Abstract
The adrenal glands are complex structures from which a variety of benign and malignant tumors may arise and are a common site of metastatic disease. Several radiopharmaceuticals are used for imaging the adrenals, including I-123/I-131 metaiodobenzylguanidine (MIBG), norcholesterol derivatives, In-111 pentetreotide and Ga-68 somatostatin analogs, [F-18]fluorodeoxyglucose, [F-18]fluorodopa, [F-18]fluorodopamine, C-11 meta hydroxyephedrine, and C-11/F-18/I-123 Metomidate (MTO) or its analogs. In this review we focus on the role of these reagents in metastatic lesions, cortical neoplasms, pheochromocytoma/paraganglioma, and neuroblastoma (NB).
Keywords: MIBG, somatostatin, FDG, fluorodopa, pheochromocytoma, neuroblastoma
INTRODUCTION
The adrenal is a complex gland composed of the cortex and the medulla. Each of these can give rise to a variety of benign or malignant tumors including adrenocortical adenoma (ACA), adrenocortical carcinoma (ACC), pheochromocytoma (PHEO), and neuroblastoma (NB). Furthermore, it is a site of metastatic spread. With the increasing use of high-resolution anatomic imaging, incidental identification of adrenal lesions is frequent. Thus, adrenal lesions require further characterization, using both biochemical evaluation and imaging. In this review, we will focus on the functional/molecular imaging of adrenal lesions.
MOLECULAR IMAGING AGENTS
Localization and characterization of adrenal tumors relies on conventional anatomic imaging modalities such as CT, MRI, and US. In addition, radiopharmaceuticals targeting metabolic pathways and receptors are extensively used. A brief review of the more common reagents including mechanism of localization follows.
Metaiodobenzylguanidine (MIBG)
MIBG is a structural analog of guanethidine that shares structural features with nor-epinephrine and is used for imaging pheochromocytomas/paragangliomas (PHEO/PGL), neuroblastoma NB, and other neuroendocrine tumors [1]. MIBG internalizes via norepinephrine transporters and moves into secretory granules via vesicular monoamine transporters [2]. For imaging, I-123 MIBG is superior to I-131 MIBG [3].
Somatostatin Receptor Scintigraphy (SRS)
Somatostatin is a small regulatory peptide that binds to somatostatin receptors [4]. In-111 pentetreotide is commercially available analog for imaging neuroendocrine tumors. Newer positron-emitting somatostatin analogs including Ga-68 DOTATOC, DOTATATE, and DOTANOC perform better than In-111 pentetreotide because of their higher affinity and superior resolution of positron emission tomography (PET) [5].
Fluorodeoxyglucose (FDG)
FDG is an analog of glucose and a surrogate marker for glucose metabolism. It enters the cell through GLUT transporters [6] and is phosphorylated by hexokinase and trapped. Procedure guidelines describing the technical aspects of FDG PET imaging have been reported [7]. FDG is extremely useful in adrenal imaging in detecting malignancy.
F-18 Dihydroxyphenylalanine (FDOPA)
Dihydroxyphenylalanine (DOPA) is an amino acid internalized by the LAT2 amino acid transporter and then follows the DOPA metabolic pathway [8]. It is commercially available in Europe, but used as an investigational PET agent in the USA. FDOPA is extremely useful in the imaging of most benign PHEO/PGL and also most malignant ones, with apparently less sensitivity in those arising from SDHB mutations, where FDG is more sensitive.
F-18 Fluorodopamine (FDA)
F-18 fluorodopamine is an analog of dopamine used as an investigational agent for imaging of PHEO/PGL at the National Institutes of Health (NIH) [9]. The mechanism of action is similar to that of MIBG. It has higher affinity for norepinephrine transporters [10] than MIBG, and as a PET reagent, is quantitative, has higher resolution, improved dosimetry, and is a 1-day study.
C-11 Meta-Hydroxyephedrine
C-11 meta-hydroxyephedrine (HED) is a catecholamine analog developed for imaging the sympathetic nervous system, that accumulates in adrenergic neurons via the norepinephrine transporter [11]. While it has the advantage of being a PET agent, it has the disadvantage of C-11 labeling, with its 20 min T ½ that requires an onsite cyclotron.
Radiocholesterol Studies
Radiolabeled analogs of cholesterol include I-131–6beta-iodomethyl-norcholesterol (NP59) and Se-75 selenomethyl-norcholesterol (Scintiadren), that are taken up by adrenocortical cells [12]. Drawbacks include the high absorbed dose to the adrenal glands, the length of time required to obtain images (up to 7–14 days), and the poor spatial resolution of the images. These agents are not commercially available in the USA, and have limited availability elsewhere.
Metomidate (MTO)
C-11 MTO is a highly specific PET imaging agent for adrenocortical tumors. MTO binds and inhibits CYP11B enzymes 11B-hydroxylase and aldosterone synthetase that are involved in synthesis of cortisol and aldosterone and expressed in adrenocortical cells [13]. It is used to characterize and localize tumors of adrenocortical origin [14]. Because of the T1/2 limitations of C-11, a F-18 labeled analog [F-18] fluoroetimodate (FETO) has been developed [15]. In addition, MTO has been labeled with I-123 for SPECT imaging [16] and I-131 MTO has been used to treat adrenocortical carcinoma (ACC) [17].
CLINICAL APPLICATIONS OF FUNCTIONAL IMAGING
Tumors of the Adrenal Cortex
Radiocholesterol studies.
Prior to the introduction of PET scanning, molecular imaging of adrenal cortical lesions for malignancy was limited to use of various radiolabeled cholesterol analogs such as NP59 and Se75 selenomethyl-nor-cholesterol. These agents are used in conjunction with anatomic imaging to assess patients with hypersecretory states and adrenal adenomas (ACA) [18,19] and to distinguish benign from malignant adrenal lesions [20,21], where concordant uptake in the adrenal lesion signifies benign adenoma, and discordant, lack of uptake in an adrenal mass is an indication of malignancy. Reports describe rare uptake in malignant lesions [20,22].
FDG PET.
The modern PET/CT era has shown rapid growth in the use of FDG in oncology. The addition of CT to PET scanners has improved detection and proper localization of adrenal tumors compared to PET alone. PET’s ability to quantitate allows for reproducible measurements in the form of standardized uptake values (SUV = fraction of the injected dose per gram, normalized to body weight).
Bagheri et al. [23] looked at the presumed normal adrenal glands (based upon normal appearance on prior CT scans) in patients undergoing FDG PET, describing adrenal FDG uptake in up to 68% of normal adrenals on PET/CT. In this group, most adrenals had uptake less than liver. Only a small percentage had uptake equal to or slightly greater than liver, with mean SUVmax of 0.9 (right) and 1.1 (left) (range 0.95 to 2.46).
As with solitary pulmonary nodules, a major role of FDG PET is in differentiating benign from malignant disease when adrenal abnormalities are detected. A common setting for this applies to that of adrenal “incidentalomas”—adrenal lesions incidentally discovered on imaging performed for other, non-adrenal reasons. The reported prevalence of incidentaloma on CT scans is 4.5–5% [24,25]. The ability to reliably separate benign from malignant in these cases is particularly important given the prevalence of adrenocortical adenomas (ACA) of up to 8.7% [26] in autopsy series.
Adrenal lesions in patients with extra-adrenal malignancies.
Unsuspected adrenal abnormalities are frequent incidental findings in patients with known extra-adrenal malignancy. In an autopsy series, the overall incidence of metastases to the adrenal glands in patients with epithelial cancer was 27% [27]. The incidence was particularly high in certain malignancies such as breast (53.9%) and lung cancer (35.6%). However, even in these patients, benign lesions are common. Fortunately, FDG has proven useful in distinguishing between benign and malignant lesions.
A recent meta-analysis by Boland et al. [28] looking at data from 1,217 patients concluded that FDG PET was highly effective in distinguishing between malignant and benign adrenal masses, with a mean sensitivity and specificity of 97% and 91%, respectively. They found no differences in accuracy between visual or quantitative analysis methods. Visually, adrenal uptake is compared to that of normal liver, with most malignant lesions demonstrating uptake > liver and most benign lesions < liver. Such an approach yields sensitivities ranging from 74 to 100% and specificities of 38.2–100%, depending on the criteria used. Table I lists the details of several of the larger reports. Quantitatively, most malignant lesions demonstrate much higher FDG uptake than benign lesions. The mean SUVmax for malignant lesions has ranged from 4.8 to 12.2, whereas for benign lesions it has ranged from 1.78 to 4.2. Tumor:Liver SUVmax ratios (T:L) yield results similar to visual analysis, depending on the cutoff values used, with sensitivities ranging from 87 to 100% and specificities of 91–100%, whereas use of an SUV cut-off may be less successful due to some overlap between benign and malignant lesions (Table I).
TABLE I.
