Abstract
The relationship between membrane damage and loss of viability following pressure treatment was examined in Escherichia coli strains C9490, H1071, and NCTC 8003. These strains showed high, medium, and low resistance to pressure, respectively, in stationary phase but similar resistance to pressure in exponential phase. Loss of membrane integrity was measured as loss of osmotic responsiveness or as increased uptake of the fluorescent dye propidium iodide. In exponential-phase cells, loss of viability was correlated with a permanent loss of membrane integrity in all strains, whereas in stationary-phase cells, a more complicated picture emerged in which cell membranes became leaky during pressure treatment but resealed to a greater or lesser extent following decompression. Strain H1071 displayed a very unusual pressure response in stationary phase in which survival decreased to a minimum at 300 MPa but then increased at 400 to 500 MPa before decreasing again. Membranes were unable to reseal after treatment at 300 MPa but could do so after treatment at higher pressures. Membrane damage in this strain was thus typical of exponential-phase cells under low-pressure conditions but of stationary-phase cells under higher-pressure conditions. Heat shock treatment of strain H1071 cells increased pressure resistance under low-pressure conditions and also allowed membrane damage to reseal. Growth in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside) increased resistance under high-pressure conditions. The mechanisms of inactivation may thus differ at high and low pressures. These studies support the view that membrane damage is an important event in the inactivation of bacteria by high pressure, but the nature of membrane damage and its relation to cell death may differ between species and phases of growth.
High hydrostatic pressure is one of the more promising nonthermal methods for inactivating microbes in food, and several food products treated by this method have now been produced commercially. It has been especially useful for extending the shelf life of fruit juices or products such as guacamole, made from avocado, where freshness of taste is particularly sensitive to conventional pasteurization (8, 11, 17). To extend the range of products that can usefully be processed by high pressure will require a thorough knowledge of the factors that affect microbial pressure resistance. Additionally, a better understanding of the way pressure kills cells would help in defining effective pressure treatments that, alone or in combination with other physical treatments or antimicrobial agents, could form alternatives to traditional heat preservation (7, 9, 10, 18).
Many attempts have been made to discover whether the changes observed after a pressure treatment, such as membrane alterations, ribosome denaturation, changes in the nucleoid, enzyme inactivation, or inhibition of transcription and protein synthesis, are responsible for the inactivation of vegetative cells by high-hydrostatic-pressure treatments (1, 5, 16, 22). However, despite much effort, the mechanisms of microbial inactivation are still not completely understood.
It is generally acknowledged that membrane damage seems to play an important role in pressure inactivation. High pressure causes tighter packing of the acyl chains and promotes membrane lipid gelation (14). There is evidence that bacterial cells become more sensitive to pressure as the membrane becomes more rigid and more resistant with a more fluid membrane (23). Physical damage to the bacterial cell membrane has been demonstrated as leakage of ATP or UV-absorbing material from bacterial cells subjected to pressure (23) or increased uptake of fluorescent dyes such as propidium iodide (PI) that do not normally penetrate the membranes of healthy cells (2, 21, 23). Loss of membrane functionality as a consequence of pressure treatment has also been described. In Lactobacillus plantarum, pressure treatment caused partial inactivation of the F0F1 ATPase such that the ability of cells to maintain a ΔpH was reduced, and the acid efflux mechanism was impaired (25). Loss of membrane ATPase activity may thus contribute to high-pressure inactivation.
Previously, we showed that different strains of Escherichia coli vary quite markedly in their resistance to pressure and presented preliminary data that this might be related to their susceptibility to membrane damage (2). If membrane damage is indeed a critical event leading to loss of viability, it might be expected that, when exposed to equivalent pressure treatments, pressure-resistant strains would be less susceptible to membrane damage than pressure-sensitive strains and, conversely, that cells of any given strain in the exponential phase of growth—that are more sensitive to pressure than cells in stationary phase—would show increased susceptibility to membrane damage. The aim of this work was to examine in more detail the relationship between membrane damage and loss of viability in sensitive and resistant strains of E. coli. Two methods were used to monitor loss of integrity of the cell membrane: measurement of uptake of the membrane-impermeant fluorescent dye PI and measurement of the loss of ability to plasmolyse in the presence of 0.75 M NaCl.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli O157:H7 strain C9490 (a clinical isolate from the Jack-in-the-Box western U.S. hamburger patty outbreak of 1993) was kindly provided by M. Doyle, University of Georgia, Griffin. E. coli O157:H7 H1071, a clinical isolate, was from M. Patterson, Queen's University, Belfast, United Kingdom. E. coli NCTC 8003 (serotype 0124) was also tested. Previous work (2) established that stationary-phase cells of strain C9490 are very pressure resistant, whereas those of strain NCTC 8003 are pressure sensitive. Strain H1071 demonstrates intermediate resistance. The following strains of E. coli K-12 were kindly provided by the E. coli Genetic Stock Center, Yale University, New Haven, Conn.: MO (parent), M7025 (lacZ90), M7044 (lacY328), and Hfr 3000 YA 694 (lacI694).
