Abstract
Cytokine storm is the most prominent hallmark in patients with coronavirus disease 2019 (COVID-19) that stimulates the free radical storm, both of which induce an overactive immune response during viral infection. We hypothesized that owning to its radical-scavenging and anti-inflammatory properties, Edaravone could reduce multi-organ injury, clinical complications, and mortality in severe COVID-19 cases. This single-center randomized clinical trial was accompanied in the intensive care units (ICUs) of the teaching hospital of Tabriz University of Medical Sciences to evaluate the effect of Edaravone on the outcome of patients with severe COVID-19. Thirty-eight patients admitted to ICU were included and randomized into two control and intervention arms. Patients in the intervention group received 30 mg Edaravone by slow intravenous infusion for three days in addition to receiving national therapy. The primary outcome was the need for intubation, the intubation length, and mortality rate. Secondary endpoints were clinical improvement. Edaravone administration improved the primary outcomes; it decreased the need for endotracheal intubation and mechanical ventilation [10.52% (n = 2) versus 42.1% (n = 8); p = 0.03] and intubation length [3 (1–7) versus 28 (4–28), p = 0.04] compared to control group. Baseline characteristics and laboratory tests were similar between the studied groups. No marked differences were observed in secondary endpoints (p > 0.05). Administration of Edaravone could decrease the need for mechanical ventilation and length of intubation in severe COVID-19 patients admitted to ICU.
Keywords: Antioxidant, Edaravone, Pneumonia; mechanical ventilation, COVID-19
Introduction
Cytokine storm is the most prominent hallmark of coronavirus disease 2019 (COVID-19), mainly in those with severe respiratory involvement. Inflammatory cytokines induce free radicals formation and the interaction between free radical storm and cytokine storm induces an overactive immune response (Wu 2020). The lung is the major involved organ in COVID-19 that is also prone to oxidative stress (Thimmulappa et al. 2020). The development of hypoxia in COVID-19 is related to COVID-associated or cytokine-related parenchymal structural damage and thrombosis (Shadyro et al. 2021). Elevated levels of free radicals (reactive oxygen species (ROS) and nitric oxide) are also among the contributors to virus-induced pneumonia and death (Akaike et al. 1996; Perrone et al. 2013). Free radicals induce oxidative stress in a positive loop that helps SARS-CoV-2 replicate and consequently, produces more inflammatory responses.
Free radicals could be a reliable therapeutic target in cases with severe COVID infection. Edaravone is lipophilic compound that has shown its in vitro activity to scavenge free radicals along with antioxidant and anti-inflammatory activity that has been used in the acute phase of stroke and amyotrophic lateral sclerosis (Kikuchi et al. 2012; Watanabe et al. 2008). It has been introduced as an intravenously administrated antioxidant in the acute phase of cerebral infarction and amyotrophic lateral sclerosis. It has also been used in chemical-induced organ damage (Kikuchi et al. 2012). It diminishes the levels of and nitric oxide, ROS, chemokines, and cytokines (tumor necrosis factor-alpha (TNF-α), IL-1β, -2, -6,) in several experimental studies (Kikuchi et al. 2012). In the setting of different diseases caused by cytokine-induced apoptosis and oxidative stress (e.g., hypertension, diabetes, atherosclerosis, or heart failure), Edaravone could be a therapeutic agent to improve endothelial dysfunction (Kikuchi et al. 2013).
There are highly effective vaccines on diverse platforms for COVID 19; however, the incidence of the new viral mutations necessitates the development of effective therapeutic agents alongside the development of vaccination programs (Rahbar Saadat et al. 2021). Since both free radical and cytokine storms are synergistically damaging pathophysiology in COVID-19, it is essential to focus on free radical protection beside antiviral therapy developments. In this regard, we examined the free radical-scavenging and anti-inflammatory properties of Edaravone to reduce multi-organ injury, clinical complications, and mortality in patients with severe COVID-19.
Materials and methods
Study design and participants
This study was conducted in the intensive care units (ICU) of the teaching hospital of Tabriz University of Medical Sciences. Tabriz, Iran. An informed consent form was signed by participants or their close family members, if they were unable to cooperate. This study was permitted by the ethics committee of Tabriz University of Medical Sciences (Ethics code: IR.TBZMED.REC.1399.128) and was registered at the Iranian Registry of Clinical Trials (IRCT) with registration code: IRCT20200317046797N6 (https://en.irct.ir/).
