Abstract
Oysters are saltwater bivalves with high nutritional and medicinal value that are consumed widely around the world. As well as being highly nutritious, oysters are a low-calorie, low-cholesterol source of protein and an exceptional source of zinc, which strengthens the immune system; and a rich source of bioactive compounds, which comprise various biological activities. The present review summarizes the biological applications and bioactive compounds from oyster shells, whole tissue, gill tissue, and mantle tissue. The various biological compounds present in an oyster shell, and their chemical constituents, have applications in the food, pharmaceutical, and medical industries. Bioactive peptides and proteins obtained from the whole, mantle, and gill tissues of oysters exhibit antioxidant, antimicrobial, antihypertensive, anticancer, antifatigue, anticoagulant, and anti-wrinkle effects, as well as enhance osteoblast differentiation. This review clearly shows that oysters have great potential for functional food production and that various compounds therein can have pharmaceutical applications.
Keywords: marine organisms, oyster, bioactive peptides, protein hydrolysates, oyster shell, the biocidal activity of oyster, oyster peptide
Introduction
Oyster is an important and extensively farmed marine resource with high commercial value (Cheng et al., 2021; Hao et al., 2021). Oyster aquaculture has been practiced for over 2,000 years (Campbell and Hall, 2018). According to the Food and Agriculture Organization (FAO, 2018), global oyster production stands at around 6.1 million tons per year, and worldwide, oyster exports increased threefold from 1997 to 2017. Oysters are highly nutritious and possess medicinal value. Oysters have high protein, active polysaccharides, taurine, vitamin, and mineral contents, and are also low in fat (Guo et al., 2020). Many researchers aim to extract bioactive materials from various oyster parts for biomedical applications.
More than 100 species of oyster are cultured worldwide, including Suminoe oyster (Crassostrea ariakensis), Zhe oyster (Crassostrea plicatula), and Pacific cupped oyster (Crassostrea gigas) (Botta et al., 2020). These oysters are excellent sources of nutrition. The protein, glycogen, and fat contents of oyster flesh (dry flesh weight) are approximately 39.1–53.1%, 21.6–38%, and 7.8–8.7%, respectively (Linehan et al., 1999). Thus, oysters are an excellent source of protein and provide vital nutrients with a wide range of bioactive effects (Guo et al., 2020).
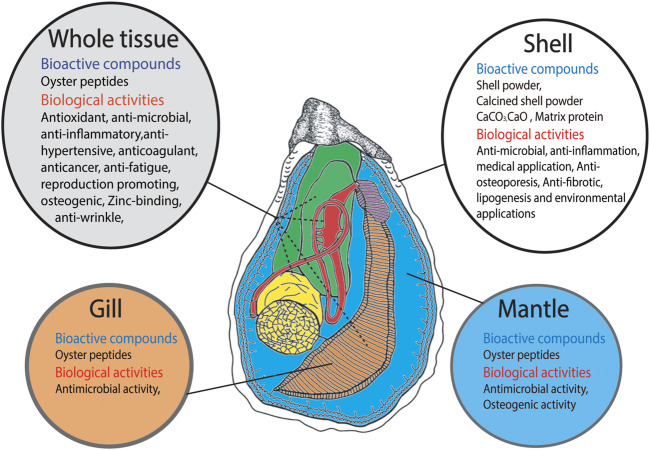
Oyster protein can be defragmented into a large number of peptides with high bioactivity. Recently, oyster protein hydrolysates (OPHs) and peptides have attracted attention due to their stability and diverse biological activities (Xie et al., 2018; Guo et al., 2020). Many studies have described the bioactive properties of oyster peptides (OPs), including antioxidant, antitumor, immunomodulatory, antimicrobial, antiviral, antihypertensive, anti-inflammatory, antifungal, anticancer, antimelanogenic, anti-wrinkle, anti-fatigue, anticoagulant, antithrombotic, and osteogenic effects. OPs may also enhance spatial learning and memory, acetylcholinesterase activity, and sexual function, as well as serve as angiotensin-converting enzyme (ACE) inhibitors (Wang et al., 2020 and references therein, Zhang et al., 2021 and references therein, Hao et al., 2021 and references therein). Oyster protein can reduce blood pressure (Achour et al., 1997; Tanaka et al., 2006). Moreover, some studies have reported bioactive effects of compounds in oyster shell, including anti-inflammatory, antiosteoporosis, antifibrotic, antimicrobial, and antifungal effects, and lipogenesis inhibition (Lee et al., 2013; Xing et al., 2013; Han et al., 2015; Latire et al., 2017; Tran et al., 2015; Feng et al., 2021; Figure 1).
FIGURE 1.
Schematic representation of Bioactive compounds derived from different parts of oyster such as shell, whole tissue, gill tissue and mantle tissue and their biological activities.
Oysters are rich in selenium, which supports a number of cellular functions including heavy metal detoxification. Additionally, oyster soft tissue has a higher zinc (Zn) content than most seafoods (Li et al., 2019). Zn-chelating peptides extracted from oysters have attracted wide attention. Chen et al. (2013) demonstrated that (Figure 1) the peptide HLRQEEKEEVTVGSLK, produced via oyster protein hydrolysis, has the ability to bind Zhang et al., 2018 demonstrated that the OPH-Zn complex enhances Zn bioaccessibility.
This review aims to provide insight into the bioactive compounds found in whole, gill, and mantle tissues of oysters, as well as the shell. We first focus on the extensive bioactivity of OPHs, peptides, and matrix proteins in an oyster shell, shell extracts, and calcified shell powder, then discuss future directions for studies on bioactive compounds in oysters.
Oyster Shell
Oysters are economically and ecologically important shellfish known for their delicious meat and calcareous shell. The oyster shell comprises an organic matrix and minerals that protect soft tissue (Upadhyay et al., 2016). The shell accounts for about 60% of the total oyster weight (Xing et al., 2013). Oyster shell disposal is associated with waste accumulation and water and marine pollution, due to improper landfill and microbial activity, as well as off-odor issues due to the use of cheap disposal methods, high management costs, and negative effects on soil pH caused by inappropriate recycling (e.g., use as fertilizer). However, oyster waste products have potential as safe and ecofriendly functional compounds. The use of these waste products as biocompatible antimicrobials could improve human health and waste management (Sadeghi et al., 2019). Oyster shells mainly consist of calcium carbonate (CaCO3; ∼95%) in addition to a small proportion of organic matrix proteins (∼0.1–5%), which are also called skeleton/shell proteins (Upadhyay et al., 2016; Figure 2).
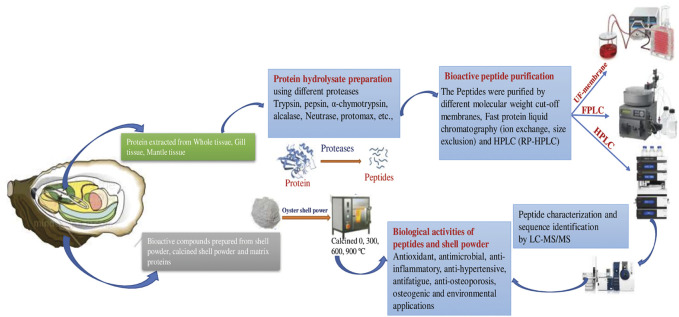
FIGURE 2.
Schematic representation of Preparation and applications of Bioactive peptides from oyster tissue and shell.
Antimicrobial and Biocidal Activities
Calcium-based compounds such as calcium oxide (CaO) and calcium hydroxide [Ca(OH)2] can be derived from oyster shell. CaO is extensively used as a catalyst in industrial research and tissue engineering. Calcined oyster shell powder has attracted considerable attention due to its biocompatibility and antimicrobial and biocidal activities. Hence, calcined oyster shell could serve as a good antimicrobial alternative in food processing and food packaging. Adding these natural antimicrobial additives to processed and fresh foods could be a safe means of ensuring food quality. The antimicrobial activity of oyster shell relies primarily on the alkalinity of CaO, which is a major compound in calcined oyster shell that increases the surrounding pH. Calcium ions derived from CaO react with cardiolipin (a major lipid in the bacterial cell membrane), leading to cell wall rupture and the generation of reactive oxygen species (ROS) and free radicals, which strongly affect cell integrity. The antifungal activities of CaO are also related to its alkalinity and ROS generation (Sadeghi et al., 2019).
Over the last 2 decades, many studies have revealed antimicrobial and antifungal activities of oyster shell powder presented in (Table 1). Oikawa et al. (2000) reported that calcined shell powder derived from various species (i.e., oyster, scallop, and clam) exerted strong antimicrobial activity by retarding aerobic bacterial growth and suppressing Escherichia coli. Choi et al. (2006) observed that 0.05% oyster shell powder significantly improved the quality of kimchi during storage by reducing and maintaining the numbers of aerobic and lactic bacteria, respectively. Similarly, Kim et al. (2007) reported that the shelf life of tofu could be extended by up to 2 days by adding 0.05–0.2% oyster shell powder, which reduced microbial expression to maintain quality and freshness. Jung et al. (2010) reported that oyster shell powder prolonged the storage time (to 80 days at 5°C) and quality of gat kimchi by reducing lactic acid bacteria, yeast, and E. coli compared to the control. A study on the antifungal activity of calcined oyster shells, with heat-treated (1,050°C) CaO as the major compound, demonstrated strong biocidal activity against Physalospora piricola and Rhizoctonia solani. That study also reported antifungal activity from non-calcined oyster shell, which could be due to the alkalinity of CaCO3 in the slurry phase (Xing et al., 2013).
TABLE 1.
