100-fold, grown to mid-exponential phase at 37°C, and induced with 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as described by Ausubel et al. (1). Uninduced controls were prepared by dividing the mid-exponential-phase (optical density at 600 nm, 0.4) culture in two before adding IPTG. Samples from both uninduced and induced cultures were removed at various time intervals after induction. Samples (1 ml) of cultures induced for 24 h were sonicated, and the protein concentration was determined by the method of Bradford (5). Volumes of denatured cell extracts representing 50 μg of protein were separated by SDS-PAGE.
Proteins were transferred from SDS-polyacrylamide gels onto nitrocellulose membranes by the method of Towbin et al. (34). Western blot analysis was carried out using the primary antibody raised against the Cry1Ac7 protein (supplied by SASEX) and goat anti-rabbit immunoglobulin G conjugated to alkaline phosphatase (Sigma) as the secondary antibody.
Bacterial transformation by electroporation and conjugation.
Broad-host-range plasmids and the integration vector carrying the ptac-cry1Ac7 cassette were electroporated into P. fluorescens 14 and H. seropedicae Nal1 using a modification of the method of Waalwijk et al. (36). Cells harvested at mid-exponential phase and washed three times in 300 mM sucrose were electroporated in 40-μl volumes with 1 to 3 μg of DNA using a Bio-Rad Gene Pulser and controller set at 25 μF, 2.5 kV, and 200 Ω. Phenotypic expression was carried out at 30°C for 2 h in 1 ml of LB or JNFb medium (for P. fluorescens and H. seropedicae, respectively). The cells were plated undiluted onto LB medium with agar or JNFb solid medium supplemented with kanamycin (100 μg/ml) and grown at 30°C. To increase the electroporation efficiency of H. seropedicae, the method described by Wirth et al. (37) was attempted.
Plasmids were also mobilized into H. seropedicae Nal1 as described by Simon et al. (30) with modifications. E. coli JM105 carrying the plasmids for mobilization (donor cells), E. coli HB101 carrying the helper plasmid pRK2013 for mobilization, and the recipient bacteria were grown overnight in 5 ml of LB (for E. coli) or JNFb (for H. seropedicae) medium supplemented with the appropriate antibiotic at 30°C with shaking. An equal volume (1.5 ml) of each strain was harvested, washed three times with LB medium, combined in a 1:1:1 ratio (500 μl of each), and resuspended in 100 μl of LB medium. The mixed culture was then spotted in 20-μl volumes onto plates containing a mixture of JNFb and LB media without antibiotics and grown overnight at 30°C. Serial dilutions of the resulting colonies were plated onto JNFb medium supplemented with nalidixic acid (100 μg/ml) and kanamycin (100 μg/ml) and incubated at 30°C overnight. Donor, recipient, and helper strains which had not been mixed were treated as already described to serve as controls. Plates were incubated at 30°C.
Plasmid stability.
The stability of the pML122-derived plasmids pMT7 and pMT11 carrying the cry1Ac7 gene in H. seropedicae Nal1 was assessed. The strains were grown in liquid JNFb medium supplemented with kanamycin (100 μg/ml) to stationary phase at 30°C, diluted to 10−6 bacteria per 20 ml of JNFb medium without antibiotics, and grown to stationary phase. This cycle of growth and dilution was performed five times. The cells from a 10−6 dilution were plated onto JNFb plates after each cycle of growth. One hundred of the resulting CFU were patched onto JNFb plates supplemented with kanamycin (100 μg/ml). The percentage of patched colonies which grew on these plates was recorded. This experiment was performed three times.
Southern blot analysis.
Southern blot analysis was used to demonstrate integration of the Omegon-Km–ptac-cry1Ac7 cassette into the chromosome of P. fluorescens 14 clones. Total bacterial DNA was isolated as described by Ausubel et al. (1). Probes were labeled with digoxigenin-11-dUTP (Boehringer Mannheim) by the random primed DNA labeling method in accordance with the manufacturer's instructions. Southern blot analysis was carried out by the method described in the digoxigenin-11-dUTP system user's guide.
Toxicity bioassays.
