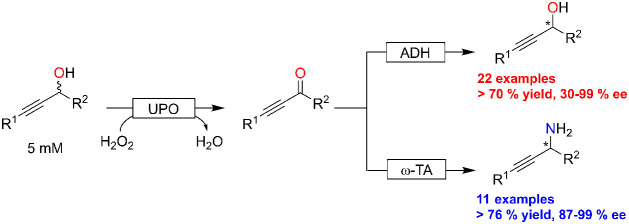
Abstract
Propargylic alcohols and amines are versatile building blocks in organic synthesis. We demonstrate a straightforward enzymatic cascade to synthesize enantiomerically pure propargylic alcohols and amines from readily available racemic starting materials. In the first step, the peroxygenase from Agrocybe aegerita converted the racemic propargylic alcohols into the corresponding ketones, which then were converted into the enantiomerically pure alcohols using the (R)-selective alcohol dehydrogenase from Lactobacillus kefir or the (S)-selective alcohol dehydrogenase from Thermoanaerobacter brokii. Moreover, an enzymatic Mitsunobu-type conversion of the racemic alcohols into enantiomerically enriched propargylic amines using (R)-selective amine transaminase from Aspergillus terreus or (S)-selective amine transaminase from Chromobacterium violaceum was established. The one-pot two-step cascade reaction yielded a broad range of enantioenriched alcohol and amine products in 70–99% yield.
Functionalized alkynes, such as propargylic alcohols and amines, are versatile building blocks in organic chemistry.1−3 The presence of at least two functional groups predestines these compounds for a broad variety of chemical transformations, explaining their importance as building blocks in pharmaceutical and agrochemical chemistry. The natural antifungal agent Capillin, for example, is chemically synthesized via carbonyl oxidation of its pendant propargylic alcohols.4 Levonorgestrel and ethinylestradiol are common therapeutic hormones containing propargylic alcohols.5 Propargylic amines such as the monoamine oxidase-B inhibitors Pargyline6 and Rasagyline7 are used in the treatment of Parkinson’s disease and are of comparable importance.
Especially for the synthesis of APIs and fine chemicals, the optical purity of propargylic alcohols and amines is crucial. Therefore, it is not astonishing that a range of enantioselective syntheses starting from aldehydes8−11 or imines12,13 have been reported. Though conceptually elegant, these methods suffer from the need for comparably high (transition) metal catalyst loadings, posing a significant economic and environmental challenge. Very recently, Arnold and co-workers proposed a biocatalytic route to enantiomerically pure propargylic amines.14
Enantioselective reduction of propargylic ketones has been reported already in 2001 by Müller and co-workers.21 Using enantiocomplementary alcohol dehydrogenases, the authors demonstrated the synthesis of both enantiomers of propargylic alcohols. As racemic propargylic alcohols are more readily accessible, synthesis routes starting from racemic propargylic alcohols are desirable. Kinetic resolution approaches appear to be a practical route to enantiomerically pure products.15−17 Compared to the kinetic resolution strategy, deracemization (or dynamic kinetic resolution) intrinsically bears the advantage of theoretically yielding excellent conversion of the starting material into the desired product isomer (Scheme 1).
Scheme 1. Synthetic Approaches to Obtain Enantiomerically Pure Propargylic Alcohols and Amines from Racemic Starting Materials.
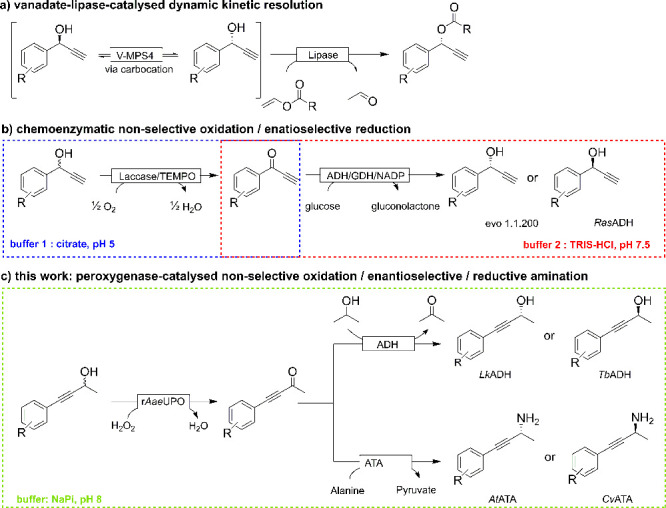
Chemoenzymatic approaches utilizing vanadium18 or TEMPO19 catalysts to enable the conversion of the racemic staring materials have been reported. The group around Lavandera and Gotor-Fernández, for example, used a laccase-TEMPO oxidation system to oxidize racemic propargylic alcohols into the corresponding ketones followed by enantioselective ADH-catalyzed reduction into the corresponding enantioenriched products.19 Unfortunately TEMPO, despite its popularity, is not a very efficient oxidation reagent, and catalyst loadings of up to 30 mol % are necessary in order to attain complete conversion of the alcohols to the corresponding ketones. Furthermore, the pH incompatibility of both reaction steps necessitated an intermittent buffer exchange.
