Abstract
Only recently histopathological studies of patients with dermatosis and concomitant SARS-Cov-2 viral infection were published. Seven months into the COVID-19 pandemic, more skin biopsies of COVID-19-positive patients are taking place. We examined the histological features of 30 skin biopsies from two groups of patients: Ten specimens of patients tested positive for COVID-19 with an active systemic infection and associated dermatosis. Twenty specimens were from patients not considered COVID-positive (due to PCR swab negativity or not tested at all) with cutaneous lesions either showing viral infection symptoms (fever, cough, ageusia and severe immunocompromised condition due to HIV infection and malignancies), or presented a high risk of being infected (such as cohabitation with COVID-19-positive parents and siblings with simultaneous chilblains). This study analyses the histological and immunohistochemical (SARS-CoV-2 2019-nCoV nucleocapsid antibody) characteristics of the two groups and identifies 4 histopathological patterns. The histopathological features of the two groups present similar features that may help to identify an ongoing COVID-19 infection even in asymptomatic carriers with dermatosis.
Key words: COVID-19 and skin histopathology, COVID-19 and skin histology, COVID-19 and skin dermatosis, COVID-19 histopathology, COVID-19 and skin immunohistochemistry
Cutaneous histopathological reports of patients with concurrent dermatoses and COVID-19 infection began to be published only 4 months after the World Health Organization (WHO) characterized COVID-19 as a global pandemic. These studies often described chilblains or erythema-like lesions, and were largely reports of single cases or groups of patients (1–3). A range of skin histopathological findings associated with various clinical aspects and degrees of severity of COVID-19 infection have been reported so far (4, 5).
Skin biopsies in COVID-19-positive patients are now being performed more frequently, and the diagnostic experience achieved is consequently improved. The histopathological knowledge gained leads us to suspect that some patients, referred to dermatologists with skin lesions between March and May 2020, may have had COVID-19-related dermatoses, even if they were asymptomatic carriers of the virus. These patients were all at high-risk of COVID-19 infection due to lock-down directives, which often resulted in their co-confinement with COVID-positive relatives or those with minor symptoms of the infection. The suspected clinical diagnoses in these patients were commonly urticaria, erythema multiforme, chilblains, or pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease) (6).
SIGNIFICANCE
We examined the histological features of 30 skin biopsies from two groups of patients: Ten specimens of patients tested positive for COVID-19 with an active systemic infection and associated dermatosis. Twenty specimens were from patients not PCR swab or serological test positive but with cutaneous lesions either showing viral infection symptoms. This study analyses the histological and immunohistochemical characteristics of the two groups and identifies four histopathological patterns. We think it is possible to suspect a COVID-19 infection-related dermatosis simply by histopathological analysis of the skin specimen because the histopathological features of the two groups present similar features.
In this paper, we describe the histopathological and clinical features of patients with test-confirmed COVID dermatoses (group N1) and patients not considered COVID-positive (due to PCR swab negativity or not tested at all) but with a reasonably high risk of being infected (group N2). Finally, we have had the possibility to perform immunohistochemical analysis on paraffin sections using the SARS-CoV-2 (2019-nCoV) nucleocapsid antibody in a small number of cases of the two groups. The aim of our work was to evaluate histopathological and immunohistochemical similarities or differences between the two groups in order to verify if it is possible to suspect a COVID infection-related dermatosis simply by histopathological analysis of the skin specimen.
MATERIALS AND METHODS
This series included 30 skin biopsies of patients presenting with different dermatoses from March to May 2020. We organized the cases in two group: Group N1 (n = 10) representing patients who were PCR SWAB test positive for COVID-19 with an active infection in different stages of severity of systemic disease. The group N2 (n = 20) comprised patient never diagnosed with COVID infection, but considered at “high risk” due to mild systemic symptoms of the disease or cohabitation with COVID-19-positive relatives.
Two patients of group N1 were hospitalized in Intensive Care Unit (ICU), 3 patients received only oxygen support, two were hospitalized for drug administration and two were treated at home in quarantine.