FDG Imaging Results in Cancer Patients With Adrenal Incidentaloma
| Study | Number of patients | Method of assessment for malignancy | Sensitivity for malignancy | Specifcity for malignancy | Accuracy for malignancy | % malignant lesion | Mean SUVmax Benign | Mean SUVmax malignant |
|---|---|---|---|---|---|---|---|---|
| [32] | 93 | SUVmax >3.2 | 95.2% | 81.3% | 88% | 35.9% | 2.4 | 8.4 |
| Adr SUVmax/Liver SUVmax > 1.6 | 92.9% | 86.7% | 79.6% | |||||
| SUVmax>2.8 and <10% of pixels <0 HU | 100% | 97.3% | 95.7% | |||||
| [31] | 81 | SUVmax ≥ 4.2 | 88.6% | 88.2% | 88.5% | 67.3% | 3.0 | 8.8 |
| Adr SUVmax/Liver SUVavg > 1.68 | 90% | 91.1% | 90.4% | |||||
| [35] | 105 | Visual ≥ liver | 94% | 83% | 90% | 61% | 2.4 | 6.0–8.4 |
| [37] | 109 | Visual >> Liver | 97% | 94% | 45% | 2.29 | 12.2 | |
| Adr SUVmax/Liver SUVmax >1 | 100% | 100% | ||||||
| [109] | 96 | Adr SUVavg/Liver SUVavg ≥1 | 83.3% | 85.4% | 85% | 26.8% | 1.9 a | 7.2 a |
| [36] b | 59 | Visual>liver | 75% | 89% | 86.4% | 20% | 4.2 | 9.2 |
| Adr SUVmax/Liver SUVavg ≥ 1.0 | 100% | 95.7% | 96.6% | |||||
| [34] | 74 | Visual > Liver | 93% | 96% | 95% | 37.5% | 2.6 | 5.5 |
| SUVmax > 3.4 | 95% | 86% | ||||||
| [29] | 150 | SUVmax>3.1 | 98.5% | 92% | 39% | 1.78 | 10.13 | |
| SUVmax >3.1 or >10 HU | 100% | 98% | ||||||
| [30] | 147c | SUVmax>3.1 | 97.3% | 75.9% | 84.2% | 39% | 3.0 | 10.4 |
| Adr SUVmax/Liver SUVavg >1 | 97.3% | 65.5% | 77.9% |
Adr, adrenal; Liv, liver.
Mean SUVavg values.
Recalculated from data provided in article.
Lung cancer only.
The current generation of scanners allow us to take full advantage of the CT for co-registration and determination of density (HU = Hounsfield units), together with visual or semiquantitative assessment of the intensity of uptake. The primary cause of false positive FDG uptake is due to lipid poor adenomas. By combining the SUV measurement with attenuation values on CT, higher specificities can be obtained [29–33].
False negative adrenal uptake on FDG is usually due to the small size, necrosis and hemorrhage, well differentiated tumors, metastases from pulmonary carcinoid, and metastases from other non-FDG avid tumors [29,34,35]. False positives have been due to uptake by ACAs, often lipid poor [33,36], myelolipomas [33], infection/inflammation [37], adrenal hyperplasia, adrenal hemorrhage with bilateral FDG uptake [38], the occasional normal adrenal [23], and uptake in perirenal brown fat. In addition, benign PHEO also frequently show FDG uptake greater than liver.
Adrenal lesions in patients without known malignancy.
When questions of possible malignancy arise in patients with adrenal lesions and no history of extra-adrenal malignancy, FDG PET can play an important role. The problem, of course, is to reliably identify the rare, unsuspected ACC, the diagnosis of which usually carries a grave prognosis unless detected at an early stage. Only a few studies have focused on this patient population, and both visual and quantitative criteria have been used. Note that these studies all focused on patients needing adrenalectomy (for hyper-secretory tumor, enlarging tumor, or indeterminate/suspicious imaging characteristics on CT), and thus used selected populations.
In two series, FDG PET/CT was used to evaluate suspicious adrenal lesions, excluding those with clear-cut benign features on anatomic imaging [39,40]. Using the visual criterion of T:L > 1.6–1.8 resulted in 100% sensitivity and 90–100% specificity for malignancy. Groussin et al. [41] prospectively performed FDG scans on 77 patients without a history of cancer who were to undergo adrenal surgery. Using a T:L cutoff value of 1.45, the sensitivity and specificity for differentiating between benign and malignant disease were 100 and 88%, respectively, compared to 82 and 100% when the cutoff was 2.63. The mean SUVmax for ACC was 11.1 (range 3.5–26.2), and for ACAs 3.3 (range 1.7–7.8). Using SUVmax alone, a cutoff value of 3.4 resulted in a sensitivity and specificity of 100 and 70%, respectively. No ACC had uptake less than liver. A negative FDG was highly predictive of a benign lesion, thus possibly helping avoid unnecessary surgery. Similarly, Ansquer et al. [42] evaluated 78 patients without history of malignancy or malignancy in remission for over 1 year. FDG was negative in 97% of the lesions deemed non-surgical with one false positive adenoma. A cutoff SUVmax of 3.3 resulted in a sensitivity of 93% and specificity of 78% for malignancy. Overall, the negative predictive value for detecting malignancy was 93% and the positive predictive value for detecting a surgical lesion was 97%.
Adrenal cortical carcinoma.
As discussed above, in addition to adrenal metastases, primary ACC also demonstrates increased FDG uptake, and FDG PET can play a role in initial diagnosis, staging, and follow-up, as well as having prognostic implications. While rare, ACC carries a grave prognosis with an overall 5-year mortality of 75 to 90% and an average survival of less than 15 months [43]. Distant metastases are present at the time of diagnosis in a large percentage of patients, and many others have locoregional spread. The primary and only curative approach to ACC is surgical resection of the primary tumor and metastases. Surgical resection is also often considered for recurrent disease, both to improve survival and for local control. To this end, preoperative imaging for staging and localization of metastases is necessary and FDG is a valuable tool.
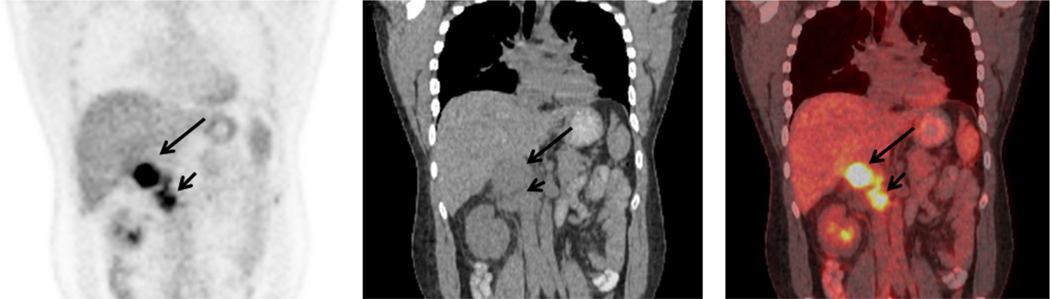
In 2001, Becherer et al. [44] reported on 10 ACC patients in whom FDG had a sensitivity and specificity for tumor detection of 100% and 95% vs. 89% and 100% with CT. Lesions had an average SUVmax of 7.4. FDG altered staging in 3/10 patients and changed therapeutic management in 2/10 patients. Mackie et al. [45] evaluated 12 patients with suspected recurrent or metastatic ACC with FDG PET. 10/11 patients subsequently shown to have recurrent or metastatic disease had positive FDG scans, with mean SUVmax of 7.8 (range 2.9–14.2). These lesions all had T:L > 2.0. One of 12 patients with metastatic ACC had a false negative PET/CT scan with mild FDG uptake in a liver lesion (SUVmax 2.9) and T:L ratio of 0.7, suggesting benign disease. Another patient had a small liver lesion with SUVmax of 1.9 with a T:L ratio of 0.9, consistent with benign disease and subsequently shown to be scar tissue. An example of a positive ACC with metastatic spread is shown Figure 1.
Fig. 1.
Patient with an 8.2 × 5.3 cm adrenal cortical carcinoma showing hypermetabolic activity on FDG in primary tumor with SUVmax of 11.9 (long arrows) and tumor thrombus SUVmax 10.2 (short arrows) confirmed at surgery. Coronal FDG (left panel), CT (middle panel), and fused PET/CT (right panel) images.
Leboulleux et al. [46] used FDG PET/CT and thoracic, abdominal, and pelvic CT to prospectively evaluate 28 patients with ACC. Individual lesion-wise (269 total), FDG had a sensitivity of 90% compared to 88% for CT. Organ-wise, FDG had a sensitivity of 93% compared to 82% for CT. Twelve percent of lesions were seen by FDG and not CT, whereas 7% were seen only on CT. In 38% of cases, local recurrences were detected only by PET. In a separate study of 51 patients where 22/51 had ACC, FDG had a 95% sensitivity and 97% specificity using a T:L ratio ≥ 1.7 for ACC [47].
A correlation between tumor aggressiveness and FDG uptake has been described [47]. Prognostically, an SUVmax > 10 and a high FDG uptake volume both correlated with a poorer survival with 54% of patients with SUVmax >10 dying within 6 months of their scan, compared to none of those with SUVmax <10 [46].
