Abstract
Background
Cardiac procedures in infants and children require a high level of skill and dexterity owing to small stature and anatomy. Lower incidence of procedure volume in this population results in fewer clinical opportunities for learning. Simulators have grown in popularity for education and training, though most existing simulators are often cost-prohibitive or model adult anatomy.
Objective
Develop a low-cost simulator for practicing the skills to perform percutaneous pericardial access and cardiac ablation procedures in pediatric patients.
Methods
We describe 2 simulators for practicing cardiac procedures in pediatric patients, with a total cost of less than $500. Both simulators are housed within an infant-size doll. The first simulator is composed of an infant-size heart and a skin-like covering to practice percutaneous pericardial access to the heart. Participants obtained sheath access to the heart under direct visualization. The second simulator houses a child-size heart with 7 touch-activated targets to practice manipulating a catheter through a small heart. This can be performed under direct visualization and with 3-dimensional mapping via CARTO. Participants manipulated a catheter to map the heart by touching the 6 positive targets, avoiding the negative target.
Results
Physicians-in-training improved their time to complete the task between the first and second attempts. Physicians experienced with the tools took less time to complete the task than physicians-in-training.
Conclusion
This inexpensive simulator is anatomically realistic and can be used to practice manipulating procedure tools and develop competency for pediatric cardiac procedures.
Keywords: Pediatric, Simulation, Training, Electrophysiology, Pericardiocentesis, Cardiac
Key Findings.
-
▪
It is possible to produce an inexpensive, pediatric-size simulator for practicing skills such as percutaneous pericardial access and cardiac ablation procedures.
-
▪
The simulator provides a safe space to practice maneuvering clinical tools and overcome learning curves associated with new skills.
-
▪
The simulator is anatomically consistent with a clinical setting.
-
▪
Training physicians saw improvement in time to complete a task after practice with the simulator.
Introduction
Procedural phantoms provide the opportunity to train and test medical devices without patient contact or animal models. These simulators allow trainees and practicing physicians to become familiar with procedural techniques and equipment and develop skills in a controlled environment.1 Because the setting is safe, learners have “permission to fail,” can practice at their own rate, and can explore the limits of each technique rather than remaining within the zone of clinical safety.2 This is especially important for procedures performed in children, as they are often performed less frequently than in adults, providing fewer opportunities for hands-on clinical learning. Surgical residents trained using a simulator achieved proficiency with fewer trials and in less time than residents who learned exclusively in the operating room.2,3
Medical training has seen a shift toward virtual reality systems, as they show promise for no-risk practice, relying on haptic feedback to replicate hands-on experience. However, poor mechanical performance of the haptic technology can have a negative training effect.4 Many clinical skills require a high level of dexterity and expertise when it comes to manipulating clinical tools such as needles, sheaths, and catheters, which is not easily replicated by virtual reality but rather is achieved through physical simulators.
While many highly realistic phantoms do exist to provide this tactile experience, they are often prohibitively expensive. Nearly two-thirds of students in a high-fidelity patient simulation study expressed enthusiasm for simulators as opportunities for active learning, practice without risk, and a smooth transition from observing to patient care, while identifying cost as the main disadvantage.5 Simulation systems can range from less than $5000 for simple laparoscopy simulators to over $100,000 for highly sophisticated simulators,6 not including additional maintenance and personnel costs.
Currently, low-cost phantoms for cardiac procedures have some drawbacks. There is an inexpensive simulator for cardiac bypass cannulation, but it relies on porcine hearts that may not be readily accessible for all institutions, requires the sacrifice of an animal, and has limited working time owing to tissue degradation.7 Several groups have attempted to develop a low-cost pericardiocentesis simulator, representing the heart with a ball inside a fluid-filled sack,8,9 yet the ball is not anatomically correct. Furthermore, these simulators have adult-size anatomy. Pantalos and colleagues10 describe a pediatric-size mock circulatory simulator for training the setup of mechanical assist devices, demonstrating the importance of designing simulators for the unique challenges of pediatric care. Thus, we identified an unmet need for a low-cost simulator built to depict pediatric anatomy more accurately and allow trainees to develop the skills, manual dexterity, and strength to maneuver tools under direct visualization.
