Abstract
Protein–protein interactions (PPI) of co-stimulatory molecules CD2-CD58 are important in the early stage of an immune response, and increased expression of these co-stimulatory molecules is observed in the synovial region of joints in rheumatoid arthritis (RA) patients. A CD2 epitope region that binds to CD58 was grafted on to sunflower trypsin inhibitor (SFTI) template structure to inhibit CD2-CD58 PPI. The peptide was incorporated with an organic moiety dibenzofuran (DBF) in its structure. The designed peptidomimetic was studied for its ability to inhibit CD2-CD58 interactions in vitro, and its thermal and enzymatic stability was evaluated. Stability studies indicated that the grafted peptidomimetic was stable against trypsin cleavage. In vivo studies using the collagen-induced arthritis (CIA) model in mice indicated that the peptidomimetic was able to slow down the progress of arthritis, an autoimmune disease in the mice model. These studies suggest that with the grafting of organic functional groups in the stable peptide template SFTI stabilizes the peptide structure, and these peptides can be used as a template to design stable peptides for therapeutic purposes.
Keywords: Rheumatoid arthritis, Protein–protein interaction, CD2, CD58, Grafted peptide
1. Introduction
Most of the biological processes, including transmembrane signal transduction, cell regulation, and immune response, involve protein–protein interactions (PPI) of signaling protein molecules.1,2 The immune response occurs by a complex network of signaling involving several PPIs. Major histocompatibility complex (MHC) of antigen-presenting cells (APC) presents the antigen epitope to T-cell receptors (TCR) of T-cells to generate the immune response.3 Generation of immune response after the presentation of antigen to T cells depends upon other co-stimulatory molecules. The co-stimulatory molecules are involved in making close contact with T-cells, with APC generating the activating signals for an immune response.4–6 One of the important co-stimulatory molecules is CD58, also called lymphocyte function-associated antigen-3 (LFA-3) present on APC is a ligand for cell adhesion molecule CD2 present on T-cells. CD2 binds to CD58 via the first portion of the extracellular domain.5,7 TCR on T cells recognizes the correct peptide major histocompatibility complex (pMHC) on APC with high efficiency if CD2-CD58 interaction is active than in the absence of CD2-CD58 interaction (with 50 to 100-fold decrease in efficiency).5 CD2-CD58 interaction leads to the generation of inflammatory cytokines, expansion of naive T helper cells, and induction of large amounts of IFN-γ in memory cells.8,9 CD2-CD58 has been found to be upregulated in autoimmune diseases such as rheumatoid arthritis (RA). Modulation of CD2-CD58 interaction by antibodies and fusion proteins has shown promise in the treatment of autoimmune disease by significantly decreasing the inflammation.10,11 This makes CD2-CD58 modulation a feasible approach in the design of immunomodulators.
We have designed and studied various peptides and peptidomimetics that can bind to CD58 and can block CD2-CD58 interaction.12–14 Previously we have reported cyclic peptides and sunflower trypsin inhibitor (SFTI) grafted peptides for immunomodulation.13,15 In the presented work, our effort has been to make a conformatinally constrained grafted peptide by the modification of the previously grafted peptide (SFTI-a1) to make it a peptidomimetic (Fig. 1). The modification was carried out on the beta-turn-inducing Pro–Pro motif in the SFTI-a1 peptide as our previous study suggested that the Pro–Pro motif might undergo cis–trans isomerization giving rise to multiple conformers.14 By modification of Pro–Pro motif by dibenzofuran moiety (DBF) in SFTI-a1 peptide,15 we were able to obtain a peptidomimetic that exhibits cell adhesion inhibition activity of 3.8 nM using lymphocyte-epithelial cell adhesion assay. Furthermore, thermal and enzymatic stability, in vivo study, and immunogenicity study of peptidomimetic SFTI-DBF were carried out. These studies suggested that modification of peptide to peptidomimetic enhanced the cell adhesion activity of the SFTI-DBF peptide compared to the SFTI-a1 peptide, and the peptidomimetic was able to suppress RA in collagen-induced arthritis mice model (CIA).
Fig. 1.