Weighed quantities of the various freeze-dried bacterial preparations expressing the cry1Ac7 and chiA genes were mixed with weighed quantities of an artificial insect diet (11) such that a known amount of bacterial preparation per gram of diet was obtained. Aliquots (0.2 g) were added to Eppendorf tubes, and five 2-week-old E. saccharina neonate larvae were placed in each tube. Each treatment comprised five tubes. Mortality of the larvae was recorded every 24 h for 5 days. An analysis of variance was done with one between-subjects variable (treatment) and one within-subjects variable (time). As the response variable, mortality, was a proportion, it had to be transformed using the usual variance-stabilizing transformation for proportions, namely, arcsine square root (22). Multiple comparisons were made using Fisher's least-significant-difference procedure (15).
RESULTS
Introduction of the cry1Ac7 gene of B. thuringiensis isolate 234 into expression, broad-host-range, and integration vectors and Cry1Ac7 expression.
In order to improve the expression of the cry1Ac7 gene, referred to as tox in this report, from pGH37D-1 (13) for subsequent biocontrol use, it was cloned into the plasmid pKK223-3 for expression under the control of the tac promoter. The 3.7-kb NdeI fragment of pGH37D-1, carrying the cry1Ac7 gene under the control of its own promoter, was cloned into the SmaI site of pKK223-3. The resulting construct was called ptac-tox. The 4-kb BamHI fragment of ptac-tox was cloned into the BamHI site of the broad-host-range plasmid pKT240, yielding pKTT (pKT240tactox). Overexpression of a chitinase gene on pKT240 was shown to be highly unstable in H. seropedicae and P. fluorescens (data not shown), and the highly expressed cry1Ac7 gene on pKTT had a detrimental effect on the latter strain (see below). It was therefore decided to clone the cry1Ac7 gene under the control of its own promoter into the broad-host-range plasmids pML122 and pKmM0 (32), a Kmr derivative of the broad-host-range plasmid pDER405 (27) shown to be stably maintained in Herbaspirillum spp. (data not shown). The 3.7-kb blunted NdeI fragment of pGH37D-1 was cloned into the blunted EcoRI site of pML122 in both orientations with respect to the Nmr promoter present on the vector, resulting in the plasmids pMT7 and pMT11 (pML122tox). In pMT7, the cry1Ac7 gene was cloned under the control of the Nmr promoter in addition to its endogenous promoter while in pMT11, the cry1Ac7 gene was under the control of its own promoter. The fragment was cloned into the EcoRV site of pKmM0 to yield pKmM0tox. For cloning into the integration vector, the BamHI ptac-cry1Ac7 fragment from ptac-tox was made blunt and cloned into the blunted NdeI site of the integration vector pJFF350, which carries the artificial interposon Omegon-Km, generating the Omegon-Km–ptac-cry1Ac7 cassette on pJTT (pJFF350tactox).
The expression of the cry1Ac7 gene in E. coli JM105 was determined by Western blot analysis, and it was evident that it is expressed from the tac promoter in ptac-tox at levels considerably higher than from its own promoter (results not shown).
Construction of the ptac-chiA cassette and introduction into P. fluorescens Rif1.
For high-level expression of the S. marcescens chiA gene in gram-negative bacteria for the biocontrol of phytopathogenic fungi, the gene was cloned under the control of the tac promoter and introduced into the chromosome of isolate P. fluorescens Rif1 as described by Downing and Thomson (8).
Construction of P. fluorescens 14 and H. seropedicae Nal1 strains expressing the cry1Ac7 gene.