Therefore, we set out to evaluate if peroxygenases may be more efficient catalysts for the first oxidation step. Though peroxygenases are mostly used for the oxyfunctionalization of (nonactivated) C–H-bonds20,21 recent experiments in our laboratories indicated that also the oxidation of alcohols to aldehydes/ketones may be an interesting application for peroxygenase catalysis.
As peroxygenase catalyst we chose the recombinant, evolved peroxygenase from Agrocybe aegerita (rAaeUPO, PaDa-I),22,23 which we recently produced on pilot scale.24 In a first set of experiments, we investigated the applicability of rAaeUPO for the complete oxidation of racemic 4-(4-fluorophenyl)but-3-yn-2-ol (1a, Figure 1). Previously, rAaeUPO has been reported as a highly enantioselective catalyst, especially for the hydroxylation of benzylic C–H-bonds;25−27 also the hydroxylation of but-1-yn-1-ylbenzene proceeded enantioselectively (85% ee). We therefore expected the rAaeUPO-catalyzed oxidation of racemic alcohol (1a) to result in a kinetic resolution and were very surprised observing that the oxidation of rac-(1a) occurred with low enantioselectivity (E-value of 3.6)28 (Figure 1), essentially converting both enantiomers.
Figure 1.
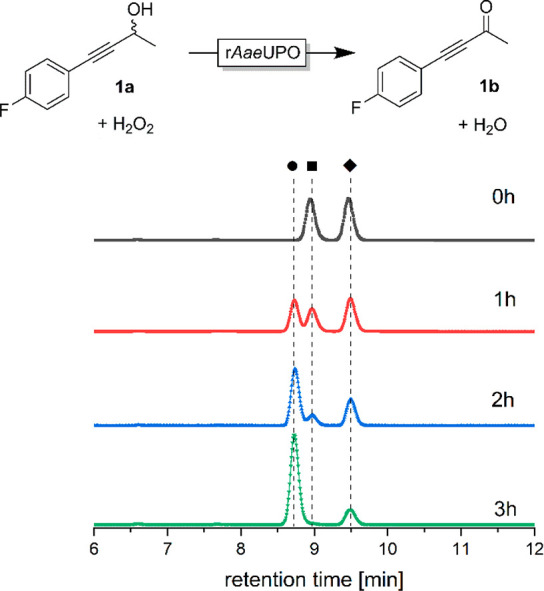
Oxidation of racemic 4-(4-fluorophenyl)but-3-yn-2-ol (1a) catalyzed by rAaeUPO monitored by chiral HPLC. Conditions: [1a] = 5 mM, [rAaeUPO] = 2 μM, [H2O2]final = 10 mM added at 2 mM h–1, NaPi buffer (100 mM, pH 8), 30% (v/v) MeCN, 30 °C, 800 rpm. The retention times of (R)-1a (■), (S)-1a (◆), and the ketone product 1b (●) were 7.8, 8.3, and 7.6 min, respectively. After 5 h, all starting material had been converted into the ketone (not shown).
To gain further insight into this unexpected low stereoselectivity of rAaeUPO toward the racemic alcohol, we modeled both enantiomers individually into the active site of rAaeUPO (Figure S1).29,30 It turned out that the binding affinity of both enantiomers was very similar, differing by only approximately 0.1 kcal × mol–1, suggesting that both enantiomers may be converted by rAaeUPO with comparable efficiency, thereby explaining the low enantioselectivity.
Complete oxidation of 1a into 1b was achieved within 5 h using 2 μM of rAaeUPO at a H2O2 addition rate of 2 mM h–1 (Figure S2). Control reactions in the absence of the biocatalyst under otherwise identical conditions did not yield in detectable conversion of any of these starting materials.
