Graphical abstract
Keywords: TBBPA, TCBPA, HepG2 cells, PPARγ, Fatty acid synthase
Highlights
-
•
TBBPA and TCBPA alone or as binary mixtures decreases cell viability.
-
•
TBBPA or TCBPA alone or as a mixture enhances effects of reference PPARγ ligands.
-
•
Overexpression of PPARγ does not mitigate nor enhance the effects of TBBPA.
-
•
TBBPA and TCBPA induce toxicity within HepG2 cells in a PPARγ-independent manner.
Abstract
Tetrabromobisphenol A (TBBPA) and tetrachlorobisphenol A (TCBPA) – both halogenated bisphenol (BPA) analogues – are suspected ligands of peroxisome proliferator-activated receptor gamma (PPARγ). While previous studies have shown that TBBPA and TCBPA activate PPARγ within cell-free assays, the downstream effects of TBBPA- and TCBPA-induced PPARγ activation on cellular transcription and physiology have not been thoroughly investigated. Therefore, the objective of this study was to determine whether exposure to TBBPA or TCBPA (either alone or in combination) alters levels of neutral lipids and fatty acid synthase (FASN) – an enzyme that catalyzes synthesis of long-chain saturated fatty acids – within intact cells in a PPARγ-dependent manner. For this study, we relied on human hepatocellular carcinoma (HepG2) cells as a model since these liver cells express basal levels of PPARγ and have been used to study lipoprotein metabolism and regulation of drug metabolizing enzymes. Although exposure to TBBPA and TCBPA alone did not affect cell viability nor neutral lipid and FASN levels in a concentration-dependent manner, exposure to binary mixtures of TBBPA and TCBPA resulted in a concentration-dependent decrease in cell viability in the absence of concentration-dependent effects on neutral lipid and FASN levels. Interestingly, exposure to TBBPA or TCBPA alone or as a mixture enhanced the effects of a reference PPARγ agonist (ciglitazone) and antagonist (GW 9662) on cell viability (but not neutral lipid levels), suggesting that these two halogenated BPA analogues may interact synergistically with ciglitazone and GW 9662 to induce cytotoxicity. However, overexpression of PPARγ did not mitigate nor enhance the effects of TBBPA – a potent PPARγ ligand predicted by ToxCast’s cell-free competitive binding assays – on cell viability, neutral lipid levels, nor the cellular transcriptome. Overall, our findings suggest that halogenated BPA analogues such as TCBPA and TBBPA induce toxicity within HepG2 cells in a PPARγ-independent manner.
1. Introduction
Although bisphenol A (BPA) is mainly used as a plasticizer in polycarbonate and epoxy resins, tetrabromobisphenol A (TBBPA) and tetrachlorobisphenol A (TCBPA) are two halogenated BPA analogs primarily used as flame retardants in electronic devices. As a result, these two halogenated BPA analogues have the potential to migrate into indoor dust (Leisewitz et al., 2001) and expose humans within the built environment, where levels of TBBPA in house dust have been previously found to range from 1 to 3600 ng/g (Wang et al., 2015). TBBPA-sulfate and TBBPA-glucuronide (Schauer et al., 2006) – the primary metabolites of TBBPA – have been found in both serum and urine samples in humans around the world (Nagayama et al., 2000, Thomsen et al., 2001, Jakobsson et al., 2002, Dirtu et al., 2008, Shi et al., 2013, Ho et al., 2017). While BPA is a weak ligand for the estrogen receptor (Gould et al., 1998, Liu et al., 2018), neither TBBPA nor TCBPA activate ER to the same magnitude as BPA (Lee et al., 2012, Cao et al., 2017). However, TBBPA and TCBPA have both been shown to activate another member of the nuclear receptor family – peroxisome proliferator activated receptor gamma (PPARγ) (Riu et al., 2011, Akiyama et al., 2015, Chappell et al., 2018).
PPARγ is a transcription factor (Issemann and Green, 1990, Tontonoz et al., 1994, Martin et al., 1998) that can be activated by both endogenous ligands such as prostaglandin PGJ2 and eicosapentaeonic acid (Forman et al., 1995, Kliewer et al., 1995, Nagy et al., 1998) or exogenous ligands such as pharmaceutical compounds (e.g., thiazolidinediones) (Nolan et al., 1994, Lehmann et al., 1995). Upon activation by ligand binding, PPARγ heterodimerizes with retinoid X receptor (RXR) and then binds to PPAR response elements (PPREs), resulting in transcription of genes involved in lipid/glucose metabolism and adipogenesis (Tontonoz et al., 1994, Martin et al., 1998, Chawla et al., 2001). While several thiazolidinediones have been developed to treat Type II diabetes and obesity within human populations, many of these compounds have severe side effects and have been discontinued from use (Nesto et al., 2003, Nissen and Wolski, 2007, Lewis et al., 2011). As several environmental chemicals have been shown to bind and activate PPARγ, there is concern about human exposure to xenobiotic PPARγ ligands and potential downstream effects (Hurst and Waxman, 2003, Riu et al., 2011, Wang et al., 2016).
Although TBBPA and TCBPA have nearly identical chemical structures (TBBPA is brominated whereas TCBPA is chlorinated), TBBPA binds to the ligand binding domain of human PPARγ with greater affinity than TCBPA (Riu et al., 2011). For example, TBBPA induces neurotoxic effects within mouse primary neuronal cells that is partially PPARγ-dependent (Wojtowicz et al., 2014). While previous studies have investigated the ability of TBBPA and TCBPA to bind to PPARγ within reporter and ligand binding assays, to our knowledge no prior studies have linked TBBPA or TCBPA exposure to PPARγ activation and subsequent downstream effects within a human liver cell model. Therefore, the overall objective of this study was to determine whether exposure of human liver cells to non-cytotoxic concentrations of TBBPA or TCBPA results in effects on transcription and other endpoints that are consistent with the known mechanism of action for PPARγ activation, such as neutral lipid staining (Wakabayashi et al., 2009). We also utilized immunohistochemical staining of fatty acid synthase (FASN) protein in situ, as FASN catalyzes synthesis of long-chain saturated fatty acids and is correlated with PPARγ expression in adipocytes (Zhao et al., 2011).
For this study, we relied on human hepatocellular carcinoma (HepG2) cells as a model since these liver cells express basal levels of PPARγ and have previously been used to study lipoprotein metabolism (Meex et al., 2011) and regulation of drug metabolizing enzymes (Wilkening et al., 2003). We also utilized a human PPARγ expression plasmid to overexpress PPARγ and determine whether the effects of halogenated BPA analogues were mitigated or enhanced in the presence of increased PPARγ levels. Our overall hypothesis was that TBBPA- and TCBPA-induced effects on cell viability, lipid homeostasis, and FASN protein levels were associated with PPARγ-mediated alterations to the cellular transcriptome. Therefore, our study is timely and significant to the field of environmental toxicology, as it 1) helps us further understand the mechanisms of action of two widely used flame retardants (TBBPA and TCBPA) within human cells and 2) challenges a long-standing assumption that PPARγ activation by environmental chemicals leads to downstream effects on cellular transcription and physiology.