Bioactive compounds derived from oyster shell and their biological applications.
| Species | Application | Bioactive compound | Results | References |
|---|---|---|---|---|
| Oyster | Antimicrobial activity | Calcined shell powder | Suppressing the growth of Escherichia coli | Oikawa et al. (2000) |
| Oyster | 0.05% of Shell powder | Improving the quality of kimchi by reducing the growth of aerobic bacteria and increasing lactic bacteria | Choi et al. (2006) | |
| Oyster | 0.05–0.2% of shell powder | Reducing he micobial number and increase the self life of tofu | Kim et al. (2007) | |
| Oyster | Shell powder additives | Reducing the microbial number and prolonged the and quality of Gat kimchi | Jung et al. (2010) | |
| Oyster | Heat trated calcined oyster shell (CaO) and non-calined oyster shell | Calcined shellpowder showed biocidal activity against the Physalospora piricola and Rhizoctonia solani and non-calcine shell powder showed antifungal activity | Xing et al. (2013) | |
| Oyster | Calcined shell powder | Inhibited the microbial growth and increased the self life of pork ham | Choi et al. (2014) | |
| Oyster | Calcined shell powder | Inhibited the food borne disease microorganisms (Staphylococcus aureus), | Chen et al. (2015) | |
| Listeria monocytogenes, Salmonella typhimurium, Enterobacter aerogenes, and Proteus vulgaris) | ||||
| Oyster | Propylene film with calcined shell powder | Enhanced antimicrobial efficiency without cytotoxicity | Tsou et al. (2019) | |
| Oyster | Anti-inflammatory activity | Oyster shell extract | Suppressed the NO production, decreased the expression of the oyster iNOS, COX-2 and NF-κB and inhibites the production of IL-1β, IL-6, and TNF-α in LPS stimulated Raw 264.7 cells | Lee et al. (2013) |
| Oyster | Bone tissue bioengineering | Nacre as bone graft sustituion | Narce binds directely with newly formed bone without any fiborous tissue formation | Atlan et al. (1999) |
| Oyster | Molecules of nacre | Activates the osteogenesis in bone marrow cells | Lamghari et al. (1999) | |
| Crassostrea gigas | Water soluble matrix protein (WSMP) | Identified protein with osteogenic activity which are responsible for the bone remodelling and biocompatibility | Oliveira et al. (2012) | |
| Oyster | Nacre powder | osteogenic differentiation of human bone marrow mesenchymal stem cells | Flausse et al. (2013) | |
| Oyster | Nacre particle and its soluble protein matrix | Induce the differentation in human bone cells (osteoinductive capacity) | Green et al. (2015) | |
| Crassostrea gigas | Anti-osteooresis activity | WSMP | Promote the osteogenesis and inhibits osteoclast absorption | Feng et al. (2021) |
| Oyster | Anti-fibrotic activity | Shell extract | Enhances the catabolic pathway of human dermal fibroblasts | |
| Oyster | Lipogenesis inhibition | Oyster shell extract | lipid-lowering effect via inhibition of lipogenesis and diminished the cellular triglyceride level | Khoi et al., 2015 |
| Oyster | Environmental application | Oyster shell | Used to remove pollutants, organics, neurotoxin and nitrogen | Bonnard. 2021 |
| Oyster | Environmental application | Oyster shell powder | Mitigate the harmful algal bloom by reducing phosphate, nitrogen and COD | Huh et al. (2016) |
Choi et al. (2014) increased the shelf life of ham by adding calcined shell powder, which inhibited microbial growth. Similarly, Chen et al. (2015) derived an antimicrobial agent through the calcination of oyster, hard clam, and sea urchin shells that suppressed the growth of foodborne microorganisms such as Staphylococcus aureus, Listeria monocytogenes, Salmonella typhimurium, Enterobacter aerogenes, and Proteus vulgaris. However, non-calcined oyster shell powder did not inhibit microbial growth. The addition of antimicrobial agents to polymer matrices enhances shelf life and prevents foodborne diseases. Tsou et al. (2019) observed that the addition of calcined oyster shells to propylene film enhanced antimicrobial efficiency without causing any cytotoxicity. Thus, calcined oyster shell powder is a good option for increasing the shelf life of food products and preventing foodborne diseases.
Lee et al. (2013) showed that oyster shell extract can significantly suppress the production of nitric oxide (NO) and decrease the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and nuclear factor (NF)-κB. Additionally, oyster shell extract significantly inhibited the production of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in lipopolysaccharide (LPS)-stimulated RAW264.7 cells, thus exerting an anti-inflammatory effect.
Medical Applications
Along with high CaCO3 content, the organic matrix network plays a vital role in biomineral formationin oyster shells. It comprises macromolecules such as polysaccharides (mostly chitin), water soluble and insoluble proteins (including glycol proteins), and lipids, in addition to smaller molecules such as pigments, free amino acids, and short peptides (Marin et al., 2012). These biomolecules are distinguished based on extraction methods and solubility, with matrix types including the water-soluble matrix (WSM), ethanol-soluble matrix (ESM), acid-soluble matrix (ASM), acid-insoluble matrix (AIM), ethylenediaminetetraacetic acid (EDTA)-soluble matrix (EDTASM), EDTA-insoluble matrix (EDTAIM), and fat-soluble matrix (FSM; (Bonnard, 2021). In addition to regulating biomineralization, the WSM also promotes nacre biological activities such as cell recruitment, differentiation, and stimulation. Due to its beneficial biological activities, nacre (and its biomatrix) is used in traditional pharmaceutical preparations to stimulate bone growth and enhance bone density (Chaturvedi et al., 2013 and references therein). Recent in vitro and in vivo studies also suggest that nacre is a biocompatible and biodegradable material with osteoinductive, osteointegrative, and osteoconductive properties. Thus, nacre (and its biomatrix) has been evaluated as a potential bone substitute. In fact, nacre has been applied in bone tissue bioengineering as early as 1931 (in the jawbone of a Mayan individual; Green et al., 2015).
A major breakthrough in bone graft substitution using nacre was achieved by Lopez et al. (1992). Atlan et al. (1999) subsequently investigated the interface between bone and nacre in sheep, showing that nacre binds directly to newly formed bone without the need for intervening fibrous tissue. In vitro and in vivo studies have demonstrated that nacre contains molecules capable of activating osteogenic bone marrow cells (Lamghari et al., 1999). Using proteomics, proteins with osteogenic activity were identified among WSM proteins (WSMPs) from C. gigas nacre. These proteins are important for bone remodeling and biocompatibility (Oliveira et al., 2012). The effects of alginate hydrogels, including nacre powder, on osteogenic differentiation of human bone marrow mesenchymal stem cells have also been investigated (Flausse et al., 2013).
Chaturvedi et al. (2013) showed that water-soluble bioactive compounds in nacre possess antioxidant activity and promote osteoblast differentiation. A study on the osteogenic potency of human mesenchymal stem cells indicated that nacre and its soluble protein matrix induce early differentiation of human bone cells via osteoinductive effects (Green et al., 2015). Studies have also been conducted on nacre powder mixed with blood, nacre chips, nacre prostheses, and nacre matrix proteins, as well as the effects of nacre WSM on alkaline phosphate activity in MRC-5 fibroblasts and osteogenic activities of nacre WSM and ESM (for a review, see Zhang et al., 2017). Recent in vitro and in vivo studies demonstrated anti-osteoporosis effects of WSMPs from C. gigas that result from the promotion of osteogenesis and inhibition of osteoclast absorption (Feng et al., 2021). Shell extracts also activate the catabolic pathway of human dermal fibroblasts and are thus used in anti-fibrotic strategies, especially in the scleroderma. Latire et al. (2017) and Khoi et al. (2015) investigated the ability of oyster shell extract to inhibit lipogenesis and thus provide a lipid-lowering effect (especially of cellular triglycerides). These studies suggest that oyster shell extracts such as those from the WSM and ESM and WSMPs have a wide range of therapeutic and medicinal effects, including anti-osteoporotic, anti-fibrotic, and osteogenic activities as well as lipogenesis inhibition. Thus, they can play a role in human tissue engineering.
Environmental and Other Apllications of Shell Waste/Byproducts
Oysters reduce the eutrophication of water bodies and may reduce the amounts of metal cations, plastic particles, and other chemicals in water. Oyster shells can be used to remove pollutants such as certain anions (phosphate [PO4 3-], F−, and NO3 −) and cations (Cu, Ni, Mn, As, U, Th, Pb, Fe, Zn, and Co), antibiotics, neurotoxins, and excess nitrogen (N) (Bonnard, 2021). Oyster shell powder has also been used to improve the water quality of lakes by facilitating the removal of algal blooms. This was achieved by reducing total PO4 3- (by 97%), N (by 91%), and chemical oxygen demand (by 51%; Huh et al., 2016). Moreover, oyster shell powder has been used to prevent the migration of N from sediment (to improve the sediment-water quality) and to suppress eutrophication and control harmful algal blooms in the marine environment (Khirul et al., 2020). Shell waste is also used as a soil conditioner and acts as a pH buffer, sorbent or fertilizer. The hydrogen carbonate content of the oyster shell made it the best neutralizer of acidic soil. Treatment of acidic soil with oyster shells neutralizes the soil as well as increases the Ca2+, Mg2+, K+ and Na+ and the stabilization of heavy metals with low solubility that is less exchangeable upon lixiviation (Du et al., 2011; Moon et al., 2014). Calcinated oyster shells are also effective in removing air pollutants such as SO2, SO3, H2S, and NO2 in dry or wet processes as well as CO2 sequestration. Combination of oyster shell and Polyvinyl chloride (PVC), neutralizes harmful hydrochloric acid which results from PVC incineration mimicking the activity of commercially available calcium carbonate and with CaCl2 as by-products. Oyster shell wastes are also used in material synthesis (Filler incomposite, foaming agent, template, support for catalysts, source of sodium, calcium, and, calcium carbonate) and used as building materials (Limestone and aggregate) and cosmetic ingredients (Bonnard, 2021).
Oyster Tissues
Oyster meat accounts for about half of the dry weight of an oyster (Linehan et al., 1999). Various enzymes have been used to digest oyster meat, and bioactive peptides have been purified from OPHs. OPs possess a wide range of bioactivities that vary according to the receptors involved. This section deals with the various bioactive effects of OPs, including antioxidant, anti-inflammatory, anticancer, antimicrobial, antihypertensive, anticoagulant, antithrombotic, and antifatigue effects; they also inhibit ACE (Figure 2).
Antioxidants
Antioxidants are free-radical scavengers that prevent cell damage by disrupting the radical chain reaction underlying lipid peroxidation (by scavenging ROS, which play a major role in many diseases) (Kim et al., 2007; Papachristoforou et al., 2020). During normal metabolic reactions, free radicals are produced in cells and tissues, adversely affecting biomolecules such as nucleic acids, lipids, and proteins. They also affect redox status and promote oxidative stress. Free radical-associated oxidative stress is involved with diabetes mellitus, neurodegenerative disorders (e.g., Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis), cardiovascular diseases (e.g., atherosclerosis and hypertension), respiratory diseases, pulmonary dysfunction, cataracts, rheumatoid arthritis, and various cancers (Phaniendra et al., 2015). Some natural antioxidants, such as ascorbic acid, tocopherol, and catechin, are common in foods, medicines, and pharmaceuticals; accordingly, they are used in industries in which the application of synthetic antioxidants (such as butylated hydroxytoluene, tert-butyl hydroquinone, and propyl gallate) is restricted due to the latter’s toxic effects.