Percutaneous pericardial access may be used for pericardiocentesis, epicardial arrhythmia mapping and ablation, atrial appendage ligation, or pericardial biopsy.11 Pericardial access requires careful performance of the modified Seldinger technique. Luboz and colleagues12 have attempted to develop a Seldinger technique trainer, but their setup relies on augmented reality and expensive sensors. We propose it is possible to develop a simple setup to allow less experienced physicians to develop the necessary skills to perform procedures such as pericardial needle access or the Seldinger technique successfully and safely. Additionally, this simulator could be used for investigating or training physicians on new medical devices, such as a port for minimally invasive delivery of cardiac pacing leads in pediatric patients.13
Catheter manipulation during ablation procedures can be challenging, requiring a high level of finger dexterity and strength. Inappropriate catheter movements can not only add procedure time but also pose serious risk to important cardiac structures such as the atrioventricular (AV) node.14 Ablation trainers exist with adult-size anatomy (Heartroid; JMC Corporation, Yokohama, Japan) yet do not address the unique challenges of pediatric anatomy. We propose it is possible to develop an inexpensive simulator to allow physicians to practice maneuvering a catheter within the small confines of a pediatric heart and develop the necessary tactile feedback, strength, and dexterity to deliberately maneuver the catheter.
In this article, we outline a low-cost simulator that does not involve tissue or animal models to train physicians for percutaneous pericardial access and cardiac catheter ablation procedures in infants and small children (Figure 1).
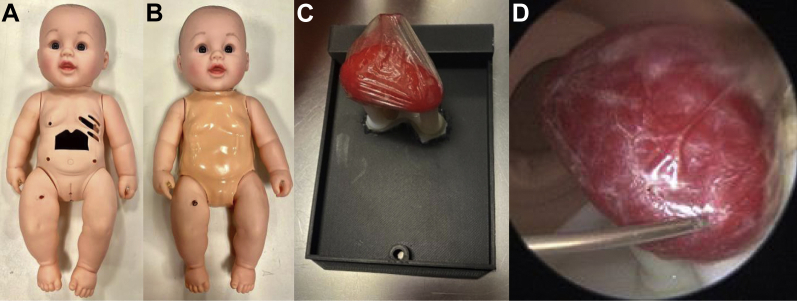
Figure 1.
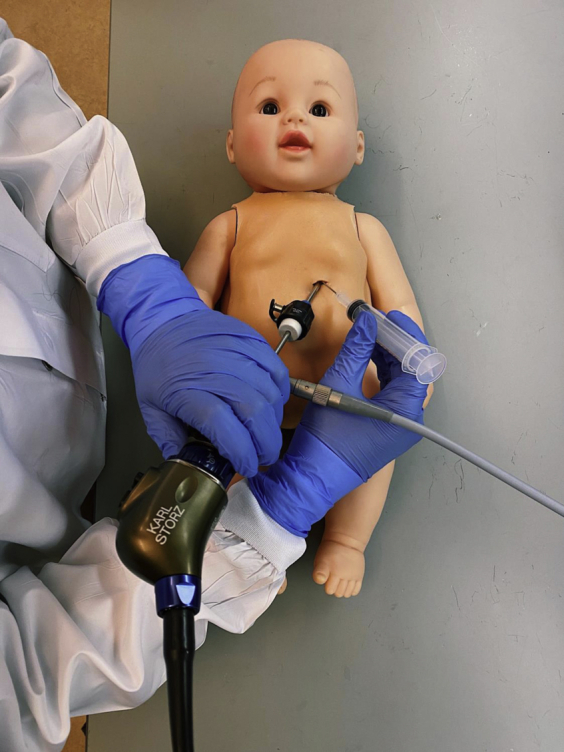
Pediatric simulator doll with operator practicing obtaining percutaneous access to the pericardium with visualization from a scope inserted through a subxiphoid approach.