2. Materials and methods
2.1. Chemicals
TBBPA (>97% purity) and TCBPA (>98% purity) were purchased from Millipore Sigma (St. Louis, MO, USA). Ciglitazone (>99.4% purity) was purchased from Tocris Biosciences (Bristol, UK) and GW 9662 (>98% purity) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). For all chemicals, stock solutions were prepared in high-performance liquid chromatography (HPLC)-grade dimethyl sulfoxide (DMSO) and stored in 2-mL amber glass vials with polytetrafluoroethylene-lined caps. Working solutions were prepared by spiking stock solutions into sterile cell culture media immediately prior to each experiment, resulting in 0.1% DMSO (single chemical exposures) or 0.2% DMSO (binary mixture exposures) within all treatment groups. For binary mixture exposures, the final concentration of DMSO used was 0.2% since an equal volume of two different stock solutions were added to working solutions, resulting in twice the volume of DMSO relative to single chemical exposures. As shown in Fig. 1, Fig. 2, exposure to 0.2% DMSO had no effect on cell viability relative to exposure to 0.1% DMSO.
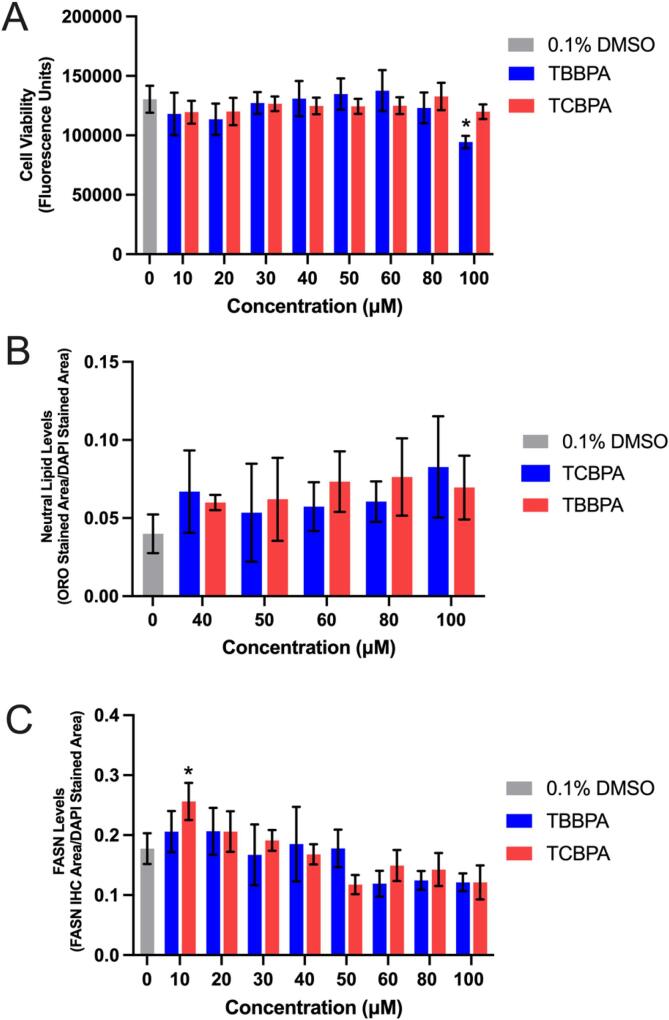
Fig. 1.
Mean (±standard deviation) fluorescence (cell viability) (A), neutral lipid staining normalized to DAPI staining (B), and fatty acid synthase protein levels normalized to DAPI staining (C) of HepG2 cells exposed to vehicle (0.1% DMSO), 10–100 µM TBBPA, or 10–100 µM TCBPA for 24 h. Asterisk (*) denotes a significant difference (p < 0.05) relative to vehicle-exposed cells.
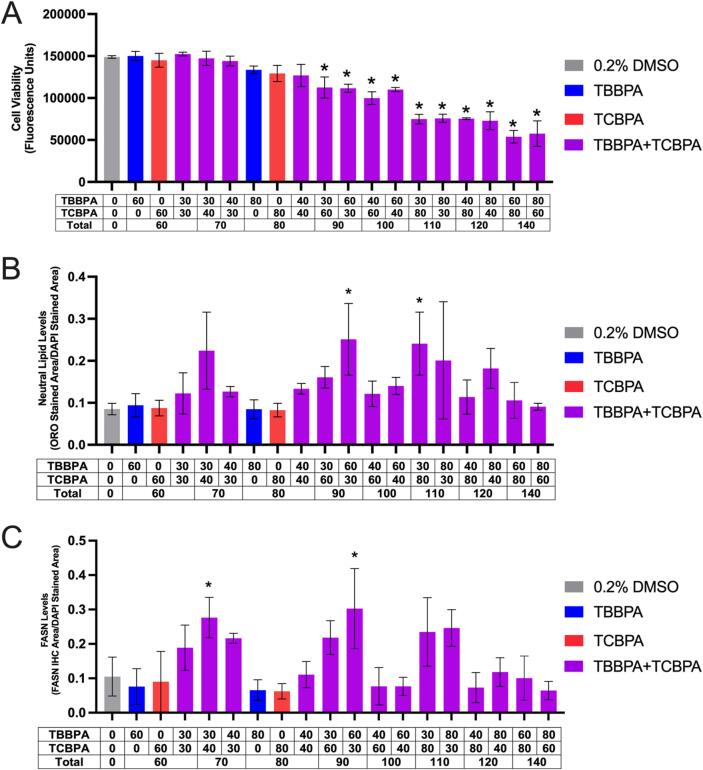
Fig. 2.
Mean (±standard deviation) fluorescence (cell viability) (A), Oil Red O neutral lipid-stained area divided by DAPI stained area (B), or FASN immunofluorescence area divided by DAPI stained area of HepG2 cells exposed to vehicle (0.2% DMSO) or TBBPA and/or TCBPA for 24 h. Asterisk (*) denotes a significant difference (p < 0.05) in cell viability, neutral lipid staining, or FASN immunofluorescence relative to vehicle-exposed cells.
2.2. PPARγ ligand exposures and cell viability assays
HepG2 cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA) and grown within T75 cell culture flasks (MilliporeSigma, St. Louis, MO, USA) containing 15 mL of Eagle’s Minimum Essential Medium supplemented with 10% fetal bovine serum (FBS) (ATCC, Manassas, VA, USA) at 37 °C and 5% CO2. Media was changed within each flask every other day and cells were split every four days using 0.25% Trypsin/0.53 mM EDTA (ATCC, Manassas, VA, USA) after reaching ∼70–90% confluency.
HepG2 cells were plated at a concentration of 0.5x104 cells per well in a clear, polystyrene 96-well plate (Thermo Fisher Scientific, Waltham, MA, USA) and allowed to adhere overnight. Media was removed and replaced with 200 µL media spiked with either vehicle (0.1% DMSO for single chemical exposures), TBBPA (10–100 µM), or TCBPA (10–100 µM) and incubated at 37 °C and 5% CO2 for 24 h (4 replicate wells per treatment). To assess whether TBBPA and TCBPA resulted in additive or synergistic effects on cell viability and FASN levels, cells were also exposed to either vehicle (0.2% DMSO for binary mixture exposures), 60 or 80 µM TBBPA alone, 60 or 80 µM TCBPA alone, or binary mixtures of 30–100 µM TBBPA and 30–100 µM TCBPA and then incubated at 37 °C and 5% CO2 for 24 h; rather than using a combination index design, we simply tested binary mixtures that reflected combinations of treatments that were tested for TBBPA and TCBPA alone. At the end of the exposure duration, treatment solution was removed and replaced with 100 µL of clean cell culture media and 20 µL of CellTiter-Blue (Promega, Madison, WI, USA), and then allowed to incubate for 2 h at 37 °C and 5% CO2. Fluorescence was then quantified using a GloMax Multi+ Detection System (Promega, Madison, WI, USA).
