Abstract
Individuals with fragile X syndrome (FXS), especially those co-diagnosed with autism spectrum disorder (ASD), face many sensory processing challenges. However, sensory processing measures informed by neurophysiology are lacking. This paper describes the development and psychometric properties of a parent/caregiver report, the Brain-Body Center Sensory Scales (BBCSS), based on Polyvagal Theory. Parents/guardians reported on 333 individuals with FXS, 41% with ASD features. Factor structure using a split-sample exploratory-confirmatory design conformed to neurophysiological predictions. Internal consistency, test-retest, and inter-rater reliability were good to excellent. BBCSS subscales converged with the Sensory Profile and Sensory Experiences Questionnaire. However, data also suggest that BBCSS subscales reflect unique features related to sensory processing. Individuals with FXS and ASD features displayed more sensory challenges on most subscales.
Keywords: fragile X syndrome, autism spectrum disorders, polyvagal theory, autonomic nervous system, psychometrics, sensory processing
Fragile X syndrome (FXS), which results from a mutation on the 5′ untranslated region of the FMR1 gene, is the most common inherited form of intellectual disability. FXS occurs when the CGG trinucleotide repeat exceeds 200; typically, individuals have approximately 30 CCG repeats. This expansion reduces or prevents the production of fragile X mental retardation protein (FMRP), which is needed for normal brain development. Given that FXS is an X-linked condition, prevalence rates are higher in males than females (Riley, Mailick, Berry-Kravis, & Bolen, 2017). Individuals with FXS have higher rates of several co-occurring psychiatric and medical conditions, including anxiety, attention problems, and hyperactivity (Bailey, Raspa, Olmsted, & Holiday, 2008) as well as poorer affiliative social behavior including social gaze aversion and social avoidance (Cohen et al., 1988; 1989; 1991; Hall et al., 2015). FXS is also the most common single-gene cause of autism spectrum disorders (ASD), with ASD prevalence estimates ranging between 30 and 50% of males and 10% of females with FXS (Raspa, Wheeler, & Riley, 2017).
Sensory processing related abnormalities are well documented in FXS. When compared with typically developing controls, children with FXS often show higher rates of sensory challenges, including tactile sensitivity, taste/smell sensitivity, stimulation seeking behaviors, and auditory filtering (Rogers, Hepburn, & Wehner, 2003). These rates are statistically similar to children with ASD (McIntosh, Miller, & Shyu, 1999; Rogers et al., 2003) and children co-diagnosed with FXS and ASD (Bailey, Mesibov, Hatton, Clark, Roberts, Mayhew, 1998). Likewise, studies of children with ASD show higher rates of sensory challenges when compared with children with other developmental delays and typically developing children using parent-reported measures (Baranek, David, Poe, Stone, & Watson, 2006; Tomcheck & Dunn, 2007) and parent interviews (Leekam, Nieto, Libby, Wing, & Gould, 2007). Similarities in sensory processing in FXS and ASD may be due to similar pathophysiological and anatomical abnormalities (Belmonte & Bourgeron, 2006; Hagerman, 2006; Feinstein & Reiss, 1998). Longitudinal studies using both observational and parent-reported measures have shown that sensory processing problems begin early in children with FXS (Baraneck et al., 2008). Other common sensory challenges are selective eating (Raspa, Bailey, Bishop, Holiday, & Olmsted, 2010) and gastrointestinal issues (Kidd et al., 2014).
To facilitate study of sensory processing abnormalities, several questionnaires are available for research and clinical applications. The most widely-used of these is the Sensory Profile (SP), available in multiple age-specific forms (Ermer & Dunn, 1998; Brown et al., 2001; Dunn & Daniels, 2002), based on Dunn’s model of sensory processing (Dunn 1997; 2007). Dunn’s model, building on the work of Ayres (1964; 1965; 1972), proposes that neurological thresholds and behavioral strategies for self-regulating sensory experiences form the basis for individual differences in sensory patterns (Dunn, 1997). This model provides the foundation for the four domain scores of the Sensory Profile, which describe the extent to which individuals have: (a) a high sensory threshold with passive behavioral responses (Low registration); (b) a high sensory threshold with active behavioral self-regulation (Sensation seeking); (c) a low threshold with passive responses (Sensory sensitivity); and (d) a low threshold with active self-regulation (Sensation avoiding).
Despite its ease of interpretation, this theoretical model and the resulting measurement tool presents challenges for researchers and clinicians. A single threshold-based model, even when thresholds may differ across sensory domains, cannot account for the concurrent hyper- and hypo-sensitivities observed in FXS and ASD populations, including poor responding to individual voices coupled with an aversion to noisy environments such as a crowded restaurant (Stackhouse et al., 2014). Indeed, Sensory Profile scores show positive correlations across high- and low-sensory threshold domains (e.g., Ben-Sasson et al., 2007; Engel-Yeger, 2012), reflecting that simultaneously elevated and dampened sensory responsivity can and do co-occur. These correlated domains of the SP are at odds with the categorical structure of the theoretical model and intervention recommendations (see Dunn, 2007; Dunn, Saiter, & Rinner, 2002), posing challenges for explaining individual differences and developing treatments for individuals with both hypo- and hyper-sensitivities in a single domain. Furthermore, this model lacks a plausible mechanism or organizing principles for the neural regulation involved in shifting sensory experience and biobehavioral state (Dunn, 1997), the lack of an integrated neural mechanism for sensory modulation has steered research toward treating sensory processing as a fixed trait (see Dunn, 2001) and interventions toward changing environments to better accommodate an individual’s sensory processing profile (see Dunn, 2007; Dunn, Saiter, & Rinner, 2002).
Another widely-used sensory processing scale is the Sensory Experiences Questionnaire (Baranek, 1999; Baranek et al., 2006; Little et al., 2011), which was developed for identifying sensory features of children with autism. It is composed of subscales that assess hyper-responsiveness, hypo-responsiveness, and sensory seeking within individual sensory domains as well as social and non-social contexts. In contrast to a single threshold model, this scale is based on a conceptual model of sensory processing problems arising from a narrowed optimal engagement band, with a higher threshold required for orientation and a decreased threshold for aversive responses (Baranek, Reinhartsen, & Wannamaker, 2001; Baranek, 1999). Although providing a conceptual explanation of concurring hyper- and hypo-sensitivities, stimuli eliciting hyper-reactivity must have stronger signals than those that result in hypo-responsivity, at least within social or non-social domains. However, the very low-amplitude auditory stimuli that can elicit intense aversive reactions in individuals with ASD, such as particularly high or low frequency appliance noise not normally noticed by others (Talay-Ongan & Wood, 2000), pose a challenge to this model. Most importantly, like Dunn’s model of sensory processing, this conceptual model provides room for state-dependent sensory modulation but lacks a proposed neural mechanism that gives rise to such differences.
An approach that provides a neurophysiological framework for the study of sensory processing is based on the Polyvagal Theory (Porges, 1995; 2001; 2007; 2011). This theoretical framework traces the evolution of the mammalian nervous system as it transitioned from optimization for defense and life-threat responses toward an affiliative, social way of life that required the dampening of primitive defense systems. Polyvagal Theory hypothesizes that the nervous system dynamically detects and evaluates sensory signals from within the body and from the environment as cues of safety, danger, or life-threat. The theory proposes that these exteroceptive and interoceptive inputs are integrated to inform a neurophysiological state that can flexibly regulate sensory information to promote vigilance for evolutionary danger cues or, conversely, attention to social affiliative cues via the motor pathways of the autonomic nervous system. This focus on state-regulated sensory processing modulation may provide a foundation for improved documentation of sensory processing problems and provide a conceptual bridge between neuroscience, physiology, and clinical approaches to studying sensory systems and their pathology. Notably, this threat-response approach is consistent with Cohen’s (1995) proposal that the behavioral phenotype of individuals with FXS, including tactile and auditory hypersensitivities, may be caused by hyperarousal.
Polyvagal Theory proposes that evolutionarily-salient cues, outside the realm of conscious awareness, reflexively trigger physiological state changes via motor pathways of the autonomic nervous system, modulating sensory processing. For instance, safety-related states may promote the regulation of the middle ear muscles to dynamically boost the frequency band in which spoken language intelligibility occurs, promoting speech orientation and comprehension for affiliative social interactions. However, as danger-responsive physiological states shifts to support fight/flight behaviors, these muscles can be regulated to dampen language-related vocal frequencies to boost salience of high frequency signals, associated with distress calls, or low frequency signals, evolutionarily associated with predator calls (see Kolacz, Lewis, & Porges, in press; Porges & Lewis, 2010). Other sensory domains can be similarly regulated to promote threat vigilance, such as heightened sensitivity for visual movement, or defense-oriented states and responses that are unsuited for affiliative social interaction, such as aversion to friendly touch. Physiological profiles have been observed to predict differences in children’s temperamental affective discomfort to sensation (Kolacz et al., 2016). These sensory physiological pathways also link with the regulation of swallowing muscles (Kolacz, Lewis, & Porges, in press), and control of gastrointestinal functions in response to metabolic needs (Porges, 2011; also see Zhu et al., 2016; Zhang et al., 2006; Herman et al., 2009).
