Abstract
Objective
To identify and summarise evaluated interventions aiming to improve the communication of palliative care (PC) and end-of-life (EoL) issues in physicians caring for cancer patients. Such interventions are needed with regard to the aim of an earlier communication of those issues in oncology daily practice, which is associated with a range of benefits for patients and caregivers but is often impeded by physicians’ communication insecurities.
Design
Systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data sources
Relevant publications were systematically searched in MEDLINE, PsycINFO, CINAHL and Web of Science databases in September 2020 with an update in July 2021.
Eligibility criteria
We included publications reporting a quantitative evaluation of a communication intervention on one or more PC/EoL issues with a communication-related main outcome. Target group had to be physicians caring for cancer patients non-specialist in PC.
Data extraction and synthesis
Two independent raters extracted intervention characteristics, publication characteristics and publication quality. Results were narratively synthesised.
Results
24 publications reporting 22 interventions were included. 13 publications reported randomised controlled trials. A majority of the interventions addressed one specific PC/EoL issue, most often breaking bad news. Teaching strategies mostly involved role-plays. Target group were mainly oncologists. In addition to self-reported outcome measurements for evaluation, most publications also reported the use of external rating data. All but one publication reported significant intervention effects on at least one outcome parameter. Publication quality was overall moderate.
Conclusions
The empirically tested communication interventions on PC/EoL issues seem to effectively improve physicians’ communication. Future interventions should focus on other issues than breaking bad news, such as preparing for the future. Target group should also be organ-specific oncologists, as all primary caring physicians are responsible for timely communication. Our risk-of-bias assessment revealed some weaknesses, indicating that more high-quality studies for evaluation are needed.
PROSPERO registration number
CRD42020191054.
Keywords: palliative care, oncology, medical education & training
Strengths and limitations of this study.
This systematic review was built on a comprehensive database search.
Intervention and publication characteristics were narratively summarised and concisely displayed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
We used the Effective Public Health Practice Project Quality Assessment Tool to assess risk of bias of the included publications.
Due to heterogeneity of the publications, a quantitative meta-analysis was not possible.
A publication bias in favour of significant results is possible and some unpublished studies might have been missed.
Introduction
Physicians caring for patients with advanced cancer should communicate early about issues related to palliative care (PC) and the end of life (EoL). Important components include, for example, talking about goals of care, advance care planning, eliciting personal values, preparing for the future or involving caregivers.1
Several studies indicate that an early communication about these issues brings a range of benefits to patients, caregivers and the healthcare system,2–7 as this is associated with improved symptom control, increased quality of life, better acceptance of their incurable illness leading to premature dying and a decrease of caregivers’ burden. Besides, EoL conversations are associated with less aggressive medical care at the very EoL, less admissions to the intensive care unit as well as earlier hospice referrals.6 7
Also patients themselves often prefer an early and clear communication and consider this as essential for their personal EoL care.8 The majority prefers an early and honest conversation about their prognosis and EoL issues.9–12 A timely communication enables them to participate more actively in treatment decisions, to avoid inadequate treatments, to set own individual priorities and to prepare themselves for death.13 It also enables them access to specialised PC services, which is essential with regard to the high PC needs of oncological patients.14
Previous studies demonstrated that conversation about PC/EoL issues usually occur too late, that is, when patients are no longer able to decide for themselves or already are in crisis.15 Although according to different guidelines—such as the ones from the American Society of Clinical Oncology Clinical Practice—the primary caring oncologist is responsible for addressing these issues,16 17 they often fail to do so in daily clinical practice.15
Major barriers to timely discussion of the aspects are communication insecurities of the caring physicians, who seem to avoid these conversations.18 19 Indeed, addressing the EoL is considered the most stressful and uncomfortable part of oncological care.20 Physicians report, for example, the fear of causing stress or destroying hope when addressing these issues.10 20–22 Additionally, previous personal traumatic experiences might be responsible,18 as well as own attitudes and fears towards death.23 24 Besides those personal and individual reasons, a lack of physicians’ knowledge about early communication of PC/EoL issues represents a barrier. A systematic scoping review on advance care planning in practice, for example, found that advance care planning often fails due to the absence of professionals’ awareness about initiating it at an early stage.25 Lastly, also a deficiency of physicians’ training in EoL communication seems to be crucial for those rare conversations.26
Hence, evidence-based interventions to reduce communication barriers regarding PC/EoL issues are needed. As in cancer care the primary caring (organ-specific) oncologist is supposed to provide primary PC and to communicate these aspects, those interventions should target non-palliative-care specialists.
A range of communication skills trainings in oncological settings already exist and previous reviews have summarised and evaluated their effectiveness.27 28 Two existing systematic reviews including studies published up to December 2015 already focused on EoL communication interventions for generalist PC providers, but without restriction to trainings explicitly targeting physicians.29 30 In fact, in these reviews only 30% of the interventions were designed for physicians29 30 and those and other reviews on such interventions do not focus on the oncological setting.29–33
So far, there is little evidence on PC/EoL communication interventions specifically designed for the oncology physicians’ perspective. With regard to the aim of an earlier communication of PC/EoL issues in oncology daily practice, which is the main responsibility of the primary caring physician, an overview on those interventions is essential. This will enable conclusions for the need, the design and the evaluation of future interventions on strengthening physicians in early communication, which will then in turn lead to a significant improvement of advanced cancer care. Against this background, the objective of this study is to systematically review the evidence on communication interventions for oncologists or organ-specific physicians who are not specialised in PC that focus one or more communication issues relevant to PC and the EoL.
Methods
In order to provide a complete and transparent reporting, our systematic review was developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (online supplemental material S1).34 35 The review protocol published in the International Prospective Register of Systematic Reviews (PROSPERO) is presented in online supplemental material S2.
bmjopen-2021-059652supp001.pdf (51KB, pdf)
bmjopen-2021-059652supp002.pdf (43.8KB, pdf)
The research questions of this systematic review were developed based on the PICO criteria (‘Participants’, ‘Interventions’, ‘Comparison’, ‘Outcome’).36 As we included publications independent of the presence or absence of a comparison group, we specified all but the criterion comparison (C). This resulted in the following research questions that this article addresses:
Which communication interventions on PC/EoL issues for physicians caring for cancer patients were evaluated and published?
What are the aims of the interventions, how are they structured and how is the content conveyed?
Which specific PC/EoL issues do they address?
Which communication-related outcome measurements are used?
What effects of the interventions are reported?
Eligibility criteria
The inclusion criteria regarding the publication characteristics were: (1) German or English language, (2) accessibility of full text, (3) published in a peer-reviewed journal, (4) primary research (eg, no intervention descriptions, study protocols or review articles), (5) studies that provide a (partly) quantitative evaluation of an intervention and (6) studies with a communication-related main outcome.
With regard to the study participants, the following criteria had to be met: (1) physicians caring for cancer patients (oncologists or organ-specific specialists such as gynaecologists or urologists), (2) not more than 20% PC specialists unless specialists and non-specialists were reported separately (defined according to procedures in the systematic reviews of Brighton et al and Selman et al29 30), (3) no medical students or healthcare professionals other than physicians unless they were reported separately, (4) no joint interventions for physicians and patients unless the results of the physicians were reported separately. The inclusion in case of a separate reporting of the physicians in the above cases was considered acceptable, as the effect of the intervention on the target group of this review could then be extracted separately.
Finally, the inclusion criteria regarding the intervention were: (1) main focus on improving communication, (2) intervention on one or more PC/EoL issues, (3) designed for oncological setting and (4) no paediatric context. Inspired by Back, who summarised central patient-clinician communication issues in PC1 from different systematic reviews and guidelines such as the ones from the American Society of Clinical Oncology,37 we defined the following PC/EoL issues:
Preparing for the future.
Talking about death and dying.
Talking about transition to PC/introducing PC.
Talking about prognosis
Discussing goals of care.
Supporting or involving family caregivers
Preparing for the future.
Eliciting values.
Dealing with emotions/giving emotional support.
Breaking bad news/discussing serious news.
Advance care planning.
Talking about advance directives.
Shared decision making (in an oncological context).
Search
We conducted our search in MEDLINE (via OVID), PsycINFO (via OVID), CINAHL (Cumulative Index to Nursing and Allied Health Literature) and Web of Science up to September 2020. An update of the search was conducted in July 2021. There were no restrictions on year of publication or geographical location. The search strategy is displayed in online supplemental material S3. We conducted additional hand searches in reference lists of relevant papers and earlier reviews to identify further suitable articles.
bmjopen-2021-059652supp003.pdf (86.5KB, pdf)
Study selection
In a first step, the first author (NH) removed duplicates and screened the titles and abstracts identified within the searches. Second, the first author (NH) and another member of the research team (HMR) independently screened the full texts with a screening form according to the predefined inclusion and exclusion criteria. Disagreements were resolved by consensus after discussion. Excluded papers were listed and reasons for exclusion were documented.
Data extraction and quality assessment
Data of the selected publications were independently extracted by two authors (NH and HMR) using a data extraction form. The form included the following information, which was sought from all articles: publication characteristics (authors, year, title, language, country), methods (study design, sample size, participants, communication-related outcome parameters), the investigated intervention (name of intervention, target group, setting, aim, content/learning activities) as well as the reported effect of the intervention. After a comparison of the two independently completed data extraction forms and a discussion of disagreements, all the above information was tabulated for each publication.
