Abstract
Background
We examined the characteristics and outcomes in a contemporary ambulatory population of patients with atrial fibrillation (AF), comparing rate control with rhythm control.
Methods
This is a post hoc analysis of a cluster-randomized trial (Integrated Management Program Advancing Community Treatment of Atrial Fibrillation [IMPACT-AF]) in ambulatory AF patients from 2016 to 2018, which compared use of a clinical decision support tool for general practitioners to usual care. This analysis compared patients managed with rate vs rhythm control, at entry into the study. Outcomes included AF-related emergency department (ED) visits, unplanned cardiovascular hospitalizations, and bleeding events at 12 months.
Results
A total of 870 patients were included in this analysis, 99 (11.4%) in the rhythm-control group, and 40% women. In the rhythm-control group, the mean age was younger (70 ± 11.4 vs 72.7 ± 9.5 years, P = 0.03), a higher number were paroxysmal (80% vs 43%, P < 0.001), and CHADS2 scores were lower. The rate of AF-related ED visits was higher in the rhythm-control group (17.2 vs 7.3%, P = 0.003), and repeat visits (rate ratio 3.03, 95% confidence interval [1.99-4.52], P < 0.001). The number of repeat ED visits was independently associated with female sex and being in the rhythm-control group.
Conclusions
Both rate- and rhythm-control patients have recurrent ED visits, with a higher rate in patients treated with rhythm control. These findings are observational, but taken in the context of current guidelines could help develop further therapies aimed at improving symptom burden in both rhythm- and rate-control patients to broadly improve healthcare utilization in the AF population.
Résumé
Contexte
Nous avons examiné les caractéristiques et le devenir de patients ambulatoires contemporains atteints de fibrillation auriculaire (FA) dans le cadre d’une comparaison entre la maîtrise de la fréquence cardiaque et la maîtrise du rythme cardiaque.
Méthodologie
Nous avons effectué une analyse a posteriori d’un essai à répartition aléatoire par grappes (IntegratedManagementProgramAdvancingCommunityTreatment ofAtrialFibrillation [IMPACT-AF]) mené de 2016 à 2018 chez des patients ambulatoires atteints de FA en vue de comparer un outil d’aide à la décision clinique destiné aux omnipraticiens avec les soins habituels. Notre analyse a permis d’établir une comparaison entre les patients pris en charge par une maîtrise de la fréquence cardiaque et ceux pris en charge par une maîtrise du rythme cardiaque lors de leur inscription à l’essai. Les paramètres d’évaluation comprenaient les consultations aux urgences liées à la FA, les hospitalisations imprévues ayant des causes cardiovasculaires et les épisodes hémorragiques à 12 mois.
Résultats
Au total, 870 patients ont été inclus dans cette analyse; 99 (11,4 %) faisaient partie du groupe pris en charge par une maîtrise du rythme cardiaque, et 40 % étaient de femmes. Dans le groupe pris en charge par une maîtrise du rythme cardiaque, l’âge moyen était moindre (70 ± 11,4 ans vs 72,7 ± 9,5 ans, P = 0,03), un plus grand nombre de patients présentaient une FA paroxystique (80 % vs 43 %, P < 0,001) et les scores CHADS2 étaient moins élevés. Le taux de consultations aux urgences liées à la FA était plus élevé dans le groupe pris en charge par une maîtrise du rythme cardiaque (17,2 vs 7,3 %, P = 0,003) tout comme le taux de consultations répétées aux urgences (rapport des taux de 3,03, intervalle de confiance à 95 % de 1,99 à 4,52, P < 0,001). Le nombre de consultations répétées aux urgences était indépendamment associé au sexe féminin et à l’inclusion dans le groupe pris en charge par une maîtrise du rythme cardiaque.
Conclusions
Des consultations répétées aux urgences ont été notées tant chez les patients pris en charge par une maîtrise de la fréquence cardiaque que chez ceux pris en charge par une maîtrise du rythme cardiaque quoique plus fréquemment chez ces derniers. Nos constats sont de type observationnel. Néanmoins, dans le contexte des lignes directrices actuelles, ils pourraient contribuer à la mise au point d’autres traitements visant à atténuer le fardeau des symptômes tant chez les patients pris en charge par une maîtrise du rythme cardiaque que chez ceux pris en charge par une maîtrise de la fréquence cardiaque et ainsi permettre globalement une meilleure utilisation des soins de santé chez les patients atteints de FA.