Clinical and histopathological data from 4 subjects of this group have been published recently (7–9). In the current study, 6 new, previously unpublished, patients were added to this group, bringing the total to 10 patients.
The group N2 comprised 20 patients with only few or mild symptoms of COVID-19 infection, such as fever, cough, and ageusia but with additional factors responsible for a higher risk of potentially undiagnosed infection such as cohabitation with COVID-19-positive relatives, simultaneous appearance of chilblains in siblings during the lockdown, or the presence of a severely immunocompromised state (e.g. previous malignant condition, HIV infection). The patients in this group were PCR swab negative or had not been tested due to the impossibility to perform PCR swabs during that particular period of the emergency (March–May 2020). All of the patients from both the groups had clinically well-described and recognized dermatoses that have recently been associated with possible SARS-Cov-2 infection (10). (See figure legends for detailed clinical and histopathological data)
The histopathological findings in the COVID-19-positive group N1 were compared with those from the “high risk” group N2. The specimen obtained was formalin-fixed paraffin embedded and stained with haematoxylin and eosin. In 3 patients of the group N1 and in 10 patients of the group N2, we performed immunohistochemical analysis on paraffin sections using the SARS-CoV-2 (2019-nCoV) nucleocapsid antibody, rabbit Mab (Rabbit Mono-clonal antibody, Sino Biological)
RESULTS
Histopathological findings
In this work we found 4 different histopathological patterns that were completely reproducible in the two groups examined. No significant differences were observed between the two groups concerning the histopathological patterns and specific histological features.
Each case was ascribable to one of the 4 described dermatopathological patterns. Using immunohistochemical technique we observed a cuticular staining of the glomerular part of the eccrine glands in all the 3 cases of the group N1. Regarding the patients of the group N2 we observed the same staining pattern in 8 of the examined cases (8/10).
Mini-chilblain-like pattern. This pattern was most frequently observed in the group N2 (10 patients out of 20, 50%). It was observed in 3 (30%) of the 10 patients with confirmed COVID-19 positivity. No histopathological differences were noted between the two groups. Although it is possible to mistake this pattern, it is also the most histologically reproducible. The histological features are shown in Fig. 1. Most of this group of patients presented with erythematous-urticarial lesions, which occasionally had a target-like appearance. In other patients the clinical lesions resembled other dermatoses, such as erythema multiforme, pigmented purpuric dermatitis, or pityriasis lichenoides et varioliformis acuta (Mucha-Habermann disease). In 4 cases, a punch biopsy was performed within 3 days of the onset of the disease (one COVID+ and 3 COVID– patients). The dermis was markedly oedematous, with highly dilated capillaries, and collections of perivascular lymphocytes were present. Throughout the dermis, dilated and vertically arranged vessels with mild interstitial infiltration of eosinophils were seen. In 9 cases, the biopsy was performed 5 days after the onset of the lesions, two COVID+ and 7 COVID– patients). The fully developed lesion had a dense coat or sleeve-like perivascular lymphoid infiltrate. Capillaries were conspicuously dilated and engorged with red blood cells. Numerous interstitial eosinophils and extravasated erythrocytes were frequently observed, together with a mild peri-glandular lymphoid infiltrate surrounding the dermal ducts and eccrine glands. Nests of intraepidermal Langerhans cells were frequently present in both COVID+ and COVID– groups. Immunohistochemical technique was performed on paraffin sections of 4 cases (group N2). We observed a cuticular staining of the glomerular part of the eccrine glands in all the examined specimens. (4/4). The same staining pattern was present in one case of the group N1 (1/1).
