Abstract
A novel nucleoside phosphorylation process using the food additive pyrophosphate as the phosphate source was investigated. The Morganella morganii gene encoding a selective nucleoside pyrophosphate phosphotransferase was cloned. It was identical to the M. morganii PhoC acid phosphatase gene. Sequential in vitro random mutagenesis was performed on the gene by error-prone PCR to construct a mutant library. The mutant library was introduced into Escherichia coli, and the transformants were screened for the production of 5′-IMP. One mutated acid phosphatase with an increased phosphotransferase reaction yield was obtained. With E. coli overproducing the mutated acid phosphatase, 101 g of 5′-IMP per liter (192 mM) was synthesized from inosine in an 88% molar yield. This improvement was achieved with two mutations, Gly to Asp at position 92 and Ile to Thr at position 171. A decreased Km value for inosine was responsible for the increased productivity.
Nucleotides are often used as food additives and as pharmaceutical intermediates. Among them, 5′-IMP and 5′-GMP are important, because they have a characteristic taste and are used as flavor potentiators in various foods. Purine nucleosides such as inosine (7, 9) and guanosine (8) can be produced efficiently by fermentation, and phosphorylation of nucleosides is a very efficient process for the large-scale production of 5′ nucleotides.
At present, there are two main phosphorylation methods. One is a chemical phosphorylation process that uses phosphoryl chloride (POCl3) (22), and the other is an enzymatic phosphorylation process that uses inosine kinase of Escherichia coli (11, 12). The chemical phosphorylation process is relatively complex, because it needs two reactors, for the fermentation and chemical reactions. The enzymatic phosphorylation process is simpler, because the enzymatic reaction can be carried out in the same reactor as the fermentation reaction. The inosine kinase reaction, however, requires ATP, and the ATP needs to be regenerated by resting cells of Corynebacterium ammoniagenes, which are used for the fermentative production of inosine. Therefore, applications of the enzymatic phosphorylation process are limited. Alternatively, an enzyme that catalyzes the synthesis of nucleotides by transfer of phosphate groups from low-energy phosphate esters to nucleosides was described by Brawerman and Chargaff (3) and Mitsugi and coworkers (10).
Prompted by these findings, we have investigated a novel nucleoside phosphorylation reaction using the food additive pyrophosphate (PPi), as shown in the following equation (9, 10): nucleoside + PPi → nucleoside 5′-monophosphate + Pi acid phosphatase/phosphotransferase (EC 3.1.3.2). We purified and characterized a C5′-position selective pyrophosphate-nucleoside phosphotransferase from a crude extract of Morganella morganii NCIMB10466 (2). The purified enzyme exhibited not only phosphotransferase activity but also phosphatase activity. On the basis of a kinetic study, it appeared to be a phosphatase with regioselective phosphotransferase activity. In order for the enzyme to be useful, it would be necessary to suppress the dephosphorylation reaction and increase the efficiency of the transphosphorylation reaction.
In this paper we describe the cloning of the phosphotransferase gene from M. morganii NCIMB10466, further optimization of the reaction conditions, and improvement of enzyme activity by random mutation.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
M. morganii NCIMB10466, which was previously selected as the strain producing the highest level of 5′ nucleotide using PPi as the phosphate source (1), was used as the DNA donor. E. coli JM109 (21) was used as the host strain for DNA manipulation and expression. Plasmids pUC18 and pUC19 (20) (Takara Shuzo, Kyoto, Japan) were used as vectors for E. coli. Luria-Bertani (LB) medium (15) was used for the culturing of M. morganii and E. coli. These microorganisms were grown aerobically at 37°C. For the selection of E. coli transformants, ampicillin (50 μg/ml) was added to the medium.
General DNA manipulations.
All basic recombinant DNA procedures, such as isolation and purification of DNA, restriction enzyme digestion, ligation of DNA, and transformation of E. coli, were performed as described by Sambrook et al. (15).
DNA was sequenced by the dideoxynucleotide chain termination method with a dye terminator cycle sequencing kit (Perkin-Elmer, Norfolk, Conn.) and a DNA sequencer (model 373A; Perkin-Elmer).
For the analysis of mutation sites, nine primers were synthesized, as follows: MP1, 5′-CTTCCGTCTGTTTCGTCACA-3′; MP2, 5′-CCACCAAGCCGGATTTATAT-3′; MP3, 5′-TGGCAACCGCATTTTCAGGGGCATTCGG-3′; MP4, 5′-ATCTTACCCGTCAGGTCATA-3′; MP5, 5′-ATCCGGCATTTCAGGCGCAG-3′; MP6, 5′-TATTGCACTGCCCTGACCAG-3′; MP7, 5′-CCCGTTCCAGAATCGCATCC-3′; MP8, 5′-AAGGTCACCGGCATCCTCAA-3′; and MP9, 5′-CTGAATACTGCCGACTTAAA-3′.
