Abstract
Objective
The objective was to evaluate the pharmacokinetics of compounding non-steroidal anti-inflammatory drugs (NSAIDs) meloxicam or flunixin meglumine with iron dextran (ID) in piglets.
Animal
Forty piglets (8 d of age) were randomly allocated into 5 groups (8 piglets/group) and received 1 intramuscular injection in the neck of the following treatments: flunixin meglumine (2.2 mg/kg) administered alone (F) or mixed with ID (F+ID); or meloxicam (0.4 mg/kg) administered alone (M) or mixed with ID (M+ID); or ID alone.
Procedure
Blood samples were collected via indwelling jugular catheters at pre-dose, and 10, 20, 30, 45, and 60 min, and 2, 4, 8, 12, 24, 36, 48, and 72 h post-treatment to determine plasma NSAIDs concentrations using liquid chromatography-tandem mass spectrometry. Pharmacokinetic parameters for plasma meloxicam and flunixin meglumine concentration-time profiles were determined for each piglet using noncompartmental analysis approaches. Statistical analyses were performed using SAS software with significance set at P < 0.05.
Results
The AUC0–tlast, AUC0–∞, Cmax, and relative bioavailability values in the M+ID and F+ID groups were lower than corresponding M and F groups. The M+ID group elimination half-life was lower, whereas λz and tmax values were greater than the corresponding M group.
Conclusion
Relative bioavailability of meloxicam and flunixin meglumine were reduced when compounded with ID in the same bottle and administered to piglets.
Clinical relevance
Further research is warranted to evaluate if decreased NSAID exposure when compounded with ID alters analgesic efficacy or drug residue depletion.
Résumé
Objectif
L’objectif était d’évaluer la pharmacocinétique de la combinaison d’anti-inflammatoires non stéroïdiens (NSAID) méloxicam ou flunixine méglumine avec du fer dextran (ID) chez les porcelets.
Animal
Quarante porcelets (âgés de 8 jours) ont été répartis au hasard en cinq groupes (8 porcelets/groupe) et ont reçu une injection intramusculaire dans le cou des traitements suivants : flunixine méglumine (2,2 mg/kg) administrée seule (F) ou mélangée avec ID (F+ID); soit du méloxicam (0,4 mg/kg) administré seul (M) ou en mélange avec ID (M+ID); ou du ID seul.
Procédure
Des échantillons de sang ont été prélevés via des cathéters jugulaires à demeure à la pré-dose, et 10, 20, 30, 45 et 60 min, et 2, 4, 8, 12, 24, 36, 48 et 72 h après le traitement pour déterminer la concentration plasmatique de NSAID par chromatographie liquide-spectrométrie de masse en tandem. Les paramètres pharmacocinétiques des profils concentration-temps du méloxicam et de la flunixine méglumine plasmatiques ont été déterminés pour chaque porcelet à l’aide d’approches d’analyse non compartimentale. Les analyses statistiques ont été effectuées à l’aide du logiciel SAS avec un seuil de signification fixé à P < 0,05.
Résultats
Les valeurs AUC0–tlast, AUC0–∞, Cmax et de biodisponibilité relative dans les groupes M+ID et F+ID étaient inférieures à celles des groupes M et F correspondants. La demi-vie d’élimination du groupe M+ID était plus faible, tandis que les valeurs λz et tmax étaient supérieures à celles du groupe M correspondant.
Conclusion
La biodisponibilité relative du méloxicam et de la méglumine de flunixine était réduite lorsqu’ils étaient combinés avec ID dans le même flacon et administrés aux porcelets.
Pertinence clinique
Des recherches supplémentaires sont nécessaires pour évaluer si une diminution de l’exposition aux NSAID lorsqu’elle est associée à une ID modifie l’efficacité analgésique ou l’épuisement des résidus de médicaments.
(Traduit par Dr Serge Messier)
Introduction
The Canadian Code of Practice for the Care and Handling of Pigs requires analgesics to be administered to piglets during processing, when tail docking and castration are to be performed (1). Currently, meloxicam, flunixin meglumine and ketoprofen are approved non-steroidal anti-inflammatory drugs (NSAIDS) for use in swine in Canada. Meloxicam, as Metacam for Swine at 5 mg/mL injectable is the only NSAID approved for use in piglets at the time of castration to control pain, with flunixin and ketoprofen being used extra label for pain control at castration. Although the benefits of providing analgesia to piglets peri-operatively or during routine processing is supported in terms of pain reduction, administration of an additional intramuscular (IM) treatment, such as an NSAID, around the time of processing can be stressful on animals and an added cost to production (2).
Neonatal pigs are routinely injected with iron dextran (ID), an injectable low-molecular-weight ferric hydroxide complex, to prevent iron deficiency anemia (2,3). To minimize animal handling and improve animal welfare, NSAIDs may be mixed with ID and the compounded product administered as a single injection at the time of processing as a convenient option. Although published studies reported that this practice could reduce post-castration pain in piglets (4,5), combining veterinary drugs for use in food-producing animals is considered a form of compounding and extra-label drug use (ELDU) (6). This is a concern due to the potential for drug-drug interactions that may impact efficacy, and pharmacokinetics including drug absorption from the injection site, and possibly clearance, that could result in violative drug residues in edible animal products.
Drug-drug interactions can occur as a result of, but not limited to, pharmacokinetic interactions and pharmaceutical incompatibility (7–9). Whether administration of a combination of injectable NSAIDs with ID to piglets impacts the pharmacokinetics of NSAIDs requires empirical evidence to support the practice. The objective of this study was to evaluate the pharmacokinetics of meloxicam and flunixin meglumine administered alone, and when mixed with ID, and administered by IM injection to piglets.