Liquid growth medium consisted of tryptone soya broth (Oxoid, Basingstoke, United Kingdom) supplemented with 0.6% yeast extract (TSBYE). The plating medium was tryptone soy agar (Oxoid) containing 0.6% yeast extract (TSAYE). Inoculum cultures were prepared by inoculating 10 ml of TSBYE with a loopful of growth taken from TSAYE and incubating the resulting culture with shaking at 37°C for 6 h. Cells in the stationary phase of growth were prepared by inoculating 100 μl of this culture into 100 ml of fresh TSBYE and incubating the resulting culture for 18 h under the same conditions. Under these conditions, cells had been in stationary phase for approximately 12 h at the time of harvest. To obtain exponential-phase cells, 100 μl of the stationary-phase culture was inoculated into 100 ml of fresh medium and incubated for approximately 3 h, which resulted in an optical density at 680 nm (OD680) of 0.2, measured with a spectrophotometer (model CE 1021; Cecil Instruments, Cambridge, United Kingdom) (equivalent to approximately 108 cells per ml).
Pressure treatment.
Cells were centrifuged at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in phosphate-buffered saline (PBS) (pH 7.0) to give viable counts of about 5 × 109 CFU/ml. Cell suspensions (2 ml each) were placed in sterile plastic pouches (4 by 5 cm) that were heat sealed and kept on ice before pressurization. Samples were treated in a 300-ml pressure vessel (model S-FL-850-9-W; Stansted Fluid Power, Stansted, United Kingdom). The pressure-transmitting fluid used was ethanol-castor oil (80:20). Cells were either exposed to a pressure of 100, 200, 300, 400, 500, or 600 for 8 min (total) or were exposed to a single pressure for various time intervals. Pressure treatment was at room temperature (approximately 20°C). The rise in temperature in the transmitting fluid, measured with a thermocouple, was about 4.3°C per 100 MPa, giving a maximum temperature of about 46°C at 600 MPa. The time spent above 40°C during compression to 600 MPa was approximately 70 s. After pressure treatment, the pouches were removed from the unit and placed on ice before viable counts were determined or other tests were performed.
Viable counts.
Cell suspensions were serially diluted in maximum recovery diluent (Oxoid) and plated onto TSAYE containing 0.1% (wt/vol) filter-sterilized sodium pyruvate added to the molten agar. Colonies were counted after the plates had been incubated at 37°C for 48 h. Data presented are mean values obtained from two to four independent experiments. The error bars on the figures indicate the mean standard deviations for the data points.
Heat shock treatment.
Cells were centrifuged at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in PBS (pH 7.0) preheated to 45°C and held at this temperature by immersion in a thermostated water bath. Heat-shocked-cell suspensions (2 ml each) were sealed in sterile plastic pouches that were kept on ice before pressurization.
Induction of β-galactosidase synthesis.
Cells were grown at 37°C for 18 h in TSBYE containing 2 mM isopropyl β-d-thiogalactoside (IPTG) (Sigma-Aldrich).
Staining cells with PI.
Pressure-treated cells were diluted in PBS to an OD680 of approximately 0.2 (corresponding to a dry weight of 95 μg (standard error, 3.9) of exponential-phase cells per ml and 76 μg (standard error, 2.7) of stationary-phase cells per ml; and PI (Sigma-Aldrich) was added to a final concentration of 2.9 μM. After incubation for 10 min, the samples were centrifuged and washed twice in PBS. Fluorescence was measured with a spectrofluorophotometer (model LS-5B; Perkin-Elmer); the excitation wavelength was set at 495 nm, and the emission wavelength was set at 615 nm. The slit width was 10 nm. Fluorescence data for cell suspension were normalized against OD680. Fluorescence values obtained for untreated cells were subtracted from all experimental values. In some experiments, cell suspensions were diluted in PBS as described above, and PI was added to a final concentration of 2.9 μM before pressure treatment. After pressure treatment, the samples were centrifuged and washed twice in PBS, and fluorescence was measured.