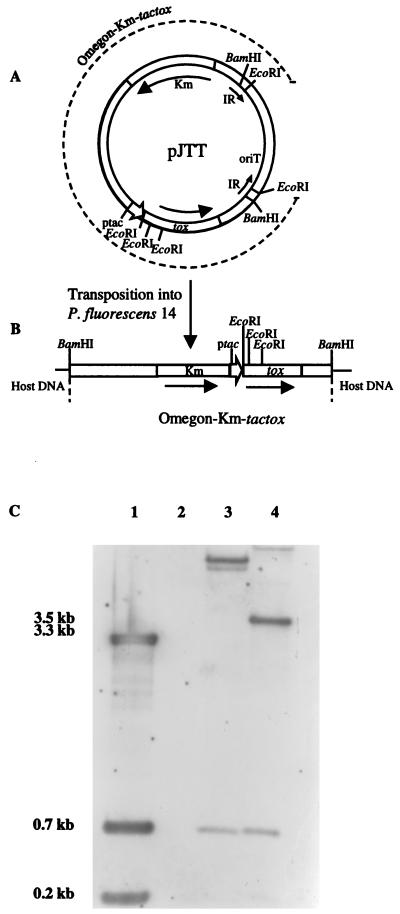
The plasmids pKTT and pJTT, carrying the ptac-cry1Ac7 cassette on pKT240 and the integration vector pJFF350, respectively, were introduced into P. fluorescens 14 by electroporation. P. fluorescens 14(pKTT) electrotransformants grew poorly in liquid medium, indicating that constitutive expression of the cry1Ac7 gene at high levels had a lethal effect on this organism. To circumvent this problem and to prevent horizontal transfer of the gene to other bacterial species, the Omegon-Km–ptac-cry1Ac7 cassette was inserted into the chromosome and integration was confirmed by Southern blot analysis. Total DNA of P. fluorescens 14::ptac-cry1Ac7 was cut with EcoRI and probed with the 4-kb BamHI fragment of ptac-tox carrying the ptac-cry1Ac7 cassette (Fig. 1). Four fragments of 3.5, 3.3, 0.7, and 0.2 kb hybridized to EcoRI-restricted pJTT. In all of the clones analyzed, two EcoRI fragments of 0.7 and 0.2 kb, corresponding to the fragments internal to the Omegon-Km–ptac-cry1Ac7 cassette, and two of different sizes greater than 3.5 and 3.3 kb, hybridized to the probe. Random, single integration of the cassette was indicated by the fact that the two larger EcoRI fragments were of different sizes and only two of the larger EcoRI fragments were detected in these clones.
FIG. 1.
Integration of Omegon-Km–ptac-cry1Ac7 into the chromosome of P. fluorescens 14. The ptac-cry1Ac7 cassette from ptac-tox was cloned into the NdeI site of pJFF350, resulting in pJTT (A). The plasmid was electroporated into P. fluorescens 14 with the transposition of the Omegon-Km–ptac-cry1Ac7 cassette into the chromosome (B). (C) Southern blot analysis of plasmid and chromosomal DNAs cut with EcoRI and probed with the 4-kb BamHI fragment of pJTT carrying the ptac-cry1Ac7 cassette. Lanes 1, pJTT; 2, P. fluorescens 14; 3 and 4, P. fluorescens 14::ptac-cry1Ac7 clones 1 and 2, respectively. IR, inverted repeat; oriT, origin of transfer.
The stability of the integrated cassette was not definitively established, but there was no evidence of decreased Cry1Ac7 expression in SDS-PAGE after 48 h (results not shown) and the growth rate of the recombinant strain did not appear to be different from that of P. fluorescens 14.
The plasmids pKT240 and pJFF350, their recombinant ptac-cry1Ac7 derivatives, and the pML122- and pKmM0-derived plasmids carrying the cry1Ac7 gene were electroporated into H. seropedicae Nal1. pKT240 and the pKmM0- and pML122-based plasmids carrying the cry1Ac7 gene were successfully introduced with an efficiency of ca. 8 × 104 transformants/μg of DNA. Electroporation of pKT240-derived pKTT carrying the ptac-cry1Ac7 cassette resulted in only a few Kmr colonies, possibly due to the instability of this construct in H. seropedicae. Plasmid stability studies showed that pMT7 carrying the cry1Ac7 gene inserted downstream of the strong Nmr promoter on pML122 was extremely unstable after overnight growth, whereas pMT11, with the gene in the opposite orientation with respect to this promoter, was stable over the 60 generations tested (results not shown). This is likely to be due to the intolerance of high levels of constitutively expressed cry1Ac7 in H. seropedicae cells. pKmM0-tox was stable over the 40 generations tested (results not shown). Efforts to introduce the ptac-cry1Ac7 cassette on the integrative construct pJTT into H. seropedicae by electroporation and conjugative transfer resulted in very low numbers of Kmr colonies, indicative of low transposition frequencies, believed to be due to inefficient transformation.
Expression of the δ-endotoxin gene.