Materials and methods
Animals
Forty crossbred piglets (Landrace × Yorkshire × Duroc, 20 males and 20 females) from several litters, approximately 3 d old at arrival, were obtained from a commercial source in Ontario. All piglets were considered clinically healthy before the study, based on physical examination. Piglets were housed individually in the Department of Animal Biosciences’ animal facilities at the University of Guelph according to standard of care for swine, for the duration of the study. Piglets were fed a milk replacer, Supp-Le-Milk (Soppe Systems, Manchester, Iowa, USA), and were monitored daily for general health and any adverse events related to the trial throughout the study. Piglets were free of drugs upon arrival at the facility. At the time of test article administration, piglets were approximately 8 d of age with a mean weight of 2.9 kg (range: 2.2 to 3.4 kg). The experimental protocols for this study were approved by the Institutional Animal Care and Use Committee of the University of Guelph (Animal Use Protocol #3030) and conformed to standards set forth by the Canadian Council on Animal Care.
Jugular vein catheterization
Indwelling jugular catheters for blood sample collection were placed in each piglet under general anesthesia 3 d following arrival and acclimation to housing and individual feeding. A premix cocktail of ketamine hydrochloride, 50 mg/mL, xylazine hydrochloride, 10 mg/mL and butorphanol tartrate, 1 mg/mL was administered IM to all piglets at a dose rate of 0.2 mL/kg. The premix was prepared by the Ontario Veterinary College pharmacy (University of Guelph, Guelph, Ontario). General anesthesia was provided with 2.5% isoflurane in oxygen delivered via a face mask. Indwelling jugular catheters were aseptically placed, secured to the right side of the piglets’ neck and protected by bandaging. After instrumentation, piglets were allowed to recover from anesthesia with buprenorphine hydrochloride, 0.01 mg/kg, IM for postoperative analgesia. Catheters were regularly flushed with heparinized physiological saline (10 IU/mL) to maintain patency, and catheter sites were examined daily for any catheter-related complications (e.g., perivascular swelling). Following jugular catheter placement, a 2-day interval before test article administration enabled anesthetic drug washout, i.e., clearance of anesthetics used for catheter placement. No other drugs were administered throughout the study.
Experimental protocols
Piglets were randomly allocated into 1 of 5 treatment groups (n = 8 piglets/group) using a parallel study design, with each group balanced for sex: flunixin meglumine (Banamine Sterile Injectable Solution; Merck Animal Health, Kirkland, Quebec), 50 mg/mL administered alone (F) or mixed with ID (Ferroforte; Bimeda-MTX Animal Health, Cambridge, Ontario), 200 mg/mL (F+ID); meloxicam (Metacam 20 mg/mL solution for injection; Boehringer Ingelheim, Burlington, Ontario), 20 mg/mL administered alone (M) or mixed with ID (M+ID); and ID alone (ID). An ID alone group was included as a control to assess possible collateral effects of the drugs, and to demonstrate that ID in incurred plasma samples did not affect the NSAID assay. At the time of the study, meloxicam as Metacam for Swine at 5 mg/mL injectable was not yet approved nor available in Canada. Piglets were studied in 4 batches of 10 animals (2 piglets per treatment group, 1 male, 1 female) to accommodate sampling times and technical constraints.
Preparation of the compounded formulations of meloxicam for the M+ID group and flunixin meglumine for the F+ID group was based on a similar approach used in a previous pharmacokinetic study with ketoprofen in young piglets (9). To ensure equal dosing of the NSAIDs when administered alone and in compounded formulations, the latter were dosed according to body weight (mg/kg) for the NSAID component of the final compounded formulation. In the M and M+ID groups, the target dose of meloxicam was 0.4 mg/kg given IM in the neck. In the F and F+ID groups the target dose flunixin meglumine was 2.2 mg/kg also given IM in the neck. The compounded formulations containing NSAIDs were prepared fresh each day of dosing in sterile 100 mL injection vials, with 6.80 mL of 20 mg/mL meloxicam added to 93.20 mL of 200 mg/mL ID and 14.96 mL of 50 mg/mL flunixin meglumine added to 85.04 mL of 200 mg/mL ID. Both compounded formulations were mixed thoroughly by repeated gentle agitation before injection, resulting in a final theoretical concentration of 1.36 mg meloxicam and 186.4 mg of ID per mL of M+ID solution and 7.48 mg of flunixin meglumine and 170.08 mg of ID per mL of F+ID solution. The final theoretical concentrations of meloxicam and flunixin meglumine in their compounded formulations were based on a desired maximum delivery volume of 1.0 mL with a single injection to a 3.4 kg piglet using a tuberculin syringe and 23-gauge needle, which ensured dosing accuracy of meloxicam at 0.4 mg/kg and flunixin meglumine at 2.2 mg/kg for the range of piglet weights studied. As such, piglets in the M+ID group received ID proportional to body weight at 54.8 mg/kg, in the F+ID group piglets received ID at 50.0 mg/kg, whereas in the ID alone group piglets received ID at 58.8 mg/kg. All treatments were administered once by IM injection in the left side of the neck.
