Abstract
Background
Thiamine supplementation may improve cardiac function in older adults with heart failure (HF). Our objectives were to determine the following: (i) the feasibility of conducting a large trial of thiamine supplementation in HF; and (ii) the effects of thiamine on clinical outcomes.
Methods
We conducted a double-blinded randomized placebo-controlled 2-period crossover feasibility study from June 2018 to April 2021. Adults aged ≥ 60 years with symptomatic HF and reduced ejection fraction (≤ 45%) were included. Participants were randomized to thiamine mononitrate 500 mg, or placebo, for 90 days and were switched to the opposite treatment for 90 days after a 6-week washout period. The primary feasibility outcome was recruitment of 24 participants in 11 months.
Results
We screened 330 patients over 21 months to recruit 24 patients. Participants’ mean age was 73.4 years. The targets for refusal rate, retention rate, and adherence rate were met. Nonsignificant improvements occurred in left ventricular ejection fraction and N-terminal pro-brain natriuretic peptide (NT-proBNP) level with thiamine. A total of 13 serious adverse events occurred in 7 patients; none were related to the study drug.
Conclusions
Although we did not reach our recruitment target, we found high-dose thiamine supplementation to be well tolerated, with potential improvements in biomarker outcomes. A larger trial of thiamine supplementation is warranted.
Résumé
Introduction
La supplémentation en thiamine peut améliorer la fonction cardiaque chez les personnes âgées atteintes d’insuffisance cardiaque (IC). Nos objectifs visaient à déterminer : (i) la faisabilité d’un essai de grande envergure sur la supplémentation en thiamine lors d’IC ; (ii) les effets de la thiamine sur les résultats cliniques.
Méthodes
Nous avons réalisé une étude de faisabilité croisée à double insu et à répartition aléatoire contre placebo sur deux périodes de juin 2018 à avril 2021. Nous avons retenu les adultes de ≥ 60 ans qui avaient une IC symptomatique et une fraction d’éjection réduite (≤ 45 %). Nous avons réparti les participants de façon aléatoire pour recevoir 500 mg de mononitrate de thiamine ou le placebo durant 90 jours, et avons inversé le traitement durant 90 jours après une période de lavage de 6 semaines. Le principal critère de faisabilité était le recrutement de 24 participants en 11 mois.
Résultats
Nous avons recruté 24 patients sur les 330 patients sélectionnés durant 21 mois. L’âge moyen des participants était de 73,4 ans. Les cibles des taux de refus, des taux de rétention et des taux d’adhésion ont été atteintes. Avec la thiamine, nous avons observé des améliorations non significatives de la fraction d’éjection ventriculaire gauche et de la concentration de propeptide natriurétique de type B N-terminal (NT-proBNP). Parmi les 13 événements indésirables sérieux qu’ont subis sept patients, aucun n’a été associé au médicament étudié.
Conclusions
Bien que nous n’ayons pas atteint notre cible de recrutement, nous avons observé que la supplémentation en thiamine à dose élevée était bien tolérée et qu’il y avait des améliorations potentielles des résultats des biomarqueurs. Un essai de plus grande envergure sur la supplémentation en thiamine est justifié.
Thiamine (vitamin B1) is an essential micronutrient that is required for energy production in the heart. Even though thiamine is an essential cofactor for 2 enzymatic steps in adenosine triphosphate synthesis, it is not synthesized in the body. If a diet is thiamine-deficient, the body will deplete its store in 2-3 weeks.1 Thiamine deficiency is common in patients with heart failure (HF), occurring in 33% of patients.2 The failing heart exhibits decreased adenosine triphosphate levels when examined using magnetic resonance spectroscopy.3 Patients with heart failure with reduced ejection fraction (HFrEF),2 older age,4 or diuretic use5 are more likely to have thiamine deficiency.