Measurement of osmotic response.
Around 30 μl of stationary-phase pressure-treated cell suspension or 100 μl of exponential-phase pressure-treated cell suspension was added in triplicate to 1 ml of PBS and 1 ml of PBS containing 0.75 M NaCl. The initial OD680 in PBS was about 0.2. Optical density was measured 2 min after mixing. The increase in OD680 was calculated by subtracting the mean value of the three measurements in PBS from the mean value of the three measurements in PBS containing 0.75 M NaCl. These OD increases were expressed as a percentage of the mean value obtained with PBS alone.
RESULTS
Pressure resistance and membrane damage in exponential-phase cells.
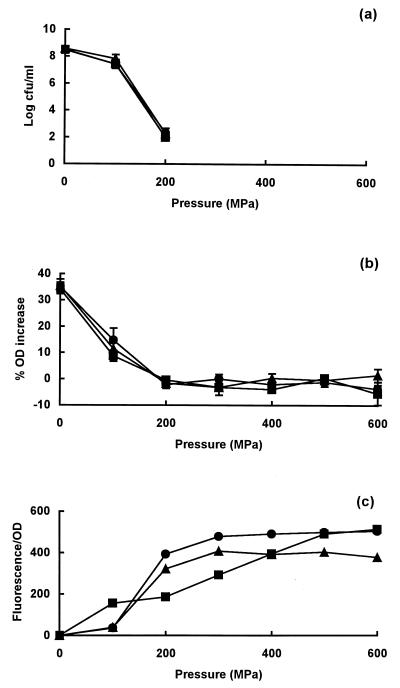
When cells of E. coli strains C9490, H1071, and NCTC 8003 were exposed to a range of pressures in the exponential phase of growth, there were no differences in resistance between them (Fig. 1a). In all cases, loss of viability was apparent after treatment at 100 MPa; after treatment at 200 MPa, viable numbers had decreased by 6 to 7 log10 units. All strains sustained membrane damage, as shown by the partial loss of ability to plasmolyse in the presence of 0.75 M NaCl after exposure to 100 MPa and a total loss of ability after exposure to 200 MPa (Fig. 1b). Membrane damage was confirmed by the uptake of PI by cells treated at pressures of 100 to 200 MPa or higher (Fig. 1c).
FIG. 1.
Pressure resistance and membrane damage in exponential-phase cells of E. coli strains C9490 (●), H1071 (▴), and NCTC 8003 (■). (a) Survivors after treatment at different pressures for 8 min; (b) osmotic responses of pressure-treated cells measured as the increase in OD of cell suspensions placed in 0.75 M NaCl; (c) permeability of pressure-treated cells to PI, measured as an increase in fluorescence relative to that of untreated cells. PI was added after pressure treatment. Viable counts following treatments at pressures above 200 MPa were below the limits of detection (50 CFU/ml).
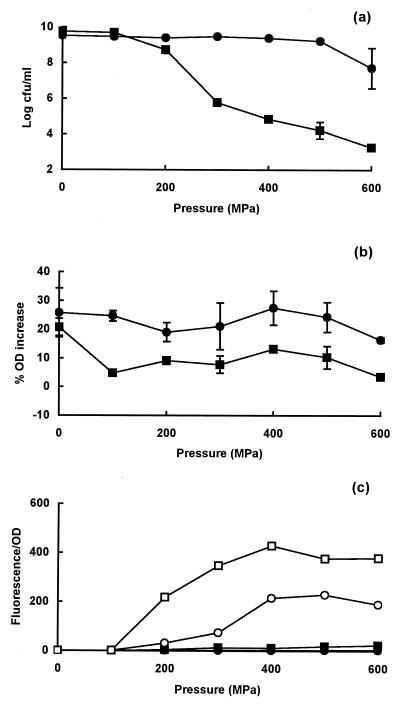
Pressure resistance and membrane damage in stationary-phase cells of strains C9490 and NCTC 8003.