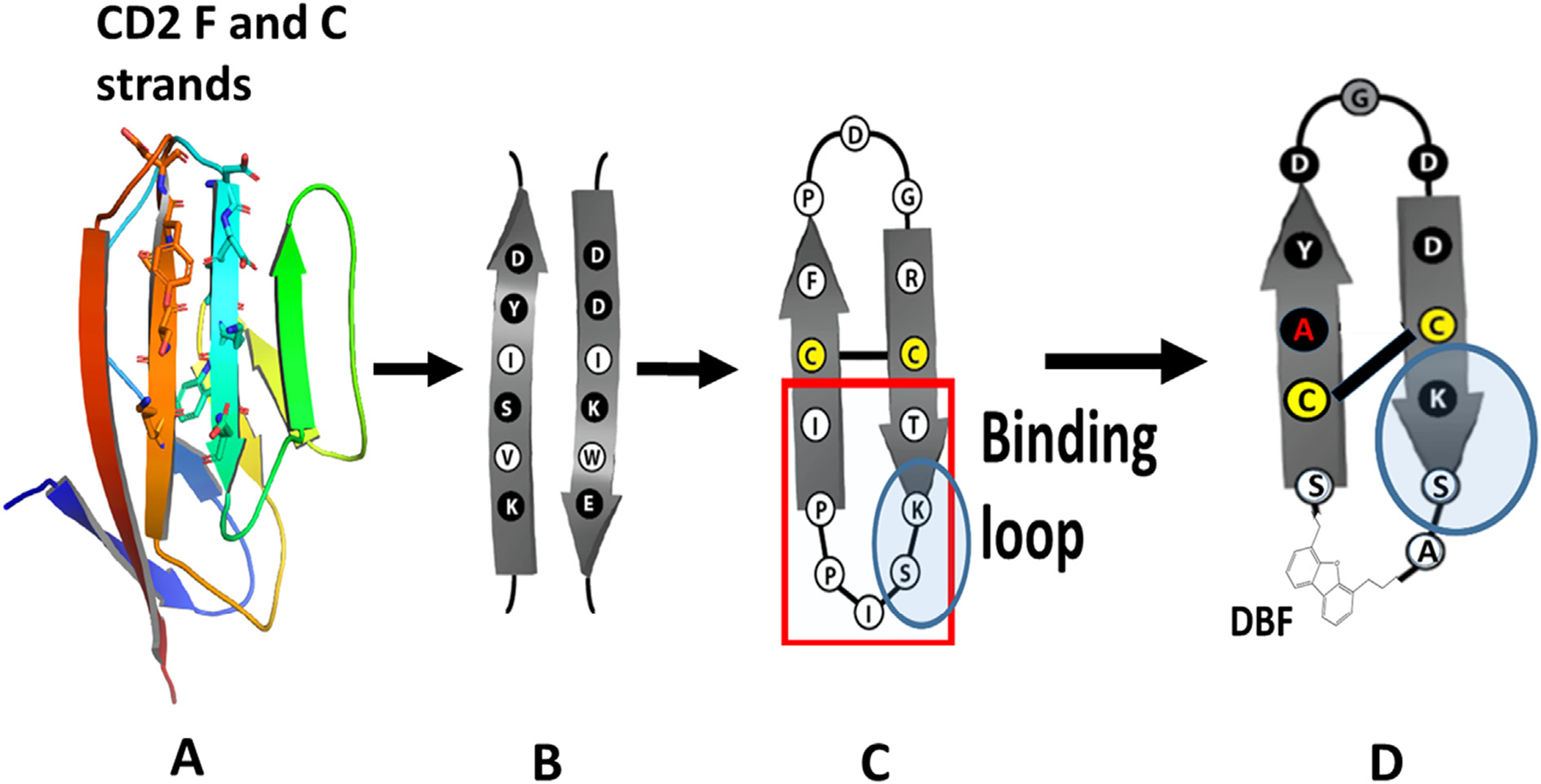
Design of peptides from CD2 protein and grafting of CD2 peptide to SFTI framework. A) Adhesion domain of CD2 with beta-strands. B) Amino acids in β-strands of CD2, C) SFTI peptide template (PDB ID: 1JBL, Uniprot: Q4GWU5, reference Sequence 1JBL-1, Organism(s): Helianthus annuus). D) Grafting β-strand amino acids onto SFTI with beta-strand structures. The new grafted peptide with DBF moiety. Pro–Pro sequence replaced with DBF. Trypsin enzyme binding loop and Ser–Lys sequence highlighted.
