Abstract
Background
The type 1 electrocardiographic (ECG) pattern diagnostic of Brugada syndrome (BrS) can be dynamic. Limited studies have rigorously evaluated the temporal stability of the Brugada ECG pattern.
Objective
We sought to evaluate fluctuations of the Brugada pattern in serial resting ECGs from BrS patients managed within a large health care system.
Methods
In our cohort of BrS patients with at least 2 standard, resting ECGs recorded on separate clinical encounters, we evaluated serial changes in the Brugada pattern and categorized patients into 1 of 3 groups: dynamic was defined as the presence of both type 1 and non–type 1 patterns in available ECGs; the provoked-only group was defined as having a non–type 1 Brugada pattern across resting ECGs; and the persistent group was defined as having a type 1 pattern on all ECGs. We also evaluated the clinical risk in this cohort according to the Shanghai risk score.
Results
In 72 patients with BrS (mean age 46 ± 15 years, 69% male), 828 standard, resting ECGs were recorded over a median duration of 30.2 (interquartile range 6.3–68.1) months. The dynamic group comprised 50 (69% of the cohort) patients, the provoked-only group consisted of 17 patients (24% of the cohort), and the persistent group included 5 patients. No significant differences were detected in the total number of ECGs evaluated during the follow-up period between any of the groups. Only sinus node dysfunction and a prior cardiac arrest were associated with the persistent type 1 group. The majority of patients had a low annualized risk of lethal arrhythmic events.
Conclusion
Most BrS patients have a dynamic Brugada pattern noted on longitudinal, resting ECGs. Expert consensus statements should provide clarity on the frequency of obtaining resting ECGs in patients suspected of having BrS during follow-up.
Keywords: Brugada syndrome, Channelopathies, Electrocardiography, Risk stratification, Sudden cardiac death
Graphical abstract
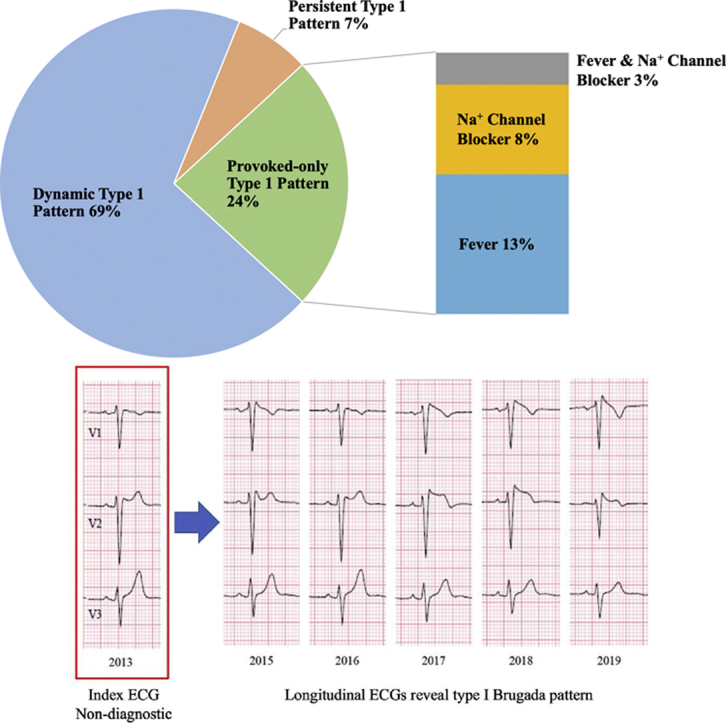
Key Findings.
-
▪
The majority of Brugada syndrome patients had a dynamic electrocardiogram (ECG) pattern in which a spontaneous type 1 pattern was present only 20% of the time.
-
▪
Of patients with a type 1 Brugada pattern provoked by fever or sodium channel blockade, nearly half presented with a spontaneous type 1 pattern at some point in follow-up.
-
▪
In Brugada syndrome patients, a persistent spontaneous type 1 ECG pattern is rarely present.
Introduction
The type 1 electrocardiogram (ECG) pattern diagnostic of Brugada syndrome (BrS) is often dynamic. Early studies evaluated serial ECGs in BrS patients and described a high prevalence of an intermittent type 1 pattern.1,2 Further, only 2% of patients had a persistent type 1 pattern. One of these studies evaluated the dynamic nature of the type 1 ECG pattern in patients who had BrS and an implantable cardioverter-defibrillator (ICD).2 This group had a higher risk for lethal arrhythmias and the findings are not generalizable to a broader BrS population without an ICD. Also, given that several of these studies are from >10 years prior, it is relevant to expand prior observations of a dynamic type 1 pattern to a contemporary, lower-risk population.