Based on the anatomical and functional organization described above, a behavioral profile marked by aversion to or neglect of social affiliative interactions, heightened sensitivity to threat cues, and digestive/ingestive difficulties – common in individuals with FXS and ASD - would be expected to be marked by a physiological withdraw of socially-supportive circuits and stronger activation of defense-supporting circuits. This physiological profile is evidenced in individuals with FXS and ASD in whom vagal regulation of the heart, which reflects the calming affiliative-promoting circuits and gives rise to respiratory sinus arrhythmia, is tonically low and lacks the normal challenge-induced regulatory pattern of their typically developing peers (Klusek, Roberts, & Losh, 2015; Heilman et al. 2011; Roberts et al., 2001). In addition, males with FXS have exaggerated sympathetic activity, a fight/flight mobilization response, during conversations involving eye contact (Belser and Sudhalter, 1995) and in response to sensory stimuli in multiple domains (Miller et al., 1999). Notably, more pronounced sympathetic responses to sensory stimuli are related to lower expression of FMRP (measured by percent of FMRP-positive lymphocytes; Miller et al., 1999).
The converging evidence reviewed above is consistent with sensory processing as linked with a neuro-physiological regulation mechanism for responding to environmental threat and safety cues. To promote research into these functions in FXS and ASD populations, there is a need for a questionnaire instrument that can assess patterns of everyday sensory responses informed by an understanding of neurophysiological processes. The Brain-Body Center Sensory Scales (BBCSS; Porges, 2012), a caregiver-reported questionnaire, was designed to address this gap. In this paper, we present results of a psychometric study evaluating its factor structure, reliability, and validity study for the BBCSS.
We hypothesized that factors would be best described by underlying threat-related neurophysiological regulation, rather than single- or dual-model sensory thresholds. Because of the exploratory goal of examining the BBCSS factor structure, our specific hypotheses about relations of the BBCSS subscales with validity instruments and differences between children with and without ASD were limited in specificity. We expected to find moderate levels of convergence with the passive subscales of the Sensory Profile forms at both ages (sensitivity and registration/bystander, low registration) and lower convergence with the active subscales, which reflect behavioral strategy responses to sensory needs. We also expected to find moderate correlations with modality-, context-, and hypo-/hyper-reactivity specific subscales of the SEQ, dependent on whether the BBCSS subscales emerge to reflect such specificity. However, we also expected that the derived subscales would demonstrate substantial unique variance and structural divergence reflecting the distinct approach posed by our organizing theoretical model. Given the general elevated severity of the ASD+FXS phenotype compared to FXS without ASD (Bailey et al., 2001; Kaufmann et al., 2004; Lewis et al., 2006; Hernandez et al., 2009), differences between individuals with FXS only and FXS and ASD were expected as well, with co-diagnosis relating to more impaired sensory behaviors.
Methods
Participants
Parents and legal guardians of individuals with full mutation FXS were recruited through the Our Fragile X World (OFXW) survey registry. Invitations were sent via e-mail and included a secure link to a web-based survey platform where respondents entered a unique ID to access the questionnaires. Informed consent was obtained from all individual participants included in the study. In total, 333 parents and guardians completed the survey. Respondents were between the ages of 26 and 85 years (M = 54.03; SD = 10.41); the majority were female (92%), married (85%), white (92%; 2% African-American or black; 4% Hispanic; 2% other or multi-racial), had at least a 4-year college degree (66%), and had a family income of $75,000 or more (68%). The average age of individuals with FXS was 24 years (SD = 10.96, Min = 5.20, Max = 58.63), 99% were the biological or step-child of the primary respondent (n = 1 was other relative), and 84% lived in the household with the primary respondent at the time of survey completion. Consistent with FXS prevalence rates, most individuals with FXS were male (90%). Females were included in all analyses because they are at high risk of many of the same symptoms as their male counterparts with FXS (Bailey et al., 2008).
The initial survey consisted of demographic questions, the BBCSS (see below), as well as an age-appropriate version of the Sensory Profile and the Sensory Experiences Questionnaire for assessing convergent validity (see below). After completing the initial survey, the original respondents were invited by e-mail to complete the BBCSS a second time to assess test-retest reliability. This second wave of data collection from the same respondents (n=138) were, on average, completed 2 months after the initial assessment (M = 8.47 weeks, SD = 5.78). To collect inter-rater reliability data, primary respondents were given the option to provide an e-mail address for a second parent or legal guardian who could report on the same individuals with FXS. Fifty-seven secondary respondents completed the BBCSS on the target child.
Measures
BBCSS.
The full item pool used for psychometric assessment of the BBCSS consisted of 59 items across auditory, visual, tactile, ingestive, and digestive domains. The questionnaire content was selected based on conversations between the questionnaire author and parents of children with sensory problems. Common complaints were organized and distilled into questionnaire items through the framework of the Polyvagal Theory to capture sensitivity and responses to evolutionary safety and threat cues. The resulting BBCSS questionnaire invites parents to report on the behaviors and observed experiences of their children using a 4-point scale (1 = Almost always, 2 = Frequently/often, 3 = Sometimes/occasionally, 4 = Almost never), a response order that reflects more optimal functioning at higher levels. Items are organized in blocks according to the auditory, visual, tactile, and ingestive/digestive domains.
Sensory Profile.
Convergent validity for the BBCSS was assessed in comparison to the Sensory Profile-2 Short Form (SP2; Dunn, 2014) for children with FXS ages 14 and younger (n = 89) and the Adolescent-Adult Sensory Profile (AASP; Brown & Dunn, 2002) for individuals ages 15 and older (n = 237). Both the SP2 and AASP are based on Dunn’s (1997) mode of sensory processing [see introduction]. Item responses are based on a 5-point Likert-type scale (1 = Almost never, 5 = Almost always) and subscales are computed across all sensory domains to derive broad, cross-domain dimensional scores.
SP2 and AASP consist of four subscales: Sensation seeking, Sensation avoiding, Low registration/bystander, and Sensory sensitivity. Sensation seeking refers to the extent that individuals actively pursue sensory experiences to meet high sensory thresholds. Sensation avoiding refers to the extent that individuals actively withdraw from sensory experiences to prevent being overwhelmed because of their low sensory thresholds. Low registration refers to the extent that individuals display sluggish or no behavioral response to sensory stimuli due to a high neurological threshold. Finally, Sensory sensitivity refers to the extent to which individuals have a low sensory threshold but react without actively avoiding sensory stimuli.
Sensory Experiences Questionnaire.
Convergent validity was also examined using the Sensory Experiences Questionnaire, version 2.1 (SEQ; Baranek, 1999), a 43-item questionnaire developed to assesses sensory features in children with autism and other developmental disabilities. Children’s typical responses are reported on a 5-point Likert-type scale (1 = Almost never, 5 = Almost always). The derived subscales are divided into sensory response pattern (hypersensitivity, hyposensitivity, sensory seeking), sensory context (social, non-social), and sensory modality (tactile, auditory, visual, gustatory & olfactory, vestibular & proprioceptive).
ASD features.
ASD features were assessed using parent responses to behavioral items using an algorithm based on the DSM-4 criteria (Wheeler et al., 2014). These data were collected for 249 individuals in the sample. For those whom features data were not available, a parent report of whether the child had received treatment or services for ASD were used. In total, 41% of the sample displayed ASD features based on the DSM-IV criteria or had received treatment or services for it.
Statistical Methods
Exploratory and confirmatory factor analysis were conducted using Mplus version 7.4 (Muthen & Muthen, 1998–2015). Data cleaning and all other analyses were conducted using SAS 9.4 (SAS Institute, Cary NC), R version 3.3.3 (R Core Team, 2017), and RStudio version 1.0.136 (RStudio, Inc., 2009–2016).
Data preparation.
BBCSS items were first examined for item-level test-retest reliability in order to limit the effects of unstable items on factor solutions. Item test-retest reliability was assessed using weighted Kappa statistics, using a threshold of .40 to indicate at least moderate or fair test-retest reliability (Landis & Koch; 1977; Fleiss, 1981). Using this cut off (Kappa > .40), we identified 55 reliably stable items of the original 59-item item pool to utilize in the factor analysis (see supplemental material table S1). The resulting item distributions were then examined for response cell sizes to assess whether item response categories were well represented. To prevent factor analysis estimation problems, we recoded items with cell sizes that were endorsed by fewer than 10 participants. All items with low cell counts (n = 23) occurred in responses at high frequency levels. For these items, the response options “almost always” were recoded into one category that reflected “almost always or frequently/often” value, resulting in 3-level variables (see supplemental material table S2).