After adrenalectomy, false positive localization in the normal remaining adrenal of patients treated with mitotane has been reported within 24 months of starting mitotane, with FDG uptake > liver in up to 14–19% [48]. This may be related to increased ACTH stimulation due to the suppression of steroidogenesis by mitotane.
MTO imaging.
The initial C-11MTO report by Bergstrom et al. [14] demonstrated promising results with MTO PET in 15 patients with adrenal masses on CT who were to undergo surgery. Normal adrenals had a mean SUVmax of 12 (range 7–17). Normal liver mean SUVmax was 11 (range 6–20). Adrenocortical lesions (six ACA, two ACC, and one nodular hyperplasia) demonstrated very high uptake of the radiotracer, in contrast to non-cortical lesions that had mean SUVmax of 1.5 (pheochromocytoma, myelolipoma, cysts, and metastases). ACAs and hyperplasia had mean SUVmax of 29 and 22, respectively, whereas ACCs had mean SUVmax of 16. Subsequent reports confirm that MTO can reliably distinguish adrenocortical from non-cortical tumors, but cannot separate malignant from benign disease [13,49–51]. In a series of 173 patients, Hennings et al. [50] found a sensitivity and specificity of 89 and 96% for identifying tumors of the adrenal cortex. There is also evidence that functional tumors have higher SUVs than non-functional ones [49].
One study to date has focused on the potential role of MTO imaging in ACC [52], demonstrating uptake in primary and metastatic sites of disease in a study of 11 patients. MTO detected two sites of disease missed by CT, and correctly identified a focus that was thought to be a liver metastasis on CT as benign. Conversely, MTO missed three CT positive lesions, but all were subsequently found to be largely necrotic at surgery. Thus, MTO may be useful in initial staging and restaging of ACC. How it compares to FDG imaging in this regard is yet to be established, although a recent case report shows better visualization of metastatic disease with MTO than with FDG [53].
In order to overcome the limitations of the short T1/2 of C-11, an F-18 analog fluoroetimodate (FETO) has been evaluated in 10 healthy volunteers, with promising results [15]. Alternatively, iodometimodate (IMTO) labeled with I-123 is suitable for SPECT imaging [16]. This agent exhibits high uptake in adrenocortical tissue and derived tumors, both primary and metastatic, including lesions missed by CT. Subsequently, this group administered I-131 IMTO to 11 patients with advanced Stage IV ACC and very high I-123 IMTO uptake by all known disease sites and observed a partial response in 1/10 and stable disease in 5/10 [17]. Mean survival was 15 months (range 3–33) with median progression free survival was 14 months (range 5–33) which was ongoing in three patients at last follow-up.
In summary, while further work is needed, initial results indicate that MTO imaging is both sensitive and specific for discriminating between cortical and non-cortical tumors of the adrenal cortex. Unfortunately, it cannot differentiate between malignant and benign tissue, although visualization of extra-adrenal sites is suggestive of malignancy and metastatic disease. This may change, however, as further experience with longer-lived agents such as F-18 FETO and I-123 IMTO is gained. In the meanwhile, C11 MTO holds promise as a more specific agent than FDG for staging of ACC, as does I-131 IMTO for therapy.
TUMORSOFTHE ADRENAL MEDULLA
Pheochromocytoma/Paraganglioma (PHEO/PGL)
PGL are rare tumors that originate from chromaffin-producing cells. Those PGLs originating in the adrenal gland are called pheochromocytoma (PHEO), whereas in all other locations they are referred to as PGLs. Differentiation between benign and malignant PGLs cannot be made histologically and requires the presence of metastatic disease to confirm malignancy. Most PGLs are sympathetic in origin and arise from the adrenal; whereas those originating in the head and neck are most often of parasympathetic origin and generally do not produce catecholamines. For the purposes of this imaging review, we will discuss PGL/PHEO together since most reports do not separate these out and imaging characteristics are somewhat similar, with the exception of head and neck PGL, that are beyond the scope of this review.
The diagnosis of PHEO/PGL is usually made biochemically. Imaging is used to localize the primary tumor and evaluate for multicentric or metastatic disease. Furthermore, it is important to localize disease prior to intervention. In addition, in patients with metastatic disease, MIBG and SRS scintigraphy may be used to select candidates for I-131 MIBG or Y-90/Lu-177 somatostatin analog therapy.
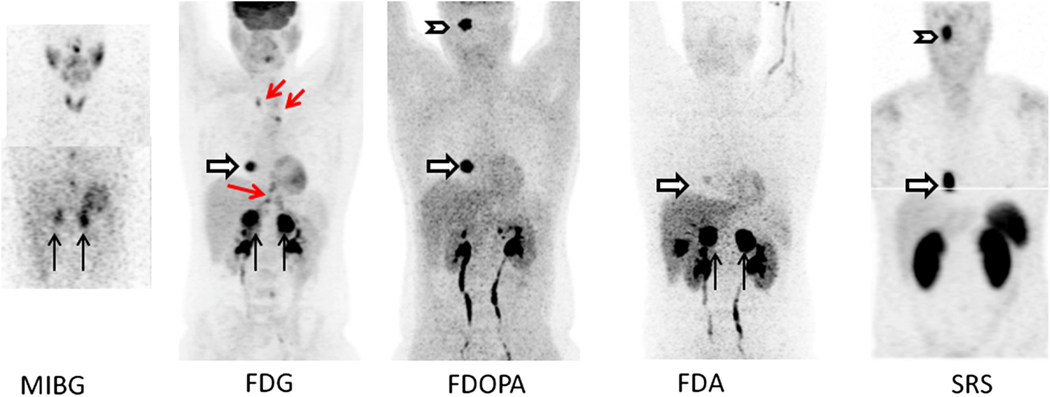
A number of familial syndromes are associated with PHEO/PGL, and these different genotypes are associated with higher rates of malignancies, extra-adrenal disease, and/or different imaging phenotypes. The major syndromes include multiple endocrine neoplasia 2 (MEN2A,B), von Hippel Lindau (VHL), neurofibromatosis 1 (NF1), and SDHx (x = B, C, and D) mutations. Unfortunately, most imaging studies have evaluated mixed populations, making it difficult to delineate the precise role of the various agents in each syndrome. Timmers et al. [54] was the first to recognize that in patients with malignant disease associated with SDHB, FDG imaging had the highest sensitivity (74% on a per lesion basis) compared to 57% for I-123 MIBG, 76% for FDA and 45% for FDOPA PET. Taieb et al. [55] also showed high uptake and sensitivity of FDG in patients with SDHx mutations. These findings are likely related to up regulation of hypoxic angiogenic responsive genes in patients with SDHx mutations [56]. In VHL patients, a study has shown that FDA localizes much better than I-131 MIBG [57]. Thus, sensitivities reported in the literature for functional imaging in PHEO/PGL may vary significantly depending on the patient population being studied, given the heterogeneity of uptake with the various radiopharmaceuticals (Fig. 2).
Fig. 2.
Patient with SDHD and metastatic PHEO/PGL. Imaging with a variety of radiopharmaceutical shows heterogeneity in uptake. MIBG panel (MIP—top, and coronal slice—bottom) shows uptake in bilateral adrenal PHEOs (black arrows). Two foci in lower midline chest are thought to be due to swallowed activity in esophagus, glomus tumor is not visualized. FDG MIP panel shows intense bilateral uptake in the adrenal PHEOs and a right atrial PGL (white arrow). Mild uptake in a right glomus jugulare PGL was also seen (not shown this projection). Foci in mediastinum and upper abdomen (red arrows) represent uptake in brown fat. FDOPA MIP panel shows intense uptake in right glomus jugulare PGL (chevron) and right atrial lesion. Mild uptake was seen in the left adrenal PHEO but not right (not seen in this view). FDA MIP shows intense uptake in bilateral adrenal PHEOs with faint uptake in atrial lesion. Physiologic gallbladder activity is also seen. No intracranial uptake was seen. SRS panel shows In-111 pentetreotide MIP images with intense uptake in the right glomus jugulare tumor and cardiac lesion but no uptake in the bilateral adrenal tumors. Images courtesy of Dr. Karel Pacak, NICHD, NIH.
I-131/I-123 MIBG
I-131/I-123 MIBG are the prototypical reagents for imaging PHEO/PGL. I-131 has high overall sensitivity (Tables II and III), and results with I-123 MIBG are superior to I-131 MIBG [3]. A phase 3 multicenter trial of 140 patients evaluated I-123 MIBG for PHEO/PGL and found the sensitivity for PHEO was 87% compared to 67% for PGL with an overall sensitivity of 81% and specificity of 75% [58]. A meta-analysis evaluating the sensitivity of I-123 MIBG for tumor detection in patients with PHEO included 395 subjects and estimated a sensitivity of 96% and specificity of 98% [59].
TABLE II.