Above that, the two raters (NH and HMR) independently assessed the quality of the included articles by means of the Effective Public Health Practice Project Quality Assessment Tool (EPHPP).38 39 The instrument assesses information on six aspects: selection bias, design, confounders, blinding, data collection methods as well as withdrawals and drop-outs. Based on the rating of these components (strong, moderate or weak), the findings were summed up to a final grade (strong, moderate or weak). Disagreements between the raters were resolved by consensus after discussion.
Information regarding study characteristics as well as intervention characteristics was tabulated.
Data synthesis
We conducted a narrative synthesis of the results, as the methodological and statistical heterogeneity of the publications allowed no quantitative synthesis.40 In doing so, the recommendations of the Cochrane Consumers and Communication Review Group were respected.41
Patient and public involvement
No patient involved.
Results
Publication selection
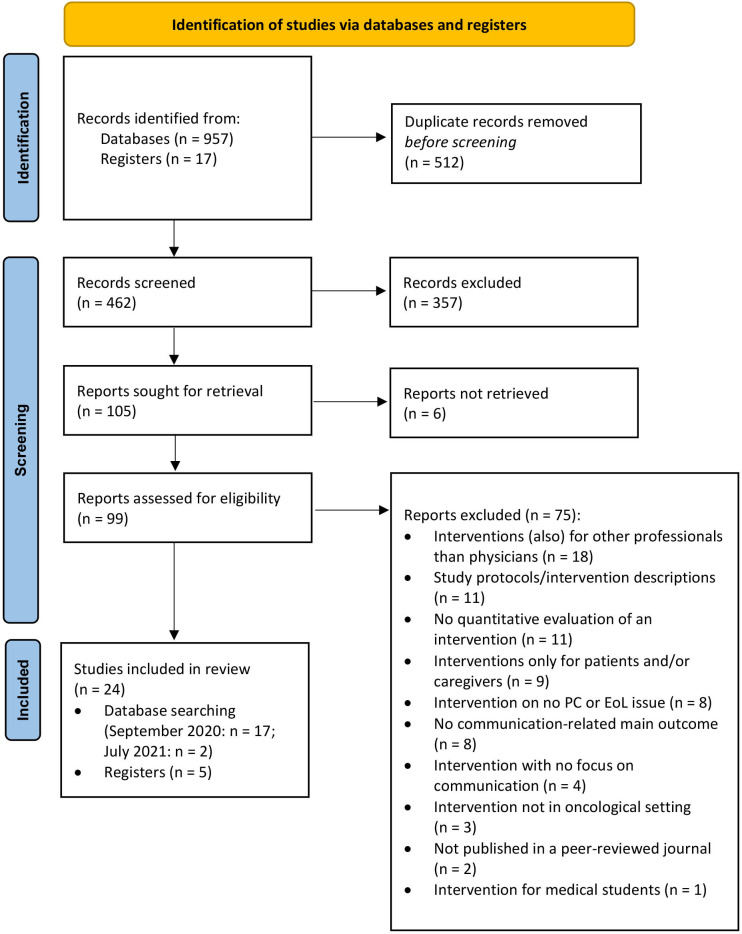
The initial database searches yielded a total of 957 records. An additional 17 publications were identified through handsearch (via reference lists, etc). After removal of duplicates, a total of 462 abstracts and titles were screened. The screening process retrieved 105 potentially relevant papers, which were subsequently full-text assessed for eligibility. This resulted in 22 articles that were initially included in this review. The reasons for exclusion of all full-text screened papers are displayed in figure 1. Five publications appeared to meet the inclusion criteria but were excluded because the reported interventions targeted more general communication skills of physicians working in oncology rather than having a focus on PC/EoL issues.42–46
Figure 1.
PRISMA 2020 flow diagram of the systematic literature search. EoL, end-of-life; PC, palliative care; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Two more articles were identified through a rerun of database searches in July 2021. Thus, a total of 24 articles were included in the qualitative synthesis. An overview of the publication selection process is illustrated in the PRISMA flow diagram (figure 1).
Publication and intervention characteristics
Across the 24 included publications, 22 different interventions were evaluated. Two interventions were evaluated in two publications each.47–50 Two times, an adapted version in form of one submodule or one workshop of an intervention reported in another publication was investigated.51 52
Publication characteristics
An overview of the relevant publication characteristics is provided in table 1. The included articles were published between 199953 and 2021.50 Half of them are from North America (n=12), followed by Europe (n=6), Asia (n=5) and Australia (n=1). Thirteen publications reported randomised controlled trials (RCTs).47–50 54–62 The remaining 11 papers reported uncontrolled intervention studies with at least two measurement time points (pre and post).51–53 63–70 The sample size ranges from n=1061 63 to n=38370 studied physicians. However, the majority (n=20 articles) reported sample sizes of less than 70 participating physicians. In the majority of the publications (n=14), the target group were oncologists (medical, surgical and radiation oncologists).49 50 53–56 59 61 64 66–70 In eight publications, the study participants were multi-specialty physicians working in oncology (oncologists and organ-specific physicians)47 48 51 52 57 60 62 65 and two exclusively targeted physicians other than oncologists.58 63 Bylund et al reported a train-the-trainer intervention, thus the target group were future facilitators of the training.65
Table 1.
Overview of publication characteristics in N=24 publications on N=22 communication interventions for physicians
| Name of the intervention | Publication (country) | Study design (groups; measurement time points); sample size | Target group |
| An Illness-Trajectory Communication Curriculum | Cannone et al 2019 (USA)66 | Uncontrolled intervention study (IG; pre, post—2 weeks after the last module)); N=22 | Multispecialty oncology residents and fellows |
| Avatar-mediated training in a virtual world | Andrade et al 2010 (USA)63 | Uncontrolled intervention study (IG; pre, post—directly after the intervention); N=10 | Geriatric and internal medicine fellows |
| Belgian Interuniversity Curriculum-communication skills training (BIC-CST) | Liénard et al 2010 (Belgium)60 | Randomised controlled trial (IG, CG; pre, post—after 8 months); N=98 | Medical residents working with cancer patients |
| Brief Breaking Bad News (BBN) CST module | Gorniewicz et al 2017 (USA)58 | Randomised controlled trial (IG, CG; pre, post—within 1 month after pre); n=38 (plus n=28 separately reported students) | Residents of family medicine and internal medicine and medical, nursing or pharmacy students (reported separately) |
| CST | Butow et al 2008 (Australia)54 | Randomised controlled trial (IG, CG; pre, post -shortly after the intervention, follow-up—12 months after pre); N=30 | Medical and radiation oncologists |
| CST | Baile et al 1999, (USA)53 | Uncontrolled intervention study (IG; pre, post—directly after the workshop); N=29 (thereof n=17 in workshop one and n=12 in workshop 2) | Oncologists and oncology fellows |
| CST53-adapted version (workshop on BBN) | Fujimori et al 2003 (Japan)51 | Uncontrolled intervention study (IG; pre, post—directly after the workshop, follow-up—3 months later); N=58 | Oncologists |
| CST based on patients preferences | Fujimori et al 2014 (Japan)56 | Randomised controlled trial (IG, CG; pre, post—2 weeks after pre); N=30 | Oncologists |
| CST workshop | Yamada et al 2018; (Japan)70 | Uncontrolled intervention study (IG; pre, post—directly after the workshop, follow-up—after 3 months); N=383 | Oncologists with three or more years of clinical experience in oncology |
| Communication training in oncology | Lenzi et al 2011 (USA)68 | Uncontrolled intervention study (IG; pre, post—directly after the training); N=57 | Senior oncologists |
| COM-ON-p (communication in oncology-transition to palliative care) | Goelz et al 2011 (Germany)57 | Randomised controlled trial (IG, CG; pre, post—5 weeks after pre); N=41 | Oncologists (haematology, oncology, gynaecology, surgery) |
| Comskil Training Curriculum | Bylund et al 2010 (USA)65 | Uncontrolled intervention study (IG; pre, post—not stated when); N=36 | Physicians and surgeons being future facilitators of the training (train-the-trainer) |
| Comskil Training Curriculum65—adapted version (module on discussing prognosis) | Brown et al 2010 (USA)52 | Uncontrolled intervention study (IG; pre, post—directly at the end of the intervention); N=142 | Multispecialty fellows and physicians working in oncology setting |
| Goals-of-Care communication skills and coaching intervention (INT) | Annadurai et al 2021 (USA)50 | Randomised controlled trial (IG, usual care; pre, post—after 6 months); N=22 | Solid tumour oncologists |
| Bickell et al 2020 (USA)49 | Randomised controlled trial (IG, usual care; pre, post—after 6 months); N=22 physicians, N=265 patients | Solid tumour oncologists and patients with a <2 years life expectancy | |
| Interact-Cancer (computer-assisted instruction programme) | Hulsman et al 2002 (Netherlands)67 | Uncontrolled intervention study (IG; participants divided into implementers vs non-implementers based on self-reported motivation; four measurement time points at intervals of 4 weeks: T1 (pre), T2, T3, T4 (intervention between T2 and T3); N=21 | Medical oncologists |
| Integrating simulation model with art-based teaching strategies | Yakhforoshha et al 2018 (Iran)69 | Uncontrolled intervention study (IG; 3 pre and three post measurements within 2 weeks intervals); N=19 | Medical oncology fellows |
| Oncotalk | Back et al 2007 (USA)64 | Uncontrolled intervention study (IG; pre, post—directly after the 4-day intervention); N=115 | Oncology fellows |
| Patient-Centred Communication Intervention (VOICE) | Epstein et al 2017 (USA)55 | Randomised controlled trial (IG, CG; pre, post—not stated when); N=38 physicians, N=265 patients | Medical oncologists and their patients |
| Posttraining Consolidation Workshops after a basic training programme | Delvaux et al 2005 (Belgium)48 | Randomised controlled trial (IG receiving basic programme and consolidation workshop, waitlist CG receiving only basic programme; baseline—before basic programme, post—after consolidation workshops of IG, 5 months after baseline); N=62 | Multispecialty physicians working with cancer patients |
| Razavi et al 2003 (Belgium)47 | Randomised controlled trial (IG receiving basic programme and consolidation workshop, waitlist CG receiving only basic programme; baseline—before basic programme, post—after consolidation workshops of IG, 5 months after baseline); N=62 | Multi-specialty physicians working with cancer patients (oncology, radiotherapy, gynaecology, etc) | |
| SCOPE (Studying Communication in Oncologist-Patient Encounters) CD-ROM | Tulsky et al 2011 (USA)62 | Randomised controlled trial (IG, CG; pre, post—within 1 month after the intervention); N=48 | Medical, gynecologic and radiation oncologists |
| Training Oncologists and Empowering Patients in Effective Communication During Medical Consultations in Singapore | Malhotra et al 2019 (Singapore)61 | Randomised controlled trial (IG, CG; pre, post—not stated when); N=10 physicians, N=60 patients | Oncologists and their patients |
| Training on Shared Decision-Making About Palliative Chemotherapy | Henselmans et al 2019 (Netherlands)59 | Randomised controlled trial (IG, CG; pre, post—after 4 months); N=31 | Medical oncologists and oncologists-in-training |
CG, control group; IG, intervention group; n. s., not significant; SP, simulated patients.