Whole-blood samples (1.5 mL) were collected into heparinized tubes at baseline (pre-dose) and 10, 20, 30, 45, and 60 min and 2, 4, 8, 12, 24, 36, 48, and 72 h post-treatment for the M, M+ID, F and F+ID groups, and in the ID group, were collected at baseline and 1 h post-dosing. Whole-blood samples were collected from jugular catheters after ensuring that heparinized saline flush in the catheter i.e., dead space, (0.4 mL) was drawn and discarded. Following collection of each blood sample, catheters were flushed with fresh heparinized saline. Collected blood samples were immediately placed on ice and subsequently centrifuged at 1400 × g for 20 min at 5°C. The plasma was separated, aliquoted into cryovials, and stored at −80°C until analyzed. Determination of flunixin meglumine and meloxicam concentrations was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). At the end of the study period, piglets were euthanized using pentobarbital sodium intravenously at 0.3 mL/kg.
Quantitation of meloxicam and flunixin meglumine using LC MS/MS
Reference standard of flunixin meglumine was purchased from United States Pharmacopeia (USP, Rockville, Maryland, USA). Meloxicam reference standard and deuterated internal standards (IS) of meloxicam-d3 and flunixin-d3 were purchased from Sigma Aldrich (Oakville, Ontario). Acetic acid, ultra LC/MS grade of acetonitrile and methanol were purchased from Caledon (Georgetown, Ontario). Stock solutions were prepared at 100 μg/mL in methanol and stored at −20°C.
To extract meloxicam and flunixin from piglet plasma, a simple protein precipitation was conducted by adding a 250 μL aliquot of the internal standard working solution to 0.5 mL plasma samples, then diluted with methanol to a final volume of 5 mL. The sample was mixed on a rotary shaker for 10 min, and then centrifuged at 1932 × g for 5 min. A volume of supernatant (2.5 mL) was evaporated under a stream of nitrogen at 40°C. The residue of fortified samples was reconstituted with 500 μL of 1 mM acetic acid in Nanopure water and acetonitrile (90:10, v/v). Calibration curves for 3 concentration ranges (2 to 400, 10 to 2000, and 20 to 4000 ng/mL) were prepared on the day of analysis by spiking working standard solutions into blank piglet plasma.
The LC MS/MS quantitation of flunixin and meloxicam was conducted by the Laboratory Services Division of the University Guelph (Guelph, Ontario). An Agilent 1100 series system (Mississauga, Ontario) was used for LC analysis. Separations were achieved on a Phenomenex Kinetex C18 column (5 μm, 2.1 × 50 mm, Torrance, California, USA) with the temperature maintained at 40°C. Mobile phase A consisted of 1 mM acetic acid in Nanopure water and acetonitrile (90:10, v/v), and mobile phase B was acetonitrile. The gradient conditions were set as follows: from 0 to 2 min ramp linearly from 1 to 80% of mobile phase B, then ramp again over 2 min to get back to 1% of mobile phase B. The flow rate was 0.5 mL/min and a sample volume of 10 μL was injected. Retention time was 2.41 min for flunixin and 2.45 min for meloxicam, with a total run time of 6 min.
Negative electrospray ionization (ESI) mass spectrometry analysis was operated in multiple reaction monitoring (MRM) mode using a SCIEX QTRAP 4000 mass spectrometer (SCIEX AB, Concord, Ontario). The instrument was equipped with a Turbo V source and the electrospray probe was set at 600°C. The source conditions were optimized as: ionspray voltage (IS) −4500 V, collision gas medium, Gas 1 50 psi and Gas 2 40 psi. Data were acquired and processed using Analyst 1.5.1 (SCIEX AB). For quantitation, MRM transitions were monitored at m/z 295.0→251.0 for flunixin with collision energy (CE) of −24 V, m/z 298.0→254.0 for flunixin-d3 (CE −14 V), m/z 349.8→285.9 for meloxicam (CE −19V) and m/z 353.0→289.0 for meloxicam-d3 (CE −15V).
The LC MS/MS method was validated under the FDA and EPA guidelines. The limits of detection (LOD) for flunixin meglumine and meloxicam were 0.42 and 0.45 ng/mL, respectively, and limits of quantification (LOQ) were 1.39 and 1.51 ng/mL. The LOD and LOQ were determined based on 3 and 10 standard deviations above the blank response. Calibration standards and quality controls were prepared and assayed on 3 separate days. Calibration curves were linear for all 3 concentration ranges: 2 to 400, 10 to 2000, and 20 to 4000 ng/mL, with coefficient of determination (R2) > 0.99 for all calibration curves. The accuracy was within 15% of the nominal concentration for all calibration levels for both drugs. The intra-day precision was 2.8 to 4.1% (CV) for flunixin, and 3.1 to 5.6% (CV) for meloxicam. The inter-day precision was 3.3 to 7.8% (CV) for flunixin, and 4.3 to 6.4% (CV) for meloxicam.
Pharmacokinetics and statistical analyses
Pharmacokinetic parameters for plasma meloxicam and flunixin meglumine concentration-time profiles were determined for each piglet using noncompartmental analysis approaches (Phoenix WinNonlin version 8.1; Certara USA, Princeton, New Jersey, USA). Pharmacokinetic parameters taken directly from the individual data sets included time to maximal plasma concentration (Tmax) and maximum plasma concentration (Cmax). Whereas calculated pharmacokinetic parameters included area under the concentration time curve from time zero to the last measured concentration (AUC0–tlast), area under the concentration time curve from time zero to infinity (AUC0–∞), the elimination rate constant (λz), elimination half-life (t1/2 λz), systemic clearance per fraction absorbed (CL/F), volume of distribution per fraction absorbed (VD/F), and mean residence time from time zero to infinity (MRT0–∞).