Sensitivity of Detection of Benign Pheochromocytoma/Paraganglioma
| Reference | Patient-based sensitivity (lesion-based sensitivity in parenthesis) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Number of patientsa | MIBG | SRS | FDG | FDA | FDOPA | CT | MRI | |
| [110] | 48 | 98%b | ||||||
| [111] | 32 | 90.6%b | 90% | 93.3% | ||||
| [66] | 17 | 87.5%b | 28.5% | 87.5% | 100%c | 100%c | ||
| [112] | 32 | (25%) | ||||||
| [68] | 12 | 83%d | 58% | |||||
| [57] | 7 VHL | 57%d | 100% | 100% | 83% | |||
| [54] | 20 | (77%)b | (88%) | (77%) | (81%) | (100%)c | (100%)c | |
| [69] | 17 | 71%b | 100% | 100% | ||||
| [71] | 24 | (52%)e | (85%) | (70%)c | (70%)c | |||
| [113] | 22 | 100% | 66% | |||||
| [63] | 25 | (53%)b | 97% | |||||
Data for benign PGL only, some studies included both benign and malignant PGL.
I-123 MIBG.
Studies used either CT or MRI.
Either I-123 or I-131 MIBG.
I-131 MIBG.
TABLE III.
Sensitivity for Detection of Malignant Pheochromocytoma/Paraganglioma
| Reference | Patient-based sensitivity (lesion-based sensitivity in parentheses) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Number of patientsa | MIBG | SRS | FDG | FDA | FDOPA | CT | MRI | |
| [114] | 64 | 92.4%b | ||||||
| [68] | 17 | 82%c | 88% | |||||
| [112] | 14 | 57%d | 87.5% | |||||
| [66] | 53 | 70.6%d | 88.9% | 91.4% | 100%e | 100%e | ||
| [73] | 9 | 75%b | 89% | 100% | ||||
| [62] | 30 SDHB | 80%c | 100% | 88% | ||||
| [54] | 28 | 84% (57%)d | 89%(74%) | 81%(76%) | 74%(45%)f | (100%)e | (100%)e | |
| [115] | 28 | 100% | ||||||
| [71] | 21 | (56%)d | (73%) | (45%) | ||||
| [72] | 12 | 75%d (38%) | 100% (98%) | |||||
Data for malignant PGL only, some studies included both benign and malignant PGL.
I-131 MIBG.
Either I-123 or I-131.
I-123 MIBG.
Studies used either CT or MRI.
This represents a mixed population, in SDHB the per lesion was sensitivity of FDOPA was 20% whereas in non-SDHB it was 93%.
Another meta-analysis found a sensitivity of 92–98% for I-123 MIBG in patients with non metastatic PGL and 79% for metastatic disease [60]. Bhatia et al. [61] have also shown higher (85%) sensitivity for detecting primary adrenal PHEO compared to 58% for extraadrenal PGL. Overall, the sensitivity of MIBG for metastatic disease appears to be slightly lower than for primary tumors [62], perhaps related to dedifferentiation or to genetically determined differences in phenotype. Recent studies have shown that lack of VMAT-1 transporters is associated with an MIBG negative imaging phenotype [63].
Visualization of normal adrenal glands with I-123 MIBG imaging occurs in 31–50% of cases, and can lead to false positive results [3,64], although in the absence of a CT abnormality and adrenal uptake ≤ liver one would favor physiologic uptake [64]. The advent of SPECT/CT may improve [65] the interpretation and accuracy of MIBG.
F-18 Fluorodopamine (FDA)
FDA PET imaging is superior to MIBG in PHEO/PGL (higher receptor affinity [10], higher resolution and sensitivity of PET and same day imaging), but has been used extensively in only one institution. The sensitivity of FDA in metastatic PHEO/PGL is high, ranging from 88 to 100% on a per patient basis or 70 to 97% on a per lesion basis [54,57,62,66]. Direct comparisons have shown higher sensitivity for FDA than MIBG [57,62,66].
C-11 Meta-Hydroxyephedrine PET
Two groups have reported on the use of C-11 HED in patients with tumor of the sympathetic nervous system including PHEO and NB [67]. In eight PHEO patients, HED detected all sites of confirmed disease compared to MIBG, which failed to localize sites in four patients [11]. Favorable results were seen in 19 patients with PHEO and NB where HED also detected more tumor lesions than MIBG [67].
FDG PET
Shulkin et al. [68] was among the first to report on the use of FDG for PHEO/PGL imaging. They observed a per patient sensitivity of 76% overall, 58% for benign PHEO/PGL compared to 88% for malignant disease. In patients who were both MIBG and FDG positive, MIBG was superior in 56% and equal or better than FDG in 88%. More recently, higher FDG sensitivities have been reported for benign lesions (88%) [54]. In malignant PHEO/PGL, FDG sensitivity ranges from 88 to 100% on a per patient basis (Table III). FDG appears to have the highest sensitivity in patients with SDHB mutations [62].
FDOPA PET
Several studies have evaluated the use of FDOPA in PHEO/PGL, with reported per patient sensitivities of 88–100% (Tables II and III). Compared to MIBG, FDOPA detected 100% of benign lesions, whereas MIBG only detected 71% [69]. Timmers et al. [54] also reported a higher sensitivity of 81% for FDOPA compared to 77% for MIBG in benign tumors. FDOPA also has high sensitivity in malignant PHEO/PGL, typically superior to MIBG [70–72] (Table III). For instance, Taieb et al. [73] had 100% sensitivity on a per patient basis with FDOPA, higher than FDG or MIBG. More recently, out of 353 lesions, FDOPA detected 98% compared to 38.5% for MIBG [72], although in one series, the per region sensitivity was only 45%, lower than MIBG (57%), due to inclusion of SDHB patients. In SDHB patients, 7/15 had falsely negative FDOPA scans, with only 20% sensitivity on a per lesion basis compared to 83% for FDG, 82% for FDA, and 57% for MIBG. In contrast, in non-SDHB patients, FDOPA had the highest sensitivity of 93% compared to 76% for FDA, 62% for FDG, and 59% for MIBG [54]. Other studies also show very high sensitivity for benign PHEO/PGL (Table II).
Somatostatin Receptor Imaging (SRS)—In-111 and Ga-68 Agents
Several studies have evaluated In-111 pentetreotide for detection of PHEO/PGL. In benign PHEO/PGL, sensitivity is low, ranging from 25 to 28.5% (Table II). In contrast, the sensitivity of SRS for malignant PHEO/PGL is usually high, ranging from 87.5 to 88.9% (Table III).
Although not officially approved, studies have shown that Ga-68 labeled DOTATOC, DOTATATE, or DOTANOC have higher sensitivity for tumor detection than In-111 pentetreotide in neuroendocrine tumors [74]. Naji et al. [75] compared I-123 MIBG to Ga-68 DOTATATE in 12 patients with neural crest tumors. Six of seven patients with PHEO were Ga-68 DOTATATE positive compared to four with I-123 MIBG. Like In-111 pentetreotide, these reagents have the potential to identify patients who may benefit from Y-90 or Lu-177 SRS directed therapy.
Neuroblastoma (NB)
Like PHEO/PGL, NB is a neuroendocrine tumor derived from the sympathetic nervous system. It is the most common extracranial solid tumor in children and among the most lethal. Approximately 81% of patients are diagnosed between ages 0–4 years [76]. With improvements in therapy, overall 5-year survival has increased from 45 to 73% from the 1970s to 2000s [77]. The most common sites of involvement are the adrenals (48%), retroperitoneum (25%), and chest (16%) [78]. Distant metastasis predominantly affect bone marrow and bone. Survival is dependent on age, stage, and other biologic parameters at presentation; initial assessment involves complex testing and imaging [79].
MIBG
A consensus report from the international Neuroblastoma Risk Group Project has indicated the importance of MIBG imaging in their guidelines [79]. MIBG is routinely used in the initial staging and follow-up of patients with NB since it will frequently upstage patients, may have prognostic implications and on follow-up, can identify recurrent or refractory disease.
The original reports using MIBG in NB date back to the early 1980s. MIBG has high sensitivity in NB, typically greater than 90% [80–82] (Table IV). A meta-analysis of I-123 MIBG in patients with NB showed an overall sensitivity of 94% (range 76–100%) [59]. A multicenter trial evaluating 93 patients found a sensitivity of 88% and specificity of 83% [83].
TABLE IV.
Sensitivity for detection of Neuroblastoma
| Patient-based sensitivity (lesion-based sensitivity in parentheses) | |||
|---|---|---|---|
|
|
|||
| Name | Patients | In-111 pentetreotide | MIBG |
| [95] | 5 | Ga-68 100% | |
| (97.2%) | (90.7%)a | ||
| [91] | 88 | 64% | 94%a |
| [116] | 7 | 57% | 86%b |
| [93] | 12 | 75% | 92%a |
I-123 MIBG.
I-131 MIBG.
Several studies have noted that 24–41% of patients imaged following therapy will have falsely negative MIBG scans [80,84,85]. Nonetheless, in a small percentage of patients, MIBG will be the only positive modality and therefore, should be included in followup [84].
Bone Scanning
Bone scanning agents (Tc-99m labeled bisphosphonates) generally have lower sensitivity for detecting bony metastases than MIBG [86,87]. At initial staging, bone scintigraphy may help localize some patients that are MIBG negative or that have cortical bone lesions [87,88], but they are not used routinely in follow-up since in most cases, they do not provide additional information beyond MIBG and are associated with a higher frequency of false positive findings.