Expression of the cry1Ac7 gene in P. fluorescens 14 and H. seropedicae Nal1 was determined by quantitative Western blot analysis. The 134-kDa Cry1Ac7 protein was not detected in P. fluorescens 14 (pKTT) clones carrying the ptac-cry1Ac7 cassette on pKT240 (Fig. 2A, lane 4). However, this gene, under the control of its endogenous promoter on pKT240 and pDER405, was expressed in P. fluorescens 14 at toxin protein levels of 3.5 and 2.2%, respectively, of the total proteins (12). This implied that constitutive expression of cry1Ac7 at high levels in P. fluorescens 14(pKTT) must have resulted in the accumulation of mutants defective in cry1Ac7 expression after overnight growth.
FIG. 2.
Western blot analysis of cry1Ac7 expression in recombinant clones. (A) Lanes: 1 and 2, E. coli(pKTT) and E. coli(pJTT) carrying the ptac-cry1Ac7 cassette on pKT240 and pJFF350, respectively; 3, P. fluorescens 14; 4, P. fluorescens 14(pKTT); 5, P. fluorescens 14::cry1Ac7 clone 2; 6 to 10, P. fluorescens 14::ptac-cry1Ac7 clones 1 and 2. (B) Lanes: 1, E. coli(pMT7); 2, E. coli(pMT11); 3, H. seropedicae Nal1; 4, H. seropedicae Nal1(pMT7); 5, H. seropedicae Nal1(pMT11).
All of the analyzed P. fluorescens 14::ptac-tox clones, carrying the integrated Omegon-Km–ptac-tox cassette, produced the 134-kDa protein at levels considerably greater than that of the previously constructed P. fluorescens 14::Omegon–Km–cry strain, referred to in this report as P. fluorescens 14::tox (Fig. 2A, compare lanes 6 to 10 with lane 5).
The Cry1Ac7 protein was not detected by Western blot assay of H. seropedicae(pKTT) carrying the ptac-cry1Ac7 cassette on pKT240 (results not shown). As in P. fluorescens 14(pKTT) clones, this is possibly due to the accumulation of Cry1Ac7− mutants resulting from high levels of the constitutively expressed cry1Ac7 gene in H. seropedicae(pKTT). In contrast, H. seropedicae(pMT7) clones with the cry1Ac7 gene downstream of the Nmr promoter on pML122 produced higher levels of the Cry1Ac7 protein than did H. seropedicae(pMT11) clones with the gene in the opposite orientation with respect to this promoter (Fig. 2B). This indicated that expression of the gene in the former clones was under the control of the Nmr promoter, which could explain the high levels of instability of this plasmid. Labes et al. (19) reported that the Nmr promoter was an efficient and more effective promoter than the tac promoter for overexpression of foreign genes in soil bacteria, including Pseudomonas spp. The Cry1Ac7 protein was also detected in strains carrying pKmM0-tox (results not shown).
Effect of Cry1Ac7+ P. fluorescens and H. seropedicae strains on E. saccharina larvae.
The biological activity of Cry1Ac7+ P. fluorescens and H. seropedicae strains was determined in toxicity bioassays using 3 mg of freeze-dried bacteria per g of diet (Table 2). The results, at the 5% significance level, showed that P. fluorescens 14::ptac-cry1Ac7 was significantly different from P. fluorescens 14::cry1Ac7. Both P. fluorescens 14::cry1Ac7 and 14::ptac-cry1Ac7 were significantly different from the parental strain, which was not significantly different from the untreated control. H. seropedicae Nal1(pMT7) was significantly different from H. seropedicae Nal1, which was not significantly different from the control. H. seropedicae Nal1(pMT11) was different from H. seropedicae Nal1, but the sample size was not large enough to declare significance at the 5% level.
TABLE 2.
Toxicity to E. saccharina neonate larvae of P. fluorescens 14 and H. seropedicae Nal1 strains expressing the cry1Ac7 gene
| Day | Avg % mortality (SE)a due to:
|
||||||
|---|---|---|---|---|---|---|---|
| Controlb | 14c | 14::cry1Ac7 | 14::ptac-cry1Ac7 | Nal1d | Nal1(pMT7) | Nal1(pMT11) | |
| 1 | 0 | 4 (4.0) | 4 (4.0) | 16 (7.5) | 0 (0) | 4 (4) | 4 (4) |
| 2 | 0 | 4 (4.0) | 32 (8.0) | 48 (8.0) | 0 (0) | 8 (4.9) | 8 (8) |
| 3 | 0 | 8 (4.9) | 40 (12.6) | 68 (4.9) | 0 (0) | 16 (7.5) | 16 (7.5) |
| 4 | 0 | 16 (7.5) | 56 (11.7) | 84 (7.5) | 12 (8) | 48 (12) | 28 (10.2) |
| 8 | 0 | 28 (8) | 84 (7.5) | 92 (4.9) | 20 (8.9) | 52 (10.2) | 36 (14.7) |
Averages of five experiments are shown. The statistical analysis was done on transformed data (see Materials and Methods; 22).