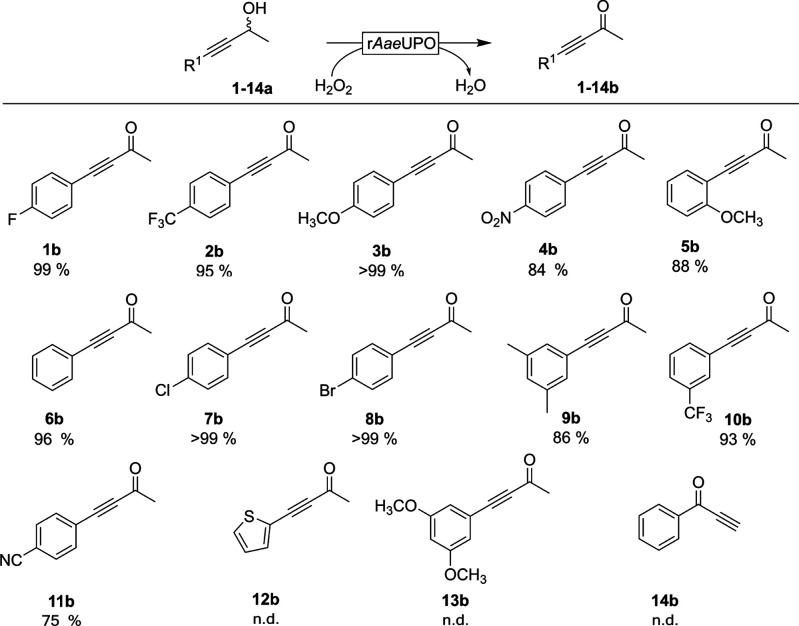
This procedure was extended to the oxidation of a range of racemic derivatives (Figure 2). With the exception of the substrates (12a–14a), all propargylic alcohols tested were oxidized into the corresponding ketones in good to excellent yields.
Figure 2.
Peroxygenase catalyzed oxidation of racemic propargylic alcohols into the corresponding ketones. Conditions: [substrate] = 5 mM, [rAaeUPO] = 2 μM, [H2O2]final = 10 mM added at 2 mM h–1, NaPi buffer (100 mM, pH 8), 30% (v/v) MeCN, 5 h, 30 °C, 800 rpm. N.D. = not detected. The GC yield was calculated via [ketone]final × ([alcohol]final + [ketone]final)−1.
Having established the rAaeUPO-catalyzed oxidation, we next investigated the enantioselective reduction of the ketone intermediate. For this, we chose the (R)-selective alcohol dehydrogenase from Lactobacillus kefir DSM 20587 (LkADH)31 as well as the (S)-selective ADH from Thermoanaerobacter brokii (TbADH).32 Both enzymes were prepared by recombinant expression in recombinant E. coli and used as lysed cells (obtained by treating the cell pellets with lysozyme and DNase I, see the SI for further details). Treating 1b with TbADH or LkADH (in the presence of 5% v/v isopropanol for in situ regeneration of NADPH) gave the expected (R)-1a and (S)-1a, respectively, in high enantiomeric purity (Figure S3).
We therefore proceeded combining both reaction steps to attain the desired deracemization procedure. Preliminary experiments performing the cascade in a one-pot one-step fashion were not successful, resulting in a painfully slow deracemization of 1a: after 7 h reaction time, the ee-value of (R)-1a had increased from 0% to 5.7%. Possibly catalase present in the ADH preparation (being a crude cell extract of the E. coli expression system) competed with rAaeUPO for the H2O2 added and thereby significantly decreased the oxidation rate of the first cascade step. Therefore, we drew our attention to a one-pot two-step procedure in which the rAaeUPO-catalyzed oxidation of racemic propargylic alcohols was followed by the enantioselective, ADH-catalyzed reduction. A representative time course is shown in Figure 3.
Figure 3.