2.3. Oil Red O staining
To determine whether exposure to TBBPA or TCBPA affected neutral lipid abundance, HepG2 cells were exposed to vehicle (0.1% DMSO), TBBPA, or TCBPA as described above and then stained for neutral lipids using Oil Red O (ORO) (MilliporeSigma, St. Louis, MO, USA) as previously described (Cheng et al., 2021). Briefly, cells were fixed using 4% paraformaldehyde for 20 min at room temperature. Cells were then rinsed with 60% isopropanol and stained with ORO working solution (1.8 mg ORO per 1 mL 60% isopropanol) for 10 min at room temperature. Cells were then rinsed four times with molecular biology-grade water for 5 min at room temperature. After the final wash, cells were counterstained with a 1:4 solution of DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL, USA) for 5 min at room temperature. Cells were washed three more times with molecular biology-grade water and then imaged (at 10× magnification) and analyzed using our ImageXpress Micro XLS Widefield High-Content Screening System (Molecular Devices, Sunnyvale, CA, USA).
2.4. FASN immunohistochemistry
To quantify FASN protein levels in situ, cells were exposed to either vehicle (0.1% DMSO), TBBPA, or TCBPA as described above for 24 h. At exposure termination, cells were fixed with 4% formaldehyde at room temperature for 10 min. Cells were then rinsed three times with 1× phosphate-buffered saline (PBS) and incubated in blocking buffer [1× PBS + 0.1% Tween-20 (PBST), 2 mg/mL bovine serum albumin, and 2% sheep serum] at room temperature for 1 h by shaking gently. Blocking buffer was then replaced with a 1:500 dilution of a human FASN-specific antibody (G-11, sc-48357; Santa Cruz Biotechnology, Dallas, TX, USA) in blocking buffer and allowed to incubate overnight at 4 °C. Cells were then incubated with a 1:500 dilution of Alexa Fluor 488-conjugated goat anti-mouse IgG1 antibody (Thermo Fisher Scientific, Waltman, MA, USA) overnight at 4 °C. Cells were then counterstained with a 1:4 solution of DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL, USA) for 5 min, rinsed with 1× PBS three times, and then imaged (at 10× magnification) and analyzed using our ImageXpress Micro XLS Widefield High-Content Screening System (Molecular Devices, Sunnyvale, CA, USA).
2.5. Pretreatment with reference PPARγ ligands
To determine whether TBBPA- or TCBPA-induced effects were mitigated or enhanced by a reference PPARγ agonist (ciglitazone) or antagonist (GW 9662), HepG2 cells were plated in 96-well plates as described above. As shown in our prior study (Cheng et al., 2021), exposure to GW 9662 resulted in decreased PPARγ protein levels after 24 h; therefore, we relied on a 24-h pretreatment with either ciglitazone or GW 9662. Following pretreatment with either vehicle (0.1% DMSO), 30–100 µM ciglitazone, or 10–100 µM GW 9662, cells were then exposed to either vehicle (0.2% DMSO), 30 µM TBBPA, 60 µM TCBPA, or 30 µM TBBPA + 60 µM TCBPA for 24 h and collected for cell viability, neutral lipid staining, and FASN protein IHC as described above.
2.6. ToxCast data mining
ToxCast assay endpoint data for TBBPA and TCBPA were downloaded from the U.S. Environmental Protection Agency’s website (https://www.epa.gov/ncct/toxcast/data/html). Half-maximal activity concentrations (AC50) for TBBPA and TCBPA were used to calculate assay hit rates and develop overall summary statistics. Since the number of assay endpoints for TBBPA and TCBPA were not identical, the percent assay hit rate for each chemical was defined as the number of assay endpoints with an AC50 of <1000 µM – the maximal concentration tested and the basis for an “inactive” activity call – relative to the total number of assay endpoints per chemical. Assays were then sorted by “Intended_Target_Family” for “Nuclear Receptor” to determine the hit rate for nuclear receptor assays. Finally, “Gene_Name” was sorted by “PPARγ” to determine the hit rate for PPARγ-specific assay endpoints.
2.7. Overexpression of PPARγ within HepG2 cells
To determine whether overexpression of PPARγ modified the effects of TBBPA (a potent PPARγ ligand based on ToxCast data), HepG2 cells were plated and transfected with either a negative control (NC) cloning plasmid (pCMV6-XL4, OriGene, Rockville, MD, USA) or an expression plasmid containing human untagged PPARγ clone (SC124177, OriGene, Rockville, MD, USA) following the manufacturer’s protocol. Briefly, 100 ng/well of either NC plasmid or PPARγ expression plasmid were diluted in Opti-MEM reduced serum media (Thermo Fisher Scientific, Waltman, MA, USA). TurboFectin 8.0 (OriGene, Rockville, MD USA) (0.3 µL/well) was added to plasmids diluted in Opti-MEM and incubated for 15 min at room temperature to form complexes. Following incubation, plasmid:TurboFectin 8.0 complexes were added to each well and gently shaken to distribute evenly. Transfected HepG2 cells were then allowed to incubate for 48 h followed by exposure to either vehicle (0.1% DMSO) or 30 µM TBBPA for 24 h as described above. PPARγ protein levels in situ across transfections and treatments were then quantified using a 1:150 dilution of a human PPARγ-specific antibody (E-8, sc-7273; Santa Cruz Biotechnology, Dallas, TX, USA) following previously described protocols (Cheng et al., 2021). Transfected cells were also collected for cell viability, ORO neutral lipid staining, and FASN IHC as described above.
2.8. mRNA-sequencing
HepG2 cells were plated, transfected with either NC plasmid or PPARγ expression plasmid, and exposed to either vehicle (0.1% DMSO) or 30 μM TBBPA (3 wells pooled per replicate; 3 replicates per treatment) as described above. After 24 h, total RNA from each replicate was isolated using a Promega SV Total RNA Isolation System (Promega, Madison, WI, USA) following the manufacturer’s protocol. RNA quantity and quality were confirmed using a Qubit 4.0 Fluorometer and Bioanalyzer 2100 system, respectively. Based on sample-specific Bioanalyzer traces, the RNA Integrity Number (RIN) was > 8.5 for all RNA samples used for library preparations.
Library preps were performed using a QuantSeq 3′ mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen, Vienna, Austria) and indexed by treatment replicate per manufacturer’s instructions. Library quantity and quality were confirmed using a Qubit 4.0 Fluorometer and 2100 BioAnalyzer system, respectively. Raw Illumina (fastq.qz) sequencing files (12 total) are available via NCBI’s BioProject database under BioProject ID PRJNA752134, and a summary of sequencing run metrics are provided in Table S1. All 12 raw and indexed Illumina (fastq.gz) sequencing files were downloaded from Illumina’s BaseSpace and uploaded to Bluebee’s genomics analysis platform (https://www.bluebee.com) to align reads against the human genome (GRCh38/hg38). After combining treatment replicate files, a DESeq2 application within Bluebee (Lexogen Quantseq DE1.4) was used to identify significant treatment-related effects on transcript abundance (relative to vehicle) based on a false discovery rate (FDR) p-adjusted value ≤0.05. To determine whether differentially expressed genes contained PPREs, we searched for PPRE consensus half-site sequences (AGGTCA) (Chawla et al., 2001) up to 5000 bases upstream of the transcription start site (TSS) using the sequence text view tool within NCBI (https://www.ncbi.nlm.nih.gov).