Atrial fibrillation (AF) has become an increasing burden on the Canadian healthcare system, due to its association with increased mortality, morbidity, high healthcare costs, and impairment of quality of life.1 Ensuring adequate management of AF entails significant complexity, owing to several factors. Many AF patients present as asymptomatic and are diagnosed while being assessed for other comorbidities.2 Primary care physicians (PCPs) have an increased burden to diagnose AF initially, with approximately 63% of cases diagnosed by PCPs.3 Emergency departments (EDs) increasingly have been managing patients with AF, as about 30% of patients will present to an ED with symptoms at some point during the course of their illness.4 Attempts to improve access to care and outcomes in ambulatory AF patients are much needed to curb the current rise in healthcare utilization associated with AF.5,6
The Canadian Cardiovascular Society (CCS) AF guidelines provide detailed guidance on the use of rate- and/or rhythm-control therapies for patients with AF.7 The driving forces behind therapies for AF are to improve quality of life, prevent life-threatening complications such as stroke and heart failure, and avoid AF-related ED visits and hospitalizations. The use of rate-control therapies is often initiated by PCPs, but may not be adequate, thereby leading to reduction in quality of life, ED visits, or hospitalizations.3 The use of rhythm control is more challenging for most physicians who are not well versed in heart rhythm disorders, as it involves antiarrhythmic drugs, which have the potential to induce life-threatening arrhythmias if not used safely. The Canadian Cardiovascular Society AF guidelines provide detail in this regard, but how well these are applied, and in whom, is unknown. Catheter ablation for AF is a highly specialized technique that is confined to heart rhythm specialists, who are currently a limited resource in Canada, meaning access to them may be difficult to obtain. The proportion of patients managed in an ambulatory care setting who might benefit from this therapy, with improvement in quality of life as well as reduction in ED visits and hospitalizations, is unknown.
We sought to examine a contemporary cohort of ambulatory AF patients managed with either rhythm or rate control, and to compare healthcare utilization in the form of cardiovascular (CV) hospitalizations and AF-related ED visits, to provide input regarding these gaps in knowledge. We hypothesize that an identifiable subset of the ambulatory AF population accounts for the majority of healthcare resource utilization, with a more significant proportion in the rhythm-control group. The cohort utilized was derived from the Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF) study. The IMPACT-AF clinical trial was a prospective, cluster-randomized, clinical trial conducted in the primary care setting to evaluate whether an integrated clinical decision support (CDS) tool could support both healthcare practitioners (HCPs) and patients in the management of AF, by providing evidence-based strategies for management.8 The study did not find that a CDS system has benefit in terms of reduced AF-related ED visits and CV hospitalizations; however, the study provides a unique opportunity to examine a contemporary ambulatory AF population regarding the use of rate- and rhythm-control therapies and the scope of resource utilization in this important population.
Methods
Study design
This study is a post hoc analysis of the IMPACT-AF trial; the detailed protocol of the IMPACT-AF study has been published previously.8 Briefly, the IMPACT-AF study was a cluster-randomized trial, with blinded endpoint evaluation of 1133 patients with ambulatory AF, comparing the use of a CDS tool to usual care, from June 2014 to December 2016 in the province of Nova Scotia. Randomization was performed at the level of the PCP (n = 203). The primary outcome was a composite of CV hospitalizations and AF-related ED visits at 12 months.
This analysis compared patients by rate vs rhythm control, at the time of study entry. The rhythm-control group comprised patients treated with any of the following at the time of enrollment: dronedarone, amiodarone, flecainide, mexiletine, propafenone, or sotalol. Patients could have been on additional atrioventricular nodal blocking agents, along with the antiarrhythmic medication. The use of cardioversion alone, which provides acute rhythm control, was not considered for inclusion in the rhythm-control group. The rate-control group was defined as those for whom any atrioventricular nodal blocking agent (acebutolol, atenolol, bisoprolol, carvedilol, labetalol, metoprolol, nadolol, pindolol, propranolol, timolol, diltiazem, verapamil, or digoxin) was used at the time of enrollment. The 2 groups were compared within randomized strata, and then secondarily as a cohort.
Health record information was aggregated from primary care medical charts, hospitalization records, and provincial databases. PCPs were allocated to use of the CDS tool vs continuing traditional/usual practice in a 1:1 manner, as previously described.8 The academic researchers independently conducted the trial and undertook the primary data analyses. The Nova Scotia Health Research Ethics Board provided ethics approval.
Study population
The IMPACT-AF study population included patients age 18 years or older who had an electrocardiographically confirmed diagnosis of AF or documented past management of AF. Patient-selection criteria were stratified by whether the PCP was in an urban population (> 10,000) or rural population (< 10,000). PCPs included in the study were those in full-time practice, managing adults, with access to the Internet. Patients were excluded if they were unable to provide informed consent or had a terminal illness such that they were not expected to be alive at the end of follow-up in the IMPACT-AF clinical trial, which in each case involved a minimum of 12 months after enrollment in the trial.