Enzyme assays.
Phosphotransferase activity was assayed in a standard reaction mixture containing 100 μmol of sodium acetate buffer (pH 5.0), 40 μmol of inosine, 100 μmol of tetrasodium pyrophosphate, and the enzyme solution in a total volume of 1 ml. Kinetic constants for inosine in the transphosphorylation reaction were measured at pH 4.0 using 100 μmol of sodium acetate buffer (pH 4.0). The reaction mixture was incubated for 10 min at 30°C, and then the reaction was stopped by adding 0.2 ml of 2 N HCl. Quantitative determination of inosine and 5′-IMP was carried out by high-pressure liquid chromatography (HPLC) using a Cosmosil 5C18-MS column (4.6 by 150 mm; Nacalai Tesque Co., Kyoto, Japan) with detection at 245 nm. The mobile phase was 5 mM potassium phosphate buffer (pH 2.8)-methanol (95:5, [vol/vol]), and the flow rate was 1 ml/min. One unit of phosphotransferase activity was defined as the amount of enzyme that produced 1 μmol of 5′-IMP per min under the assay conditions.
5′-Nucleotidase activity was assayed in a standard reaction mixture containing 100 μmol of sodium acetate buffer (pH 4.0), 10 μmol of 5′-IMP, and the enzyme solution in a total volume of 1 ml. The reaction mixture was incubated for 5 min at 30°C. The reaction was stopped by adding 0.2 ml of 2 N HCl, and then released inosine was measured by HPLC. One unit of 5′-nucleotidase activity was defined as the amount of enzyme that produced 1 μmol of inosine per min under the assay conditions.
Phosphatase activity was assayed by monitoring the rate of hydrolysis of p-nitrophenyl phosphate (p-NPP). The reaction mixture contained 100 μmol of morpholineethanesulfonic acid (MES)-NaOH buffer (pH 6.0), 10 μmol of p-NPP, and the enzyme solution in a total volume of 1 ml. The reaction mixture was incubated for 1 min at 30°C, and then the reaction was stopped by adding 0.2 ml of 2 N KOH. The release of p-nitrophenol was measured at 410 nm. One unit of phosphatase activity was defined as the amount of enzyme that produced 1 μmol of p-nitrophenol per min under the assay conditions.
Kinetic constants for inosine in the transphosphorylation reaction and for 5′-IMP in the dephosphorylation reaction were measured at pH 4.0, at which 5′-IMP synthesis was carried out. Kinetic constants for p-NPP in the dephosphorylation reaction were measured at pH 6.0, at which the enzyme has optimal activity. The initial velocities were determined under the assay conditions described above, and the steady-state kinetic constants were calculated by using a Lineweaver-Burk plot. As the solubility of inosine was limited, kinetic constants for inosine were determined with a substrate concentration ranging from 0.5 to 80 mM.
Preparation of internal peptides of the phosphotransferase from M. morganii.
The purified phosphotransferase from M. morganii (1 mg) (2) was digested with 15 μg of lysyl endopeptidase (Wako, Kyoto, Japan) in 1 ml of 15 mM Tris-HCl buffer (pH 9.0) at 30°C for 16 h. The reaction mixture was subsequently eluted by HPLC on a TSK gel octadecyl silane-80Ts column (4.5 by 150 mm; Tosoh, Tokyo, Japan) with a 20 to 70% linear gradient of CH3CN containing 0.1% trifluoroacetic acid at a flow rate of 0.5 ml/min, and each peptide fragment was collected. The amino acid sequences of the peptides and of the amino-terminal region were analyzed with an automated protein sequencer (Prosequencer 6625; Millipore Corp.).
Cloning and nucleotide sequencing of the phosphotransferase gene.
An M. morganii chromosomal DNA library was constructed by inserting partial Sau3AI-digested fragments of 4 to 8 kb into the BamHI site of pUC18. E. coli JM109 transformants were grown on LB plates containing 50 μg of ampicillin per ml and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 16 h. To visualize colonies of transformants showing acid phosphatase activity, 2 ml of 0.1 M MES-NaOH buffer (pH 6.0) containing 4 mM p-NPP was poured onto the surface of the plates. We then screened for phosphotransferase-positive clones among the phosphatase-positive candidates. Each candidate was cultured in 4 ml of LB medium containing 50 μg of ampicillin per ml and 1 mM IPTG at 37°C for 16 h. Recombinant cells were harvested by centrifugation, and phosphotransferase activity was measured as described. Recombinants that produced 5′-IMP were selected and used for further study.