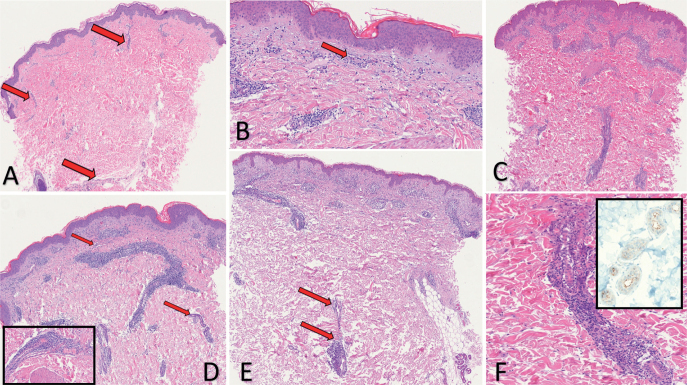
Fig. 1.
Histopathological findings in mini-chilblain-like pattern. (Haematoxylin & Eosin (H&E), A: 4x, B: 20x) COVID-19-positive 22-year-old patient. Presenting clinically with urticarial lesions at an early stage (biopsy 2 days after dermatosis onset). Diffuse oedema of the dermis, mild lymphocytic perivascular and interstitial infiltration were observed (arrows). Swollen superficial dermal vessels with eosinophils (arrow). (H&E, C: 4x, F: 20x) Patient at high risk of COVID-19, PCR swab-negative, 10 years of age. Under lockdown at home with COVID-19-positive mother. Presenting clinically with pityriasis lichenoides like lesions. Histology showed a dense periductal lymphocytic cuff. Dense perivascular and periductal lymphocytic cuff. (H&E, D: 4x) Patient at high risk of COVID-19, PCR swab-negative, 34 years of age. Under lockdown at home with COVID-19-positive wife. Presenting clinically with urticarial target-like lesions. Dense perivascular and periductal lymphocytic cuff (arrows). Inset: abnormally dilated and engorged large vessel. Swollen lymphatic-like vessel and periductal lymphocytic cuff. (H&E, E: 4x) COVID-19-positive patient, 45 years old. Presenting clinically with target-like lesions and chilblain-like ulcerated plaque. (F, same patient as in Fig. C. H&E, 10x). Dense perivascular and periductal lymphocytic cuff (arrows). Inset: Cuticular positivity of the eccrine gland using the SARS-CoV-2 (2019-nCoV) nucleocapsid antibody.
Chilblain-like pattern. This histological pattern was observed in 5/20 (25%) patients in the second “high risk” group and in 2/10 (20%) of the first COVID-19-positive patients. This histological and clinical pattern is currently the most frequently described in COVID positive patients (2, 3, 11, 12). In all cases, a punch biopsy was performed within 5 days of the onset of the skin manifestation. No histopathological differences were detected between the two groups. Histological features were those of chilblain-like erythema with a substantial lymphocytic vasculopathy around medium- and small-calibre vessels associated with a dense peri-glandular lymphocytic infiltration. In chilblain erythema associated with COVID-19 infection, the substantial difference from typical chilblain erythema (pre-COVID-19) was the severity of the vasculitis, which was so intense that it induced detachment at the dermo–epidermal junction and necrosis of the epidermis. Occasional microthromboses of small deep dermal vessels were observed both in the COVID-19-positive group and in the second group of COVID-19-negative patients at high risk of infection (even those who had a negative nasopharyngeal swab). Furthermore, in both groups, occasional dense lymphoid CD8+ lymphocytes around acrosyringeal ducts and the almost constant presence of eosinophils in the infiltration were observed. Immunohistochemical technique was performed on paraffin sections of 3 cases (group N2). We observed a cuticular staining of the glomerular part of the eccrine glands in two specimens (2/3). The same staining pattern was present in one case of the group N1 (1/1).
