Abstract
Innate lymphoid cells 2 (ILC2s) are novel lymphocytes that both promote and suppress anti-tumor immunity. Jou and colleagues (1) now report in colorectal tumorigenesis that the cytokine IL-25 activates ILC2s to induce myeloid cells that suppress anti-tumor immunity.
Since their discovery, group 2 innate lymphoid cells (ILC2s) – an enigmatic lymphocyte initially identified as the primary producer of the canonical Th2 cytokine IL-13 that protects hosts against helminth infections – have been spooling out a dizzying array of functional roles in tissue physiology. ILC2s communicate with a wide array of immune and non-immune cells to coordinate a broad range of tissue functions including homeostasis, metabolism, immunity, repair, and neuronal dialog. Given this broad and continuously expanding role for ILC2s as an apex cell that coordinates and maintains optimal tissue health, it logically follows that ILC2s would be similarly poised to modulate disruptive tissue processes such as cancer. Further evidence reported by Jou et al. now supports such a role (1).
ILC2s appear to act as sentinels of early tissue damage. Indeed, in both infections and cancer, ILC2s recognize the tissue alarmin IL-33 that is released by damaged cells. Yet, unlike in acute models of infection and inflammation where IL-33 stimulates ILC2s to activate Th2 immunity, multiple studies have now uncovered a parallel pathway that is active in chronically transformed tissues such as cancer. Here, ILC2s activate anti-cancer CD8+ T cells (2, 3), and eosinophils (4, 5) to suppress cancer (Fig. 1).
Figure 1:
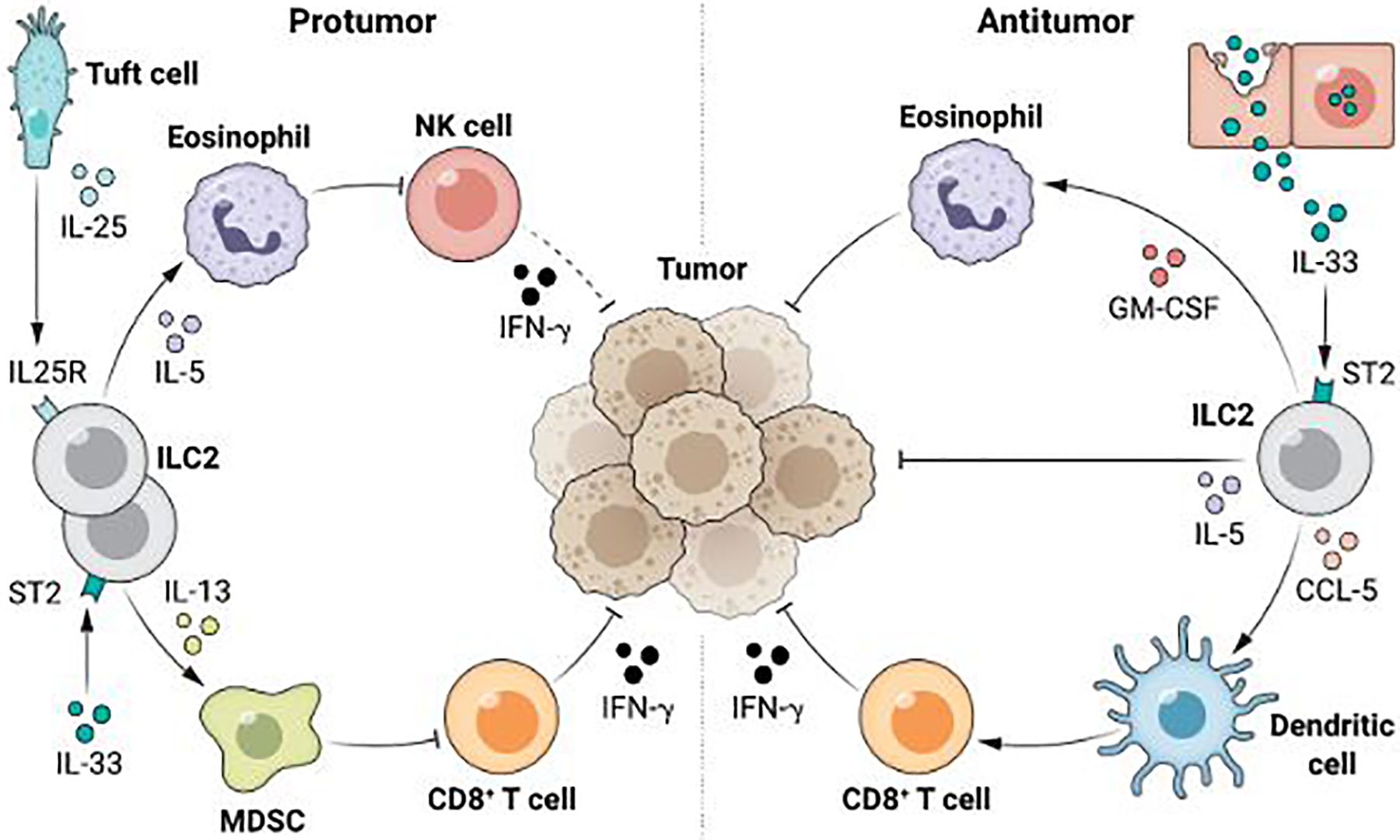
Schematic of select pro and anti-tumor functions of ILC2s in cancer. IL-33-activates ILC2s to produce chemokines that induce NK cells and CD8+ T cells to indirectly, and cytokines to directly suppress cancer. In contrast, IL-25 activates ILC2s to produce cytokines that stimulate myeloid cells and eosinophils to promote cancer. MDSC – Myeloid Derived Suppressor Cell; NK – Natural Killer.