Patients with a documented spontaneous type 1 pattern have greater arrhythmic risk than those with a provoked-only phenotype.3 Holter monitoring studies have demonstrated that one-third of patients with a drug-provoked type 1 pattern had a spontaneous type 1 pattern over a 24-hour period.4 However, the prevalence of a spontaneous type 1 pattern on 12-lead ECGs in patients who have had a provoked phenotype remains unknown.5
In this study, we sought to evaluate the prevalence of a dynamic type 1 Brugada pattern across serial resting ECGs from a contemporary cohort of BrS patients. In addition, among those patients who had a provoked Brugada pattern, we evaluated the presence of a spontaneous type 1 phenotype across the longitudinal follow-up period.
Methods
Study population
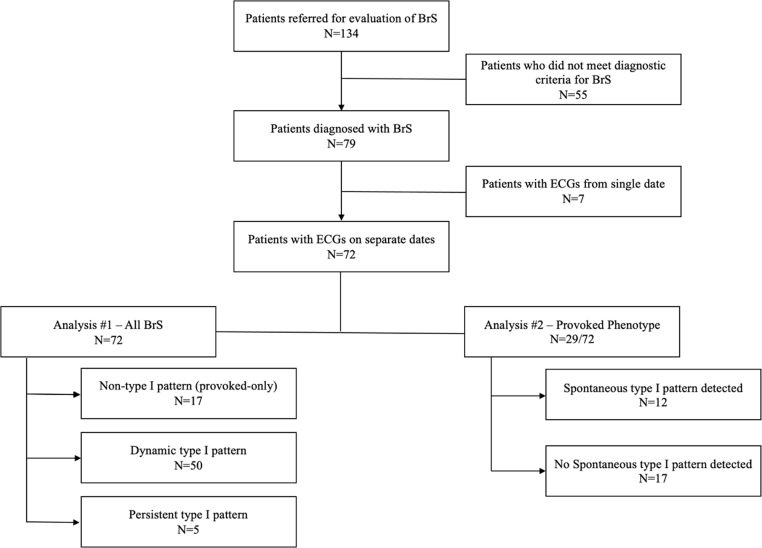
Our primary cohort was composed of 134 patients, who were referred to the University of Pennsylvania electrophysiology program for evaluation of suspected BrS between 2005 and 2019 (Figure 1). Patients were referred for either symptomatic arrhythmic events, cascade family screening of suspected or confirmed BrS, and/or a Brugada pattern on the 12-lead ECG. We only included those patients who had at least 2 ECGs on separate clinical encounters. Phenocopies were excluded with the use of echocardiography and cardiac magnetic resonance imaging. Any equivocal case with overlapping conditions was excluded. All patients provided written informed consent for inclusion in our registry, which was approved by the University of Pennsylvania Health System’s Institutional Review Board.
Figure 1.
Study design. BrS = Brugada syndrome; ECG = electrocardiogram.
Electrocardiographic analysis
For each patient, we evaluated all standard, 12-lead ECGs that were obtained at rest and had been stored in the electronic health record. ECGs were reviewed by at least 2 cardiac electrophysiologists for the presence of a spontaneous type 1 pattern, which was defined according to expert recommendations as coved ST-segment elevation greater than 2 mm in at least 1 right precordial lead.6 P-wave morphology was confirmed to be the same across ECG tracings to ensure similar positioning of the precordial leads. ECG tracings obtained on the same day as a reported fever (>100.5°F) or administration of a sodium channel blocker were considered a provoked phenotype and were not included in the longitudinal analysis evaluating the presence of a spontaneous type 1 Brugada pattern in the resting state.
We initially categorized patients into 1 of 3 groups: (1) the dynamic group, defined as the presence of both type 1 and non–type 1 patterns in available ECGs; (2) the persistent group, defined as patients who had a type 1 pattern on all ECGs; and (3) the provoked-only group, defined as having a non–type 1 Brugada pattern across all standard, resting ECGs. In the provoked-only group, a spontaneous type 1 pattern was never observed on the resting ECGs. Instead, the diagnosis of BrS in this group was established only after provocative testing was performed in the electrophysiology lab or in a febrile state. Patients who had a provoked phenotype and were then found to have a spontaneous type 1 pattern at some later time point were part of the dynamic group.
Clinical risk assessment
A comprehensive clinical profile was documented at the time of the initial evaluation. In addition to basic demographics such as age, sex, and race, we assessed for a history of syncope that was considered secondary to an arrhythmia based on the absence of a prodrome or triggering circumstance. A history of syncope also required a complete loss of consciousness or severe trauma. We also recorded any history of cardiac arrest, atrial fibrillation or atrial flutter, nocturnal agonal respiration, or sinus node dysfunction. Each patient’s family history was reviewed thoroughly for relatives with definite BrS or sudden cardiac death (SCD) in the setting of fever, sleep, or medications that have been implicated in triggering arrhythmic events in BrS.7,8
When available, we recorded the results of genetic testing, including the presence of a pathogenic, unknown, or benign variant in the SCN5A gene. We also evaluated the results of electrophysiology study, including inducibility of ventricular fibrillation (VF). Finally, we documented echocardiographic-based right and left ventricular function.