Small, randomized trials have shown inconsistent benefits of thiamine supplementation in HF. A meta-analysis of 2 small placebo-controlled trials (pooled n = 38) showed a small improvement (absolute increase of 3.28%, 95% confidence interval [CI] 0.64-5.93) in left ventricular ejection fraction (LVEF) with thiamine doses of 200-300 mg/d.6 Another trial of 50 stable HFrEF patients (mean age: 61.4 years) showed no change to LVEF after 1 month of thiamine supplementation (300 mg/d), but there was an improvement in patient-reported peripheral edema.7 A recent trial of 69 stable HFrEF patients (mean age: 56.7 years) found no changes in LVEF or N-terminal pro-brain natriuretic peptide (NT-proBNP) level after 6 months of thiamine supplementation (200 mg/d).8
One reason for the inconsistent results in the literature may be related to the age of participants. Older adults aged ≥ 65 years are more susceptible to thiamine deficiency4 and may be more likely to benefit from thiamine supplementation. The 2 negative trials of thiamine supplementation did not enrich their sample for older age.7,8 In addition, older adults are often underrepresented in randomized trials of pharmacologic agents in HF, as they may not tolerate the target doses of HF medications or may have comorbidities that lead to exclusion.9 As thiamine is safe and well tolerated, it is a potential adjunct for HF treatment in older adults. Our objective was to conduct a pilot trial of thiamine in older adults with HFrEF to determine the feasibility of recruiting this population for a larger trial.
Materials and Methods
Study design, population, and setting
We conducted a randomized placebo-controlled 2-period crossover feasibility study of thiamine supplementation in HF. The study was conducted from June 2018 to April 2021 in Hamilton, Canada. The protocol and rationale for this study have been published elsewhere.10 Briefly, we included adults age ≥ 60 years with symptomatic HF (New York Heart Association [NYHA] class II-IV), LVEF ≤ 45%, and either of the following: (i) a HF-related hospitalization in the past 12 months; or (ii) an NT-proBNP level ≥ 600 ng/L within 60 days of screening. Due to challenges with recruiting stable patients with recent HF-related hospitalization, we added the option of using NT-proBNP level as an alternative to requiring a recent hospitalization, similar to the approach used in other recent HF trials.11,12 Patients also were optimized medically on guideline-directed medical therapy10 and were stable on medications without hospitalization in the month immediately preceding enrollment. Exclusion criteria included taking > 2.5 mg/d of a thiamine supplement (typical content in a multivitamin tablet), having end-stage renal disease on dialysis, having severe mitral valve disease (which affects strain echocardiography interpretation), having cognitive impairment (defined by chart documentation) without a caregiver administering medications, or having an expected survival period of < 1 year due to noncardiac disease. Patients with symptomatic thiamine deficiency (Wernicke’s encephalopathy, severe malnutrition, or refeeding syndrome) or heavy alcohol use (> 15 drinks per day in men and ≥ 10 drinks per day in women) also were excluded. A full list of eligibility criteria is available in the protocol.10 We followed the Consolidated Standards of Reporting Trials (CONSORT) statement adapted for crossover trials.13
Procedures
Patients were recruited from the Heart Function Clinic at the Hamilton General Hospital (Hamilton, Ontario, Canada) by a research assistant. Eligible participants provided written consent for the study. Participants were then randomly assigned to start with either thiamine mononitrate 500 mg orally each day, or placebo, in a blinded fashion. The bottles of study drug and placebo were pre-randomized and sequentially labelled by the manufacturer using a randomization assignment list to which only the statistician and pharmacy had access. Study investigators were blinded to the assignments. Each participant took the study medication (thiamine or placebo) for 90 days (phase 1), which was followed by a 6-week washout period. Participants then switched to the other study medication (placebo or thiamine) for 90 days (phase 2). Each participant had a total of 4 study visits, occurring at the start and end of each medication phase. Each study visit included a medication review, study medication adherence assessment by pill count, an echocardiogram, bloodwork (erythrocyte thiamine pyrophosphate [TPP] and NT-proBNP), a quality-of-life questionnaire, and a review for adverse events and hospital visits. A pre-planned external review by a cardiologist was done midway through the study to assess for safety concerns. The external reviewer was not blinded to drug allocation, but the investigators remained blinded.
Outcomes
The primary outcome of the study was the feasibility of recruiting 24 participants in 11 months. Secondary outcomes included additional feasibility outcomes and exploratory clinical outcomes. Secondary feasibility outcomes included refusal rate (defined as number of eligible patients who refused to participate; feasibility threshold < 40%), retention rate (defined as number of enrolled patients completing the study; feasibility threshold > 80%), and adherence rate (a patient was considered adherent when they took at least 80% of the study drug; feasibility threshold > 90% of participants being adherent to the drug). We also measured TPP level at each visit, to detect biochemical evidence of supplementation.