2.9. Statistical analyses
For cell viability, ORO staining, and immunohistochemistry data, a general linear model (GLM) analysis of variance (ANOVA) (α = 0.05) was performed using SPSS Statistics 27 (IBM, Armonk, NY, USA), as data did not meet the equal variance assumption for non-GLM ANOVAs. Treatment groups were compared with vehicle controls using pair-wise Tukey based multiple comparisons of least square means to identify significant treatment-specific differences.
3. Results
3.1. Exposure to TBBPA and TCBPA alone or as binary mixtures decreases cell viability in the absence of effects on neutral lipid and FASN levels
Relative to cells exposed to vehicle (0.1% DMSO) for 24 h, cells exposed to 100 µM TBBPA for 24 h resulted in a significant decrease in cell viability (Fig. 1A), whereas exposure to concentrations of TBBPA and TCBPA between 40 and 100 µM resulted in a slight (albeit non-significant) increase in neutral lipid staining (Fig. 1B) and decrease in FASN protein levels (Fig. 1C). Following exposure to binary mixtures of TBBPA and TCBPA, cell viability decreased in a concentration-dependent manner (based on the total sum concentration of TBBPA and TCBPA), with significant decreases in cell viability occurring at 90 µM and higher (Fig. 2A). However, effects on cell viability occurred in the absence of consistent, concentration-dependent effects on neutral lipid (Fig. 2B) and FASN levels (Fig. 2C). Since neutral lipid staining has previously been used as a readout for PPARγ activation (Wakabayashi et al., 2009), ORO was used in subsequent experiments. However, since FASN is not a downstream PPARγ-activated protein, FASN was not used as an endpoint for subsequent experiments.
3.2. Exposure to TBBPA or TCBPA alone or as a mixture enhances the effects of reference PPARγ ligands on cell viability
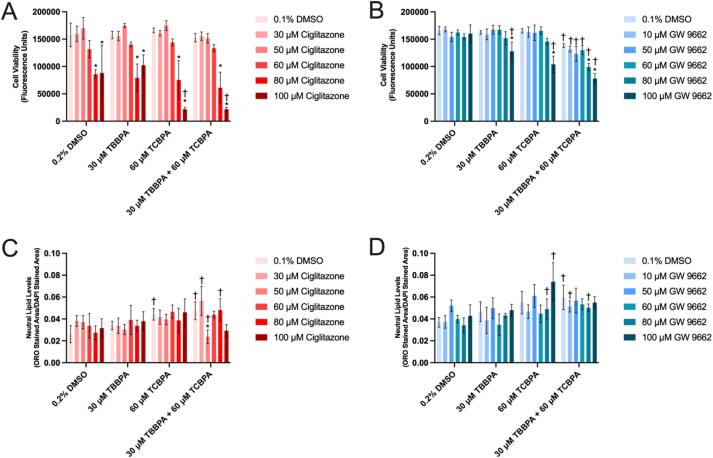
Pretreatment with ciglitazone alone at 80 µM or higher resulted in a significant decrease in cell viability within all treatment groups (Fig. 3A). Interestingly, pretreatment with increasing ciglitazone concentrations followed by exposure to 60 µM TCBPA or 30 µM TBBPA + 60 µM TCBPA resulted in a concentration-dependent decrease in cell viability (Fig. 3A), whereas this concentration-dependent effect was not observed following pretreatment with ciglitazone and exposure to 0.2% DMSO or 30 µM TBBPA alone. Following pretreatment with 100 µM ciglitazone, exposure to 60 µM TCBPA or 30 µM TBBPA + 60 µM TCBPA resulted in a significant decrease in cell viability relative to vehicle (0.2% DMSO) and 100 µM ciglitazone alone even though exposure to 60 µM TCBPA or 30 µM TBBPA + 60 µM TCBPA alone (i.e., in the absence of ciglitazone pretreatment) did not affect cell viability relative to vehicle-treated cells (Fig. 3A).
Fig. 3.
Mean (±standard deviation) fluorescence (cell viability) (A, B) or Oil Red O neutral lipid staining normalized to DAPI stained area (C, D) of HepG2 cells after 24 h pretreatment with ciglitazone or GW 9662 followed by exposure to vehicle (0.2% DMSO), 30 µM TBBPA, 60 µM TCBPA, or 30 µM TBBPA + 60 µM TCBPA for 24 h. Asterisk (*) denotes a significant PPARγ ligand-driven difference (p < 0.05) in cell viability or neutral lipid staining relative to vehicle (0.1% DMSO)-pretreated cells. Cross (†) denotes a significant TBBPA- and/or TCBPA-driven difference (p < 0.05) in cell viability or neutral lipid staining relative to vehicle (0.2% DMSO)-exposed cells pretreated with the same PPARγ ligand concentration.
Exposure to 30 µM TBBPA or 60 µM TCBPA resulted in a significant decrease in cell viability relative to vehicle (0.2% DMSO) and GW 9662 alone even though GW 9662 alone did not affect cell viability at all concentrations tested (Fig. 3B). Similar to ciglitazone, pretreatment with increasing GW 9662 concentrations followed by exposure to 30 µM TBBPA + 60 µM TCBPA resulted in a concentration-dependent decrease in cell viability (Fig. 3B), whereas this concentration-dependent effect was not as significant following pretreatment with GW 9662 and exposure to 0.2% DMSO, 30 µM TBBPA, or 60 µM TCBPA alone. However, pretreatment with ciglitazone or GW 9662 did not consistently enhance nor mitigate the effects on neutral lipid levels after TBBPA or TCBPA exposure (Fig. 3C and 3D).