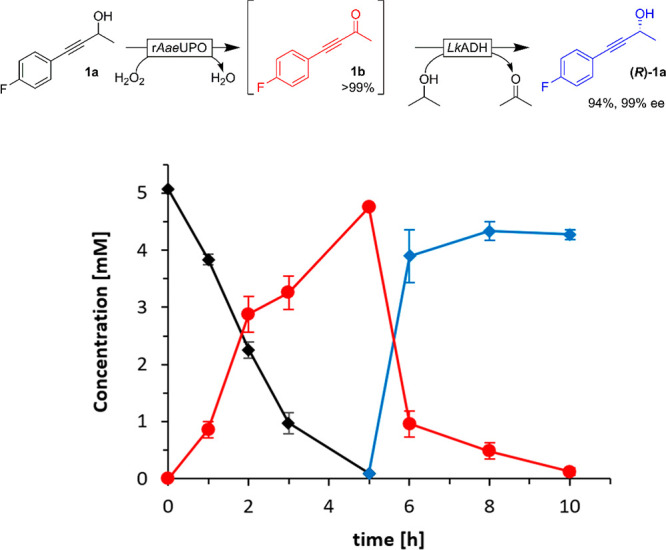
Representative time course of a one-pot two-step deracemization (1a (black ◆), 1b (red ●), (R)-1a (blue ◆)). The rAaeUPO-catalyzed oxidation step was performed first, followed by addition of the ADH, isopropanol after 5 h. Conditions: [1a] = 5 mM, [rAaeUPO] = 2 μM; [H2O2]final = 10 mM added at 2 mM h–1 (for 5 h), [LkADH] = 60 μM, NaPi buffer (100 mM, pH 8), 30% (v/v) MeCN, 5% isopropanol, [lysozyme] = 1 mg mL–1, [DNase I] = 6 U mL–1, 30 °C, 800 rpm.
Almost complete conversion of the racemic starting material (1a) into the ketone (1b) was achieved within 5 h. After addition of, e.g., LkADH and 5% (v/v) of isopropanol (serving as sacrificial reductant for the LkADH-catalyzed reduction reaction), smooth reduction into (R)-1a (94% yield and 99% ee) was observed. Similarly, using TbADH the corresponding (S)-1a was obtained in 77% yield and 98% ee (Figure S4–S6).
Encouraged by these results, we further synthesized a range of racemic propargylic alcohols (Figure S7–S45) and subjected them to the bienzymatic deracemization cascade (Figure 4). Depending on the substitution pattern of the starting material, the deracemization reaction proceeded between mediocre to excellent yields and optical purities of the products (Figure S46–S75). The ADH-selectivities were as expected with the notable exception of the TbADH-catalyzed reduction of 10b, which resulted in the formation of the (R)-alcohol (R)-10a instead of the expected (S)-10a.
Figure 4.
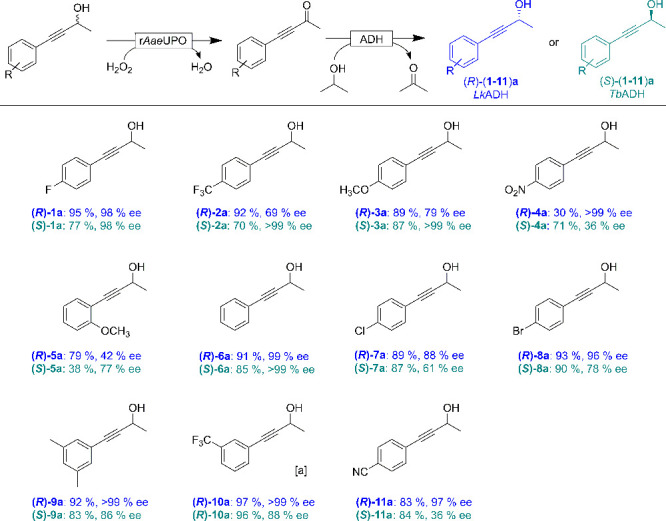
Biocatalytic cascade reaction for the synthesis of enantiomerically pure propargylic alcohols. Reaction conditions: [1a–11a] = 5 mM, [rAaeUPO] = 2 μM, [H2O2]final = 10 mM added at 2 mM h–1, [LkADH] = 60 μM, or [TbADH] = 25 μM, NaPi buffer (100 mM, pH 8), 30% (v/v) MeCN, 5% isopropanol, [lysozyme] = 1 mg mL–1, [DNase I] = 6 U mL–1, 30 °C, 5 h, 800 rpm. n.d. = Not detected. aNote that using TbADH yielded in formation of the (R)-alcohol.