Methods and materials
The phantom is housed within an 18-inch hollow plastic baby doll (Triokid; Apexcel Co, New Taipei City, Taiwan). A 3-dimensional (3D) surface rendering of the doll was created by 3D scanning with a FaroArm (Faro Technologies, Lake Mary, FL) and imported into 3-matic (Materialise, Leuven, Belgium). A 3.25” × 4” portion of the back of the doll was cut out, and 2 removable backplates were designed and 3D printed in plastic to securely fit into the contours of the doll. One phantom was designed for percutaneous access to the pericardial space; the other phantom was developed for manipulating cardiac ablation catheters within the heart and adapted to be compatible with the CARTO 3 platform (Biosense Webster Inc, Irvine, CA). All phantoms are designed to provide direct visualization of the practice space through a small endoscope inserted through the chest wall.
Pericardial access phantom
A 1.25” × 2.25” window was cut into the abdomen of the doll with the xiphoid process and lower ribcage shaping the upper edge of the window. Openings at the fourth through sixth intercostal spaces were created (Figure 2A). A 4-mm-thick silicone rubber skin was created using Dragon Skin™ FX-Pro™ (Smooth-On, Inc, Macungie, PA) to place over the doll and emulate skin effects when percutaneously inserting tools (Figure 2B). A mold of the doll’s chest was generated with a thermoforming machine (Vaquform, Inc, Burbank, CA) using clear polyethylene terephthalate glycol. A 3D heart model was segmented from a computed tomography scan using Mimics Research software (Materialise), printed using a plastic and rubber material blend (VeroWhite and Tango+; Stratasys Inc, Rehovot, Israel). The heart was placed inside a lambskin condom (Naturalamb; Trojan, Ewing, NJ) to mimic the pericardial lining and fixated to a stand on the doll’s backplate using magnets (Figure 2C). A portable 5.5 mm endoscope connected to a cellphone was inserted through the side of the doll’s chest at approximately the level of the fifth intercostal space to visualize the heart and the tools within the space (Figure 2D).
Figure 2.
A: Simulator doll without skin. Opening in fourth, fifth, and sixth intercostal spaces and abdominal window with lower ribcage and xiphoid process are shown. B: Simulator doll dressed in skin overlay. C: Removable backplate with infant-size 3D-printed heart, magnetically attached to a stand to hold its orientation, with a lambskin condom over the heart to act as the pericardium. D: Internal view of the 3D-printed rubber heart and needle approaching the pericardial space.
Pediatric cardiology fellows and attendings (n = 3) were provided a 7F access kit (Merit Medical Systems, South Jordan, UT) and asked to obtain sheath access to the pericardium through a subxiphoid approach using the modified Seldinger technique. The participants were also given a deflectable endoscope (Endocamelon; Karl Storz, Tuttlingen, Germany) to be inserted alongside the access tools to view the tools and heart. For this study, the chest wall endoscope was hidden from the participants and used by the investigator to monitor their progress. This task was repeated twice by each participant. Time to complete the task is reported in minutes and seconds for each participant. No statistical analysis was performed given the small sample size.