Secondary clinical outcomes included echocardiographic measurements (peak global longitudinal strain [GLS] and LVEF), NYHA functional class, NT-proBNP level, quality-of-life questionnaire score (the Kansas City Cardiomyopathy Questionnaire [KCCQ]),14 and clinical events. Clinical events included all-cause mortality, HF hospitalizations, HF emergency room visits, and other adverse events.
Sample size and statistical analysis
A sample size of 24 patients (12 per arm) was determined to be sufficiently large to inform feasibility outcomes and to gather estimates of precision of clinical outcomes.15,16 The feasibility outcomes were analyzed by comparing estimates to predefined thresholds. Changes in clinical outcomes were tested using analysis of variance (ANOVA) models accounting for period and carryover effects. Mean differences, 95% confidence intervals, and P values are reported. The level of significance was set at α = 0.05. We conducted a secondary analysis using a linear mixed-effects model adjusting for baseline values and incorporating several timepoints. Statistical analysis was done using SPSS version 26 (IBM, Armonk, NY).
Results
Baseline characteristics
In the 24 patients enrolled (Table 1), the mean age was 73.4 years (standard deviation [SD] 7.4 years), and 7 were female (29.2%). The baseline NYHA class was II in 18 patients (75.0%), and III in 6 patients (25.0%). The etiology of the HF was ischemic in 16 patients (66.7%). All patients were on a beta blocker; 19 patients (79.2%) were on an angiotensin-converting enzyme inhibitor/angiotensin receptor blocker or angiotensin receptor-neprilysin inhibitor; 12 (50.0%) were on an aldosterone antagonist; and 19 (79.2%) were on a loop diuretic. Only one patient had documented cognitive impairment, and that patient had a caregiver administering medications. HF-related quality of life as measured by mean KCCQ overall score was 56.4 (SD 21.1), which indicates fair-to-good health status.17 Half of the patients (n = 12) had a HF hospitalization in the 12 months prior to enrollment. The median baseline NT-proBNP was 3898 mg/L (interquartile range [IQR] 144-26861 mg/L). The mean LVEF was 33.1% (SD 10.5%), and peak global longitudinal strain was -8.1% (SD 3.1%).
Table 1.
Baseline characteristics of enrolled patients (n = 24).
| Variables | Sequence |
Total (n = 24) | |
|---|---|---|---|
| Thiamine, then placebo (n = 12) | Placebo, then thiamine (n = 12) | ||
| Age, y | 72.6 (± 7.0) | 74.1 (± 8.0) | 73.4 (± 7.4) |
| Sex, female | 5 (41.7) | 2 (16.7) | 7 (29.2) |
| NYHA class | |||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 2 | 9 (75.0) | 9 (75.0) | 18 (75.0) |
| 3 | 3 (25.0) | 3 (25.0) | 6 (25.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hospitalizations in past 12 months | 5 (41.7) | 7 (58.3) | 12 (50.0) |
| Emergency room visits in past 12 months | 2 (16.7) | 6 (50.0) | 8 (33.3) |
| Ischaemic heart failure | 9 (75.0) | 7 (58.3) | 16 (66.7) |
| Comorbidities | |||
| Diabetes | 5 (41.7) | 4 (33.3) | 9 (37.5) |
| Hypertension | 6 (50.0) | 9 (75.0) | 15 (62.5) |
| Dyslipidemia | 5 (41.7) | 7 (58.3) | 12 (50.0) |
| Smoking history | 7 (58.3) | 6 (50.0) | 13 (54.2) |
| Atrial fibrillation | 6 (50.0) | 7 (58.3) | 13 (54.2) |
| Stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cognitive impairment | 0 (0.0) | 1 (8.3) | 1 (4.2) |
| KCCQ overall score | 54.5 (± 20.0) | 58.3 (± 22.8) | 56.4 (± 21.1) |
| KCCQ clinical score | 57.2 (± 22.9) | 66.2 (± 20.3) | 61.7 (± 21.6) |