Erythema multiforme-like pattern. This is the most interesting histopathological pattern, since it implies a possible direct interaction of COVID-19 with the keratinocytes in the epidermis and acrosyringium. This pattern was present in 3/20 (15%) patients in the second “high risk” group and in 3/10 (30%) patients with confirmed COVID-19 PCR swab positivity. Fig. 2A–E. Clinically, an erythematous-maculopapular rash or a targetoid appearance, similar to that seen in classical erythema multiforme, was observed. In all cases, a punch biopsy was performed within 8 days of the onset of the dermatoses. Histopathological examination revealed a diffuse interface dermatitis, consisting of cytotoxic CD8+ lymphocytes that diffusely infiltrate the epithelium inducing scattered necrosis of keratinocytes with lymphocyte accumulation, particularly around the acrosyringeal ducts and along the dermal eccrine gland ducts. Again, in these cases, abnormally dilated capillaries engorged with red blood cells were often associated with nests of Langerhans cells in the epidermis. In one case in the control group, a change closely resembling herpetic cytopathic damage to keratinocytes of the hair follicles was noted. Moreover, microthrombi were observed in the deep dermis in 2/20 (10%) patients in the second group (Fig. 2C). Immunohistochemical technique was performed on paraffin sections of 3 cases (group N2). We observed a cuticular staining of the glomerular part of the eccrine glands in two specimens (2/3). The same staining pattern was present in one case of the group N1 (1/1).
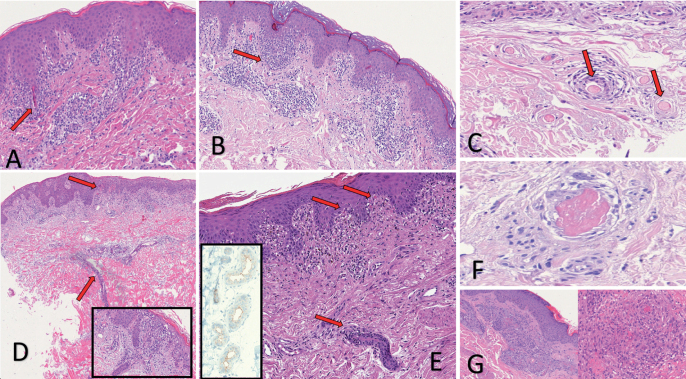
Fig. 2.
Histopathological findings in erythema multiforme like and livedoid like patterns. (A, Haematoxylin & Eosin (H&E) 20x) COVID-19-positive patient, 65 years old. Erythema multiforme-like pattern. Presenting clinically with multiple targetoid lesions on the trunk. Lichenoid dermatitis composed of CD8+ lymphocytes showing marked epidermotropism around necrotic acrosyringeal ducts (arrows). (B, H&E 10x) Patient at high risk of COVID-19, PCR swab-negative, 33 years old. Under lockdown at home with COVID-19-positive wife. Presenting clinically with target-like lesions on elbows and hands. Patchy perivascular band-like infiltration, particularly around acrosyringeal ducts and sparse necrotic keratinocytes. (C, H&E 40x) Patient at high risk of COVID-19, PCR swab-negative, 25 years old. Under lockdown at home with COVID-19-positive partner. Target-like lesions on elbows and hands. Multiple small thrombi in vessels of the deep dermis (arrows). (D, H&E 4x) COVID-19-positive 64-year-old patient. Erythema multiforme-like pattern. Presenting clinically with multiple papulo-squamous lesions on the trunk. Band-like infiltration involving the epidermis (arrow). In the dermis, vertically arranged and swollen dermal ducts surrounded by lymphocytes (arrow). Inset: diffuse epidermotropic infiltration with necrotic keratinocytes. (E, H&E 20x) Patient at high risk of COVID-19, 51 years old. Fever cough, ageusia, PCR swab not performed. Presenting clinically with polymorphic rash (targetoid, livedoid and maculo-purpuric). Band-like diffuse epidermotropic lymphocytic infiltration. Necrotic keratinocytes in the epidermis (arrows), lymphocytic infiltration involving eccrine dermal ducts (arrow). Inset: Cuticular positivity of the eccrine gland using the SARS-CoV-2 (2019-nCoV) nucleocapsid antibody. (F, H&E 50x) COVID-19 PCR swab-positive intubated 49-year-old patient. Livedoid thrombotic-like pattern. Presenting clinically with diffuse livedo vasculitis on the trunk and legs. A medium-sized thrombosed vessel in the deep dermis. (G, H&E 10x and 40x) Patient at high risk of COVID-19, 8 years old, PCR swab-negative. Under lockdown at home with COVID-19-positive mother. Livedoid thrombotic-like pattern. Presenting clinically with diffuse target-like lesions. Extreme vascular proliferation of endothelial cells with small thrombi.