Overall, the bienzymatic deracemization of a range of propargylic alcohols was established. The catalytic performance of the individual catalysts in terms of turnover numbers (TON, [product]final × [catalysts]−1) was calculated to be 2500, 80, and 190 for rAaeUPO, LkADH, TbADH, respectively. The concentration of the nicotinamide cofactor (endogenously contained in the E. coli crude extract) can be estimated using a NADP content of approximately 0.51 μmol × gwet E. coli–1 to be around 51 μM,33,34 translating into an approximate TONNADP around 100.
Next, we enlarged the bienzymatic deracemization concept with a reductive amination step yielding an enantioselective, biocatalytic variant of the Mitsunobu reaction.35−37 For this, we simply substituted the previously used ADHs in the sequential cascade by the (R)-selective amine transaminase from Aspergillus terreus (AtATA)38 or the (S)-selective amine transaminase from Chromobacterium violaceum DSM30191 (CvATA).39 Again, the reaction was realized as one-pot two-step procedure (Figure 5). A representative time course converting rac-6a into (R)-6c is shown in Figure S76. Under partially optimized reaction conditions (Figure S77–S79) a range of enantiomerically pure (R)- and (S)-propargylic amines could be obtained in reasonable to excellent yields and enantiomeric excess from the corresponding racemic alcohols (see experimental details in the SI, Figures S80–S100 and Figures S101–S108). The TONs observed for AtATA and CvATA were 62 and 122.
Figure 5.
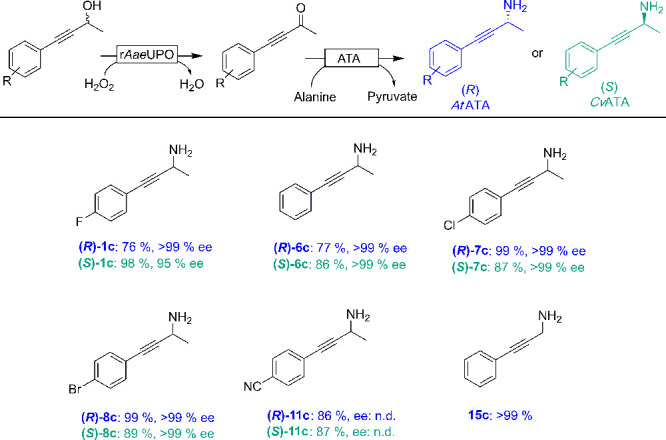
Biocatalytic cascade reaction for the synthesis of enantiomerically pure propargylic amines from racemic alcohols. Reaction conditions: [substrate] = 5 mM, [rAaeUPO] = 2 μM, [H2O2] = 2 mM h–1 for 5 h, [H2O2]final = 10 mM, [AtATA] = 80 μM or [CvATA] = 40 μM, [PLP] = 0.1 mM, [d/l-alanine] = 1 M, NaPi buffer (100 mM, pH 8), 30% (v/v) MeCN, 30 °C, 800 rpm, 24 h. n.d. = not determined.
Finally, we performed a semipreparative scale synthesis. Both propargylic alcohol and amine were performed at 3 mmol scale (20 mM of 1a in 150 mL a representative time course is shown in Figure S109). The peroxygenase enabled complete oxidation of 1a in the first step. The overall cascade reaction gave (S)-1a and (R)-1c in 76% (91.1% ee) and 19.5% (>99% ee) isolated yield, respectively (Figure S110–115). The low isolated yield of (R)-1c was due to issues with the chromatographic purification.
In summary, we have established a catalytic platform transforming readily accessible racemic propargylic alcohols into enantiomerically pure propargylic alcohols and amines. We are convinced that the simplicity of the procedure will provide preparative organic and medicinal chemists with a practical tool for their synthesis planning. Apparently, the catalytic performance of the enzymes used has to be increased considerably to attain economic feasibility. We are, however, convinced that further reaction engineering measures can increase the TONs considerably. For example, external addition of the ADH-cofactor may accelerate the ADH-catalyzed reduction reaction. Also the efficiency of the reductive amination can be improved upon application of suitable coupled reactions to shift the equilibrium.40 Efforts broadening the substrate scope (e.g., for starting materials 12a–14a) via engineered rAaeUPO variants, expanding the amine product scope and increasing the substrate loading to preparatively relevant scales, are currently ongoing in our laboratories.