SRS
Studies have shown that NB cells exhibit predominantly somatostatin receptors (SSTR) 1 and 2 [89], and quantitation of In-111 pentetreotide uptake is related to SSRT levels [90]. Several studies have compared the utility of SRS to that of MIBG (Table IV). In most, MIBG detected more lesions with higher contrast than In-111 pentetreotide. In the largest series of 88 patients, the sensitivity of MIBG was much higher than SRS, 94% vs. 64%, respectively [91]. In line with other studies [92–94], prognosis was better for SRS positive patients who had a survival probability of 95% at 4 years compared to 62% for SRS negative patients. Furthermore, the event free survival was 86% and 37%, respectively at 4 years.
Overall, SRS is not routinely used in the evaluation of NB [79]. Preliminary studies have shown that the newer Ga-68 DOTATOC labeled SRS PET agents appear to have higher sensitivity than In-111 pentetreotide, similar to MIBG [95], with 97% sensitivity compared to 90.7% for MIBG. Further studies with these newer PET reagents need to be performed to evaluate their role, if any, in NB.
FDG PET
Some NB do not have or lose their ability to concentrate MIBG; this is generally ascribed to changes in differentiation, although this has not been corroborated histologically [96]. Studies evaluating the use of FDG at presentation, recurrence, or evaluation of response to treatment have been reported [97–102], and there appears to be a correlation between the degree of FDG uptake and shorter survival [99].
A comparison between FDG and MIBG in primary/residual NB prior to systemic therapy was performed by Shulkin et al. [97]. In their report, FDG and MIBG identified all primary/residual lesions, but MIBG was equal or superior in 5/7 patients. In a retrospective study in patients with stage 1 and 2 disease, FDG depicted more primary or locoregional metastases in 6/10 patients than MIBG, although SUVs were not very high (mean 2.8) [98].
Similarly, in evaluation patients with refractory or recurrent NB, MIBG outperformed/equaled FDG in 11/13 patients in Shulkin’s study [97]. In another study of 20 patients, MIBG was superior to FDG in depicting tumor load in 43%, equal in 43%, and inferior in 14% [99]. In stage 4 disease, MIBG depicted more lesions in 52% (44/85) scans, whereas FDG detected more in 13% (11/85) and was equal in 15% (13/85) [98]. In all of these studies, detection of bone/marrow disease was problematic with FDG because of its physiologic distribution in bone marrow in young patients and the normal increased marrow uptake following growth factor therapy.
A report by Taggart et al. [103] evaluated FDG and I-123 MIBG for assessment of response to therapy in 14 patients treated with I-131 MIBG. Out of 139 distinct lesions, 51% were MIBG positive/FDG negative, 9.4% were MIBG negative/FDG positive, and 39.6% were concordantly positive. In the post therapy setting, FDG positive/MIBG negative lesions were infrequent (3.9%). In both the pre- and post-treatment setting, the incidence of FDG positive/MIBG negative lesions was low (<6.6%). Complete metabolic response observed by FDG did not always correlate with MIBG scanning. Thus, these and other studies show that FDG cannot replace MIBG for follow-up [104].
Other PET Reagents
FDOPA proved promising in a group of 19 patients with suspected primary or recurrent NB [105]. FDOPA correctly identified 94% with disease, in contrast to 65% on MIBG. All MIBG positive patients were FDOPA positive. Overall, on a scan-based analysis, FDOPA showed a sensitivity of 95% and specificity of 96% compared to 68% and 64%, respectively, for MIBG. FDOPA resulted in patient management changes 32% of the time. FDOPA appeared to have an advantage in identifying primary/residual tumor and locoregional soft tissue recurrence, but no significant difference in bone/ bone marrow disease was noted, although when a per lesion analysis was performed, FDOPA also outperformed MIBG in the bone/bone marrow.
A preliminary study in seven patients evaluated C-11 hydroxyephedrine PET imaging of patients with NB [106]. Tumors were positive in all cases and imaging could be started within the first five minutes post injection. A possible alternative is the use of FDA, that has been used extensively in patients with PHEO and has a longer T1/2 [107].
In summary, I-123 MIBG imaging remains the most important functional imaging modality in patients with NB given its sensitivity, specificity, and prognostic implications. Nonetheless, some NBs are not MIBG avid and in these cases or in cases with equivocal/discordant findings, FDG PET is helpful, although its use in lieu of MIBG is not endorsed [108]. New modalities that have not been adequately evaluated include FDOPA, FDA, C-11 hydroxyephedrine, and Ga-68 labeled somatostatin analogs.
SUMMARY
There are a number of molecular imaging agents that have been used for imaging the adrenal. These include MIBG, radiolabeled nor-cholesterol derivatives, and In-111 pentetreotide. Newer reagents include FDG, FDA, HED, FDOPA, Ga-68 SRS, and MTO. Some of these reagents such as the nor-cholesterol derivatives are rarely used these days, while PET reagents other than FDG are only available in a few centers with on-site cyclotron and/or radiochemistry facilities and have not been used in large number of patients.
In the evaluation of adrenal incidentalomas, FDG plays an important role with established high sensitivity and specificity in determining malignancy, particularly in the setting of known extra-adrenal malignancy. FDG is also useful in patients with ACC in evaluating the extent of disease. Furthermore, it appears to have prognostic implications. MTO reagents can differentiate cortical from non-cortical adrenal tumors, but cannot separate benign from malignant disease. Because its mechanism of binding, it may have advantage in ACC compared to FDG, but this is yet to be determined.
I-123 MIBG plays an established role in imaging of enterochromaffin-derived tumors including PHEO/PGL and NB. MIBG remains an indispensable tool in the management of NB patients as an imaging agent and for selecting patients for therapeutic applications. FDG and FDOPA also show promise in NB. While SRS agents have been used in NB, their limited sensitivity in advanced disease has limited their utility. The newer Ga-68 labeled SRS reagents require further studies in this setting.
There are multiple competing reagents for evaluation of patients with PHEO/PGL. MIBG, because of availability, is the most frequently used and has an important role, including selecting patients for I-131 MIBG therapy. Nonetheless, it is clear that newer reagents with similar mechanisms of uptake such as FDA have better performance characteristics—unfortunately, this reagent has been used extensively in only one center. FDOPA, approved in Europe, has shown high sensitivity for PHEO/PGL, although it is not commercially available in the USA. FDG is also extremely useful in PHEO/ PGL, particularly in the setting of malignant disease. SRS reagents are less sensitive for metastatic PHEO/PGL, although sensitivity is much higher for malignant than benign disease. The newer Ga-68 SRS analogs may exhibit better performance characteristics, but their role, particularly with respect to selection for therapy with Y-90/Lu-177 SRS, remains to be defined.
ACKNOWLEDGMENTS
Millie Whatley CNMT for assistance with image selection.
Footnotes
Conflicts of interest: None.
Staff Physician.
Attending Physician.