No treatment.
P. fluorescens 14.
H. seropedicae Nal1.
Effect of P. fluorescens strains expressing the cry1Ac7 and chiA genes on E. saccharina larvae.
P. fluorescens 14::ptac-cry1Ac7 and P. fluorescens Rif1::ptac-chiA were combined at different concentrations and used in toxicity bioassays (Table 3). Mortality was determined after 2 and 5 days. The results, at the 5% level of significance, show that when the chitinase-expressing strain was added at either 0.3 or 30 mg/g of diet along with the Cry1Ac7-expressing strain at 0.3 mg/g of diet, there was a significantly increased toxic effect. The reason for the lack of increased toxicity when the chitinase-expressing strain was added at 3.0 mg/g of diet along with the Cry1Ac7-expressing strain at 0.3 mg/g of diet is most likely the smaller sample size of this experiment (n = 4). Although the chitinase-expressing strain showed toxicity at 30 mg/g of diet, it did not do so at 0.3 or 3 mg/g of diet. However, there was a significant increase in toxicity when it was mixed with the Cry1Ac7-expressing strain at 0.3 mg/g of diet.
TABLE 3.
Toxicity to E. saccharina neonate larvae of P. fluorescens 14::ptac-cry1Ac7 and P. fluorescens Rif1::ptac-chiA
| Concn (mg/g of diet) of:
|
Avg % mortality (SE)a
|
||
|---|---|---|---|
| P. fluorescens 14::ptac-cry1Ac7 | P. fluorescens Rif1::ptac-chiA | Day 2 | Day 5 |
| 0 | 0 | 5.5 (2.2) | 7.6 (2.8) |
| 0.3 | 0 | 12.5 (3.7) | 33.8 (10.3) |
| 3.0 | 0 | 30.8 (8.1) | 42.7 (9.9) |
| 0 | 0.3 | 8.2 (3.9) | 20 (7.6) |
| 0 | 3.0 | 7.7 (2.8) | 21.5 (8.0) |
| 0 | 30.0 | 21.8 (9.1) | 42.7 (7.8) |
| 0.3 | 0.3 | 39.3 (7.7) | 55.7 (6.6) |
| 0.3 | 30.0 | 37.5 (6.0) | 68.3 (5.8) |
Averages of 11 to 16 experiments are shown. The statistical analysis was done on transformed data (see Materials and Methods; 22).
DISCUSSION
B. thuringiensis cry genes have been introduced into bacteria other than B. thuringiensis, such as the root colonizers P. fluorescens and Agrobacterium radiobacter and Ancylobacter aquaticus, a bacterium isolated from aquatic habitats. These strains were toxic against the larvae of the tobacco hornworm (Manduca sexta), the malaria mosquito Anopheles stephensi, and the leatherjacket (Tipula oleracea) (16, 23, 24, 35). The introduction of the cryIA(c) gene from B. thuringiensis subsp. kurstaki into the chromosome of Clavibacter xyli subsp. cynodontis, which naturally colonizes the xylem of Bermuda grass, is the only report of the use of a genetically modified endophyte as a biocontrol agent. This recombinant endophyte was shown to colonize corn and was tested for its effectiveness against the European corn borer (Ostrinia nubilalis). Moderate control of this pest was achieved by expression of the toxin gene chromosomally integrated into the endophyte (21). However, integration of endotoxin gene sequences into the chromosome of C. xyli subsp. cynodontis was unstable and segregant colonies made up 15% of the colonies isolated from corn at the end of the growing season. The authors suggested that the loss of the integrated gene could serve as an environmental safety feature (35). As little research had been done on the suitability of endophytic bacteria as biocontrol agents to date, it was of interest to investigate the potential of the sugarcane endophytes A. diazotrophicus and H. seropedicae, in addition to the phylloplane bacterium P. fluorescens 14, engineered to express a B. thuringiensis cry gene against the sugarcane borer E. saccharina.