Acknowledgments
We thank Prof. Qiaqing Wu from Tianjin Institute of Industrial Biotechnology (CAS) for the guidance of preparing the (S)-selective amine transaminase. The authors acknowledge financial support from the National Natural Science Foundation of China (No. 32171253 and No. 31601675), the European Union (Horizon 2020 Research and Innovation Programme under Grant Agreement No. 886567), the PhD Start-up Fund of Hubei University of Science and Technology (BK201821), and Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (No. TSBI-CIP-CXRC-032).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c01547.
Author Contributions
All authors have given approval to the final version of the manuscript. X.S., F.T., and B.Y. prepared the materials and performed the reactions. Z.Z. supervised the study, analyzed the experimental results, and corrected the draft. M.W. and Z.S. analyzed the results. X.S., G.Q., and M.A. performed the docking results. F.H. and W.Z. promoted the concept and wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Zhu Y.; Sun L.; Lu P.; Wang Y. Recent advances on the lewis acid-catalyzed cascade rearrangements of propargylic alcohols and their derivatives. ACS Catal. 2014, 4 (6), 1911–1925. 10.1021/cs400922y. [DOI] [Google Scholar]
- Lauder K.; Toscani A.; Scalacci N.; Castagnolo D. Synthesis and reactivity of propargylamines in organic chemistry. Chem. Rev. 2017, 117 (24), 14091–14200. 10.1021/acs.chemrev.7b00343. [DOI] [PubMed] [Google Scholar]
- Ding C.-H.; Hou X.-L. Catalytic asymmetric propargylation. Chem. Rev. 2011, 111 (3), 1914–1937. 10.1021/cr100284m. [DOI] [PubMed] [Google Scholar]
- Nash B. W.; Thomas D. A.; Warburton W. K.; Williams T. D. The preparation of capillin and some related compounds, and of some substituted pent-4-en-2-yn-1-ones. J. Chem. Soc. (Resumed) 1965, 2983–2988. 10.1039/jr9650002983. [DOI] [PubMed] [Google Scholar]
- McGrath N. A.; Brichacek M.; Njardarson J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ. 2010, 87 (12), 1348–1349. 10.1021/ed1003806. [DOI] [Google Scholar]
- Langston J. W.; Irwin I.; Langston E. B.; Forno L. S. Pargyline prevents mptp-induced parkinsonism in primates. Science 1984, 225 (4669), 1480–1482. 10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- Chen J. J.; Swope D. M. Clinical pharmacology of rasagiline: a novel, second-generation propargylamine for the treatment of parkinson disease. J. Clin. Pharmacol. 2005, 45 (8), 878–894. 10.1177/0091270005277935. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Tan Y.; Harms K.; Marsch M.; Riedel R.; Zhang L.; Meggers E. Octahedral ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis. J. Am. Chem. Soc. 2017, 139 (12), 4322–4325. 10.1021/jacs.7b01098. [DOI] [PubMed] [Google Scholar]
- Turlington M.; Yue Y.; Yu X.-Q.; Pu L. Catalytic asymmetric synthesis of chiral propargylic alcohols for the intramolecular pauson–khand cycloaddition. J. Org. Chem. 2010, 75 (20), 6941–6952. 10.1021/jo101545v. [DOI] [PubMed] [Google Scholar]
- Takita R.; Yakura K.; Ohshima T.; Shibasaki M. Asymmetric alkynylation of aldehydes catalyzed by an In(III)/BINOL complex. J. Am. Chem. Soc. 2005, 127 (40), 13760–13761. 10.1021/ja053946n. [DOI] [PubMed] [Google Scholar]
- Barozzino-Consiglio G.; Yuan Y.; Fressigné C.; Harrison-Marchand A.; Oulyadi H.; Maddaluno J. Enantioselective alkynylation of aldehydes by mixed aggregates of 3-aminopyrrolidine lithium amides and lithium acetylides. Organometallics 2015, 34 (18), 4441–4450. 10.1021/acs.organomet.5b00647. [DOI] [Google Scholar]