Outcomes
The primary study outcome for this study was defined as the composite of any AF-related ED visit or unplanned CV hospitalization over 12 months (admission with at least 1 overnight stay in the hospital). The prespecified definition of an AF-related ED visit included any presentation, with palpitations, rapid heart rate, presyncope or syncope, shortness of breath, transient chest discomfort, or hemodynamic instability resolving with AF cardioversion or rate-control, that does not result in hospitalization. The prespecified main causes for unplanned CV hospitalization were as follows: acute coronary syndrome, presyncope/syncope, transient ischemic attack/stroke, AF, atrial flutter, pulmonary embolism/deep vein thrombosis / systemic embolism, and worsening congestive heart failure including pulmonary edema or dyspnea of cardiac origin. Patients may have had recurrent events, but the primary outcome counted the first event only.
The primary safety outcome was major bleeding, per the definition provided by the modified International Society on Thrombosis and Haemostasis, as follows: fatal bleeding and/or bleeding in a critical area or organ (such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in hemoglobin level of 1.24 mmol/L [20 g/L], and/or requiring transfusion of 2 or more units of whole blood or red cells, and/or the use of a rapid-acting reversal agent [excluding vitamin K]).9 Secondary outcomes include AF-related ED visits, unplanned CV hospitalizations, stroke, and all-cause mortality.
Clinical data at the level of primary care were obtained through a complete review of patient charts (including both primary care and hospital-based). Clinical data related to ED visits, hospitalization, or death were identified through administrative datasets corresponding to the Discharge Abstract Database, the National Ambulatory Care Reporting System, review of provider medical records, and hospital-based health systems. All clinical events were blinded to the assigned treatment arm and independently reviewed by 2 members of an adjudication committee, with any disagreement reviewed by a third member. Mortality was identified through review of provider medical records and hospital-based health systems, and via provincial department of health and wellness datasets (vital statistics and the medical social insurance client registry). PCP charts were audited for any letters, discharge summaries, or other documentation pertaining to such events. Lists of patient events and reasons (most responsible diagnosis and discharge details) for ED and hospital encounters relating to the population of ambulatory AF patients were reviewed, and those that were clearly unrelated to AF were excluded from further analysis. The patients' charts were reviewed from respective hospital sites by a trained abstractor, and relevant case report form data were extracted.
Statistical analysis
The baseline characteristics of the ambulatory AF patient population were reported by group as mean (standard deviation) or median (first quartile, third quartile) for continuous variables, and count (percent) for categorical variables. Clinical characteristics were compared between rhythm and rate using the Fisher’s exact or χ2 test for categorical variables and t-tests for continuous data. Clinical endpoints were assessed between usual care and CDS within rhythm and rate separately using Fisher’s exact or χ2 tests. Primary and safety outcomes between rhythm and rate were also summarized as frequency and percentage, and comparisons made using Fisher’s exact or χ2 tests. The effect of rhythm vs rate on recurrent AF-related ED visits was assessed using a multivariable logistic regression model adjusted for age, sex, type of AF, hypertension, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled) (CHADS2) score, and sleep apnea. The criterion for significance used was P < 0.05.
Results
The IMPACT-AF study enrolled 1133 patients across Nova Scotia; of these. 99 patients (9%) were classified as receiving rhythm-control therapy at the time of entry into the study, 771 patients (68%) were classified as receiving rate-control therapy, and 263 patients (23%) were on no rate- or rhythm-control therapy. The patients receiving neither rate- nor rhythm-control therapy were excluded from the analysis. Baseline characteristics of the ambulatory AF population, comparing rhythm-control patients (n = 99) to rate-control patients (n = 771), are shown in Table 1. Rhythm-control patients differed from rate-control patients at baseline in that they were younger in age (70 ± 11.4 vs 72.7 ± 9.5 years, P = 0.03), more likely to be paroxysmal (79.5% vs 43%, P < 0.0001), had fewer patients with a CHADS2 score ≥ 2 (60.6% vs 72.6%, P = 0.01), and had a higher proportion of obstructive sleep apnea (29.3% vs 18.5%, P = 0.02). Rhythm-control patients had more ablations for atrial flutter or AF (13.1% vs 4.7%, P = 0.0002) and more cardioversions (13.1% vs 2.9%, P < 0.0001). No significant differences were present in men vs women in either of the 2 groups, or in those from an urban vs a rural location. Of patients with known paroxysmal AF (n = 235), 58 (24.6%) received rhythm control, and 177 (75.3%) received rate control (Table 1).
Table 1.