To improve expression of the cry1Ac7 gene, we cloned it under the control of the strong tac promoter and used the vector pJFF350, which carries the artificial interposon Omegon-Km (9), to integrate it into the chromosome of P. fluorescens 14. On this plasmid, the Ω interposon is flanked by two synthetic inverted 28-bp repeats of IS1, which can transpose if IS1 gene products are supplied. The vector has an origin of transfer which allows mobilization into gram-negative bacteria. It also carries a disabled IS1 element which enables transposition of the Omegon-Km cassette although it cannot transpose itself, resulting in stably integrated genes. Although the stability of the ptac-cry1Ac7 cassette in P. fluorescens 14 was not investigated, Herrera (12) showed that the Omegon-Km–cry cassette in P. fluorescens 14 was stably integrated for at least 100 generations and stable integration of cry genes into root-colonizing P. fluorescens strains using a transposon Tn5-mediated system or suicide vectors for integration by homologous recombination have been described in the literature (23, 24, 35).
Our results proved that the tac promoter is capable of operating efficiently in Pseudomonas and is responsible for the increased levels of expression of the gene. We are unaware of any reports of a cry1A(c) gene under the control of the tac promoter having been integrated into the chromosome of a Pseudomonas sp. Quantitative analysis of the δ-endotoxin by enzyme-linked immunosorbent assay in P. fluorescens 14:: ptac-cry1Ac7 clones was not determined, but Herrera et al. (13) showed that P. fluorescens 14::cry1Ac7 clones produced high levels of Cry1Ac7 protein similar to those produced by pKT240-cry1Ac7 clones, representing 3.7 and 3.5% of the total protein, respectively. These levels were comparable to those of 0.5 to 1% reported by Obuckowicz (23) for a similar cry gene in root-colonizing pseudomonads. Ge et al. (10) reported that expression of the cry1A(c)73 gene from its own promoter in E. coli was 0.24% of the total cellular protein and that from the tac promoter after induction was about 50% of the total cellular protein. In another system, expression of a B. thuringiensis subsp. aizawai 130-kDa protein gene from the tac promoter in E. coli was 38% of the total cellular protein compared to 3% of the total cellular protein when expressed from its own promoter (25). Therefore, it is believed that the levels of the Cry1Ac7 protein expressed from the tac promoter in P. fluorescens would be considerably greater than 3.7%. This would only result if the ptac-cry1Ac7 cassette were present in the cell as a single integrated copy, as it is clear from the nonexpressing mutants of P. fluorescens carrying the cassette on the multicopy plasmid pKTT that only a certain level of expression is tolerated by these cells.
Toxicity bioassays showed that increased expression of the cry1Ac7 gene in both P. fluorescens 14 and H. seropedicae improves the control of E. saccharina larvae. However, it is important to consider that although increased expression leads to increased toxicity, it can also be a burden on bacterial cells, resulting in the accumulation of nonexpressing mutants or in lethality. All of these factors need to be taken into account when planning strategies for biological control of E. saccharina in sugarcane.
Synergistic insecticidal effects with combined B. thuringiensis suspensions and chitinase or chitinase-producing bacteria, as well as the combined effects of a Cry1C protein and S. marcescens ChiA, have been demonstrated previously (28, 31). The addition of both B. thuringiensis and chitinase increased the insecticidal effect on Chonstoneura fumiferana larvae significantly. Perforation of the peritrophic membrane by ChiA caused an increase in the toxicity of Cry1C, possibly due to an increase in the numbers of Cry1C toxin molecules binding to the membrane receptors present in the epithelium of the insect larvae. A Cry1C concentration of 20 μg/ml was required for a maximum toxic effect on larvae in the absence of chitinase, whereas only 3 μg of Cry1C per ml was needed for the same toxic effect in the presence of ChiA.
Our results demonstrate that by cointroduction of cry1Ac7 and chitinase genes into strains of P. fluorescens, increased biocontrol of insect pests could be achieved, requiring lower levels of Cry1Ac7 protein expression. This is advantageous, since lower expression may enable the bacteria to compete better in the environment with a diminished risk of generation of resistant larval populations resulting from exposure to high levels of Cry protein. The optimum, effective concentrations of the recombinant strains, as well as the synergistic toxic effect of H. seropedicae strains producing the Cry1Ac7 protein and chitinase, need to be investigated.