Subclones were prepared with the pUC18 and pUC19 vectors by transformation into E. coli JM109 by standard methods (15). To generate shorter clones suitable for sequencing, the exonuclease III deletion method (Kilo-Sequencing kit; Takara Shuzo) was used.
In vitro random mutagenesis and screening of mutants.
Random mutagenesis of the PhoC gene was performed by error-prone PCR according to the method of Cadwell and Joyce (4, 5). The mutagenic reaction mixtures contained 20 fmol of plasmid pMPI501 as a template, 30 pmol of M13 primer RV, 30 pmol of M13 primer M4 (Takara Shuzo), 50 mM KCl, 10 mM Tris-HCl buffer (pH 8.3), 7 mM MgCl2, 0.5 mM MnCl2, 0.2 mM each dATP and dGTP, 1 mM each dTTP and dCTP, and 5 U of Taq DNA polymerase (Pharmacia) in a total volume of 100 μl. PCR was carried out with programs of 30 cycles of 94°C for 1 min, 42°C for 2 min, and 72°C for 3 min. PCR products were purified by chloroform-isoamyl alcohol extraction and ethanol precipitation. A mixture of EcoRI-HindIII fragments including the mutagenized PhoC gene was cut out and religated into the pUC18 plasmid to generate a mutant library.
The constructed mutant library was transformed with E. coli JM109. Each transformant was isolated and cultivated in LB broth as described above. A phosphotransferase reaction was then carried out with each of the clones. The reaction mixture contained 20 g of inosine per liter (75 mM), 100 g of tetrasodium pyrophosphate decahydrate per liter (224 mM), 0.1 M sodium acetate buffer (pH 4.0), and approximately 50 mg (wet weight, harvested from 3 ml of culture broth) of E. coli JM109 transformants in 0.5 ml. After incubation for 2, 6, and 16 h at 30°C, the amount of 5′-IMP produced was measured. Candidates that produced a larger amount of 5′-IMP and showed lower nucleotidase activity than E. coli JM109 harboring wild-type phoC were selected.
The second round of random mutagenesis was performed by the same procedure, using as a template a plasmid derived from an improved variant produced in the first round of mutagenesis and chosen as the parent for the next generation.
Site-directed mutagenesis.
To prepare a PhoC acid phosphatase mutant with the G92D mutation, the following mutagenic primer was synthesized: 5′-GCGGTTGCCACA(C to T)CCCCTGCG-3′. The target mutation was introduced into plasmid pMPI501 with the MUT1 primer (Takara Shuzo) and the mutagenic primer (for the first PCR) and with M13 primer RV and M13 primer M4 (for the first and second PCRs) via heteroduplex formation between the first two PCR products (6). We used an in vitro mutagenesis kit (Takara Shuzo). PCR was carried out with programs of 25 cycles of 94°C for 30 s, 55°C for 2 min, and 72°C for 3 min (for the first PCR) and 10 cycles under the same conditions as those used for the first PCR (for the second PCR). The single-stranded region of the heteroduplex was filled in by the second PCR, followed by double digestion with EcoRI and HindIII. The double-stranded DNA fragment carrying the mutation could, in theory, be selectively digested with both enzymes and cloned into the same restriction site of plasmid pUC18. The 1.2-kb EcoRI-HindIII fragment religated into pUC18 was designated pMPI502, and the mutation was confirmed by sequencing.
Synthesis of 5′-IMP by E. coli overproducing wild-type and mutated PhoC.
Each E. coli JM109 transformant subcultured on LB agar containing 50 μg of ampicillin per ml was inoculated into 200 ml of LB medium containing 50 μg of ampicillin per ml and 1 mM IPTG in 500-ml flasks and cultured aerobically on a reciprocal shaker at 37°C for 20 h. The cells were harvested from the culture broth by centrifugation at 10,000 × g for 10 min. The harvested cells were washed with 10 mM potassium phosphate buffer (pH 7.0) and then suspended in the same buffer. Cell growth was estimated turbidimetrically by means of a dry-cell calibration curve for the absorbance at 610 nm: 0.24 mg of dry cell weight per ml was equivalent to 1.0 U of optical density at 610 nm. Approximately 200 mg (dry weight) of cells was obtained from 200 ml of culture broth. The standard reaction mixture for 5′-IMP synthesis contained various concentrations of inosine, 150 g of disodium hydrogen pyrophosphate per liter (200 mM), 1 mM sodium acetate buffer (pH 4.0), and 1 g (dry weight) of cells per liter in a 10-ml volume. The reaction was carried out at 30°C with moderate shaking and stopped by adding 1 ml of 2 N HCl. Synthesized 5′-IMP was calculated as IMP · 2Na · 7.5H2O (molecular weight, 527).
Enzyme purification.