Baseline characteristics of ambulatory atrial fibrillation (AF) population by treatment group
| Characteristic | Rhythm control (n = 99) | Rate control (n = 771) | P |
|---|---|---|---|
| Age, y | 70 ± 11.4 | 72.7 ± 9.5 | 0.03 |
| Creatinine, mmol/L) | 94.1 ± 34.5 | 90.6 ± 28.9 | 0.35 |
| Women | 47 (47.5) | 303 (39.3) | 0.13 |
| Rural location | 59 (59.6) | 431 (55.9) | 0.52 |
| Paroxysmal AF | 58 (79.5) | 177 (43.0) | < 0.0001 |
| Persistent AF | 14 (19.2) | 185 (44.9) | < 0.0001 |
| First episode of AF | 1 (1.4%) | 50 (12.1) | < 0.0001 |
| Hypertension | 74 (74.7) | 641 (83.1) | 0.05 |
| Alcohol abuse∗ | 3 (3.0) | 62 (8.0) | 0.1 |
| CHADS2 score | |||
| 0 | 15 (15.2) | 58 (7.5) | 0.01 |
| 1 | 24 (24.2) | 153 (19.8) | |
| ≥ 2 | 60 (60.6) | 560 (72.6) | |
| Previous stroke, systemic embolism, or transient ischemic attack | 14 (14.1) | 151 (19.6) | 0.22 |
| Previous myocardial infarction | 11 (11.1) | 113 (14.7) | 0.44 |
| Obstructive sleep apnea | 29 (29.3) | 143 (18.5) | 0.02 |
| Ablation (for atrial flutter or AF) | 13 (13.1) | 36 (4.7) | 0.002 |
| Pacemaker/ICD | 9 (9.1) | 101 (13.1) | 0.33 |
| Cardioversion | 13 (13.1) | 22 (2.9) | < 0.0001 |
| Prior echocardiogram | 62 (62.6) | 457 (59.3) | 0.59 |
Values are mean ± standard deviation, or n (%), unless otherwise indicated.
CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); ICD, implantable cardioverter defibrillator.
Alcohol abuse was defined as ≥ 3 drinks per day or > 11 drinks per week for women, and as ≥ 4 drinks per day or > 16 drinks per week for men.
Outcomes by randomized groups
Within the rhythm-control group, 48 patients were assigned to the usual care arm, and 51 patients were assigned to the CDS arm. No significant differences were present between the arms in patients reaching the primary composite endpoint of any AF-related ED visit or unplanned CV hospitalization (16.7% vs 19.6%, usual care arm vs CDS arm, P = 0.8) within the rhythm-control group (Table 2). Within the rate-control group, 350 patients were assigned to the usual care arm, and 421 patients were assigned to the CDS arm. Within the rate-control group, no significant difference was seen between arms in patients reaching the primary composite endpoint (12.0 vs 12.1%, usual care arm vs CDS arm, P = 1.0).
Table 2.
Rate and rhythm control by randomized groups
| Variable | Rate control |
P | Rhythm control |
P | ||
|---|---|---|---|---|---|---|
| Usual care (n = 350) | CDS system (n = 421) | Usual care (n = 48) | CDS system (n =51) | |||
| Composite of any AF-related ED visit or unplanned CV hospitalization | 42 (12) | 51 (12.1) | 0.8 | 8 (16.7) | 10 (19.6) | 1.00 |
| AF-related ED visits | 28 (8) | 28 (6.7) | 0.6 | 7 (14.6) | 10 (19.6) | 0.49 |
| Heart failure | 0 (0) | 0 (0) | 1.00 | 0 (0) | 0 (0) | 1.00 |
| Syncope | 3 (0.9) | 1 (0.2) | 0.61 | 2 (4.2) | 1 (2) | 0.33 |
| TIA/stroke | 0 (0) | 1 (0.2) | 1.00 | 0 (0) | 0 (0) | 1.00 |
| ACS (UA/MI) | 0 (0) | 4 (1) | 1.00 | 0 (0) | 1 (2) | 0.13 |
| Palpitations | 25 (7.1) | 17 (4) | 0.76 | 5 (10.4) | 7 (13.7) | 0.08 |
| Unplanned CV hospitalization | 18 (5.1) | 27 (6.4) | 1.00 | 1 (2.1) | 2 (3.9) | 0.54 |
| Heart failure | 7 (2) | 11 (2.6) | 0.48 | 1 (2.1) | 0 (0) | 0.64 |
| Syncope | 0 (0) | 1 (0.2) | 1.00 | 0 (0) | 0 (0) | 1.00 |
| TIA/stroke/SE | 3 (0.9) | 6 (1.4) | 1.00 | 0 (0) | 0 (0) | 0.52 |
| ACS | 3 (0.9) | 5 (1.2) | 1.00 | 0 (0) | 1 (2) | 0.73 |
| Rate/rhythm | 10 (2.9) | 8 (1.9) | 1.00 | 1 (2.1) | 1 (2) | 0.47 |
| All-cause mortality | 16 (4.6) | 23 (5.5) | 1.00 | 0 (0) | 1 (2) | 0.62 |
Values are n (%), unless otherwise indicated.
ACS, acute coronary syndrome; AF, atrial fibrillation; CDS, clinical decision support; CV, cardiovascular; MI, myocardial infarction; PE, pulmonary embolism; SE, systemic embolism; TIA, transient ischemic attack; UA, unstable angina.