Data analysis.
The full sample was randomly divided into an exploratory and confirmatory sample (exploratory n = 168; confirmatory n = 165). We applied a robust weighted least squares estimator (WLSMV), as recommended by Barendse, Oort, and Timmerman (2015) for models with categorical responses. This method uses a diagonal weight matrix with standard errors as well as a mean and variance-adjusted chi-square test statistic that utilizes a full weight matrix (Muthén & Muthén, 1998–2015). EFA factor retention was guided by examination of the scree plot (Cattell, 1966), model fit, simple structure of factor loadings, and theoretical utility. Goodness of fit to the data was evaluated using the root mean squared error of approximation (RMSEA; Steiger & Lind, 1980; Steiger, 1990), the Tucker-Lewis Index (TLI; Tucker & Lewis, 1973); and the Comparative Fit Index (CFI; Bentler, 1990). As suggested by Hu and Bentler (1999), we considered good fit to be evidenced by an RMSEA value near .06 or lower as well as CFI and TLI values near .95 or greater. Scree plots were examined for substantial drops in eigenvalue magnitude (Fabrigar et al., 1999). EFA results were subject to oblique rotation according to the geomin criterion (Yates, 1987), a rotation that minimizes variable complexity and produces easily interpretable rotations when factor structure is not highly complex (Sass & Schmitt, 2010). Oblique rotation methods permit solutions with correlated factors but can reproduce uncorrelated factor structures if such factor relations are implied by the data (Fabrigar et al., 1999).
Internal consistency was assessed using the reliability coefficient proposed by Bentler (ρxx; 1972; 2009), which represents the ratio of common variance to the total variance among the items. These values were estimated using a polychoric covariance matrix implemented in the semTools R package (semTools Contributors, 2016). This method was used in lieu of Cronbach’s alpha because items were discrete andfactor loadings were variable (Revelle & Zinbarg, 2009; Trizano-Hermosilla & Alvarado, 2016; McNeish, 2017). High internal consistency values represent items with high shared common variance that can be explained by a single factor while lower values represent greater variance attributable to unique influences or other factors. Although there is no consensus on cut off values, there is evidence that explained common variance estimates below .70 may signal non-ignorable multi-dimensionality (Quinn, 2014).
Test-retest and inter-rater reliability were assessed using the intraclass correlation coefficient (ICC). Based on the categories and nomenclature proposed by Shrout & Fleiss (1979), Model 1 [ICC(1,1) and ICC(1,k)] estimates were used because all targets were assessed by their unique caregivers. Test-retest reliability was computed as a one-way ANOVA fixed effects model assessing absolute agreement (ICC(1,1)) and inter-rater reliability was assessed as the average of absolute agreement across raters (ICC(1,k)). Comparisons between individuals with FXS only and FXS with ASD features were conducted using the Wilcoxon-Mann-Whitney rank sum test. Effect sizes were calculated using Cliff’s d (Cliff, 1993), implemented in the orddom R package (Rogmann, 2013), which are included for relative comparison of effect strength in FXS/FXS+ASD comparisons.
Results
Exploratory factor analysis.
EFA was conducted on sensory and ingestive/digestive items separately. Items that resulted in estimation problems were dropped (see Table S1 in the supplementary materials). The sensory item scree plot contained several substantial drops after the 1st, 4th, and 6th eigenvalues, indicating that any of these solutions may describe the data well (Figure 1, top panel). In examining fit indices, we noted that the 5- and 6-factor solutions may fit the data well (Table 1, top rows). However, because the TLI was slightly low in the 5-factor solution, the scree plot favored the 6- rather than 5-factor solution, and underfactoring is a more serious problem than overfactoring (Fabrigar et al., 1999), we chose the 6-factor solution. The ingestive/digestive item scree plot demonstrated one high value followed by another small but substantial drop following the 5th eigenvalue (Figure 1, bottom panel). Fit indices showed that the model fit reasonably well with three factors (Table 1, center rows). Thus, three factors were retained. Upon examination of the loading patterns, we identified the following factors: Auditory Threat Hypersensitivity, Auditory Hyposensitivity to Voices, Visual Hypersensitivity, two Tactile Hypersensitivity factors that shared many of the same items, Affiliative Touch Aversion, Selective Eating, Ingestive Problems, and Digestive Problems (see tables S3 and S4 in the supplementary materials for EFA factor loadings, see table S5 for factor correlations). Based on these interpretations, we devised a testable factor structure for confirmatory analysis.
Figure 1.
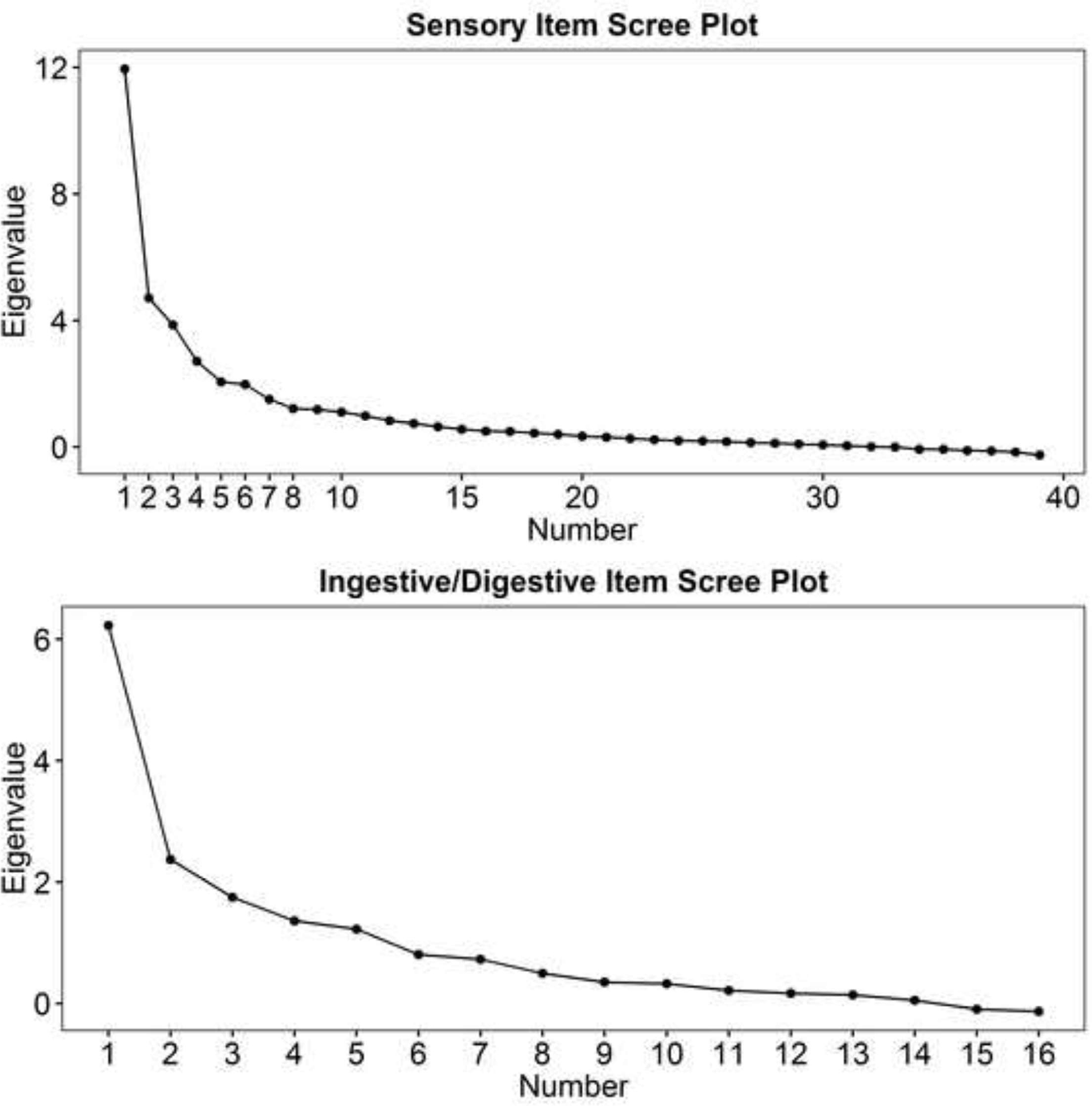
Exploratory factor analysis scree plots
Table 1.