Patients and randomization
Patients who were diagnosed with SARS-CoV-2-related pneumonia were recruited in this study from April to September 2021. The inclusion criteria were: (1) age ≥ 18 and < 80 years; (2) positive real time-PCR for SARS-CoV-2; (3) chest imaging-confirmed pneumonia (Computed tomography (CT) of the chest scan); (4) patients with acute respiratory distress syndrome (ARDS) according to Berlin ARDS criteria. Exclusion criteria were history of drug allergy, pregnancy or lactating, patients on mechanical ventilation, and expected survival duration < 24 h. Enrolled patients in other clinical trials were also excluded. Patients with severe COVID-19 were divided into two intervention and non-intervention (control) arms by block randomization. The study was blinded to the patients and researchers, but unblinded for the clinical team. The patients in the intervention arm in addition to their standard treatments protocols of COVID-19, received Edaravone (Alsava, 1.5 mg/ml, 20 ml vial, Zistdaru Danesh Co., Tehran, Iran) by slow intravenous infusion 30 mg daily for three successive days. Control groups just received the standard therapeutic protocol of the center. The infusion was immediately stopped when any adverse events were seen during infusion and the patients were carefully monitored.
Outcome and data collection
Baseline data, including vital signs, comorbid conditions, demographics, laboratory data, PaO2/FiO2 (arterial pO2/fraction of inspired O2 expressed as a decimal), sequential Organ Failure Assessment (SOFA), and Glasgow Coma Scale (GCS) scores were recorded during the follow-up period (28 days). The primary outcomes were the need for invasive mechanical ventilation, intubation length, and 28‐day mortality. Secondary endpoints including ICU admission length, inflammatory parameters including C-reactive protein (CRP), PO2, erythrocyte sedimentation rate (ESR), platelet (PLT), white blood cell, lymphocyte, and neutrophil counts, urea, creatinine, prothrombin time (PT), partial PT (PTT), along with SOFA and GCS scores were recorded for both groups. The outcome of this trial was assessed on days 1, 3, 7, and 28.
Statistical analysis
Quantitative variables were presented as mean ± standard deviation or median (min–max) and were compared respectively via independent t-test or Mann–Whitney U test between the groups. In addition, one-way ANOVA test followed by post-hoc test (Tukey) was utilized for comparing the intergroup variables. The frequency of qualitative variables was compared between the groups via Fisher’s exact test. SPSS software version 23.0 (Chicago, USA) was utilized for data analysis. A P value < 0.05 was considered significant.
Results and discussion
Baseline characteristics of patients with severe COVID-19
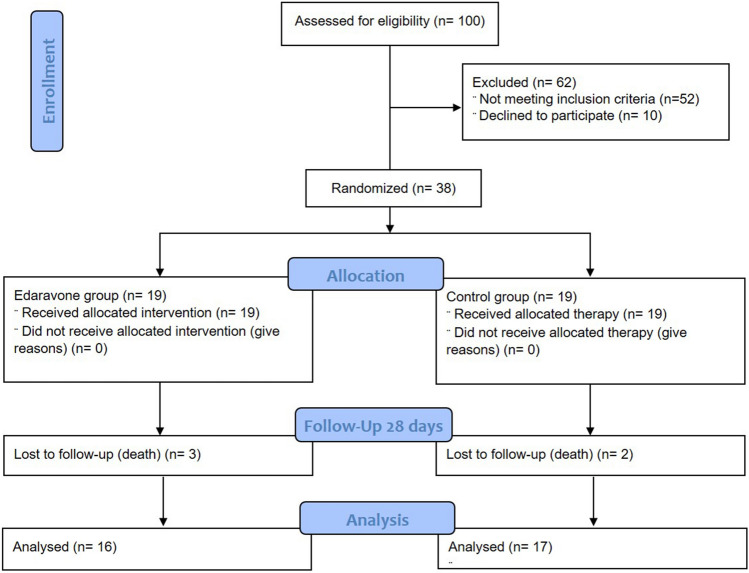
To conduct this single-center randomized controlled trial (RCT), which was conducted during 5 months (April–September, 2021), 100 patients with severe COVID-19 admitted to ICUs were assessed for enrollment criteria, of which 38 cases were eligible for entering the study. Patients were randomized equally to Edaravone (n = 19) and control (n = 19) groups (Fig. 1). Baseline features were similar in both studied groups. In intervention group, 52.63% of patients were women (n = 10) and 47.36% were men (n = 9). The mean age of patients in the control and intervention groups was 59.35 ± 18.16 and 62.09 ± 9.55, respectively (Table 1).