Statistical analysis
Categorical data are presented as frequencies and proportions; continuous data are presented as mean ± standard deviation or medians and interquartile range. Comparisons were then made between the dynamic, persistent, and provoked-only groups using the χ2 or Fisher exact test for categorical variables and the 1-way ANOVA for continuous variables. We calculated each individual’s Shanghai risk score,9 which was proposed in the 2016 HRS J-Waves Syndromes Expert Consensus Conference Report for diagnosing and risk-stratifying patients.10
In secondary analyses, we created a separate categorization scheme in which we evaluated the subgroup of patients who had a provoked phenotype diagnostic of BrS. Provocation could have occurred either in the febrile state or after sodium channel blockade. Among this group, we evaluated the prevalence of ECGs demonstrating a spontaneous type 1 pattern at any point during follow-up.
Results
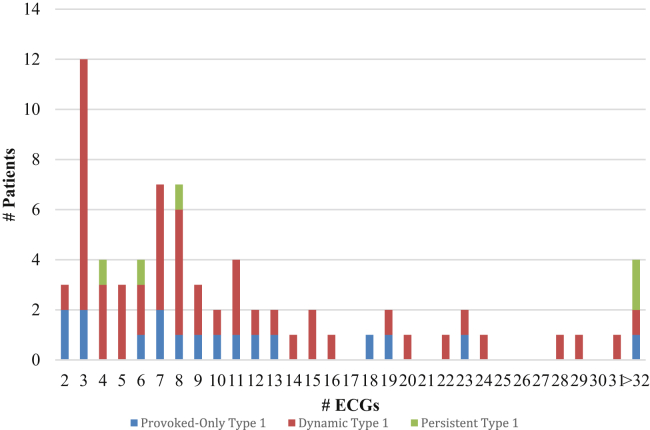
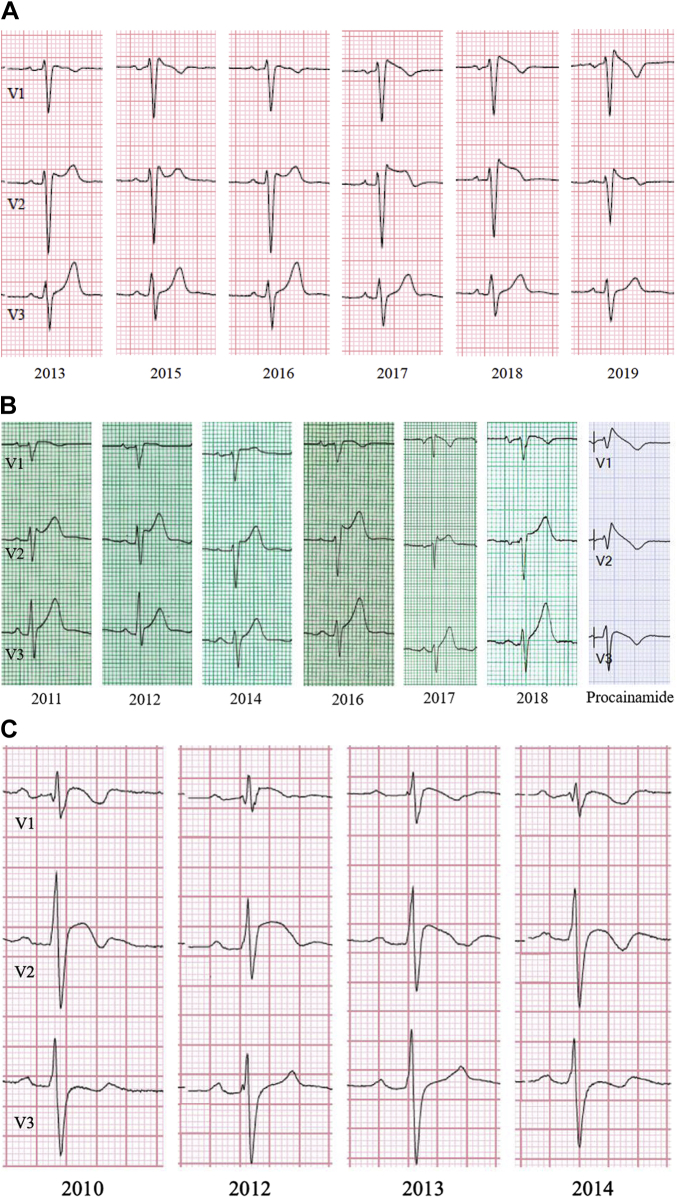
A total of 828 ECGs obtained over a median duration of 30.2 (interquartile range 6.3–68.1) months were evaluated in our final cohort of 72 BrS patients (Table 1). No significant differences were detected in the mean or median number of ECGs evaluated during the follow-up period between any of the ECG groups (Figure 2). The majority of BrS patients had a dynamic, type 1 ECG pattern (50 patients, 69% of cohort; Figure 3, Panel A). This group contributed 553 ECGs, and a spontaneous type 1 Brugada pattern was present only 20% of the time (n = 112 ECGs). There were 17 patients (24% of cohort) who were provoked-only for type 1 pattern and had a non–type 1 ECG pattern on serial, resting ECGs (Figure 3, Panel B). Among these patients, a type 1 pattern was induced via fever in 11 patients or a sodium channel blocker in the electrophysiology laboratory in 8 patients. Only 5 patients (7% of cohort) had a persistent type 1 ECG pattern (Figure 3, Panel C).