EFA and CFA fit indices
| Factors | χ2 | df | RMSEA | RMSEA 90% Confidence Intervals | CFI | TLI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Sensory Items EFA | |||||||
| 1 | 1995.52 | 702 | .105 | .099 | .110 | .77 | .76 |
| 2 | 1544.06 | 664 | .089 | .083 | .095 | .85 | .83 |
| 3 | 1158.88 | 627 | .071 | .065 | .077 | .91 | .89 |
| 4 | 967.40 | 591 | .062 | .055 | .068 | .93 | .92 |
| 5 | 794.90 | 556 | .051 | .042 | .058 | .96 | .94 |
| 6 | 653.38 | 522 | .039 | .028 | .048 | .98 | .97 |
| 7 | 572.83 | 489 | .032 | .019 | .042 | .99 | .98 |
| Ingestive/Digestive Items EFA | |||||||
| 1 | 318.15 | 104 | .113 | .099 | .127 | .87 | .85 |
| 2 | 177.92 | 89 | .079 | .062 | .096 | .95 | .93 |
| 3 | 120.97 | 75 | .062 | .040 | .081 | .97 | .96 |
| 4 | 74.89 | 62 | .036 | .000 | .062 | .99 | .99 |
| CFA (full item set) | |||||||
| 8 | 1544.665 | 1246 | .038 | .032 | .044 | .96 | .96 |
EFA = exploratory factor analysis; CFA = confirmatory factor analysis; RMSEA = root mean square error of approximation; CFI = comparative fit index; TLI = Tucker-Lewis index
Confirmatory factor analysis.
In the interest of maximizing the theoretical interpretability of the factor structure, we used the exploratory factor analysis as a starting point and adjusted the factor loading patterns for use in the CFA. We dropped items that resulted in estimation problems (See Table S1 in the supplementary materials) and a single item that did not have substantial loadings on any factor [Item E15, “Eats (or wants to eat) significantly more than I think is appropriate for his/her size or age”]. In addition, we adjusted items to fit the theoretical factors and combined the two similar tactile hypersensitivity factors from the EFA into a single factor. We found the resulting adjusted factor loading pattern to fit well (Table 1, bottom row) and that the factor loadings were substantial for all items (Table 2). Most factors had low or moderate positive correlations (Table 3).
Table 2.
Confirmatory factor analysis loadings
| Auditory Threat Hypersensitivity | Loading |
|
| |
| responds negatively to unexpected or loud noises (for example, hides or cries at noise from ambulance, train, fire or car alarm, fireworks) | 0.628 |
| becomes distracted, or has difficulty following verbal instructions when there is a lot of noise around | 0.737 |
| holds his/her hands over the ears | 0.618 |
| has trouble working with background noise (for example, air conditioner, traffic noises, airplanes) | 0.785 |
| is unusually angry or frightened or appears in pain when others cry or scream | 0.581 |
| seems overly aware, distracted, or disturbed by continuous noise in the environment (for example, TV, stereo) | 0.429 |
| startles easily at sound, compared to other children the same age, with loud or high- pitched noises (for example, vacuum, blender, fire alarms) | 0.728 |
| is distracted by sounds not normally noticed by other people (for example, air conditioning fans, trains or planes outside) | 0.718 |
| responds negatively (i.e. tantrums, becomes distracted or anxious) when entering places with continuous background noises (for example, grocery stores, schools, shopping malls) | 0.895 |
|
| |
| Auditory Hyposensitivity to Voices | Loading |
|
| |
| appears not to hear what I say (for example, does not seem to pay attention to what I say, appears to ignore me) | 0.815 |
| does not respond when his/her name is called, even though I know my child’s hearing is not a problem | 0.864 |
| I have to speak loudly or get very close to my child’s face to get my child’s attention | 0.821 |
| seems unaware of continuous noise in the environment (for example, TV, stereo) | 0.460 |
| takes a long time to respond when spoken to, even to familiar voices | 0.785 |
|
| |
| Visual Hypersensitivity | Loading |
|
| |
| is bothered by bright lights after my eyes or other children’s eyes have adapted to the same light | 0.939 |
| covers his/her eyes or squints | 0.890 |
| seems unable to tolerate bright lights | 0.951 |
| seems unable to tolerate flashing lights | 0.914 |
| gets fussy when exposed to bright lights | 0.941 |
| seems sensitive to bright lights (for example, cries or closes eyes) | 0.967 |
| seems sensitive to flashing lights (for example, cries or closes eyes) | 0.920 |
| hesitates to go outside when sunny | 0.714 |
| seems easily distracted by movement he/she can see | 0.704 |
| seems easily distracted by movements of objects (i.e. mechanical toys or cars) | 0.671 |
|
| |
| Tactile Hypersensitivity | Loading |
|
| |
| seems distressed by tooth-brushing | 0.737 |
| seems distressed by face-washing | 0.828 |
| seems distressed by fingernail-cutting | 0.742 |
| seems distressed by hair-brushing | 0.868 |
| insists that labels or tags be removed from most clothing | 0.681 |
| refuses to wear certain fabrics or cries or fusses in response to wearing certain fabrics | 0.880 |
| complains that certain garments are too tight or scratchy | 0.945 |
| prefers to not wear clothing | 0.656 |
| reacts emotionally or aggressively when touching very cold objects with his/her hands | 0.934 |
| reacts emotionally or aggressively when very cold objects touch his/her face | 0.913 |
|
| |
| Affiliative Touch Aversion | Loading |
|
| |
| resists hugging | 0.772 |
| reacts negatively or aggressively to hand-holding | 0.855 |
| reacts emotionally or aggressively to being touched | 0.858 |
|
| |
| Selective Eating | Loading |
|
| |
| avoids certain tastes | 0.916 |
| resists certain textures of food | 0.945 |
| avoids certain food smells | 0.919 |
| resists certain temperatures of food | 0.890 |
| sucks on objects other than food (for example, pacifier, own tongue, thumb) | 0.517 |
| eats (or wants to eat) significantly less than I think is appropriate for his/her size or age | 0.505 |
|
| |
| Ingestive Problems | Loading |
|
| |
| gags | 0.950 |
| vomits | 0.866 |
| seems to have difficulty swallowing solid foods | 0.770 |
|
| |
| Digestive Problems | Loading |
|
| |
| has acid reflux | 0.877 |
| has excessive intestinal gas | 0.824 |
| becomes constipated | 0.704 |
| experiences stomach or intestinal cramping | 0.857 |
Table 3.
Confirmatory factor correlations
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1 | Auditory Threat Hypersensitivity | .479* | .645* | .557* | .407* | .406* | .327* | .336* |
| 2 | Auditory Hyposensitivity to Voices | .433* | .293* | .334* | .338* | .294* | .170 | |
| 3 | Visual Hypersensitivity | .498* | .607* | .429* | .163† | .285* | ||
| 4 | Tactile Hypersensitivity | .552* | .518* | .437* | .270* | |||
| 5 | Affiliative Touch Aversion | .166† | .021 | .186 | ||||
| 6 | Selective Eating | .646* | .290* | |||||
| 7 | Ingestive Problems | .371* | ||||||
| 8 | Digestive Problems | |||||||
p < .05
p<.10
Reliability.
Reliability results are presented in Table 4. Internal consistency estimates demonstrated estimated common variances that supported each subscale as unidimensional (ρxx(SEM) range: .77 – .93). Two-month test-retest reliability measures, assessed using the intra-class correlation coefficient, ranged from .71–.83, indicating good to excellent reliability as described by Cicchetti (1994). Inter-rater reliability ranged from .57 to .83, indicating fair to excellent reliability between caregivers.
Table 4.
Internal consistency (ρxx; Bentler 1972; 2009), test-retest, and inter-rater reliability (intra-class correlation coefficient; ICC)
| Internal Consistency ρxx | Test-Retest ICC | Inter-Rater ICC | |
|---|---|---|---|
|
| |||
| Auditory Threat Hypersensitivity | .86 | .82 | .57 |
| Auditory Hyposensitivity to Voices | .77 | .72 | .69 |
| Visual Hypersensitivity | .93 | .71 | .65 |
| Tactile Hypersensitivity | .92 | .82 | .80 |
| Affiliative Touch Aversion | .80 | .82 | .65 |
| Selective Eating | .89 | .75 | .70 |
| Ingestive Problems | .87 | .78 | .83 |
| Digestive Problems | .84 | .83 | .73 |
Convergent validity.
Spearman correlations (Rho) of the BBCSS with the SP2 and AASP subscales are presented in Table 5. Eighty-nine primary respondents completed the SP2 for children age 14 and younger. Correlations between the BBCSS subscales and the SP2 Sensory Sensitivity subscale (range of Rho: .309 – .526) and Sensory Avoidance subscale (range of Rho: .313 – .422) were moderate. The other SP2 subscales were largely uncorrelated with BBCSS sensory subscales, with the exception of BBCSS Tactile Hyperensitivity, which was positively correlated with the SP2 Low Registration/Bystander subscale.
Table 5.