Statistical analyses were performed using SAS (SAS Institute, Version 9.4; Cary, North Carolina, USA). The AUC0–∞, AUC0–tlast, Cmax, and CL/F were expressed as the mean ± SD, whereas t1/2 λz, λz, and MRT0–∞ were expressed as the harmonic mean with 95% confidence intervals (CI). Tmax was expressed as the median with lower and upper range limits. Tmax, t1/2 λz, and MRT0–∞ data obtained from M and M+ID treatment groups were not normally distributed; the normality test also failed on log-transformed data. This data set, therefore, was analyzed for differences by a non-parametric test, a Wilcoxon 2-sample test. The rest of the pharmacokinetic data were analyzed for differences by a 2-sample Student’s t-test. Relative bioavailability of the compounded formulations for each NSAID compared to the NSAID alone was determined from estimates of geometric least squares means and 90% CI for the ratio of the means using respective AUC0–tlast and AUC0–∞ values. The differences in the ratio of least squares means from 1 were analyzed by a 2-sample t-test. In all statistical analyses, P < 0.05 was considered significant.
Results
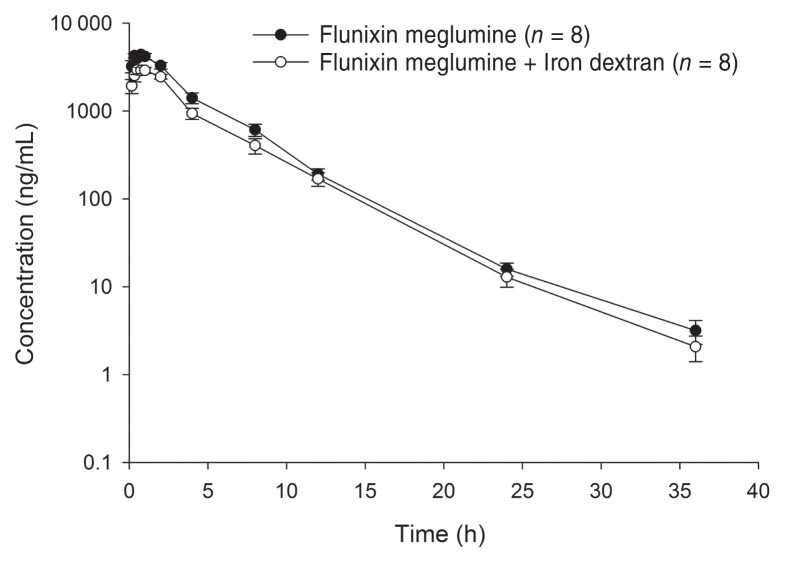
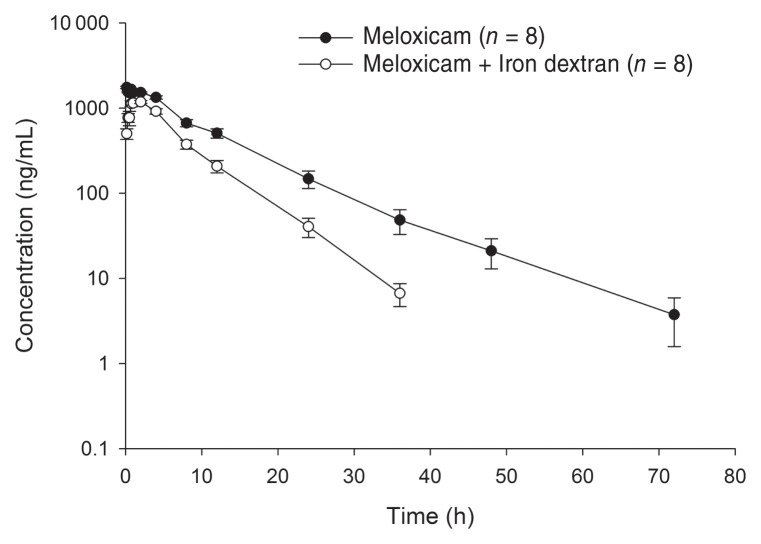
All piglets completed the study, and no adverse effects were noted from administration of the test articles to completion of the study. Plasma concentration-time curves of compounded (F+ID, M+ID) and reference (F, M) formulations of flunixin meglumine and meloxicam are shown in Figures 1 and 2, respectively. Plasma NSAID concentrations were detected out to 36 h post-dosing for the flunixin meglumine alone group, and both compounded formulations, whereas plasma concentrations in the meloxicam alone group were detected out to 72 h post-dosing. Pharmacokinetic parameters determined for compounded and reference formulations for flunixin meglumine and meloxicam are presented in Tables 1 and 2, respectively. The overall drug exposure for both NSAIDs was significantly lower when compounded with ID compared to NSAID administration alone. The relative bioavailability of the compounded formulations compared to the reference formulations for each NSAID is presented in Table 3. The relative bioavailability of meloxicam and flunixin meglumine in the compounded formulations i.e., M+ID and F+ID treatment groups were significantly lower than corresponding reference formulations, i.e., M and F treatment groups, with a ratio of least square means between formulations for both AUC0–tlast and AUC0–∞ of 0.53 (90% CI: 0.42 to 0.68) for meloxicam and 0.70 (90% CI: 0.56 to 0.89) for flunixin, respectively. This was based on the 90% confidence intervals not including 1, indicating the ratios were significantly different from equality. The percentage of the AUC0–∞ that was extrapolated from the last measurable concentration was < 20% for the F, M, M+ID and F+ID treatment groups. All baseline (pre-dosing) blood samples and blood samples taken from piglets in the ID group recorded no detectable concentrations of meloxicam or flunixin meglumine.