Outcomes by treatment group
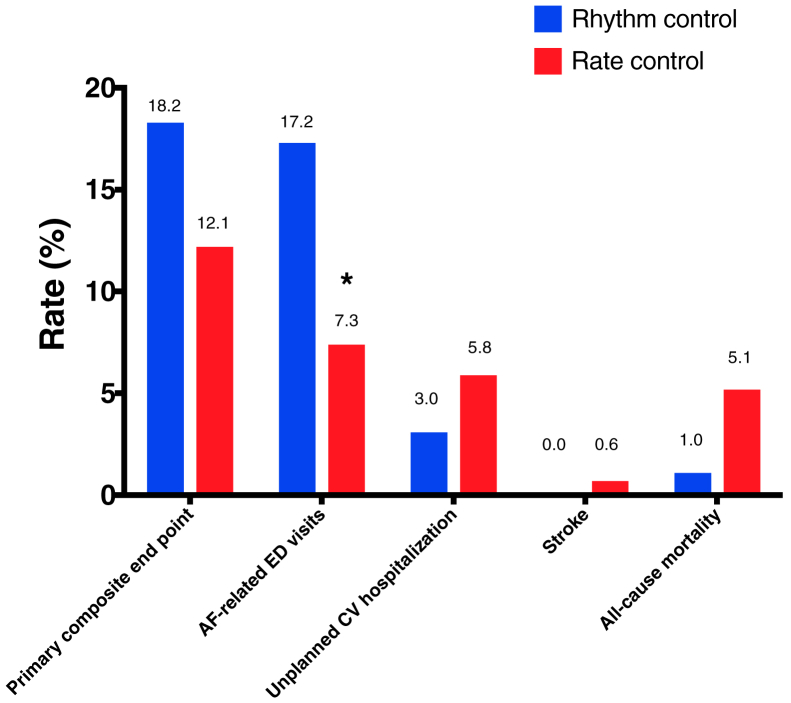
At 12 months, a trend occurred,toward a higher proportion of patients in the rhythm-control group reaching the primary end point (18.2% vs 12.1%, P = 0.11). A significant difference was seen in AF-related ED visits, with a higher proportion occurring in the rhythm-control group vs the rate-control group (17.2% vs 7.3%, P = 0.0003). A trend toward increased mortality occurred in the rate-control group, but this was not statistically significant (1.0 vs 5.1%, P = 0.08). Additionally, no significant differences were seen between the rhythm- and rate-control groups for safety outcomes and unplanned CV hospitalizations (P = 0.11; Fig. 1; Table 3).
Figure 1.
Rhythm- vs rate-control drug use for treatment of atrial fibrillation (AF): primary and secondary outcomes at 12 months. ∗P < 0.05. CV, cardiovascular; ED, emergency department.
Table 3.
Rhythm vs rate control: outcomes at 12 months
| Variable | Rhythm (n = 99) | Rate (n = 771) | P |
|---|---|---|---|
| Composite of any AF-related ED visit or unplanned CV hospitalization | 18 (18.2) | 93 (12.1) | 0.11 |
| Major bleeding | 0 (0) | 4 (0.5) | 1.00 |
| Hemoglobin decrease < 2 g/dL | 0 (0) | 2 (0.3) | 1.00 |
| Transfusion > 2 units | 0 (0) | 0 (0) | |
| Fatal bleeding | 0 (0) | 1 (0.1) | 1.00 |
| Intracranial hemorrhage | 0 (0) | 1 (0.1) | 1.00 |
| Reversal agent received | 0 (0) | 0 (0) | |
| Minor bleeding | 0 (0) | 1 (0.1) | 1.00 |
| AF-related ED visits | 17 (17.2) | 56 (7.3) | 0.003 |
| Heart failure | 0 (0) | 0 (0) | |
| Syncope | 3 (3) | 4 (0.5) | 0.04 |
| TIA/stroke | 0 (0) | 1 (0.1) | 1.00 |
| ACS | 1 (1) | 4 (0.5) | 0.45 |
| Palpitations | 12 (12.1) | 42 (5.4) | 0.01 |
| Unplanned CV hospitalization | 3 (3) | 45 (5.8) | 0.35 |
| Heart failure | 1 (1) | 18 (2.3) | 0.71 |
| Syncope/presyncope | 0 (0) | 1 (0.1) | 1.00 |
| TIA/stroke/SE | 0 (0) | 9 (1.2) | 0.61 |
| ACS (UA/MI) | 1 (1) | 8 (1) | 1.00 |
| Uncontrolled rate | 2 (2) | 18 (2.3) | 1.00 |
| All-cause mortality | 1 (1) | 39 (5.1) | 0.08 |
Values are n (%), unless otherwise indicated.