Electrophysiology study phantom
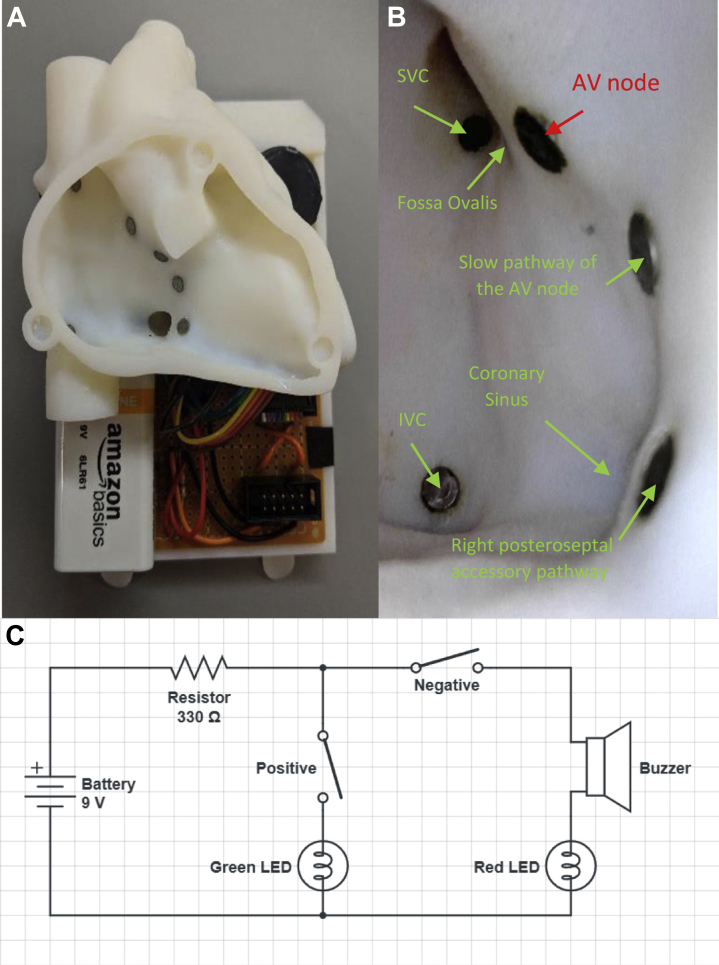
An infant computed tomography scan was segmented using Mimics Research software (Materialise) to create a hollowed myocardial heart model scaled to the average size of a small child that may be a candidate for a cardiac ablation procedure but designed to fit within the removable backplate of the infant phantom (Figure 3A). The heart was 3D printed in VeroWhite plastic (Stratasys). Seven metal targets were placed at the following locations: superior vena cava (SVC), inferior vena cava (IVC), fossa ovalis (FO), AV node, slow pathway of the AV node, and a right posteroseptal accessory pathway (Figure 3B). Additionally, the coronary sinus (CS) was modeled in the heart and a target was placed approximately 5 mm inside the entrance to the CS. The heart was placed inside the doll with 5 mm inner diameter tubing connected from the IVC to the right femoral region and from the SVC to the left subclavian region (Figure 4A). A lightweight swivel joint was inserted through the chest wall, left laterally approximately at the level of the fifth intercostal space. The portable 5.5 mm endoscope connected to a cellphone was inserted through the swivel joint and oriented toward the right atrium, providing direct visualization of the heart in a left anterior oblique orientation, similar to the common clinical view provided during 3D electroanatomic mapping (Figure 3B), and allowing users to directly visualize how catheter manipulation translates to catheter tip movement.
Figure 3.
A: Removable backplate with pediatric-size heart and circuitry. B: Internal view of the cardiac electrophysiology heart as viewed by the endoscope inserted through the chest wall. C: Circuit diagram with the positive terminal of a 9V battery connected through a 330 ohm resister to the catheter, with the catheter tip representing the first half of the switch. The second half of the switch represents the target of interest within the heart. The positive targets are wired through the green LED to close the circuit and the negative target is wired through a buzzer and the red LED. AV = atrioventricular; IVC = inferior vena cava; SVC = superior vena cava.
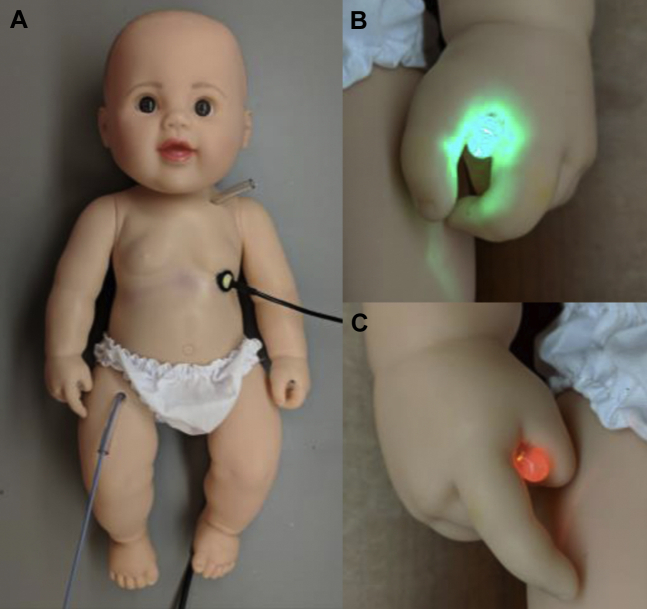
Figure 4.