Table 1.
Clinical characteristics of provoked-only, dynamic, and persistent type 1 electrocardiogram patterns
| Provoked-only type 1 (n=17) | Dynamic type 1 (n=50) | Persistent type 1 (n=5) | P value C1 vs C2 | P value C1 vs. C3 | P value C2 vs C3 | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) ± SD | 42 ± 16 | 48 ± 15 | 37 ± 11 | .16 | .56 | .12 |
| Male sex, n (%) | 12 (71) | 36 (72) | 2 (40) | 1.00 | .31 | .17 |
| Self-identified race or ethnic group | ||||||
| White, n (%) | 10 (59) | 40 (80) | 3 (60) | .11 | 1.00 | .30 |
| African American, n (%) | 4 (24) | 3 (6) | 1 (20) | .06 | 1.00 | .32 |
| Asian, n (%) | 3 (18) | 2 (4) | 0 (0) | .10 | 1.00 | 1.00 |
| ECG data | ||||||
| No. of ECGs | 198 | 553 | 77 | .85 | .51 | .40 |
| Mean ECGs ± SD | 12 ± 11 | 11 ± 11 | 15 ± 13 | |||
| Median ECGs [Q1–Q3] | 9 [6–13] | 8 [4–14] | 8 [6–29] | |||
| Fever-induced type 1 pattern, n (%) | 11 (65) | 3 (6) | 0 (0) | |||
| Drug-induced type 1 pattern, n (% of tested patients) | 8 (89) | 9 (64) | 0 (0) | |||
| Arrhythmia phenotyping | ||||||
| Syncope, n (%) | 4 (24) | 25 (50) | 2 (40) | .09 | .59 | 1.00 |
| Cardiac arrest / VF / PMVT, n (%) | 0 (0) | 8 (16) | 2 (40) | .10 | .04 | .22 |
| Atrial fibrillation / atrial flutter <30 years, n (%) | 0 (0) | 2 (4) | 0 (0) | 1.00 | 1.00 | 1.00 |
| Nocturnal agonal respiration, n (%) | 2 (12) | 5 (10) | 0 (0) | 1.00 | 1.00 | 1.00 |
| SND, n (%) | 2 (12) | 5 (10) | 3 (60) | 1.00 | .05 | .02 |
| ICD implant, n (%) | 7 (32) | 31 (62) | 3 (60) | .16 | .62 | 1.00 |
| Family history† | ||||||
| Definite BrS, n (%) | 0 (0) | 6 (12) | 1 (20) | .33 | .23 | .51 |
| SCD, n (%) | 3 (18) | 15 (30) | 1 (20) | .53 | 1.00 | 1.00 |
| SCD <45 years, n (%) | 3 (18) | 8 (16) | 0 (0) | 1.00 | 1.00 | 1.00 |
| Genetics | ||||||
| Pathogenic BrS variant, n (% of tested patients) | 1 (14) | 4 (16) | 2 (100) | 1.00 | .12 | .09 |
| Electrophysiology study | ||||||
| Inducible VF with PES, n (% of tested patients) | 5 (83) | 15 (75) | 3 (100) | 1.00 | 1.00 | 1.00 |
| Imaging | ||||||
| LVEF %, mean ± SD | 61 ± 4 | 61 ± 5 | 56 ± 5 | .86 | .03 | .06 |
| RV dysfunction moderate to severe, n (%) | 0 (0) | 1 (2) | 0 (0) | 1.00 | 1.00 | 1.00 |
BrS = Brugada syndrome; ECG = electrocardiogram; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; PES = programmed electrical stimulation; PMVT = polymorphic ventricular tachycardia; RV = right ventricular; SCD = sudden cardiac death; SND = sinus node dysfunction; VF = ventricular fibrillation.