REFERENCES
- 1.Wieland DM, Swanson DP, Brown LE, et al. : Imaging the adrenal-medulla with an 1–131-labeled antiadrenergic agent. J Nucl Med 1979;20:155–158. [PubMed] [Google Scholar]
- 2.Kolby L, Bernhardt P, Levin-Jakobsen AM, et al. : Uptake of meta-iodobenzylguanidine in neuroendocrine tumours is mediated by vesicular monoamine transporters. Br J Cancer 2003; 89:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta N, Kiyota H, Yoshigoe F, et al. : Diagnosis of pheochromocytoma using [I-123]-compared with [I-131]-metaiodobenzylguanidine scintigraphy. Int J Urol 1999;6:119–124. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer D, Bell GI, Berelowitz M, et al. : Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci 1995;16:86–88. [DOI] [PubMed] [Google Scholar]
- 5.Khan MU, Khan S, Ei-Refaie S, et al. : Clinical indications forGallium-68 positron emission tomography imaging. Eur J Surg Oncol 2009;35:561–567. [DOI] [PubMed] [Google Scholar]
- 6.Song YS, Lee WW, Chung JH, et al. : Correlation between FDG uptake and glucose transporter type 1 expression in neuroendocrine tumors of the lung. Lung Cancer 2008;61:54–60. [DOI] [PubMed] [Google Scholar]
- 7.Delbeke D, Coleman RE, Guiberteau MJ, et al. : Procedure guideline for tumor imaging with F-18-FDG PET/CT 1.0. J Nucl Med 2006;47:885–895. [PubMed] [Google Scholar]
- 8.Jager PL, Chirakal R, Marriott CJ, et al. : 6-L-F-18-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: Basic aspects and emerging clinical applications. J Nucl Med 2008; 49:573–586. [DOI] [PubMed] [Google Scholar]
- 9.Pacak K, Eisenhofer G, Carrasquillo JA, et al. : 6- F-18 fluorodopamine positron emission tomographic (PET) scanning for diagnostic localization of pheochromocytoma. Hypertension 2001;38:6–8. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhofer G: The role of neuronal and extraneuronal plasmamembrane transporters in the inactivation of peripheral catecholamines. Pharmacol Ther 2001;91:35–62. [DOI] [PubMed] [Google Scholar]
- 11.Mann GN, Link JM, Pham P, et al. : [C-11]Metahydroxyephedrine and [F-18]fluorodeoxyglucose positron emission tomography improve clinical decision making in suspected pheochromocytoma. Ann Surg Oncol 2006;13:187–197. [DOI] [PubMed] [Google Scholar]
- 12.Rubello D, Bui C, Casara D, et al. : Functional scintigraphy ofthe adrenal gland. Eur J Endocrinol 2002;147:13–28. [DOI] [PubMed] [Google Scholar]
- 13.Zettinig G, Mitterhauser M, Wadsak W, et al. : Positron emission tomography imaging of adrenal masses: F-18-fluorodeoxyglucose and the 11 beta-hydroxylase tracer C-11-metomidate. Eur J Nucl Med Mol Imaging 2004;31:1224–1230. [DOI] [PubMed] [Google Scholar]
- 14.Bergstrom M, Juhlin C, Bonasera TA, et al. : PET imaging of adrenal cortical tumors with the 11beta-hydroxylase tracer 11C-metomidate. J Nucl Med 2000;41:275–282. [PubMed] [Google Scholar]
- 15.Wadsak W, Mitterhauser M, Rendl G, et al. : [18F]FETO for adrenocortical PET imaging: A pilot study in healthy volunteers. Eur J Nucl Med Mol Imaging 2006;33:669–672. [DOI] [PubMed] [Google Scholar]
- 16.Hahner S, Stuermer A, Kreissl M, et al. : [123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab 2008;93:2358–2365. [DOI] [PubMed] [Google Scholar]
- 17.Hahner S, Kreissl MC, Fassnacht M, et al. : [131I]Iodometomidate for targeted radionuclide therapy of advanced adrenocortical carcinoma. J Clin Endocrinol Metab 2012;97:914–922. [DOI] [PubMed] [Google Scholar]
- 18.Gross MD, Djekidel M, Hay RV, et al. : Scintigraphic localization of adrenal tumors. Minerva Endocrinol 2009;34:171–184. [PubMed] [Google Scholar]
- 19.Maurea S, Klain M, Caraco C, et al. : Diagnostic accuracy of radionuclide imaging using I-131 nor-cholesterol or meta-iodobenzylguanidine in patients with hypersecreting or non-hypersecreting adrenal tumours. Nucl Med Commun 2002;23:951–960. [DOI] [PubMed] [Google Scholar]
- 20.Fig LM, Gross MD, Shapiro B, et al. : Adrenal localization in the adrenocorticotropic hormone-independent Cushing syndrome. Ann Intern Med 1988;109:547–553. [DOI] [PubMed] [Google Scholar]
- 21.Park JR, Stewart CF, London WB, et al. : A topotecan-containing induction regimen for treatment of high risk neuroblastoma. J Clin Oncol 2006;24:505s–505s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barzon L, Zucchetta P, Boscaro M, et al. : Scintigraphic patterns of adrenocortical carcinoma: Morpho-functional correlates. Eur J Endocrinol 2001;145:743–748. [DOI] [PubMed] [Google Scholar]
- 23.Bagheri B, Maurer AH, Cone L, et al. : Characterization of the normal adrenal gland with F-18-FDG PET/CT. J Nucl Med 2004;45:1340–1343. [PubMed] [Google Scholar]
- 24.Song JH, Chaudhry FS, Mayo-Smith WW: The incidental adrenal mass on CT: Prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. Am J Roentgenol 2008;190:1163–1168. [DOI] [PubMed] [Google Scholar]
- 25.Hammarstedt L, Muth A, Wangberg B, et al. : Adrenal lesion frequency: A prospective, cross-sectional CT study in a defined region, including systematic re-evaluation. Acta Radiol 2010;51:1149–1156. [DOI] [PubMed] [Google Scholar]
- 26.Hedeland H, Ostberg G, Hokfelt B: On the prevalence of adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. Acta Med Scand 1968;184:211–214. [DOI] [PubMed] [Google Scholar]
- 27.Abrams HL, Spiro R, Goldstein N: Metastases in carcinoma - analysis of 1000 autopsied cases. Cancer 1950;3:74–85. [DOI] [PubMed] [Google Scholar]
- 28.Boland GWL, Dwamena BA, Sangwaiya MJ, et al. : Characterization of adrenal masses by using FDG PET: A systematic review and meta-analysis of diagnostic test performance. Radiology 2011;259:117–126. [DOI] [PubMed] [Google Scholar]
- 29.Metser U, Miller E, Lerman H, et al. : F-18-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med 2006;47:32–37. [PubMed] [Google Scholar]
- 30.Brady MJ, Thomas J, Wong TZ, et al. : Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: A proposal for an efficient Diagnostic algorithm. Radiology 2009;250:523–530. [DOI] [PubMed] [Google Scholar]
- 31.Ozcan Kara P, Kara T, Gedik GK, et al. : The role of fluorodeoxyglucose-positron emission tomography/computed tomography in differentiating between benign and malignant adrenal lesions. Nucl Med Commun 2011;32:106–112. [DOI] [PubMed] [Google Scholar]
- 32.Perri M, Erba P, Volterrani D, et al. : Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. Am J Roentgenol 2011;197:209–216. [DOI] [PubMed] [Google Scholar]
- 33.Boland GWL, Blake MA, Holalkere NS, et al. : PET/CT for the characterization of adrenal masses in patients with cancer: Qualitative versus quantitative accuracy in 150 consecutive patients. Am J Roentgenol 2009;192:956–962. [DOI] [PubMed] [Google Scholar]
- 34.Jana S, Zhang T, Milstein DM, et al. : FDG-PET and CT characterization of adrenal lesions in cancer patients. Eur J Nucl Med Mol Imaging 2006;33:29–35. [DOI] [PubMed] [Google Scholar]
- 35.Han SJ, Kim TS, Jeon SW, et al. : Analysis of adrenal masses by F-18-FDG positron emission tomography scanning. Int J Clin Practice 2007;61:802–809. [DOI] [PubMed] [Google Scholar]
- 36.Caoili EM, Korobkin M, Brown RKJ, et al. : Differentiating adrenal adenomas from nonadenomas using F-18-FDG PET/CT: Quantitative and qualitative evaluation. Acad Radiol 2007;14:468–475. [DOI] [PubMed] [Google Scholar]
- 37.Gratz S, Kemke B, Kaiser W, et al. : Incidental non-secreting adrenal masses in cancer patients: Intra-individual comparison of (18)F-Fluorodeoxyglucose positron emission tomography/computed tomography with computed tomography and shift magnetic resonance imaging. J Int Med Res 2010;38:633–644. [DOI] [PubMed] [Google Scholar]
- 38.Repko BM, Tulchinsky M: Increased F-18 FDG uptake in resolving atraumatic bilateral adrenal hemorrhage (hematoma) on PET/CT. Clin Nucl Med 2008;33:651–653. [DOI] [PubMed] [Google Scholar]
- 39.Tessonnier L, Sebag F, Palazzo FF, et al. : Does F-18-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours? Eur J Nucl Med Mol Imaging 2008;35:2018–2025. [DOI] [PubMed] [Google Scholar]
- 40.Nunes ML, Rault A, Teynie J, et al. : 18F-FDG PET for the identification of adrenocortical carcinomas among indeterminate adrenal tumors at computed tomography scanning. World J Surg 2010;34:1506–1510. [DOI] [PubMed] [Google Scholar]
- 41.Groussin L, Bonardel G, Silvera S, et al. : (18)F-Fluorodeoxy glucose positron emission tomography for the diagnosis of adrenocortical tumors: A prospective study in 77 operated patients. J Clin Endocrinol Metab 2009;94:1713–1722. [DOI] [PubMed] [Google Scholar]
