Abstract
Receptors are important pharmacological targets on cells. The Triggering Receptor Expressed on Myeloid Cells (TREM) – Like Transcript – 1 is an abundant, yet little understood, platelet receptor. It is a single Ig domain containing receptor isolated in the α-granules of resting platelets and brought to the platelet surface upon activation. On platelets, the integrin αIIbβ3 is the major receptor having roughly 80,000 copies. αIIbβ3 is a heterodimeric multidomain structure that mediates platelet aggregation through its interaction with the plasma protein fibrinogen. Anti-platelet drugs have successfully targeted αIIbβ3 to control thrombosis. Like αIIbβ3, TLT-1 also binds fibrinogen, making its role in platelet function somewhat obscure. In this review, we highlight the known structural features of TLT-1 and present the challenges of understanding TLT-1 function. In our analysis of the dynamics of the platelet surface after activation we propose a model in which TLT-1 supports αIIbβ3 function as a mechanoreceptor that may direct platelets toward immune function.
Keywords: Blood platelets, fibrinogen, lungs, neutrophils, platelet receptors, trem-Like transcript-1 (TLT-1), αIIbβ3
Introduction
Receptors are important targets for the pharmacological manipulation of cellular functions and disease. Even though platelets are considered, by many, as simple cells because of their lack of a nucleus, their functions are very complex. Platelets probe our vasculature, identify breaches, and restore vessel integrity [1,2]. In recent years, the role of platelets beyond hemostasis has taken center stage, especially in the field of innate immunology, where they have been shown to be key determinates in disease severity and outcomes [1,3]. As such, understanding the pathways that are regulated by platelet receptors is paramount, if we are to be able to manipulate the diseases in which their function is key.
In this review we will focus on the enigmatic platelet receptor Triggering Receptor Expressed in Myeloid cells (TREM) – Like Transcript −1 or TLT-1. TLT-1 is a paradigmatic type-1 single immunoglobulin domain membrane protein receptor, similar in structure to other immunoglobulin-like variable domains, particularly those of triggering receptor expressed on myeloid cells-1 (TREM-1), the polymeric immunoglobulin receptor (plgR), and the natural killer cell-activating receptor of 44 kD (NKp44) [4–6]. TLT-1 was identified in a screen of the Celera database using the sequence of NKp46 as a probe. It was surprising when TLT-1 was identified as an abundant receptor unique to platelets [5,7]. Given its abundance on activated platelets, its localization in the platelet α-granules of resting platelets explains how it had remained undiscovered for so long; however now that it has been identified, TLT-1 holds its secrets tightly guarded.
Here, we will look at the structural aspects of TLT-1 in relation to the functional and pathophysiological evidence that has been presented. From this we hope to derive a clearer picture of the niche that TLT-1 fills in platelet function and present a road map to uncovering the secrets to platelet function that it guards.
The TREM-Like Transcript-1 Structure
TLT-1 lies within the TREM gene cluster [6,8]. Cloning and expression studies have defined TLT-1 as a membrane bound protein consisting of 311 amino acids and a mass of 32,679 Da [4,5]. Topologically it is characterized by an extracellular domain (147 amino acids in length), a transmembrane domain (21 amino acids in length) and a cytoplasmic domain (127 amino acids in length) that is found exclusively in platelets and megakaryocytes (MKs) [9].
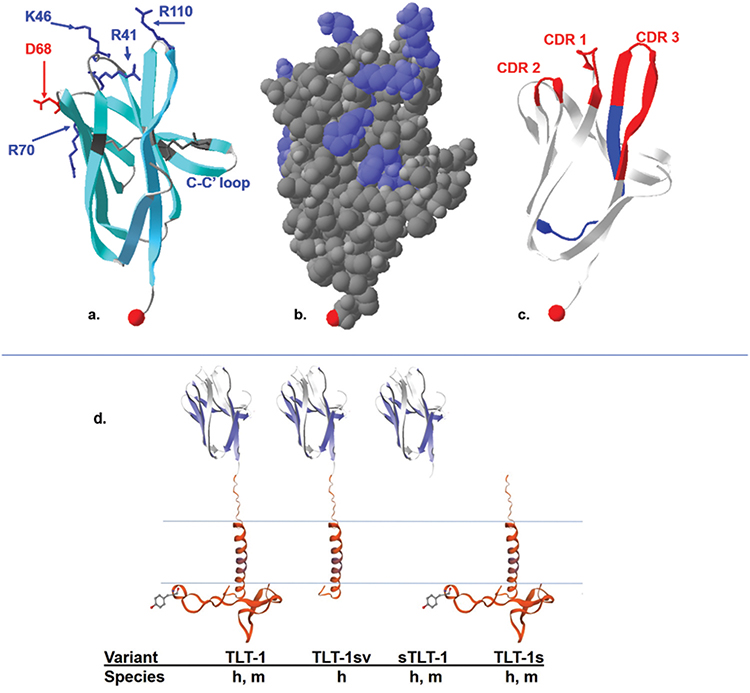
The immunoglobulin-like domain of hTLT-1 is typical of V-type immunoglobulin like folds. It consists of 105 amino acids forming 9 beta strands and it has conserved CDR loop regions (CDR1, CDR2 and CDR3) [4]. Several charged patches run across the molecular electrostatic surface of hTLT-1 (Figure 1b) [4]. The hypervariable CDR regions are of particular interest because their tips contain an abundance of lysine and arginine residues, thus determining a predominantly positive charge in these regions. Some interesting structural features to highlight these CDR loops are the R41 & K46 extending from the center of CDR1, the D68 containing negative path of CDR2 proceeding the positive charge from R70, R110 extending from CDR3, and the short 4 residue loop of CRD3 containing hydrogen bonds that are not characteristic of B-strands (Figure 1a &). CRD3 is the most variable loop, giving the greatest conformational flexibility in comparison to similar ligands with immunoglobulin like domains such as TREM-1, and NKp44 [10,11]. On the other hand, like NKp44 and plgR, hTLT-1 has two disulfide bonds connecting the β-strands:C to C’, and B to F [12]. The disulfide bond between C38-C104 is conserved in human and murine TREM-1, NKp44 and plgR. The second disulfide bond between C52 and C59 in strand BC is conserved in NKp44 and plgR but not TREM-1(Figure 1) [4,11]. The immunoglobulin-like domain is attached to the membrane spanning helix by a 37-amino acid stalk containing a negatively charge patch of 5 consecutive glutamic acids.
Figure 1.