Family history was evaluated in first- and second-degree relatives.
Figure 2.
Distribution of the number of electrocardiograms (ECGs) according to phenotype classification: provoked-only type 1 group; dynamic type 1 group; and persistent type 1 group.
Figure 3.
A: Serial electrocardiograms (ECGs) from a patient showing a dynamic type 1 Brugada pattern. A type 1 Brugada pattern was observed in this patient but was not persistent across all ECGs obtained from the clinical encounters. B: Serial ECGs from a patient with static non–type 1 Brugada pattern. A type 1 Brugada pattern was only inducible through provocative testing or in the setting of fevers for patients in this group. C: Serial ECGs from a patient with persistent type 1 Brugada pattern. A type 1 Brugada pattern was observed across all ECGs from all clinical encounters for this patient.
The mean age across the entire cohort was 46 ± 15 years, 69% were men, and no significant differences in these demographics were observed between the 3 groups (Table 1). A history of syncope was absent in the majority of patients in our BrS cohort. Half of the individuals in the dynamic type 1 group and approximately one-quarter of those in the provoked-only group had a history of syncope. The prevalence of other arrhythmic conditions including prior cardiac arrest/VF/ventricular tachycardia, atrial arrhythmias, and sinus node dysfunction was low. Further, 41 patients (57% of study population) had received an ICD. Although our cohort only had 5 patients with a persistent type 1 pattern, these individuals had a higher likelihood of having a prior cardiac arrest or sinus node dysfunction. Family history of SCD was present in approximately 20%–30% of patients across the 3 groups.
Of the 72 patients in our Brugada cohort, 34 patients elected to undergo commercial genetic screening for pathogenic variants implicated in BrS. A SCN5A mutation was found in the minority of patients from the provoked-only and dynamic ECG groups. Only 2 patients from the persistent type 1 group had genetic testing and both had SCN5A mutations. There were 29 patients who underwent electrophysiology study, and the majority of them were inducible for VF. Left ventricular ejection fraction at index clinical presentation was lower statistically in the persistent type 1 patients compared to the other 2 ECG groups. In 1 patient, BrS had been diagnosed nearly 2 decades prior after an aborted cardiac arrest, and moderate-to-severe right ventricular dysfunction (cor pulmonale) occurred in follow-up and was present at the time of the initial ECGs ascertained in this analysis.
In the exploratory, secondary analysis of all patients diagnosed for BrS based on provocative testing, a spontaneous type 1 ECG pattern was documented in 12 of 29 patients at some point in the follow-up period (Table 2). A spontaneous type 1 pattern was not detected in the other 17 patients of this subgroup. Although the overall number of patients is limited, no significant differences were observed in patient demographics. Also, patients with a spontaneous type 1 pattern appeared to have higher rates of syncope, cardiac arrest, and family history of BrS and SCD; however, these differences were not statistically significant.
Table 2.
Comparison of electrocardiograms and clinical characteristics in Brugada syndrome patients with provoked phenotype
| Spontaneous type 1 not detected (n = 17) | Spontaneous type 1 present (n = 12) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) ± SD | 42 ± 16 | 48.8 ± 15.7 | .26 |
| Male sex, n (%) | 12 (71) | 7 (58) | .69 |
| Race or ethnic group | |||
| White, n (%) | 10 (59) | 10 (83) | .23 |
| African American, n (%) | 4 (24) | 1 (8) | .37 |
| Asian, n (%) | 3 (18) | 1 (8) | .62 |
| ECG data | |||
| No. of ECGs | 198 | 164 | .70 |
| Mean ECGs ± SD | 12 ± 11 | 14 ± 17 | |
| Median ECGs [Q1–Q3] | 9 [6–13] | 8 [3–16] | |
| Fever-provoked type 1 pattern, n (%) | 11 (65) | 3 (25) | .06 |
| Drug-provoked type 1 pattern, n (% of tested patients) | 8 (89) | 9 (100) | 1.00 |
| Arrhythmia phenotyping | |||
| Syncope, n (%) | 4 (24) | 5 (42) | .42 |
| Cardiac arrest / VF / PMVT, n (%) | 0 (0) | 2 (17) | .16 |
| Atrial fibrillation / atrial flutter <30 years, n (%) | 0 (0) | 0 (0) | 1.00 |
| Nocturnal agonal respiration, n (%) | 2 (12) | 1 (8) | 1.00 |
| SND, n (%) | 2 (12) | 0 (0) | .50 |
| Family history† | |||
| Definite BrS, n (%) | 0 (0) | 3 (25) | .06 |
| SCD, n (%) | 3 (18) | 6 (50) | .11 |
| SCD <45 years, n (%) | 3 (18) | 3 (25) | 1.00 |
| Genetics | |||
| Pathogenic BrS variant, n (% of tested patients) | 1 (14) | 1 (8) | 1.00 |
| Electrophysiology study | |||
| Inducible VF with PES, n (% of tested patients) | 5 (83) | 5 (83) | 1.00 |
Abbreviations as in Table 1.