In stationary-phase cells of strain C9490, no loss of viability occurred below 500 MPa, whereas in strain NCTC 8003, the onset of cell death began at 100 MPa, confirming the difference in resistance previously described by Benito et al. (2) (Fig. 2a). The pressure-resistant strain C9490 completely retained its ability to plasmolyse in a high-salt solution, but strain NCTC 8003 showed a partial loss of plasmolysis response (Fig. 2b). When PI was added after decompression, no uptake was observed in either strain (Fig. 2c). However, when PI was present in the suspending medium during pressurization, both strains took up the dye at pressures above 100 MPa, but the degree of staining was greater in pressure-sensitive strain NCTC 8003 (Fig. 2c).
FIG. 2.
Pressure resistance and membrane damage in stationary-phase cells of E. coli strains C9490 and NCTC 8003. (a) Survivors after treatment of C9490 (●) and NCTC 8003 (■) at different pressures for 8 min; (b) osmotic responses of pressure-treated cells of C9490 (●) and NCTC 8003 (■), measured as the increase in OD of cell suspensions placed in 0.75 M NaCl; (c) uptake of PI, present during pressure treatment of C9490 (○) or NCTC 8003 (□) or after pressure treatment of C9490 (●) or NCTC 8003 (■).
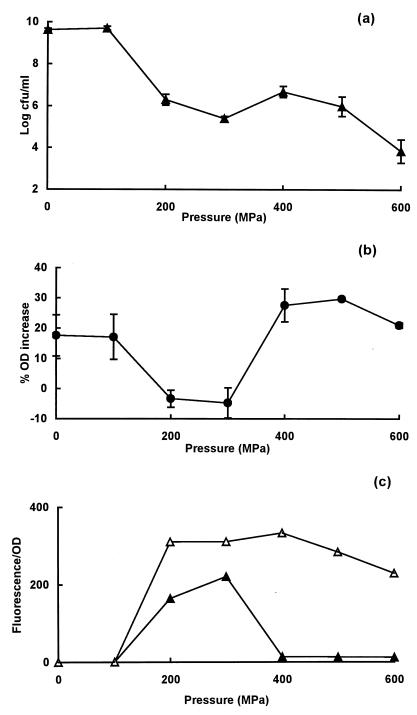
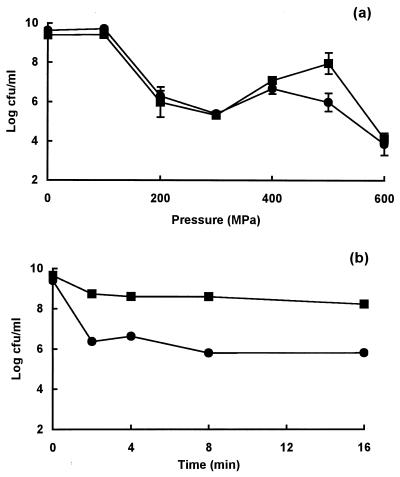
Pressure resistance and membrane damage in stationary-phase cells of strain H1071.
Strain H1071 showed an unusual response to pressure (of a type never seen before), and its behavior is therefore described separately. The onset of cell death began at 100 MPa, and the number of survivors decreased further between 200 and 300 MPa but then increased between 400 and 500 MPa before decreasing again at 600 MPa (Fig. 3a). At 400 MPa, the number of survivors per milliliter was approximately 1.6 log10 units higher than at 300 MPa. This unusual pattern of survival was observed consistently in many experiments.
FIG. 3.
Pressure resistance and membrane damage in stationary-phase cells of E. coli strain H1071. (a) Survivors after treatment at different pressures for 8 min; (b) osmotic responses of pressure-treated cells measured as the increase in OD of cell suspensions placed in 0.75 M NaCl; (c) uptake of PI present during (▵) or after (▴) pressure treatment.
The corresponding ability of cells to plasmolyse decreased at pressures above 100 MPa, reaching a minimum at 200 to 300 MPa before increasing again at higher pressures (Fig. 3b). Uptake of PI (added after pressure treatment) began at pressures above 100 Pa and increased to a maximum at 300 MPa before decreasing to basal levels at 400 MPa (Fig. 3c). Thus, in this strain, the dip in survival observed with pressures between 200 and 400 MPa coincided with maximum membrane damage. However, as with the other strains tested, the membrane of H1071 became transiently leaky at higher pressures, because the uptake of PI present during pressurization increased from a pressure of 100 MPa at the onset to a maximum at 200 MPa, thereafter remaining at a similar level at higher pressures (Fig. 3c).