Intervention characteristics
The interventions were conducted in very different settings. While four interventions were carried out only virtual,58 61 63 67 a majority (n=18) was carried out at least partly in person47–57 59 60 62 64–66 68–70 (table 2; detailed description in online supplemental material S4). The length ranged from short and individual computer-assisted trainings or videos58 61 63 67 to multiple hour51–53 or multiple day workshops.56 64 68 Some interventions (n=6) consisted of basic group sessions plus follow-up appointments such as individual coaching sessions, consolidation workshops, video conferences or phone calls.47–50 54 55 57 59 Half of the reported interventions (n=10) lasted more than 1 day or consisted of several hours spread over a longer period of time, that is, weeks or months.47 48 54 56 57 60 64–66 68 70 Two interventions included also a patients’ coaching or a communication aid for them.55 61 Delvaux et al and Razavi et al—reporting on the same study—tested explicitly the efficacy of consolidation workshops following a basic training programme.47 48
Table 2.
Overview of intervention characteristics (N=22 interventions evaluated in N=24 publications)
| Name of the intervention | Setting | Duration | Addressed PC/EoL issue | Learning activities/didactics | |||||||||
| Virtual | In person | Up to 1 day | More than 1 day | Spread over weeks/ months | Breaking Bad News (BBN) | Dealing with emotions/ managing reaction | Prognosis | Other | Role play | Didactic lecture (by staff or computer-based) | Example videos | Other | |
| No of trainings fulfilling the criteria (n) | 5 | 18 | 10 | 11 | 9 | 14 | 10 | 4 | 9 | 16 | 19 | 7 | 16 |
| An Illness-Trajectory Communication Curriculum66 | x | x | x | x | x | x | x | x | |||||
| Avatar-mediated training in a virtual world63 | x | x | x | x | x | ||||||||
| Belgian Interuniversity Curriculum-communication skills training (CST)60 | x | x | x | x | x | x | |||||||
| Brief BBN CST module58 | x | x | x | x | x | ||||||||
| CST54 | x | x | x | x | x | x | x | x | |||||
| CST53 | x | x | x | x | x | x | |||||||
| CST53-adapted version of the workshop on BBN51 | x | x | x | x | x | ||||||||
| CST based on patients preferences56 | x | x | x | x | x | ||||||||
| CST workshop70 | x | x | x | x | x | x | |||||||
| Communication training in oncology68 | x | x | x | x | x | x | x | ||||||
| COM-ON-p (communication in oncology-transition to palliative care)57 | x | x | x | x | x | ||||||||
| Comskil Training Curriculum65 | x | x | x | x | x | x | x | x | x | x | |||
| Comskil Training Curriculum65 – adapted version (module about discussing prognosis)52 | x | x | x | x | x | x | x | ||||||
| Goals-of-Care communication skills and coaching intervention49 50 | x | x | x | x | x | x | x | ||||||
| Interact-Cancer (computer-assisted instruction programme)67 | x | x | x | x | x | x | x | x | |||||
| Integrating simulation model with art-based teaching strategies69 | x | x | x | x | x | x | |||||||
| Oncotalk64 | x | x | x | x | x | x | x | ||||||
| Patient-Centred Communication Intervention (VOICE)55 | x | x | x | x | x | x | x | ||||||
| Posttraining Consolidation Workshops after a basic training programme47 48 | x | x | x | x | x | x | x | x | x | ||||
| SCOPE (Studying Communication in Oncologist-Patient Encounters) CD-ROM62 | x | x | x | x | x | x | |||||||
| Training Oncologists and Empowering Patients in Effective Communication During Medical Consultations in Singapore61 | x | N/A | N/A | N/A | x | x | x | x | x | x | |||
| Training on Shared Decision-Making About Palliative Chemotherapy59 | x | x | x | x | x | x | x | x | |||||
EoL, end-of-life; N/A, not available; PC, palliative care.
bmjopen-2021-059652supp004.pdf (212.9KB, pdf)
The interventions focused on communication about a variety of PC/EoL issues. While 10 interventions targeted more than one PC/EoL issue,47 48 53 55 61 64–68 70 12 interventions focused on just one issue.49–52 54 56–60 62 63 69 The most frequently addressed PC/EoL issue was breaking bad news (n=14),47 48 51 53 56 58 60 63–70 followed by dealing with emotions (including subtopics such as showing empathy, managing reactions to illness, dealing with denial, anger; n=10).47 48 53–55 61 62 65 67 68 70 Talking about prognosis was addressed four times52 55 61 65 and other issues, such as goals of care discussions49 50 61 or interacting with relatives,47 48 66 were targeted in three or less interventions.
The didactic approach and the content of the described interventions vary widely. The largest overlap regarding the theoretical basis of the curriculum represented the SPIKES protocol, a protocol suggesting a six-step approach to deliver bad news.71 SPIKES stands for (1) ‘Setting up the interview’, (2) ‘assessing the patient’s Perception’, (3) ‘making an Invitation to disclose the news’, (4) ‘sharing the Knowledge about the news’, (5) ‘responding to patient’s Emotion’, (6) ‘Summarise the plan’.71 In six interventions, the communication skills were taught based on this approach.49–51 53 63 66 69 With regard to learning activities, the most frequently used training method was the conduction of practical role-plays, which were part of 16 of the reviewed interventions.47–54 56 57 59 60 64–66 68–70 Those were carried out with simulated patients in eleven interventions,.52 54 56 57 59 64–66 68–70 Fifteen interventions included a lecture held by a facilitator,47–54 59–62 64–66 68 69 whereas in four interventions the theoretical input was computer-based.56 63 67 70 In about one-third of the interventions (n=7), videos of ‘ideal’ conversations were presented.52 54 58 59 61 65 67 In some interventions, the facilitators gave individual feedback on real patient encounters, for example, in form of a coaching or by discussing a taped conversation (n=4).49 50 59 61 62 Two times the facilitators conducted face-to-face meetings following the intervention to discuss and transfer individual learning goals into daily routine.57 66
Outcome measurements and intervention effects
Outcome measurements
The reviewed articles reported different communication-related outcome measurements and methods to evaluate the intervention. Those are displayed in table 3. While six of the included publications describe only self-reported data for evaluation,51–53 63 68 70 the majority (n=18) also included objective, externally assessed outcome measurements.47–50 54–62 64–67 69 With one exception,69 all objective outcome measurements were assessed by an external rating of pre and post videotaped or audiotaped clinical encounters of the participating physicians.47–50 54–62 64–67 In four articles, the rating was conducted based on transcripts of the audiotapes.47 48 60 61 Only Yakhforoshha et al carried out the rating simultaneously during an outpatient consultation.69 Gorniewicz et al and Cannone et al used videotaped objective structured clinical exams for their ratings, a commonly used evaluation tool for physicians.58 66 The clinical encounters were either conducted with simulated patients (n=9),24 54 56–58 60 64 66 69 real patients (n=7)49 50 55 61 62 65 67 or both (n=2).47 48 Rating was mostly conducted through study staff (n=17).47–50 54–57 59–62 64–67 69 In six articles, the authors also collected rating data from of the (simulated) patients’ perspective47–49 62 66 69 and in one case only the simulated patients represented the raters.58
Table 3.