The crystal structure of the TLT-1 extracellular domain (aa 20–125). a: ribbon model highlighting the negative residues in red, positive residues in blue, the two cysteine bonds characteristic of an Ig fold in dark gray. One of the disulfide bonds is from C38–C104, the second disulfide bond, connects C52 located in strand βC to C59 in strand βC’ (C-C’ loop). The C terminal region is represented by the red space filled proline. b: Space filled model showing the positively charged surface molecules (blue) over a relatively electrostatically neutral surface gray, the C-terminal region is represented by the light red space filed proline. c: The ribbon structure showing the CDR loops 1, 2, and 3 in red and the regions representing the LR12 peptide in blue. The C terminal region is represented by the red space filled proline. d: A schematic representation of the published TLT-1 isoforms with names listed below and designating that the TLT-1-sv is only found in humans (h); compared to the other forms that have been identified in mice(m) and humans. Figure was generated with the Swiss-PdbViewer 4.1.0, using data generated in DOI: 10.2210/pdb2FRG/pdb.
The putative transmembrane segment of hTLT-1 is 20 amino acids long and lacks the positively charged amino acid that other TREM family members use to interact with a negatively charged side chain of the adaptor molecule DNAX activation protein (DAP) 12 [5,13,14]. However, TLT-1 has been shown to be palmitoylated on amino acid cysteine 196 [15], meaning that it should be found in lipid rafts with αIIbβ3 and may influence αIIbβ3 function directly.
TLT-1 conveys its versatility in the four isoforms that have been isolated to date [16]. In platelets, three isoforms have been reported: TLT-1 full length (TLT-1), TLT-1 splice variant (TLT-1 sv); the soluble isoform that is released upon platelet activation (sTLT-1), and a form that contains a very short extracellular domain (TLT-1s; see Figure 1d) [4,7,17]. TLT-1 sv has not been identified in mice and the specific function of this form is yet to be determined. The soluble isoform corresponds to the extracellular sequence of the protein with a distinct C-terminus containing an additional 2 cysteines that lead to sTLT-1 multimerization [18]. The fourth isoform (TLT-1s) has only been identified in preosteoclasts [17]. TLT-1s has an alternative translational start sight and contains a very short extracellular domain consisting of the 37 amino acid stalk region, the transmembrane and the complete intracellular domain [17]. The full-length form of TLT-1 (37 kDa) and isoform TLT-1 sv (25k Da) both contain a transmembrane domain (TMD) but the TLT-1 full length form harbors an immunoreceptor tyrosine-based inhibition motif (ITIM). The cleaved form of TLT-1 which is also called soluble TLT-1 (sTLT-1;17 kDa), is distinct from the soluble splice variant [5,6]. The exact cleavage site of cleaved form is not known but it lacks the extra cysteines allowing multimerization like the spliced soluble form. The full-length form (311 amino acids) has only been found on platelets and megakaryocytes to date.
The Soluble Fragment
The soluble fragment of the TLT-1 extracellular domain (sTLT-1) is found in serum of humans and mice [4]. In humans there is a splice variant that multimerizes. However, based on sequencing reads from 16 volunteers, only represents an average 5% of the available TLT-1 transcript in platelets [19]. The majority of soluble fragment found in human serum, however, is believed to be derived from proteolytic cleavage of either the full length or TLT-1 sv from the platelet surface [19]. In mice, sTLT-1 seems to be cleaved whole from the surface, releasing a single 25-kDa fragment that is not further proteolyzed. Soluble TLT-1 corresponds roughly to 100 and 110 amino acids of the extracellular domain, or approximately the complete immunoglobulin-like domain (105 residues total, 20–125) [4]. The recombinant form has been demonstrated to enhance platelet aggregation in a dose-dependent manner [18]. Although the smaller forms of sTLT-1 may result from alternative transcription, release of recombinant human TLT-1 extracellular domain from HEK293 cells strongly suggests that the stalk region of TLT-1 is susceptible to hydrolysis [16]. Interestingly, sTLT-1 is the platelet’s fourth most abundantly released molecule upon platelet activation [20] and has been detected in the serum but not in plasma of healthy mice and humans [18]. TLT-1 has been demonstrated to be a better marker of platelet activation than P-selectin in mice [21], while sTLT-1 has been shown to be a biomarker for disease severity in acute respiratory distress syndrome (ARDS) in humans [19].
The TLT-1 Ligand
Key to understanding the function of any receptor is the identification of its ligand. In our early studies we proposed fibrinogen as a ligand of TLT-1 and suggested that during platelet aggregation, TLT-1 cross-links extracellular fibrinogen, stabilizing higher-order platelet aggregates [18]. In this study, lysates generated from purified human platelets were applied to AminoLink columns preloaded with either sTLT-1 or sTREM-1. This approach revealed specific binding of 3 proteins with molecular masses between 50 and 80 kDa on a Coomassie stained gel. Mass spectroscopy identified these proteins as the α, β, and γ chains of fibrinogen. To confirm these findings, fibrinogen was also eluted from His-tagged TLT-1 but not TREM-1 bound to nickel columns and resolved by PAGE in either native or reduced conditions. Consistent with disulfide-linked multimers of fibrinogen, TLT-1 column specifically bound a high molecular weight complex that when reduced resolved into the same 3 bands detected with AminoLink columns. Immunoblotting with anti-fibrinogen confirmed the identity of these TLT-1–interacting proteins as fibrinogen. This was further supported by ELISA, confirming fibrinogen as a TLT-1 ligand. While the identification of fibrinogen as a ligand was expected to bring answers, it only generated larger questions.
TLT-1/αIIbβ3/Fibrinogen
There is broad agreement that platelet aggregation is generally dependent on fibrinogen binding to the glycoprotein (GP) alpha 2b beta 3a integrin (αIIbβ3) receptor expressed on the platelet surface (Figure 2) [22]. Mutations, in αIIbβ3 effectively reduce platelet aggregation and individuals lacking functional αIIbβ3 are afflicted with a disease called Glanzmann’s thrombasthenia [23], characterized by a severe bleeding disorder. Mice lacking either the αIIb or the β3 subunits of the integrin display a similar bleeding diathesis as patients with Glanzmann’s thrombasthenia [24,25]. Accordingly, pharmacological agents, such as Integrilin (Eptifibatide acetate) [26,27] and Abciximab [28], directed toward the αIIbβ3/fibrinogen interaction site are effective at blocking thrombosis, thereby substantiating the importance of αIIbβ3 to platelet/platelet interaction.
Figure 2.