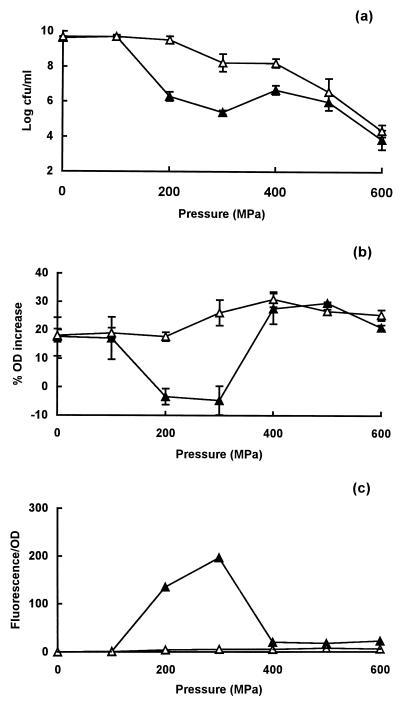
Effect of heat shock on pressure resistance and membrane damage in strain H1071.
A heat shock treatment at 45°C for 45 min increased the pressure resistance of strain H1071 at pressures between 200 and 400 MPa (Fig. 4a). The increase in pressure resistance was greater at 200 and 300 MPa than at 400 MPa; at the higher pressures of 500 and 600 MPa, no significant protective effect was seen. In parallel with this increase in pressure resistance, heat-shocked cells no longer lost the ability to plasmolyse after treatment at 200 or 300 MPa (Fig. 4b), and no uptake of PI was observed (Fig. 4c).
FIG. 4.
Influence of a heat shock treatment at 45°C for 45 min on pressure resistance and membrane damage in E. coli strain H1071. (a) Survivors after exposure to different pressures for 8 min without (▴) or with (▵) a prior heat shock treatment; (b) osmotic response of cells pressure treated without (▴) or with (▵) a prior heat shock treatment; (c) uptake of PI after decompression in cells pressure treated without (▴) or with (▵) a prior heat shock treatment.
Effect of inducing β-galactosidase synthesis during growth on pressure resistance of cells of strain H1071.
During studies of the inactivation of β-galactosidase in pressure-treated cells, we noticed that growth in the presence of the inducer IPTG affected the pattern of pressure resistance in strain H1071. The principal effect was to increase pressure resistance specifically at pressures around 500 MPa (Fig. 5a). This had the effect of increasing the difference in pressure resistance between cells treated at 200 MPa and those treated at 500 MPa. To examine this effect in more detail, the time course of inactivation was followed at 200 and 500 MPa. Figure 5b shows unequivocally that stationary-phase cells of strain H1071 were inactivated to a greater extent at the lower pressure than at the higher.
FIG. 5.
Effect of growth in the presence of IPTG on pressure resistance of E. coli strain H10171. (a) Survival after exposure to different pressures for 8 min in cells grown in medium with (■) or without (●) 2 mM IPTG; (b) time course of inactivation at 200 (●) or 500 (■) MPa for cells grown in medium containing IPTG.
To investigate this phenomenon further, we examined the pressure resistance of strains carrying mutations in the lactose operon. However, when strains were tested over the same range of pressures, no differences in resistance were seen between E. coli K-12 strain MO (parent) and M7025 (lacZ), M7044 (lacY), and M3000 (lacI) strains (data not shown).
DISCUSSION
There are large differences in pressure resistance between E. coli strains C9490, H1071, and NCTC 8003, but these differences are observed only in stationary-phase cells. These strains therefore provide a good opportunity for investigating the relationship between membrane damage and loss of viability under conditions in which cells of the different strains display similar or different resistances to pressure inactivation, depending on the growth phase.
There was a good relationship between the onset of membrane damage and loss of viability in exponential-phase cells of all three strains. After 8 min of pressure treatment at 100 MPa, a 75% decrease in the ability to plasmolyse in exponential-phase cells in the three strains was observed, which was consistent with a similar decrease in the number of survivors. Above this pressure, more than 99% of the cells were dead, and the ability to plasmolyse had been reduced to nil. On the other hand, the uptake of PI seems to be a later event occurring at slightly higher pressures. This seems to indicate that, for exponential-phase cells, the loss of ability to plasmolyse is more closely related to cell inactivation than uptake of PI. The simplest interpretation of the data for exponential-phase cells is that pressure causes physical membrane damage of a type that cannot be resealed and that such a permanent loss of membrane integrity is inimical to the maintenance of homeostasis and leads ultimately to the death of the cell.