Overview of outcome measurements and intervention effects in N=24 publications
| Publication | (1) Communication-related outcome measurements; (2) effects of the intervention |
| Cannone et al 201966 | (1) External rating of 6 domains of communication skills in OSCE-scenarios by faculty members and SP via a self-developed instrument based on SPIKES protocol, self-reported perceived readiness and comfort level; (2) Sign. improvement in global communication skills and positive changes in some subcategories rated by faculty members (‘emotion and empathy’, ‘delivering phase of breaking bad news’ (BBN), isolated items of other domains), increased comfort level in all areas. |
| Andrade et al 201063 | (1) Self-reported self-efficacy via the self-efficacy Affective Competency Score;86 (2) Sign. improvement of self-efficacy. |
| Liénard et al 201060 | (1) Quantitative analyses of physicians’ utterances regarding assessment, support and information type in transcripts of audiotaped SP encounters via a communication content analysis software, external rating of 3 phases of the BBN-process; (2) Signficantly more open questions, open directive questions and empathy as well as a sign. decrease in the amount of given information in IG; BBN process: IG allocated more time to the predelivery phase and less time to the delivery phase and delivered bad news more precisely. |
| Gorniewicz et al 201758 | (1) External rating of 5 domains of BBN skills in videotaped SP-OSCE-sessions via a BBN rating form checklist by SP, external rating of 5 general communication skills via the Common Ground Assessment Summary form83 by SP; (2) Sign. intervention effect on 3 BBN domains: ‘BBN’, ‘communication related to emotions’ and ‘after BBN, determines patient readiness to proceed and communication preferences’, significant intervention effect on four general communication skills (‘active listening’, ‘addressing feelings with patients’, ‘closing the interview’ and ‘global interview performance’). |
| Butow et al 200854 | (1) External rating of 10 key doctor behaviours and the number of predetermined patient concerns plus the degree to which they were adequately addressed in videotaped SP encounters via a self-developed instrument; (2) Trend of IG to show more creating environment and fewer blocking behaviours than the CG (n.s.). |
| Baile et al 199953 | (1) Self-reported confidence in communication regarding BBN and difficult patient situations via self-developed items; (2) Workshop 1 (BBN): significant improvement of confidence in 18 of 21 items; workshop 2 (managing difficult patient situations): sign. improvement of confidence in 11 of 45 items. |
| Fujimori et al 200351 | (1) Self-reported confidence in communication with patients regarding BBN via items developed by Baile et al;53 (2) Sign. improvement of confidence in 20 of 21 items at post and follow-up. |
| Fujimori et al 201456 | (1) External rating of 4 communication domains in videotaped SP encounters via a self-developed rating system based on the SHARE protocol on BBN,85 self-reported confidence in communication via items related to SHARE and the confidence questionnaire by Baile et al,53 self-reported patients’ satisfaction with consultation and trust in oncologist via self-developed items; (2) Sign. intervention effect on ‘setting up supportive environment for interview’, ‘considering how to deliver bad news’ and ‘providing reassurance/addressing patient’s emotions with empathic responses’, sign. effect on confidence; no change in satisfaction. |
| Yamada et al 201870 | (1) Self-reported intrapersonal empathy via the Jefferson Scale of Physician Empathy (JSPE)87 and the Interpersonal Reactivity Index (IRI);88 (2) Sign. improvement of JSPE total empathy-score and all subscale scores (‘perspective taking’, ‘compassionate care’, ‘standing in the patient’s shoes’) at post and follow-up, sign. improvement in 2 of 3 IRI subscales (‘perspective taking’ and ‘personal distress’) from pre to follow-up. |
| Lenzi et al 201168 | (1) Self-reported data on self-efficacy, use of BBN and communication skills, knowledge on communication skills as well as attitudes via not specified questionnaires; (2) Sign. improvement in 14 of 15 items on used BBN-skills, most of the communication skills items, knowledge questions, attitudes and self-efficacy. |
| Goelz et al 201157 | (1) External rating of 3 domains of communication behaviour in videotaped SP encounters via a rating system developed for this purpose (COM-ON-Checklist (communication in oncology-transition); (2) Sign. intervention effect on all domains: transition to palliative care, global communication skills and involvement of sign. others. |
| Bylund et al 201065 | (1) External rating of 6 communication domains in videotaped real patient encounters via the self-developed Comskil Coding System; (2) Sign. improvement in two communication domains (‘establishing the consultation framework’, ‘checking skills’) and in five individual items; mediated by amount of modules participated in. |
| Brown et al 201052 | (1) Self-reported confidence about discussing prognosis via two self-developed items; (2) Sign. improvement in both items. |
| Annadurai et al 202150 | (1) External rating of 7 core communication skills via an assessment tool based on SPIKES71 and NURSE72 statements plus some additional skills in pre and post audio recordings of real clinical encounters; (2) Sign. intervention effect on eliciting patient values, no increase in overall and other communication skills. |
| Bickell et al 202049 | (1) Perception and quality of Goals-of-Care (GoC) discussions rated by patients via two self-developed items, external rating of 7 core communication skills via an assessment tool based on SPIKES71 and NURSE72 statements (detailed description by Annadurai et al50) and some additional skills in pre and post audio recordings of real clinical encounters; (2) Sign. intervention effect on eliciting patient values, prevalence/quality of GoC communication n.s., overall and other communication skills n.s. |
| Hulsman et al 200267 | (1) External rating of 7 domains of communication behaviour in videotaped real patient encounters via the self-developed Communication Rating System, self-reported patients’ satisfaction via the Medical Interview Satisfaction Scale;89 (2) Sign. intervention effect on observed general communication behaviour only in the group identified as ‘implementers’ (no change in non-implementers); no change in patients’ satisfaction. |
| Yakhforoshha et al 201869 | (1) External rating of 7 domains of BBN performance during SP encounters in real outpatient setting via the modified BBN-checklist90 (Iranian version of the SPIKES-protocol;71 (2) Sign. level changes in three domains of BBN checklist: strategy, knowledge and invitation; longitudinal effects n.s. |
| Back et al 200764 | (1) External rating of quality of BBN (based on SPIKES model,71 quality of discussing transition to palliative care (based on self-developed 6-step-approach) and empathy (five skills based on NURSE model72 in 2 pre-SP and two post-SP encounters; (2) Sign. improvement in 4 SPIKES-steps regarding BBN, 4 steps regarding the transition to palliative care and 4-5 empathic skills |
| Epstein et al 201755 | (1) External rating of 4 communication domains in audio recorded real physician visits via a self-developed instrument (a combination of scales from different existing instruments), self-reported patient-physician relationship, healthcare climate and perceived efficacy in patient-physician interactions by patients and physicians via standardised questionnaires; (2) Sign. intervention effect on three domains: ‘engaging patients in discussions’, ‘responding to emotions’ and ‘discussions of prognosis and treatment choices’, self-reported outcomes n. s. |
| Delvaux et al 200548 | (1) External rating of form, function and emotional level of each utterance in transcripts of simulated and real audiotaped three-person-interviews (with patient and relative) via the adapted Cancer Research Campaign Workshop Evaluation Manual with a new scale to identify the addressee of utterances, self-reported retrospective perception of the interview by patient, relative and physician via the Perception of the Interview Questionnaire (unpublished dissertation); (2) Sign. intervention effect on 2 of 16 communication skills (‘openness toward patient’s and relative’s concerns and needs’ and ‘open assessment skills’; changes toward relatives more modest in actual than in simulated interviews), difference in the number of utterance-addressees n. s., sign. intervention effect on patients’ (but not in relatives’) perception of the physician’s performance. |
| Razavi et al 200347 | (1) External rating of form, function and emotional level of each utterance in transcripts of simulated and real audiotaped patient encounters via the adapted Cancer Research Campaign Workshop Evaluation Manual, retrospective perception of the interview via the Perception of the Interview Questionnaire (unpublished dissertation); (2) Basic training effect mainly observable in simulated interviews; consolidation workshops: sign. intervention effect on 3 of 22 communication skills in simulated interviews (‘open and open directive questions’, ‘utterances alerting patients to reality’, decrease in ‘premature reassurance’) and in 4 of 22 skills in actual interviews (‘acknowledgments’, ‘empathic statements’, ‘educated guesses’, ‘negotiations’); patients view: physicians’ of IG showed significantly better understanding of disease. |
| Tulsky et al 201162 | (1) External rating of number of empathic statements in audiotaped real clinic visits via NURSE statement72 and responses to empathic opportunities via a model by Suchman et al,91 postmeasurement of patients’ trust and perceptions of their oncologist; (2) IG shows significantly more empathic statements and better responding to empathic opportunities; greater trust of patients whose oncologists were in IG. |
| Malhotra et al 201961 | (1) External rating of the number of negative emotion expressions via the model of empathic communication by Suchmann et al91 and number of empathic responses via self-developed items in pre and post transcripts of real patient encounters, proportion of consultations discussing prognosis and goals of care; (2) Sign. more empathic responses and more discussions about prognosis in IG. |
| Henselmans et al 201959 | (1) External rating of shared desicion making (SDM) in videotaped SP encounters via the Observing Patient Involvement Sclae 12,84 external rating of SDM per stage via a self-developed instrument, external rating of 2 communication skills via self-developed items, self-reported oncologists’ satisfaction with communication via oncologist-version of the 5-item Patient Satisfaction Questionnaire; (2) Sign. intervention effect on amount of SDM, improvement in all SDM stages and improvement in both communication skills (‘responsiveness to emotions’ and ‘information provision skills’), no effect on satisfaction with the consultation. |
CG, control group; IG, intervention group; n.s., not significant; OSCE, objective structured clinical exams; sign., significant; SP, simulated patients.