Oyster peptides exhibit significant antioxidant activity, and several bioactive peptides have been purified from oyster tissue via enzymatic degradation. Presented in Table 2. DNA is the ultimate target of ROS-related oxidative damage. The antioxidative peptide LKQELEDLLEKQE, isolated from the gastrointestinal digestive system of C. gigas, scavenged cellular and hydroxyl radicals (Qian et al., 2008). OPs were isolated from Crassostrea talienwhannensis meat via the action of digestive proteases including papain, neutrase, and alcalase. Hydroxylates derived via alcalase digestion exhibited high antioxidant activity (Dong et al., 2010). A study on OPs isolated from Ostrea plicatula meat using neutral proteinase reported scavenging effects against hydroxyl radicals (e.g., 1,1-diphenyl-2-picrylhydrazyl) and superoxide anion radicals (Hao et al., 2013). In a similar study, the tissue of Saccostrea cucullata was digested using protease enzyme, and seven peptides were obtained (SCAP 1–7). These OPHs exhibited potential for donating hydrogen atoms and scavenging hydrogen peroxide, hydroxyl, and diphenyl-picrylhydrazyl (DPPH) radicals. Among SCAP 1–7, SCAP 1, 3, and 7 exhibited the highest scavenging ability for DPPH radicals (Umayaparvathi et al., 2014). Wang et al. (2014) isolated two OPs (PVMGA and QHGV) from the meat of C. talienwhanensis with high antioxidative activities, as reflected in their hydroxyl and DPPH radical scavenging activities. Crassostrea gigas meat can be digested by food-grade enzymes such as alcalase, bromelin, and neutral proteases. Among OPs, those derived from hydrolysates exhibited the strongest scavenging activity against DPPH and hydroxyl radicals (Zhang et al., 2015). OPs from enzymatic hydrolysates of Crassostrea madrasensis exhibited excellent antioxidant activity and inhibited lipid peroxidation (Asha et al., 2016). Interestingly, OPs extracted from Ostrea rivularis by proteases exhibited oxygen radical absorbance capacity (ORAC) and cellular antioxidant activity in a HepG2 cell model (Miao et al., 2018). In a similar study, the antioxidant peptide YA, extracted from oysters, exhibited dose-dependent DPPH and 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity (Xie et al., 2018). Moreover, Zhang et al. (2019) reported higher antioxidant activity in alcalase-hydrolyzed oyster meat (Crassostrea rivularis) compared to meat not subjected to gastrointestinal digestion. In vivo, alcalase-hydrolyzed oyster meat contained high levels of antioxidant enzymes such as glutathione peroxidase, superoxide dismutase, and hydrogen peroxidase, which increased and decreased the levels of T-AOC and malonaldehyde in mice, respectively (Zhang et al., 2019). In a recent study, oyster (C. talienwhanensis) protein was hydrolyzed with trypsin under various conditions to obtain peptides in an optimized manner using response surface methodology (Wang et al., 2020). Hydrophobic OP fractions (PEP-1, PEP-2, TRYP-2, MIX-2, and TRYP-2) were extracted from C. talienwhanensis and exhibited high antioxidant and DPPH scavenging activities in vitro, according to in vitro ferric reducing ability of plasma and ORAC assays.
TABLE 2.
Antioxidant peptides derived from oyster whole tissue.
| Species | Enzyme used | Oyster peptides and/or MW | Results | References |
|---|---|---|---|---|
| Crassostrea gigas | Pepsin, trypsin, α-chymotrypsin | LKQELEDLLEKQE (1600 Da) | Scavenged cellular radicals and protective effect on hydroxyl radicals generated DNA damage | Qian et al. (2008) |
| Crassostrea talienwhannensis | papain, neutrase, and alcalase | (<1 kDa) | Alcalase derived hydrolysates showed the strongest overall antioxidant activity | Dong et al. (2010) |
| Ostrea plicatula Gmelin | neutral proteinase | scavenging effects against the hydroxyl radicals | Hao et al. (2013) | |
| DPPH, and superoxide anion radicals | ||||
| Saccostrea cucullata | Protease | SCAP 1–7 | Peptides (SCAP 1, 3, and 7) had the highest scavenging ability on DPPH radicals | Umayaparvathi et al. (2014) |
| Crassostrea talienwhanensis | Subtilisin | PVMGA (518 Da) and QHGV | DPPH and hydroxyl radical scavenging activity | Wang et al. (2014) |
| Crassostrea gigas | Food grade proteases, alcalase, bromelin, and neutral protease | Alcalase derived hydrolysates showed the maximum scavenging asainst DPPH and hydroxyl radicles | Zhang et al. (2015) | |
| Crassostrea madrasensis | Papain | ISIGGQPAGRIVM (1297.72 Da) | DPPH and hydroxyl radical-scavenging activity, | Asha et al. (2016) |
| Ferric reducing and iron chelating activity and lipid peroxidation inhibition | ||||
| Ostrea rivularis | Protease | (<6 kDa) | ABTS and DPPH radical-scavenging activity oxygen radical absorbance capacity (ORAC) and cellular antioxidant activity (CAA) in a HepG2 cells | Miao et al. (2018) |
| oyster | Protamex, Neutrase | YA | Dose dependent DPPH and ABTS radical scavenging activity | Xie et al. (2018) |
| Microbial transglutaminase | ||||
| Crassostrea rivularis | Crassostrea rivularis | Alcalase | In-vivo study showed the increased activity of antioxidant enzymes (GSH-Px, SOD and CAT) | Zhang et al. (2019) |
| and the T-AOC levels and reduced MDA level | ||||
| Crassostrea talienwhanensis | Trypsin | PVMGA (518 Da), QHGV (440 Da) | DPPH and hydroxyl radical-scavenging activity | Wang et al. (2020) |
| Crassostrea talienwhanensis | Pepsin, trypsin, Maxipro PSP | PEP-1, PEP-2, TRYP-2, and MIX-2 | DPPH and hydroxyl radical-scavenging activity | Qian et al. (2020) |
Antimicrobial Activity
Antimicrobial peptides (AMPs) constitute a small class of naturally abundant peptides that play a vital role in the innate immunity of various organisms. The discovery of antibiotic-resistant microorganisms prompted the development of synthetic AMPs with applications in medicine, the food industry, animal husbandry, and aquaculture. Many studies have demonstrated that AMPs have inhibitory effects against bacteria, fungi, parasites, and viruses (Huan et al., 2020). AMPs consist of amino acids with a positive net charge that form an α helix or β sheet. Based on their structure and components, AMPs promote pore formation and disrupt the negatively charged bacterial membrane via ionic and hydrophobic interactions. Hundreds of AMPs have been isolated from various organisms, from fungi to humans, and are major components of the innate immune defense of many marine invertebrates including crustaceans, chelicerates, and urochordates (Hao et al., 2021).
Oyster blood cells, gills, and mantle tissue contain abundant AMPs. AMPs from oysters affect Gram-positive (Bacillus subtilis, Listeria monocytogenes, Staphylococcus aureus, etc.) and Gram-negative bacteria (E. coli, Vibrio parahaemolyticus, and Vibrio harveyi) as well as fungi (Fusarium oxysporum, Botrytis cinerea, and Penicillium expansum) presented in Table 3. Some studies have shown that the oyster protein peptides LLEYSI and LLEYSL inhibit HIV-1 protease, which is a crucial enzyme for viral maturation and a major target of HIV-1 treatment (Bednasz et al., 2019). These peptides showed superiority over pepstatin A as HIV-1 protease inhibitors (Lee and Maruyama 1998). Vero cells are model organisms for studying the antiviral activities of oyster AMPs. Previous studies have demonstrated cytotoxic effects of AMPs on Vero cells carrying pseudorabies virus and herpes simplex virus type 1 (Olicard et al., 2005; Zeng et al., 2008). OPs are classified as AMPs, antimicrobial proteins (>50 kDa), and ubiquitous proteins, and exhibit antimicrobial activity among other functions. Liu et al. (2007) reported that CgPep33, a novel and cysteine-rich AMP isolated from enzymatic hydrolysates of Pacific oyster (C. gigas), exhibited high inhibitory activity against the fungus Botrytis cinerea. A similar study by Liu et al. (2008) showed that CgPep33 exhibited activity against Gram-positive and -negative bacteria and fungi. Cysteine and aromatic residues were also posited to play a crucial role in CgPep33 antimicrobial activity. Interestingly, chemically synthesized AMPs with short amino-acid chains, referred to as Cg-Prp (found in C. gigas), exhibited antimicrobial activity against Gram-positive and -negative bacteria and fungi (Gueguen et al., 2006). Rosa et al. (2011) identified so-called “big defensin form” AMP sequences involved in genomic organization and the regulation of gene expression. CgMolluscidin is an oyster dibasic residue repeat-rich AMP comprising 23 basic (lysine) and 15 hydrophobic amino acids that exhibits activity against Gram-positive and -negative bacteria. The amino-acid sequence cgTβ, isolated from C. gigas, is similar to β-thymosin in terms of its antibacterial activity. Moreover, protein hydrolysates from digestion by food-grade proteases were shown to exhibit antimicrobial activity against human pathogenic bacteria (Nam et al., 2015; Zhang et al., 2015).
TABLE 3.
Antimicrobial peptide derived from oyster whole Tissue.