Spearman correlations (Rho) of BBCSS subscales with relevant subscales of the Sensory Profile-2 (SP2; Age 14 and younger) and the Sensory Profile Adolescent
| BBCSS | SP2 Age 14 and Younger | AASP Age 15+ | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Registration/Bystander | Sensation Seeking | Sensory Sensitivity | Sensory Avoidance | Low Registration | Sensation Seeking | Sensory Sensitivity | Sensory Avoidance | |
|
| ||||||||
| Auditory Threat Hypersensitivity | 0.186 | 0.178 | 0.526* | 0.422* | 0.370* | −0.124† | 0.396* | 0.430* |
| Auditory Hyposensitivity to Voices | 0.205† | 0.188 | 0.369* | 0.405* | 0.451* | −0.02 | 0.183* | 0.215* |
| Visual Sensitivity | 0.151 | 0.082 | 0.363* | 0.398* | 0.322* | −0.072 | 0.380* | 0.354* |
| Tactile Hypersensitivity | 0.253* | 0.204† | 0.330* | 0.345* | 0.374* | −0.119† | 0.526* | 0.483* |
| Affiliative Touch Aversion | 0.205† | 0.089 | 0.309* | 0.313* | 0.269* | −0.223* | 0.404* | 0.364* |
p < .05
p<.10
The AASP was completed by 237 primary respondents (target individual age 15+ years). As with the younger age group, all BBCSS sensory subscales were positively correlated with the AASP Sensory Sensitivity (range of Rho: .183–.526) and Sensory Avoidance subscales (range of Rho: .215–.483). Likewise, AASP Sensation Seeking scores were largely uncorrelated with BBCSS sensory scales, with the exception of a negative correlation with the BBCSS Tactile Social Sensitivity subscale. In contrast to the younger age group, AASP Low Registration scores were positively correlated with all BBCSS sensory subscales. Because the SP2 and AASP do not include ingestive or digestive subscales, they could not be used for convergent validity testing of those BBCSS subscales.
Forty-three primary respondents completed the SEQ on the target child (age 12 years and younger). The SEQ is organized to decompose sensory experiences into three dimensions: responsivity (hypo- and hyper-), context (social, non-social), and modality (auditory, visual, tactile, and gustatory/olfactory). Although such a dimensional structure did not describe the BBCSS subscales, we assessed convergent validity of the BBCSS subscales to the SEQ subscales as applicable (Table 6). All BBCSS subscales except digestive problems had at least one conceptually-related SEQ subscale. BBCSS Auditory Threat Hypersensitivity scores were positively correlated with SEQ Hyper-responsivity and Auditory Modality scores. BBCSS Auditory Hyposensitivity to Voices was positively correlated with SEQ Hypo-responsivity and Auditory Modality scores. BBCSS Visual Sensitivity was positively correlated with SEQ Hyper-responsivity, but not Visual Modality scores. BBCSS Tactile Hyperensitivity was positively correlated with SEQ Hyper-responsivity and Tactile Modality, but not Non-Social Context. Lastly, the BBCSS Affiliative Touch Aversion was positively correlated with SEQ Hyper-responsivity and Tactile Modality, as well as showing a trending association with Social Context scores.
Table 6.
Spearman correlations (Rho) of BBCSS subscales with relevant subscales of the Sensory Experiences Questionnaire (Age 12 and younger; n = 43)
| BBCSS | SEQ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Hypo-responsivity | Hyper-responsivity | Social Context | Non-Social Context | Auditory Modality | Visual Modality | Tactile Modality | Gustatory and Olfactory Modality | Selective Eating Item | |
|
| |||||||||
| Auditory Threat Hypersensitivity | 0.690* | 0.804* | |||||||
| Auditory Hyposensitivity to Voices | 0.725* | 0.499* | |||||||
| Visual Sensitivity | 0.552* | 0.138 | |||||||
| Tactile Hypersensitivity | 0.487* | 0.231 | 0.407* | ||||||
| Affiliative Touch Aversion | 0.440* | 0.279† | 0.371* | ||||||
| Selective Eating | 0.538* | 0.348* | 0.667* | ||||||
| Ingestive Problems | 0.580* | ||||||||
| Digestive Problems | |||||||||
p < .05
p<.10
The SEQ did not include a subscale score that corresponded to BBCSS Selective Eating; however, this subscale converged well the SEQ item “Is your child selective in food preferences? (e.g., eating only a narrow variety of foods)” (Rho = .667, p < .05). BBCSS Selective Eating scores also positively correlated with SEQ Hyper-responsivity and Gustatory/Olfactory Modality scores. Finally, the BBCSS Ingestive Problems were also positively correlated with SEQ Gustatory/Olfactory Modality scores.
Comparison of FXS and FXS+ASD feature subscale scores.
Figure 2 displays a comparison of BBCSS subscale scores in individuals with FXS only and those with FXS+ASD features or treatment. Scores were higher for those with ASD features or treatment on all subscales except ingestive and digestive problems.
Figure 2.
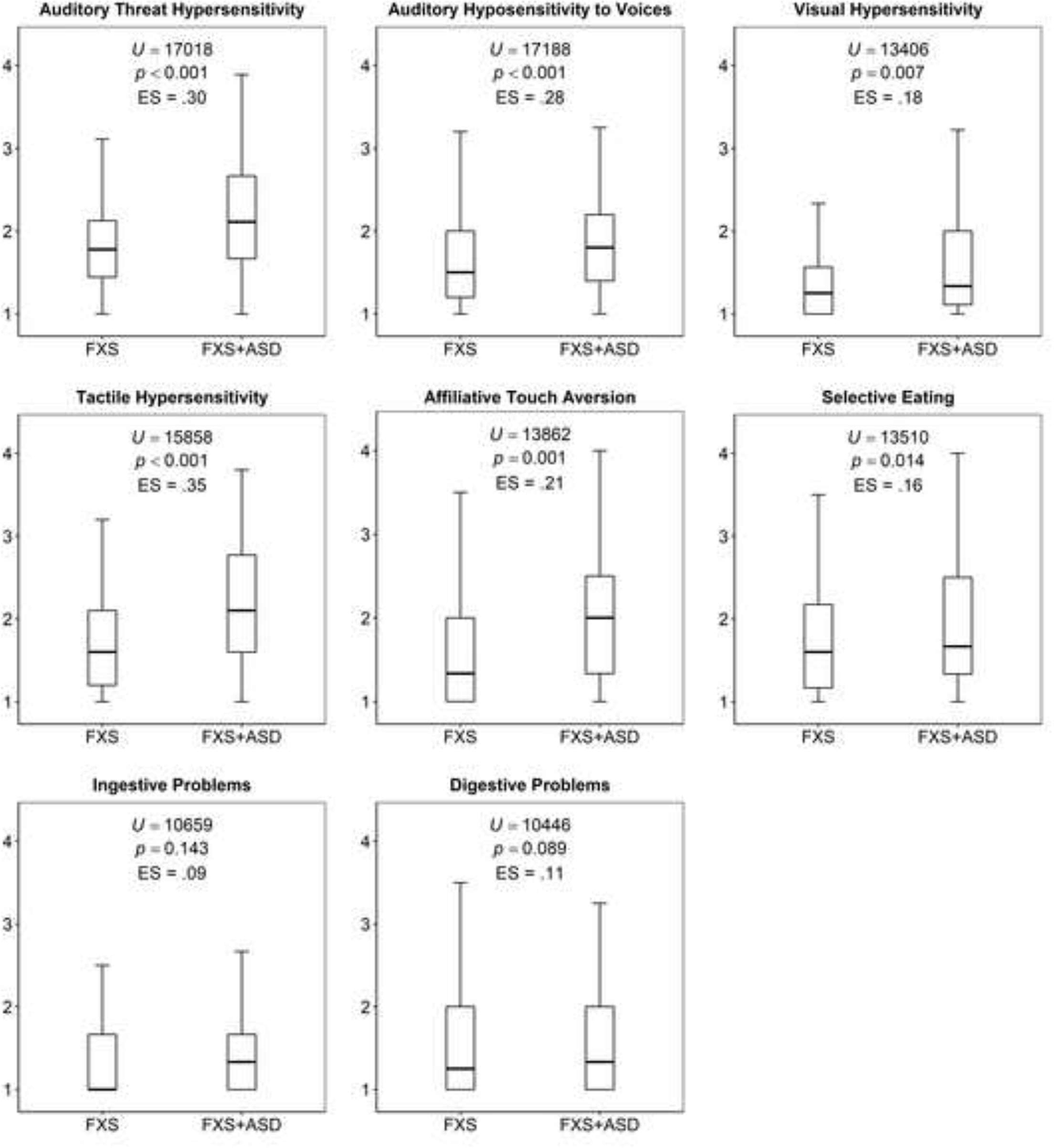
Comparison of BBCSS subscales in individuals with Fragile X (FXS) only and those with concurrent Autism Spectrum
Discussion
This study described the factor structure, reliability, and convergent validity of the BBCSS, a caregiver report for assessing sensory processing problems in a sample of individuals with FXS and FXS+ASD features across a range of ages from early childhood to adulthood. Internal consistency estimates were consistent with unidimensionality for each subscale. Two-month test-retest and inter-rater reliabilities were largely good to excellent.