With regard to the applied measurement instruments and outcome parameters, the publications varied widely. The most frequently assessed outcome parameter was interpersonal empathy or responsiveness to emotions, which was assessed with at least one scale in 21 articles (88 %).47–51 53–62 64–67 69 70 In four papers, the authors rated empathy based on the NURSE-statement,72 a commonly used approach to measure empathic expressions (‘Naming’, ‘Understanding’, ‘Respecting’, ‘Supporting’ and ‘Exploring’).49 50 62 64 Over one-third of the articles reported an external rating based on the steps of the SPIKES protocol49 50 64 66 69 or other protocols on breaking bad news.56 58 60 69 In five publications, the authors used self-developed coding systems on specific communication skills such as ‘transition to PC’57 or ‘informing about prognosis’.54 55 57 65 67 The self-assessment data mostly referred to constructs like perceived confidence or comfort level,51–53 56 66 self-efficacy in communicating the respective topic63 68 or a retrospective evaluation of a patient encounter.47 48 59
Taken together, in less than half of the publications (n=10), the authors made use of existing, valid and reliable measurement instruments,47–50 58 59 63 67 69 70 which were then in most cases combined with further self-developed items or questionnaires.47–50 58 59 67 In eight papers, the authors created a study-specific rating system and reported acceptable inter-rater reliabilities54 56 57 64 65 67 or other reliability data.55 66
In seven publications, also outcome parameters non-related to communication were assessed.47 49 51 54–56 61 Those were among others patient-related outcomes such as distress, anxiety or quality of life or physician-related outcomes such as stress or burnout levels. Bickell et al also assessed the utilisation of aggressive care at the EoL.49
Intervention effects
The reported effects of the interventions are displayed in table 3. With regard to the externally assessed outcome measurements, 11 of the 24 articles reported a significant improvement in externally rated empathy or responsiveness to emotions.47 55 56 58–62 64 66 70 Eight articles, on the other hand, reported no significant improvement in this regard.48–50 54 57 65 67 69 Almost half of the publications (n=11) reported a significant improvement in global communication skills (such as question type, assessment skills or engaging patients in consultations).47 48 55 57–60 65–67 69 The externally rated quality of the breaking bad news process according to different protocols improved in five of eight publications in at least some steps.56 58 64 66 69
Also, some papers reported significant effects on specific communication skills related to the PC/EoL issues that the intervention addresses. Two publications on the same intervention, for example, reported a positive impact of their goals-of-care intervention on eliciting patient values.49 50 Goelz et al found a significant positive effect of their intervention targeting the transition to PC on communicating the transition to PC and involving significant others.57 Two articles reported an increase in the discussion of prognosis after the intervention,55 61 one of which also reported an increase in discussing treatment choices.55 Henselmans et al reported a positive impact of their intervention targeting shared decision making about palliative chemotherapy on the emergence and the quality of shared decision making within the consultations.59
With regard to self-reported outcome measures, significant improvements were reported on empathy,51 53 69 confidence/comfort level in communication51 53 56 66 as well as the perceived self-efficacy.63 One publication reported only trends but no significant effects of their intervention.54
The effects on the outcome parameters non-related to communication can only be described exemplarily within this review, as we focus on the communication-related outcomes. Here it is worth mentioning that in the articles reporting physician-related outcomes, the interventions did not succeed in reducing burnout or stress level51 54 or aggressive care at the EoL.49
Risk of bias
The quality of the studies reported in the 24 publications was assessed using the EPHPP Quality Assessment Tool.38 39 Table 4 provides an overview of the ratings according to the seven categories as well as the global ratings. A great majority of the articles achieved the final grade moderate (n=20) and the remaining four articles achieved the final grade weak. The category with the most frequent weak ratings was the selection bias, which is determined by the representativeness and the participation rate of the reported study. With the exception of one strong rating,70 all articles were rated weak in this regard. In most of those papers, it was the small sample size that was crucial to the poor representability. More than half of the publications received moderate or weak ratings regarding the applied measurement instruments. In eight articles, this was due to unstandardised, self-developed questionnaires or rating systems without evidence on reliability and/or validity. In other publications, the authors used existing instruments or items with insufficient information on quality criteria (n=5). None of the papers was rated weak in the category study design, as all of the reported studies were either RCTs (n=11; receiving the rating strong) or cohort studies/interrupted time series (n=11; receiving the rating medium).
Table 4.
Methodological quality of the included publications (N=24) via the effective public health practice project quality assessment tool
| Publication | Selection bias | Design | Confounders* | Blinding* | Data collection methods | Withdrawals and drop-outs† | Global rating |
| Andrade et al 201063 | WEAK | MODERATE | N/A | N/A | STRONG | N/A‡ | MODERATE |
| Annadurai et al 202150 | WEAK | STRONG | STRONG | MODERATE | STRONG | STRONG | MODERATE |
| Back et al 200764 | WEAK | MODERATE | N/A | N/A | STRONG | STRONG | MODERATE |
| Baile et al 199953 | WEAK | MODERATE | N/A | N/A | WEAK | STRONG | WEAK |
| Bickell et al 202049 | WEAK | STRONG | STRONG | MODERATE | STRONG | STRONG | MODERATE |
| Brown et al 201052 | WEAK | MODERATE | N/A | N/A | WEAK | N/A‡ | WEAK |
| Butow et al 200854 | WEAK | STRONG | STRONG | WEAK | MODERATE | STRONG | MODERATE |
| Bylund et al 200965 | WEAK | MODERATE | N/A | N/A | STRONG | MODERATE | MODERATE |
| Cannone et al 201966 | WEAK | MODERATE | N/A | N/A | MODERATE | STRONG | MODERATE |
| Delvaux et al 200548 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Epstein et al 201755 | WEAK | STRONG | STRONG | MODERATE | STRONG | STRONG | MODERATE |
| Fujimori et al 200351 | WEAK | MODERATE | N/A | N/A | WEAK | STRONG | MODERATE |
| Fujimori et al 201456 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Goelz et al 201157 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Gorniewicz et al 201758 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Henselmans et al 201959 | WEAK | STRONG | STRONG | MODERATE | STRONG | STRONG | MODERATE |
| Hulsman et al 200267 | WEAK | MODERATE | N/A | N/A | STRONG | MODERATE | MODERATE |
| Lenzi et al 201168 | WEAK | MODERATE | N/A | N/A | WEAK | STRONG | WEAK |
| Liénard et al 201060 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Malhotra et al 201961 | WEAK | STRONG | STRONG | MODERATE | WEAK | STRONG | WEAK |
| Razavi et al 200347 | WEAK | STRONG | STRONG | MODERATE | MODERATE | STRONG | MODERATE |
| Tulsky et al 201162 | WEAK | STRONG | STRONG | MODERATE | STRONG | STRONG | MODERATE |
| Yakhforoshha et al 201869 | WEAK | MODERATE | N/A | N/A | STRONG | STRONG | MODERATE |
| Yamada et al 201870 | STRONG | MODERATE | N/A | N/A | STRONG | MODERATE | MODERATE |
*For studies with only one group confounders and blinding was set N/A.
†For studies with only one measurement time point withdrawals and drop-outs was set N/A.
‡No drop-outs possible, as the second measurement point was at the end of the intervention and at the same day as the first measurement point.
N/A, not available; RCT, randomised controlled trial.
The most frequent strong ratings (n=19) were given for withdrawals and drop-outs, a category determined by the follow-up rate. For two articles this rating was set ‘N/A’, as in their reported study second measurement time point was conducted directly after the intervention on the same day as the first measurement time point, so that a dropout in this case was unlikely.
Discussion
To our knowledge, this is the first systematic review of evaluated communication interventions on PC/EoL issues for physicians caring for cancer patients. We identified the relevant publications and conducted a narrative synthesis with regard to publication characteristics, setting and didactics of the interventions, the addressed PC/EoL issues, the communication-related outcome measurements as well as the reported effectiveness. Further, the methodological quality of the studies was systematically assessed. We focused on interventions explicitly designed for physicians, as there is profound evidence that a timely and adequate communication of PC/EoL issues via the primary caring (organ-specific) oncologist is of great importance.2–7 13 16 17
We identified 24 publications evaluating 22 different communication interventions. The results revealed a great variety, but also similarities between the publications regarding the reported interventions and the evaluation methods. We found that in a majority of the articles the target group were oncologists, while only one-third of the studies included both oncologists and organ-specific specialists. We consider this to be too few, as the primary caring physicians of cancer patients in many cases are not specialised oncologists but physicians with other specialisations. Goulart et al, for example, found that in 55% of 28.977 studied lung cancer patients in the USA the primary caring physician was specialised in internal or family medicine.73
The most frequently addressed PC/EoL issue in communication interventions is breaking bad news. As guidelines demand for the use of predefined, published frameworks when discussing serious news,37 the high number of existing interventions teaching those is a positive result. Further, it is favourable that dealing with patients’ emotions has also been addressed in several interventions, as dealing with emotions represents an important communication deficiency of physicians and at the same time is considered to be one of the most central components of communication in PC.1 37 Other crucial PC/EoL communication issues, such as goals of care discussions, eliciting values, involving family caregivers or preparing for the future (including talking about death and dying)1 have received little attention so far. Notably, none of the evaluated interventions for oncologists addressed the topic of discussing advanced directives. Since important communication guidelines in oncology, such as the consensus guideline from the American Society of Clinical Oncology, strongly recommend to timely discuss those,37 this lack of training is a clinically relevant finding. Further, a majority of the interventions focused on just one specific topic, while only a few covered a wider range of communication issues in the field of PC. Moreover, the intervention setting as well as the length of the interventions differed considerably. A majority of the interventions was time intensive and lasted more than 1 day and one-third of the studies the training included follow-up sessions or individual coaching for consolidation. There is no consistent evidence on the optimal length of communication interventions in oncology.27 Nonetheless, Moore et al demand to take into account the high time pressure in healthcare professionals and therefore to conduct communication skills trainings in less on-site time.27
Despite large differences with regard to the setting, the didactics and learning activities used within the interventions were similar. A very commonly used technique were role-plays, often performed with simulated patients. This is in concordance with guidelines and strong empirical evidence confirming the effectiveness of role-plays as a teaching strategy in communication skills trainings.74–76 Another commonly applied teaching method was to present example videos of ideal communication behaviour.