| Species | Name of AMP | Enzyme used | Oyster peptides and/or MW | Results | References |
|---|---|---|---|---|---|
| Crassostrea gigas | Thermolysin | LLEYSI, LLEYSL | Inhibit the HIV-1 protease | Lee & Maruyama (1998) | |
| Crassostrea virginica | Plasma peptides | (<10 kDa) | Against the Gram-positive bacteria (B. megaterium) | Anderson and Beaven (2001) | |
| Crassostrea gigas | SPE | Cytotoxic activity against vero cells | Olicard et al. (2005) | ||
| Crassostrea gigas | Cg-Defh1 and Cg-Defh2 | GFGCPRDQYKCNSHCQSIGCRAGYCDAVTLWLRCTCTDCNGKK and GFGCPGDQYECNRHCRSIGCRAGYCDAVTLWLRCTCTGCSGKK | Recombinant AMP’s based on prokaryotic expression of Escherichia coli | Gonzalez et al. (2007) | |
| Crassostrea gigas | CgPep33 | Alcalase | Antifungul activity against Botrytis cinerea | Liu et al. (2007) | |
| Crassostrea gigas | CgPep33 | Alcalase | Antibacterial activity against Gram-positive bacteria (Bacillus subtilis, Streptococcus aureus) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), fungi (B. cinerea, Penicillium expansum) | Liu et al. (2008) | |
| Crassostrea gigas | Cg-Prp | Alcalase | ILENLLARSTNEDREGSIFDTGPIRRPKPRPRPRPEG | Against Gram-positive bacteria (Micrococcus lysodeikticus, Brevibacterium stationis, Microbacterium maritypicum), Gram-negative bacteria (E. coli SBS363, Enterobacter cloacae, Erwinia carotovora, Klebsiella pneumonia), fungi (Fusarium oxysporum, Botrytis cinerea) | Gueguen et al. (2009) |
| Crassostrea gigas | Cg-BigDef1 | QAQALLPIASYAGLTVSAPVFAALVTVYGAYALYRYNIRRRENSYQRIRSDHDSHSCANNRGWCRPTCFSHEYTDWFNNDVCGSYRCCRPGRSG (10.7 kDa) | Against gram positive and negative bacteria | Rosa et al. (2011) | |
| Cg-BigDef1 | QAQALLPIASYAGLAVSPPVFAALVTAYGVYALYRYNIRRENSDHDSHSCANNRGWCRPTCYSYEYTDWFNNDVCGSYRCCRPGRRG | ||||
| Cg-BigDef1 | QAQILLPIASYAGLTVTAPVFAALVAAYGIYAVTRYAIRKRRIVMYSDSHSCANNRGWCRESCFSHEYTDWANTFGVCGSYFCCRPY | ||||
| Crassostrea gigas | cgTβ | Trypsin | (4656.4 Da) | Against Gram-positive bacteria (B. subtilis KCTC | Nam et al. (2015) |
| 1021) and Gram-negative bacteria (E. coli D31) | |||||
| Crassostrea gigas | Alcalase, bromelin, neutrase | (6500 Da) | Against Gram-positive bacteria (Listeria monocytogenes, Staphylococcus aureus) and Gram-negative bacteria (E. coli, Pseudomonas aeruginosa, Vibriyo parahaemolytius, V. harveyi) | Zhang et al. (2015) |
Antihypertensive Activity
Hypertension is reaching epidemic proportions and currently affects 15–20% of adults worldwide. Hypertension is a severe chronic health issue that increases the risk of arteriosclerosis, stroke, myocardial infarction, and end-stage renal disease. ACE is a Zn-metallopeptidase that plays a crucial role in regulating blood pressure. ACE catalyzes angiotensin conversion from an inactive decapeptide (angiotensin I) into a potent vasoconstrictor octapeptide (angiotensin II) and inactivates the antihypertensive vasodilator bradykinin (Wang et al., 2008). High angiotensin II levels and low NO production elevate the risk of high blood pressure (Oparil et al., 2018). Many studies have been carried out on the synthesis of ACE inhibitors, such as captopril, enalapril, alacepril, and lisinopril, which are used in the treatment of hypertension and heart failure. However, side effects led to the search for ACE inhibitors from other sources, including milk, hemoglobin, fish, buckwheat, beef, soybean, and fermented foods (Wang et al., 2008). Rich sources of ACE-inhibitory peptides include squid and jellyfish, among other invertebrates. Similarly, ACE-inhibitory peptides derived from oysters exert significant antihypertensive effects. Oyster extracts containing low-molecular-weight Ops were also found to inhibit ACE and reduce systolic blood pressure in spontaneously hypertensive rats (Hao et al., 2021 and references therein).
A peptide sequence (VVYPWTQRF) purified from protein hydrolysates of the oyster C. talienwhanensis was observed to exert antihypertensive activity (Wang et al., 2008). An in vivo study demonstrated competitive inhibition of spontaneously hypertensive rats by peptides (592.9 Da) isolated from fermented oyster sauce, as well as a reduction in blood pressure in the rats (Je et al., 2005). Two novel peptides (HLHT and GWA) isolated from the meat of pearl oyster exhibited high ACE-inhibitory activity, and protein hydrolysates exerted a strong antihypertensive effect in SRH rats. These results indicate that OPs could serve as ingredients of functional foods for hypertension (Liu et al., 2019). In silico and in vitro studies of protein hydrolysates produced from the pepsins bromelain and papain, isolated from the Portuguese oyster Crassostrea angulata, revealed high DPP-IV activity in pepsin hydrolysates. Bioactivity assays also indicated higher activity of low-molecular-weight pepsin hydrolysate compared to crude hydrolysate (Gomez et al., 2019). Another interesting study showed that heat treatment of C. gigas yielded more OPs with ACE-inhibiting effects. In fact, that study considered oyster protein to be the best natural source of ACE-inhibitory peptides, such that those peptides could be incorporated into functional foods for hypertension (Guo et al., 2020).
Anti-Inflammatory Activity
Inflammation is generally caused by the release of chemicals from tissues and migrating cells, and serves as defense against noxious stimuli and microbial infections. Inflammation promotes rapid tissue repair but also plays a role in diseases such as rheumatoid arthritis, chronic bronchitis, asthma, and cancer (Hwang et al., 2019). Bacterial endotoxins present in the cell wall of bacteria, such as LPS, activate the mitogen-activated protein kinase (MAPK) pathway, downstream NF-κB, and cyclic AMP-responsive element. This leads to macrophage proliferation and upregulates inducible NO and COX-2 expression (Choi et al., 2009). COX-2 plays a major role in converting arachidonic acid into prostaglandins, which regulate immune functions. iNOS is regulated by cytokines and transcriptional activation of macrophage cells (Hwang et al., 2019); elevated levels of iNOS cause NO accumulation and promote the production of cytokines (TNF-α and ILs), among other inflammatory factors (Mollace et al., 2005).
Recent studies have shown that OPs exert robust anti-inflammatory effects by suppressing proinflammatory cytokines. For example, a purified peptide sequence (Gln-Cys-Gln-Cys-Ala-Val-Glu-Gly-Gly-Leu at the N-terminal position) from C. gigas exhibits strong anti-inflammatory activity. RAW264.7 cell lines are generally used in studies on anti-inflammatory effects with LPS. In one study on protein hydrolysates of C. gigas, an NO assay with RAW264.7 cells was performed (Hwang et al., 2012). YA, a multifunctional oyster-derived peptide, exhibited anti-inflammatory activity (Xie et al., 2018). Oyster-derived β-thymosin, a ubiquitous, low-molecular-weight polypeptide, inhibited NO production in LPS-induced RAW264.7 cells to the same extent as human β-thymosin. Oyster β-thymosin also inhibited the expression of inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and suppressed the nuclear translocation of phosphorylated NF-κB and the degradation of inhibitory κB in LPS-induced RAW264.7 cells (Hwang et al., 2019). Qian et al. (2020) demonstrated anti-inflammatory effects of four peptides (PEP-1, PEP-2, TRYP-2, and MIX-2) isolated from oyster soft tissue, based on downregulation of TNF-α and mRNA expression of proinflammatory mediators (IL-1β, IL-6, and iNOS) in LPS-stimulated RAW264.7 cells.
Anticoagulant Activity
Cardiovascular diseases (CVDs) are the biggest threat to human health. CVDs include deep vein thrombosis, pulmonary embolism, stroke, and ischemic heart disease. Thrombosis and hypercoagulability are the primary drivers of CVD. Thrombosis can present as primary or secondary hemostasis; in the latter condition, soluble fibrinogen is converted into insoluble fibrin by thrombin, a serine protease and major component of the coagulation cascade. Hence, thrombin is a major target for anticoagulant agents (Cheng et al., 2021). Recent studies have demonstrated anticoagulant effects of OPs. For instance, the antithrombotic peptides DFEEIPEEYLQ and LSKEEIEEAKEV were isolated from oysters, with the former exhibiting thrombin-inhibiting activity including prolongation of partial thromboplastin and thrombin times. Meanwhile, LSKEEIEEAKEV interacts with thrombin (via carbon-hydrogen and conventional hydrogen bonds) through a salt bridge involving various amino acids (Cheng et al., 2018; Chen et al., 2019). Other OPs, such as TARNEANVNIY and P-3-CG, also exhibit anticoagulant activity. P-3-CG inhibits the binding of fibrinogen and thrombin, thus limiting coagulation (Cheng et al., 2018; Cheng et al., 2021).
Anticancer Activity
Multiple compounds from marine organisms, including bioactive peptides, exhibit antioxidant properties. Recent studies have demonstrated that bioactive peptides from marine organisms, such as fish and mussels, inhibit cancer cell growth while being of low toxicity. Moreover, peptides from tuna dark muscle and sepia ink exhibited cytotoxicity against human breast and prostate cancer cells, respectively (Hao et al., 2021 and references therein). Similarly, OPs have exhibited anticancer activity, including preventing the proliferation of cancer cells. An in vivo study of oligopeptide-enriched hydrolysates from C. gigas reported inhibition of the growth of sarcoma S-180 in BALB/c mice in a dose-dependent manner (Wang et al., 2010). Peña-Ramos et al., 2004 reported anticancer activities of OPs in relation to hydrophobic amino acids, such as leucine, isoleucine, methionine, and tryptophan. Moreover, Cheong et al. (2013) revealed that the OP HFNIGNRCLC causes apoptosis of prostate, breast, and lung cancer cells but not normal liver cells. The OP SCAP1-LANAK, isolated from Saccostrea cucullata, exhibited anticancer activity against human colon carcinoma cell lines (Umayaparvathi et al., 2014).
Other Potential Biological Activities
Oyster protein and peptides exhibited various biological activities which is presented in Table 4. Fatigue can prevent an individual from performing their usual activities of daily living and can also be the initial symptom of Parkinson’s disease (Kluger et al., 2016). In vivo studies showed that mice treated with OPs exhibited strong swimming performance, high endurance, and elevated levels of glycogen in the muscle and liver during vigorous exercise (Miao et al., 2018). Lactic acid and blood urea levels, as indicators of anti-fatigue effects and endurance in humans, are regulated by OPs (Hao et al., 2013; Xiao et al., 2020). OPs additionally mitigate skin damage caused by oxidative stress, inflammation, and collagen degradation. The ability of OPs to potentiate the anti-wrinkle effects of human fibroblasts has been well-studied (Kim et al., 2015; Bang and Choung, 2019; Peng et al., 2020).
TABLE 4.