Initial studies revealed that ILC2-derived IL-5 (4), and subsequently GM-CSF (5), can activate anti-tumor eosinophils in lung cancer (4) and melanoma (5). IL-33-activated ILC2s can also produce apoptotic ligands to suppress melanoma, lymphoma, and colon cancer (6). In lung (2) and pancreatic cancer (3), IL-33 stimulated ILC2s recruit CD103+ dendritic cells to activate anti-tumor CD8+ T cells. Interestingly, studies also show that these anti-tumor ILC2s are inhibited by the PD-1 pathway that inhibits other anti-cancer lymphocytes (3, 5). Naturally, this raises the possibility that one could block such inhibitory pathways to co-activate ILC2s along with other anti-cancer lymphocytes, as shown in pancreatic cancer (3) and melanoma (5, 7). A report (7) has even suggested that PD-1 blockade on ILC2s may broaden ILC2’s functional repertoire, to elicit production of TNF-α, and induce ILC2s to acquire direct anti-cancer cellular potential. Though intriguing and consistent with the significant functional plasticity of the ILC2 lineage, this finding must be confirmed in future studies. Notably, IL-33 can also induce ILC2s to suppress cancer (8), highlighting the need for further mechanistic studies to disentangle the contextual differences that promote these dual roles.
In contrast to these ”first responder” sentinel functions of ILC2s triggered by likely damage-induced passive IL-33 release from tissues and tumors, ILC2s also appear to be activated by a parallel pathway that actively senses and responds to microenvironmental cues. This pathway is initiated by the tuft cell – a rare, chemosensory, secretory epithelial cell that senses an array of luminal signals at barrier surfaces, and then assumes the sole responsibility to produce IL-25 - another canonical cytokine that activates ILC2s. In infection and inflammatory states, the tuft cell-IL-25 axis recognizes luminal stimuli to activate ILC2s to produce IL-13, IL-4, and IL-5. Jou and colleagues (1) use a combination of human samples and genetic mouse models to add to the evidence that this axis is active in cancer. The authors find that higher IL-25 expression in human colorectal tumors correlates with worse prognosis. In addition, the authors show that IL-25R+ ILC2s infiltrate human colorectal cancers and their frequency correlates positively with higher frequencies of myeloid cells yet negatively with CD8+ T cells.