Family history was evaluated in first- and second-degree relatives.
Distribution of risk
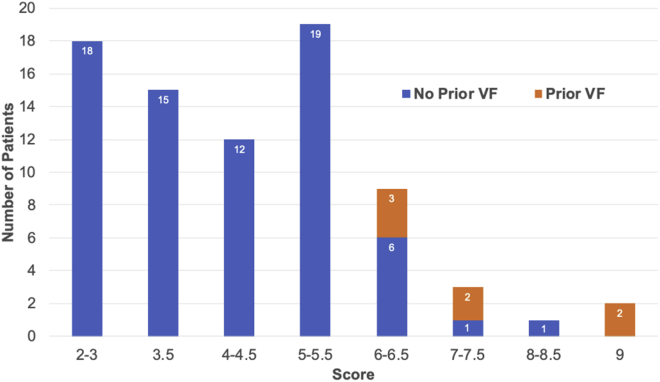
As part of the primary analysis, the mean Shanghai score was 3.3 ± 0.8 for the provoked-only group, who never had any evidence of a spontaneous type 1 ECG pattern; 5.0 ± 1.5 for the dynamic ECG group; and 5.7 ± 2.4 for the persistent type 1 group. Differences in mean Shanghai score were significant between the provoked-only group and dynamic group (P < .001) and between the provoked-only group and persistent type 1 group (P < .005). No difference in risk score was detected between the dynamic and persistent type 1 group (P = .313). The overall distribution in our cohort is depicted in Table 3 and Figure 4. All 7 patients who had a history of ventricular fibrillation at initial presentation scored 6 points or greater. No patient scored less than 2 points, which is nondiagnostic for BrS according to the Shanghai scoring system.
Table 3.
Clinical characteristics of the study cohort and proposed Shanghai score system
| Characteristic | N (%) (N = 79 subjects) |
|---|---|
| I. ECG (12-lead/ambulatory) | |
|
52 (66) |
|
15 (19) |
|
12 (15) |
| II. Clinical history | |
|
10 (12) |
|
7 (9) |
|
24 (30) |
|
11 (14) |
|
3 (4) |
| III. Family History | |
|
7 (9) |
|
9 (11) |
|
11 (14) |
| IV. Genetic test result | |
|
7 (19) |
BrS = Brugada syndrome; ECG = electrocardiogram; SCD = sudden cardiac death; VF = ventricular fibrillation; VT = ventricular tachycardia.
Figure 4.
Distribution of risk according to the Shanghai risk score. The maximum score possible is 9. A score of <2 points is nondiagnostic of Brugada syndrome, a score of 2–3 indicates possible Brugada syndrome, and a score of ≥3.5 points suggests definite or probable Brugada syndrome. VF = ventricular fibrillation.
Discussion
Our study evaluated 72 BrS patients, who contributed 828 ECGs over a 30-month average follow-up period, to provide a contemporary understanding on the fluctuation between type 1 and non–type 1 ECGs. The majority of patients from our practice had a dynamic ECG pattern in which a spontaneous, unprovoked type 1 Brugada pattern was present only 20% of the time. In addition, approximately 25% of our cohort had a static, non–type 1 pattern on routine ECGs and required provocation with sodium channel blockers or an increase in body temperature. In further analysis, we also noted that 41% of all patients with a provoked, type 1 pattern presented with a spontaneous type 1 pattern at some point in follow-up. A persistent type 1 pattern was present in <10% of our cohort. These patients had a higher prevalence of sinus node dysfunction and aborted SCD or VF.
Overall, these findings extend prior observations related to ECG fluctuations in BrS.1,2,4 Compared to 2 prior studies, our contemporary cohort did not require clinical symptoms for the diagnosis of BrS.1,4 One additional study from 2008 reported similar findings in 89 patients with BrS and an implanted ICD.2 The risk of lethal arrhythmic events in our cohort is likely less than that of an ICD population, particularly as diagnostic criteria evolved since 2008 and more patients are diagnosed with BrS without the presence of clinically significant symptoms or history of arrhythmic events.6,10, 11, 12 However, their observation of a persistent type 1 pattern in a minority of patients and approximately one-quarter of all ECGs with a type 1 pattern is similar to the findings of our analysis.
Compared to a higher-risk BrS population with ICDs, our results highlight the implications of the variable type 1 pattern for screening and diagnosis of the BrS in a broader, lower-risk population. A single patient visit in our analysis would have been insufficient to confirm or “rule out” the diagnosis of BrS based on a standard, 12-lead ECG. Instead, “at-risk” patients should be advised to undergo routine follow-up ECG screening, especially when a diagnosis is suspected but not confirmed upon initial evaluation.