Figure 1.
Mean flunixin meglumine plasma concentration (± SE) following intramuscular administration of 2.2 mg/kg of flunixin meglumine alone or flunixin meglumine mixed with iron dextran in piglets. n — Number of piglets per treatment group.
Figure 2.
Mean meloxicam plasma concentration (± SE) following intramuscular administration of 0.4 mg/kg of meloxicam alone or meloxicam mixed with iron dextran in piglets. n — Number of piglets per treatment group.
Table 1.
Pharmacokinetic parameters for flunixin meglumine and flunixin meglumine compounded with iron dextran determined by noncompartmental analysis in piglets.
| Parameter | Unit | Flunixin meglumine n = 8 |
Flunixin meglumine + Iron dextran n = 8 |
|---|---|---|---|
| AUC0–tlast | h*μg/mL | 18.21 ± 5.03 | 12.70 ± 2.88* |
| AUC0–∞ | h*μg/mL | 18.24 ± 5.03 | 12.72 ± 2.88* |
| t1/2 λz (HM) | h | 3.73 (LL 3.11, UL 4.66) | 3.42 (LL 2.89, UL 4.19) |
| λz (HM) | 1/h | 0.17 (LL 0.14, UL 0.21) | 0.19 (LL 0.16, UL 0.26) |
| CL/F | mL/h/kg | 129.19 ± 36.02 | 182.19 ± 48.65* |
| Cmax | μg/mL | 4.58 ± 0.74 | 3.22 ± 0.66* |
| Tmax | h | 0.57 (LL 0.36, UL 0.89) | 0.68 (LL 0.43, UL 1.07) |
| VD/F | mL/kg | 750.10 ± 287.56 | 919.02 ± 245.44 |
| MRT0–∞ (HM) | h | 3.89 (LL 3.43, UL 4.51) | 3.92 (LL 3.45, UL 4.54) |
AUC0–tlast — Area under the concentration time curve from time zero to last measured concentration; AUC0–∞ — AUC from time zero to infinity; t1/2λz — Elimination half-life; λz — Elimination rate constant; CL/F — Systemic clearance per fraction absorbed; Cmax — Maximum plasma concentration; Tmax — Time to maximal plasma concentration; VD/F — Volume of distribution per fraction absorbed; MRT0–∞ — Mean residence time from time zero to infinity. AUC0–tlast, AUC0–∞, CL/F, Cmax, and VD/F are expressed as mean ± SD. HM — Harmonic mean with 95% CI. Tmax is presented as median with lower and upper range limits.
Versus corresponding reference value (P < 0.05). n — Number of piglets per treatment group; LL — Lower limit; UL — Upper limit.
Table 2.
Pharmacokinetic parameters for meloxicam and meloxicam compounded with iron dextran determined by noncompartmental analysis in piglets.
| Parameter | Unit | Meloxicam n = 8 |
Meloxicam + Iron dextran n = 8 |
|---|---|---|---|
| AUC0–tlast | h*μg/mL | 17.19 ± 4.61 | 9.11 ± 2.11* |
| AUC0–∞ | h*μg/mL | 17.28 ± 4.68 | 9.15 ± 2.12* |
| t1/2 λz (HM) | h | 6.56 (LL 5.03, UL 9.44) | 4.41* (LL 3.66, UL 5.55) |
| λz (HM) | 1/h | 0.09 (LL 0.07, UL 0.13) | 0.15* (LL 0.13, UL 0.18) |
| CL/F | mL/h/kg | 24.90 ± 7.89 | 46.03 ± 11.56* |
| Cmax | μg/mL | 1.79 ± 0.14 | 1.28 ± 0.13* |
| Tmax | h | 0.28 (LL 0.17, UL 0.47) | 1.27* (LL 0.77, UL 2.10) |
| VD/F | mL/kg | 249.29 ± 48.43 | 292.25 ± 28.74* |
| MRT0–∞ (HM) | h | 8.75 (LL 6.73, UL 12.53) | 6.14 (LL 5.07, UL 7.79) |
AUC0–tlast — Area under the concentration time curve from time zero to last measured concentration; AUC0–∞ — AUC from time zero to infinity; t1/2λz — Elimination half-life; λz — Elimination rate constant; CL/F — Systemic clearance per fraction absorbed; Cmax — Maximum plasma concentration; Tmax — Time to maximal plasma concentration; VD/F — Volume of distribution per fraction absorbed; MRT0–∞ — Mean residence time from time zero to infinity. AUC0–tlast, AUC0–∞, CL/F, Cmax, and VD/F are expressed as mean ± SD. HM — Harmonic mean with 95% CI. Tmax is presented as median with lower and upper range limits.
Versus corresponding reference value (P < 0.05). n — Number of piglets per treatment group; LL — Lower limit; UL — Upper limit.
Table 3.
Relative bioavailability of flunixin meglumine compounded with iron dextran (F+ID) and meloxicam compounded with iron dextran (M+ID) versus flunixin meglumine (F) and meloxicam (M) alone in piglets.
| Parameter | Unit | Ratio of least-squares means (F+ID/F) | 90% CI | |
|---|---|---|---|---|
|
| ||||
| LL | UL | |||
| Flunixin meglumine | ||||
| AUC0–tlast | h*μg/mL | 0.70* | 0.56 | 0.89 |
| AUC0–∞ | h*μg/mL | 0.70* | 0.56 | 0.89 |
|
| ||||
| Parameter | Unit | Ratio of least-squares means (M+ID/M) | 90% CI | |
|
| ||||
| LL | UL | |||
|
| ||||
| Meloxicam | ||||
| AUC0–tlast | h*μg/mL | 0.53* | 0.42 | 0.68 |
| AUC0–∞ | h*μg/mL | 0.53* | 0.42 | 0.68 |
AUC0–tlast — area under the concentration time curve from time zero to last measured concentration. AUC0–∞ — AUC from time zero to infinity. The differences in the ratio of least squares means were analyzed by 2-sample t-test.