With regard to the outcome measurements, 75% of the publications did not only rely on self-reported data, but also used externally assessed, objective outcome measurements for evaluation. This is commendable, since using different sources of data is an important quality criterion of evaluating complex interventions.77 The objective data were usually assessed by external ratings of the physicians’ communication behaviour in videotaped or audiotaped clinical conversations either with simulated or real patients. The most frequently rated parameter was intrapersonal empathy, which seems reasonable, as this is important for all communication issues due to the high level of emotionality in these consultations.1
All but one article reported significant positive effects of the intervention on at least one outcome parameter, which indicates that the reviewed interventions in general seem to be effective, even though the areas of improvement differ. While almost half of the papers reported a positive impact of the intervention on the competence of empathy, eight of them reported no effect in this regard. A possible explanation for these contradictory results is the great variance in the measurement instruments and the lack of standardised instruments reported within the publications. Several articles reported further improvements in general communication behaviour not directly connected to the PC/EoL issue, such as asking open questions or establishing a framework for the consultation. With regard to more specific aspects of communication, the most frequently reported effects referred to the application of a stepwise approach to deliver bad news, such as the SPIKES protocol.71 This might be due to the high predefined structure of those protocols and thus an easier operationalisation of the outcome measurements. Numerous further specific changes in subscales were reported. Overall, outcomes assessed by self-reported data improved in almost all of the publications, whereas the effects of externally assessed outcomes were not that unequivocal. In most cases, the improvement was only observed in some of the communication domains or subscales. In about one-third of the articles, less than half of the assessed outcomes revealed a significant change. It can be concluded that the interventions may more easily have a positive impact on self-reported outcomes such as confidence or self-efficacy than on externally rated communication behaviour. This finding is in concordance with other systematic reviews.32 78 However, previous findings indicate that self-assessed confidence data are no reliable indicator for competence.79 Tulsky et al reported a large gap between the self-assessed confidence and the real ability in EoL discussions.80 81 Hence, self-report questionnaires seem to be a limited outcome measurement.
The methodological quality of the publications was overall moderate. A majority of the included articles reported RCTs, which reflects a high quality and represents the most robust method to evaluate interventions.77 Due to mostly very small sample sizes and low response rates, a frequent methodological weakness was the representability. This indicates a selection bias, probably in favour of physicians that are already more interested in communication. Another frequent methodological limitation was the quality of the assessment tools. Instead of standardised and validated instruments, many publications reported the use of self-developed items or rating instruments. These findings are consistent with previous reviews claiming methodological weaknesses of the studies and demanding more RCTs and more valid and reliable instruments when evaluating communication interventions in the setting of EoL care.27 29–32 78
Implications
Characteristics and design of future interventions
This systematic review reveals several implications for future communication interventions on PC/EoL issues for physicians. Content wise, those should focus on other aspects than breaking bad news, such as discussing goals of care, preparing for the future or introducing advance directives, because breaking bad news is already sufficiently covered by existing interventions. In addition, interventions need to focus more on strengthening physicians’ awareness for the adequate time to communicate the topics. In order to address all primary caring physicians of cancer patients, the target group should be oncologists as well as physicians with other specialisations caring for patients with cancer. Due to the time pressure physicians face, there as well is a need of shorter interventions that are easier to integrate into daily work. Those might include booster sessions for consolidation, as proposed by guidelines on effective communication training strategies in oncology.76 Also, Razavi et al and Delvaux et al found consolidation workshops to be effective47 48 and Niglio de Figueiredo et al indicate that a higher amount of individual coaching sessions after a communication workshop positively affects the effectiveness of the intervention.82
Characteristics of future studies
Future studies to evaluate the interventions should be designed as methodologically high-quality RCTs. Authors should make use of different sources of data to assess outcomes, but set their focus on the external rating data. Rating data should be assessed via valid, reliable and standardised rating instruments, such as the Common Ground Assessment Summary form83 for general communication skills or the Observing Patient Involvement scale 1284 for shared decision making. BBN should be rated via standardised protocols on breaking bad news like SPIKES71 or SHARE.85 However, since the observed communication challenges are often very specific, where appropriate, additional study-specific rating systems should be created. Ideally, those should incorporate or adapt existing rating scales or items, like the developed systems of Fujimori et al56 or Back et al64 do. To enhance validity, the study-specific rating systems should be built on an extensive literature research and the development should be presented transparently. An acceptable interrater-reliability can be established through double ratings. Additional self-report questionnaires should be valid, reliable and standardised, such as the self-efficacy Affective Competency Score86 to assess changes in self-efficacy through the intervention.
Strengths and limitations
Our systematic review has strengths and some limitations. By publishing a review protocol in advance, we provided transparency of the review process. Also, we conducted and reported our review based on the PRISMA guidelines. To increase objectivity, two independent raters carried out the assessment of full texts for eligibility, the data extraction as well as the quality assessments of the included papers.
Since the methodology of the included publications was too heterogeneous, an important limitation is that we could not conduct a quantitative meta-analysis. Further, we conducted our search in four relevant databases and added a few articles via hand searches in reference lists. Thus, it is possible that we might have missed some publications that were not covered by these databases and which we did not find by additional searches. As we restricted our search to articles published in English or German, we might as well have missed studies published in other languages. Besides, a publication bias in favour of significant results is possible. Lastly, by excluding publications with no communication-related main outcome, it might be that some relevant evaluated interventions in this field were not included. However, setting this focus enabled a more detailed view on how existing interventions actually affect the physicians’ communication behaviour. Future systematic reviews should separately report communication-related and non-communication-related outcome measurements.
Conclusion
This systematic review provides a detailed overview of existing communication interventions on PC/EoL issues for physicians working in oncology. We found several interventions that seem to effectively improve physicians’ communication behaviour. Our results are an important resource for researchers and clinicians planning to develop and evaluate further interventions in this area. An important future focus should be to develop feasible interventions on other PC/EoL issues than breaking bad news, such as goals-of-care discussions or preparing for the future. Due to the benefits of an early communication of PC/EoL issues in oncological care, interventions should also emphasise the adequate timing of communicating these aspects. Target group should be oncologists and organ-specific specialists, as all primary caring physicians are responsible for earlier communication and the latter have not been sufficiently considered in existing interventions. It is important that the effectiveness of the interventions is empirically evaluated within high-quality RCTs using validated instruments and different sources of data.