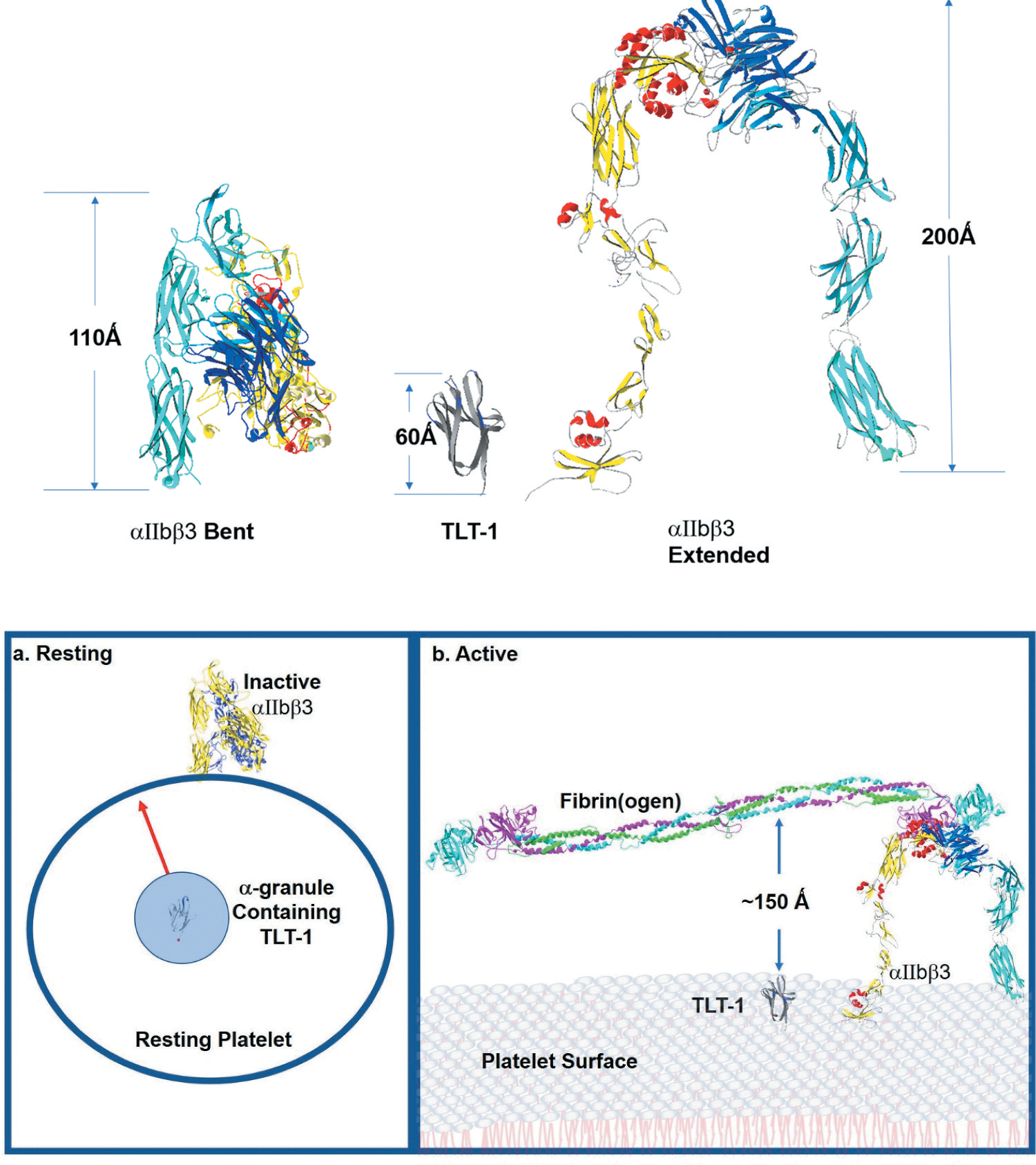
The platelet membrane landscape changes after platelet activation. Top - αIIbβ3 is the major platelet receptor. αIIbβ3 is a heterodimeric integrin receptor that binds fibrinogen. The α-subunit has 1039 amino acids and the β-subunit has 788 amino acids. On the resting platelets current models suggest that αIIbβ3 maintains an inactive bent (left) confirmation and upon platelet activation it changes from the bent form reaching to 110 Ǻ above the platelet surface to the extended form which towers to 200 Ǻ above the platelet surface (right). TLT-1 (middle) is estimated to reach between 50 and 60 Ǻ over the platelet surface. Bottom -. a: resting shows the relationship of TLT-1 to αIIbβ3 in resting platelets. The greater part of αIIbβ3 is found in the bent formation on the surface of resting platelets. There is some αIIbβ3 stored in the granules (not shown for clarity). TLT-1 on the other hand is sequestered in the α-granules and brought to the surface upon activation. On the resting platelet, neither receptor normally interacts with fibrinogen. Active(b) shows the surface of activated platelets. αIIbβ3 interacting with fibrin on the surface of a platelet showing TLT-1 for size comparison. Once on the platelet surface TLT-1 seems dwarfed in comparison to αIIbβ3. αIIbβ3 stands approximately 200 Ǻ over the platelet surface where TLT-1 is between 50–60 Ǻ including the stalk (not shown). Figure was generated with the Swiss-PdbViewer 4.1.0, using data published in DOI: 10.2210/pdb2FRG/pdb, DOI: 10.2210/pdb3FCS/pdb, DOI: 10.2210/pdb3GHG/pdb, and DOI: 10.2210/pdb4G1M/pdb.
However, just like αIIbβ3, TLT-1 binds fibrinogen [18,19], and herein lies the challenge.
On resting platelets, αIIbβ3 assumes an inactive conformation with greatly reduced ability to bind fibrinogen [29]. TLT-1, on the other hand is stored in the α-granules, sequestered from fibrinogen [9] (Figure 2 bottom). As such, neither receptor has the predilection to bind fibrinogen.
Activation, however, changes the platelet landscape and presents a seemingly inhospitable environment for TLT-1/fibrinogen interactions (Figure 2 bottom). αIIbβ3 is the most abundant integrin on the platelet surface with approximately 80,000 complexes on the surface of each platelet [30]. Activation changes αIIbβ3 from the low affinity bent conformation to the high affinity extended conformation (Figure 2) [29]. It is well established that αIIbβ3 is able to bind both fibrinogen and fibrin, and monomeric fibrin displays a higher probability of interacting with αIIbβ3 and a greater binding strength (kD) than fibrinogen (<1 nM vs 70 – 100 nM) [31,32]. At present, the binding specificity of TLT-1 to fibrin vs fibrinogen have not been determined. Measurement of fibrinogen deposition after LPS challenge in the lungs of mice suggest that TLT-1 may be more specific to fibrinogen than fibrin because there is reduced fibrinogen deposition in the TLT-1 null (treml1−/−) when compared to the wild type [19]. However, clearer insights will come once the exact molecular interactions between the two molecules are known.