3.3. Overexpression of PPARγ does not mitigate nor enhance the effects of TBBPA on cell viability, neutral lipid levels, nor the cellular transcriptome
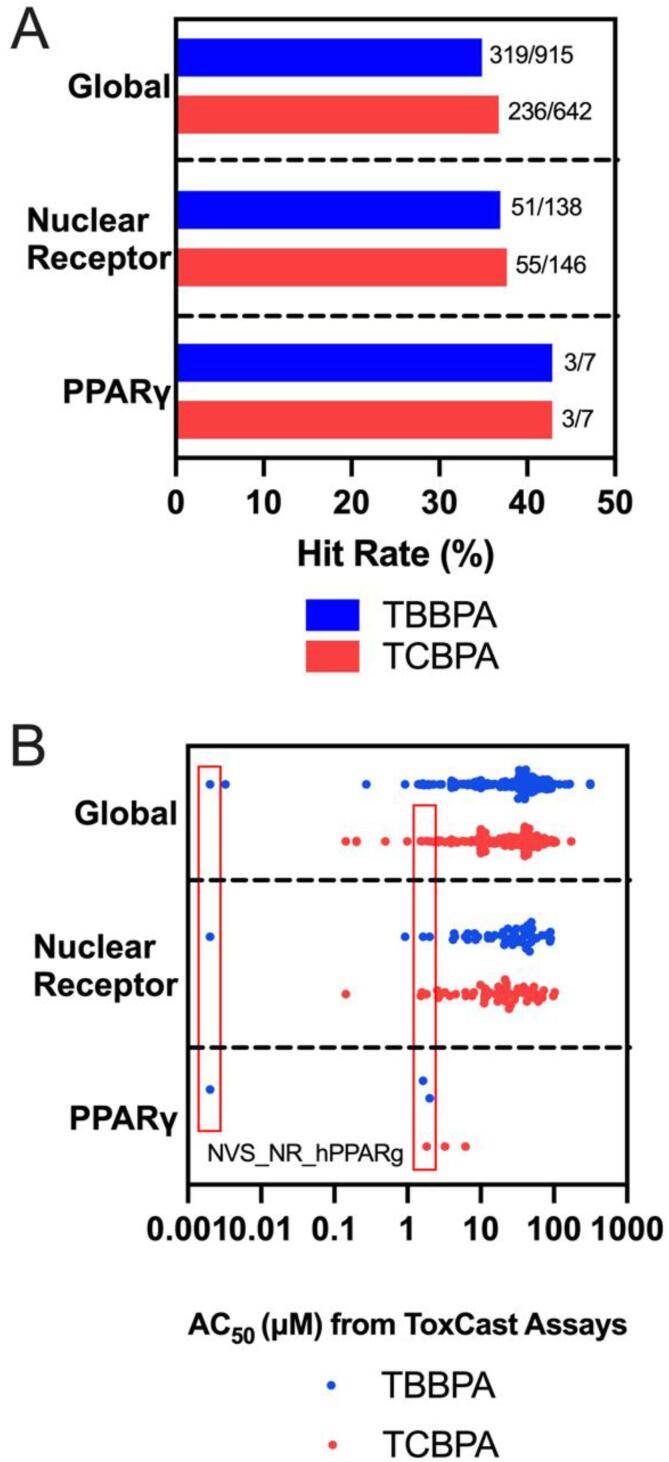
When comparing available ToxCast assays for TBBPA and TCBPA, both compounds had similar hit rates (active assay endpoint divided by total available assays) within the global, nuclear receptor, and PPARγ data sets (Fig. 4A). When comparing the range of AC50 values across active assays, TBBPA and TCBPA also had very similar distributions (Fig. 4B). Of all ToxCast assays, TBBPA was the most potent (AC50 = 0.002 µM) within a cell-free, human-specific PPARγ ligand binding assay (NVS_NR_hPPARg), whereas the AC50 for TCBPA was 1.83 µM based on the same assay. Therefore, as a ToxCast-based human-specific PPARγ ligand binding assay predicted TBBPA to be ∼900× more potent relative to TCBPA, we focused on the potential effects of PPARγ overexpression on TBBPA-induced toxicity.
Fig. 4.
Hit rate (A) and summary statistics (B) based on AC50 values for TBBPA and TCBPA screened within ToxCast. AC50 values based on a cell-free, human-specific PPARγ ligand binding assay (NVS_NR_hPPARg) are boxed in red for both TBBPA and TCBPA.
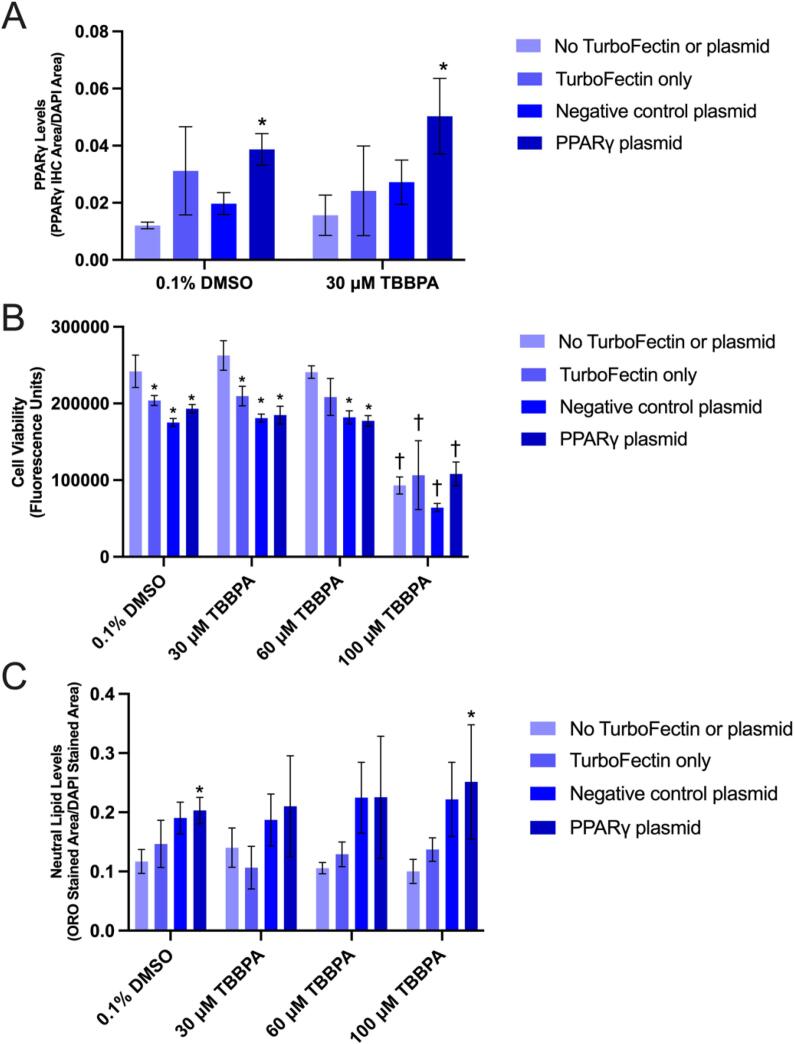
A human PPARγ-specific antibody was used to determine whether cells transfected with a PPARγ expression plasmid increased PPARγ protein in situ within HepG2 cells. Relative to cells transfected to NC plasmid, PPARγ levels were approximately doubled within PPARγ-transfected cells exposed to either vehicle (0.1% DMSO) or 30 µM TBBPA (Fig. 5A). While transfection with Turbofectin 8.0 alone decreased cell viability, the viability of cells transfected with either NC or PPARγ expression plasmid was not affected across all treatment groups. As expected, exposure to 100 µM TBBPA resulted in significant decrease in cell viability across all transfection groups (Fig. 5B). Interestingly, within the vehicle (0.1% DMSO) or 100 µM TBBPA treatment groups, cells transfected with PPARγ expression plasmid slightly increased neutral lipid levels relative to non-transfected cells; however, this effect was not observed following exposure to 30 µM nor 60 µM TBBPA (Fig. 5C).
Fig. 5.
Mean (±standard deviation) PPARγ immunofluorescence area divided by DAPI stained area (A), cell viability (fluorescence) (B), and Oil Red O neutral lipid staining divided by DAPI stained area (C) of HepG2 cells transfected with either no TurboFectin 8.0 or plasmid, TurboFectin 8.0 only, negative control plasmid, or PPARγ expression plasmid and then exposed to either vehicle (0.1% DMSO), 30, 60, or 100 µM TBBPA for 24 h. Asterisk (*) denotes a significant difference (p < 0.05) in cell viability or neutral lipid staining relative to no TurboFectin or plasmid-transfected cells. Cross (†) denotes a significant difference (p < 0.05) in cell viability or neutral lipid staining relative to vehicle-exposed cells.