| Systolic blood pressure | 106.7 (± 16.1) | 114.5 (± 13.9) | 110.6 (± 15.3) |
| Diastolic blood pressure | 66.4 (± 9.0) | 69.5 (± 9.7) | 67.9 (± 9.3) |
| BMI | 33.2 (± 7.6) | 33.0 (± 7.7) | 33.1 (± 7.4) |
| NT-proBNP, mg/L, median (IQR) | 4664 (144, 26,861) | 2744 (406, 17,945) | 3898 (144, 26,861) |
| Erythrocyte TPP, nmol/L | 186.3 (±45.5) | 169.0 (±30.7) | 177.6 (±39.0) |
| Medications | |||
| ACEi/ARB/ARNI | 8 (66.7) | 11 (91.7) | 19 (79.2) |
| β-blocker | 12 (100.0) | 12 (100.0) | 24 (100.0) |
| Aldosterone antagonist | 4 (33.3) | 8 (66.7) | 12 (50.0) |
| Nitrate | 0 (0.0) | 2 (16.7) | 2 (8.3) |
| Calcium channel blocker | 1 (8.3) | 0 (0.0) | 1 (4.2) |
| Thiazide | 1 (8.3) | 0 (0.0) | 1 (4.2) |
| Loop diuretic | 11 (91.7) | 8 (66.7) | 19 (79.2) |
| Hydralazine | 0 (0.0) | 1 (8.3) | 1 (4.2) |
| Digoxin | 0 (0.0) | 1 (8.3) | 1 (4.2) |
| Amiodarone | 3 (25.0) | 3 (25.0) | 6 (25.0) |
| Acetylsalicylic acid | 3 (25.0) | 2 (16.7) | 5 (20.8) |
| Anticoagulation | 7 (58.3) | 9 (75.0) | 16 (66.7) |
| Multivitamins | 2 (16.7) | 1 (8.3) | 3 (12.5) |
| Echography | |||
| LVEF, % | 34.2 (±8.1) | 31.9 (±12.8) | 33.1 (±10.5) |
| Peak GLS, % | –7.7 (±3.6) | –8.6 (±2.3) | –8.1 (±3.1) |
Values are n (%), or mean (±standard deviation), unless otherwise indicated. Patients were randomized to starting with thiamine or placebo during the first study phase.
ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; BMI, body mass index; GLS, global longitudinal strain; IQR, interquartile range; LVEF, eft ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; KCCQ, Kansas City Cardiomyopathy Questionnaire; TPP, thiamine pyrophosphate.
Patients starting on thiamine or placebo (phase 1) were similar in most characteristics (Table 1). There were more female patients in the group starting with thiamine (n = 5 of 12 [41.7%] vs 2 of 12 [16.7%] in the placebo phase 1 group). The NT-proBNP level was also higher in the thiamine-first group, but the IQR was wide and overlapping (4664 mg/L [IQR 144-26,861] vs 2744 mg/L [IQR 406-17,945] in the placebo group). More patients were on a loop diuretic in the thiamine-first group (n = 11 of 12 [91.7%] vs 8 of 12 [66.7%] in the placebo group).
Feasibility outcomes
We screened 330 patients over 21 months to recruit 24 patients (Fig. 1), which did not meet our primary feasibility outcome of recruitment within 11 months (Table 2). Of the 100 patients who met inclusion criteria, 37 declined to participate. Two patients dropped out due to personal reasons, and one died after dropping out. Three other participants died of HF during the study. A total of 19 patients completed the trial. COVID-19 affected our ability to recruit patients, due to restrictions on in-person research. The overall refusal rate was 37% (feasibility threshold < 40%), the retention rate was 92% (feasibility threshold > 80%), and the adherence rate was 92.5% (feasibility threshold > 90%).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. Eligible participants were randomized to start with either thiamine or placebo in a blinded fashion. After 3 months on the study drug, they were crossed over to the other intervention for another 3 months. A 6-week washout period separated the 2 treatment phases. For those not meeting inclusion criteria (n = 230), reasons for exclusion included age (n = 64), ejection fraction (n = 92), medical instability (n = 52), no hospitalization prior to N-terminal pro-brain natriuretic peptide criteria (n = 13), and New York Heart Association class I (n = 9).
Table 2.