Fig. 1.
CONSORT 2010 flow diagram. Patients’ randomization was represented during the study
Table 1.
The effect of Edaravone in patients with severe COVID-19 infection
| Control (n = 19) | Intervention (n = 19) | P value | |
|---|---|---|---|
| Clinical parameters | |||
| Age (years) | 59.35 ± 18.16 | 62.09 ± 9.55 | 0.65 |
| Gender; men/women | 9 (47.4%)/10 (52.6%) | 9 (47.36%)/10 (52.63%) | 0.749 |
| Primary outcomes | |||
| Need for intubation | 8 (42.1%) | 2 (10.52%) | 0.034 |
| Intubation length (days) | 28 (4–28) | 3 (1–7) | 0.047 |
| ICU admission length (days) | 10 (7–26) | 11 (2–28) | 0.917 |
| Mortality | 2 (10.5%) | 3 (15.78%) | 0.66 |
Quantitative variables were reported as mean ± SD or median (min–max) and qualitative variables were reported as number (percentage). Independent t-test or Mann–Whitney U test were utilized for data analysis and p value < 0.05 was considered significant
Primary outcomes of patients with severe COVID-19
Regarding to defined primary outcomes, patients who received Edaravone did differ in the necessity of intubation in comparison to the control group [10.52% (n = 2) versus 42.1% (n = 8); p = 0.034]. Additionally, mean days of intubation was significantly more in the control group than the intervention group (median of 28 (4–28) versus 3 (1–7) days, p = 0.047). In both groups, mortality was almost equal (p = 0.66): 2 patients in the control arm (10.5%) and 3 in the Edaravone arm (15.78%) (Table 1). The median duration of ICU admission in the intervention and control groups were 11 (2–43) versus 10 (7–26) days, respectively that was not different between the two groups (p = 0.917). Regarding symptom alleviation, considered symptoms were not significantly different between the studied groups on day 28.
Secondary outcomes of patients with severe COVID-19
At the time of ICU admission, the P/F ratio was un-markedly higher in the control arm in comparison to the intervention arm (156.36 ± 98.71 versus 184.33 ± 88.29, p = 0.455). SOFA and GCS were equal in both groups (p ≥ 0.79). Hemoglobin, urea, serum creatinine, alanine transaminase (ALT), and aspartate transaminase (AST) levels were not different between the intervention and control groups during the mentioned followed-up period (p > 0.05). However, it was observed that the hemoglobin levels of patients in the control group were significantly decreased during the follow-up period compared to the baseline (p = 0.001). P/F ratio and platelets levels of patients in the intervention group were slightly higher compared to the controls (p > 0.05). In contrast, the increased levels of the P/F ratio in the control group after routine treatment were statistically significant (p = 0.026). International normalized ratio (INR) [(PTtest/PTnormal)ISI], alkaline phosphatase (ALP), serum total bilirubin levels were slightly lower in the intervention group than the control group (p > 0.05). Total number of WBCs of patients in the intervention group were lower than the control group and this increase was statistically significant at day 1 (p = 0.006). In addition, ESR levels at third (p = 0.018) and seventh (p = 0.032) days of hospitalization were significantly lower in the intervention group compared to the control group. PT was lower in the intervention group of patients with severe COVID-19 infection than the control group at day 28 that was statistically significant (p = 0.044), Table 2.
Table 2.