Livedoid-like pattern. This pattern was observed in 2/20 (10%) of the second “high risk” group, who were aged 8 and 25 years, respectively. Clinically, they had multiple purpuric target-like lesions on the elbows, hands and legs. A punch biopsy was performed within 7 days of the onset of the clinical skin manifestations (Fig. 2G). The same histopathological features were present in two patients (age 49 and 65) with COVID-19 positivity and severe systemic symptoms (Fig. 2F). No histopathological differences were reported between the two groups. The two COVID-19-positive patients were hospitalized in the ICU with systemic and pulmonary involvement. A livedoid exanthematous eruption appeared in both patients during ICU treatment. Histologically, mild spongiosis with minimal lymphocytic exocytosis was noted, together with intraepidermal Langerhans nests. Microthrombi were evident in the superficial and deep dermis. Nuclear and eosinophilic debris were not always seen. Diffuse livedoid exanthematous eruption appears to be related to multi-organ involvement in COVID-positive patients. The two patients hospitalized in the ICU recovered from the disease and are currently in good health.
DISCUSSION
The high incidence of COVID-19 infections in northern Italy allowed to examine a large number of skin biopsies related to the pandemic. However, the pathogenic mechanisms of SARS-CoV-2 are currently largely hypothetical. Published data indicate two possible forms of viral-induced intravascular diffuse coagulopathy. The first is via the induction of diffuse thrombosis with the presence of eosinophils (13, 14), and the second is through activation of the mannose-binding-lectin (MBL) pathway bound by the SARS-CoV-2 spike glycoprotein (15).
A direct cytopathic action on keratinocytes has been postulated, based on the observation of numerous patients with histopathological aspects similar to those found in erythema multiforme, in which many necrotic keratinocytes are present (1, 9, 16). In the examined histopathological specimens of the present work, no substantial histopathological differences were seen between the two groups studied. In contrast, there were notable histological similarities between the COVID-positive group and the second “high risk” group in the current study.
In the mini-chilblain-like pattern, the similarities observed were constantly reproducible in both the two groups. Particularly the presence of a perivascular cuff-like lymphocytic infiltrate and the vertical arrangement of dilated capillaries, flanked by a dermal sleeve-like lymphocytic infiltrate around the dermal secretory gland ducts.
In the chilblain-like pattern, there were also no significant differences between the two groups showing a severe lymphocytic vasculopathy around medium- and small-calibre vessels together with a dense peri-glandular lymphocytic infiltration.
In the erythema multiforme-like pattern, in both groups a constant finding was necrosis of keratinocytes with lymphocytic aggregates, particularly around the acrosyringeal ducts.
In the livedoid-like pattern the only difference found was demographic; the young age of the 2 patients from the second “high risk” compared to the 2 middle-aged ICU patients in the COVID-19-positive group. Hamming et al. (11) identified in 2004 the metallopeptidase named angiotensin-converting enzyme 2 (ACE2) as the functional receptor for SARS-CoV responsible for an epidemic outbreak during 2003–2004. Immunohistochemistry demonstrated the surface expression of ACE2 protein in pneumocytes, macrophages and enterocytes of the small intestine. In the skin the basal layer of the epidermis, the endothelial cells in the dermis and eccrine adnexal glands expressed strongly ACE2. In our cases the presence of lymphocytic infiltration around verticalized dermal vessels and eccrine excretory ducts leads us to consider the hypothesis that ACE2 is the gateway for the virus from the bloodstream to the deep eccrine glands which showed a clear cuticular labelling for the proteins of the viral core.