Feasibility outcomes
| Measure | Target | Observed | Description |
|---|---|---|---|
| Recruitment rate | 24 patients in 11 months | 24 patients in 21 months | Challenges in recruitment, due to comorbidities, illness severity, no full-time research assistant, COVID-19 |
| Refusal rate | < 40% | 37% | 37 of 100 eligible patients declined to participate |
| Retention rate | > 80% | 92% | 2 of 24 patients dropped out for personal reasons |
| Adherence rate | > 90% | 92.5% | Participants were adherent to the medication in 37 of 40 study periods. We excluded those who died or dropped out before taking the study drug. |
Exploratory clinical outcomes
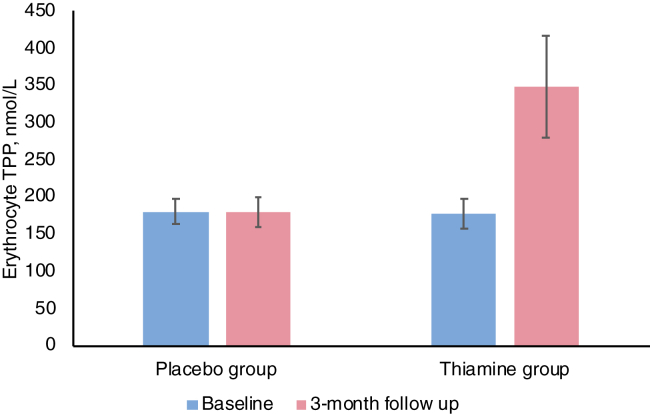
We did not find period or carryover effects between the groups. The mean erythrocyte TPP level increased in the thiamine group but was unchanged in the placebo group (Fig. 2). Comparing thiamine and placebo groups (Table 3), nonsignificant improvements occurred in the thiamine group in mean peak GLS (-8.4% vs -8.0%, P = 0.451), LVEF (39.4% vs 36.7%, P = 0.173), and NT-proBNP level (2805 mg/L vs 3781 mg/L, P = 0.113). Quality of life as measured by mean KCCQ overall score was lower in the thiamine group (60.1 vs 67.0, P = 0.046). When we analyzed the KCCQ clinical score, the difference was not significant (69.3 vs 72.3, P = 0.338). A secondary analysis of only the first study phase (before crossover) showed no differences in all outcomes (Supplemental Table S1). Reasons for missing clinical outcomes mainly included COVID-19 research restrictions and uninterpretable echocardiography images (for GLS), due to study quality (Supplemental Table S2). The mean baseline, 3-month, and within-period changes in outcome measures are shown in Supplemental Table S3. A secondary analysis of LVEF with adjustment for baseline values using a linear mixed-effects model did not reveal differences from pairwise comparisons.
Figure 2.
Erythrocyte thiamine pyrophosphate (TPP) level in the placebo and thiamine groups. TPP levels increased in the thiamine group but not the placebo group, indicating that patients were adherent to the study drug. See Supplemental Figure S1 for an alternative version of this figure with lines showing change in mean TPP level across the 4 timepoints.
Table 3.
Exploratory clinical outcomes for thiamine vs placebo
| Outcome | n | Thiamine (n = 24) | Placebo (n = 24) | Mean difference (95% CI)∗ | P† |
|---|---|---|---|---|---|
| Peak GLS, % | 10 | –8.4 (3.4) | –8.0 (3.7) | 0.42 (–0.8, 1.6) | 0.451 |
| LVEF, % | 13 | 39.4 (11.5) | 36.7 (12.7) | –2.8 (–7.0, 1.3) | 0.173 |
| KCCQ overall score | 18 | 60.1 (19.5) | 67.0 (19.6) | –6.8 (13.3, –4.0) | 0.046 |
| KCCQ clinical score | 18 | 69.3 (16.4) | 72.3 (16.9) | –2.9 (–3.3, 9.3) | 0.338 |
| NYHA class, mean (SD) | 19 | 1.79 (0.5) | 1.68 (0.6) | 0.1 (–1.7, 0.4) | 0.414 |
| NYHA class, median (IQR) | 19 | 2 (1.25–2) | 2 (1–2) | N/A | 0.414 |
| NT-proBNP, mean (SD) | 14 | 2805.2 (3099.8) | 3781.1 (4533.9) | –975.8 (–2124.5, 172.7) | 0.113 |
| NT-proBNP, median (IQR) | 14 | 2033.5 (1265.3–3475.0) | 4144.0 (1225.5–4858) | N/A | 0.140 |
Values are mean (standard deviation), unless otherwise indicated. N-terminal pro-brain natriuretic peptide (NT-proBNP) and New York Heart Association (NYHA) class also compared with Wilxocon test because of skew (P = 0.140 and P = 0.414, respectively)
GLS, global longitudinal strain; KCCQ, Kansas City Cardiomyopathy Questionnaire (quality of life); LVEF, left ventricular ejection fraction; N/A, not applicable.