A: Cardiac electrophysiology simulator doll with catheter inserted through femoral tubing. Additional entrance point is located in the left subclavian area. A small swivel joint in the chest wall holds a camera simulating the left anterior oblique view of the heart that the operator would see during an ablation procedure. The catheter is electrically connected through a jack in the left foot. B: A green LED in the left hand of the doll is illuminated when the catheter contacts the superior vena cava, inferior vena cava, fossa ovalis, coronary sinus, slow pathway of the atrioventricular (AV) node, or the right posteroseptal accessory pathway. C: A red LED in the right had of the doll is illuminated when the catheter contacts the AV node, and a buzzer is also activated.
A small circuit (Figure 3C) was assembled on the backplate connecting a 9V battery, 2 LEDs, and a buzzer such that the targets at the SVC, IVC, FO, CS, and both pathways illuminated a green light bulb when the catheter tip contacted the target (positive reinforcement). The AV node was connected to a red light bulb and a buzzer (signaling negative simulated result) to alert the user when inadvertent catheter contact occurs. A 3.5 mm jack was wired to the circuit board with a removable plug and fixed via the doll’s left foot. A fixture was developed to connect the corresponding pin of the tip electrode of the catheter to a 3.5 mm plug that could be inserted into the jack to connect the catheter to the circuit. The green and red LEDs were placed in the left and right hand of the doll, respectively (Figure 4B and 4C), and wired to the circuit board with a removable plug. The goal of the simulation was for the trainee to manipulate the catheter tip to touch the 6 positive targets within the heart without touching the AV node.
Thirty-two pediatric and adult cardiology fellows and attendings were asked to navigate a 7F ablation catheter (Biosense Webster) under direct visualization with the portable endoscope. The objective was to locate and contact the tip electrode of the catheter to all 6 positive targets in the order of SVC/IVC, FO/CS, and pathways, avoiding the AV node. Deliberate contact with the correct target was orally confirmed by the participant calling out the target when contact was made and visually confirmed by the study investigator via the endoscope. The time to complete the task was recorded. Each person participated in the study twice, completing 4 tasks in total—a first and second trial once each for 2 different catheter tools.
Time to complete the task is reported in seconds. Descriptive statistics (means and standard deviations) and Wilcoxon matched-pairs signed rank tests comparing the time to complete the task from the first trial to the second trial for each study were performed in Prism 9.3.1 (GraphPad, San Diego, CA).
Compatibility with CARTO
The setup was also adapted to allow 3D electroanatomic mapping, using the CARTO 3 platform (Biosense Webster) for visualization of the heart. A standard mapping catheter CARTO connector was modified to include a split on the wire for the tip electrode to simultaneously connect the catheter to the CARTO setup and the simulator circuitry. A frame was laser cut from acrylic to hold the CARTO skin patches above and below the simulator at the minimum distance necessary for CARTO sensing. The current wires were removed from the skin patches and placed in a saline solution to produce an impedance reading. The doll was placed inside the acrylic frame on the bed in the electrophysiology laboratory (Figure 5). An operator was then able to maneuver a catheter through the heart with both 3D electroanatomic mapping and direct visualization via the endoscope inserted through the chest wall. To demonstrate functionality and compatibility, a user was asked to generate a 3D map of the simulator heart using CARTO. No further testing for learning objectives was performed.
Figure 5.