Platelet activation also brings TLT-1 to the platelet surface (Figure 2 bottom) [9]. Strength of activation seems to determine the amount of surface expressed TLT-1. Activation with 5 μM ADP yielded an average of ~50,000 copies of TLT-1 on the platelet surface. Using plate-bound fibrinogen, sTLT-1 interacts with fibrinogen with a kD of 50 nM [33]. The size difference between αIIbβ3 and TLT-1 may also be a factor. The height difference between extracellular domains of αIIbβ3 and TLT-1 is ~3:1. The extracellular domain of TLT-1 is estimated to be about 50–60 angstroms compared to αIIbβ3 which towers approximately 200 angstroms over the platelet surface [34] (Figure 2 top). If you can envision the singer Shakira (1.57 M height) pitted against Michael Jordan (1.98 M height) in a basketball game; would Shakira ever see the ball? Similarly, the question arises, “at what point does TLT-1 interact with fibrinogen.
The in vitro and in vivo studies of TLT-1 further support the complexity of this scenario. To date there are no known TLT-1 bleeding disorders. While antibodies to TLT-1 can be used to inhibit platelet aggregation in vitro [35,36], there is a very narrow range of activator concentrations in which antibodies are effective. Moreover, the treml1−/− mouse does not display an overt bleeding phenotype. We have demonstrated reduced aggregation with ADP or collagen [18] and reduced clotting in the pulmonary embolism model [37] and inhibited platelet aggregation using anti - TLT-1 antibodies [35,38]. Taken together, these studies demonstrate the subtlety of TLT-1 role in classical hemostasis.
All things considered, it is difficult to understand why there are two high-density fibrinogen receptors on platelets and even more difficult to delineate what the contribution of each one has to platelet function. When do they overlap and co-regulate platelet function, and when is there strict regulation of fibrinogen binding by one or the other?
In 2015 it was demonstrated that GpVI, the major signaling molecule on platelets for collagen, binds fibrin and stimulates platelet activation pathways [39]. Interestingly, GpVI couples to the ITAM containing FcRγ and early models of TLT-1 function suggested that the ITIM of TLT-1 may regulate GpVI/FcRγ ITAM signaling. However, this model was soon abandoned once TLT-1 was shown contribute to activation pathways [7]. The fact that TLT-1 and GpVI bind fibrinogen and physiologically that they both have a demonstrated role in immune-derived bleeding [18,40,41] opens the possibility that these two fibrinogen binding receptors have either additive or synergistic affects during the inflammatory response. Fibrinogen has binding sites for the integrin MAC-1 which mediates transmigration of leukocytes such as neutrophils and mutation of this binding site impairs bacterial clearance [42,43]. In a murine model, the presence of TLT-1 on platelets is associated with increased fibrinogen deposition [19] and the absence of TLT-1 delays neutrophil transmigration. Interestingly, αIIbβ3, which is critical for platelet aggregation, is not considered necessary for immune-derived bleeding [44]. Taken together a model begins to develop where platelets coordinate neutrophil transmigration and regulation of immune derived bleeding using GpVI, fibrinogen, and TLT-1 independently of αIIbβ3.
Insights to role for TLT-1 in hemostasis came from studies using the reverse Arthus reaction. In the reverse Arthus reaction, immune complexes develop sub dermally and lead to complement deposition and neutrophil infiltration. In normal mice it leaves a bruise, in thrombocytopenic mice it leads to overt bleeding. In these studies, by Goerge et al [44] the β3 mice did not bleed, suggesting that the αIIbβ3/fibrinogen interaction was not important for hemostasis derived from immune triggers. Treml1−/− mice show a distinct petechia when compared to their wild type counterparts [37] suggesting that the TLT-1/fibrinogen interaction may regulate immune functions of platelets.
Pathophysiological Role of TLT-1
In sepsis and more recent ARDS studies, we have demonstrated a role for TLT-1 in the regulation of immune-mediated hemostasis. In studies using lipopolysaccharide (LPS) to induce sepsis in mice, TLT-1 null mice (treml1−/−) have a significantly greater rate of mortality than their wild-type counterparts. Studies using the Shwartzman reaction, which correlates with the severity of sepsis and mimics the mechanisms of disseminated intravascular coagulation (DIC) in a localized lesion, demonstrate that the treml1−mice bleed from the inflammatory insult, whereas the wild-type mice do not [18]. A longitudinal study allowed a comparison of plasma sTLT-1 with D-dimer levels in patients diagnosed with severe sepsis, interestingly, sTLT-1 levels correlated with the presence of DIC better than D-dimers, suggesting sTLT-1 as a new candidate biomarker to test for DIC [18]. These results implicate TLT-1 in the regulation of immune-derived hemostasis.
Over the last decade, a novel concept that platelets are critical regulators of the lung inflammatory processes has been emerging [45–47]. In a study of ARDS patients, Morales-Ortiz et. al demonstrated that plasma levels of sTLT-1 of ARDS patients were significantly elevated compared to those in a control group. In a subsequent study using a cohort of 799 ARDS patients, we demonstrated the utility of sTLT-1 as a specific platelet marker in the development of ARDS due to sepsis. It was found that very low baseline platelet counts (<80 000/μL) and elevated plasma sTLT-1 concentrations (>1200 pg/mL) are predictors of mortality and poor prognosis [19]. This work was further supported in a murine model where LPS was used to induce acute lung injury. In these studies, TLT-1 was shown to be important for controlling bleeding into the alveolar sacs [19]. Treml1−/− mice demonstrate substantial bleeding in the lungs after LPS treatment whereas their wild-type counterparts did not. It was demonstrated that in the treml1−/− mice, that there was a delay of neutrophil transmigration with a subsequent influx of three times the neutrophils into the alveoli compared to wild type mice. Consistent with a TLT-1 interaction with fibrinogen, treml1−/− mice displayed significantly reduced fibrinogen deposition and increased tissue damage in the lung compared to wile type mice. The addition of intravenous sTLT-1 in these studies increased the fibrinogen deposition and reduced tissue damage.
Neutrophil extravasation from the vessels is a common feature of the Arthus, sepsis, and acute lung injury models and suggests that TLT-1 may directly affect neutrophil function. Studies by Derive et al [48] demonstrate that TLT-1 plays a crucial role in regulating leukocyte activation and modulating sepsis-induced acute inflammatory response. A 17-amino acid sequence (LR17 – 94-LQEEDAGEYGCMVDGAR-110) of the TLT-1 extracellular domain that has great structural similarity but only 35% homology with the TREM family member TREM-1 was identified. These studies demonstrate that LR17 mediates a protective effect in murine sepsis models, reduces organ damage, and mortality in the treml1−/− mouse sepsis model. The authors interpret these results as the LR17 competes with the ligand of TREM-1, thereby providing a blockade of TREM-1 as an amplifier of the inflammatory response. A recent study using LR12, a truncated LR17 (94-LQEEDAGEYGCM-105; Figure 1c blue ribbon), with a greater structural similarity and 50% homology reproduces the LR 17 results in wild type septic mice [49]. These results argue for the importance of this 12 amino acid region in TLT-1, however caution should be taken in the interpretation of TREM-1 ligand competition. While LR17 and 12 may block TREM-1 function directly, neither platelets nor fibrinogen were carefully evaluated and there is no way to determine if TREM-1 activation was secondary to platelet function in these studies.