Supplementary Material
Footnotes
Contributors: NH is the guarantor. NH, KO and CB conceptualised and designed the review. NH and HMR extracted the data and assessed the quality of the articles. NH interpreted and analysed the data in consultation with HMR and under supervision of CB. NH drafted the manuscript, which was modified and supplemented by all HMR, KO and CB. All authors were involved in revising the manuscript substantively, and read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No additional data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Back AL. Patient-clinician communication issues in palliative care for patients with advanced cancer. J Clin Oncol 2020;38:866–76. 10.1200/JCO.19.00128 [DOI] [PubMed] [Google Scholar]
- 2.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the enable III randomized controlled trial. J Clin Oncol 2015;33:1438–45. 10.1200/JCO.2014.58.6362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721–30. 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 4.Rugno FC, Paiva BSR, Paiva CE. Early integration of palliative care facilitates the discontinuation of anticancer treatment in women with advanced breast or gynecologic cancers. Gynecol Oncol 2014;135:249–54. 10.1016/j.ygyno.2014.08.030 [DOI] [PubMed] [Google Scholar]
- 5.Vanbutsele G, Pardon K, Van Belle S, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol 2018;19:394–404. 10.1016/S1470-2045(18)30060-3 [DOI] [PubMed] [Google Scholar]
- 6.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and Gi cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41. 10.1200/JCO.2016.70.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–73. 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284:2476–82. 10.1001/jama.284.19.2476 [DOI] [PubMed] [Google Scholar]
- 9.Nyborn JA, Olcese M, Nickerson T, et al. Don’t try to cover the sky with your hands: parents’ experiences with prognosis communication about their children with advanced cancer. J Palliat Med 2016;19:626–31. 10.1089/jpm.2015.0472 [DOI] [PubMed] [Google Scholar]
- 10.Brighton LJ, Bristowe K. Communication in palliative care: talking about the end of life, before the end of life. Postgrad Med J 2016;92:466–70. 10.1136/postgradmedj-2015-133368 [DOI] [PubMed] [Google Scholar]
- 11.Hagerty RG, Butow PN, Ellis PA, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 2004;22:1721–30. 10.1200/JCO.2004.04.095 [DOI] [PubMed] [Google Scholar]
- 12.Collins A, McLachlan S-A, Philip J. Communication about palliative care: a phenomenological study exploring patient views and responses to its discussion. Palliat Med 2018;32:133–42. 10.1177/0269216317735247 [DOI] [PubMed] [Google Scholar]
- 13.Clayton JM, Hancock KM, Butow PN, et al. Clinical practice guidelines for communicating prognosis and end-of-life issues with adults in the advanced stages of a life-limiting illness, and their caregivers. Med J Aust 2007;186:S77–105. 10.5694/j.1326-5377.2007.tb01100.x [DOI] [PubMed] [Google Scholar]
- 14.Mayland CR, Ho QM, Doughty HC, et al. The palliative care needs and experiences of people with advanced head and neck cancer: a scoping review. Palliat Med 2021;35:27–44. 10.1177/0269216320963892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: a cohort study. Ann Intern Med 2012;156:204–10. 10.7326/0003-4819-156-3-201202070-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc 2017;92:280–6. 10.1016/j.mayocp.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of clinical oncology clinical practice guideline update. J Clin Oncol 2017;35:96–112. 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 18.Granek L, Nakash O, Cohen M, et al. Oncologists' communication about end of life: the relationship among secondary traumatic stress, compassion satisfaction, and approach and avoidance communication. Psychooncology 2017;26:1980–6. 10.1002/pon.4289 [DOI] [PubMed] [Google Scholar]
- 19.Almack K, Cox K, Moghaddam N, et al. After you: conversations between patients and healthcare professionals in planning for end of life care. BMC Palliat Care 2012;11:15. 10.1186/1472-684X-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baile WF, Lenzi R, Parker PA, et al. Oncologists’ attitudes toward and practices in giving bad news: an exploratory study. J Clin Oncol 2002;20:2189–96. 10.1200/JCO.2002.08.004 [DOI] [PubMed] [Google Scholar]
- 21.Granek L, Krzyzanowska MK, Tozer R, et al. Oncologists’ strategies and barriers to effective communication about the end of life. J Oncol Pract 2013;9:e129–35. 10.1200/JOP.2012.000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi WI, Smith TJ. Early integration of palliative care into oncology: evidence, challenges and barriers. Ann Palliat Med 2015;4:122–31. 10.3978/j.issn.2224-5820.2015.07.03 [DOI] [PubMed] [Google Scholar]
- 23.Cripe L, Frankel RM. Understanding what influences oncology clinicians’ communicating with dying patients: Awareness of one’s own mortality may be one key. Patient Educ Couns 2016;99:307–9. 10.1016/j.pec.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 24.Draper EJ, Hillen MA, Moors M, et al. Relationship between physicians’ death anxiety and medical communication and decision-making: a systematic review. Patient Educ Couns 2019;102:266–74. 10.1016/j.pec.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 25.Kuusisto A, Santavirta J, Saranto K, et al. Advance care planning for patients with cancer in palliative care: a scoping review from a professional perspective. J Clin Nurs 2020;29:2069–82. 10.1111/jocn.15216 [DOI] [PubMed] [Google Scholar]
- 26.Buss MK, Lessen DS, Sullivan AM, et al. Hematology/oncology fellows' training in palliative care: results of a national survey. Cancer 2011;117:4304–11. 10.1002/cncr.25952 [DOI] [PubMed] [Google Scholar]
- 27.Moore PM, Rivera S, Bravo-Soto GA, et al. Communication skills training for healthcare professionals working with people who have cancer. Cochrane Database Syst Rev 2018;7:CD003751. 10.1002/14651858.CD003751.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer F, Helmer S, Rogge A, et al. Outcomes and outcome measures used in evaluation of communication training in oncology - a systematic literature review, an expert workshop, and recommendations for future research. BMC Cancer 2019;19:808. 10.1186/s12885-019-6022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman LE, Brighton LJ, Hawkins A, et al. The effect of communication skills training for generalist palliative care providers on patient-reported outcomes and clinician behaviors: a systematic review and meta-analysis. J Pain Symptom Manage 2017;54:404–16. 10.1016/j.jpainsymman.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 30.Brighton LJ, Koffman J, Hawkins A, et al. A systematic review of end-of-life care communication skills training for generalist palliative care providers: research quality and reporting guidance. J Pain Symptom Manage 2017;54:417–25. 10.1016/j.jpainsymman.2017.04.008 [DOI] [PubMed] [Google Scholar]
- 31.Walczak A, Butow PN, Bu S, et al. A systematic review of evidence for end-of-life communication interventions: who do they target, how are they structured and do they work? Patient Educ Couns 2016;99:3–16. 10.1016/j.pec.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 32.Chung H-O, Oczkowski SJW, Hanvey L, et al. Educational interventions to train healthcare professionals in end-of-life communication: a systematic review and meta-analysis. BMC Med Educ 2016;16:131. 10.1186/s12909-016-0653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakke KE, Miranda SP, Castillo-Angeles M, et al. Training surgeons and anesthesiologists to facilitate end-of-life conversations with patients and families: a systematic review of existing educational models. J Surg Educ 2018;75:702–21. 10.1016/j.jsurg.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 36.Richardson WS, Wilson MC, Nishikawa J, et al. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12–13. [PubMed] [Google Scholar]
- 37.Gilligan T, Coyle N, Frankel RM, et al. Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol 2017;35:3618–32. 10.1200/JCO.2017.75.2311 [DOI] [PubMed] [Google Scholar]
- 38.Armijo-Olivo S, Stiles CR, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract 2012;18:12–18. 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 39.Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004;1:176–84. 10.1111/j.1524-475X.2004.04006.x [DOI] [PubMed] [Google Scholar]
- 40.Dixon-Woods M, Agarwal S, Jones D, et al. Synthesising qualitative and quantitative evidence: a review of possible methods. J Health Serv Res Policy 2005;10:45–53. 10.1177/135581960501000110 [DOI] [PubMed] [Google Scholar]
- 41.Cochrane consumers and communication review group: data synthesis and analysis, 2016. Available: https://community.cochrane.org/ [Accessed Sep 2021].
- 42.Fallowfield L, Lipkin M, Hall A. Teaching senior oncologists communication skills: results from phase I of a comprehensive longitudinal program in the United Kingdom. J Clin Oncol 1998;16:1961–8. 10.1200/JCO.1998.16.5.1961 [DOI] [PubMed] [Google Scholar]
- 43.Fallowfield L, Jenkins V, Farewell V, et al. Efficacy of a cancer research UK communication skills training model for oncologists: a randomised controlled trial. Lancet 2002;359:650–6. 10.1016/S0140-6736(02)07810-8 [DOI] [PubMed] [Google Scholar]
- 44.Jenkins V, Fallowfield L. Can communication skills training alter physicians' beliefs and behavior in clinics? J Clin Oncol 2002;20:765–9. 10.1200/JCO.2002.20.3.765 [DOI] [PubMed] [Google Scholar]
- 45.Shilling V, Jenkins V, Fallowfield L. Factors affecting patient and clinician satisfaction with the clinical consultation: can communication skills training for clinicians improve satisfaction? Psychooncology 2003;12:599–611. 10.1002/pon.731 [DOI] [PubMed] [Google Scholar]
- 46.Stewart M, Brown JB, Hammerton J, et al. Improving communication between doctors and breast cancer patients. Ann Fam Med 2007;5:387–94. 10.1370/afm.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razavi D, Merckaert I, Marchal S, et al. How to optimize physicians' communication skills in cancer care: results of a randomized study assessing the usefulness of posttraining consolidation workshops. J Clin Oncol 2003;21:3141–9. 10.1200/JCO.2003.08.031 [DOI] [PubMed] [Google Scholar]
- 48.Delvaux N, Merckaert I, Marchal S, et al. Physicians' communication with a cancer patient and a relative: a randomized study assessing the efficacy of consolidation workshops. Cancer 2005;103:2397–411. 10.1002/cncr.21093 [DOI] [PubMed] [Google Scholar]