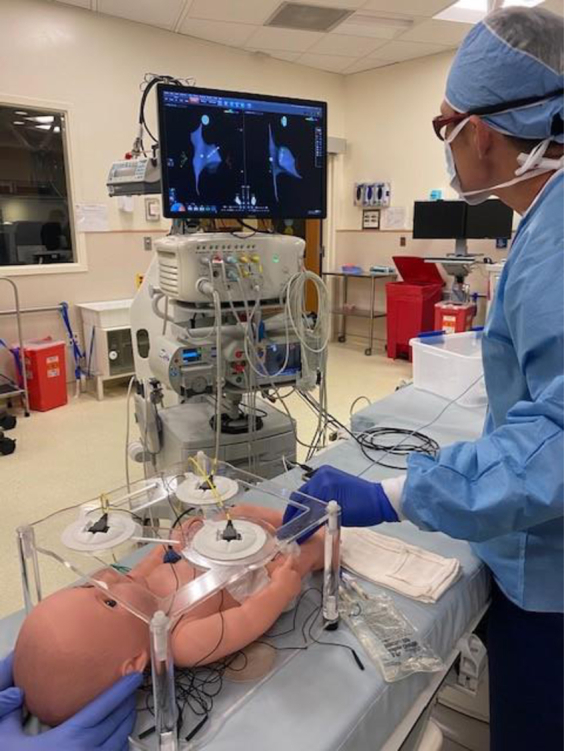
Cardiac ablation simulator setup in cardiac catheterization laboratory. Mapping catheter is electrically connected to the simulator and CARTO mapping (Biosense Webster Inc, Irvine, CA). Simulator is placed inside an acrylic frame to place the CARTO chest and back patches.
Results
Pericardial access phantom
Three participants (n = 3) of varying experience were asked to participate in a study that uses the pericardial access module: a second-year pediatric cardiology fellow, a fourth-year pediatric cardiology fellow specializing in electrophysiology, and a pediatric electrophysiology attending (approximately 10 years of experience). The cardiology fellow reported to have limited experience with performing the modified Seldinger technique independently and spent the most time performing the tasks using the simulator doll. However, the second attempt had a noticeable improvement in time to complete the task, improving by 2:30 minutes. The electrophysiology fellow also saw an improvement in time to complete the task, reducing procedure time by 6 minutes on the second trial. The participant credited this improvement to learning how to manipulate the instruments within the simulator doll. The experienced electrophysiologist did not show any improvements in time between trials (-5 seconds). Furthermore, there was a reduction in average time to complete the task with increasing experience level (Table 1).
Table 1.
Summary of the total time for each participant to gain needle access to the pericardium, average time to complete the tasks, and the difference in time between the first and second trial
| Second-year fellow | Fourth-year fellow | Attending | |
|---|---|---|---|
| First trial | 18:30 | 9:40 | 5:05 |
| Second trial | 16:00 | 3:23 | 5:10 |
| Average | 17:15 | 6:31 | 5:07 |
| Difference | 2:30 | 6:17 | -0:05 |
Results are presented in minutes:seconds.
Electrophysiology study phantom
Thirty-two participants consisting of cardiology fellows, pediatric interventional cardiologists, adult and pediatric electrophysiologists, and noninvasive pediatric cardiology attendings completed the study twice each (n = 64 studies). The fellow group (n = 30 studies, 15 participants), being the least experienced group, took the most time to complete the tasks, averaging 193 seconds across all trials, and showed a significant improvement (P = .027) of 51 seconds from the first trial to the second trial. The attending group not experienced with manipulating catheters (n = 8 studies, 4 participants) took an average of 120 seconds across all trials to complete the task, while the electrophysiology (n = 18 studies, 9 participants) and cardiac catheterization (n = 8 studies, 4 participants) attendings experienced with manipulating catheters took considerably less time to complete the task than the other 2 groups, averaging 84 seconds across all trials (87 and 78 seconds, respectively) (Table 2). Neither the combined group of attendings experienced with manipulating catheters nor the attendings not experienced with manipulating catheters showed a significant improvement from the first trial to the second trial.
Table 2.
Summary of the total time (in seconds) for each group of participants to touch the 6 targets, average time to complete the tasks, and the mean difference in time between the first and second trial, with standard deviation
| Not experienced with catheters |
Experienced with catheters |
|||
|---|---|---|---|---|
| Fellows (15 participants) | Noninvasive attendings (4 participants) | EP attendings (9 participants) | Cath attendings (4 participants) | |
| First trial | 219 | 113 | 98 | 80 |
| Second trial | 168 | 167 | 77 | 77 |
| Average | 193 | 140 | 87 | 79 |
| Mean difference | 50.8 | -53.8 | 20.8 | 2.3 |
| Standard deviation | ±155.4 | ±92.08 | ±33.96 | ±67.32 |
EP = electrophysiology.