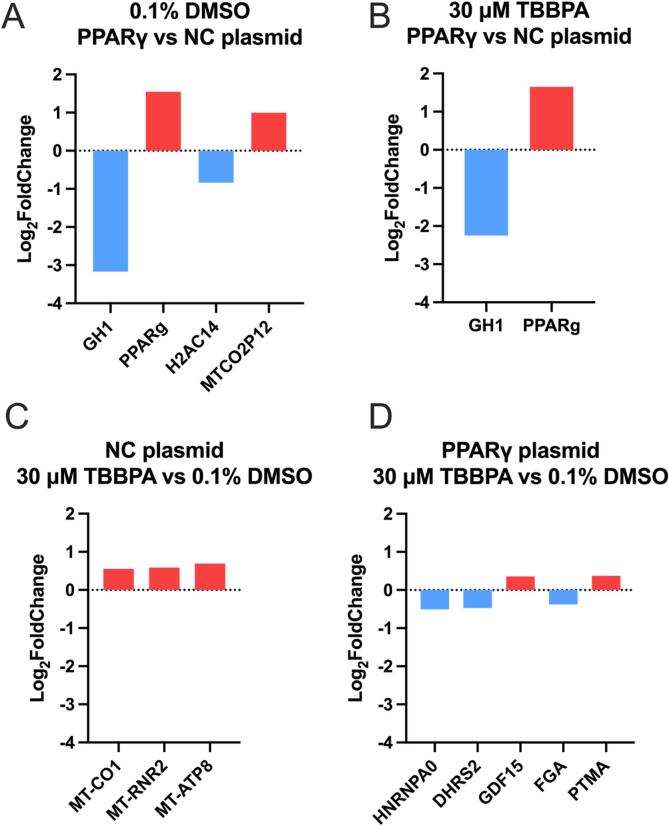
Relative to cells transfected with NC plasmid, exposure of cells transfected with PPARγ expression plasmid to vehicle (0.1% DMSO) or 30 µM TBBPA resulted in significant effects on the abundance of four and two transcripts, respectively. Interestingly, growth hormone 1 (GH1) and PPARγ were significantly decreased and increased, respectively, in both groups (Fig. 6A and B; Tables S2 and S3). Relative to vehicle-treated cells, exposure of cells transfected with NC plasmid to 30 µM TBBPA – a concentration that did not affect cell viability – resulted in a significant increase in the abundance of three transcripts (MT-CO1, MT-RNR2, and MT-ATP8), all of which were mitochondrially encoded genes (Fig. 6C; Table S4). Relative to vehicle-treated cells, exposure of cells transfected with PPARγ expression plasmid to 30 µM TBBPA resulted in significant effects on the abundance of five transcripts – HNRNPA0, DHRS2, GDF15, FGA, and PTMA (Fig. 6D; Table S5). Of the transcripts that were significantly affected across all four comparisons, there were 1–4 PPRE consensus half-site sequences within 5000 bases upstream of the respective TSS within corresponding genes, whereas mitochondrially encoded genes did not have any PPRE consensus half-site sequences within 5000 bases upstream of the respective TSS (Table 1).
Fig. 6.
Significantly affected transcripts for cells transfected with negative control (NC) plasmid transfected cells or PPARγ plasmid transfected cells and then exposed to vehicle (0.1% DMSO) or 30 µM TBBPA. Panels A and B are based on within-treatment group comparisons relative to NC plasmid, whereas Panels C and D are based on within-transfection group comparisons relative to vehicle-exposed cells.
Table 1.
Number of PPRE consensus half-site sequences (AGGTCA) within 5000 base pairs upstream of the transcription start site for differentially expressed genes.
| Gene Name | Gene Symbol | GO Annotations* | # of PPRE consensus half-site sequences |
|---|---|---|---|
| Mitochondrially encoded cytochrome c oxidase 1 | MT-CO1 | Iron ion binding; electron transfer activity | 0 |
| Mitochondrially encoded 16s rRNA | MT-RNR2 | Not available | 0 |
| Mitochondrially encoded ATP synthase membrane subunit 8 | MT-ATP8 | ATP hydrolysis activity; proton transmembrane transporter activity | 0 |
| Heterogeneous nuclear ribonucleoprotein A0 | HNRNPA0 | Nucleic acid binding; RNA binding | 4 |
| Dehydrogenase reductase 2 | DHRS2 | Oxidoreductase activity; carbonyl reductase activity | 4 |
| Growth differentiation factor 15 | GDF15 | Cytokine activity; transforming growth factor beta receptor binding | 3 |
| Fibrinogen alpha chain | FGA | Signaling receptor binding; protein-macromolecule adaptor activity | 1 |
| Prothymosin alpha | PTMA | Not available | 2 |
| Growth hormone 1 | GH1 | Growth factor activity; growth hormone receptor binding | 1 |
| Peroxisome Proliferator activated Receptor Gamma | PPARγ | DNA-binding transcription factor activity; chromatin binding | 2 |
| H2A clustered histone 14 | H2AC14 | Not available | 1 |
| MT-CO2 pseudogene 12 | MTCO2P12 | Not available | 0 |
*Gene Ontology (GO) annotations related to each gene were retrieved from the GeneCards Summary at https://www.genecards.org. If GO annotations were not provided, then GO annotations were listed as “Not available”.
4. Discussion
Within this study, we found that TBBPA and TCBPA (either alone or as binary mixtures) induce cytotoxicity within HepG2 cells at high µM (ppm) concentrations that are well above environmentally relevant human exposures (ng/g or ppb concentrations in house dust) (Wang et al., 2015). In part, this may be driven by the sensitivity of HepG2 cells (an immortalized cell line) to chemically-induced toxicity, as overexpression of PPARγ did not alter the toxicity of TBBPA within HepG2 cells. When cells were exposed to binary mixtures of TBBPA and TCBPA, cell viability decreased in a concentration-dependent manner based on the total sum concentration of TBBPA and TCBPA, suggesting that these effects were driven by simple additive toxicity. Similarly, cell-based studies using TBBPA have previously identified cytotoxic (LC50) concentrations ranging from 21 µM in mouse TM4 Sertoli cells (Ogunbayo et al., 2008) to 200 µM in Cal-62 human thyroid cells (Strack et al., 2007). Likewise, following a 24-h exposure, TBBPA and TCBPA are cytotoxic in mouse embryonic stem cells at 150 and 200 µM, respectively (Yin et al., 2018). Interestingly, based on pretreatment experiments with reference PPARγ ligands (ciglitazone and GW 9662), we found that TCBPA enhanced the cytotoxic effects of ciglitazone whereas exposure to GW 9662 and TBBPA or TCBPA resulted in synergistic toxicity (based on cell viability) relative to cells exposed to GW 9662, TBBPA or TCBPA alone. However, overexpression of PPARγ did not alter TBBPA-induced cytotoxicity, suggesting that the cytotoxic effects of halogenated BPA analogues within HepG2 cells may be PPARγ-independent and driven by other mechanisms such as oxidative stress.
To determine whether TBBPA or TCBPA exposure resulted in effects on lipid homeostasis, ORO neutral lipid staining was used to quantify potential changes in neutral lipid abundance. Neutral lipid staining by ORO has previously been linked to PPARγ activation and activity during 3T3-L1 adipocyte differentiation (Wakabayashi et al., 2009). While reliable and reproducible within adipocytes that express higher levels of PPARγ relative to hepatocytes (Elbrecht et al., 1996), neutral lipid staining by ORO appears to lack sensitivity in HepG2 cells, as PPARγ reference ligands (ciglitazone and GW 9662) only alter neutral lipid abundance at higher concentrations (>100 µM) (Cheng et al., 2021). As a result, pretreatment with ciglitazone and GW 9662 did not enhance nor mitigate TBBPA- or TCBPA-induced effects on neutral lipid abundance. However, we found that overexpression of PPARγ within HepG2 cells increased neutral lipid staining, suggesting that PPARγ transfection in combination with ORO staining may enable HepG2 cells to be a more sensitive model system for PPARγ activation using neutral lipid abundance as a readout.