Indicates significant difference (P < 0.05) based on the 90% confidence intervals (CI) not including 1.
Discussion
Painful procedures such as tail docking and castration are often performed at the time of routine iron supplementation to piglets. Non-steroidal anti-inflammatory drugs are widely used to manage pain and inflammation in food-producing animals, including swine, with meloxicam and flunixin meglumine commonly prescribed in pigs (10,11). The practice of mixing NSAIDs with ID and administering the compounded product as a single injection at the time of processing has the potential to maximize code compliance as well as minimize animal handling and stress. Although pharmacokinetics of these NSAIDs following their administration to piglets or adult pigs have been investigated (11–16), studies examining the pharmacokinetics of meloxicam and flunixin meglumine, when mixed with ID and administered to piglets, are lacking. The results of the current pharmacokinetic study are important for veterinarians and producers to make informed decisions regarding whether to compound NSAIDs with ID by combining them in the same dosing bottle, while ensuring optimal piglet care and welfare.
The pharmacokinetic parameters obtained from flunixin meglumine and meloxicam administered alone in the current study were comparable to those in previous studies in similar aged piglets and older pigs (11,13–15). However, in the current study, there were significant differences between pharmacokinetic parameters and relative bioavailability for compounded formulations versus reference NSAID formulations. Bioavailability of a drug is defined as the extent and rate to which the active pharmaceutical ingredient or active moiety from the drug formulation is absorbed into the systemic circulation and becomes available at the site of drug action (17). The extent, i.e., exposure, is usually measured by the AUC (17). In this study, comparison of AUC0–last, AUC0–∞, and Cmax values obtained with the M+ID and F+ID treatment groups demonstrated that all these parameters, for both NSAIDs, were significantly lower than corresponding parameters obtained in the M and F treatment groups, respectively. Comparison of the ratio of the least square means of AUC0–last and AUC0–∞ for the compounded formulations versus the corresponding reference formulations indicated significantly lower relative bioavailability. Taken together, it is possible that reduced plasma drug concentrations noted with the compounded formulations may result in reduced efficacy following compounding of these NSAIDs with ID in the same bottle and administered to pigs at the time of castration. However, it is important to note that plasma NSAID concentrations do not correlate well with NSAID concentrations obtained at sites of inflammation, and therefore efficacy (18–20). The results of an efficacy study by Reynolds et al (4) evaluating analgesia in similar aged piglets receiving meloxicam alone versus meloxicam compounded with ID in the same bottle suggested similar efficacy of the 2 formulations. The Reynolds et al (4) study used the same dosage levels as used in this study, given approximately 1 h before castration with pain control measured quantitatively by a chute navigation test post-castration with results indicating no difference in chute run times between the 2 formulations, supporting similar analgesic efficacy.
Levionnois et al (21) investigated the pharmacokinetics and pharmacodynamic modeling of NSAIDs in a kaolin-induced inflammation model in piglets. They reported a plasma concentration of flunixin meglumine producing half of the maximum inhibitory effect (IC50) of 6.78 μg/mL for pressure-induced pain when flunixin meglumine was given at 2.2 mg/kg, IV to similar aged piglets as the current study. It should be noted that this study administered flunixin meglumine after induction of inflammation, which may have produced differing results than pre-emptive use of the NSAID. In the current study, piglets were treated with 2.2 mg/kg of flunixin meglumine given IM, and plasma concentrations did not reach the median IC50 reported in the Levionnois et al (21) study in any treated piglets, at any study time point. However, it should also be noted that the optimal percentage of cyclooxygenase-2 enzyme inhibition required for maximal analgesia is not known, and that efficacy may be achieved with less than complete enzyme inhibition (20). Although we could not find published information on IC50 values for meloxicam in pigs, its effectiveness at reducing inflammation and producing analgesia in a similar inflammation model was shown to be inadequate following IV and IM administration at dosages of 0.4 mg/kg and 0.6 mg/kg, respectively (12,13). However, these studies noted the kaolin-induced inflammation model used is more valid for assessing somatic pain versus visceral pain, as would occur with castration.
In the current study, absolute bioavailability was not determined, requiring drugs to be given by the intravenous route. As such, the pharmacokinetic parameters clearance and volume of distribution reported are dependent on the fraction (% F) of drug absorbed from the injection site into the systemic circulation following extravascular administration. This could explain differences noted between these values with the compounded versus reference formulations. Measurement of injection site drug levels would have provided additional valuable information regarding drug absorption, but unfortunately were not measured in the current study.
The reduced AUC and Cmax values reported with the compounded formulations may be attributed to reduced absorption of NSAIDs from the injection site due to interactions with ID, thus reducing bioavailability and increasing volume of distribution (22,23). It is also possible that compounding of ID with the NSAIDs reduced stability of the NSAID. Finally, it is also possible that the extraction ratio and clearance of the NSAIDs may also have been affected, if NSAID and ID were bound or chelated and absorbed together into the systemic circulation. Flunixin meglumine and meloxicam have a low hepatic extraction ratio (16,24,25). It is possible that binding of these NSAIDs to ID may have increased their extraction ratio, which could account for the higher clearance rates (CL/F) obtained with the M+ID and F+ID treatment groups compared to the M and F groups, respectively (26,27).