Paired difference.
P values for treatment effect from analysis of variance that includes period and carryover effect.
Adverse events
A total of 13 serious adverse events occurred in 7 patients (29.2%); none were related to the study drug (Table 4). A total of 4 deaths (16.7%) occurred over the study period, all related to HF and occurring during both the placebo and thiamine phases. A total of 9 hospitalizations occurred in 5 patients (20.8%). Most of the hospitalizations occurred in the washout or placebo phases, with only 2 occurring in the thiamine phase. Admissions were HF related in 3 patients. Nonserious adverse events occurred in 6 patients (25.0%), with one patient having 2 episodes of atrial fibrillation in both phases of the trial. One patient had diarrhea after starting the study medication —the only adverse event related to the study drug, which resolved after discontinuation of the medication.
Table 4.
Adverse events by phase of the trial
| Adverse events | Thiamine phase | Washout phase | Placebo phase | Total† |
|---|---|---|---|---|
| Serious∗ | 5 (38.5) | 3 (23.1) | 6 (46.2) | 13 (100.0) |
| Death | 2 (50.0) | 0 (0.0) | 2 (50.0) | 4 (100.0) |
| HF–related death | 2 (50.0) | 0 (0.0) | 2 (50.0) | 4 (100.0) |
| Hospitalizations | 2 (22.2) | 3 (33.3) | 4 (44.4) | 9 (100.0) |
| HF | 0 (0.0) | 1 (33.3) | 2 (66.7) | 3 (100.0) |
| Pneumonia | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| COPD exacerbation | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Hip fracture | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Atrial fibrillation | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Hernia | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| AKI | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Nonserious | 3 (42.9) | 2 (28.6) | 2 (28.6) | 7 (100.0) |
| Atrial fibrillation | 1 (50) | 1 (50) | 0 (0.0) | 2 (100.0) |
| Nausea/vomiting | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) |
| Pneumonia | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Swollen hands | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) |
| Back pain | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Diarrhea | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
Values are n (%).
AKI, acute kidney injury; COPD, chronic pulmonary obstructive disease; HF, heart failure.
Serious adverse events included hospitalization and death.
The percentages are calculated by row.
Discussion
In this pilot trial of thiamine supplementation in older adults with HFrEF, we were unable to recruit the target number of patients within 11 months, but our other feasibility targets were reached, including retention, refusal, and adherence rates. Similar to a recent parallel group thiamine trial,8 our study did not identify statistically significant benefits of thiamine supplementation, despite using a higher daily dose (500 mg) than previous trials (100-300 mg).7,8,18, 19, 20 The use of sensitive markers, such as peak GLS on echocardiography and NT-proBNP level, reveal nonsignificant improvement with supplementation over 3 months.
Our trial was unique because we included older and sicker patients, with lower baseline thiamine levels, compared with those in previous thiamine trials. The mean baseline thiamine level was 178 nmol/L in our trial, whereas the level in other trials8,19 ranged from 216 to 321 nmol/L. Our mean age was 73 years, whereas the other chronic HF thiamine trials7,8,18, 19, 20 had a lower mean age (range: 56.7-69.5 years). HF-related deaths occurred in 16.7% of patients during our 7.5-month trial, whereas HF deaths varied from 0% to 10% in previous trials of up to 6 months in length.7,8,18, 19, 20 The inclusion of older patients with more cardiovascular events allowed us to better understand how thiamine impacts this population. Recent publications show that older patients are just as likely as younger patients to respond to HF therapy.21,22 However, this population also posed challenges to recruitment, as patients were often too sick or frail to participate. This feasibility trial highlights why older adults with multimorbidity are often excluded from HF trials,23 which sometimes raises question around the application of evidence to this population.