The size and distribution differences between αIIbβ3 and TLT-1 allow the conjecture that TLT-1 may play a mechanosensory role. In this speculative model, compression of cells, or shear stress increases the probability of fibrinogen clustering TLT-1 on the platelet surface (Figure 3). This hypothesis is supported by the transmigration models in which neutrophils from either wild-type or treml1−/− mice (TLT-1−/− null mice) fail to release attached platelets during transmigration over fibrinogen in the presence of platelets. Wild-type platelets, with either neutrophils from either wild-type or treml1−/− mice transmigrated without attached platelets [19]. Studies using Mn++ to activate αIIbβ3, suggest that even in the bent form, αIIbβ3 is capable of binding fibrin(ogen) [34]. It is possible that transmigration induces the bent form of αIIbβ3 and increases fibrinogen interaction with TLT-1, inducing stronger platelet/fibrinogen interactions. The neutrophils transmigrate and the platelets seal the gap [50]. Going back to the comparison of Shakira to Michael Jordon, if the competition were singing and not basketball, how the voices worked together would be more important than height.
Figure 3.
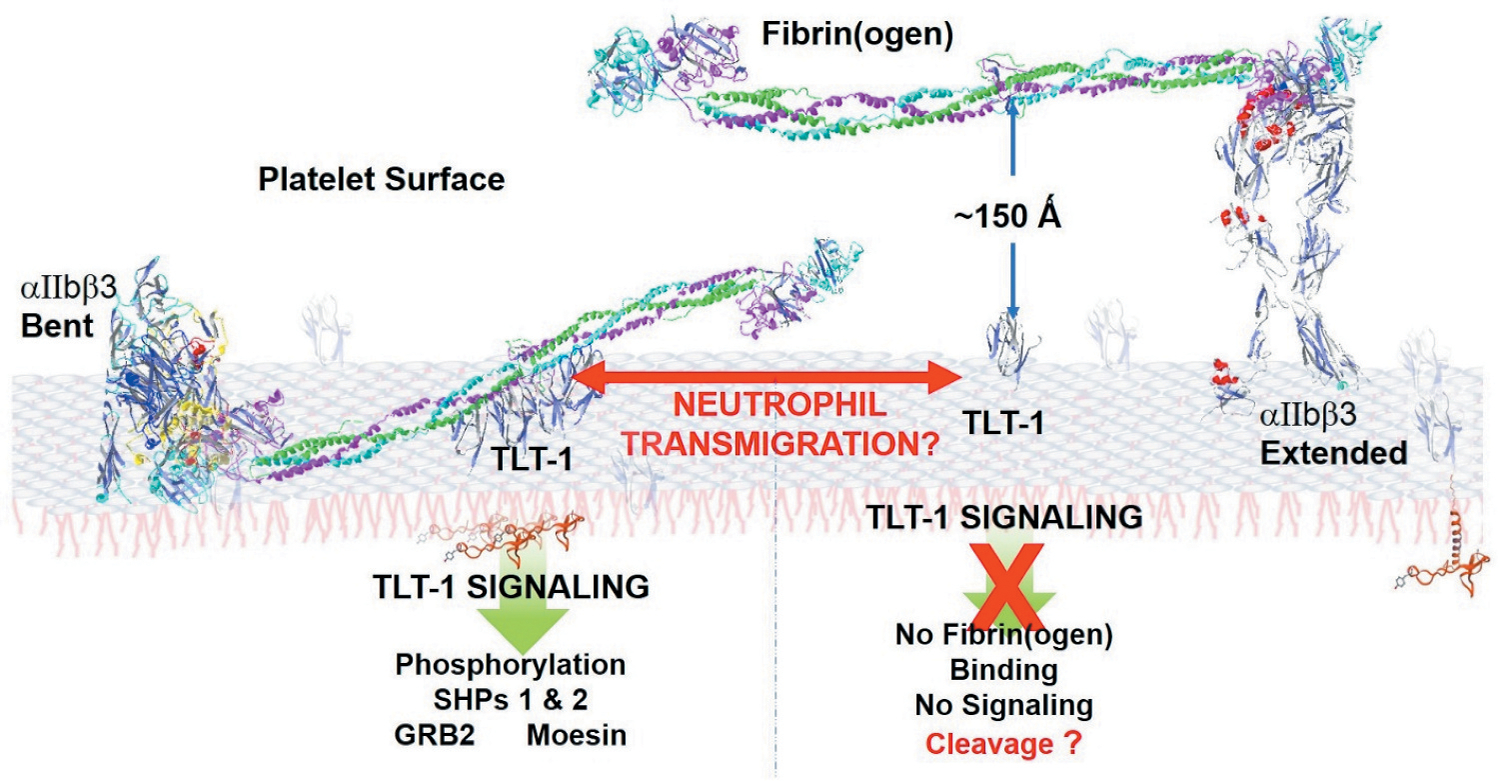
Mechanistic Model for TLT-1/Fibrinogen interaction. When in the extended confirmation, the extreme height of αIIbβ3 may prevent TLT-1 interaction with fibrin(ogen). Transmigration, however, may induce the bent confirmation of αIIbβ3, allowing fibrin(ogen) to interact with TLT-1 more readily. TLT-1 - fibrinogen interaction may induce intercellular signaling that does not happen under conditions of classic hemostasis. TLT-1-fibrinogen interactions would induce TLT-1 clustering (left side), a tighter binding of fibrinogen to the platelet surface, and intercellular signaling. Without the fibrinogen interaction TLT-1 may be cleaved off of the surface more readily (right side). Figure was generated with the Swiss-PdbViewer 4.1.0, using data published in DOI: 10.2210/pdb2FRG/pdb, DOI: 10.2210/pdb3FCS/pdb, DOI: 10.2210/pdb3GHG/pdb, and DOI: 10.2210/pdb4G1M/pdb.
Cell Signaling & TLT-1
Despite the known importance of TLT-1 in platelet biology and several potentially interesting motifs in its cytoplasmic domain, our knowledge of TLT-1 mechanistic signaling is limited. The cytoplasmic tail contains proline rich domains, two tyrosines (Y245 and Y281) shown to be phosphorylated [7] and a string of serines demonstrated to be phosphorylated in human platelets [51] (Figure 4). While Y281 is central to a canonical immunoreceptor tyrosine-based activation motif (ITIM) and shown to be important to binding SHP-1 [7], not much is known about the importance of Y245. In the membrane proximal regions, there are a series of serines that may be of great interest. There are models that suggest that the cytoplasmic tails of ITIM containing proteins in the resting state are unstructured but remain buried in the membrane in a way that sequesters access of key residues from interaction partners [52–54]. PECAM-1, also found in platelets, is an example of one of these receptors [55]. It has been demonstrated that upon platelet activation, serines become phosphorylated, unzippering the tail from the membrane granting access to key motifs such as the ITIM. The structure of the TLT-1 cytoplasmic tail containing 7 serines that are phosphorylated and a distal ITIM, is very reminiscent of the PECAM-1 cytoplasmic tail suggesting that TLT-1 may work in a similar fashion to PECAM-1 upon platelet activation.