As predicted by the Polyvagal Theory, auditory and tactile sensitivities were best described by their relation to evolutionary threat and affiliative cues, rather than as singular hyper- or hypo-sensitivities. Auditory hyposensitivities to voices correlated positively with auditory hypersensitivities to largely low and high frequency sounds, outside of the band boosted by middle ear bone resonance and in which language comprehension occurs. This supports the proposition that auditory sensory processing is regulated to heighten or blunt responses to specific acoustic features, which is evolutionarily related to orienting to and processing of voices during states of safety and to predator or distress calls during states of mobilization to threat (see Kolacz, Lewis, & Porges, in press). Ingestive and digestive problems also showed low but substantial correlations with sensory problems, supporting the assertion that the physiological control of ingestion is coordinated with sensory processing. However, some digestive problems may be due to organic causes and will need to be distinguished from those that may be caused by neural brain-body regulation.
The item “Eats (or wants to eat) significantly more than I think is appropriate for his/her size or age” was excluded from the subscales due to its lack of shared variance with other items. It is possible that lack of shared variance with the rest of the item pool may be due to its wording, which invites parent judgement about on “appropriate” food intake. However, it may also be that that the item reflects a construct that has little conceptual overlap with the other subscales. Though overeating may in some cases be a manifestation of low selective eating, it is possible that even children who are highly picky about type of food may consume high quantities of the foods they enjoy. Future work is needed to develop a subscale based on multiple items that reflect food consumption amount.
Convergent validity with the Sensory Profile and Sensory Experiences Questionnaire showed a range of correlation magnitudes from low to high, suggesting some conceptual overlap between scales and some unique measurement qualities of the BBCSS. Convergence with the child and adolescent/adult version of the SP was consistent across ages and BBCSS subscales for Sensory Sensitivity, which suggests that the BBCSS subscales share some broad measurement overlap with general sensory hypersensitivities. Also, as expected, BBCSS subscales had low to non-significant correlations with the SP Sensation Seeking subscale, which reflects an active behavioral strategy that is not assessed by the BBCSS, in both child and adolescents/adults. However, the BBCSS also had consistent correlations with the SP Sensory Avoidance subscale, which measures an active behavioral strategy, possibly because sensory avoidance behavior would be expected in children with high sensitivities. Finally, the AASP Low Registration/Bystander subscale, which reflects hypo-sensitivities, was positively correlated with all BBCSS sensory subscales, suggesting overlap between the sensory processing problems and broad hypo-sensitivities in adolescence and adulthood. However, there was little evidence of this in the age 14 and younger cohort. This may reflect the slightly different items that compose these two SP subscales.
BBCSS convergence with the SEQ supported some conceptual overlap between these questionnaires, with nearly all subscales showing a moderate to strong positive correlation with their conceptually-overlapping counterparts. However, the SEQ Social and Non-social Context subscales did not significantly correlate with the BBCSS Affiliative Touch Aversion and Tactile Hypersensitivity, respectively. This may be due to the general, multi-domain nature of the social and non-social SEQ subscales, which contrasts with the stimuli- and sensory domain specificity of the BBCSS. Furthermore, the Visual Modality subscale of the SEQ did not converge with the BBCSS Visual Sensitivity, as expected. This divergence may be caused by the SEQ visual domain inclusion of social interaction items, including gaze toward faces and acknowledging the presence of new persons that enter the room, which differ from the general movement and light sensitivity measured by the BBCSS Visual Hypersensitivity subscale.
BBCSS subscale scores were elevated for individuals with FXS who also had ASD features or had received ASD treatment. This builds on earlier work by Bailey and colleagues (1998) and provides additional evidence for the severity of sensory processing in the FXS+ASD phenotype. These sensory processing severity differences were observed in all subscales except ingestive and digestive problems, with strongest effects in the auditory and tactile domains. Although this study did not include a typically-developing comparison group, these data also are in keeping with earlier research that showed higher rates of sensory challenges in FXS and ASD populations compared to typically developing children (Rogers et al, 2003). In addition, preliminary evidence suggests that ASD severity is positively related to physiological dysfunction in individuals with Fragile X (Roberts et al., 2012a; 2012b; though see Klusek, Martin, & Losh, 2013 for a study that did not find the association). Thus, it is possible that the greater sensory challenges associated with combined FXS and ASD may be due to more extreme physiological dysfunction in this group. Though objective measures of autonomic function were not assessed in this study, future work is needed to test this possibility.
Notably, the FXS+ASD phenotype is associated with poorer cognitive and adaptive function compared to FXS without a concurrent ASD diagnosis (Bailey et al., 2001; Kaufmann et al., 2004; Lewis et al., 2006; Hernandez et al., 2009). Cognitive functioning was not assessed in the present study but it is possible that greater sensory challenges may be related to these greater intellectual impairments that are often observed in ASD populations. It has been proposed that this relation may be due to sensory problems resulting from impaired cognitive function or cognitive function problems resulting in impaired sensory processing (see review in Robertson & Baron-Cohen, 2017). However, due to the integrated nature of sensory processing and physiological state regulation problems, it is likely that it may be the regulation of the coordinated brain-body sensory-motor system that may cause interference with cognitive development and higher-level intellectual abilities (Cohen, 1995; Porges, 1996). Evidence from human brain imaging and evolutionary comparisons suggest that sensory processing and fluid intelligence share common neural circuits (e.g., the anterior insular and cingulate cortices; Yuan et al., 2012; Craig, 2015), and the impairment of these circuits may result in both sensory challenges and diminished cognitive function. As a questionnaire instrument informed by evolutionary neurophysiology, the BBCSS may be uniquely poised to assess such models.
When devising questionnaires, Likert-type item responses may be arranged in either the positive or negative direction. In the case of the BBCSS, items were organized from “Almost always” to “Almost never”, reflecting more optimal function at the highest levels, and thus are reverse scored to assess degree of sensory challenges. Systematic assessments of Likert and Likert-type scales so far do not support one response direction as systematically performing better than another. For individual items, there is mixed evidence for biases in means and frequencies of response types and when these are present they tend to vary in magnitude and direction based on data collection method (Dillman et al., 1995; Christian et al., 2008; Stapleton, 2013). For construct measurement, two studies have found no substantial psychometric effects of response direction on latent variable measurement properties (Weng & Cheng, 2000; Krebs and Hoffmeyer-Zlotnik, 2010) while one study that did observe an effect found that reversing response options can both positively and negatively influence measurement properties (Chan, 1991). Thus, given the available evidence, it is not known whether reversing the BBCSS response options could affect the instrument’s measurement properties and it is recommended that the scale be used with the response option direction with which it was developed.
Limitations and Future Directions
Though the observed factor structure and subscale clustering were consistent with predictions based on neurophysiological mechanisms, empirical evidence for the relation of BBCSS subscale scores with dampened vagal regulation and greater sympathetic activation is needed. Further research also needs additional digestion subscale validation, which did not include a convergent validity measure in our study. In addition, the factor structure drawn from our item pool resulted in some subscales with low item counts, which may lead to decreased prevision of subscale scores. Thus, future development of the BBCSS may include expansion of item counts for the observed constructs (effects of these changes on reliability, validity, and factor structure will need to be examined).
Importantly, our study was limited to caregiver reports. Firstly, this may provide bias in ASD features measurement, which may not necessarily reflect clinical assessment. Further work is needed to assess whether the sensory processing differences we observed between children with FXS only and those who also have ASD features can be replicated with a clinician-provided diagnosis. Secondly, this design limits sensory processing reporting to observers. Future studies will need to be examine the psychometric properties of the BBCSS when collected via self-reports of adolescents and adults.
Several demographic characteristics of the study sample need to be considered in relation to the findings. First, our sample included too few females to test gender differences; future studies with a higher number of full mutation females are needed to assess gender differences in BBCSS scores. In addition, online methods often bias samples toward higher SES families, as was likely the case in our study. Future work will need to assess the BBCSS scales across a more diverse range of respondents. Furthermore, we view the broad age range used here as a first step for the refinement of the BBCSS. Sensory processing has been shown to demonstrate age-related changes in childhood (Baranek et al., 2008) and further work is needed to determine the nature of BBCSS age-related differences in subscale scores and item loadings. Finally, future work will need to assess whether BBCSS scores are related to hearing and vision impairments or IQ differences, which were not measured in this study, as these may be related to sensory sensitivities.
Conclusion
Despite these considerations, this study demonstrates several strengths. Firstly, in comparison to typical studies of individuals with FXS, the sample in this study was relatively large and provided an opportunity for psychometric assessment targeted to this specific population. Furthermore, to our knowledge, this is the first sensory processing questionnaire developed from an evolutionary neurophysiological foundation. Thus, the development of the BBCSS and its foundation in the Polyvagal Theory provides a novel direction for understanding the evolutionary and neurophysiological mechanisms that give rise to sensory processing difficulties and points the way for the development of more effective interventions.