Each acid phosphatase was purified from harvested cells of E. coli JM109 transformants harboring wild-type or mutated phoC by ammonium sulfate fractionation and ion-exchange, hydrophobic, and gel filtration column chromatographies as described previously (2). The purity of the recovered samples was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (14% polyacrylamide).
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession number AB35805.
RESULTS
Isolation of an M. morganii pyrophosphate-nucleoside phosphotransferase gene.
For mutagenesis, an M. morganii gene encoding a pyrophosphate-nucleoside phosphotransferase was isolated by a shotgun cloning strategy. As the enzyme appeared to be a phosphatase (2), phosphatase-positive clones were selected, and their phosphotransferase activity was examined. Of about 10,000 transformants examined, 42 were phosphatase positive. Among these 42 candidates, 3 transformants showed phosphotransferase activity. These three may overlap, because a 1.2-kb EcoRI-HindIII fragment was contained in the plasmids rescued from each candidate.
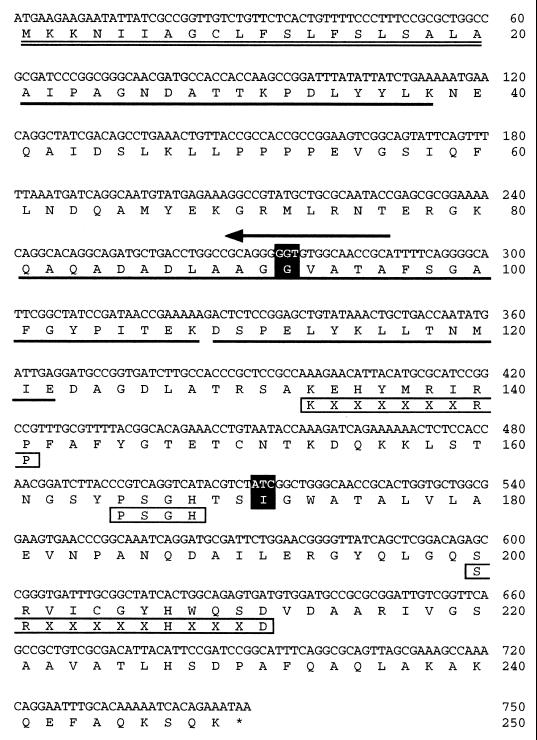
The results of subcloning showed that phosphotransferase activity was retained on a 1.2-kb EcoRI-HindIII fragment. The 1.2-kb fragment was subcloned into pUC18 and designated pMPI501. The nucleotide sequence of the fragment (given in the database) revealed an open reading frame (ORF) encoding the 747-bp phosphotransferase gene (Fig. 1). The amino-terminal and internal amino acid sequences of the purified enzyme were also detected in the deduced amino acid sequence (Fig. 1). The amino-terminal segment actually appeared to function as a signal sequence that was cleaved by a signal peptidase after the alanine residue at position 18. The calculated molecular mass of 25,004 Da (calculated from 693 bp coding for 231 amino acids, excluding the signal peptide that was removed by posttranslational modification) is in good agreement with the value of 25,000 estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified protein. From these results, we concluded that the ORF encoded the phosphotransferase gene. Computer sequence alignment using the SWISS-PROT and NBRF-PIR databases revealed that the gene appeared to be identical to the M. morganii PhoC acid phosphatase gene (accession no. P28581) (18). Seven bases in the ORFs were different, but these differences did not lead to differences in the predicted amino acid sequences: 54 A (in this report) to G (in phoC), 72 A to G, 276 A to T, 378 C to T, 420 T to G, 525 G to C, and 531 A to G.
FIG. 1.
Nucleotide sequence of the M. morganii PhoC acid phosphatase gene and predicted amino acid sequence. A 747-bp ORF is shown with the deduced 249-amino-acid sequence. Sequences confirmed by protein sequencing are underlined. The signal sequence of the PhoC protein is doubly underlined. The amino acid residues substituted in the course of random mutagenesis are shaded in black. The mutagenic primer is indicated by an arrow. Three phosphatase motif domains are indicated in boxes under the deduced amino acid sequence.
Reaction conditions for the synthesis of 5′-IMP by E. coli overproducing wild-type PhoC acid phosphatase.
Phosphotransferase activity was hardly detectable in E. coli JM109(pUC18). On the other hand, the specific activity of phosphotransferase in a cell extract of E. coli JM109(pMPI501) cells grown in LB broth with induction by IPTG was 1.25 ± 0.01 U/mg of protein; this value was 1.5- and 120-fold higher than those of cells grown without IPTG induction [(8.50 ± 0.10) × 10−1 U/mg] and of M. morganii [(1.04 ± 0.02) × 10−2 U/mg, without IPTG induction], respectively. As the transformant showed high phosphotransferase activity without IPTG induction, phoC appeared to be expressed under the control of both its own promoter and the lac promoter.