To investigate potential causal links between IL-25 and ILC2s, the authors use a series of tools to delete, block or boost IL-25 and ILC2s in an autochthonous mouse model of intestinal polyposis and colitis-driven intestinal cancer. The authors use the Apc1322T/+ mouse model, an APC tumor suppressor gene driven model of intestinal polyposis on a Roraf/fIl7rCre/+ ILC2-deficient background, to show that ILC2s depletion reduces myeloid cells, increases CD8+, and CD4+ Th1 T cells, is accompanied by smaller and fewer intestinal tumors, and prolongs mouse survival. The authors then show that these cancer supportive ILC2s are IL25-responsive by demonstrating that recombinant IL25 administration, or genetic IL25 deficiency expands or contracts both ILC2s, and myeloid cells with reciprocal changes in tumor burden. Furthermore, the authors note that these tumor ILC2s produce IL13, and intratumoral myeloid cells upregulate the IL13R within tumors. To link IL13 as the ILC2-derived cytokine that modulates this myeloid cell-T cell axis, the authors then show ILC2-derived IL13 expands myeloid and T cells through global and ILC2-cell specific IL13 deficiency, and in vitro experiments. Finally, to test a possible strategy to therapeutically modulate this axis, the authors show that an antibody that blocks the IL-25R decreases ILC2s, myeloid cells, and concomitantly expands CD8+ and CD4+ Th1 cells to reduce tumorigenesis in both polyposis and cancer models. Overall, this report adds to the burgeoning evidence that ILC2s communicate with myeloid cells (9) to promote cancer. More broadly, it hints that the emerging dual role of ILC2s in cancer may be driven by fundamental differences in how ILC2s respond to distinct ILC2 cytokine inputs. Interestingly, as the authors confirm tuft cells to be the primary source of IL-25, this hints that this duality may more broadly reflect how ILC2s respond to tissue damage versus sensory cell driven input signals.
How do ILC2s achieve such duality in cancer? Certainly, variable compositions of the cells that produce these inciting cytokines (IL-25 and IL-33) in tumors is one possibility. This seems plausible as tuft cells are not ubiquitous in all tissues, and thus different tumors that arise from different tissues may contain variable compositions of IL25-producing tuft cells and IL-33+ cells. Not exclusive to this possibility, it is now also clear that ILC2s have significant functional heterogeneity reflected by different cellular subsets and tissue-specific functions. Specifically, ILC2 subsets and ILC2s in different tissues express distinct combinations of cytokine receptors (10), and exhibit subtype- and tissue-specific cytokine response patterns. This further raises the possibility that the ILC2 anti- and pro-tumor functions may, at least in part, be mediated by heterogeneous compositions of ILC2s in different tumors that arise in distinct tissues. In support of this, Jou and colleagues (1) use genetic loss and molecular gain-of-function tools to show that IL-25, but not IL33, can stimulate ILC2’s immune suppressive function in colorectal cancer. It is possible that these differences may not only extend to different tumors arising in different tissues, but also to identical tumors metastasized to different tissues. More mechanistic studies in both human tumors and mouse models that capture these heterogeneous variables are undoubtedly needed to unravel these interesting complexities of ILC2s in cancer.
Why might ILC2s exert such a dual role in cancer? One could envision this may be fundamentally linked to how a chemosensory axis versus a damage response axis induce distinct ILC2 functions in cancer. A further interesting possibility remains that the downstream functional specification of the ILC2 might be toggled by the nature of the signals both inputted into and transmitted by the tuft cell. As tuft cells have demonstrated the capability to sense diverse stimuli from the environment, how this diversification affects ILC2s in cancers remains to be seen.
ILCs have emerged as a remarkable new population of lymphocytes within the past 15 years. Among them, ILC2s are perhaps the most functionally diverse and fascinating. In the last decade, important progress has helped dissect and define ILC2 biology as pertains to their roles in infection and inflammation. However, our understanding of ILC2s in cancer is nascent. Early clues suggest ILC2 function depends on phenotypic subsets, tissue sites, upstream cytokine signals, and thus remains strongly contextual. Yet, it is clear that ILC2s represent a critical cell with diverse functions in cancer. Further rigorous studies are thus needed to unveil the full breadth of their functions in cancer and highlight possible strategies to harness these functions with immunotherapies.