Compatibility with CARTO
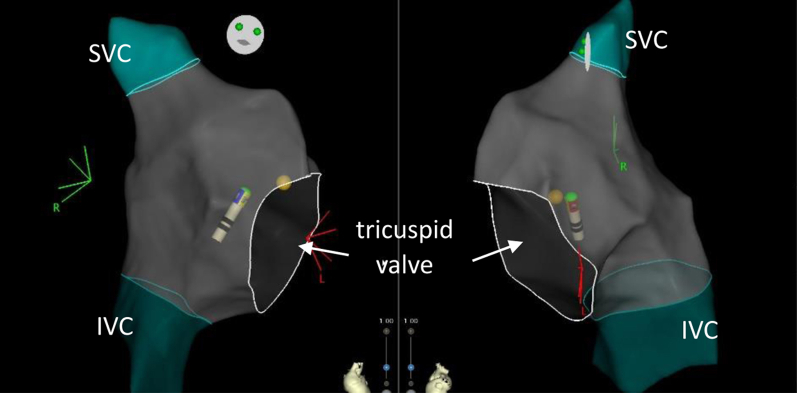
The electrophysiology phantom was set up with connection to the CARTO 3 electromagnetic mapping system. The catheter was maneuvered through the right atrium to successfully produce a 3D map of the right atrium (Figure 6). As illustrated, the SVC, IVC, and tricuspid valve are readily imaged, and the mapping catheter is clearly visualized within the heart.
Figure 6.
Electronic 3-dimensional map of the right atrium produced by manipulating the mapping catheter through the electrophysiology phantom heart.
Discussion
The designed simulator is pediatric-size and portable, housed within an infant-size doll. The participants were able to successfully complete the tasks with standard tools used in a clinical setting. When comparing the electronic map of the simulator heart to that of a patient, the results were comparable in size and appearance. Furthermore, we demonstrate that training phantoms do not need to be complex or expensive. Both models of the simulator presented are housed in an inexpensive doll that was purchased for less than $50 and built with low-cost items and simple prototyping tools that are generally available at most research facilities. The addition of a portable endoscope allows training to occur when more expensive equipment (ie, CARTO or Storz visualization system) is not available. We estimate a total of less than $500 in materials and parts to assemble the 2 simulators.
For both experiments, fellows-in-training saw a greater improvement in time to complete the task from the first trial to the second trial than physicians more experienced with the task. These results suggest that practicing with a simulator doll can aid in learning complex procedures and build proficiency for fellows- and physicians-in-training. Furthermore, it was observed that more experienced physicians took less time overall to complete the task than training physicians, suggesting that the model is similar to the clinical environment observed and there was not a long learning curve associated with using the simulator. It is important to note that sample size is low for all groups and further studies would be necessary to confirm results, especially given the large variability, which is to be expected given the nature of training.
The participants in the study stated manipulating the tools in the simulator felt similar to a clinical setting, though the simulator would be improved with the addition of movement and pulsation of the heart to mimic a more realistic experience. Additionally, we identified issues with friction in the rubber skin and the tubing to navigate the catheter to the heart. This was mitigated by lubricating the tubing and tools that were inserted percutaneously. The electrophysiology heart was oversized for the doll; however, we felt the heart anatomical size and shape was more important than the simulated size of the patient for catheter procedures. This design contributes to the simulator’s portability, allowing for additional patient anatomies to be used in the same setup to model other unique anatomies that may be desirable to train on, such as dextrocardic hearts or personalized models from individual patients with complex congenital heart disease.
Conclusion
The simulator doll provides a tactile experience similar to a pediatric procedure owing to its real-life sizing and anatomical accuracy of cardiac structures. This infant phantom is an affordable way to learn pediatric cardiac techniques such as percutaneous pericardial access and cardiac ablations. The simulator provides a safe environment to practice manipulating tools and gain proficiency outside of a clinical setting. The setup is portable and does not require animal tissues, allowing for the training to be readily available. We successfully demonstrated that the simulator could be used for practicing percutaneous pericardial access and manipulating cardiac ablation catheters inside the heart, including with CARTO mapping. Simple and low-cost simulators such as this are a valuable tool for training and education.