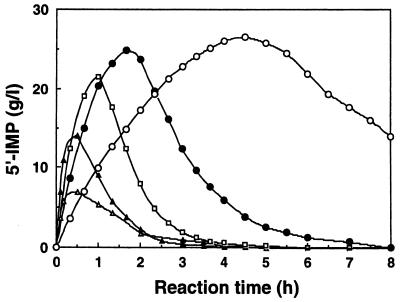
The effect of pH on the synthesis of 5′-IMP was studied using resting cells of E. coli JM109(pMPI501) overproducing PhoC acid phosphatase. The optimum pH for the phosphorylation reaction was found to be 5.2, and the rate of 5′-IMP synthesis decreased as the reaction pH decreased. At a lower pH, the rate of hydrolysis of 5′-IMP also decreased and the amount of 5′-IMP synthesized increased (Fig. 2). However, at any pH, dephosphorylation of the synthesized 5′-IMP occurred and all of the IMP was hydrolyzed to inosine as the reaction time was prolonged. On the basis of these results, further investigations of the phosphorylation reaction were performed at pH 4.0 and 30°C.
FIG. 2.
Effect of pH on the synthesis of 5′-IMP. The time course of 5′-IMP synthesis was measured at different pHs with 0.1 M sodium acetate buffer: pH 3.5 (○), pH 4.0 (●), pH 5.0 (□), pH 5.5 (▴), and pH 6.0 (▵). The reaction was carried out at 30°C in a reaction mixture consisting of 0.1 M sodium acetate buffer containing (per liter) 20 g of inosine (74.6 mM), 150 g of disodium hydrogen pyrophosphate (200 mM), and 10 g (dry weight) of E. coli JM109(pMPI501) cells.
Random mutation of PhoC acid phosphatase and synthesis of 5′-IMP by E. coli overproducing mutated PhoC acid phosphatase.
In order to suppress the dephosphorylation reaction and increase the efficiency of the transphosphorylation reaction, a random mutagenesis approach was used. By error-prone PCR, random mutations were introduced into the PhoC acid phosphatase gene. About 2,000 transformants that overexpressed mutated phoC were screened for increased yield of the phosphotransferase reaction. One candidate mutant, which showed almost the same phosphotransferase activity as E. coli(pMPI501) harboring wild-type phoC as well as lower 5′-nucleotidase activity, was successfully obtained in the first round. This improved variant, derived from E. coli JM109(pMPI600), was chosen as the parent for the second generation. The second round of random mutagenesis was performed on the mutated gene using the same procedure. About 3,000 transformants were screened, and a more improved mutant, E. coli JM109(pMPI700), was obtained. This candidate showed lower phosphotransferase activity but much lower 5′-nucleotidase activity than E. coli JM109(pMPI600).
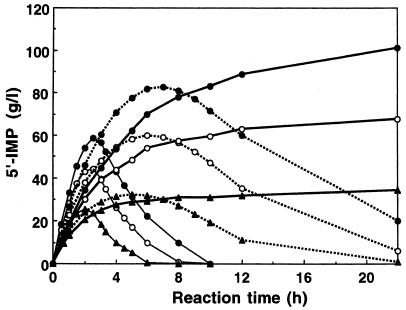
The time course of 5′-IMP synthesis using E. coli overproducing the wild-type and mutated phoC gene products is shown in Fig. 3. Inosine was phosphorylated to 5′-IMP, along with the hydrolysis of PPi. Using E. coli JM109(pMPI501), 58.5 g of 5′-IMP per liter (111 mM) was synthesized, with a maximum molar yield of approximately 49%, from inosine. As the reaction time was prolonged, dephosphorylation was directed toward 5′-IMP and all of the synthesized 5′-IMP was hydrolyzed to inosine. With E. coli JM109(pMPI600), dephosphorylation of the synthesized 5′-IMP was suppressed to some extent, and a maximum of 82.9 g of 5′-IMP per liter (157 mM) was synthesized, with a molar yield of approximately 70%, from inosine.
FIG. 3.
5′-IMP synthesis using E. coli overproducing wild-type or mutated acid phosphatases. The time course of 5′-IMP synthesis by resting cells of E. coli JM109(pMPI501) (thin lines), E. coli JM109(pMPI600) (broken lines), and E. coli JM109(pMPI700) (thick lines) was measured. The reaction was carried out at pH 4.0 and 30°C in a reaction mixture consisting of 0.1 M sodium acetate buffer (pH 4.0) containing various concentration of inosine, 150 g of disodium hydrogen pyrophosphate per liter (200 mM), and 10 g (dry weight) of each type of cell per liter. Inosine was added to the reaction mixture at 20 g/liter (74.6 mol/liter) (▴), 40 g/liter (149 mol/liter) (○), and 60 g/liter (224 mol/liter) (●).