Sensory interventions are one of the most common requests by parents of children with FXS and ASD (Green et al., 2006; Stackhouse et al., 2014) but the effectiveness of current sensory interventions is poor to modest (Case-Smith, Weaver, & Fristad, 2015). By refocusing theoretical approaches away from general hypo- and hyper-sensitivity frameworks and assessments, researchers and clinicians can move toward an understanding of the specific environmental features that cue neurophysiological mechanisms for modulating sensory processing. This theoretical and methodological reframing may point toward techniques that aim to utilize these environmental features, optimizing natural safety and threat cues to “retune” the nervous system’s sensory processing from threat-related vigilance and mobilization states toward supporting affiliative social interactions.
Supplementary Material
Acknowledgments
This study was supported by grant #550KR111516 from the North Carolina Translational & Clinical Sciences Institute. We wish to thank all families who dedicated their time to make this study possible.
Funding:
This study was funded by the North Carolina Translational & Clinical Sciences Institute grant # 550KR111516.
Footnotes
Conflict of Interest: Jacek Kolacz declares that he has no conflict of interest. Melissa Raspa declares that she has no conflict of interest. Keri J. Heilman declares that she has no conflict of interest. Stephen W. Porges declares that he has no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- Ayres AJ (1964). Tactile functions: Their relation to hyperactive and perceptual–motor behavior. American Journal of Occupational Therapy, 18, 6–11. [PubMed] [Google Scholar]
- Ayres AJ (1965). Patterns of perceptual–motor dysfunction in children: A factor analytic study. Perceptual and Motor Skills, 20, 335–368. [DOI] [PubMed] [Google Scholar]
- Ayres AJ (1972). Sensory integration and the child. Los Angeles, CA: Westem Psychological Services. [Google Scholar]
- Bailey DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, & Mayhew L (1998). Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders, 28(6), 499–508. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, & Mesibov G (2001). Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of autism and developmental disorders, 31(2), 165–174. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, & Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A, 146(16), 2060–2069. [DOI] [PubMed] [Google Scholar]
- Baranek GT (1999). Sensory Experiences Questionnaire Version 2.1.
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Reinhartsen D, & Wannamaker S (2001). Play: Engaging children with autism. In Heubner R (Ed.), Autism: A sensorimotor approach to management (pp. 311–351). Philadelphia: F. A. Davis. [Google Scholar]
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, & Bailey DB (2008). Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Physical & Occupational Therapy in Pediatrics, 28(1), 79–98. [DOI] [PubMed] [Google Scholar]
- Barendse MT, Oort FJ, & Timmerman ME (2015). Using exploratory factor analysis to determine the dimensionality of discrete responses. Structural Equation Modeling, 22(1), 87–101. [Google Scholar]
- Belmonte MK, & Bourgeron T (2006). Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience, 9(10), 1221–1225. [DOI] [PubMed] [Google Scholar]
- Belser RC, & Sudhalter V (1995). Arousal difficulties in males with fragile X syndrome: A preliminary report. Developmental Brain Dysfunction, S(4–6), 270–279. [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, & Tager-Flusberg H (2007). Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. The American Journal of Occupational Therapy, 61(5), 584. [DOI] [PubMed] [Google Scholar]
- Bentler PM (1972). A lower-bound method for the dimension-free measurement of internal consistency. Social Science Research, 1, 343–357. [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238. [DOI] [PubMed] [Google Scholar]
- Bentler PM (2009). Alpha, dimension-free, and model-based internal consistency reliability. Psychometrika, 74, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, & Dunn W (2002). Adolescent-Adult Sensory Profile: User’s Manual. Therapy Skill Builders. [Google Scholar]
- Brown C, Tollefson N, Dunn W, Cromwell R, & Filion D (2001). The adult sensory profile: Measuring patterns of sensory processing. American Journal of Occupational Therapy, 55(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Case-Smith J, Weaver LL, & Fristad MA (2015). A systematic review of sensory processing interventions for children with autism spectrum disorders. Autism, 19(2), 133–148. [DOI] [PubMed] [Google Scholar]
- Cattell RB (1966). The scree test for the number of factors. Multivariate Behavioral Research, 1(2), 245–276. [DOI] [PubMed] [Google Scholar]
- Chan JC (1991). Response-order effects in Likert-type scales. Educational and Psychological Measurement, 51(3), 531–540. [Google Scholar]
- Christian LM, Dillman DA, & Smyth JD (2008). The effects of mode and format on answers to scalar questions in telephone and web surveys. Advances in telephone survey methodology, 12, 250–275. [Google Scholar]
- Cicchetti DV (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological assessment, 6(4), 284. [Google Scholar]
- Cliff N (1993). Dominance statistics: Ordinal analyses to answer ordinal questions. Psychological Bulletin, 114(3), 494. [Google Scholar]
- Cohen IL (1995). A theoretical analysis of the role of hyperarousal in the learning and behavior of fragile X males. Developmental Disabilities Research Reviews, 1(4), 286–291. [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, ... & Brown WT (1988). Social gaze, social avoidance, and repetitive behavior in fragile X males: a controlled study. American Journal of Mental Retardation, 92(5), 436–46. [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, & Brown WT (1989). Parent- Child Dyadic Gaze Patterns in Fragile X Males and in Non- fragile X Males with Autistic Disorder. Journal of Child Psychology and Psychiatry, 30(6), 845–856. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Vietze PM, Sudhalter V, Jenkins EC, & Brown WT (1991). Effects of age and communication level on eye contact in fragile X males and non- fragile X autistic males. American Journal of Medical Genetics Part A, 38(2– 3), 498–502. [DOI] [PubMed] [Google Scholar]
- Craig AD (2015). How do you feel?: An interoceptive moment with your neurobiological self. Princeton University Press. [Google Scholar]
- Dillman DA, Brown TL, Carlson JE, Carpenter EH, Lorenz FO, Mason R, Saltiel J, & Songster RL (1995). Effects of category order on answers in mail and telephone surveys. Rural Sociology, 60(4), 674–687. [Google Scholar]
- Dunn W (2001). The sensations of everyday life: Empirical, theoretical, and pragmatic considerations. American Journal of Occupational Therapy, 55(6), 608–620. [DOI] [PubMed] [Google Scholar]
- Dunn W (2014). Sensory Profile 2 manual. San Antonio, TX: Pearson. [Google Scholar]
- Dunn W, & Daniels DB (2002). Initial development of the infant/toddler sensory profile. Journal of Early Intervention, 25(1), 27–41. [Google Scholar]
- Dunn W, Saiter J, & Rinner L (2002). Asperger syndrome and sensory processing: A conceptual model and guidance for intervention planning. Focus on Autism and Other Developmental Disabilities, 17(3), 172–185. [Google Scholar]
- Engel-Yeger B (2012). Validating the Adolescent/Adult Sensory Profile and examining its ability to screen sensory processing difficulties among Israeli people. British Journal of Occupational Therapy, 75(7), 321–329. [Google Scholar]
- Ermer J, & Dunn W (1998). The Sensory Profile: A discriminant analysis of children with and without disabilities. American Journal of Occupational Therapy, 52(4), 283–290. [DOI] [PubMed] [Google Scholar]
- Fabrigar LR, Wegener DT, MacCallum RC, & Strahan EJ (1999). Evaluating the use of exploratory factor analysis in psychological research. Psychological Methods, 4, 272–299. [Google Scholar]
- Feinstein C, & Reiss AL (1998). Autism: the point of view from fragile X studies. Journal of Autism and Developmental Disorders, 28(5), 393–405. [DOI] [PubMed] [Google Scholar]
- Fleiss JL (1981). Statistical methods for rates and proportions (2nd ed.). New York: John Wiley. [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, ... & Silva AJ (2004). Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Molecular Psychiatry, 9(4), 417–425. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ (2006). Lessons from fragile X regarding neurobiology, autism, and neurodegeneration. Journal of Developmental & Behavioral Pediatrics, 27(1), 63–74. [DOI] [PubMed] [Google Scholar]
- Hall SS, Frank MC, Pusiol GT, Farzin F, Lightbody AA, & Reiss AL (2015). Quantifying naturalistic social gaze in fragile X syndrome using a novel eye tracking paradigm. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 168(7), 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Zageris DM, Berry-Kravis E, & Porges SW (2011). Autonomic regulation in fragile X syndrome. Developmental psychobiology, 53(8), 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Cruz MT, Sahibzada N, Verbalis J, & Gillis RA (2009). GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. American Journal of Physiology-Gastrointestinal and Liver Physiology, 296(1), G101–G111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, & Kaufmann WE (2009). Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. American Journal of Medical Genetics Part A, 149(6), 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Berry-Kravis E, Cordeiro L, Yuhas J, Ornitz EM, Campbell A, ... & Hagerman RJ (2009). Prepulse inhibition in fragile X syndrome: feasibility, reliability, and implications for treatment. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150(4), 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. [Google Scholar]