ACS, acute coronary syndrome; AF, atrial fibrillation; CV, cardiovascular; DVT, deep vein thrombosis; ED, emergency department; MI, myocardial infarction; PE, pulmonary embolism; SE, systemic embolism; TIA, transient ischemic attack; UA, unstable angina.
At 12 months, there were 35 AF-related ED visits in 17 patients (17.2%) in the rhythm-control group, compared with 90 in 56 patients (7.3%) in the rate-control group, corresponding to a rate ratio of 3.03 (95% confidence interval [CI] 1.99, 4.52), P < 0.0001. Symptoms upon presentation to the ED were more often due to presyncope/syncope (40.8% vs 24.8%, P < 0.05) in the rhythm-control group as compared to the rate-control group, whereas dyspnea was more often present in the rate-control group (16.3% vs 32.8%, P = 0.04). Chest pain (28.8% vs 32.7%) and palpitations (81.6% vs 79.6%) occurred with the same frequency.
On multivariate analysis, rhythm control remained significantly associated with AF-related ED visits (odds ratio 2.16, 95% CI [1.17, 3.98], P = 0.0141). Female sex was found to be independently associated with a higher rate of AF-related visits (odds ratio 2.05, 95% CI [1.24, 3.37], P = 0.0050) (Table 4). A low rate of referral for specialty care was seen in both groups, although the rate of referral was higher in the rhythm-control group (33% vs 21%, P < 0.01), with no difference between urban vs rural location.
Table 4.
Multivariate analysis: rhythm- vs rate-control drug use for treatment of atrial fibrillation (AF): primary and secondary outcomes at 12 months
| Variable (multivariate) | Recurrent AF-related ED visits |
|
|---|---|---|
| OR (95% CI) | P | |
| Rhythm-control group | 2.16 (1.17, 3.98) | 0.0141 |
| Age (per y) | 0.98 (0.95, 1.01) | 0.1909 |
| Female | 2.05 (1.24, 3.37) | 0.0050 |
| CHADS2 score | 0.87 (0.70, 1.07) | 0.1867 |
| Sleep apnea | 1.09 (0.60, 2.00) | 0.7775 |
| Hypertension | 0.86 (0.45, 1.65) | 0.6552 |
CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); CI, confidence interval; ED, emergency department; OR, odds ratio.
Discussion
This study provides a contemporary cohort study of ambulatory patients with AF managed with either rate or rhythm control over 12 months. The CDS tool had no impact on CV hospitalizations or AF-related ED visits in either of the 2 groups. As a cohort, however, the patients in the rhythm-control group were found to have more AF-related ED visits, as well as a greater overall burden. A small proportion of patients (7%) in the rate-control group had recurrent ED visits as well.
The rhythm-control group utilized a significantly greater proportion of healthcare resources than the rate-control group, as represented by AF-related ED visits. Interestingly, only one-third of patients in the rhythm-control group had been referred to specialists. An even smaller proportion had undergone prior AF or atrial flutter ablation. The rate-control group utilized a lower proportion of healthcare resources, but a small proportion of patients (7.3%) had recurrent visits to the ED. These patients with recurrent ED visits could conceivably have benefited from either rhythm-control (if in the rate-control group) or other advanced therapies. Prior studies have demonstrated consistent benefits of AF reduction with ablation over antiarrhythmic drugs, as well as significant decreases in cost and healthcare utilization.10, 11, 12, 13 Ladapo et al. demonstrated a 45% relative risk reduction in the number of ED visits after ablation.14 Prior observational studies have compared healthcare utilization in rhythm- and rate-control groups and have findings concordant with ours. The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) study demonstrated no difference in mortality between rate- and rhythm-control groups but did find an increase in CV hospitalizations on adjusted analysis (hazard ratio 1.24, 95% CI 1.10, 1.39, P = 0.0003), as well as higher utilization of cardioversion and catheter ablation, similar to the findings in this study.15 The Registry of Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation (RECORDAF) demonstrated a higher rate of hospitalizations for arrhythmic events but not for overall CV events in the rhythm-control arm. Both of these registries were derived from specialist-based practices, rather than primary care, where access to advanced therapies may have been more readily available.16 This level of specialist treatment is in contrast to that in our study, in which only one-third of patients were referred to specialists. Access to specialty care in the province of Nova Scotia, where the study was performed, may have been perceived as a barrier for family physicians.