The productivity of E. coli JM109(pMPI700) was superior to that of either of these strains. With E. coli JM109(pMPI501), the molar yield of 5′-IMP decreased as the concentration of inosine increased. In contrast, such a decrease in yield did not occur in the reaction with E. coli JM109(pMPI700); 101 g of 5′-IMP per liter (192 mM) was synthesized, with a molar yield of 88%, from inosine. Furthermore, dephosphorylation of the synthesized 5′-IMP was considerably depressed. These observations confirm that the mutated enzyme phosphorylates nucleosides to a useful extent at a practical level.
Characterization of mutated acid phosphatases.
Gene sequencing of 1.2-kb EcoRI-HindIII fragments cloned into pMPI501, pMPI600, and pMPI700 showed that the only mutation site in phoC from pMPI600 was at Ile-171 (ATC), which was altered to Thr (ACC). In the gene from pMPI700, a further mutation was observed at Gly-92 (GGT), which was altered to Asp (GAT). In order to investigate the effect of a single G92D mutation, a G92D recombinant was constructed by site-directed mutagenesis.
The wild-type and I171T, I171T-G92D, and G92D mutant enzymes were purified from crude extracts of each E. coli JM109 transformant and analyzed. No changes in the levels of production of the wild-type and mutant enzymes in each E. coli transformant were observed under the culture conditions used. The kinetic constants of these enzymes in the transphosphorylation and dephosphorylation reactions are summarized in Table 1.
TABLE 1.
Kinetic constants for transphosphorylation and dephosphorylation reactionsa
| Activity | Substrate | Strain (plasmid) | Km (μM) | Vmax (U/mg) |
|---|---|---|---|---|
| Phosphotransferase | Inosine | Wild type (pMPI501) | 117,000 ± 1,000 | 6.09 ± 0.10 |
| I171T (pMPI600) | 73,900 ± 2,800 | 2.77 ± 0.11 | ||
| G92D (pMPI502) | 114,000 ± 3,000 | 0.983 ± 0.050 | ||
| I171T-G92D (pMPI700) | 42,600 ± 1,800 | 2.67 ± 0.11 | ||
| 5′-Nucleotidase | 5′-IMP | Wild type (pMPI501) | 836 ± 26 | 30.3 ± 0.9 |
| I171T (pMPI600) | 1,630 ± 46 | 11.6 ± 0.4 | ||
| G92D (pMPI502) | 1,490 ± 20 | 4.70 ± 0.19 | ||
| I171T-G92D (pMPI700) | 1,350 ± 14 | 5.67 ± 0.15 | ||
| Acid phosphatase | p-NPP | Wild type (pMPI501) | 41.2 ± 1.0 | 82.9 ± 1.8 |
| I171T (pMPI600) | 64.2 ± 2.1 | 97.4 ± 3.8 | ||
| G92D (pMPI502) | 116 ± 5 | 42.3 ± 4.9 | ||
| I171T-G92D (pMPI700) | 50.6 ± 3.1 | 30.6 ± 1.8 |
The enzyme activities were assayed as described in Materials and Methods. The initial velocities were determined, and the steady-state kinetic constants were calculated by using a Lineweaver-Burk plot. Km and Vmax values are given as means ± standard deviations.
The Km value for inosine of the wild-type enzyme in the transphosphorylation reaction was 117 mM, which was extremely high. In contrast, the Km value for 5′-IMP of the wild-type enzyme in the dephosphorylation reaction was much lower. In addition, the Vmax of the enzyme for the dephosphorylation reaction was higher than that for the transphosphorylation reaction. One key distinction of the improved I171T-G92D mutant was a reduction in the Km value for inosine in the phosphotransferase reaction to approximately one-third that of the wild type. However, a similar reduction in the Vmax of the phosphotransferase reaction was also observed. Another key distinction of the evolved enzymes was a reduction in the Vmax of 5′-nucleotidase activity to approximately one-sixth that of the wild type. On the other hand, there were only small changes in the kinetic parameters for the dephosphorylation of p-NPP.
DISCUSSION
The M. morganii phosphotransferase gene was identical to the PhoC acid phosphatase gene (18). Although several phosphatase genes were also cloned from the M. morganii chromosomal DNA library in the course of the gene cloning, only the PhoC acid phosphatase exhibited phosphotransferase activity when PPi was used as the phosphate donor.