It is possible that either dextran and/or iron present in ID could interact with the NSAIDs when compounded in the same formulation (9). Iron dextran consists of dextran, a negatively charged polysaccharide derived from glucose, combined with iron as ferric (Fe3+) hydroxide. Interactions between ID and meloxicam or flunixin meglumine have not been explored to the authors’ knowledge. However, hydroxamic acid derivatives of several NSAIDs, including ketoprofen, have shown chelation activity with iron when mixed in-vitro (28); that may have also occurred in the current study. In addition, both meloxicam and flunixin meglumine have the potential to have their pharmacokinetic profiles altered when administered simultaneously with other drugs, suggesting the potential for drug-drug interactions (29,30).
In the present study, relative bioavailability of meloxicam and flunixin meglumine were reduced when compounded with ID in the same bottle and administered to piglets. In recent studies in similar aged piglets using the same compounded formulation of meloxicam and ID, and a compounded formulation of ketoprofen and ID, there were no differences with analgesic efficacy when compared to meloxicam and ketoprofen alone (4); however, no studies evaluating efficacy with flunixin meglumine compounded with ID have been performed. There is no “gold standard” for pain assessment in animals, including pigs. Behavioral and physiological measures interpreted as indicative of pain have been used to evaluate post-operative analgesia produced by NSAIDs in treated piglets (4,31–33). Further studies assessing the analgesic effects of meloxicam and flunixin meglumine when compounded with ID in piglets are still warranted.
Compounding of multiple approved veterinary drugs in a single formulation for use in food-producing animals is a form of extra label drug use that requires modification to withdrawal times and is concerning due to the potential for drug-drug interactions that could result in violative drug residues and possible human food safety concerns. In the current study, there were reduced AUC and Cmax values for both compounded formulations compared to the reference formulations. Taken together with the mean t1/2 λz value obtained in the M+ID treatment group being lower than the M treatment group value, and the F+ID treatment group t1/2 λz not differing from that obtained in the F treatment group, violative residues in the edible tissues of muscle, liver, and kidney may not be a concern. However, injection site violative residues could be a concern, supporting the need for further food safety research when meloxicam and flunixin meglumine are mixed with ID and administered to piglets.
Acknowledgments
This project was supported by Ontario Pork, University of Guelph — OMAFRA Research Partnership (Ontario Agri-Food Innovation Alliance), and the Ontario Veterinary College scholarship program. The authors thank Julia Zhu (Department of Animal Biosciences, University of Guelph) for assistance with jugular vein catheterization and William Sears (Department of Population Medicine, University of Guelph) for assistance with the statistical analyses. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.National Farm Animal Care Council. Code of Practice for the Care and Handling of Pigs. 2014. [Last accessed October 03, 2021]. Available from: http://www.nfacc.ca/pdfs/codes/pig_code_of_practice.pdf. Last accessed May 3, 2022.
- 2.Perri AM, Friendship RM, Harding JCS, O’Sullivan TL. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J Swine Health Prod. 2016;24:10–20. [Google Scholar]
- 3.Loh T, Leong K, Too H, Mah C, Choo P. The effects of iron supplementation in preweaning piglets. Malays J Nutr. 2001;7:41–49. [PubMed] [Google Scholar]
- 4.Reynolds K, Johnson R, Friendship R, Brown J, O’Sullivan TL. Assessing pain control efficacy of meloxicam and ketoprofen when compounded with iron dextran in nursing piglets using a navigation chute. Animals. 2020;10:1–14. doi: 10.3390/ani10071237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barz A, Ritzmann M, Breitinger I, et al. Examination of different options for combined administration of an NSAID (meloxicam) and iron for piglets being castrated. Tierärztl Prax Großtiere. 2010;38:23–30. [Google Scholar]
- 6.Grignon-Boutet R, Ireland M-J, Adewoye L, Mehrotra M, Russell S, Alexander I. Health Canada’s policy on extra-label drug use in food-producing animals in Canada. Can Vet J. 2008;49:689–693. [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman MD. Drug interactions: Classification and systematic approach. Am J Ther. 1995;2:433–443. [PubMed] [Google Scholar]
- 8.Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug–drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–1075. doi: 10.2147/TCRM.S79135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds KJ, Johnson R, Friendship RM, et al. Pharmacokinetics and bioavailability of ketoprofen when compounded with iron dextran for use in nursing piglets. Can Vet J. 2021;62:1211–1218. [PMC free article] [PubMed] [Google Scholar]