- 42.Ansquer C, Scigliano S, Mirallie E, et al. : (18)F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: A prospective multicentre evaluation. Eur J Nucl Med Mol Imaging 2010;37:1669–1678. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramaniam S, Fojo T: Practical considerations in the evaluation and management of adrenocortical cancer. Semin Oncol 2010;37:619–626. [DOI] [PubMed] [Google Scholar]
- 44.Becherer A, Vierhapper H, Potzi C, et al. : FDG-PET in adrenocortical carcinoma. Cancer Biother Radiopharm 2001;16:289–295. [DOI] [PubMed] [Google Scholar]
- 45.Mackie GC, Shulkin BL, Ribeiro RC, et al. : Use of (18)F fluorodeoxyglucose positron emission tomography in evaluating locally recurrent and metastatic adrenocortical carcinoma. J Clin Endocrinol Metab 2006;91:2665–2671. [DOI] [PubMed] [Google Scholar]
- 46.Leboulleux S, Dromain C, Bonniaud G, et al. : Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: A prospective comparison with computed tomography. J Clin Endocrinol Metab 2006;91:920–925. [DOI] [PubMed] [Google Scholar]
- 47.Gust L, Taieb D, Beliard A, et al. : Preoperative 18F-FDG uptake is strongly correlated with malignancy, weiss score, and molecular markers of aggressiveness in adrenal cortical tumors. World J Surg 2012;36:1406–1410. [DOI] [PubMed] [Google Scholar]
- 48.Leboulleux S, Deandreis D, Escourrou C, et al. : Fluorodesoxyglucose uptake in the remaining adrenal glands during the follow-up of patients with adrenocortical carcinoma: Do not consider it as malignancy. Eur J Endocrinol 2011;164:89–94. [DOI] [PubMed] [Google Scholar]
- 49.Minn H, Salonen A, Friberg J, et al. : Imaging of adrenal incidentalomas with PET using C-11-metomidate and F-18-FDG. J Nucl Med 2004;45:972–979. [PubMed] [Google Scholar]
- 50.Hennings J, Lindhe O, Bergstrom M, et al. : [11C]metomidatepositron emission tomography of adrenocortical tumors in correlation with histopathological findings. J Clin Endocrinol Metab 2006;91:1410–1414. [DOI] [PubMed] [Google Scholar]
- 51.Hennings J, Hellman P, Ahlstrom H, et al. : Computed tomography, magnetic resonance imaging and 11C-metomidate positron emission tomography for evaluation of adrenal incidentalomas. Eur J Radiol 2009;69:314–323. [DOI] [PubMed] [Google Scholar]
- 52.Khan TS, Sundin A, Juhlin C, et al. : C-11-metomidate PET imaging of adrenocortical cancer. Eur J Nucl Med Mol Imaging 2003;30:403–410. [DOI] [PubMed] [Google Scholar]
- 53.Mitterhauser M, Dobrozemsky G, Zettinig G, et al. : Imaging of adrenocortical metastases with [11C]metomidate. Eur J Nucl Med Mol Imaging 2006;33:974. [DOI] [PubMed] [Google Scholar]
- 54.Timmers H, Chen CC, Carrasquillo JA, et al. : Comparison of(18)F-fluoro-L-DOPA, (18)F-fluoro-deoxyglucose, and (18)F-fluorodopamine PET and (123)I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009;94:4757–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taieb D, Sebag F, Barlier A, et al. : F-18-FDG avidity of pheochromocytomas and paragangliomas: A new molecular imaging signature? J Nucl Med 2009;50:711–717. [DOI] [PubMed] [Google Scholar]
- 56.Gimenez-Roqueplo AP, Favier J, Rustin P, et al. : Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab 2002;87:4771–4774. [DOI] [PubMed] [Google Scholar]
- 57.Kaji P, Carrasquillo JA, Linehan WM, et al. : The role of 6-F-18 fluorodopamine positron emission tomography in the localization of adrenal pheochromocytoma associated with von Hippel-Lindau syndrome. Eur J Endocrinol 2007;156: 483–487. [DOI] [PubMed] [Google Scholar]
- 58.Wiseman GA, Pacak K, O’Dorisio MS, et al. : Usefulness of (123)I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: Results from a prospective multicenter trial. J Nucl Med 2009;50:1448–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacobson AF, Deng HW, Lombard J, et al. : (123)I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: Results of a meta-analysis. J Clin Endocrinol Metab 2010;95:2596–2606. [DOI] [PubMed] [Google Scholar]
- 60.Van Der Horst-Schrivers ANA, Jager PL, Boezen HM, et al. : Iodine-123 metaiodobenzylguanidine scintigraphy in localising phaeochromocytomas - Experience and meta-analysis. Anti-cancer Res 2006;26:1599–1604. [PubMed] [Google Scholar]
- 61.Bhatia KSS, Ismail MM, Sahdev A, et al. : I-123-metaiodobenzylguanidine (MIBG) scintigraphy for the detection of adrenal and extra-adrenal phaeochromocytomas: CT and MRI correlation. Clin Endocrinol 2008;69:181–188. [DOI] [PubMed] [Google Scholar]
- 62.Timmers H, Kozupa A, Chen CC, et al. : Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol 2007;25:2262–2269. [DOI] [PubMed] [Google Scholar]
- 63.Fottner C, Helisch A, Anlauf M, et al. : 6-(18)F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to (123)I-metaiodobenzyl-guanidine Scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: With vesicular monoamine transporter expression. J Clin Endocrinol Metab 2010;95:2800–2810. [DOI] [PubMed] [Google Scholar]
- 64.Mozley PD, Kim CK, Mohsin J, et al. : The efficacy of iodine-123-MIBG as a screening-test for pheochromocytoma. J Nucl Med 1994;35:1138–1144. [PubMed] [Google Scholar]
- 65.Meyer-Rochow GY, Schembri GP, Benn DE, et al. : The utility of metaiodobenzylguanidine single photon emission computed tomography/computed tomography (MIBG SPECT/CT) for the diagnosis of pheochromocytoma. Ann Surg Oncol 2010; 17:392–400. [DOI] [PubMed] [Google Scholar]
- 66.Ilias I, Chen CC, Carrasquillo JA, et al. : Comparison of 6-(18) F-fluorodopamine PET with (123)I-metaiodobenzylguanidine and (111)in-pentetreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. J Nucl Med 2008;49:1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franzius C, Hermann K, Weckesser M, et al. : Whole-body PET/CT with C-11-meta-hydroxyephedrine in tumors of the sympathetic nervous system: Feasibility study and comparison with I-123-MIBG SPECT/CT. J Nucl Med 2006;47:1635–1642. [PubMed] [Google Scholar]
- 68.Shulkin BL, Thompson NW, Shapiro B, et al. : Pheochromocytomas: Imaging with 2-[fluorine-18] fluoro-2-deoxy-D-glucose PET. Radiology 1999;212:35–41. [DOI] [PubMed] [Google Scholar]
- 69.Hoegerle S, Nitzsche E, Altehoefer C, et al. : Pheochromocytomas: Detection with F-18 DOPA whole-body PET - Initial results. Radiology 2002;222:507–512. [DOI] [PubMed] [Google Scholar]
- 70.Timmers H, Hadi M, Carrasquillo JA, et al. : The effects of carbidopa on uptake of 6-F-18-fluoro-L-DOPA in PET of pheochromocytorna and extraadrenal abdominal paraganglioma. J Nucl Med 2007;48:1599–1606. [DOI] [PubMed] [Google Scholar]
- 71.Fiebrich HB, Brouwers AH, Kerstens MN, et al. : 6- F-18 fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with I-123-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab 2009;94:3922–3930. [DOI] [PubMed] [Google Scholar]
- 72.Rufini V, Treglia G, Castaldi P, et al. : Comparison of (123)IMIBG SPECT-CT and (18)F-DOPA PET-CT in the evaluation of patients with known or suspected recurrent paraganglioma. Nucl Med Commun 2011;32:575–582. [DOI] [PubMed] [Google Scholar]
- 73.Taieb D, Tessonnier L, Sebag F, et al. : The role of F-180-FDOPA and F-18-FDG-PET in the management of malignant and multifocal phaeochromocytomas. Clin Endocrinol 2008; 69:580–586. [DOI] [PubMed] [Google Scholar]
- 74.Krausz Y, Freedman N, Rubinstein R, et al. : (68)Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: Comparison with (111)In-DTPA-Octreotide (OctreoScan (R)). Mol Imaging Biol 2011;13:583–593. [DOI] [PubMed] [Google Scholar]
- 75.Naji M, Zhao CL, Welsh SJ, et al. : (68)Ga-DOTA-TATE PET vs. (123)I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol 2011;13:769–775. [DOI] [PubMed] [Google Scholar]
- 76.Grovas A, Fremgen A, Rauck A, et al. : The national cancer data base report on patterns of childhood cancers in the United States. Cancer 1997;80:2321–2332. [DOI] [PubMed] [Google Scholar]
- 77.Navalkele P, O’Dorisio MS, O’Dorisio TM, et al. : Incidence, survival, and prevalence of neuroendocrine tumors versus neuroblastoma in children and young adults: Nine standard SEER registries, 1975–2006. Pediatr Blood Cancer 2011;56:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohn SL, Pearson ADJ, London WB, et al. : The international neuroblastoma risk group (INRG) classification System: An INRG task force report. J Clin Oncol 2009;27:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brisse HJ, McCarville MB, Granata C, et al. : Guidelines forimaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group Project. Radiology 2011;261:243–257. [DOI] [PubMed] [Google Scholar]