Figure 4.
The TLT-1 cytoplasmic tail. The serines demonstrated to be phosphorylated are shown in green, the proline rich region is shown in blue, the membrane proximal tyrosine and the ITIM are shown in red, both tyrosines have been shown to be phosphorylated. Known cytoplasmic interacting partners are shown in their regions of suspected interaction. GRB2 is believed to interact with the proline rich domain but this interaction has not been localized and is represented by the “?”, SHP 1 & 2 have been demonstrated to bind the ITIM, and Moesin is believed to bind the distal region of cytoplasmic tail last 25 amino acids.
The first characterized interacting partners of TLT-1 were the SH2 domain-containing protein phosphatases SHP-1 and SHP-2 [5,7]. They have been shown to co-immunoprecipitate with TLT-1 following induction of tyrosine phosphorylation. Barrow, et.al. demonstrated that phosphorylation of both Y245 and Y281 was regulated by tyrosine phosphatases, however, only phosphorylation of Y281 induced the TLT-1/SHP-2 interaction [7]. Furthermore, phosphorylation of Y281 within the TLT- 1 ITIM was demonstrated to enhance FcεRI mediated calcium mobilization through SHP-2 in rat basophilic leukemia cells which suggests a potential co-stimulatory role in platelets [7]. Interestingly the T280 and the S276 adjacent to the Y281 are also phosphorylated, which may affect the phosphorylation of the Y281 and ultimately affect its binding of SHP-1, and -2 [51].
The scaffolding protein moesin was shown to interact with the TLT-1 cytoplasmic tail. Moesin is known to link membrane proteins via its ERM (Ezrin, Radixin, and Moesin) domain to the actin cytoskeleton [18]. This data postulates that the mechanism by which TLT-1 facilitates platelet aggregation involves linking fibrinogen to the platelet cytoskeleton via the ERMs. However, none of the ERM protein deficient mice have a demonstrated bleeding diathesis [56,57] and therefore the importance of these interactions is yet to be determined.
TLT-1 has also been seen to stabilize clot formation by facilitating outside-in signaling of αIIbβ3. β3Y773 phosphorylation (pβ3Y773) in the αIIbβ3 tail was examined over time in WT and treml1−/− platelets. pβ3Y773 is an indicator of early outside-in signaling [37]. Consistent with published data [37] it was found that within 5 minutes of platelet activation, pβ3Y773 increased in WT mice. Treml1−/− platelets demonstrated ~2X less pβ3Y773 at 5 minutes and a greater deficiency over a 20-minute period. This data suggest that TLT-1 may enhance αIIbβ3 stability, although an association with increased phosphorylation is inconsistent with TLT-1 binding to SHP phosphatases.
Schmoker et. al recently published a proteomic analysis to define the TLT-1 protein-protein interactions in both resting and activated platelets [51]. Several novel TLT-1 interacting partners were identified including several RAB proteins and RACK1, the integrin interacting protein. Of great interest, however, are the two proteins validated in platelets, αIIbβ3 and GRB2. The amount of αIIbβ3 that associates with TLT-1 on activated platelets is greatly reduced compared to resting platelets. Thus, platelet activation seems to separate αIIbβ3 from TLT-1 suggesting that TLT-1 may play a role in maintaining αIIbβ3 in its resting or bent form. In line with a mechanosensory role for TLT-1, transmigration may release a subset of αIIbβ3 increasing the amount available to bind fibrinogen. It should be mentioned that both αIIbβ3 and TLT-1 are found in the α-granules and the reduced interaction seen upon activation may reflect release from the α-granules. At the same time TLT-1 engagement of fibrinogen would lead to the unzippering of the TLT-1 cytoplasmic tail allowing interaction with scaffolding protein moesin thereby further strengthening platelet – platelet interactions and ultimately platelet release from the neutrophil.
GRB2 on the other hand is a linker protein that contains two SH3 and a single SH2 domain, either of which could mediate interactions with TLT-1. GRB2 has been shown to play an important role in T-cell activation through interactions with SLP-76. It would be of interest to see if GRB2 mediates similar interaction in platelets, since SLP-76 has been implicated in immune-derived bleeding [40].
Conclusions
Current data suggest that the TLT-1/sTLT-1 axis is important for immune hemostasis. To understand how, or even if, fibrinogen directs platelets toward immune function will take a greater understanding of the TLT-1 structure function relationship. Given the size difference and disparity in copy number between TLT-1 and αIIbβ3, a better appreciation of the landscape of the platelet surface may also become an important metric to better understand this association. TLT-1 peptide studies suggest that the β-sheet leading to the CDR3 loop of TLT-1 may be the key to understanding the mechanism behind TLT-1 function [48,49]. Crystallographic studies suggest that this region may have flexibility for allosteric change allowing for a peptide to affect TLT-1 function [4]. Even thoughTLT-1 remains an enigmatic platelet receptor, the foundations have been laid to dissect its unique role in platelet function. Given the abundance of TLT-1 in our system we hope to use our knowledge on the structural aspects of TLT-1 to develop interventions designed reducing cardiovascular risk and/or improving survival for those who suffer from lung injury.
Acknowledgements
We would like to thank Dr Angela Gibson and Julia Warrick for their careful review and insightful comment during the preparation of this manuscript.
Sources of funding: NIH R01HL090933 and R21HL140268
Footnotes
Disclosure
A.V. Washington has been granted a patent on TLT-1 antibodies and therapeutic uses thereof.