Dermatopathologists must be alert when examining skin biopsies from patients with the “mini-chilblain-like” and “multiforme-like” patterns, as there is increasing evidence to suspect these lesions of being COVID-19 infection-related, even in asymptomatic individuals. Young age, SARS-CoV-2-negative swabs, and the absence of other symptoms appear to be common features of the minichilblain and chilblain-like patterns, as reported recently (3, 16, 17). Torrelo et al. (12) reported that immunohistochemical analysis of SARS-CoV antigen expression showed that the SARS-CoV/SARS-CoV-2 spike protein was present with a granular positivity in endothelial cells and epithelial cells of eccrine acrosyringeal ducts and glands even in swab-negative young patients. The SARS-CoV-2-negative swabs could be explained by the early disappearance of detectable viral presence in the upper airways, after a brief asymptomatic course in young patients (3, 17). Testing for immunoglobulins IgA, IgM and IgG in the sera of these patients should be performed routinely.
Further studies, using PCR analysis for SARS-CoV gene sequences in paraffin sections and in situ hybridization, are necessary in the high-risk SARS-CoV-2 PCR swab-negative patient group.
REFERENCES
- 1.Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, Suarez-Valle A, Saceda-Corralo D, Moreno-Garcia Del Real C, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings. Clin Exp Dermatol 2020. May 9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 2.Cordoro KM, Reynolds SD, Wattier R, McCalmont TH. Clustered cases of acral perniosis: clinical features, histopathology and relationship to COVID-19. Pediatr Dermatol 2020; 37: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Recalcati S, Barbagallo T, Frasin LA, Prestinari F, Cogliardi A, Provero MC, et al. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol 2020; 34: e346–e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahidi Dadras M, Zargari O, Abolghasemi R, Bahmanjahromi A, Abdollahimajd F. A probable atypical skin manifestation of COVID–19 infection. J Dermatolog Treat 2020. Jul 13 [Epub ahead of print]. [DOI] [PubMed]
- 5.Shanshal M. COVID-19 related anagen effluvium. J Dermatolog Treat 2020. Jul 16. [Epub ahead of print]. [DOI] [PubMed]
- 6.Elmas OF, Demirbas A, Ozyurt K, Atasoy M, Tursen U. Cutaneous manifestations of COVID-19: A review of the published literature. Dermatol Ther 2020. May 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Gianotti R, Veraldi S, Recalcati S, Cusini M, Ghislanzoni M, Boggio F, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol 2020; 100: adv00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianotti R, Zerbi PGianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the northern part of Italy. J Dermatol Sci 2020; 98: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianotti R, Recalcati S, Fantini F, Rica C, Milani M, Dainese E, et al. Histopathological study of a broad spectrum of skin dermatoses in patients affected or highly suspected of infection by COVID-19 in the northern part of Italy. Analysis of the many faces of the viral-induced skin diseases in previous and new reported cases. Am J Dermatopathol 2020; Jun 10 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 10.Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van-Goor H. Tissue distribution of ACE2 protein, the functional receptorfor SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrelo A, Andina D, Santonja C, Noguera-Morel L, Bascuas-Arribas M, Gaitero, et al. . Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol 2020; 37: 442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzano AV, Tedeschi A, Berti E, Fanoni D, Crosti C, Cugno M. Activation of blood coagulation in bullous pemphigoid: role of eosinophils, and local and systemic implications. Br J Dermatol 2009; 160: 266–272. [DOI] [PubMed] [Google Scholar]
- 14.Lucchese . From HSV infection to erythema multiforme through autoimmune crossreactivity. Autoimmun Rev 2018; 17: 576–581. [DOI] [PubMed] [Google Scholar]
- 15.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020; 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020; 49: 411–417. [DOI] [PubMed] [Google Scholar]
- 17.Colonna C, Genovese G, Monzani NA, Picca M, Boggio F, Gianotti R, et al. Outbreak of chilblain-like acral lesions in children in the metropolitan area of Milan, Italy, during the COVID-19 pandemic. J Am Acad Dermatol 2020. Jun 10. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]