- Garrido LE, Abad FJ, & Ponsoda V (2016). Are fit indices really fit to estimate the number of factors with categorical variables? Some cautionary findings via Monte Carlo simulation. Psychological methods, 21(1), 93. [DOI] [PubMed] [Google Scholar]
- Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, & Sigafoos J (2006). Internet survey of treatments used by parents of children with autism. Research in developmental disabilities, 27(1), 70–84. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, ... & Stanard P (2004). Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A, 129(3), 225–234. [DOI] [PubMed] [Google Scholar]
- Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, ... & Berry-Kravis E (2014). Fragile X syndrome: A review of associated medical problems. Pediatrics, 134(5), 995–1005. [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, & Losh M (2013). Physiological arousal in autism and fragile X syndrome: Group comparisons and links with pragmatic language. American Journal on Intellectual and Developmental Disabilities, 118, 475–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JE, & Losh M (2015). Cardiac autonomic regulation in autism and Fragile X syndrome: A review. Psychological Bulletin, 141(1), 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolacz J, Holochwost SJ, Gariépy JL, & Mills- Koonce WR (2016). Patterns of joint parasympathetic, sympathetic, and adrenocortical activity and their associations with temperament in early childhood. Developmental psychobiology, 58(8), 990–1001. [DOI] [PubMed] [Google Scholar]
- Kolacz J, Lewis GF, & Porges SW (in press). The integration of vocal communication and biobehavioral state regulation in mammals: A polyvagal hypothesis. In Brudzynski SM (Ed.) Handbook of Ultrasonic Vocalization. [Google Scholar]
- Krebs D & Hoffmeyer-Zlotnik JHP (2010). Positive first or negative first?: Effects of the order of answering categories on response behavior. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences, 6, 118–127. [Google Scholar]
- Landis J, & Koch G (1977). The Measurement of Observer Agreement for Categorical Data. Biometrics, 33(1), 159–174. [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, & Gould J (2007). Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders, 37(5), 894–910. [DOI] [PubMed] [Google Scholar]
- Lewis P, Abbeduto L, Murphy M, Richmond E, Giles N, Bruno L, & Schroeder S (2006). Cognitive, language and social- cognitive skills of individuals with fragile X syndrome with and without autism. Journal of Intellectual Disability Research, 50(7), 532–545. [DOI] [PubMed] [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, & Baranek GT (2011). Psychometric validation of the sensory experiences questionnaire. American Journal of Occupational Therapy, 65(2), 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, & Shyu V (1999). Development and validation of the Short Sensory Profile. In Dunn W (Ed.), Sensory Profile manual (pp. 59–73). San Antonio, TX: Psychological Corporation. [Google Scholar]
- McNeish D (2017). Thanks coefficient alpha, we’ll take it from here. Psychological Methods. Advance online publication [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, ... & Hagerman RJ (1999). Electrodermal responses to sensory stimuli in individuals with fragile X syndrome. American Journal of Medical Genetics, 83(4), 268–279. [PubMed] [Google Scholar]
- Muthén LK & Muthén BO (1998–2015). Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW (1996). Physiological regulation in high-risk infants. A model for assessment and potential intervention. Development and Psychopathology, 8, 43–58. [Google Scholar]
- Porges SW (2001). The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology, 42, 123–146. [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74, 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2011). The polyvagal theory: Neurophysiological foundations of emotions, attachment, communication, and self-regulation. WW Norton, New York. [Google Scholar]
- Porges SW (2012). The Brain-Body Center Sensory Scales (BBCSS). The Brain-Body Center. University of Illinois at Chicago. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW & Lewis GF (2009). The polyvagal hypothesis: Common mechanisms mediating autonomic regulation, vocalizations, and listening. In Brudzynski SM (Ed.), Handbook of Mammalian Vocalizations: An Integrative Neuroscience Approach. Amsterdam: Academic Press, 255–264. [Google Scholar]
- Quinn HO (2014). Bifactor models, explained common variance (ECV), and the usefulness of scores from unidimensional item response theory analyses. Unpublished Master’s thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC. [Google Scholar]
- R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Raspa M, Bailey DB Jr, Bishop E, Holiday D, & Olmsted M (2010). Obesity, food selectivity, and physical activity in individuals with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 115(6), 482–495. [DOI] [PubMed] [Google Scholar]
- Raspa M, Wheeler AC, & Riley C (2017). Public health literature review of fragile X syndrome. Pediatrics, 139(Supplement 3), S153–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelle W, & Zinbarg RE (2009). Coefficients alpha, beta, omega, and the glb: Comments on Sijtsma. Psychometrika, 74(1), 145–154. [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Hatton DD, & Skinner M (2001). Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology, 39(2), 107–123. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Long AC, Anello V, & Colombo J (2012). Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders, 42, 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen B, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117, 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, & Baron-Cohen S (2017). Sensory perception in autism. Nature Reviews Neuroscience, 18(11), 671. [DOI] [PubMed] [Google Scholar]
- Rogmann JJ (2013). Ordinal dominance statistics (orddom): An R project for statistical computing package to compute ordinal, nonparametric alternatives to mean comparison (version 3.1). Available online from the CRAN website; http://cran.r-project.org/. [Google Scholar]
- Riley C, Mailick M, Berry-Kravis E, & Bolen J (2017). The Future of fragile X syndrome: CDC stakeholder meeting summary. Pediatrics, 139(Supplement 3), S147–S152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, & Wehner E (2003). Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders, 33(6), 631–642. [DOI] [PubMed] [Google Scholar]
- Sass DA, & Schmitt TA (2010). A comparative investigation of rotation criteria within exploratory factor analysis. Multivariate Behavioral Research, 45(1), 73–103. [DOI] [PubMed] [Google Scholar]
- semTools Contributors. (2016). semTools: Useful tools for structural equation modeling. R package version 0.4–14. Retrived from http://cran.r-project.org/web/packages/semTools/index.html
- Shrout PE, & Fleiss JL (1979). Intraclass correlations: uses in assessing rater reliability. Psychological bulletin, 86(2), 420. [DOI] [PubMed] [Google Scholar]
- Stackhouse TM, Scharfenaker SK, Lachiewicz AM, Burgess D, Hessl D, Blitz R, Burgess K, Rohlik D, Hess LG, Kidd SA, & Berry-Kravis E (2014). Sensory processing and integration issues in individuals with fragile X syndrome. Retrieved June 14, 2014 from https://fragilex.org/wp-content/uploads/2012/08/Sensory-Integration-Issues-In-Fragile-X-Syndrome-2014-May.pdf
- Stapleton C (2013). The Smart (phone) Way to Collect Survey Data. Survey Practice 6(2). [Google Scholar]
- Steiger JH (1990). Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research, 25(2), 173–180. [DOI] [PubMed] [Google Scholar]
- Steiger JH & Lind JC (1980). Statistically-based tests for the number of common factors. Paper presented at the annual spring meeting of the Psychometric Society, Iowa City, IA. [Google Scholar]
- Tomchek SD, & Dunn W (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61(2), 190–200. [DOI] [PubMed] [Google Scholar]
- Talay-Ongan A, & Wood K (2000). Unusual sensory sensitivities in autism: A possible crossroads. International Journal of Disability, Development and Education, 47(2), 201–212. [Google Scholar]
- Trizano-Hermosilla I, & Alvarado JM (2016). Best alternatives to Cronbach’s alpha reliability in realistic conditions: Congeneric and asymmetrical measurements. Frontiers in psychology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LR, & Lewis C (1973). A reliability coefficient for maximum likelihood factor analysis. Psychometrika, 38, 1–10. [Google Scholar]
- Weng LJ, & Cheng CP (2000). Effects of response order on Likert-type scales. Educational and psychological measurement, 60(6), 908–924. [Google Scholar]
- Wheeler AC, Mussey J, Villagomez A, Bishop E, Raspa M, Edwards A, ... & Bailey DB Jr (2014). DSM-5 Changes and the Prevalence of Parent-Reported Autism Spectrum Symptoms in Fragile X Syndrome. Journal of Autism and Developmental Disorders, 1–14. [DOI] [PubMed] [Google Scholar]
- Yates A (1987). Multivariate exploratory data analysis: A perspective on exploratory factor analysis. Albany: State University of New York Press. [Google Scholar]
- Yuan Z, Qin W, Wang D, Jiang T, Zhang Y, & Yu C (2012). The salience network contributes to an individual’s fluid reasoning capacity. Behavioural brain research, 229(2), 384–390. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Ai HB, & Cui XY (2006). Effects of nuclei ambiguus and dorsal motor nuclei of vagus on gastric H+ and HCO3-secretion in rats. World journal of gastroenterology: WJG, 12(20), 3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chang L, Xie J, & Ai H (2016). Arginine vasopressin injected into the dorsal motor nucleus of the vagus inhibits gastric motility in rats. Gastroenterology research and practice, 4618672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