Acid phosphatases are further divided into three classes, designated A, B, and C, on the basis of amino acid sequence similarity. M. morganii PhoC acid phosphatase is classified as a class A nonspecific acid phosphatase; these enzymes have a polypeptide component of 25 to 27 kDa (19). To date, enzymes of this class have been isolated from several species, including Zymomonas mobilis, Salmonella typhimurium, and Shigella flexneri (14). Stukey and Carman have found that class A nonspecific acid phosphatases have a conserved sequence motif, KXXXXXXRP - (X12–54) - PSGH - (X31–54) - SRXXXXXHXXXD, which is shared by several lipid phosphatases and the mammalian glucose-6-phosphatases, and a mechanistic relationship among these enzymes has been suggested (17). Glucose-6-phosphatase is well known to be a multifunctional enzyme with potent phosphotransferase activity as well as phosphohydrolase activity (13). It catalyzes the six-position selective transfer of a phosphoryl group from PPi to glucose. It is interesting that the M. morganii PhoC acid phosphatase, which catalyzes the C-5′-position selective transfer of a phosphoryl group from PPi to nucleoside, shares a conserved sequence motif with glucose-6-phosphatase, as indicated in Fig. 1.
The reaction of phosphotransferase activity catalyzed by a phosphatase is thought to operate via a phosphate-enzyme intermediate. It involves the formation of binary enzyme-phosphoryl substrate complexes, which then dissociate to yield a common phosphoryl-enzyme intermediate. Transfer of the phosphoryl group to a phosphate acceptor leads to the production of a binary enzyme-phosphate acceptor-phosphate complex that ultimately dissociates to yield phosphorylated acceptor and free enzyme. Therefore, the phosphate acceptor competes with water, and a rather high concentration of acceptor is required for the transphosphorylation reaction.
As the Km value of the wild-type enzyme for inosine in the transphosphorylation reaction was extremely high, the efficiency of the transphosphorylation reaction was highly dependent on the concentration of inosine. The solubility of inosine in our reaction conditions was about 80 mM, reaching only two-thirds the Km value. Therefore, the efficiency of the transphosphorylation reaction of the wild-type enzyme was very low. On the other hand, the Km value for inosine of the I171T-G92D mutant enzyme was decreased to approximately one-third that of the wild-type enzyme, well below the achievable inosine concentration of 80 mM. As the affinity of the mutated enzyme for inosine increases, the efficiency of the transphosphorylation reaction should increase; additionally, the transphosphorylation reaction catalyzed by the mutated enzyme will proceed at a lower concentration of inosine than will that catalyzed by the wild-type enzyme. The mutations also led to a reduction in the Vmax of phosphotransferase activity, but the effect of the decrease in the Km was to allow a higher reaction rate under these conditions than would be expected from the reduced Vmax. In addition, as the Vmax of the 5′-nucleotidase activity of the mutated enzyme was decreased to approximately one-sixth the wild-type value, dephosphorylation of the synthesized 5′-IMP was suppressed. As the result of these changes in catalytic properties, the productivity of the mutated enzyme appeared to be much improved.
The I171T mutation was found to contribute to the decrease in the Km value for inosine and to the increase in the Km value for 5′-IMP. The G92D mutation did not seem to contribute to the decreased Km for inosine, but it contributed to the decreased Vmax values of both the phosphatase and the phosphotransferase activities. There was a synergistic effect of G92D combined with I171T: the Km value for inosine of the I171T-G92D mutant was reduced to about one-half that of the I171T single mutant.
We have demonstrated the potential of a new method of phosphorylating nucleosides using a mutated acid phosphatase. The 5′-nucleotidase activity of wild-type PhoC acid phosphatase rehydrolyzed the synthesized 5′ nucleotide to a nucleoside, making the wild-type enzyme unsuitable for the phosphorylation of nucleosides. However, the catalytic properties of the enzyme could be much improved by a random mutagenesis approach, and the improved enzyme synthesized 5′-IMP at a practical level.
PPi is a safe and inexpensive compound that can be used in large excess. Also, PPi is easily synthesized from phosphate (16); therefore, an efficient phosphorylation process could be achieved by recycling PPi from phosphate formed as a by-product in the phosphorylation reaction. In addition, this enzyme shows broad substrate specificity for the phosphate acceptor (2) and could be applied to the synthesis of various 5′ nucleotides. It was very interesting that the productivity of the enzyme in E. coli was much improved by the substitution of only two amino acid residues. Further studies on the structure-activity relationships of wild-type and mutated acid phosphatases are in progress.
ACKNOWLEDGMENT
We thank T. Dairi, Toyama Prefectural University, for advice concerning this work.