2. Materials and methods
2.1. Cell lines
Human epithelial ovary adenocarcinoma (OVCAR-3) human T-lymphocyte cells (Jurkat, E6–1) were purchased from American Type Culture Collection (ATCC). Jurkat cells were cultured in RPMI-1640 medium (ATCC) with 10% FBS, 0.01 mg/mL bovine insulin and 0.1 mg/mL penicillin/streptomycin. OVCAR-3 cells were cultured in RPMI-1640 medium from ATCC, with 20% FBS, 0.01 mg/mL bovine insulin, and 0.1 mg/mL penicillin/streptomycin. Human fibroblast-like synoviocytes rheumatoid arthritis (HFLS-RA) cells were purchased from Cell Applications, Inc., San Diego, CA, USA, and maintained in a human synoviocyte growth medium purchased from Cell Applications, Inc. All cell lines were maintained at 37 °C and 5% CO2.
2.2. Chemicals
Fmoc-protected amino acids, piperidine, trifluoroacetic acid (TFA), 2-chlorotrityl chloride resin, 2 - (6 - chloro - 1H - benzotriazole - 1 - yl) - 1,1,3,3 - tetramethylaminium hexafluorophosphate (HCTU), and 1, 1-[bis(dimethylamino) methylene]-1H-1,2,3-triazolo[4,5-b] pyridinium 3-oxide hexafluorophosphate (HATU) were purchased from Advanced ChemTech (Louisville, KY). Diisopropylethylamine (DIEA), triisopropylsilane (TIPS), and N-methylmorpholine (NMM) were purchased from Sigma Aldrich (St. Louis, MO). Dimethylformamide (DMF) was purchased from Protein Technologies (Tucson, AZ). Dichloromethane (DCM), methanol (MeOH), and acetonitrile (CH3CN) were purchased from VWR International, LLC (Radnor, PA). 4-(Fmoc-2-aminoethyl)-6-dibenzofuranpropionic acid (Fmoc-DBF) was purchased from Chem-Implex International Inc. (Wood Dale, IL).
2.3. Peptide synthesis
All the grafted peptides were synthesized using Fmoc based solid-phase peptide synthesis method. Briefly, chain assembly was carried out on an automated peptide synthesizer (Symphony; Protein Technologies, Inc.) as described in our earlier publication.16
2.4. Cell adhesion inhibition assay
Approximately 104 HFLS-RA cells per well were coated in 96 well plates, and cell adhesion assay was performed as described in our previous publication.14 Different concentrations of SFTI-DBF were prepared in serum-free RPMI 1640 medium, and the cells were treated with 100 μl of different concentrations of peptides (0.00001–10 μM). Jurkat cells were fluorescently tagged by incubating it with [2′,7′-Bis-(2-Carboxyethyl)-5-(and-6)-Carboxy-fluorescein, Acetoxymethyl Ester] (BCECF-AM) dye, then the fluorescently labelled Jurkat cells were incubated with peptide pretreated and non-treated HFLS-RA cells for 1 h. Then, the cells were washed, lysed, and the fluorescence was measured. The observed fluorescence was due to the adhesion of fluorescently labeled Jurkat cells to CD58 expressing OVCAR-3/HFLS-RA cells. The fluorescence reading was taken with a microplate fluorescence analyzer (Synergy H1 Hybrid plate reader Biotek, Winooski, VT, USA) with excitation wavelength at 485 nm and emission wavelength at 528 nm. A plot of percentage of inhibition of adhesion vs concetration was obtained to calculate the IC50 value.17
2.5. Effect of SFTI-DBF on BCECF-AM labeling
Approximately 5 × 105 Jurkat T cells in 500 μl phosphate buffer saline (PBS) were taken in different eppendorf tubes for control and treatment groups. These cells were fluorescently tagged with 2.5 μL (1 mg/mL) BCECF-AM dye. Different concentrations of SFTI-DBF were prepared in PBS. Then the cells were treated with varying concentrations of peptides (0.1–100 μM) in the treatment group, whereas in control, 100 μl of PBS was added. Cells were then incubated at 37 °C, 5% CO2 for different time intervals (1 and 3 h). For 1 h of treatment, the cells were centrifuged and washed, the supernatant was discarded. The cell pellets obtained were then dispersed in PBS solution, and 100 μl of cell suspension from each group was kept in 96 well plates. The cells were lysed, and the fluorescence readings were taken with a microplate fluorescence analyzer (Synergy H1 Hybrid plate reader Biotek, Winooski, VT, USA) with excitation wavelength at 485 nm and emission wavelength at 528 nm. The fluorescence reading was compared with the control and treatment groups. Finally, the same procedure was also repeated after 3 h of treatment. Experiments were in triplicate, and data were represented as mean ± standard deviation.