- Wei C.; Mague J. T.; Li C.-J. Cu(I)-catalyzed direct addition and asymmetric addition of terminal alkynes to imines. Pro. Natl. Acad. Sci. U.S.A 2004, 101 (16), 5749–5754. 10.1073/pnas.0307150101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel M.; Qu J.; Schnitzer T.; Helmchen G. Addition of organometallic reagents to chiral n-methoxylactams: enantioselective syntheses of pyrrolidines and piperidines. Eur. J. Chem. 2013, 19 (49), 16746–16755. 10.1002/chem.201302735. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Qin Z.-Y.; Zhu L.; Athavale S. V.; Sengupta A.; Jia Z.-J.; Garcia-Borràs M.; Houk K. N.; Arnold F. H. An enzymatic platform for primary amination of 1-aryl-2-alkyl alkynes. J. Am. Chem. Soc. 2022, 144 (1), 80–85. 10.1021/jacs.1c11340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birman V. B.; Guo L. Kinetic resolution of propargylic alcohols catalyzed by benzotetramisole. Org. Lett. 2006, 8 (21), 4859–4861. 10.1021/ol061906y. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang W.; Wu P.; Huang C.; Zheng Y.; Zheng W.-F.; Qian H.; Ma S. Chiral tertiary propargylic alcohols via Pd-catalyzed carboxylative kinetic resolution. Org. Chem. Front. 2020, 7 (23), 3907–3911. 10.1039/D0QO01106A. [DOI] [Google Scholar]
- Ma G.; Xu Z.; Zhang P.; Liu J.; Hao X.; Ouyang J.; Liang P.; You S.; Jia X. A Novel synthesis of rasagiline via a chemoenzymatic dynamic kinetic resolution. Org. Process Res. Dev. 2014, 18 (10), 1169–1174. 10.1021/op500152g. [DOI] [Google Scholar]
- Kawanishi S.; Oki S.; Kundu D.; Akai S. Lipase/Oxovanadium co-catalyzed dynamic kinetic resolution of propargyl alcohols: competition between racemization and rearrangement. Org. Lett. 2019, 21 (9), 2978–2982. 10.1021/acs.orglett.9b00334. [DOI] [PubMed] [Google Scholar]
- González-Granda S.; Méndez-Sánchez D.; Lavandera I.; Gotor-Fernández V. Laccase-mediated oxidations of propargylic alcohols. application in the deracemization of 1-arylprop-2-yn-1-ols in combination with alcohol dehydrogenases. ChemCatChem. 2020, 12 (2), 520–527. 10.1002/cctc.201901543. [DOI] [Google Scholar]
- Sigmund M.-C.; Poelarends G. J. Current state and future perspectives of engineered and artificial peroxygenases for the oxyfunctionalization of organic molecules. Nat. Catal. 2020, 3 (9), 690–702. 10.1038/s41929-020-00507-8. [DOI] [Google Scholar]
- Hobisch M.; Holtmann D.; Gomez de Santos P.; Alcalde M.; Hollmann F.; Kara S. Recent developments in the use of peroxygenases - Exploring their high potential in selective oxyfunctionalisations. Biotechnol. Adv. 2021, 51, 107615. 10.1016/j.biotechadv.2020.107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Espeja P.; Ma S.; Mate D. M.; Ludwig R.; Alcalde M. Tandem-yeast expression system for engineering and producing unspecific peroxygenase. Enz. Microb. Technol. 2015, 73–74, 29–33. 10.1016/j.enzmictec.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Molina-Espeja P.; Garcia-Ruiz E.; Gonzalez-Perez D.; Ullrich R.; Hofrichter M.; Alcalde M.; Cullen D. Directed evolution of unspecific peroxygenase from Agrocybe aegerita. Appl. Environ. Microbiol. 2014, 80 (11), 3496–3507. 10.1128/AEM.00490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin F.; Tieves F.; Willot S.; van Troost A.; van Oosten R.; Breestraat S.; van Pelt S.; Alcalde M.; Hollmann F. Pilot-scale production of peroxygenase from Agrocybe aegerita. Org. Process Res. Dev. 2021, 25 (6), 1414–1418. 10.1021/acs.oprd.1c00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge M.; Ullrich R.; Scheibner K.; Hofrichter M. Stereoselective benzylic hydroxylation of alkylbenzenes and epoxidation of styrene derivatives catalyzed by the peroxygenase of Agrocybe aegerita. Green Chem. 2012, 14 (2), 440–446. 10.1039/C1GC16173C. [DOI] [Google Scholar]