ACKNOWLEDGMENTS
We thank Imke Hansen-Wester for construction of pKmM0-tox and its expression in H. seropedicae, Gillian Mimmack for statistical analysis of the data, Barbara Huckett for assistance with bioassays, and Helena Boshoff for helpful discussions.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley & Sons; 1992. [Google Scholar]
- 2.Bagdasarian M M, Amann E, Lurz R, Rückert B, Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983;26:273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 3.Baldani J I, Baldani V L D, Seldin L, Döbereiner J. Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol. 1986;36:86–93. [Google Scholar]
- 4.Boddey R M. Biological nitrogen fixation in sugar cane: a key to energetically viable biofuel production. Crit Rev Plant Sci. 1995;14:263–279. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Döbereiner J, Reis V M, Paula M A, Olivares F. Endophytic diazotrophs in sugar cane, cereals and tuber plants. In: Palacios R, et al., editors. New horizons in nitrogen fixation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 671–676. [Google Scholar]
- 7.Döbereiner J, Baldani V L D, Reis V M. Endophytic occurrence of diazotrophic bacteria in non-leguminous crops. In: Fendrik I, et al., editors. Azospirillum VI and related microorganisms. Berlin, Germany: Springer-Verlag; 1995. pp. 3–14. [Google Scholar]
- 8.Downing K J, Thomson J A. Introduction of the Serratia marcescens chiA gene into an endophytic Pseudomonas fluorescens for the biocontrol of phytopathogenic fungi. Can J Microbiol. 2000;46:1–7. doi: 10.1139/w99-147. [DOI] [PubMed] [Google Scholar]
- 9.Fellay R, Krisch H M, Prentki P, Frey J. Omegon-Km: a transposable element designed for in vivo insertional mutagenesis and cloning of genes in Gram-negative bacteria. Gene. 1989;76:215–226. doi: 10.1016/0378-1119(89)90162-5. [DOI] [PubMed] [Google Scholar]
- 10.Ge A Z, Pfister R M, Dean D H. Hyperexpression of a Bacillus thuringiensis delta-endotoxin-encoding gene in Escherichia coli: properties of the product. Gene. 1990;93:49–54. doi: 10.1016/0378-1119(90)90134-d. [DOI] [PubMed] [Google Scholar]
- 11.Graham D Y, Conlong D E. Improved laboratory rearing of Eldana saccharina (Lepidoptera: Pyralidae) and its indigenous parasitoid Goniozus natalensis (Hymenoptera: Bethylidae) Proc. S Afr Sugar Technol Assoc Annu Congr. 1988;62:116–119. [Google Scholar]
- 12.Herrera G. Biological control of Eldana saccharina Walker using a cloned Bacillus thuringiensis cry gene. Ph.D. thesis. Cape Town, South Africa: University of Cape Town; 1994. [Google Scholar]
- 13.Herrera G, Snyman S J, Thomson J A. Construction of a bioinsecticidal strain of Pseudomonas fluorescens active against the sugarcane borer, Eldana saccharina. Appl Environ Microbiol. 1994;60:682–690. doi: 10.1128/aem.60.2.682-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höfte H, Whiteley H R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989;53:242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell D C. Statistical methods for psychology, third edition. Belmont, Calif: Duxbury Press; 1996. [Google Scholar]
- 16.Ho Yap W, Thanabalu T, Porter A G. Expression of mosquitocidal toxin genes in a gas-vacuolated strain of Ancylobacter aquaticus. Appl Environ Microbiol. 1994;60:4199–4202. doi: 10.1128/aem.60.11.4199-4202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs S J. Micro-organisms as potential biological control agents of Eldana saccharina Walker (Lepidoptera: Pyralidae) Proc S Afr Sugar Technol Assoc Annu Congr. 1989;63:186–188. [Google Scholar]
- 18.Kleiner K W, Ellis D D, McCown B H, Raffa K F. Field evaluation of transgenic poplar expressing a Bacillus thuringiensis cryIA(a) d-endotoxin gene against forest tent caterpillar (Lepidoptera: Lasiocampidae) and gypsy moth (Lepidoptera: Lymantriidae) following winter dormancy. Biol Control. 1996;24:1358–1364. [Google Scholar]