REFERENCES
- 1.Asano Y, Mihara Y, Yamada H. A new enzymatic method of selective phosphorylation of nucleosides. J Mol Catal B Enzymat. 1999;6:271–277. [Google Scholar]
- 2.Asano Y, Mihara Y, Yamada H. A novel selective nucleoside phosphorylation enzyme from Morganella morganii. J Biosci Bioeng. 1999;87:732–738. doi: 10.1016/s1389-1723(99)80145-5. [DOI] [PubMed] [Google Scholar]
- 3.Brawerman G, Chargaff E. On the synthesis of nucleotides by nucleoside phosphotransferase. Biochim Biophys Acta. 1954;15:549–559. doi: 10.1016/0006-3002(54)90013-x. [DOI] [PubMed] [Google Scholar]
- 4.Cadwell R C, Joyce G F. Randomization of genes by PCR mutagenesis. PCR Methods Applic. 1992;2:28–33. doi: 10.1101/gr.2.1.28. [DOI] [PubMed] [Google Scholar]
- 5.Cadwell R C, Joyce G F. Mutagenic PCR. PCR Methods Applic. 1994;3:S136–S140. doi: 10.1101/gr.3.6.s136. [DOI] [PubMed] [Google Scholar]
- 6.Ito W, Ishiguro H, Kurosawa Y. A general method for introducing a series of mutants into cloned DNA using the polymerase chain reaction. Gene. 1991;102:67–70. doi: 10.1016/0378-1119(91)90539-n. [DOI] [PubMed] [Google Scholar]
- 7.Kotani Y, Yamaguchi K, Kato F, Furuya A. Inosine accumulation by mutants of Brevibacterium (Corynebacterium) ammoniagenes: strain improvement and culture conditions. Agric Biol Chem. 1978;42:399–405. [Google Scholar]
- 8.Matsui H, Sato K, Enei H, Hirose Y. Production of guanosine by pscicofuranine and decoinine resistant mutants of Bacillus subtilis. Agric Biol Chem. 1979;43:1739–1744. [Google Scholar]
- 9.Matsui H, Sato K, Enei H, Takinami K. 5′-Nucleotidase activity in improved inosine-producing mutants of Bacillus subtilis. Agric Biol Chem. 1982;46:2347–2352. [Google Scholar]
- 10.Mitsugi K, Komagata K, Takahashi M, Iizuka H, Katagiri H. Bacterial synthesis of nucleotides. Part II. Distribution of nucleoside phosphotransferase in bacteria. Agric Biol Chem. 1964;28:586–600. [Google Scholar]
- 11.Mori H, Iida A, Teshiba S, Fujio T. Cloning of a guanosine-inosine kinase gene of Escherichia coliand characterization of the purified gene product. J Bacteriol. 1995;177:4921–4926. doi: 10.1128/jb.177.17.4921-4926.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori H, Iida A, Fujio T, Teshiba S. A novel process of inosine 5′-monophosphate production using overexpressed guanosine/inosine kinase. Appl Microbiol Biotechnol. 1997;48:693–698. doi: 10.1007/s002530051117. [DOI] [PubMed] [Google Scholar]
- 13.Nordlie R C. Glucose-6-phosphatase, hydrolytic and synthetic activities. In: Boyer P D, editor. The enzymes. 3rd ed. Vol. 4. New York, N.Y: Academic Press, Inc.; 1972. pp. 543–609. [Google Scholar]
- 14.Rossolini G M, Schippa S, Riccio M L, Berlutti F, Macaskie L E, Thaller M C. Bacterial nonspecific acid phosphohydrolases: physiology, evolution and use as tools in microbial biotechnology. Cell Mol Life Sci. 1998;54:833–850. doi: 10.1007/s000180050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 16.Staffel T. Phosphoric acid and phosphates. In: Elvers B, Hawkins S, Schulz G, editors. Ullmann's encyclopedia of industrial chemistry. 5th ed. A19. Weinheim, Germany: VCH Verlag; 1991. pp. 485–494. [Google Scholar]
- 17.Stukey J, Carman G M. Identification of a novel phosphatase sequence motif. Protein Sci. 1997;6:469–472. doi: 10.1002/pro.5560060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaller M C, Berulutti F, Schippa S, Lombordi G, Rossolini G M. Characterization and sequence of PhoC, the principal phosphate-irrepressible acid phosphatase of Morganella morganii. Microbiology. 1994;140:1341–1350. doi: 10.1099/00221287-140-6-1341. [DOI] [PubMed] [Google Scholar]
- 19.Thaller M C, Schippa S, Rossolini G M. Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci. 1998;7:1647–1652. doi: 10.1002/pro.5560070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 21.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa M, Kato T, Takenishi T. Studies of phosphorylation. III. Selective phosphorylation of unprotected nucleosides. Bull Chem Soc Jpn. 1969;42:3505–3508. [Google Scholar]