References
- 1.Guo L, Rondina MT. The era of thromboinflammation: platelets are dynamic sensors and effector cells during infectious diseases. Front Immunol 2019;10:2204. 10.3389/fimmu.2019.02204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gremmel T, Frelinger AL 3rd, Michelson AD. Platelet Physiology. Semin Thromb Hemost 2016;42(03):191–204. 10.1055/s-0035-1564835 [DOI] [PubMed] [Google Scholar]
- 3.Delabranche X, Helms J, Meziani F. Immunohaemostasis: a new view on haemostasis during sepsis. Ann Intensive Care 2017;7 (1):117. 10.1186/s13613-017-0339-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattis JL, Washington AV, Chisholm MM, Quigley L, Szyk A, McVicar DW, Lubkowski J. The Structure of the Extracellular Domain of Triggering Receptor Expressed on Myeloid Cells Like Transcript-1 and Evidence for a Naturally Occurring Soluble Fragment. J Biol Chem 2006;281(19):13396–13403. 10.1074/jbc.M600489200 [DOI] [PubMed] [Google Scholar]
- 5.Washington AV, Quigley L, McVicar DW. Initial characterization of TREM-like transcript (TLT)–1: a putative inhibitory receptor within the TREM cluster. Blood 2002;100(10):3822–3824. 10.1182/blood-2002-02-0523 [DOI] [PubMed] [Google Scholar]
- 6.Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol 2003;33(2):567–577. 10.1002/immu.200310033 [DOI] [PubMed] [Google Scholar]
- 7.Barrow AD, Astoul E, Floto A, Brooke G, Relou IA, Jennings NS, Smith KG, Ouwehand W, Farndale RW, Alexander DR, et al. Cutting edge: TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J Immunol 2004;172:5838–5842. 10.4049/jimmunol.172.10.5838 [DOI] [PubMed] [Google Scholar]
- 8.Virmani R, Farb A, Kolodgie FD. Histopathologic alterations after endovascular radiation and antiproliferative stents: similarities and differences. Herz 2002;27(1):1–6. 10.1007/s00059-002-2341-3 [DOI] [PubMed] [Google Scholar]
- 9.Washington AV, Schubert RL, Quigley L, Disipio T, Feltz R, Cho EH, McVicar DW. A TREM family member, TLT-1, is found exclusively in the α-granules of megakaryocytes and platelets. Blood 2004;104(4):1042–1047. 10.1182/blood-2004-01-0315 [DOI] [PubMed] [Google Scholar]
- 10.Cantoni C, Ponassi M, Biassoni R, Conte R, Spallarossa A, Moretta A, Moretta L, Bolognesi M, Bordo D. The three-dimensional structure of the human NK cell receptor NKp44, a triggering partner in natural cytotoxicity. Structure 2003;11(6):725–734. 10.1016/S0969-2126(03)00095-9 [DOI] [PubMed] [Google Scholar]
- 11.Colonna M TREMs in the immune system and beyond. Nat Rev Immunol 2003;3(6):445–453. 10.1038/nri1106 [DOI] [PubMed] [Google Scholar]
- 12.Hamburger AE, West AP Jr., Bjorkman PJ. Crystal structure of a polymeric immunoglobulin binding fragment of the human polymeric immunoglobulin receptor. Structure 2004;12(11):1925–1935. 10.1016/j.str.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 13.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 2000;164 (10):4991–4995. 10.4049/jimmunol.164.10.4991 [DOI] [PubMed] [Google Scholar]
- 14.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 2001;410(6832):1103–1107. 10.1038/35074114 [DOI] [PubMed] [Google Scholar]
- 15.Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. Proteomic analysis of palmitoylated platelet proteins. Blood 2011;118(13):e62–73. 10.1182/blood-2011-05-353078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer-Acosta Y, Gonzalez M, Fernandez M, Washington AV. Emerging Roles for Platelets in Inflammation and Disease. J Infect Dis Ther 2014;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon S-H, Lee YD, Ha J, Lee Y, Kim H-H. TLT-1s, alternative transcripts of triggering receptor expressed on myeloid cell-like transcript-1 (TLT-1), Inhibits the triggering receptor expressed on myeloid cell-2 (TREM-2)-mediated signaling pathway during osteoclastogenesis. J Biol Chem 2012;287(35):29620–29626. 10.1074/jbc.M112.351239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, De La Mota A, Schubert RL, Gomez-Rodriguez J, Cheng J. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest 2009;119(6):1489–1501. 10.1172/JCI36175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales-Ortiz J, Deal V, Reyes F, Maldonado-Martinez G, Ledesma N, Staback F, Croft C, Pacheco A, Ortiz-Zuazaga H, Yost CC, et al. Platelet-derived TLT-1 is a prognostic indicator in ALI/ARDS and prevents tissue damage in the lungs in a mouse model. Blood 2018;132(23):2495–2505. 10.1182/blood-2018-03-841593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang H-Y, Speicher KD, Blair IA, Speicher DW, Grosser T, et al. Deciphering the human platelet sheddome. Blood 2011;117(1):e15–e26. 10.1182/blood-2010-05-283838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith CW, Raslan Z, Parfitt L, Khan AO, Patel P, Senis YA, Mazharian A. TREM-like transcript 1: a more sensitive marker of platelet activation than P-selectin in humans and mice. Blood Adv 2018;2:2072–2078. 10.1182/bloodadvances.2018017756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadokoro S Talin Binding to Integrin Tails: a Final Common Step in Integrin Activation. Science 2003;302(5642):103–106. 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- 23.Nurden AT, Pillois X. ITGA2B and ITGB3 gene mutations associated with Glanzmann thrombasthenia. Platelets 2018;29 (1):98–101. 10.1080/09537104.2017.1371291 [DOI] [PubMed] [Google Scholar]
- 24.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 1999;103:229–238. 10.1172/JCI5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tronik-Le Roux D, Roullot V, Poujol C, Kortulewski T, Nurden P, Marguerie G. Thrombasthenic mice generated by replacement of the integrin alpha(IIb) gene: demonstration that transcriptional activation of this megakaryocytic locus precedes lineage commitment. Blood 2000;96:1399–1408. 10.1182/blood.V96.4.1399 [DOI] [PubMed] [Google Scholar]
- 26.Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol 1997;80(4):11B–20B. 10.1016/S0002-9149(97)00572-9 [DOI] [PubMed] [Google Scholar]
- 27.Scarborough RM. Development of eptifibatide. Am Heart J 1999;138(6):1093–1104. 10.1016/S0002-8703(99)70075-X [DOI] [PubMed] [Google Scholar]
- 28.Adgey AA. An overview of the results of clinical trials with glycoprotein IIb/IIIa inhibitors. Am Heart J 1998;135(4):S43–55. 10.1016/S0002-8703(98)70297-2 [DOI] [PubMed] [Google Scholar]
- 29.Dai A, Ye F, Taylor DW, Hu G, Ginsberg MH, Taylor KA. The Structure of a Full-length Membrane-embedded Integrin Bound to a Physiological Ligand. J Biol Chem 2015;290(45):27168–27175. 10.1074/jbc.M115.682377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogstad GO, Brosstad F, Krutnes MB, Hagen I, Solum NO. Fibrinogen-binding properties of the human platelet glycoprotein IIb-IIIa complex:astudy using crossed-radioimmunoelectrophoresis. Blood 1982;60:663–671. 10.1182/blood.V60.3.663.663 [DOI] [PubMed] [Google Scholar]