The relationship between membrane damage and loss of viability of stationary-phase cells after pressurization was different from that seen in exponential-phase cells. In cells of strains C9490 and NCTC 8003, there was no uptake of PI in cells stained after decompression, whereas staining did occur when PI was present during the pressure treatment. We interpret this as showing that membranes of stationary-phase cells become leaky during pressure treatment but reseal after decompression, at least to the extent of excluding PI. Because membrane leakage under pressure occurred in strain C9490 without cell death, we can conclude that a transient increase in membrane permeability is not necessarily lethal. It is noteworthy that resealing of cell membranes in strain NCTC 8003 occurred after all pressure treatments, despite the fact that more than 99.9% of cells were killed by pressures above 100 MPa.
A direct role for membrane damage in the death of stationary-phase cells is thus more questionable. While both pressure-sensitive and pressure-resistant strains NCTC 8003 and C9490 appeared to reseal membranes after decompression, the membranes of sensitive strain NCTC 8003 evidently became more permeable to sodium chloride, as shown by the large decrease in the osmotic response. The death of stationary-phase cells could thus be due to a more subtle loss of the semipermeable properties of the membrane than in membranes of exponential-phase cells. Although the membranes appear to be able to reseal physically, death may result from loss of vital membrane functions, for example, those to do with energy conservation or ion flux.
The increase in OD observed when cell suspensions are placed in hypotonic salt solutions, known as the optical effect, is caused by an increase in light scattering that is related to the extent of plasmolysis (15). In cold-shocked cells of Klebsiella (formerly Aerobacter) aerogenes, there was a good correlation between loss of osmotic response and cell death (24). Korber et al. (12) developed a microscopic method for measuring plasmolysis in single cells and showed that loss of osmotic response was associated with cell death. However, in starved K. aerogenes cells, cell death preceded loss of permeability control (19); E. coli cells frozen in water at freezing rates lower than 6°C/min died with no loss of membrane integrity (3).
In pressure-treated cells, the correlation between loss of osmotic response and death was not absolute because stationary-phase cells of NCTC 8003 retained up to 50% of their ability to plasmolyse even when more than 99.9% of cells were dead. Conversely, at 100 MPa, some loss of response was detected with no loss of viability. Similarly, cells of strain H1071 treated at pressures above 400 MPa completely retained their ability to plasmolyse when more than 99.9% had died. While the osmotic response is a useful test of membrane integrity, it is not necessarily a good indicator of viability under all circumstances. The nature of the permanent damage sustained by stationary-phase cells whose membranes have resealed requires further investigation. Recent work by Ritz et al. (20) has shown that the protein content of the membranes of Salmonella enterica serovar Typhimurium was modified by pressure, and it would thus be instructive to investigate possible differences in membrane protein composition in the E. coli strains used in this work. Alternatively, some other form of damage could be responsible for cell death in stationary-phase cells, e.g., ribosome denaturation (16).
The behavior of strain H1071 under pressure was different from anything previously described. It has never been reported before that vegetative cells were more resistant to higher- than to lower-pressure treatments. A superficially similar phenomenon occurs with spores of Bacillus and Clostridium species, in which inactivation is typically greater between 200 and 300 MPa than at higher pressures. In this case, pressure treatment causes spores to germinate and lose their resistance to heat and pressure, and the extent and/or completeness of germination is greatest at intermediate pressures (6, 26).
The two methods used to assess loss of membrane integrity allowed us to demonstrate major differences in the nature of membrane damage in strain H1071 at pressures below and above 300 MPa. Cells treated at pressures between 200 and 300 MPa lost their ability to plasmolyse and became permeable to PI added either during or after pressure treatment. In this pressure range, therefore, stationary-phase cells of strain H1071 sustained permanent membrane damage and thus behaved like exponential-phase cells. At pressures of 400 MPa and above, cells became leaky but resealed after decompression and retained their ability to respond osmotically to high-salt solutions. In this higher-pressure range, cells showed the typical behavior of stationary-phase cells.
Exposure of stationary-phase cells of strain H1071 to a mild heat shock at 45°C increased pressure resistance in the 200 to 300 MPa range and had the associated effects of enabling cell membranes to reseal and protecting against the loss of ability to plasmolyse. In this respect, the response of cells to pressures in this intermediate region changed from that typically seen in the exponential phase to that typical of the stationary phase.