The diagnostic uncertainties posed by a single non–type 1 ECG pattern adds to the enigma of BrS—an inherited condition in which the genetics remain poorly understood, and incomplete penetrance is a well-known feature limiting the reliability of clinical disease in family members.13, 14, 15 Provocative testing can be helpful to identify patients; however, limitations exist, as nearly 25% of drug-induced tests might be false-negatives.16, 17, 18 Further, the “provoked phenotype” might be somewhat of a misnomer, especially from the standpoint of risk stratification, as many of these patients develop a spontaneous type 1 pattern during follow-up. Over the course of our follow-up period, we identified a higher rate of spontaneous type 1 ECG findings than a prior Holter monitoring study that analyzed ECG changes over a significantly shorter duration.4 Given the higher risk of cardiac events associated with a spontaneous type 1 pattern, expert panels and clinical guidelines should provide clarity on the importance of longitudinal ECG assessment of suspected patients. Although specific therapies for asymptomatic BrS patients are not available, patients, families, and caregivers would be interested to know if a type 1 ECG pattern has been documented so that appropriate risk counseling and family screening can be pursued.
Routine ECG screening in individuals suspected of having BrS will increase the number of asymptomatic patients. A spontaneous type 1 Brugada pattern contributes at least 3.5 points to the Shanghai risk score and correlates to a 1.2% annual risk of lethal arrhythmic events.9 Other studies have observed similar rates of adverse events that range from 0.5% to 1.2% per year in asymptomatic BrS patients.19, 20, 21 These estimates translate to a nontrivial accumulated lifetime risk of arrhythmic events, especially in a young cohort with a mean age of 46 years. They also highlight the need to evaluate novel therapies and interventions. Management strategies for BrS, such as ICD implantation, quinidine, and radiofrequency ablation, have been limited to high-risk patients who have had syncope, cardiac arrest, or recurrent ICD shocks. However, better phenotyping of what are conventionally deemed to be lower-risk BrS patients might pave the way for future pharmacologic or interventional studies that go beyond just close observation with routine clinical follow-up.
Several limitations of our study should be considered. Our study was not powered to evaluate the long-term risk of ventricular arrhythmias, cardiac arrest, or SCD. As such, our study cannot explain the difference in prognosis for patients with a spontaneous vs drug-induced Brugada pattern. Future studies with large, diverse patient populations should evaluate outcomes according to various risk markers and validate the Shanghai scoring system. In addition, although we reviewed all prescribed medications at each ECG encounter, it is possible that patients were taking medications that had sodium channel blocking properties and were either over-the-counter or prescribed by an out-of-network provider. Another limitation relates to our Brugada patient population as part of a large, academic tertiary referral center. Patients who experience severe arrhythmic events and resuscitated cardiac arrest are more likely to be referred to our institution for further management than lower-risk patients. This referral bias could influence the frequency of detecting a type 1 ECG pattern. In addition, we had limited information available using other methods of risk stratification such as the signal-averaged ECGs, which is known to provide prognostic information.22 However, we sought to eliminate the likelihood of a Brugada pattern phenocopy through detailed assessment of symptoms, family history, advanced imaging, and genetic testing where indicated. Further, exercise testing was not routinely performed in our cohort. Exercise testing is known to elicit a type 1 pattern in some SCN5A mutation–positive patients,23 and hence we may have underdiagnosed type 1 BrS using ECG obtained only in the resting state. Placement of the right precordial leads in the second and third intercostal spaces is also commonly used to elicit the type 1 pattern, and future studies should include these tracings.
Conclusion
Our study demonstrates that the majority of patients with BrS have a dynamic type 1 Brugada pattern. While a spontaneous type 1 pattern confers a higher risk for SCD, our findings show that a single ECG may be insufficient to detect this diagnostic ECG pattern when it is transient in nature, even if pharmacological provocation with a sodium channel blocker is negative. Further, a persistent type 1 pattern was rarely observed in our population. These characteristics may reflect greater inclusivity of a contemporary cohort owing to purely ECG-based diagnostic criteria and expanded BrS phenotyping.
Acknowledgments
Funding Sources
Partial support for this project was provided by the Winkelman Family Fund in Cardiovascular Innovation.
Disclosures
The authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
All patients provided written informed consent for inclusion in the registry.
Ethics Statement
The research reported in this study was conducted according to the principles of the Declaration of Helsinki. The registry was approved by the University of Pennsylvania Health System’s Institutional Review Board.
Disclaimer
Given his role as Associate Editor, Saman Nazarian had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Editors Steven Lubitz and Jeanne E. Poole.