The clinical outcome of Edaravone in patients with severe COVID-19 infection
| Parameters | Groups | First day | 3rd day | 7th day | 28th day | P value* |
|---|---|---|---|---|---|---|
| WBC (109/L) | Control | 12,826.32 ± 5606.72 | 11,655.56 ± 5890.19 | 10,250 ± 5491.62 | 10,220 ± 5145.39 | 0.484 |
| Intervention | 8444.44 ± 2999.25 | 9800 ± 4339.21 | 11,614.29 ± 4382.8 | 7350 ± 1909.18 | 0.125 | |
| P value | 0.006 | 0.299 | 0.454 | 0.469 | ||
| Hb (mg/dl) | Control | 13.23 ± 2.13 | 12.69 ± 2.02 | 11.85 ± 2.46 | 9.81 ± 1.58 | 0.001 |
| Intervention | 13.05 ± 1.56 | 12.72 ± 1.71 | 13.21 ± 1.75 | 12.75 ± 2.47 | 0.866 | |
| P value | 0.764 | 0.964 | 0.09 | 0.182 | ||
| PLT (109/L) | Control | 213,578.95 ± 126,171.53 | 184,133.33 ± 135,862.52 | 221,444.44 ± 122,607.56 | 231,900 ± 137,522.07 | 0.764 |
| Intervention | 205,277.78 ± 113,609.13 | 243,411.76 ± 166,113.68 | 257,230.77 ± 153,421.07 | 252,500 ± 118,086.83 | 0.764 | |
| P value | 0.835 | 0.255 | 0.476 | 0.849 | ||
| PT (seconds) | Control | 13 (12–17.1) | 14.95 (12.5–20.1) | 15.1 (12–23) | 14.5 (13.5–25) | 0.018 |
| Intervention | 13 (11.1–18) | 15 (13–42.2) | 14.75 (13.2–20.3) | 12.8 (12.6–13) | 0.134 | |
| P value | 0.48 | 0.716 | 0.726 | 0.044 | ||
| PTT (seconds) | Control | 33.95 ± 5.75 | 35.94 ± 10.54 | 35.17 ± 8.07 | 42.56 ± 29.6 | 0.488 |
| Intervention | 35.5 ± 5.943 | 32.09 ± 3.7 | 31 ± 3.46 | 37 ± 9.899 | 0.087 | |
| P value | 0.425 | 0.259 | 0.146 | 0.806 | ||
| CRP (mg/l) | Control | 66 (14–158) | 54 (26–171) | 22 (12–206) | 46 (22–70) | 0.638 |
| Intervention | 106 (7–186) | 80 (3–194) | 20 (1–145) | 0.219 | ||
| P value | 0.247 | 0.937 | 0.545 | 0.564 | ||
| Urea (mg/dL) | Control | 54 (18–136) | 52 (20–206) | 45 (15–238) | 30 (17–245) | 0.743 |
| Intervention | 47.5 (24–93) | 56 (20–157) | 62 (21–157) | 44.5 (28–61) | 0.313 | |
| P value | 0.15 | 0.757 | 0.843 | 0.606 | ||
| Cr (mg/dl) | Control | 1.37 (0.7–6.92) | 1.07 (0.6–7.66) | 1.01 (0.56–8.68) | 0.91 (0.6–5.7) | 0.889 |
| Intervention | 1.15 (0.8–1.7) | 0.91 (0.72–2) | 0.98 (0.51–2.26) | 1.17 (0.95–1.4) | 0.758 | |
| P value | 0.129 | 0.483 | 0.512 | 0.364 | ||
| P/F ratio | Control | 156.36 ± 98.71 | 161 ± 89.99 | 245.55 ± 47.72 | 256 ± 62.68 | 0.026 |
| Intervention | 184.33 ± 88.29 | 217.91 ± 98.29 | 255.45 ± 83.94 | 290 ± 14.14 | 0.166 | |
| P value | 0.455 | 0.176 | 0.757 | 0.504 | ||
| SOFA | Control | 4.63 ± 1.53 | 4 ± 1.29 | 4.29 ± 1.79 | 4.55 ± 2.35 | 0.944 |
| Intervention | 4.53 ± 2.89 | 4.84 ± 3.02 | 3.27 ± 2.57 | 4 ± 2.82 | 0.574 | |
| P value | 0.9 | 0.666 | 0.226 | 0.775 | ||
| GCS | Control | 15 (14–15) | 15 (6–15) | 15 (5–15) | 0.644 | |
| Intervention | 15 (6–15) | 15 (7–15) | 15 (13–15) | 0.829 | ||
| P value | 0.796 | 0.851 | 0.879 |
Quantitative variables were reported as mean ± SD or median (min–max)
AST aspartate transaminase, ALT alanine transaminase, ALP alkaline phosphatase, Cr creatinine, ESR erythrocyte sedimentation rate, CRP C-reactive protein, GCS glasgow coma scale, Hb hemoglobin, INR international normalized ratio, P/F ratio PaO2/FiO2 ratio, PT prothrombin time, PLT platelets, PTT partial P, SOFA sequential organ failure assessment, WBC white-blood cell
*One-way ANOVA test was utilized for comparing intragroup values
Discussion
In this single-center RCT, there were significant differences regarding the primary outcomes including need for mechanical intubation and intubation length between those who received Edaravone compared with controls. Secondary endpoints including ICU admission length, and general clinical characteristics were quietly equal in the studied groups.