While FASN activity has been shown to be strongly correlated to PPARγ mRNA levels in adipocytes (Schmid et al., 2005), exposure to TBBPA and TCBPA (either alone or as binary mixtures) did not increase FASN protein levels in a concentration-dependent manner within HepG2 cells. Additionally, TBBPA did not result in differential expression of the FASN transcript based on our mRNA-seq data, further confirming that FASN transcription was not affected by exposure to TBBPA and TCBPA. Since FASN inhibitors are able to reduce PPARγ mRNA levels (Schmid et al., 2005), this suggests that FASN activity is upstream of PPARγ and, as such, may explain the lack of response of TBBPA and TCBPA on FASN transcript and protein levels within HepG2 cells. If FASN activity is upstream of PPARγ, future studies should focus on whether pretreatment with FASN inhibitors decreases PPARγ mRNA levels and alters the toxicity of TBBPA and TCBPA within HepG2 cells.
While TBBPA has been shown in several ToxCast assays to activate PPARγ with relatively strong potency, we found no evidence of PPARγ activation and transcription of target genes following TBBPA exposure. Despite using a non-cytotoxic concentration of TBBPA (30 µM) to minimize possible off-target effects, we were unable to detect significant PPARγ-related transcriptional effects even after overexpression of PPARγ. Instead, we found that three mitochondrially encoded genes (MT-CO1, MT-RNR2, and MT-ATP8) – none of which have PPRE consensus half-site sequences within 5000 bases upstream of the TSS – were the only significantly altered transcripts within TBBPA- vs. vehicle-exposed cells transfected with the NC plasmid, suggesting that exposure to 30 µM TBBPA resulted in a mitochondrial-specific stress response (Zhao et al., 2002) in the absence of PPARγ-mediated transcription. These findings are consistent with previous studies that have shown that TBBPA exposure to L02 human hepatocytes increased reactive oxygen species, induced mitochondrial apoptosis, and altered transcripts related to oxidative stress within the Nrf2 pathway (Zhang et al., 2019). Within cells transfected with PPARγ expression plasmid, exposure to TBBPA resulted in significant effects on the abundance of five transcripts (HNRNPA0, DHRS2, GDF15, FGA, and PTMA), all of which have at least one PPRE consensus half-site sequence within 5000 bases upstream of the TSS. Of these transcripts, DHRS2 is part of the short-chain dehydrogenase reductase enzyme family involved in the metabolism of steroids, prostaglandins, lipids, and xenobiotics (Gabrielli et al., 1995). However, no studies have found any of these transcripts to be directly regulated by PPARγ.
5. Conclusions
Overall, our study found that, while TBBPA and TCBPA affected cell viability at a similar magnitude and in an additive manner, non-cytotoxic concentrations of TBBPA and TCBPA did not significantly affect FASN protein levels nor neutral lipid abundance in a PPARγ-dependent manner. Moreover, TBBPA and TCBPA enhanced the adverse effects of reference PPARγ ligands on cell viability, but non-cytotoxic concentrations of TBBPA and TCBPA did not consistently affect downstream neutral lipid abundance after reference PPARγ ligand pretreatment. Although ToxCast assays identified PPARγ as a target for TBBPA and previous studies have confirmed TBBPA binding within cell-free assays, we were unable to link TBBPA-induced effects on the transcriptome to PPARγ-dependent downstream effects even after overexpression of PPARγ. However, there is a possibility that TBBPA-induced effects may have been PPARγ-dependent since we did not knock down PPARγ (e.g., using siRNAs) within cells overexpressing PPARγ. Nevertheless, our findings suggest that halogenated BPA analogues such as TBBPA and TCBPA induce toxicity within HepG2 cells in a PPARγ-independent manner, and further studies are needed to 1) identify other targets or mechanisms of action for TBBPA and TCBPA within HepG2 cells and 2) determine whether TBBPA and TCBPA induce PPARγ-dependent toxicity with human cells (e.g., adipocytes) that express higher baseline levels of PPARγ.
Funding
This work was supported by a NRSA T32 Training Program [T32ES018827] to V.C, and a National Institutes of Health grant [R01ES027576] and USDA National Institute of Food and Agriculture Hatch Project [1009609] to D.C.V.
CRediT authorship contribution statement
Vanessa Cheng: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. David C. Volz: Conceptualization, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Thomas B. Knudsen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2022.100079.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akiyama E., Kakutani H., Nakao T., Motomura Y., Takano Y., Sorakubo R., Mizuno A., Aozasa O., Tachibana K., Doi T., Ohta S. Facilitation of adipocyte differentiation of 3T3-L1 cells by debrominated tetrabromobisphenol A compounds detected in Japanese breast milk. Environ. Res. 2015;140:157–164. doi: 10.1016/j.envres.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Cao H., Wang F., Liang Y., Wang H., Zhang A., Song M. Experimental and computational insights on the recognition mechanism between the estrogen receptor α with bisphenol compounds. Arch. Toxicol. 2017;91:3897–3912. doi: 10.1007/s00204-017-2011-0. [DOI] [PubMed] [Google Scholar]
- Chappell V.A., Janesick A., Blumberg B., Fenton S.E. Tetrabromobisphenol-A promotes early adipogenesis and lipogenesis in 3T3-L1 cells. Toxicol. Sci. 2018;166:332–344. doi: 10.1093/toxsci/kfy209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A., Boisvert W.A., Lee C.-H., Laffitte B.A., Barak Y., Joseph S.B., Liao D., Nagy L., Edwards P.A., Curtiss L.K., Evans R.M., Tontonoz P. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–171. doi: 10.1016/S1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Cheng V., Reddam A., Bhatia A., Hur M., Kirkwood J.S., Volz D.C. Utilizing systems biology to reveal cellular responses to peroxisome proliferator-activated receptor γ ligand exposure. Curr. Res. Toxicol. 2021;2:169–178. doi: 10.1016/j.crtox.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirtu A.C., Roosens L., Geens T., Gheorghe A., Neels H., Covaci A. Simultaneous determination of bisphenol A, triclosan, and tetrabromobisphenol A in human serum using solid-phase extraction and gas chromatography-electron capture negative-ionization mass spectrometry. Anal. Bioanal. Chem. 2008;391:1175–1181. doi: 10.1007/s00216-007-1807-9. [DOI] [PubMed] [Google Scholar]
- Elbrecht A., Chen Y., Cullinan C.A., Hayes N., Leibowitz M.D., Moller D.E., Berger J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors γ1 and γ2. Biochem. Biophys. Res. Commun. 1996;224:431–437. doi: 10.1006/bbrc.1996.1044. [DOI] [PubMed] [Google Scholar]
- Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B.M., Evans R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Gabrielli F., Donadel G., Bensi G., Heguy A., Melli M. A nuclear protein, synthesized in growth-arrested human hepatoblastoma cells, is a novel member of the short-chain alcohol dehydrogenase family. Eur. J. Biochem. 1995;232:473–477. doi: 10.1111/j.1432-1033.1995.473zz.x. [DOI] [PubMed] [Google Scholar]
- Gould J.C., Leonard L.S., Maness S.C., Wagner B.L., Conner K., Zacharewski T., Safe S., McDonnell D.P., Gaido K.W. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol. Cell. Endocrinol. 1998;142:203–214. doi: 10.1016/S0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Ho K.-L., Yuen K.-K., Yau M.-S., Murphy M.B., Wan Y., Fong B.-M.-W., Tam S., Giesy J.P., Leung K.-S.-Y., Lam M.-H.-W. Glucuronide and sulfate conjugates of tetrabromobisphenol A (TBBPA): Chemical synthesis and correlation between their urinary levels and plasma TBBPA content in voluntary human donors. Environ. Int. 2017;98:46–53. doi: 10.1016/j.envint.2016.09.018. [DOI] [PubMed] [Google Scholar]
- Hurst C.H., Waxman D.J. Activation of PPARα and PPARγ by Environmental Phthalate Monoesters. Toxicol. Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jakobsson K., Thuresson K., Rylander L., Sjödin A., Hagmar L., Bergman Å. Exposure to polybrominated diphenyl ethers and tetrabromobisphenol A among computer technicians. Chemosphere. 2002;46:709–716. doi: 10.1016/S0045-6535(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Kliewer S.A., Lenhard J.M., Willson T.M., Patel I., Morris D.C., Lehmann J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Kim T.S., Kim C.Y., Kang I.H., Kim M.G., Kyung Jung K., Kim H.S., Han S.Y., Yoon H.J., Rhee G.S. Evaluation of in vitro screening system for estrogenicity: comparison of stably transfected human estrogen receptor-α transcriptional activation (OECD TG455) assay and estrogen receptor (ER) binding assay. J. Toxicol. Sci. 2012;37:431–437. doi: 10.2131/jts.37.431. [DOI] [PubMed] [Google Scholar]
- Lehmann J.M., Moore L.B., Smith-Oliver T.A., Wilkison W.O., Willson T.M., Kliewer S.A. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Leisewitz, A., Kruse, H., Schramm, E. 2001. Substituting environmentally relevant flame retardants: Assessment fundamentals. Federal Environmental Agency Umweltbundesamt, Berlin Postfach 33 00 22 14191.