Biomarkers, including NT-proBNP level, peak GLS, and LVEF, did not differ significantly between groups in our trial, although all 3 outcomes showed a nonsignificant improvement in the thiamine group. In previous studies, one small trial found an improvement in LVEF,20 and another trial found an improvement in peripheral edema7 with thiamine, but other outcomes were not different. Studies of acute decompensated HF also did not find improvement in LVEF with intravenous thiamine.18 Our study revealed a lower HF-related quality-of-life score with thiamine, as measured by the KCCQ overall score. The sample size was small, and the P value was just under 0.05, so the finding may be spurious. A separate analysis of the KCCQ clinical score did not show a significant difference, suggesting that the overall score difference was likely spurious. Looking at the exploratory clinical outcomes from our study in the context of published literature indicates that thiamine supplementation does not consistently improve outcomes in patients with HFrEF.
Several lessons gleaned from this pilot trial are applicable to a definitive larger trial. First, recruitment strategies should be improved so that patients outside of the heart-function clinic can be successfully included. Establishing partnerships with local cardiology clinics, in both hospital and external sites, may help reach the recruitment target faster. Second, travel arrangements for trial visits should accommodate the needs of older adults. Many patients needed family or others to drive them to visits, so we arranged and paid for transportation to ensure that patients could attend. Third, the unprecedented research restrictions due to COVID-19 led to missed lab tests and echocardiography. A future trial protocol can consider virtual follow-up for certain visits, and mobile in-home lab tests where possible, which would both improve convenience for patients and mitigate risks due to pandemics.
Our pilot trial has several limitations. First, we did not meet the primary feasibility threshold, so optimization of the trial recruitment process is needed if a definitive trial is to be done. Second, the crossover design limited interpretation of clinical endpoints, such as death, because patients received both the study drug and placebo sequentially. Third, we included only one patient with cognitive impairment, despite its prevalence in the HF population, which limits generalizability. Fourth, only 79.2% of patients were on a loop diuretic. Thiamine deficiency has been reported to be more common in patients taking loop diuretics,24 so perhaps this limited the effectiveness of the administered thiamine. We also did not collect data on renal function in our assessments. Fifth, interpretation of LVEF, and to a lesser extent GLS, was dependent on image quality and reader technique. Although we had dedicated cardiologists or echocardiography fellows interpreting the images, we were not able to use consistent technicians or echocardiography machines, due to resource limitations. Sixth, challenges with echocardiographic image quality and test availability during COVID-19 led to missing data for GLS, LVEF, and NT-proBNP level, thereby affecting interpretation of these outcomes.
Our trial also has several strengths. First, we included older adults, the most vulnerable population. Despite challenges with recruitment, we were able to complete the trial with a suitable retention rate. Second, we included HF-related quality of life, a patient-centred outcome. Third, we included a team of cardiologists, geriatricians, and internists in the design and conduct of the trial. This interdisciplinary approach allowed optimization of trial procedures specifically for older adults with HF, including creating a trial drug that could be administered once daily instead of twice daily, ensuring that the capsule could be swallowed easily by older adults, and engaging family and informal caregivers in trial visits. Fourth, we used the highest daily dose of thiamine used in any chronic HF trial, which provides data on tolerability and increases potential effectiveness. Future trials can use a 500-mg daily dose, which was well tolerated in this population.
Conclusion
Based on our pilot trial, high-dose thiamine administration appears well tolerated by older adults with HF. Potential benefits were identified based on biomarker outcomes. Although our recruitment rate was not met, a definitive trial of thiamine supplementation in older adults with HF may be possible if recruitment strategies are enhanced.
Acknowledgements
We thank Accurex Health Care Manufacturing for providing the study medication and placebo in kind; Tanya Reading for technical and logistic support for our study medication; and Gita Sobhi and Diane Lourenco for medication dispensing and storage. We also thank Dr Michael Farkouh for reviewing our safety data.
Funding Sources
This work was funded by the Hamilton Health Sciences New Investigator Fund and supplemental funding from McMaster/St. Peter’s Hospital Chair of Aging. E.K.C.W. was funded by the Vanier Scholarship (Canadian Institutes of Health Research) and the Clinician Scientist Training Program from the University of Toronto. C.D. received funding from Novartis for other research. Sharon Straus was funded by a Tier 1 Canada Research Chair.
Disclosures
The authors have no conflicts of interests to disclose.
Footnotes
Ethics Statement: Eligible participants provided written consent for the study.
Trial registration: ClinicalTrials.gov Identifier NCT03228030.
See page 538 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.02.007.