References
- 1.Veltmann C., Schimpf R., Echternach C., et al. A prospective study on spontaneous fluctuations between diagnostic and non-diagnostic ECGs in Brugada syndrome: implications for correct phenotyping and risk stratification. Eur Heart J. 2006;27:2544–2552. doi: 10.1093/eurheartj/ehl205. [DOI] [PubMed] [Google Scholar]
- 2.Richter S., Sarkozy A., Veltmann C., et al. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J Cardiovasc Electrophysiol. 2009;20:69–75. doi: 10.1111/j.1540-8167.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 3.Adler A., Rosso R., Chorin E., Havakuk O., Antzelevitch C., Viskin S. Risk stratification in Brugada syndrome: clinical characteristics, electrocardiographic parameters, and auxiliary testing. Heart Rhythm. 2016;13:299–310. doi: 10.1016/j.hrthm.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Gray B., Kirby A., Kabunga P., et al. Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: potential diagnostic and prognostic implications. Heart Rhythm. 2017;14:866–874. doi: 10.1016/j.hrthm.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S., Hochstadt A., Rosso R. Type-I paradox of Brugada syndrome. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori S.G., Wilde A.A., Horie M., et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Krahn A.D., Healey J.S., Chauhan V., et al. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–285. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 8.Somani R., Krahn A.D., Healey J.S., et al. Procainamide infusion in the evaluation of unexplained cardiac arrest: from the Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) Heart Rhythm. 2014;11:1047–1054. doi: 10.1016/j.hrthm.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Kawada S., Morita H., Antzelevitch C., et al. Shanghai score system for diagnosis of Brugada syndrome: validation of the score system and system and reclassification of the patients. JACC Clin Electrophysiol. 2018;4:724–730. doi: 10.1016/j.jacep.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Antzelevitch C., Gan-Xin Y., Ackerman M.J., et al. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 2016;13:296–324. doi: 10.1016/j.hrthm.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilde A.A., Antzelevitch C., Borggrefe M., et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C., Brugada P., Borggrefe M., et al. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2:429–440. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Priori S.G., Napolitano C., Gasparini M. Clinical and genetic heterogeneity of right bundle branch block and ST-segment elevation syndrome: a prospective evaluation of 52 families. Circulation. 2000;102:2509–2515. doi: 10.1161/01.cir.102.20.2509. [DOI] [PubMed] [Google Scholar]
- 14.Benito B., Sarkozy A., Mont L. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52:1567–1573. doi: 10.1016/j.jacc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Yokokawa M., Noda T., Okamura H. Comparison of long-term follow-up of electrocardiographic features in Brugada syndrome between the SCN5A-positive probands and the SCN5A-negative probands. Am J Cardiol. 2007;100:649–655. doi: 10.1016/j.amjcard.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 16.Hong K., Brugada J., Oliva A., et al. Value of electrocardiographic parameters and ajmaline test in the diagnosis of Brugada syndrome caused by SCN5A mutations. Circulation. 2004;110:3023–3027. doi: 10.1161/01.CIR.0000144299.17008.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung C.C., Mellor G., Deyell M.W., et al. Comparison of ajmaline and procainamide provocation tests in the diagnosis of Brugada syndrome. JACC Clin Electrophysiol. 2019;5:504–512. doi: 10.1016/j.jacep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Chauveau S., Le Vavasseur O., Chevalier P. Delayed diagnosis of Brugada syndrome in a patient with aborted sudden cardiac death and initial negative flecainide challenge. Clin Case Rep. 2017;5:2022–2024. doi: 10.1002/ccr3.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieira J., Ciconte G., Conte G., et al. Asymptomatic Brugada syndrome: clinical characterization and long-term prognosis. Circ Arrhythm Electrophysiol. 2015;8:1144–1150. doi: 10.1161/CIRCEP.114.003044. [DOI] [PubMed] [Google Scholar]
- 20.Sieira J., Conte G., Ciconte G., et al. Clinical characterisation and long-term prognosis of women with Brugada syndrome. Heart. 2016;102:452–458. doi: 10.1136/heartjnl-2015-308556. [DOI] [PubMed] [Google Scholar]
- 21.Sieira J., Conte G., Ciconte G., et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol. 2015;8:777–784. doi: 10.1161/CIRCEP.114.002647. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z., Patel C., Li W., et al. Role of signal-averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: a prospective study. Heart Rhythm. 2009;6:1156–1162. doi: 10.1016/j.hrthm.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Amin A.S., De Groot E.A., Ruijter J.M., Wilde A.A., Tan H.L. Exercise-induced changes in Brugada syndrome. Circ Arrhythm Electrophysiol. 2009;2:531–539. doi: 10.1161/CIRCEP.109.862441. [DOI] [PubMed] [Google Scholar]