Recent studies propose that oxidative stress, an imbalance condition between the prooxidants and antioxidants, has a considerable role in SARS-CoV-2 viral infections (Schönrich et al. 2020). Respiratory viral infections are linked with inhibition of antioxidant defenses pathways (Nuclear factor erythroid 2–related factor 2 (Nrf2)-mediated) and activation of nuclear factor-kappa B (NF-κB) signaling that drives inflammation, oxidative injury, and cytokine storm during these infections (Komaravelli and Casola 2014). In line with this, evidence indicates that there is a connection between disease severity and reduced antioxidant enzymes, and lung injury development in COVID-19 patients (Abouhashem et al. 2020).
SARS-CoV-2 enters the host cells through angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 converts angiotensin II (AngII) into angiotensin 1–7 (Touyz et al. 2020) that can be inactivated by the viral action. Elevated levels of AngII have been observed in serum samples of severe COVID-19 patients (Wu et al., 2020). Intracellular signaling of AngII includes an elevated levels of ROS (Dikalov and Nazarewicz, 2013) to be used in signaling mechanisms (Laurindo et al. 2002). However, excessive ROS results in cell apoptosis or necrosis. Therefore, targeting the oxidative stress and free radicals in COVID-19 could be a useful therapeutic target (Nasi et al. 2020). It is reported that vitamin C level in patients with COVID-19 was considerably low and its supplementation (100 mg/kg/day) is useful (Xing et al., 2021). An open-label, randomized, controlled trial that was conducted on 60 patients with severe COVID-19 infection did not find significantly better outcomes in patients who received high-dose intravenous vitamin C (6 g daily) in addition to their standard therapies (JamaliMoghadamSiahkali et al. 2021). N-acetylcysteine (NAC), a glutathione precursor, is also proposed to reestablish redox homeostasis and improve the outcome in COVID-19 patients. This antioxidant was also tested in a patient with G6PD (glucose 6-phosphate dehydrogenase) deficiency that because of glutathione depletion was vulnerable to coronavirus infection (Ibrahim et al., 2020). In a double-blind, placebo-controlled RCT, high doses (about 300 mg/kg) administration of NAC for 20 h did no significant impact on the clinical course of patients with severe COVID-19 (de Alencar et al., 2021).
This study had some limitations; the small number of enrolled patients was the main limitation of this study. Since the distribution and duration of infected patients were unpredictable, the number of enrolled patients may be low at such this clinical centers. Therefore, several clinical trials in different centers are required to supply an adequate number of patients for a larger studies in the future. Moreover, the trial was unblinded for clinical staff who were in charge of enrolling participants; however, to minimize bias, patients and the research team were blinded. Additionally, the effect of different therapeutic regimens that studied groups received before their ICU admission were not erasable and may affect our results.
Conclusion
Considering the radical-scavenging and anti-inflammatory properties of Edaravone, the observed clinical benefit in our preliminary study is promising for the effective role of Edaravone on the clinical outcome of severe COVID-19 patients. It requires further studies with adequate patient numbers and observation time to confirm this protective effect.
Acknowledgements
Drugs were donated by Zistdaru Co. (Tehran, Iran) for conducting this study. Authors acknowledge the Company.
Author contributions
MA: design the work/final approval of the version to be published and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MM, MSh, FJ, AM, AS, HV, ASh, and MH: Data collection, MA, SMH, MM and SZV: Data analysis, drafting and revising the work.
Funding
This work was supported by the Kidney Research Center at Tabriz University of Medical Sciences, Tabriz, Iran (Grant # 65395).
Data availability
Not applicable.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammadreza Ardalan, Email: ardalan34@yahoo.com, Email: ardalanm@tbzmed.ac.ir.
Sepideh Zununi Vahed, Email: zununivahed@tbzmed.ac.ir.