Bioactive peptides derived from oyster whole tissue.
| Biological activity | Species | Enzyme used | Oyster peptides and/or MW | Results | References |
|---|---|---|---|---|---|
| Antihypertensive activity | Crassostrea talienwhanensis | Pepsin | VVYPWTQRF (1195 Da) | ACE inhibitory activity and decrease the systolic blood pressure (SBP) | Wang et al. (2008) |
| Crosse | |||||
| Crassostrea gigas (FOS) | (592.9 Da) | ACE-inhibitory activity and decrease the SBP by 12 mmHg after 3 h | Je et al. (2005) | ||
| Crassostrea gigas | Trypsin | DLTDY | ACE inhibitory activity and decrease the SBP | Shiozaki et al. (2010) | |
| Pinctada fucata | HLHT and GWA | ACE inhibitory activity and efficient antihypertensive effect on SRH rats | Liu et al. (2019) | ||
| Crassostrea angulata | Pepsin, bromelain, and papain | Higher ACE and DPP-IV activity observed in pepsin hydrolysates | Gomez et al. (2019) | ||
| Anti-inflammatory activity | Crassostrea gigas | Protamex | QCQCAVEGGL | Inhibit NO production in RAW264.7 cells and Decrease and increase the serum IgE and spleen CD4+/CD8+ levels in Male BALB/c mice, respectively | Hwang et al. (2012) |
| Microbial transglutaminase, protamex and neutrase | YA | Inhibit NO production in RAW264.7 cells | Xie et al. (2018) | ||
| Crassostrea gigas | Trypsin | β-thymosin (4656.4 Da) | Inhibits NO production in LPS-induced RAW264.7 cells and PGE2, iNOS and COX-2 expression; Inhibits the inflammatorycytokines (TNF-α, IL-1β, IL-6), repress the nucleartranslocationof phosphorylated nuclear factor-κB and degradation of inhibitor of nuclear factor-κB | Hwang et al. (2019) | |
| Crassostrea talienwhanensis | Pepsin | Repress inflammatory cytokines and mediators (TNF-α, IL-1β, IL-6, iNOS) at transcription level in LPS-induced RAW264.7 cells | Qian et al. (2020) | ||
| Anticoagulant activity | Crassostrea gigas | Pepsin, trypsin papain and nuetral protease | DFEEIPEEYLQ and TARNEANVNIY (1264.36) | Thrombin inhibition including prolonged the activated partial thromboplastin | Cheng et al. (2018) |
| Crassostrea gigas | Pepsin and pancreatin | LSKEEIEEAKEV | Prolong the APTT and TT, inhibit thrombin | Chen et al. (2018) | |
| Crassostrea gigas | Pepsin | P-3-CG | Prolong the APTT and decrease the mortality in male Kumming mice | Cheng et al. (2021) | |
| Anticancer activity | Crassostrea gigas | Protease | Oligopeptide-enriched hydrolysates (<3 kDa) | Inhibition against the growth of sarcoma-s-180 in BALB/c mice | Wang et al. (2010) |
| Crassostrea gigas | Flavourzyme | HFNIGNRCLC | Cytotoxicity including apoptosis of prostate, breast, and lung cancer cells but not normal liver cells | Cheong et al. (2013) | |
| Saccostrea cucullata | Protease isolated from Bacillus cereus SU12 | LANAK | Activity against the HT-29 cell lines | Umayaparvathi et al. (2014) | |
| Anti-fatigue | Ostrea plicatula | Nutrease | prolonged swimming time and incresed glycogen in liver and gastrocnemius muscle; decrease the serum lactic acid and BUN male Kumming mice | Hao et al. (2013) | |
| Gmelin | |||||
| Ostrea rivulari | Compound protease | (<6 kDa) | Increase in exercise tolerance, liver glycogen and muscle glycogen, decrease the levels of BUN in male Kumming mice | Miao et al. (2018) | |
| Nutrease | Prolong the swimming time, increase the levels of liver glycogen, | Xiao et al. (2020) | |||
| decrease the levels of lactic acid | |||||
| Anti-wrinkle | Crassostrea gigas | Protamex, Neutrase and AMG | Anti-melanogenic activities via downregulation of cAMP signaling in B16F10 cells and decreased the number of active melanocytes and melanin granule in UVB-irradiated mice group | Han et al., 2019 | |
| Reproduction enhancing | |||||
| Neutrase, papain, bromelain | (343 Da) | Rats with reproductive dysfunction induced by cyclophosphamide exhibited Increase the level of key regulatory protein expression and androgen in the blood | Li et al. (2020) | ||
| Crassostrea gigas | SPE | Cg-GnRH-a (pQNYHFSNGWQPa) and CgGnRH-G (pQNYHFSNGWQPG) | Regulate the neurological and endocrine factors and supports the nutrition and energy demand during reproduction | Bigot et al. (2012) | |
| Crassostrea iredalei | Ethanol extract showed the aphrodisiac property | Ridzwan et al. (2013) | |||
| Osteogenic | Crassostrea gigas | Pepsin, pancreatin | YRGDVVPK (992.40 Da) | Promote the proliferation of MC3T3-E1 cells | Chen et al. (2019) |
| Crassostrea gigas (fermented oyster extract) | Osteoclastogenesis by inactivating the NF-κB-mediated NFATc1 and c-Fos signaling pathways and scavenging the ROS generation in RAW 264.7 Cells | ||||
| Zinc-binding | Crassostrea gigas | Pepsin | Peptide-zinc complex promoted the intestinal absorption of zinc via the zinc ion channel and the small peptide transport pathway | Li et al. (2019) |
Moreover, protein hydrolysates inhibit procollagen and matrix metalloproteinase-1 production, thereby improving skin health and the appearance of wrinkles (Kim et al., 2015). The OP SSDNNDEAK downregulates genes and proteins associated with the cAMP signaling pathway in B16F10 cells, by reducing melanin production and tyrosinase activity (Han et al., 2019). Recent studies on OPs also revealed that they have collagen-restoring capabilities (i.e., anti-wrinkle properties) (Hao et al., 2021). Further, OPs promote sexual reproduction by increasing the expression of key regulatory proteins and androgens, thereby increasing serum estrogen levels and improving male sexual function (Li et al., 2020). For instance, GnRH-like peptides (Cg-GnRH-a and Cg-GnRH-G) isolated from the visceral ganglion of oysters regulate neurological and endocrine factors and provide nutrition and energy for reproduction (Bigot et al., 2012). Ridzwan et al. (2013) demonstrated the aphrodisiac properties of an ethanol extract of Crassostrea iredalei in mice and suggested that it could serve as an alternative therapy to enhance male sexual performance.
A fermented oyster extract inhibited osteoclastogenesis by inactivating the NF-κB-mediated NFATc1 and c-Fos signaling pathways and scavenging ROS in RAW264.7 cells (Jeong et al., 2019). A novel peptide (YRGDVVPK) derived from C. gigas protein hydrolysates promoted MC3T3-E1 proliferation, possibly by activating the MAPK signaling pathway (Chen et al., 2019). Zinc is an essential nutrient involved in many enzymatic and metabolic processes in humans. A peptide-Zn complex derived from C. gigas promoted intestinal absorption of Zn via the Zn ion channel and a so-called “small peptide transport pathway” (Li et al., 2019).
Bioactive Compounds From Oyster Gill and Mantle Tissues
The oyster gill is a complex ciliated organ with major roles in feeding, respiration, and excretion. Cilia of the gill are involved in the generation of strong water currents that pass through numerous branchial chambers to ensure gas exchange between organs and the surrounding medium, as well as the transport of food particles. OPs isolated from the gill exhibit various biological activities Table 5. Many AMPs and proteins have been detected in oyster gill tissue, including defensin (Seo et al., 2017). Oyster defensin (FGCPWNRYQCHSHCRSIGRLGGYCAGSLRLTCTCYRS) was detected in the gill of the American oyster Crassostrea virginica (Seo et al., 2005).
TABLE 5.
Bioactive peptides derived from oyster gill tissue and mantle tissue.
| Biological activity | Species | Enzyme used | Oyster peptides and/or MW | Results | References |
|---|---|---|---|---|---|
| Anti-microbial | Crassostrea virginica (Gill tissue) | GFGCPWNRYQCHSHCRSIGRLGGYCAGSLRLTCTCYRS (4265.0 Da) | Against Gram-positive bacteria (Lactococcus lactis subsp. lactis, S. aureus) and Gram-negative | Seo et al. (2005) | |
| bacteria (E. coli D31, Vibriyo parahemolyticus) | |||||
| Crassostrea gigas | Trypsin | cgUbiquitin (8471 Da) | Against Gram-positive bacteria (Streptococcus iniae), Gram-negative bacteria (V. parahemolyticus) | Seo et al. (2013a) | |
| (Gill tissue) | |||||
| cgMolluscidin, AATAKKGAKKADAPAKPKKATKPKSPKKAA | Against Gram-positive bacteria (B. subtilis, | Seo et al. (2013b) | |||
| KKAGAKKGVKRAGKKGAKKTTKAKK (5.5 kDa) | Micrococcus luteus, S. aureus) and Gram-negative bacteria (E. coli, Salmonella enterica,V. parahaemolyticus) | ||||
| AKSKNHTSHNQNRKQHRNGIHRPKTYRYPSMKGVDPKFL KNLKFSKKHNKNTKK(6484.6 Da) | Against Gram-positive bacteria (Bacillus subtilis | Seo et al. (2017) | |||
| KCTC 1021) and Gram-negative bacteria | |||||
| (Escherichia coli D31, Vibrio parahaemolyticus) | |||||
| Crassostrea gigas | Cg-Defm, GFGCPGNQLKCNNHCKSISCRAGYCDAATLWL | Against Gram-positive bacteria (M. lysodeikticus, | Gueguen et al. (2006) | ||
| (Mantle tissue) | RCTCTDCNGKK | S. aureus, S. hemeolyticus, B. megaterium, | |||
| B. stationis, M. maritypicum) | |||||
| Osteogenic | Pinctada fucata (Mantle tissue) | PFMG4 | Homology with C1q protein in different species, mainly in MC3T3-E1 osteoblast cell | Wang et al. (2015) |
The antimicrobial polypeptides cgUbiquitin and cgMolluscidin were purified from an acidified extract of Pacific oyster (C. gigas) gill tissue and exhibited antimicrobial activity against Gram-positive and -negative bacteria (Seo et al., 2013a and; Seo J.-K. et al., 2013). Seo et al. (2017) additionally reported antimicrobial effects (against Gram-positive and -negative bacteria) of 60S ribosomal protein of C. gigas. Oyster mantle tissue promotes the formation of oyster shell and its nacre lining. Gueguen et al. (2006) discovered a novel defensin gene in the mantle tissue of C. gigas, referred to as Cg-Def, and found that this defensin exhibited intrinsic activity against Gram-positive bacteria but had no effect on Gram-negative bacteria. The mantle gene Pinctada fucata mantle gene 4 is highly expressed in mantle tissue and homologous with C1q protein in various species. It is mainly observed to be expressed in MC3T3-E1 osteoblast cultures, suggesting an ability to enhance osteoblast differentiation (Wang et al., 2015).
Conclusion and Future Perspectives
Oyster is an abundant marine resource, which contains high protein content. Documentation of bioactive compounds which exhibits various biological activities is an emerging and substitute for artificial drug and food additives. Thus, exploring such compound from the marine edible organism will be biocompatible and less toxic. Oyster shell, considered as a major food waste, comprised of the bioactive compounds with biocidal, bioengineering and biological activities, such usages of these food waste will be beneficial to the environmental and waste management. Oyster proteins obtained from oyster tissue, yields proteins hydrolysates when digested by different proteases, which is consisting of low molecular weight peptides and amino acids and their biological applications are well studied. However structural analysis of oyster peptide is still very limited. Therefore, extensive studies on therapeutic proteins and peptides from oysters and their structural and functional characterization using bioinformatics analysis will be beneficial. This review cumulatively documented numerous isolation and biological activity of oyster peptides such as antioxidant, antimicrobial, anticancer, antihypertensive, anticoagulant etc., Besides, most of the experiments have studied the activity of oyster peptides in vitro, but not in vivo, which will not provide a reference data for the clinical research of oyster peptides. Hence we suggest that the in vivo studies of oyster peptides will be advantageous in clinical trial and drug development.