- 80.Feine U, Mullerschauenburg W, Treuner J, et al. : Metaiodobenzylguanidine (metaiodobenzylguanidine) labeled with I-123/I-131 in neuroblastoma diagnosis and follow-up treatment with a review of the diagnostic results of the international workshop of pediatric oncology held in rome, september 1986. Med Pediatr Oncol 1987;15:181–187. [DOI] [PubMed] [Google Scholar]
- 81.Abrahamsen J, Lyck B, Helgestad J, et al. : The impact of I-123 metaiodobenzylguanidine scintigraphy on diagnostics and follow-up of neuroblastoma. Acta Oncol 1995;34:505–510. [DOI] [PubMed] [Google Scholar]
- 82.Rufini V, Fisher GA, Shulkin BL, et al. : Iodine-123-MIBG imaging of neuroblastoma: Utility of SPECT and delayed imaging. J Nucl Med 1996;37:1464–1468. [PubMed] [Google Scholar]
- 83.Vik TA, Pfluger T, Kadota R, et al. : (123)I-mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatr Blood Cancer 2009;52:784–790. [DOI] [PubMed] [Google Scholar]
- 84.Kushner BH, Yeh SDJ, Kramer K, et al. : Impact of metaiodobenzylguanidine scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol 2003;21:1082–1086. [DOI] [PubMed] [Google Scholar]
- 85.Troncone L, Rufini V, Danza FM, et al. : Radioiodinated meta-iodobenzylguanidine (I-metaiodobenzylguanidine) scintigraphy in neuroblastoma - a review of 160 studies. J Nucl Med Allied Sci 1990;34:279–288. [PubMed] [Google Scholar]
- 86.Osmanagaoglu K, Lippens M, Benoit Y, et al. : A comparison of I-123 metaiodobenzylguanidine scintigraphy and single bone-marrow aspiration biopsy in the diagnosis and follow-up of 26 children with neuroblastoma. Eur J Nucl Med 1993;20:1154–1160. [DOI] [PubMed] [Google Scholar]
- 87.Gordon I, Peters AM, Gutman A, et al. : Skeletal assessment in neuroblastoma - the pitfalls of iodine-123-mIBG scans. J Nucl Med 1990;31:129–134. [PubMed] [Google Scholar]
- 88.Sautterbihl ML, Bihl H, Heinze HG: The value of TC-99MMDP bone- scintigraphy in patients with neuroblastoma. Nuklearmedizin 1991;30:7–12. [PubMed] [Google Scholar]
- 89.Albers AR, O’Dorisio MS, Balster DA, et al. : Somatostatin receptor gene expression in neuroblastoma. Regul Pept 2000;88:61–73. [DOI] [PubMed] [Google Scholar]
- 90.Briganti V, Sestini R, Orlando C, et al. : Imaging of somatostatin receptors by indium-111-pentetreotide correlates with quantitative determination of somatostatin receptor type 2 gene expression in neuroblastoma tumors. Clin Cancer Res 1997;3:2385–2391. [PubMed] [Google Scholar]
- 91.Schilling FH, Bihl H, Jacobsson H, et al. : Combined In-111-pentetreotide scintigraphy and I-123-mIBG scintigraphy in neuroblastoma provides prognostic information. Med Pediatr Oncol 2000;35:688–691. [DOI] [PubMed] [Google Scholar]
- 92.Moertel CL, Reubi JC, Scheithauer BS, et al. : Expression of somatostatin receptors in childhood neuroblastoma. Am J Clin Pathol 1994;102:752–756. [DOI] [PubMed] [Google Scholar]
- 93.Kropp J, Hofmann M, Bihl H: Comparison of MIBG and pentetreotide scintigraphy in children with neuroblastoma. Is the expression of somatostatin receptors a prognostic factor? Anticancer Res 1997;17:1583–1588. [PubMed] [Google Scholar]
- 94.Raggi CC, Maggi M, Renzi D, et al. : Quantitative determination of sst2 gene expression in neuroblastoma tumor predicts patient outcome. J Clin Endocrinol Metab 2000;85:3866–3873. [DOI] [PubMed] [Google Scholar]
- 95.Kroiss A, Putzer D, Uprimny C, et al. : Functional imaging in phaeochromocytoma and neuroblastoma with (68)Ga-DOTA-Tyr(3)-octreotide positron emission tomography and (123)I-metaiodobenzylguanidine. Eur J Nucl Med Mol Imaging 2011;38:865–873. [DOI] [PubMed] [Google Scholar]
- 96.Brans B, Laureys G, Schelfhout V, et al. : Activity of iodine-123 metaiodobenzylguanidine in childhood neuroblastoma: Lack of relation to tumour differentiation in vivo. Eur J Nucl Med 1998;25:144–149. [DOI] [PubMed] [Google Scholar]
- 97.Shulkin BL, Hutchinson RJ, Castle VP, et al. : Neuroblastoma: Positron emission tomography with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose compared with metaiodobenzylguanidine scintigraphy. Radiology 1996;199:743–750. [DOI] [PubMed] [Google Scholar]
- 98.Sharp SE, Shulkin BL, Gelfand MJ, et al. : (123)I-MIBG scintigraphy and (18)F-FDG PET in neuroblastoma. J Nucl Med 2009;50:1237–1243. [DOI] [PubMed] [Google Scholar]
- 99.Papathanasiou ND, Gaze MN, Sullivan K, et al. : (18)F-FDG PET/CT and (123)I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: Diagnostic comparison and survival analysis. J Nucl Med 2011;52:519–525. [DOI] [PubMed] [Google Scholar]
- 100.Melzer HI, Coppenrath E, Schmid I, et al. : (123)I-MIBG scintigraphy/SPECT versus (18)F-FDG PET in paediatric neuroblastoma. Eur J Nucl Med Mol Imaging 2011;38:1648–1658. [DOI] [PubMed] [Google Scholar]
- 101.Kushner BH, Yeung HWD, Larson SM, et al. : Extending positron emission tomography scan utility to high-risk neuroblastoma: Fluorine-18 fluorodeoxyglucose positron emission tomography as sole imaging modality in follow-up of patients. J Clin Oncol 2001;19:3397–3405. [DOI] [PubMed] [Google Scholar]
- 102.Colavolpe C, Guedj E, Cammilleri S, et al. : Utility of FDG-PET/CT in the follow-up of neuroblastoma which became MIBG-negative. Pediatr Blood Cancer 2008;51:828–831. [DOI] [PubMed] [Google Scholar]
- 103.Taggart DR, Han MM, Quach A, et al. : Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and (18)F fluorodeoxyglucose positron emission tomography to Evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol 2009;27:5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kushner BH, Kramer K, Modak S, et al. : Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol 2009;27:1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piccardo A, Lopci E, Conte M, et al. : Comparison of (18)F-dopa PET/CT and (123)I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: A pilot study. Eur J Nucl Med Mol Imaging 2012;39:57–71. [DOI] [PubMed] [Google Scholar]
- 106.Shulkin BL, Wieland DM, Baro ME, et al. : PET hydroxyephedrine imaging of neuroblastoma. J Nucl Med 1996;37:16–21. [PubMed] [Google Scholar]
- 107.Pacak K, Eisenhofer G, Carrasquillo JA, et al. : Diagnostic localization of pheochromocytoma - The coming of age of positron emission tomography. In: Pacak K, Eisenhofer G, Editors: “Ann Ny Acad Sci.”. 2002;970:170–176. [DOI] [PubMed] [Google Scholar]
- 108.Matthay KK, Shulkin B, Ladenstein R, et al. : Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 2010;102:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vikram R, Yeung HDW, Macapinlac HA, et al. : Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. Am J Roentgenol 2008;191:1545–1551. [DOI] [PubMed] [Google Scholar]
- 110.Miskulin J, Shulkin BL, Doherty GM, et al. : Is preoperative iodine 123 meta-iodobenzylguanidine scintigraphy routinely necessary before initial adrenalectomy for pheochromocytoma? Surgery 2003;134:918–922. [DOI] [PubMed] [Google Scholar]
- 111.Lumachi F, Tregnaghi A, Zucchetta P, et al. : Sensitivity and positive predictive value of CT, MRI and I-123-MIBG scintigraphy in localizing pheochromocytomas: A prospective study. Nucl Med Commun 2006;27:583–587. [DOI] [PubMed] [Google Scholar]
- 112.van der Harst E, de Herder WW, Bruining HA, et al. : [I-123]metaiodobenzylguanidine and [In-111]octreotide uptake in benign and malignant pheochromocytomas. J Clin Endocrinol Metab 2001;86:685–693. [DOI] [PubMed] [Google Scholar]
- 113.Luster M, Karges W, Zeich K, et al. : Clinical value of F-18-fluorodihydroxyphenylalanine positron emission tomography/computed tomography (F-18-DOPA PET/CT) for detecting pheochromocytoma. Eur J Nucl Med Mol Imaging 2010;37:484–493. [DOI] [PubMed] [Google Scholar]
- 114.Shapiro B, Copp JE, Sisson JC, et al. : I-131 metaiodobenzyl-guanidine for the locating of suspected pheochromocytoma - experience in 400 cases. J Nucl Med 1985;26:576–585. [PubMed] [Google Scholar]
- 115.Taieb D, Tessonnier L, Mundler O: Positron emission tomography in digestive neuroendocrine tumors: Choice of the tracer. Medecine Nucleaire-Imagerie Fonctionnelle Et Metabolique 2009;33:712–717. [Google Scholar]
- 116.ShalabyRana E, Majd M, Andrich MP, et al. : In-111 pentetreotide scintigraphy in patients with neuroblastoma - Comparison with I-131 MIBG, N-Myc oncogene amplification, and patient outcome. Clin Nucl Med 1997;22:315–319. [DOI] [PubMed] [Google Scholar]