- 10.Viscardi AV, Turner PV. Use of meloxicam or ketoprofen for piglet pain control following surgical castration. Front Vet Sci. 2018;5:299. doi: 10.3389/fvets.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nixon E, Mays TP, Routh PA, et al. Plasma, urine and tissue concentrations of flunixin and meloxicam in pigs. BMC Vet Res. 2020;16:1–10. doi: 10.1186/s12917-020-02556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosse TK, Haga HA, Hormazabal V, Haugejorden G, Horsberg TE, Ranheim B. Pharmacokinetics and pharmacodynamics of meloxicam in piglets. J Vet Pharmacol Ther. 2008;31:246–252. doi: 10.1111/j.1365-2885.2008.00958.x. [DOI] [PubMed] [Google Scholar]
- 13.Fosse TK, Spadavecchia C, Horsberg TE, Haga HA, Ranheim B. Pharmacokinetics and pharmacodynamic effects of meloxicam in piglets subjected to a kaolin inflammation model. Vet Pharmacol Ther. 2011;34:367–375. doi: 10.1111/j.1365-2885.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 14.Pairis-Garcia MD, Karriker LA, Johnson AK, et al. Pharmacokinetics of flunixin meglumine in mature swine after intravenous, intramuscular and oral administration. BMC Vet Res. 2013;9:1–7. doi: 10.1186/1746-6148-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nixon E, Almond GW, Baynes RE, Messenger KM. Comparative plasma and interstitial fluid pharmacokinetics of meloxicam, flunixin, and ketoprofen in neonatal piglets. Front Vet Sci. 2020;7:1–9. doi: 10.3389/fvets.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kittrell HC, Mochel JP, Brown JT, et al. Pharmacokinetics of intravenous, intramuscular, oral, and transdermal administration of flunixin meglumine in pre-wean piglets. Front Vet Sci. 2020;7:1–13. doi: 10.3389/fvets.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow Shein-Chung. Bioavailability and bioequivalence in drug development. Wiley Interdiscip Rev Comput Stat. 2014;6:304–312. doi: 10.1002/wics.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brune K, Furst DE. Combining enzyme specificity and tissue selectivity of cyclooxygenase inhibitors: Towards better tolerability? Rheumatology. 2007;46:911–919. doi: 10.1093/rheumatology/kem070. [DOI] [PubMed] [Google Scholar]
- 19.Messenger KM, Wofford JA, Papich MG. Carprofen pharmacokinetics in plasma and in control and inflamed canine tissue fluid using in vivo ultrafiltration. J Vet Pharmacol Ther. 2016;39:32–39. doi: 10.1111/jvp.12233. [DOI] [PubMed] [Google Scholar]
- 20.Lees P, Landoni MF, Giraudel J, Toutain PL. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther. 2004;27:479–490. doi: 10.1111/j.1365-2885.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 21.Levionnois OL, Fosse TK, Ranheim B. PK/PD modeling of flunixin meglumine in a kaolin-induced inflammation model in piglets. J Vet Pharmacol Ther. 2018;41:314–323. doi: 10.1111/jvp.12468. [DOI] [PubMed] [Google Scholar]
- 22.Lin JH. Tissue distribution and pharmacodynamics: A complicated relationship. Curr Drug Metab. 2006;7:39–65. doi: 10.2174/138920006774832578. [DOI] [PubMed] [Google Scholar]
- 23.Hochman J, Tang C, Prueksaritanont T. Drug-drug interactions related to altered absorption and plasma protein binding: Theoretical and regulatory considerations, and an industry perspective. J Pharm Sci. 2015;104:916–929. doi: 10.1002/jps.24306. [DOI] [PubMed] [Google Scholar]
- 24.Warner R, Ydstie JA, Wulf LW, et al. Comparative pharmacokinetics of meloxicam between healthy postpartum vs. mid-lactation dairy cattle. Front Vet Sci. 2020;7:548. doi: 10.3389/fvets.2020.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies NM, Skjodt NM. Clinical pharmacokinetics of meloxicam. A cyclo-oxygenase-2 preferential nonsteroidal anti-inflammatory drug. Clin Pharmacokinet. 1999;36:115–126. doi: 10.2165/00003088-199936020-00003. [DOI] [PubMed] [Google Scholar]
- 26.Toutain PL, Bousquet-Melou A. Free drug fraction vs free drug concentration: A matter of frequent confusion. J Vet Pharmacol Ther. 2002;25:460–463. doi: 10.1046/j.1365-2885.2002.00442.x. [DOI] [PubMed] [Google Scholar]
- 27.Toutain PL, Bousquet-Mélou A. Plasma clearance. J Vet Pharmacol Ther. 2004;27:415–425. doi: 10.1111/j.1365-2885.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- 28.Končić MZ, Rajić Z, Petrić N, Zorc B. Antioxidant activity of NSAID hydroxamic acids. Acta Pharm. 2009;59:235–242. doi: 10.2478/v10007-009-0017-8. [DOI] [PubMed] [Google Scholar]
- 29.Busch U, Heinzel G, Narjes H. The effect of cholestyramine on the pharmacokinetics of meloxicam, a new non-steroidal anti-inflammatory drug (NSAID), in man. Eur J Clin Pharmacol. 1995;48:269–272. doi: 10.1007/BF00198310. [DOI] [PubMed] [Google Scholar]
- 30.Abo-EL-Sooud K, AL-Anati L. Pharmacokinetic study of flunixin and its interaction with enrofloxacin after intramuscular administration in calves. Vet World. 2011;4:449–454. [Google Scholar]
- 31.Keita A, Pagot E, Prunier A, Guidarini C. Pre-emptive meloxicam for postoperative analgesia in piglets undergoing surgical castration. Vet Anaesth Analg. 2010;37:367–374. doi: 10.1111/j.1467-2995.2010.00546.x. [DOI] [PubMed] [Google Scholar]
- 32.Cassar G, Amezcua R, Tenbergen R, Friendship RM. Preoperative ketoprofen administration to piglets undergoing castration does not affect subsequent growth performance. Can Vet J. 2014;55:1250–1252. [PMC free article] [PubMed] [Google Scholar]
- 33.Zöls S, Ritzmann M, Heinritzi K. Effect of analgesics on the castration of male piglets. Berl Munch Tierarztl Wochenschr. 2006;119:193–196. [PubMed] [Google Scholar]