- 49.Bickell NA, Back AL, Adelson K, et al. Effects of a communication intervention randomized controlled trial to enable goals-of-care discussions. JCO Oncol Pract 2020;16:OP2000040:e1015–28. 10.1200/OP.20.00040 [DOI] [PubMed] [Google Scholar]
- 50.Annadurai V, Smith CB, Bickell N, et al. Impact of a novel goals-of-care communication skills coaching intervention for practicing oncologists. J Palliat Med 2021;24:838–45. 10.1089/jpm.2020.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujimori M, Oba A, Koike M, et al. Communication skills training for Japanese oncologists on how to break bad news. J Cancer Educ 2003;18:194–201. 10.1207/s15430154jce1804_6 [DOI] [PubMed] [Google Scholar]
- 52.Brown R, Bylund CL, Eddington J, et al. Discussing prognosis in an oncology setting: initial evaluation of a communication skills training module. Psychooncology 2010;19:408–14. 10.1002/pon.1580 [DOI] [PubMed] [Google Scholar]
- 53.Baile WF, Kudelka AP, Beale EA, et al. Communication skills training in oncology. description and preliminary outcomes of workshops on breaking bad news and managing patient reactions to illness. Cancer 1999;86:887–97. [PubMed] [Google Scholar]
- 54.Butow P, Cockburn J, Girgis A, et al. Increasing oncologists' skills in eliciting and responding to emotional cues: evaluation of a communication skills training program. Psychooncology 2008;17:209–18. 10.1002/pon.1217 [DOI] [PubMed] [Google Scholar]
- 55.Epstein RM, Duberstein PR, Fenton JJ, et al. Effect of a patient-centered communication intervention on Oncologist-Patient communication, quality of life, and health care utilization in advanced cancer: the voice randomized clinical trial. JAMA Oncol 2017;3:92–100. 10.1001/jamaoncol.2016.4373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimori M, Shirai Y, Asai M, et al. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: a randomized controlled trial. J Clin Oncol 2014;32:2166–72. 10.1200/JCO.2013.51.2756 [DOI] [PubMed] [Google Scholar]
- 57.Goelz T, Wuensch A, Stubenrauch S, et al. Specific training program improves oncologists' palliative care communication skills in a randomized controlled trial. J Clin Oncol 2011;29:3402–7. 10.1200/JCO.2010.31.6372 [DOI] [PubMed] [Google Scholar]
- 58.Gorniewicz J, Floyd M, Krishnan K, et al. Breaking bad news to patients with cancer: a randomized control trial of a brief communication skills training module incorporating the stories and preferences of actual patients. Patient Educ Couns 2017;100:655–66. 10.1016/j.pec.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henselmans I, van Laarhoven HWM, de Haes HCJM, et al. Training for medical oncologists on shared decision-making about palliative chemotherapy: a randomized controlled trial. Oncologist 2019;24:259–65. 10.1634/theoncologist.2018-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liénard A, Merckaert I, Libert Y, et al. Is it possible to improve residents breaking bad news skills? A randomised study assessing the efficacy of a communication skills training program. Br J Cancer 2010;103:171–7. 10.1038/sj.bjc.6605749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malhotra C, Rajasekaran T, Kanesvaran R, et al. Pilot trial of a combined oncologist-patient-caregiver communication intervention in Singapore. JCO Oncol Pract 2020;16:e190–200. 10.1200/JOP.19.00412 [DOI] [PubMed] [Google Scholar]
- 62.Tulsky JA, Arnold RM, Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program: a randomized trial. Ann Intern Med 2011;155:593–U64. 10.7326/0003-4819-155-9-201111010-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrade AD, Bagri A, Zaw K, et al. Avatar-mediated training in the delivery of bad news in a virtual world. J Palliat Med 2010;13:1415–9. 10.1089/jpm.2010.0108 [DOI] [PubMed] [Google Scholar]
- 64.Back AL, Arnold RM, Baile WF, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Arch Intern Med 2007;167:453–60. 10.1001/archinte.167.5.453 [DOI] [PubMed] [Google Scholar]
- 65.Bylund CL, Brown R, Gueguen JA, et al. The implementation and assessment of a comprehensive communication skills training curriculum for oncologists. Psychooncology 2010;19:583–93. 10.1002/pon.1585 [DOI] [PubMed] [Google Scholar]
- 66.Cannone D, Atlas M, Fornari A. Delivering challenging news: an illness-trajectory communication curriculum for multispecialty oncology residents and fellows. Mededportal Publications;15:10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hulsman RL, Ros WJG, Winnubst JAM, et al. The effectiveness of a computer-assisted instruction programme on communication skills of medical specialists in oncology. Med Educ 2002;36:125–34. 10.1046/j.1365-2923.2002.01074.x [DOI] [PubMed] [Google Scholar]
- 68.Lenzi R, Baile WF, Costantini A, et al. Communication training in oncology: results of intensive communication workshops for Italian oncologists. Eur J Cancer Care 2011;20:196–203. 10.1111/j.1365-2354.2010.01189.x [DOI] [PubMed] [Google Scholar]
- 69.Yakhforoshha A, Emami SAH, Shahi F, et al. Effectiveness of integrating simulation with art-based teaching strategies on oncology fellows' performance regarding breaking bad news. J Cancer Educ 2019;34:463–71. 10.1007/s13187-018-1324-x [DOI] [PubMed] [Google Scholar]
- 70.Yamada Y, Fujimori M, Shirai Y, et al. Changes in physicians’ intrapersonal empathy after a communication skills training in Japan. Acad Med 2018;93:1821–6. 10.1097/ACM.0000000000002426 [DOI] [PubMed] [Google Scholar]
- 71.Baile WF, Buckman R, Lenzi R, et al. SPIKES-A six-step protocol for delivering bad news: application to the patient with cancer. Oncologist 2000;5:302–11. 10.1634/theoncologist.5-4-302 [DOI] [PubMed] [Google Scholar]
- 72.Back AL, Arnold RM, Baile WF, et al. Approaching difficult communication tasks in oncology. CA Cancer J Clin 2005;55:164–77. 10.3322/canjclin.55.3.164 [DOI] [PubMed] [Google Scholar]
- 73.Goulart BHL, Reyes CM, Fedorenko CR, et al. Referral and treatment patterns among patients with stages III and IV non-small-cell lung cancer. J Oncol Pract 2013;9:42–50. 10.1200/JOP.2012.000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berkhof M, van Rijssen HJ, Schellart AJM, et al. Effective training strategies for teaching communication skills to physicians: an overview of systematic reviews. Patient Educ Couns 2011;84:152–62. 10.1016/j.pec.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 75.Barrows HS. An overview of the uses of standardized patients for teaching and evaluating clinical skills. AAMC. Acad Med 1993;68:443–51. 10.1097/00001888-199306000-00002 [DOI] [PubMed] [Google Scholar]
- 76.Stiefel F, Barth J, Bensing J, et al. Communication skills training in oncology: a position paper based on a consensus meeting among European experts in 2009. Ann Oncol 2010;21:204–7. 10.1093/annonc/mdp564 [DOI] [PubMed] [Google Scholar]
- 77.Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 2012;66:1182–6. 10.1136/jech-2011-200375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lord L, Clark-Carter D, Grove A. The effectiveness of communication-skills training interventions in end-of-life noncancer care in acute hospital-based services: a systematic review. Palliat Support Care 2016;14:433–44. 10.1017/S1478951515001108 [DOI] [PubMed] [Google Scholar]
- 79.Norman G, Eva KW. Quantitative research methods in medical education. In: Swanwick T, ed. Understanding medical education. Oxford, UK: Wiley-Blackwell, 2010: 301–22. [Google Scholar]
- 80.Tulsky JA, Chesney MA, Lo B. How do medical residents discuss resuscitation with patients? J Gen Intern Med 1995;10:436–42. 10.1007/BF02599915 [DOI] [PubMed] [Google Scholar]
- 81.Tulsky JA, Chesney MA, Lo B. See one, do one, teach one? House staff experience discussing do-not-resuscitate orders. Arch Intern Med 1996;156:1285–9. 10.1001/archinte.156.12.1285 [DOI] [PubMed] [Google Scholar]
- 82.Niglio de Figueiredo M, Krippeit L, Ihorst G, et al. ComOn-Coaching: the effect of a varied number of coaching sessions on transfer into clinical practice following communication skills training in oncology: results of a randomized controlled trial. PLoS One 2018;13:e0205315. 10.1371/journal.pone.0205315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lang F, McCord R, Harvill L, et al. Communication assessment using the common ground instrument: psychometric properties. Fam Med 2004;36:189–98. [PubMed] [Google Scholar]
- 84.Elwyn G, Edwards A, Wensing M, et al. Shared decision making: developing the OPTION scale for measuring patient involvement. Qual Saf Health Care 2003;12:93–9. 10.1136/qhc.12.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujimori M, Parker PA, Akechi T, et al. Japanese cancer patients’ communication style preferences when receiving bad news. Psychooncology 2007;16:617–25. 10.1002/pon.1102 [DOI] [PubMed] [Google Scholar]
- 86.Quest TE, Ander DS, Ratcliff JJ. The validity and reliability of the affective competency score to evaluate death disclosure using standardized patients. J Palliat Med 2006;9:361–70. 10.1089/jpm.2006.9.361 [DOI] [PubMed] [Google Scholar]
- 87.Hojat M, Gonnella JS, Nasca TJ, et al. Physician empathy: definition, components, measurement, and relationship to gender and specialty. Am J Psychiatry 2002;159:1563–9. 10.1176/appi.ajp.159.9.1563 [DOI] [PubMed] [Google Scholar]
- 88.Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol 1983;44:113–26. 10.1037/0022-3514.44.1.113 [DOI] [Google Scholar]
- 89.Wolf MH, Putnam SM, James SA, et al. The medical interview satisfaction scale: development of a scale to measure patient perceptions of physician behavior. J Behav Med 1978;1:391–401. 10.1007/BF00846695 [DOI] [PubMed] [Google Scholar]
- 90.Farokhyar N, Shirazi M, Bahador H. Assessing the validity and reliability of spikes questionnaires regard in of medical residents awareness breaking bad news in TUMS 2012. Razi J Med Sci 2014;21:29–36. [Google Scholar]
- 91.Suchman AL, Markakis K, Beckman HB, et al. A model of empathic communication in the medical interview. JAMA 1997;277:678–82. 10.1001/jama.1997.03540320082047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059652supp001.pdf (51KB, pdf)
bmjopen-2021-059652supp002.pdf (43.8KB, pdf)
bmjopen-2021-059652supp003.pdf (86.5KB, pdf)
bmjopen-2021-059652supp004.pdf (212.9KB, pdf)
Data Availability Statement
No additional data are available.