Author Contributions
Conceptualization, Y-HC and SU; formal analysis, SU and SK; investigation, SU and T-JN; data curation, SU and S.K; writing—original draft preparation, SU and SK; writing—review and editing, Y-HC and T-JN; supervision, Y-HC and T-JN; project administration, T-JN; funding acquisition, T-JN. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted as part of a project of the Future Fisheries Food Research Center funded by the Ministry of Oceans and Fisheries, Korea (project number: 201803932).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Achour A., Lachgar A., Astgen A., Chams V., Bizzini B., Tapiero H., et al. (1997). Potentialization of IL-2 Effects on Immune Cells by Oyster Extract (JCOE) in Normal and HIV-Infected Individuals. Biomed. Pharmacother. 51, 427–429. 10.1016/s0753-3322(97)82320-7 [DOI] [PubMed] [Google Scholar]
- Anderson R. S., Beaven A. E. (2001). Antibacterial Activities of Oyster (Crassostrea virginica) and mussel (Mytilus edulis and Geuken- sia demissa) Plasma. Aquat. Living Resour. 14, 343–349. 10.1016/S0990-7440(01)01143-3 [DOI] [Google Scholar]
- Asha K. K., Remya Kumari K. R., Ashok Kumar K., Chatterjee N. S., Anandan R., Mathew S. (2016). Sequence Determination of an Antioxidant Peptide Obtained by Enzymatic Hydrolysis of Oyster Crassostrea Madrasensis (Preston). Int. J. Pept. Res. Ther. 22, 421–433. 10.1007/s10989-016-9521-0 [DOI] [Google Scholar]
- Atlan G., Delattre O., Berland S., LeFaou A., Nabias G., Cot D., et al. (1999). Interface between Bone and Nacre Implants in Sheep. Biomaterials 20, 1017–1022. 10.1016/s0142-9612(98)90212-5 [DOI] [PubMed] [Google Scholar]
- Bang J. S., Choung S.-Y. (2020). Inhibitory Effect of Oyster Hydrolysate on Wrinkle Formation against UVB Irradiation in Human Dermal Fibroblast via MAPK/AP-1 and TGFβ/Smad Pathway. J. Photochem. Photobiol. B Biol. 209, 111946. 10.1016/j.jphotobiol.2020.111946 [DOI] [PubMed] [Google Scholar]
- Bednasz C. J., Venuto C. S., Ma Q., Daar E. S., Sax P. E., Fischl M. A., et al. (2019). Race/Ethnicity and Protease Inhibitor Use Influence Plasma Tenofovir Exposure in Adults Living With HIV-1 in AIDS Clinical Trials Group Study A5202. Antimicrob. Agents Chemother. 63, 1–11. 10.1128/AAC.01638-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot L., Zatylny-Gaudin C., Rodet F., Bernay B., Boudry P., Favrel P. (2012). Characterization of GnRH-Related Peptides from the Pacific Oyster Crassostrea gigas . Peptides 34, 303–310. 10.1016/j.peptides.2012.01.017 [DOI] [PubMed] [Google Scholar]
- Bonnard M. (2021). Identification of Valuable Compounds from the Shell of the Edible Oyster Crassostrea Gigas : HAL Id : Tel-03282617 DE L’UNIVERSITÉ DE MONTPELLIER En Chimie Séparative, Matériaux Et Procédés École Doctorale Sciences Chimiques Balard. Montpellier: HAL Open Science. [Google Scholar]
- Botta R., Asche F., Borsum J. S., Camp E. V. (2020). A Review of Global Oyster Aquaculture Production and Consumption. Mar. Policy 117, 103952. 10.1016/j.marpol.2020.103952 [DOI] [Google Scholar]
- Campbell M. D., Hall S. G. (2018). Hydrodynamic Effects on Oyster Aquaculture Systems: a Review. Rev. Aquacult 11, 896–906. 10.1111/raq.12271 [DOI] [Google Scholar]
- Chaturvedi R., Singha P. K., Dey S. (2013). Water Soluble Bioactives of Nacre Mediate Antioxidant Activity and Osteoblast Differentiation. PLOS ONE 8, e84584–10. 10.1371/journal.pone.0084584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Liu Z., Huang W., Zhao Y., Dong S., Zeng M. (2013). Purification and Characterisation of a Zinc-Binding Peptide from Oyster Protein Hydrolysate. J. Funct. Foods 5, 689–697. 10.1016/j.jff.2013.01.012 [DOI] [Google Scholar]
- Chen H., Cheng S., Fan F., Tu M., Xu Z., Du M. (2019). Identification and Molecular Mechanism of Antithrombotic Peptides from Oyster Proteins Released in Simulated Gastro-Intestinal Digestion. Food Funct. 10, 5426–5435. 10.1039/c9fo01433k [DOI] [PubMed] [Google Scholar]
- Chen H., Xu Z., Fan F., Shi P., Tu M., Wang Z., et al. (2019). Identification and Mechanism Evaluation of a Novel Osteogenesis Promoting Peptide from Tubulin Alpha-1C Chain in Crassostrea gigas. Food Chem. 272, 751–757. 10.1016/j.foodchem.2018.07.063 [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Lin C.-L., Li C.-T., Hwang D.-F. (2015). Structural Transformation of Oyster, Hard Clam, and Sea Urchin Shells after Calcination and Their Antibacterial Activity against Foodborne Microorganisms. Fish. Sci. 81 (4), 787–794. 10.1007/s12562-015-0892-5 [DOI] [Google Scholar]
- Cheng S., Tu M., Chen H., Xu Z., Wang Z., Liu H., et al. (2018). Identification and Inhibitory Activity against α-thrombin of a Novel Anticoagulant Peptide Derived from Oyster (Crassostrea gigas) Protein. Food Funct. 9, 6391–6400. 10.1039/c8fo01635f [DOI] [PubMed] [Google Scholar]
- Cheng S., Tu M., Liu H., An Y., Du M., Zhu B. (2021). A Novel Heptapeptide Derived from Crassostrea gigas Shows Anticoagulant Activity by Targeting for Thrombin Active Domain. Food Chem. 334, 127507. 10.1016/j.foodchem.2020.127507 [DOI] [PubMed] [Google Scholar]
- Cheong S. H., Kim E.-K., Hwang J.-W., Kim Y.-S., Lee J.-S., Moon S.-H., et al. (2013). Purification of a Novel Peptide Derived from a Shellfish, Crassostrea gigas, and Evaluation of its Anticancer Property. J. Agric. Food Chem. 61, 11442–11446. 10.1021/jf4032553 [DOI] [PubMed] [Google Scholar]
- Choi J.-S., Lee H.-J., Jin S.-K., Lee H.-J., Choi Y.-I. (2014). Effect of Oyster Shell Calcium Powder on the Quality of Restructured Pork Ham. Korean J. Food Sci. Animal Resour. 34, 372–377. 10.5851/kosfa.2014.34.3.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-H., Aid S., Bosetti F. (2009). The Distinct Roles of Cyclooxygenase-1 and -2 in Neuroinflammation: Implications for Translational Research. Trends Pharmacol. Sci. 30, 174–181. 10.1016/j.tips.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. M., Whang J. H., Kim J. M., Suh H. J. (2006). The Effect of Oyster Shell Powder on the Extension of the Shelf-Life of Kimchi. Food control. 17, 695–699. 10.1016/j.foodcont.2005.04.005 [DOI] [Google Scholar]
- Dong X.-P., Zhu B.-W., Zhao H.-X., Zhou D.-Y., Wu H.-T., Yang J.-F., et al. (2010). Preparation Andin Vitroantioxidant Activity of Enzymatic Hydrolysates from Oyster (Crassostrea Talienwhannensis) Meat. Int. J. Food Sci. Technol. 45, 978–984. 10.1111/j.1365-2621.2010.02223.x [DOI] [Google Scholar]
- Du Y., Lian F., Zhu L. (2011). Biosorption of Divalent Pb, Cd and Zn on Aragonite and Calcite Mollusk Shells. Environ. Pollut. 159, 1763–1768. 10.1016/j.envpol.2011.04.017 [DOI] [PubMed] [Google Scholar]
- FAO (2018). Available at: https://www.fao.org/fishery/en/statistics/en . [Google Scholar]
- Feng X., Jiang S., Zhang F., Wang R., Zhang T., Zhao Y., et al. (2021). Extraction and Characterization of Matrix Protein from Pacific Oyster (Crassostrea Gigs) Shell and its Anti-osteoporosis Properties In Vitro and In Vivo . Food Funct. 12, 9066–9076. 10.1039/d1fo00010a [DOI] [PubMed] [Google Scholar]
- Flausse A., Henrionnet C., Dossot M., Dumas D., Hupont S., Pinzano A., et al. (2013). Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells in Hydrogel Containing Nacre Powder. J. Biomed. Mat. Res. 101, 3211. 10.1002/jbm.a.34629 [DOI] [PubMed] [Google Scholar]
- Gomez H. L. R., Peralta J. P., Tejano L. A., Chang Y. W. (2019). In Silico and In Vitro Assessment of Portuguese Oyster (Crassostrea Angulata) Proteins as Precursor of Bioactive Peptides. Int. J. Mol. Sci. 20, 5191. 10.3390/ijms20205191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M., Gueguen Y., Desserre G., de Lorgeril J., Romestand B., Bachère E. (2007). Molecular Characterization of Two Isoforms of Defensin from Hemocytes of the Oyster Crassostrea gigas . Dev. Comp. Immunol. 31, 332–339. 10.1016/j.dci.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Green D. W., Kwon H.-J., Jung H.-S. (2015). Osteogenic Potency of Nacre on Human Mesenchymal Stem Cells. Mol. Cells 38, 267–272. 10.14348/molcells.2015.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen Y., Bernard R., Julie F., Paulina S., Delphine D. G., Franck V., et al. (2009). Oyster Hemocytes Express a Proline-Rich Peptide Displaying Synergistic Antimicrobial Activity With a Defensin. Mol. Immunol. 46, 516–522. 10.1016/j.molimm.2008.07.021 [DOI] [PubMed] [Google Scholar]
- Gueguen Y., Herpin A., Aumelas A., Garnier J., Fievet J., Escoubas J.-M., et al. (2006). Characterization of a Defensin from the Oyster Crassostrea gigas. J. Biol. Chem. 281, 313–323. 10.1074/jbc.m510850200 [DOI] [PubMed] [Google Scholar]
- Guo Z., Zhao F., Chen H., Tu M., Tao S., Wang Z., et al. (2020). Heat Treatments of Peptides from Oyster (Crassostrea gigas) and the Impact on Their Digestibility and Angiotensin I Converting Enzyme Inhibitory Activity. Food Sci. Biotechnol. 29 (7), 961–967. 10.1007/s10068-020-00736-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.-H., Ahmed R., Chun B.-S. (2015). Evaluation of Antimicrobial Activity of Allyl Isothiocyanate (AITC) Adsorbed in Oyster Shell on Food-Borne Bacteria. Clean. Technol. 21, 241–247. 10.7464/ksct.2015.21.4.241 [DOI] [Google Scholar]