Growth in the presence of IPTG had the converse effect of increasing resistance to a pressure treatment at 500 MPa but not to pressures between 200 and 400 MPa. The mechanism of this effect is not known. It is conceivable that proteins of the lac operon might play a direct role in pressure resistance; for example, an alteration in the membrane content of the lactose permease might affect membrane susceptibility to damage. We were unable to detect any differences between the pressure response of various lactose negative mutants (lacY, lacI, and lacZ) and their otherwise isogenic parent, but these results do not necessarily rule out a direct role for proteins of the lac operon because the parent K-12 strain did not show the unusual pressure response of H1071. To investigate this further will require isolating the relevant lactose-negative mutants in strain H1071. An alternative possibility is that a stress response is involved, because IPTG is a weak inducer of a subset of the heat shock proteins (4, 13).
The greater sensitivity of strain H1071 to lower pressures than to some higher pressures suggests that the effects of pressure on cells are not the same under different treatment conditions. Perhaps the nature of cell damage at pressures between 200 and 300 MPa is different from that at 400 MPa or higher; only when a strain is especially sensitive to low-pressure conditions are we able to detect this difference, seen here as the odd behavior of strain H1071. We have examined several other strains but so far have not seen similar behavior.
The phenotype of strain H1071 appears in some respects as a strange mixture of exponential- and stationary-phase attributes. Sequencing studies have revealed that this strain has a stop codon in its rpoS gene that could give rise to a truncated RpoS protein (M. Robey et al., unpublished observation). We speculate that some RpoS-regulated functions that affect membrane composition and development of pressure resistance are absent in this strain but that heat shock allows these changes to occur by a separate regulatory pathway. If the increase in pressure resistance caused by IPTG is also caused by a stress response, it would appear to involve a separate mechanism.
We have described for the first time important differences in the way that pressure affects the membranes of exponential- and stationary-phase cells and have also presented evidence that the nature of membrane damage may depend on the intensity of the pressure treatment. This in turn implies that the mechanisms of cell death may be different at high and low pressures. These conclusions were possible because of the unusual but fortuitous properties of strain H1071. Our studies broadly support the widely held view that membrane damage is an important event in the inactivation of bacterial cells by high pressure, with the caveat that the nature of membrane damage and its relation to cell death may differ depending on the strain and the phase of growth. In exponential-phase cells, loss of viability appears to be directly related to a permanent loss of physical integrity, whereas in stationary-phase cells, a more complicated picture emerges in which cell membranes become leaky during pressure treatment but can reseal to a greater or lesser extent following decompression. Although death of stationary-phase cells may also be related to loss of membrane integrity, other factors, such as ribosome denaturation, could also be involved (16). Confirmation of the central role of membrane damage in cell death will require a better understanding of its precise nature.
ACKNOWLEDGMENTS
We are grateful to the Spanish Ministry of Education and Science who provided R. Pagán with a grant to carry out this investigation.
We also thank Mary K. B. Berlyn of the E. coli Genetic Stock Center, Yale University, for providing strains of E. coli K-12.
REFERENCES
- 1.Bartlett D H. Microbial life at high pressures. Sci Progr (Oxford) 1992;76:479–496. [PubMed] [Google Scholar]
- 2.Benito A, Ventoura G, Casadei M A, Robinson T P, Mackey B M. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat, and other stresses. Appl Environ Microbiol. 1999;65:1565–1569. doi: 10.1128/aem.65.4.1564-1569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcott P H, Macleod R A. The survival of Escherichia coli from freeze-thaw damage: permeability damage and viability. Can J Microbiol. 1975;21:1724–1732. doi: 10.1139/m75-253. [DOI] [PubMed] [Google Scholar]