- 19.Labes M, Pühler A, Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990;89:37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lampel J S, Canter G L, Dimock M B, Kelley J L, Anderson J J, Uratani B B, Foulke J S, Jr, Turner J T. Integrative cloning, expression, and stability of the cryIA(c) gene from Bacillus thuringiensis subsp. kurstaki in a recombinant strain of Clavibacter xyli subsp. cynodontis. Appl Environ Microbiol. 1994;60:501–508. doi: 10.1128/aem.60.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendenhall W, Beaver R J. A brief course in business statistics. Belmont, Calif: Duxbury Press; 1995. [Google Scholar]
- 23.Obukowicz M G, Perlak F J, Kusano-Kretzmer K, Mayer E J, Watrud L S. Integration of the delta-endotoxin gene from Bacillus thuringiensis into the chromosome of root colonizing pseudomonads using Tn5. Gene. 1986;45:327–331. doi: 10.1016/0378-1119(86)90031-4. [DOI] [PubMed] [Google Scholar]
- 24.Obukowicz M G, Perlak F J, Kusano-Kretzmer K, Mayer E J, Bolten S L, Watrud L S. Tn5-mediated integration of the delta-endotoxin gene from Bacillus thuringiensis into the chromosome of root-colonizing pseudomonads. J Bacteriol. 1986;168:982–989. doi: 10.1128/jb.168.2.982-989.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oeda K, Oshie K, Shimizu M, Nakamura K, Yamamoto H, Nakayama I, Ohkawa H. Nucleotide sequence of the insecticidal protein gene of Bacillus thuringiensis strain aizawai IPL7 and its high-level expression in Escherichia coli. Gene. 1987;53:113–119. doi: 10.1016/0378-1119(87)90098-9. [DOI] [PubMed] [Google Scholar]
- 26.Perlak F J, Fuchs R L, Dean D A, McPherson S L, Fischhoff D A. Modification of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci USA. 1991;88:3324–3328. doi: 10.1073/pnas.88.8.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawlings D E, Pretorius I-M, Woods D R. Expression of Thiobacillus ferrooxidans plasmid functions and the development of genetic systems for the thiobacilli. Biotechnol Bioeng Symp. 1986;16:281–287. [Google Scholar]
- 28.Regev A, Keller M, Strizhov N, Sneh B, Prudovsky E, Chet I, Ginzberg I, Koncz-Kalman Z, Koncz C, Schell J, Zilberstein A. Synergistic activity of a Bacillus thuringiensis δ-endotoxin and a bacterial endochitinase against Spodoptera littoralis larvae. Appl Environ Microbiol. 1996;62:3581–3586. doi: 10.1128/aem.62.10.3581-3586.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 31.Smirnoff W A. Effect of chitinase on the action of Bacillus thuringiensis. Can Entomol. 1971;103:1829–1831. [Google Scholar]
- 32.Smith A S G, Rawlings D E. The poison-antidote stability system of the broad-host-range Thiobacillus ferrooxidans plasmid pTF-FC2. Mol Microbiol. 1997;26:961–970. doi: 10.1046/j.1365-2958.1997.6332000.x. [DOI] [PubMed] [Google Scholar]
- 33.Snyman S J, Black K G, Herrera G, Thomson J A. Pseudomonas fluorescens genetically engineered to produce an insect toxin: a culmination of five years of collaborative research. Proc S Afr Sugar Technol Assoc Annu Congr. 1993;67:78–81. [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner J T, Lampel J S, Stearman R S, Sundin G W, Gunyuzlu P, Anderson J J. Stability of the δ-endotoxin gene from Bacillus thuringiensis subsp. kurstaki in a recombinant strain of Clavibacter xyli subsp. cynodontis. Appl Environ Microbiol. 1991;57:3522–3528. doi: 10.1128/aem.57.12.3522-3528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waalwijk C, Dullemans A, Maat C. Construction of a bioinsecticidal rhizosphere isolate of Pseudomonas fluorescens. FEMS Microbiol Lett. 1991;77:257–264. [Google Scholar]
- 37.Wirth R, Friesenegger A, Fiedler S. Transformation of various species of Gram-negative bacteria belonging to 11 different genera by electroporation. Mol Gen Genet. 1989;216:175–177. doi: 10.1007/BF00332248. [DOI] [PubMed] [Google Scholar]
- 38.Yanish-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]