Acknowledgments
We would like to acknowledge Paige Botie and Hannah Osborne from Biosense Webster for their technical assistance and the Team Trace Foundation and the Van Metre Companies Professorship for support of our work.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.Consent:All participants consented to the study and voluntarily enrolled.
Ethics Statement
This study was approved by the institutional review board at Children’s National Hospital, Nationwide Children’s Hospital, and the Albert Einstein College of Medicine/Montefiore Medical Center. The research in this study was conducted in accordance with the Declaration of Helsinki.
References
- 1.Rock B.G., Leonard A.P., Freeman S.J. A training simulator for ultrasound-guided percutaneous nephrostomy insertion. Br J Radiol. 2010;83:612–614. doi: 10.1259/bjr/42026587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Good M.L. Patient simulation for training basic and advanced clinical skills. Med Educ. 2003;37:14–21. doi: 10.1046/j.1365-2923.37.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamson S., Denson J.S., Wolf R.M. Effectiveness of a simulator in training anesthesiology residents. Qual Saf Health Care. 2004;13:395–397. doi: 10.1136/qhc.13.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Våpenstad C., Hofstad E.F., Bø L.E., et al. Lack of transfer of skills after virtual reality simulator training with haptic feedback. Minim Invasive Ther Allied Technol. 2017;26:346–354. doi: 10.1080/13645706.2017.1319866. [DOI] [PubMed] [Google Scholar]
- 5.Gordon J.A., Wilkerson W.M., Shaffer D.W., Armstrong E.G. ‘Practicing’ medicine without risk. Acad Med. 2001;76:469–472. doi: 10.1097/00001888-200105000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Issenberg S.B., McGaghie W.C., Hart I.R., et al. Simulation technology for health care professional skills training and assessment. JAMA. 1999;282:861. doi: 10.1001/jama.282.9.861. [DOI] [PubMed] [Google Scholar]
- 7.Schiralli M.P., Hicks G.L., Angona R.E., Gangemi J.J. An inexpensive cardiac bypass cannulation simulator: facing challenges of modern training. Ann Thorac Surg. 2010;89:2056–2057. doi: 10.1016/j.athoracsur.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Campo Dell’orto M., Hempel D., Starzetz A., et al. Assessment of a low-cost ultrasound pericardiocentesis model. Emerg Med Int. 2013;2013:1–7. doi: 10.1155/2013/376415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly R., Planas J.H., Edens M.A. Adapting gel wax into an ultrasound-guided pericardiocentesis model at low cost. West J Emerg Med. 2017;18:114–116. doi: 10.5811/westjem.2016.10.31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantalos G.M., Ionan C., Koenig S.C., et al. Expanded pediatric cardiovascular simulator for research and training. ASAIO J. 2010;56:67–72. doi: 10.1097/MAT.0b013e3181c838ae. [DOI] [PubMed] [Google Scholar]
- 11.Ebrille E., Killu A.M., Anavekar N.S., et al. Successful percutaneous epicardial access in challenging scenarios. Pacing Clin Electrophysiol. 2014;38:84–90. doi: 10.1111/pace.12503. [DOI] [PubMed] [Google Scholar]
- 12.Luboz V., Zhang Y., Johnson S., et al. ImaGiNe Seldinger: first simulator for Seldinger technique and angiography training. Comput Methods Programs Biomed. 2013;111:419–434. doi: 10.1016/j.cmpb.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Opfermann J.D., Clark B.C., Davis T.D., Berul C.I., Krieger A. A single-incision delivery tool for epicardial pacing and defibrillation. J Med Devices. 2016;10 [Google Scholar]
- 14.Olson M.D., Phreaner N., Schuller J.L., et al. Effect of catheter movement and contact during application of radiofrequency energy on ablation lesion characteristics. J Interv Card Electrophysiol. 2013;38:123–129. doi: 10.1007/s10840-013-9824-4. [DOI] [PubMed] [Google Scholar]