- 4.Cha H J, Srivastava R, Vakharia V N, Rao G, Bentley W E. Green fluorescent protein as a noninvasive stress probe in resting Escherichia coli cells. Appl Environ Microbiol. 1999;65:409–414. doi: 10.1128/aem.65.2.409-414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheftel J C. Review: high pressure, microbial inactivation and food preservation. Food Sci Technol Int. 1995;1:75–90. [Google Scholar]
- 6.Gould G W, Sale A J H. Role of pressure in the stabilization and destabilization of bacterial spores. Symp Soc Exp Biol. 1972;26:147–157. [PubMed] [Google Scholar]
- 7.Hauben K J A, Wuytack E Y, Soontjens C C F, Michiels C W. High-pressure transient sensitisation of Escherichia coli to lysozyme and nisin by disruption of outer-membrane permeability. J Food Prot. 1996;59:350–355. doi: 10.4315/0362-028X-59.4.350. [DOI] [PubMed] [Google Scholar]
- 8.Hoover D. Minimally processed fruits and vegetables: reducing microbial load by non-thermal physical treatments. Food Technol. 1997;51:66–71. [Google Scholar]
- 9.Kalchayanand N, Sikes A, Dunne C P, Ray B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl Environ Microbiol. 1994;60:4174–4177. doi: 10.1128/aem.60.11.4174-4177.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalchayanand N, Sikes A, Dunne C P, Ray B. Factors influencing death and injury of foodborne pathogens by hydrostatic pressure-pasteurization. Food Microbiol. 1998;15:207–214. [Google Scholar]
- 11.Knorr D. Effects of high-hydrostatic-pressure processes on food safety and quality. Food Technol. 1993;47:156–161. [Google Scholar]
- 12.Korber D R, Choi A, Wolfaardt G M, Caldwell D E. Bacterial plasmolysis as a physical indicator of viability. Appl Environ Microbiol. 1996;62:3939–3947. doi: 10.1128/aem.62.11.3939-3947.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosinski M J, Rinas U, Bailey J E. Isopropyl-β-d-thiogalactopyranoside influences the metabolism of Escherichia coli. Appl Microbiol Biotechnol. 1992;36:782–784. [Google Scholar]
- 14.Macdonald A G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Philos Trans R Soc Lond B Biol Sci. 1984;304:47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- 15.Mager J, Kuczynski M, Schatzberg G, Avi-Dor Y. Turbidity changes in bacterial suspensions in relation to osmotic pressure. J Gen Microbiol. 1956;14:69–75. doi: 10.1099/00221287-14-1-69. [DOI] [PubMed] [Google Scholar]
- 16.Niven G, Miles C A, Mackey B M. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology. 1999;145:419–425. doi: 10.1099/13500872-145-2-419. [DOI] [PubMed] [Google Scholar]
- 17.Patterson M. High-pressure treatment of foods. In: Robertson R K, Batt C A, Patel P D, editors. The encyclopedia of food microbiology. London, United Kingdom: Academic Press; 1999. pp. 1059–1065. [Google Scholar]
- 18.Patterson M F, Kilpatrick D J. The combined effect of high hydrostatic pressure and mild heat on inactivation of pathogens in milk and poultry. J Food Prot. 1998;61:432–436. doi: 10.4315/0362-028x-61.4.432. [DOI] [PubMed] [Google Scholar]
- 19.Postgate J R, Hunter J R. The survival of starved bacteria. J Gen Microbiol. 1962;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- 20.Ritz M, Pilet M F P, Tholozan J L, Federighi M. High hydrostatic pressure effects on Salmonella typhimurium. Physiological and morphological damages. In: Tuijetlaars A C J, Samson R A, Rombouts F M, Notermans S, editors. Food microbiology and food safety into the next millennium. Proceedings of the Seventeenth International Conference of the International Committee on Food Microbiology and Hygiene (ICFMH). Zeist, The Netherlands: TNO Nutrition and Food Research Institute; 1999. pp. 295–298. [Google Scholar]
- 21.Shigehisa T, Ohmori T, Saito A, Taji S, Hayashi R. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat products. Int J Food Microbiol. 1991;12:207–216. doi: 10.1016/0168-1605(91)90071-v. [DOI] [PubMed] [Google Scholar]
- 22.Smelt J P P M. Recent advances in the microbiology of high-pressure processing. Trends Food Sci Technol. 1998;9:152–158. [Google Scholar]
- 23.Smelt J P P M, Rijke A G F, Hayhurst A. Possible mechanism of high-pressure inactivation of microorganisms. High Pressure Res. 1994;12:199–203. [Google Scholar]
- 24.Strange R E. Effect of magnesium on permeability control in chilled bacteria. Nature (London) 1964;203:1304–1305. doi: 10.1038/2031304a0. [DOI] [PubMed] [Google Scholar]
- 25.Wouters P C, Glaasker E, Smelt J P P M. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl Environ Microbiol. 1998;64:509–514. doi: 10.1128/aem.64.2.509-514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuytack E, Boven S, Michiels C W. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl Environ Microbiol. 1998;64:3220–3224. doi: 10.1128/aem.64.9.3220-3224.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]