Several explanatory factors that may contribute to our findings for patients in this study need to be considered. The lack of rhythm-control medication in those patients with recurrent ED visits in the rate-control arm may have been due to not only a lack of access to specialty care to provide advice regarding advanced therapies, but also a reluctance of PCPs to prescribe this class of medications, with known risks of torsades de pointe, bradyarrhythmias, and other ventricular arrhythmias.17 Those patients in the rhythm-control arm had a higher rate of comorbidities, and they may have had a higher recurrence of AF due to untreated triggers, ischemic symptoms, or heart failure symptoms, thereby leading to more ED visits.7 Patients who had been to the ED for cardioversion may have an expectation of returning for similar management in the event of recurrence, and they may have been provided with instructions to do so, owing to a perceived higher symptom burden. Patients in the rhythm-control group had lower CHADS2 scores and may have presented to the ED due to the lack of oral anticoagulation use, where cardioversion is recommended to be performed within 12-24 hours of AF onset.
The patients in this study were managed primarily by general practitioners, with a minority of patients receiving specialty care. None of these patients were managed by a multidisciplinary approach, such as in an AF clinic. Good evidence supports the use of AF clinics to improve management of patients with AF, resulting in decreased healthcare utilization, and in one study, a decrease in mortality.18, 19, 20 Previous study indicates that a small minority of the population accounts for the majority of healthcare spending, a pattern similar to that seen here in which a small minority of patients with AF account for the bulk of healthcare utilization.21
Reduction of costs associated with AF care is challenging. AF clinics may be cost-effective, but they require an investment up front in order to reap benefits over time; in addition, this model may not be implementable in many jurisdictions in Canada and has been shown to achieve desired outcomes in those with a higher level of experience, making its use less generalizable.22 Additionally, individualization of AF therapies is imperative, and their management can be challenging, particularly to improve symptoms. Alcohol use, smoking, obesity, exercise, hypertension, diabetes, and sleep apnea are among some lifestyle and risk factors that can be modified to improve AF-related symptoms.23,24 Such modification can be challenging in an ambulatory care setting, emphasizing the need for access to multidisciplinary care, as well as improved coordination at the primary care level to target the many lifestyle and risk factors that trigger and maintain AF. Our study demonstrates the potential for gaps in care for patients with a greater symptom burden, in both the rate-control and rhythm-control groups, for whom access to multidisciplinary care may help reduce healthcare utilization and improve patient-related outcomes and quality of life for this chronic condition. The best mode of delivery of a multidisciplinary approach, and identification of those who could benefit most, required study in large-scale randomized trials. This issue also was highlighted as a research priority in the recent National Heart, Lung and Blood Institute review of secondary prevention of AF.25 Further study of the reasons and barriers behind access to care is required, as patient or physician factors could influence these. In this study, an urban vs rural setting had no influence on specialty referral, but no further information on barriers to access could be ascertained. Improved implementation of guidelines, patient education, alternative multidisciplinary models of care, and digital health utilizing therapies targeted at AF patients with a greater symptom burden may reduce higher levels of healthcare utilization. Improving access to advanced rhythm-control therapies, or using alternative models of care for such patients, may translate into improved outcomes as well as reduced resource utilization.
In line with prior studies, we demonstrated that women were more likely to have recurrent AF-related ED visits than men.26, 27, 28 Prior studies have found that women are more likely to have symptoms with AF, as compared to men, as well as a greater symptom burden and lower quality of life. This finding is important as it suggests that current management should recognize the potential for sex differences. Management of women with AF may require greater emphasis on improving access to therapies, such as oral anticoagulation and catheter ablation for AF, as appropriate.
Important limitations to the study must be considered in interpretation of the results. This was a post hoc, nonrandomized comparison, with differences in baseline characteristics between the 2 groups. Multivariate analysis was performed to reduce this bias, but unrecognized confounders may not have been accounted for. The assignment to the rhythm- or rate-control group was based on medical therapy at entry into the study, and the maintenance of sinus rhythm in the rhythm-control group was not taken into account. Furthermore, any treatments initiated after enrollment, which may have influenced outcomes, were not accounted for in the analysis. Finally, the enrollment and management of patients in the study were performed by individual family physicians, whose approaches may differ according to geographic regions and may not be generalizable across jurisdictions. Bias in the enrollment of patients could have affected the type of patients enrolled into the trial.
Conclusion
Patients assigned to receive rhythm-control therapy comprised approximately one-tenth of this ambulatory AF population, but they utilized a significantly greater proportion of healthcare resources compared to those patients managed with rate control. Our findings are purely observational but they highlight the need for improving access and opportunities for provision of care for AF patients.
Acknowledgments
Funding Sources
IMPACT-AF was funded by Bayer Inc.
Disclosures
Dr Cox has received research grant funding from Bayer. Dr Parkash has received research grant funding from Medtronic, Abbott, Novartis, and Bayer. The other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The Nova Scotia Health Research Ethics Board provided ethics approval.
Trial Registration: Clinical Trials NCT01927367
See page 557 for disclosure information.