- Han J. H., Bang J. S., Choi Y. J., Choung S.-Y. (2019). Anti-melanogenic Effects of Oyster Hydrolysate in UVB-Irradiated C57BL/6J Mice and B16F10 Melanoma Cells via Downregulation of cAMP Signaling Pathway. J. Ethnopharmacol. 229, 137–144. 10.1016/j.jep.2018.09.036 [DOI] [PubMed] [Google Scholar]
- Hao G., Cao W., Hao J., Zhang C. (2013). In Vitro antioxidant Activity and In Vivo Anti-fatigue Effects of Oyster (Ostrea Plicatula Gmelin) Peptides Prepared Using Neutral Proteinase. Fstr 19, 623–631. 10.3136/fstr.19.623 [DOI] [Google Scholar]
- Hao L., Wang X., Cao Y., Xu J., Xue C. (2021). A Comprehensive Review of Oyster Peptides: Preparation, Characterisation and Bioactivities. Rev. Aquacult 14, 120–138. 10.1111/raq.12588 [DOI] [Google Scholar]
- Huan Y., Kong Q., Mou H., Yi H. (2020). Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 11, 582779. 10.3389/fmicb.2020.58277911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J.-H., Choi Y.-H., Lee H.-J., Choi W. J., Ramakrishna C., Lee H.-W., et al. (2016). The Use of Oyster Shell Powders for Water Quality Improvement of Lakes by Algal Blooms Removal. J. Korean Ceram. Soc. 53, 1–6. 10.4191/kcers.2016.53.1.1 [DOI] [Google Scholar]
- Hwang D., Kang M.-j., Jo M., Seo Y., Park N., Kim G.-D. (2019). Anti-Inflammatory Activity of β-thymosin Peptide Derived from Pacific Oyster (Crassostrea gigas) on NO and PGE2 Production by Down-Regulating NF-Κb in LPS-Induced RAW264.7 Macrophage Cells. Mar. Drugs 17, 129. 10.3390/md17020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.-W., Lee S.-J., Kim Y.-S., Kim E.-K., Ahn C.-B., Jeon Y.-J., et al. (2012). Purification and Characterization of a Novel Peptide with Inhibitory Effects on Colitis Induced Mice by Dextran Sulfate Sodium from Enzymatic Hydrolysates of Crassostrea gigas . Fish Shellfish Immunol. 33, 993–999. 10.1016/j.fsi.2012.08.017 [DOI] [PubMed] [Google Scholar]
- Je J., Park J. Y., Jung W. K., Park P. J., Kim S. K. (2005). Isolation of Angiotensin I Converting Enzyme (ACE) Inhibitor from Fermented Oyster Sauce, Crassostrea gigas . Food Chem. 90, 809–814. 10.1016/j.foodchem.2004.05.028 [DOI] [Google Scholar]
- Jeong J.-W., Choi S., Han M., Kim G.-Y., Park C., Hong S., et al. (2019). Protective Effects of Fermented Oyster Extract against RANKL-Induced Osteoclastogenesis through Scavenging ROS Generation in RAW 264.7 Cells. Ijms 20 (6), 1439. 10.3390/ijms20061439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B. M., Jung S. J., Kim E. S. (2010). Quality Characteristics and Storage Properties of Gat Kimchi Added with Oyster Shell Powder and Salicornia Herbacea Powder. Korean J. Food Cook. Sci. 26, 188–197. [Google Scholar]
- Khirul M. A., Kim B.-G., Cho D., Yoo G., Kwon S.-H. (2020). Effect of Oyster Shell Powder on Nitrogen Releases from Contaminated Marine Sediment. Environ. Eng. Res. 25, 230–237. 10.4491/eer.2018.395 [DOI] [Google Scholar]
- Kim H.-A., Park S.-H., Lee S.-S., Choi Y. J. (2015). Anti-wrinkle Effects of Enzymatic Oyster Hydrolysate and its Fractions on Human Fibroblasts. J. Korean Soc. Food Sci. Nutr. 44 (11), 1645–1652. 10.3746/jkfn.2015.44.11.1645 [DOI] [Google Scholar]
- Kim Y. S., Choi Y. M., Noh D. O., Cho S. Y., Suh H. J. (2007). The Effect of Oyster Shell Powder on the Extension of the Shelf Life of Tofu. Food Chem. 103, 155–160. 10.1016/j.foodchem.2006.07.040 [DOI] [Google Scholar]
- Kluger B. M., Herlofson K., Chou K. L., Lou J.-S., Goetz C. G., Lang A. E., et al. (2016). Parkinson's Disease-Related Fatigue: A Case Definition and Recommendations for Clinical Research. Mov. Disord. 31, 625–631. 10.1002/mds.26511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamghari M., Almeida M. J., Berland S., Huet H., Laurent A., Milet C., et al. (1999). Stimulation of Bone Marrow Cells and Bone Formation by Nacre: In Vivo and In Vitro Studies. Bone 25, 91S–94S. 10.1016/s8756-3282(99)00141-6 [DOI] [PubMed] [Google Scholar]
- Latire T., Legendre F., Bouyoucef M., Marin F., Carreiras F., Rigot-Jolivet M., et al. (2017). Shell Extracts of the Edible Mussel and Oyster Induce an Enhancement of the Catabolic Pathway of Human Skin Fibroblasts, In Vitro . Cytotechnology 69, 815–829. 10.1007/s10616-017-0096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-Y., Kim H.-J., Han J.-S. (2013). Anti-inflammatory Effect of Oyster Shell Extract in LPS-Stimulated RAW 264.7 Cells. Jfn 18, 23–29. 10.3746/pnf.2013.18.1.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-G., Maruyama S. (1998). Isolation of HIV-1 Protease-Inhibiting Peptides from Thermolysin Hydrolysate of Oyster Proteins. Biochem. biophysical Res. Commun. 253, 604–608. 10.1006/bbrc.1998.9824 [DOI] [PubMed] [Google Scholar]
- Li J., Gong C., Wang Z., Gao R., Ren J., Zhou X., et al. (2019). Oyster-derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 17, 341. 10.3390/md17060341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhou M., Wei Y., Jia F., Yan y., Zhang R., et al. (2020). The Beneficial Effect of Oyster Peptides and Oyster Powder on Cyclophosphamide‐induced Reproductive Impairment in Male Rats: A Comparative Study. J. Food Biochem. 44, 1–11. 10.1111/jfbc.13468 [DOI] [PubMed] [Google Scholar]
- Linehan L. G., O'Connor T. P., Burnell G. (1999). Seasonal Variation in the Chemical Composition and Fatty Acid Profile of Pacific Oysters (Crassostrea gigas). Food Chem. 64, 211–214. 10.1016/s0308-8146(98)00144-7 [DOI] [Google Scholar]
- Liu P., Lan X., Yaseen M., Wu S., Feng X., Zhou L., et al. (2019). Purification, Characterization and Evaluation of Inhibitory Mechanism of Ace Inhibitory Peptides from Pearl Oyster (Pinctada Fucata Martensii) Meat Protein Hydrolysate. Mar. Drugs 17, 463. 10.3390/md17080463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Dong S., Xu J., Zeng M., Song H., Zhao Y., et al. (2008). Production of Cysteine-Rich Antimicrobial Peptide by Digestion of Oyster (Crassostrea gigas) with Alcalase and Bromelin. Food control. 19, 231–235. 10.1016/j.foodcont.2007.03.004 [DOI] [Google Scholar]
- Liu Z., Zeng M., Dong S., Xu J., Song H., Zhao Y. (2007). Effect of an Antifungal Peptide from Oyster Enzymatic Hydrolysates for Control of Gray Mold (Botrytis Cinerea) on Harvested Strawberries. Postharvest Biol. Technol. 46, 95–98. 10.1016/j.postharvbio.2007.03.013 [DOI] [Google Scholar]
- Lopez E., Vidal B., Berland S., Camprasse S., Camprasse G., Silve C. (1992). Demonstration of the Capacity of Nacre to Induce Bone Formation by Human Osteoblasts Maintained In Vitro . Tissue Cell. 24, 667–679. 10.1016/0040-8166(92)90037-8 [DOI] [PubMed] [Google Scholar]
- Marin F., Roy N. L., Marie B. (2012). The Formation and Mineralization of Mollusk Shell. Front. Biosci. S4, 1099–1125. 10.2741/s321 [DOI] [PubMed] [Google Scholar]
- Miao J., Liao W., Kang M., Jia Y., Wang Q., Duan S., et al. (2018). Anti-fatigue and Anti-oxidant Activities of Oyster (Ostrea Rivularis) Hydrolysate Prepared by Compound Protease. Food Funct. 9, 6577–6585. 10.1039/c8fo01879k [DOI] [PubMed] [Google Scholar]
- Mollace V., Muscoli C., Masini E., Cuzzocrea S., Salvemini D. (2005). Modulation of Prostaglandin Biosynthesis by Nitric Oxide and Nitric Oxide Donors. Pharmacol. Rev. 57, 217–252. 10.1124/pr.57.2.1 [DOI] [PubMed] [Google Scholar]
- Moon D. H., Chang Y.-Y., Ok Y. S., Cheong K. H., Koutsospyros A., Park J.-H. (2014). Amelioration of Acidic Soil Using Various Renewable Waste Resources. Environ. Sci. Pollut. Res. 21, 774–780. 10.1007/s11356-013-2138-3 [DOI] [PubMed] [Google Scholar]
- Nam B.-H., Seo J.-K., Lee M. J., Kim Y.-O., Kim D.-G., An C. M., et al. (2015). Functional Analysis of Pacific Oyster (Crassostrea gigas) β-thymosin: Focus on Antimicrobial Activity. Fish Shellfish Immunol. 45, 167–174. 10.1016/j.fsi.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Oikawa K., Asada T., Yamamoto K., Wakabayashi H., Sasaki M., Sato M., et al. (2000). Antibacterial Activity of Calcined Shell Calcium Prepared from Wild Surf Clam. J. Health Sci. 46, 98–103. 10.1248/jhs.46.98 [DOI] [Google Scholar]
- Olicard C., Didier Y., Marty C., Bourgougnon N., Renault T. (2005). In Vitro research of Anti-HSV-1 Activity in Different Extracts from Pacific Oysters Crassostrea gigas. Dis. Aquat. Org. 67, 141–147. 10.3354/dao067141 [DOI] [PubMed] [Google Scholar]