Supplementary Material
s and Figure
References
- 1.Thomson A.D. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35:2–7. doi: 10.1093/alcalc/35.supplement_1.2. [DOI] [PubMed] [Google Scholar]
- 2.Hanninen S.A., Darling P.B., Sole M.J., Barr A., Keith M.E. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354–361. doi: 10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson T.J., Hanger H.C., George P.M., Sainsbury R. Is thiamine deficiency in elderly people related to age or co-morbidity? Age Ageing. 2000;29:111–116. doi: 10.1093/ageing/29.2.111. [DOI] [PubMed] [Google Scholar]
- 5.Rieck J., Halkin H., Almog S., et al. Urinary loss of thiamine is increased by low doses of furosemide in healthy volunteers. J Lab Clin Med. 1999;134:238–243. doi: 10.1016/s0022-2143(99)90203-2. [DOI] [PubMed] [Google Scholar]
- 6.Dinicolantonio J.J., Lavie C.J., Niazi A.K., O’Keefe J.H., Hu T. Effects of thiamine on cardiac function in patients with systolic heart failure: systematic review and metaanalysis of randomized, double-blind, placebo-controlled trials. Ochsner J. 2013;13:495–499. [PMC free article] [PubMed] [Google Scholar]
- 7.Mousavi M., Namazi S., Avadi M., Amirahmadi M., Salehifar D. Thiamine supplementation in patients with chronic heart failure receiving optimum medical treatment. J Cardiol Curr Res. 2017:9. 00316. [Google Scholar]
- 8.Keith M., Quach S., Ahmed M., et al. Thiamin supplementation does not improve left ventricular ejection fraction in ambulatory heart failure patients: a randomized controlled trial. Am J Clin Nutr. 2019;110:1287–1295. doi: 10.1093/ajcn/nqz192. [DOI] [PubMed] [Google Scholar]
- 9.Azad N., Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol. 2014;11:329–337. doi: 10.11909/j.issn.1671-5411.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong E.K.C., Lee J.Y., Leong D.P., et al. Thiamine versus placebo in older heart failure patients: study protocol for a randomized controlled crossover feasibility trial (THIAMINE-HF) Pilot Feasibility Stud. 2018;4:149. doi: 10.1186/s40814-018-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurray J.J.V., Krum H., Abraham W.T., et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N Engl J Med. 2016;374:1521–1532. doi: 10.1056/NEJMoa1514859. [DOI] [PubMed] [Google Scholar]
- 12.McMurray J.J.V., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 13.Dwan K., Li T., Altman D.G., Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366 doi: 10.1136/bmj.l4378. l4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 15.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–291. [Google Scholar]
- 16.Hertzog M.A. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 17.Spertus J.A., Jones P.G., Sandhu A.T., Arnold S.V. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 18.Smithline H.A. Thiamine for the treatment of acute decompensated heart failure. Am J Emerg Med. 2007;25:124–126. doi: 10.1016/j.ajem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Schoenenberger A.W., Schoenenberger-Berzins R., der Maur C.A., et al. Thiamine supplementation in symptomatic chronic heart failure: a randomized, double-blind, placebo-controlled, cross-over pilot study. Clin Res Cardiol. 2012;101:159–164. doi: 10.1007/s00392-011-0376-2. [DOI] [PubMed] [Google Scholar]
- 20.Shimon I., Almog S., Vered Z., et al. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995;98:485–490. doi: 10.1016/s0002-9343(99)80349-0. [DOI] [PubMed] [Google Scholar]
- 21.Floria V.G., Rector T.S., Anand I.S., Cohn J.N. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the Valsartan Heart Failure Trial. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.116.003123. e003123. [DOI] [PubMed] [Google Scholar]
- 22.Albakri A. Heart failure with improved ejection fraction: a review and pooled analysis of pathophysiology, diagnosis and clinical management. Rev Artic Intern Med Care. 2020;4:2020. [Google Scholar]
- 23.Bourgeois F.T., Orenstein L., Ballakur S., Mandl K.D., Ioannidis J.P.A. Exclusion of elderly persons in randomized clinical trials of drugs for ischemic heart disease. J Am Geriatr Soc. 2017;65:2354. doi: 10.1111/jgs.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suter P.M., Haller J., Hany A., Vetter W. Diuretic use: a risk for subclinical thiamine deficiency in elderly patients. J Nutr Health Aging. 2000;4:69–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
s and Figure