References
- Abouhashem AS, Singh K, Azzazy HME, Sen CK. Is low alveolar type II cell SOD3 in the lungs of elderly linked to the observed severity of COVID-19? Antioxid Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, et al. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alencar Julio Cesar Garcia, Moreira Claudia de Lucena, Müller Alicia Dudy, Chaves Cleuber Esteves, Fukuhara Marina Akemi, da Silva Elizabeth Aparecida, Miyamoto Maria de Fátima Silva, Pinto Vanusa Barbosa, Bueno Cauê Gasparotto, Lazar Neto Felippe, Gomez Gomez Luz Marina, Menezes Maria Clara Saad, Marchini Julio Flavio Meirelles, Marino Lucas Oliveira, Brandão Neto Rodrigo Antônio, Souza Heraldo Possolo. Double-blind, Randomized, Placebo-controlled Trial With N-acetylcysteine for Treatment of Severe Acute Respiratory Syndrome Caused by Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2021;72(11):e736–e741. doi: 10.1093/cid/ciaa1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim H, Perl A, Smith D, Lewis T, Kon Z, Goldenberg R, Yarta K, Staniloae C, Williams M. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JamaliMoghadamSiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi SA, Zendehdel A, Tabarestani M, Sekhavati Moghadam E, Abbasian L, Manshadi SAD, Salehi M, Hasannezhad M, Ghaderkhani S, Meidani M, Salahshour F, Jafari F, Manafi N, Ghiasvand F. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021 doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Takeshige N, Miura N, Morimoto Y, Ito T, Tancharoen S, et al. Beyond free radical scavenging: beneficial effects of edaravone (radicut) in various diseases (review) Exp Ther Med. 2012;3:3–8. doi: 10.3892/etm.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, et al. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci. 2013;14:13909–13930. doi: 10.3390/ijms140713909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaravelli N, Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. J Pharmacogenomics Pharmacoproteomics. 2014 doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurindo FR, de Souza HP, Pedro MA, Janiszewski M. Redox aspects of vascular response to injury. Methods Enzymol. 2002;352:432–454. doi: 10.1016/s0076-6879(02)52039-5. [DOI] [PubMed] [Google Scholar]
- Nasi A, McArdle S, Gaudernack G, Westman G, Melief C, Rockberg J, et al. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone LA, Belser JA, Wadford DA, Katz JM, Tumpey TM. Inducible nitric oxide contributes to viral pathogenesis following highly pathogenic influenza virus infection in mice. J Infect Dis. 2013;207:1576–1584. doi: 10.1093/infdis/jit062. [DOI] [PubMed] [Google Scholar]
- Rahbar SY, Hosseiniyan Khatibi SM, Zununi VS, Ardalan M. Host serine proteases: a potential targeted therapy for COVID-19 and influenza. Front Mol Biosci. 2021;8:725528. doi: 10.3389/fmolb.2021.725528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönrich G, Raftery MJ, Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biolog Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadyro O, Samovich S, Edimecheva I, Novitsky R, Khrutskin V, Ihnatovich L, et al. Potential role of free-radical processes in biomolecules damage during COVID-19 and ways of their regulation. Free Radic Res. 2021;55:745–756. doi: 10.1080/10715762.2021.1938024. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Chattopadhyay I, Rajasekaran S. Oxidative stress in lung diseases. UK: Springer, Nature Publishing Group; 2020. Oxidative stress mechanisms in the pathogenesis of environmental lung diseases; pp. 103–137. [Google Scholar]
- Touyz RM, Li H, Delles C. ACE2 the Janus-faced protein—from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clin Sci (London, England: 1979) 2020;134:747–750. doi: 10.1042/cs20200363. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26:101–114. doi: 10.1111/j.1527-3466.2008.00041.x. [DOI] [PubMed] [Google Scholar]
- Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide Biol Chem. 2020;102:39–41. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Hu R, Zhang C, Ren W, Yu A, Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically Ill COVID-19 patients. Crit Care (London, England) 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Zhao B, Yin L, Guo M, Shi H, Zhu Z, Zhang L, He J, Ling Y, Gao M, Lu H, Mao E, Zhang L. Vitamin C supplementation is necessary for patients with coronavirus disease: An ultra-high-performance liquid chromatography-tandem mass spectrometry finding. J Pharm Biomed Anal. 2021;196:113927. doi: 10.1016/j.jpba.2021.113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.