- 31.Litvinov RI, Farrell DH, Weisel JW, Bennett JS. The Platelet Integrin alphaIIbbeta3 Differentially Interacts with Fibrin Versus Fibrinogen. J Biol Chem 2016;291:7858–7867. 10.1074/jbc.M115.706861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber W, Hurst J, Schlatter D, Barner R, Hubscher J, Kouns WC, Steiner B. Determination of kinetic constants for the interaction between the platelet glycoprotein IIb-IIIa and fibrinogen by means of surface plasmon resonance. Eur J Biochem 1995;227(3):647–656. 10.1111/j.1432-1033.1995.tb20184.x [DOI] [PubMed] [Google Scholar]
- 33.Washington AV, Esponda O, Gibson A. Platelet biology of the rapidly failing lung. Br J Haematol 2020;188(5):641–651. 10.1111/bjh.16315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye F, Liu J, Winkler H, Taylor KA. Integrin alpha IIb beta 3 in a membrane environment remains the same height after Mn2+ activation when observed by cryoelectron tomography. J Mol Biol 2008;378:976–986. 10.1016/j.jmb.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giomarelli B, Washington VA, Chisholm MM, Quigley L, McMahon JB, Mori T, McVicar DW. Inhibition of thrombin-induced platelet aggregation using human single-chain Fv antibodies specific for TREM-like transcript-1. Thromb Haemost 2007;97(06):955–963. 10.1160/TH06-08-0456 [DOI] [PubMed] [Google Scholar]
- 36.Manfredi B, Morales-Ortiz J, Díaz-Díaz L, Hernandez-Matias L, Barreto-Vázquez D, Menéndez-Pérez J, Rodríguez-Cordero J, Villalobos-Santos J, Santiago-Rivera E, Rivera-Dompenciel A, et al. Characterization of monoclonal antibodies to mouse TLT-1 suggests that TLT-1 plays a role in wound healing. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy In press 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales-Ortíz J, Reyes F, Santiago O, Rivera L, Ledesma N, Manne BK, Madera B, Rondina M, Washington AV. TREM Like Transcript-1 controls early thrombus formation and stability by facilitating αIIbβ3 outside-in signaling. Int J Appl Res 2018;6:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glembotsky AC, Sliwa D, Bluteau D, Balayn N, Marin Oyarzun CP, Raimbault A, Bordas M, Droin N, Pirozhkova I, Washington V, et al. Downregulation of TREM-like transcript-1 and collagen receptor α2 subunit, two novel RUNX1-targets, contributes to platelet dysfunction in familial platelet disorder with predisposition to acute myelogenousleukemia.Haematologica 2019;104 (6):1244–1255. 10.3324/haematol.2018.188904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood 2015;126(13):1601–1608. 10.1182/blood-2015-04-641654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP III, Ware J, Kahn ML, Bergmeier W. Platelet ITAM signaling is critical for vascular integrity in inflammation.. J Clin Invest 2013;123(2):908–916. 10.1172/JCI65154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangin PH, Onselaer M-B, Receveur N, Le Lay N, Hardy AT, Wilson C, Sanchez X, Loyau S, Dupuis A, Babar AK, et al. Immobilized fibrinogen activates human platelets through glycoprotein VI.Haematologica 2018;103(5):898–907. 10.3324/haematol.2017.182972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forsyth CB, Solovjov DA, Ugarova TP, Plow EF. Integrin αMβ2-Mediated Cell Migration to Fibrinogen and Its Recognition Peptides. J Exp Med 2001;193(10):1123–1133. 10.1084/jem.193.10.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest 2004;113(11):1596–1606. 10.1172/JCI20741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O’Donnell E, Zhao BQ, Cifuni SM, Wagner DD. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008;111:4958–4964. 10.1182/blood-2007-11-123620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefrançais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017;544(7648):105–109. 10.1038/nature21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortiz-Munoz G, Yu MA, Lefrancais E, Mallavia B, Valet C, Tian JJ, Ranucci S, Wang KM, Liu Z, Kwaan N, et al. Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation. J Clin Invest 2020;130 (4):2041–2053. 10.1172/JCI129635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Branfield S, Washington AV. Control the platelets, control the disease: a novel cystic fibrosis hypothesis. J Thromb Haemost 2020;18 (7):1531–1534. 10.1111/jth.14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, Washington V, Massin F, Max J-P, Ford J, Alauzet C, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol 2012;188(11):5585–5592. 10.4049/jimmunol.1102674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi R, Zhang J, Peng Z, Yuan S, Gao S, Chen L, Yuan Y. Expression level of 12-amino acid triggering receptor on myeloid cells-like transcript 1 derived peptide alleviates lipopolysaccharide-induced acute lung injury in mice.. Int J Mol Med 2018;41(4):2159–2168. 10.3892/ijmm.2018.3443 [DOI] [PubMed] [Google Scholar]
- 50.Gros A, Syvannarath V, Lamrani L, Ollivier V, Loyau S, Goerge T, Nieswandt B, Jandrot-Perrus M, Ho-Tin-Noe B. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex–mediated inflammation in mice. Blood 2015;126 (8):1017–1026. 10.1182/blood-2014-12-617159 [DOI] [PubMed] [Google Scholar]
- 51.Schmoker AM, Perez Pearson LM, Cruz C, Colon Flores LG, Branfeild S, Pagan Torres FD, Fonseca K, Cantres YM, Salgado Ramirez CA, Melendez LM, et al. Defining the TLT-1 interactome from resting and activated human platelets. J Proteomics 2020;215:103638. 10.1016/j.jprot.2020.103638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T Cell Receptor Activation by Dynamic Membrane Binding of the CD3? Cytoplasmic Tyrosine-Based Motif. Cell 2008;135(4):702–713. 10.1016/j.cell.2008.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duchardt E, Sigalov AB, Aivazian D, Stern LJ, Schwalbe H. Structure Induction of the T-Cell Receptorζ-Chain upon Lipid Binding Investigated by NMR Spectroscopy. Chembiochem 2007;8(7):820–827. 10.1002/cbic.200600413 [DOI] [PubMed] [Google Scholar]
- 54.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol 2000;7:1023–1026. 10.1038/80930 [DOI] [PubMed] [Google Scholar]
- 55.Paddock C, Lytle BL, Peterson FC, Holyst T, Newman PJ, Volkman BF, Newman DK. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood 2011;117(22):6012–6023. 10.1182/blood-2010-11-317867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doi Y, Itoh M, Yonemura S, Ishihara S, Takano H, Noda T, Tsukita S. Normal development of mice and unimpaired cell adhesion/cell motility/actin-based cytoskeleton without compensatory up-regulation of ezrin or radixin in moesin gene knockout. J Biol Chem 1999;274:2315–2321. 10.1074/jbc.274.4.2315 [DOI] [PubMed] [Google Scholar]
- 57.Kawaguchi K, Yoshida S, Hatano R, Asano S. Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins. Biol Pharm Bull 2017;40 (4):381–390. 10.1248/bpb.b16-01011 [DOI] [PubMed] [Google Scholar]