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet. 2017;390:1873–1887. doi: 10.1016/S0140-6736(17)31072-3. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P., Schmalowsky J., Pittrow D., et al. Management of patients with atrial fibrillation by primary-care physicians in Germany: 1-year results of the ATRIUM registry. Clin Cardiol. 2014;37:277–284. doi: 10.1002/clc.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald A.J., Pelletier A.J., Ellinor P.T., Camargo C.A., Jr. Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med. 2008;51:58–65. doi: 10.1016/j.annemergmed.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Valderrama A.L., Dunbar S.B., Mensah G.A. Atrial fibrillation: public health implications. Am J Prev Med. 2005;29(5 Suppl 1):75–80. doi: 10.1016/j.amepre.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Wattigney W.A., Mensah G.A., Croft J.B. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 7.Andrade J.G., Aguilar M., Atzema C., et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Cox J.L., Parkash R., Abidi S.S., et al. Optimizing primary care management of atrial fibrillation: the rationale and methods of the Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF) study. Am Heart J. 2018;201:149–157. doi: 10.1016/j.ahj.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 10.Parkash R., Tang A.S.L., Sapp J.L., Wells G. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J Cardiovasc Electrophysiol. 2011;22:729–738. doi: 10.1111/j.1540-8167.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 11.Khaykin Y., Morillo C.A., Skanes A.C., et al. Cost comparison of catheter ablation and medical therapy in atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:907–913. doi: 10.1111/j.1540-8167.2007.00902.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarman J.W.E., Hussain W., Wong T., et al. Resource use and clinical outcomes in patients with atrial fibrillation with ablation versus antiarrhythmic drug treatment. BMC Cardiovasc Disord. 2018;18:211. doi: 10.1186/s12872-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds M.R., Gunnarsson C.L., Hunter T.D., et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 14.Ladapo J.A., David G., Gunnarsson C.L., et al. Healthcare utilization and expenditures in patients with atrial fibrillation treated with catheter ablation. J Cardiovasc Electrophysiol. 2012;23:1–8. doi: 10.1111/j.1540-8167.2011.02130.x. [DOI] [PubMed] [Google Scholar]
- 15.Noheria A., Shrader P., Piccini J.P., et al. Rhythm control versus rate control and clinical outcomes in patients with atrial fibrillation: results from the ORBIT-AF Registry. JACC Clin Electrophysiol. 2016;2:221–229. doi: 10.1016/j.jacep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Camm A.J., Breithardt G., Crijns H., et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation: RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) J Am Coll Cardiol. 2011;58:493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Nattel S., Sager P.T., Hüser J., Heijman J., Dobrev D. Why translation from basic discoveries to clinical applications is so difficult for atrial fibrillation and possible approaches to improving it. Cardiovasc Res. 2021;117:1616–1631. doi: 10.1093/cvr/cvab093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks J.M., de Wit R., Crijns H.J., et al. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–2699. doi: 10.1093/eurheartj/ehs071. [DOI] [PubMed] [Google Scholar]
- 19.Carter L., Gardner M., Magee K., et al. An integrated management approach to atrial fibrillation. J AmHeart Assoc. 2016;5 doi: 10.1161/JAHA.115.002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraswat M.K., Carter L., Berrigan P., et al. Integrated management approach to atrial fibrillation care: a cost utility analysis. Can J Cardiol. 2019;35:1142–1148. doi: 10.1016/j.cjca.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Wodchis W.P., Austin P.C., Henry D.A. A 3-year study of high-cost users of health care. Can Med Assoc J. 2016;188:182–188. doi: 10.1503/cmaj.150064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijtvliet E., Tieleman R.G., van Gelder I.C., et al. Nurse-led vs. usual-care for atrial fibrillation. Eur Heart J. 2020;41:634–641. doi: 10.1093/eurheartj/ehz666. [DOI] [PubMed] [Google Scholar]
- 23.Parkash R. Does lifestyle impact risk, burden and symptomatology of atrial fibrillation? CJGIM. 2018;13(SP1) doi: 10.22374/cjgim.v13iSP1.310. [DOI] [Google Scholar]
- 24.Chung M.K., Eckhardt L.L., Chen L.Y., et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. doi: 10.1161/CIR.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin E.J., Al-Khatib S.M., Desvigne-Nickens P., et al. Research priorities in the secondary prevention of atrial fibrillation: a National Heart, Lung, and Blood Institute virtual workshop report. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade J.G., Deyell M.W., Lee A.Y.K., Macle L. Sex differences in atrial fibrillation. Can J Cardiol. 2018;34:429–436. doi: 10.1016/j.cjca.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Humphries K.H., Kerr C.R., Connolly S.J., et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 28.Gillis A.M. Atrial fibrillation and ventricular arrhythmias: sex differences in electrophysiology, epidemiology, clinical presentation, and clinical outcomes. Circulation. 2017;135:593–608. doi: 10.1161/CIRCULATIONAHA.116.025312. [DOI] [PubMed] [Google Scholar]