- Oliveira D. V., Silva T. S., Cordeiro O. D., Cavaco S. I., Simes D. C. (2012). Identification of Proteins with Potential Osteogenic Activity Present in the Water-Soluble Matrix Proteins fromCrassostrea gigasNacre Using a Proteomic Approach. Sci. World J. 2012, 1–9. 10.1100/2012/765909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparil S., Acelajado M. C., Bakris G. L., Berlowitz D. R., Cífková R., Dominiczak A. F., et al. (2018). Hypertension. Nat. Rev. Dis. Prim. 4, 18014. 10.1038/nrdp.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristoforou E., Lambadiari V., Maratou E., Makrilakis K. (20202020). Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 1–17. 10.1155/2020/7489795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Ramos E. A., Xiong Y. L., Arteaga G. E. (2004). Fractionation and Characterisation for Antioxidant Activity of Hydrolysed Whey Protein. J. Sci. Food Agric. 84, 1908–1918. 10.1002/jsfa.1886 [DOI] [Google Scholar]
- Peng Z., Chen B., Zheng Q., Zhu G., Cao W., Qin X., et al. (2020). Ameliorative Effects of Peptides from the Oyster (Crassostrea Hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 18, 288. 10.3390/md18060288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A., Jestadi D. B., Periyasamy L. (2015). Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 30, 11–26. 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B., Zhao X., Yang Y., Tian C. (2020). Antioxidant and Anti‐inflammatory Peptide Fraction from Oyster Soft Tissue by Enzymatic Hydrolysis. Food Sci. Nutr. 8, 3947–3956. 10.1002/fsn3.1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z.-J., Jung W.-K., Byun H.-G., Kim S.-K. (2008). Protective Effect of an Antioxidative Peptide Purified from Gastrointestinal Digests of Oyster, Crassostrea gigas against Free Radical Induced DNA Damage. Bioresour. Technol. 99, 3365–3371. 10.1016/j.biortech.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Ridzwan B. H., Hanani M., Siti Norshuhadaa M. P., Farah Hanis Z., Aileen T. S. H. (2013). Screening for Aphrodisiac Property in Local Oyster of Crassostrea Iredalei . World Appl. Sci. J. 26 (12), 1546–1551. 10.5829/idosi.wasj.2013.26.12.81103 [DOI] [Google Scholar]
- Rosa R. D., Santini A., Fievet J., Bulet P., Destoumieux-Garzón D., Bachère E. (2011). Big Defensins, a Diverse Family of Antimicrobial Peptides that Follows Different Patterns of Expression in Hemocytes of the Oyster Crassostrea gigas . PLoS One 6, e25594. 10.1371/journal.pone.0025594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi K., Park K., Park K., Seo J. (2019). Oyster Shell Disposal: Potential as a Novel Ecofriendly Antimicrobial Agent for Packaging : a Mini Review. Korean. J. packag. Sci. Technol. 25, 57–62. 10.20909/kopast.2019.25.2.57 [DOI] [Google Scholar]
- Seo J.-K., Crawford J. M., Stone K. L., Noga E. J. (2005). Purification of a Novel Arthropod Defensin from the American Oyster, Crassostrea virginica. Crassostrea virginicaBiochemical Biophysical Res. Commun. 338, 1998–2004. 10.1016/j.bbrc.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Seo J.-K., Kim D.-G., Oh R., Park K.-S., Lee I.-A., Cho S.-M., et al. (2017). Antimicrobial Effect of the 60S Ribosomal Protein L29 (cgRPL29), Purified from the Gill of Pacific Oyster, Crassostrea gigas. Fish Shellfish Immunol. 67, 675–683. 10.1016/j.fsi.2017.06.058 [DOI] [PubMed] [Google Scholar]
- Seo J.-K., Lee M. J., Nam B.-H., Park N. G. (2013b). cgMolluscidin, a Novel Dibasic Residue Repeat Rich Antimicrobial Peptide, Purified from the Gill of the Pacific Oyster, Crassostrea gigas . Fish Shellfish Immunol. 35, 480–488. 10.1016/j.fsi.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Seo J. K., Lee M. J., Go H. J., Kim G. D., Jeong H. D., Nam B. H., et al. (2013a). Purification and Antimicrobial Function of Ubiquitin Isolated from the Gill of Pacific Oyster, Crassostrea gigas . Mol. Immunol. 53, 88–98. 10.1016/j.molimm.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Shiozaki M., Masuda J., Yamauchi A., Ohwada S., Nakano T., et al. (2010). Identification of Oyster-Derived Hypotensive Peptide Acting as Angiotensin-l-Converting Enzyme Inhibitor. Fish. Sci. 76, 865–872. 10.3390/md14060110 [DOI] [Google Scholar]
- Tanaka K., Nishizono S., Kugino K., Tamari M., Kurumiya M., Abe N., et al. (2006). Effects of Dietary Oyster Extract on Lipid Metabolism, Blood Pressure, and Blood Glucose in SD Rats, Hypertensive Rats, and Diabetic Rats. Biosci. Biotechnol. Biochem. 70, 462–470. 10.1271/bbb.70.462 [DOI] [PubMed] [Google Scholar]
- Tran N. K., Kwon J. E., Kang S. C., Shim S. M., Park T. S. (2015). Crassaostrea Gigas Oyster Shell Extract Inhibits Lipogenesis via Suppression of Serine Palmitoyltransferase. Nat. Prod. Commun. 10, 349–352. 10.1177/1934578x1501000236 [DOI] [PubMed] [Google Scholar]
- Tsou C.-H., Wu C.-S., Hung W.-S., De Guzman M. R., Gao C., Wang R.-Y., et al. (2019). Rendering Polypropylene Biocomposites Antibacterial through Modification with Oyster Shell Powder. Polymer 160, 265–271. 10.1016/j.polymer.2018.11.048 [DOI] [Google Scholar]
- Umayaparvathi S., Meenakshi S., Vimalraj V., Arumugam M., Sivagami G., Balasubramanian T. (2014). Antioxidant Activity and Anticancer Effect of Bioactive Peptide from Enzymatic Hydrolysate of Oyster (Saccostrea Cucullata). Biomed. Prev. Nutr. 4, 343–353. 10.1016/j.bionut.2014.04.006 [DOI] [Google Scholar]
- Upadhyay A., Thiyagarajan V., Thiyagarajan V. (2016). Proteomic Characterization of Oyster Shell Organic Matrix Proteins (OMP). Bioinformation 12, 266–278. 10.6026/97320630012266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Hu J., Cui J., Bai X., Du Y., Miyaguchi Y., et al. (2008). Purification and Identification of a ACE Inhibitory Peptide from Oyster Proteins Hydrolysate and the Antihypertensive Effect of Hydrolysate in Spontaneously Hypertensive Rats. Food Chem. 111, 302–308. 10.1016/j.foodchem.2008.03.059 [DOI] [PubMed] [Google Scholar]
- Wang Q., Li W., He Y., Ren D., Kow F., Song L., et al. (2014). Novel Antioxidative Peptides from the Protein Hydrolysate of Oysters (Crassostrea Talienwhanensis). Food Chem. 145, 991–996. 10.1016/j.foodchem.2013.08.099 [DOI] [PubMed] [Google Scholar]
- Wang X., Harimoto K., Fuji R., Liu J., Li L., Wang P., et al. (2015). Pinctada Fucata Mantle Gene 4 (PFMG4) from Pearl Oyster Mantle Enhances Osteoblast Differentiation. Biosci. Biotechnol. Biochem. 79, 558–565. 10.1080/09168451.2014.987206 [DOI] [PubMed] [Google Scholar]
- Wang X., Yu H., Xing R., Liu S., Chen X., Li P. (2020). Optimization of Oyster (Crassostrea Talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules 25, 2844. 10.3390/molecules25122844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-K., He H.-L., Wang G.-F., Wu H., Zhou B.-C., Chen X.-L., et al. (2010). Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice. Mar. Drugs 8, 255–268. 10.3390/md8020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M., Lin L., Chen H., Ge X., Huang Y., Zheng Z., et al. (2020). Anti-fatigue Property of the Oyster Polypeptide Fraction and its Effect on Gut Microbiota in Mice. Food Funct. 11, 8659–8669. 10.1039/D0FO01713B [DOI] [PubMed] [Google Scholar]
- Xie C.-L., Kang S. S., Lu C., Choi Y. J. (20182018). Quantification of Multifunctional Dipeptide YA from Oyster Hydrolysate for Quality Control and Efficacy Evaluation. BioMed Res. Int. 2018, 1–10. 10.1155/2018/8437379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R., Qin Y., Guan X., Liu S., Yu H., Li P. (2013). Comparison of Antifungal Activities of Scallop Shell, Oyster Shell and Their Pyrolyzed Products. Egypt. J. Aquatic Res. 39, 83–90. 10.1016/j.ejar.2013.07.003 [DOI] [Google Scholar]
- Zeng M., Cui W., Zhao Y., Liu Z., Dong S., Guo Y. (2008). Antiviral Active Peptide from Oyster. Chin. J. Ocean. Limnol. 26, 307–312. 10.1007/s00343-008-0307-x [DOI] [Google Scholar]
- Zhang G., Brion A., Willemin A.-S., Piet M.-H., Moby V., Bianchi A., et al. (2017). Nacre, a Natural, Multi-Use, and Timely Biomaterial for Bone Graft Substitution. J. Biomed. Mat. Res. 105, 662–671. 10.1002/jbm.a.35939i [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Y., Tian X., Tian Z. (2015). Antimicrobial Capacity and Antioxidant Activity of Enzymatic Hydrolysates of Protein from Rushan Bay Oyster (C Rassostrea Gigas ). J. Food Process. Preserv. 39, 404–412. 10.1111/jfpp.12245 [DOI] [Google Scholar]
- Zhang W., Wei Y., Cao X., Guo K., Wang Q., Xiao X., et al. (2021). Enzymatic Preparation of Crassostrea Oyster Peptides and Their Promoting Effect on Male Hormone Production. J. Ethnopharmacol. 264, 113382. 10.1016/j.jep.2020.113382 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Su G., Zhou F., Lin L., Liu X., Zhao M. (2019). Alcalase-hydrolyzed Oyster (Crassostrea Rivularis) Meat Enhances Antioxidant and Aphrodisiac Activities in Normal Male Mice. Food Res. Int. 120, 178–187. 10.1016/j.foodres.2019.02.033 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhou F., Liu X., Zhao M. (2018). Particulate Nanocomposite from Oyster ( Crassostrea Rivularis ) Hydrolysates via Zinc Chelation Improves Zinc Solubility and Peptide Activity. Food Chem. 258, 269–277. 10.1016/j.foodchem.2018.03.030 [DOI] [PubMed] [Google Scholar]