References and notes
- 1.Jou E, Rodriguez-Rodriguez N, Ferreira A-CF, Jolin HE, Clark PA, Sawmynaden K, Ko M, Murphy JE, Mannion J, Ward C, Matthews DJ, Buczacki SJA, McKenzie ANJ, An innate IL-25-ILC2-MDSC axis creates a cancer-permissive microenvironment for Apc-mutation-driven intestinal tumorigenesis. Science Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saranchova I, Han J, Zaman R, Arora H, Huang H, Fenninger F, Choi KB, Munro L, Pfeifer CG, Welch I, Takei F, Jefferies WA, Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis. Sci Rep-uk 8, 2924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moral JA, Leung J, Rojas LA, Ruan J, Zhao J, Sethna Z, Ramnarain A, Gasmi B, Gururajan M, Redmond D, Askan G, Bhanot U, Elyada E, Park Y, Tuveson DA, Gönen M, Leach SD, Wolchok JD, DeMatteo RP, Merghoub T, Balachandran VP, ILC2s amplify PD-1 blockade by activating tissue-specific cancer immunity. Nature 579, 130–135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K, Identification of Innate IL-5–Producing Cells and Their Role in Lung Eosinophil Regulation and Antitumor Immunity. J Immunol 188, 703–713 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Jacquelot N, Seillet C, Wang M, Pizzolla A, Liao Y, Hediyeh-zadeh S, Grisaru-Tal S, Louis C, Huang Q, Schreuder J, Souza-Fonseca-Guimaraes F, de Graaf CA, Thia K, Macdonald S, Camilleri M, Luong K, Zhang S, Chopin M, Molden-Hauer T, Nutt SL, Umansky V, Ciric B, Groom JR, Foster PS, Hansbro PM, McKenzie ANJ, Gray DHD, Behren A, Cebon J, Vivier E, Wicks IP, Trapani JA, Munitz A, Davis MJ, Shi W, Neeson PJ, Belz GT, Blockade of the co-inhibitory molecule PD-1 unleashes ILC2-dependent antitumor immunity in melanoma. Nat Immunol 22, 851–864 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Kim W, Moon UJ, Kim HJ, Choi H-J, Sin J-I, Park NH, Cho HR, Kwon B, Intratumorally Establishing Type 2 Innate Lymphoid Cells Blocks Tumor Growth. J Immunol 196, 2410–2423 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Howard E, Hurrell BP, Helou DG, Quach C, Painter JD, Shafiei-Jahani P, Fung M, Gill PS, Soroosh P, Sharpe AH, Akbari O, PD-1 Blockade on Tumor Microenvironment-Resident ILC2s Promotes TNF-α Production and Restricts Progression of Metastatic Melanoma. Front Immunol 12, 733136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuijs MJ, Png S, Richard AC, Tsyben A, Hamm G, Stockis J, Garcia C, Pinaud S, Nicholls A, Ros XR, Su J, Eldridge MD, Riedel A, Serrao EM, Rodewald H-R, Mack M, Shields JD, Cohen ES, McKenzie ANJ, Goodwin RJA, Brindle KM, Marioni JC, Halim TYF, ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat Immunol, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trabanelli S, Chevalier MF, Martinez-Usatorre A, Gomez-Cadena A, Salomé B, Lecciso M, Salvestrini V, Verdeil G, Racle J, Papayannidis C, Morita H, Pizzitola I, Grandclément C, Bohner P, Bruni E, Girotra M, Pallavi R, Falvo P, Leibundgut EO, Baerlocher GM, Carlo-Stella C, Taurino D, Santoro A, Spinelli O, Rambaldi A, Giarin E, Basso G, Tresoldi C, Ciceri F, Gfeller D, Akdis CA, Mazzarella L, Minucci S, Pelicci PG, Marcenaro E, McKenzie ANJ, Vanhecke D, Coukos G, Mavilio D, Curti A, Derré L, Jandus C, Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat Commun 8, 593 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricardo-Gonzalez RR, Dyken SJV, Schneider C, Lee J, Nussbaum JC, Liang H-E, Vaka D, Eckalbar WL, Molofsky AB, Erle DJ, Locksley RM, Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 19, 1093–1099 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