- Schmermund L.; Reischauer S.; Bierbaumer S.; Winkler C. K.; Diaz-Rodriguez A.; Edwards L. J.; Kara S.; Mielke T.; Cartwright J.; Grogan G.; Pieber B.; Kroutil W. Chromoselective photocatalysis enables stereocomplementary biocatalytic pathways. Angew. Chem. Int. Ed. 2021, 60 (13), 6965–6969. 10.1002/anie.202100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Brasselet H.; Jongkind E. P. J.; Alcalde M.; Paul C. E.; Hollmann F. A Peroxygenase-Alcohol Dehydrogenase cascade reaction to transform ethylbenzene derivatives into enantioenriched phenylethanols. ChemBioChem 2022, 23 (6), e202200017. 10.1002/cbic.202200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof A. J. J.; Jongejan J. A. The enantiomeric ratio: origin, determination and prediction. Enz. Microb. Technol. 1997, 21 (8), 559–571. 10.1016/S0141-0229(97)00066-5. [DOI] [Google Scholar]
- Piontek K.; Strittmatter E.; Ullrich R.; Gröbe G.; Pecyna M. J.; Kluge M.; Scheibner K.; Hofrichter M.; Plattner D. A. Structural basis of substrate conversion in a new aromatic peroxygenase: cytochrome P450 functionality with benefits. J. Biol. Chem. 2013, 288 (48), 34767–34776. 10.1074/jbc.M113.514521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek K.; Ullrich R.; Liers C.; Diederichs K.; Plattner D. A.; Hofrichter M. Crystallization of a 45 kDa peroxygenase/peroxidase from the mushroom Agrocybe aegerita and structure determination by SAD utilizing only the haem iron. Acta Crystallogr. F-Struct. Biol. Cryst. Commun. 2010, 66 (6), 693–698. 10.1107/S1744309110013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckbecker A.; Hummel W. Cloning, expression, and characterization of an (R)-specific alcohol dehydrogenase from Lactobacillus kefir. Biocatal. Biotransf. 2006, 24 (5), 380–389. 10.1080/10242420600893827. [DOI] [Google Scholar]
- Keinan E.; Hafeli E. K.; Seth K. K.; Lamed R. Thermostable enzymes in organic synthesis. 2. Asymmetric reduction of ketones with alcohol dehydrogenase from Thermoanaerobium brockii. J. Am. Chem. Soc. 1986, 108 (1), 162–169. 10.1021/ja00261a026. [DOI] [Google Scholar]
- Andersen K. B.; von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J. Biol. Chem. 1977, 252 (12), 4151–4156. 10.1016/S0021-9258(17)40245-6. [DOI] [PubMed] [Google Scholar]
- Loferer-Krößbacher M.; Klima J.; Psenner R. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl. Environ. Microbiol. 1998, 64 (2), 688–694. 10.1128/AEM.64.2.688-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacs J.; Zhang W.; Knaus T.; Mutti F. G.; Arends I. W. C. E.; Hollmann F. A photo-enzymatic cascade to transform racemic alcohols into enantiomerically pure amines. Catalysts 2019, 9 (4), 305. 10.3390/catal9040305. [DOI] [Google Scholar]
- Grogan G. Synthesis of chiral amines using redox biocatalysis. Curr. Opin. Chem. Biol. 2018, 43, 15–22. 10.1016/j.cbpa.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Musa M. M.; Hollmann F.; Mutti F. G. Synthesis of enantiomerically pure alcohols and amines via biocatalytic deracemization methods. Catal. Sci. Technol. 2019, 9 (20), 5487–5503. 10.1039/C9CY01539F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schätzle S.; Steffen-Munsberg F.; Thontowi A.; Höhne M.; Robins K.; Bornscheuer U. T. Enzymatic asymmetric synthesis of enantiomerically pure aliphatic, aromatic and arylaliphatic amines with (R)-selective amine transaminases. Adv. Synth. Catal. 2011, 353 (13), 2439–2445. 10.1002/adsc.201100435. [DOI] [Google Scholar]
- Kaulmann U.; Smithies K.; Smith M. E. B.; Hailes H. C.; Ward J. M. Substrate spectrum of ω-transaminase from Chromobacterium violaceum DSM30191 and its potential for biocatalysis. Enz. Microb. Technol. 2007, 41 (5), 628–637. 10.1016/j.enzmictec.2007.05.011. [DOI] [Google Scholar]
- Kohls H.; Steffen-Munsberg F.; Höhne M. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr. Opin. Chem. Biol. 2014, 19, 180–192. 10.1016/j.cbpa.2014.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.