- Lewis J.D., Ferrara A., Peng T., Hedderson M., Bilker W.B., Quesenberry C.P., Vaughn D.J., Nessel L., Selby J., Strom B.L. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Qu K., Hai Y., Zhao C. Bisphenol A (BPA) binding on full-length architectures of estrogen receptor. J. Cell. Biochem. 2018;119:6784–6794. doi: 10.1002/jcb.26872. [DOI] [PubMed] [Google Scholar]
- Martin G., Schoonjans K., Staels B., Auwerx J. PPARγ activators improve glucose homeostasis by stimulating fatty acid uptake in the adipocytes. Atherosclerosis. 1998;137:S75–S80. doi: 10.1016/S0021-9150(97)00315-8. [DOI] [PubMed] [Google Scholar]
- Meex S.J.R., Andreo U., Sparks J.D., Fisher E.A. Huh-7 or HepG2 cells: which is the better model for studying human apolipoprotein-B100 assembly and secretion? J. Lipid Res. 2011;52:152–158. doi: 10.1194/jlr.D008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama J., Tsuji H., Takasuga T. Comparison between brominated flame retardants and dioxins or organochlorine compounds in blood levels of Japanese adults. Organohalogen Compd. 2000;48:27–30. [Google Scholar]
- Nagy L., Tontonoz P., Alvarez J.G., Chen H., Evans R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- Nesto R.W., David B., Bonow R.O., Vivian F., Grundy S.M., Horton E.S., Martin L.W., Daniel P., Semenkovich C.F., Sidney S., Young L.H., Richard K. Thiazolidinedione use, fluid retention, and congestive heart failure. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Nolan J.J., Ludvik B., Beerdsen P., Joyce M., Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N. Engl. J. Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- Ogunbayo O.A., Lai P.F., Connolly T.J., Michelangeli F. Tetrabromobisphenol A (TBBPA), induces cell death in TM4 Sertoli cells by modulating Ca2+ transport proteins and causing dysregulation of Ca2+ homeostasis. Toxicol. In Vitro. 2008;22:943–952. doi: 10.1016/j.tiv.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Riu A., Grimaldi M., le Maire A., Bey G., Phillips K., Boulahtouf A., Perdu E., Zalko D., Bourguet W., Balaguer P. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environ. Health Perspect. 2011;119:1227–1232. doi: 10.1289/ehp.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer U.M.D., Völkel W., Dekant W. Toxicokinetics of tetrabromobisphenol A in humans and rats after oral administration. Toxicol. Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- Schmid B., Rippmann J.F., Tadayyon M., Hamilton B.S. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem. Biophys. Res. Commun. 2005;328:1073–1082. doi: 10.1016/j.bbrc.2005.01.067. [DOI] [PubMed] [Google Scholar]
- Shi Z., Jiao Y., Hu Y., Sun Z., Zhou X., Feng J., Li J., Wu Y. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ. 2013;452–453:10–18. doi: 10.1016/j.scitotenv.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Strack, S., Detzel, T., Wahl, M., Kuch, B., Krug, H.F., 2007. Cytotoxicity of TBBPA and effects on proliferation, cell cycle and MAPK pathways in mammalian cells. Chemosphere, Halogenated Persistent Organic Pollutants Dioxin 2004 67, S405–S411. https://doi.org/10.1016/j.chemosphere.2006.05.136. [DOI] [PubMed]
- Thomsen C., Lundanes E., Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J. Environ. Monit. 2001;3:366–370. doi: 10.1039/B104304H. [DOI] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Okamura M., Tsutsumi S., Nishikawa N.S., Tanaka T., Sakakibara I., Kitakami J., Ihara S., Hashimoto Y., Hamakubo T., Kodama T., Aburatani H., Sakai J. The peroxisome proliferator-activated receptor γ/retinoid X receptor α heterodimer targets the histone modification enzyme PR-Set7/Setd8 Gene and regulates adipogenesis through a positive feedback loop. Mol. Cell. Biol. 2009;29:3544–3555. doi: 10.1128/MCB.01856-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Abualnaja K.O., Asimakopoulos A.G., Covaci A., Gevao B., Johnson-Restrepo B., Kumosani T.A., Malarvannan G., Minh T.B., Moon H.-B., Nakata H., Sinha R.K., Kannan K. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015;83:183–191. doi: 10.1016/j.envint.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Wang Y., Kwon G., An L., Holmes C.N., Haeba M., LeBlanc G.A. Differential interactions of the flame retardant triphenyl phosphate within the PPAR signaling network. MOJ Toxicol. 2016;2 doi: 10.15406/mojt.2016.02.00039. [DOI] [Google Scholar]
- Wilkening S., Stahl F., Bader A. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab. Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- Wojtowicz A.K., Szychowski K.A., Kajta M. PPAR-γ agonist GW1929 but not antagonist GW9662 reduces TBBPA-induced neurotoxicity in primary neocortical cells. Neurotox. Res. 2014;25:311–322. doi: 10.1007/s12640-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin N., Liang S., Liang S., Yang R., Hu B., Qin Z., Liu A., Faiola F. TBBPA and its alternatives disturb the early stages of neural development by interfering with the NOTCH and WNT pathways. Environ. Sci. Technol. 2018;52:5459–5468. doi: 10.1021/acs.est.8b00414. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang X., Chen C., An J., Shang Y., Li H., Xia H., Yu J., Wang C., Liu Y., Guo S. Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere. 2019;226:463–471. doi: 10.1016/j.chemosphere.2019.03.167. [DOI] [PubMed] [Google Scholar]
- Zhao J., Sun X.-B., Ye F., Tian W.-X. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol. Cell. Biochem. 2011;351:19–28. doi: 10.1007/s11010-010-0707-z. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Wang J., Levichkin I.V., Stasinopoulos S., Ryan M.T., Hoogenraad N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.