2.6. Competitive binding experiment
To validate the binding of SFTI-DBF to CD58 protein, a competitive binding experiment was performed. Around 1 million OVCAR-3 cells which express CD58 were taken and fixed with formaldehyde. After fixing, the cells were incubated with different concentrations of peptides (100 μM, 25 μM, 10 μM, 0.5 μM) for 15 min. After 15 min, 20 μl of FITC-conjugated CD58 antibody (Santa Cruz Biotechnology, TX) was added to each sample and incubated further for 45 min. Antibody non-treated sample (cells only) was taken as a negative control. Upon completion of 45 min incubation, the mixture was centrifuged at 2500 rpm for 10 min, and pellets were resuspended in 2 mL of chilled PBS. Cell suspensions obtained were analyzed by flow-cytometry. Cell count vs. fluorescence unit histograms was plotted after analyzing 10,000 cells from each sample.
2.7. Thermal stability
JASCO J-815 Circular Dichroism (CD) spectrometer (Jasco, Easton MD) was used for the thermal stability of SFTI-DBF in methanol–water (50:50) by monitoring CD spectra of the peptidomimetic at different temperatures. The thermal stability of SFTI-DBF (40 μM) was performed by 5 °C increases in temperature from 25 to 60 °C. Four scans were taken for each temperature, and an average spectra was represented from 195 to 320 nm wavelength. Samples of SFTI-DBF that were heated to 25 and 60 °C were subjected to mass spectrometry for analysis.
2.8. Disulfide bond stability
Changes in the conformation of SFTI-DBF was used to analyze the disulfide bond stability using CD spectroscopy. SFTI-DBF at a concentration of 40 μM was incubated for 30 min in the presence of 0.1, 1 mM, and 5 mM dithiothreitol (DTT) solution. Four scans of CD spectra were taken for each temperature, and graphs were plotted against wavelength from 190 to 320 nm with an average of four scans. Changes in DTT addition were also analyzed by mass spectrometry. The mass spectrum was acquired on an Applied Bio-systems 5600 MALDI-TOF proteomics analyzer in positive reflectron mode. α-cyano-4-hydroxycinnamic acid (CHCA) (8 mg/mL in 50% acetonitrile/water, containing 0.05% formic acid) was used as a matrix.
2.9. In vitro human serum stability
Stability of SFTI-DBF in pooled human serum (Innovative Research, Inc, Novi MI) was analyzed for 24 h. 2 mM stock solution of the peptide was prepared in PBS, and 150 μl of the stock solution of the peptide was incubated with 1350 μl pooled human serum in the ratio of 1:9 as described previously.15 At each time point, 150 μl of the solution was taken, and the enzyme activity was terminated by adding ice-cold acetonitrile and was centrifuged at 10,000 rpm for 10 min. The supernatant was passed through a Sep-Pak column (Waters) and lyophilized. Freeze-dried samples were reconstituted with 300 μl of acetonitrile: water (50:50) and analyzed by HPLC (Shimadzu) for change in peptide peak intensity.
2.10. Trypsin stability assay
SFTI-DBF was dissolved in a 0.1 M NH4HCO3 buffer (pH 6.5) to yield a final peptide concentration of 1 mg/mL (675 μM). Trypsin solution consists of the following: 1 mg of trypsin (Sigma) in 50 mL of 0.1 M NH4HCO3 buffer (pH 8.2). A mixture of 250 μl of freshly made trypsin solution and 250 μl of prepared peptide solution were incubated in 2 mL of 0.1 M NH4HCO3 buffer (pH 8.6) at 37 °C on a shaker. Aliquots of 0.5 mL were sampled at different time intervals (0–6 h). The sample was diluted with 0.5 mL of water-acetonitrile (60:40 v/v) containing 1% TFA and analyzed by RP-HPLC at 274 nm.18 A linear peptide was used as a control for the assay.
2.11. Calcium flux assay
Calcium flux was taken as an indication of T-cell activation. To compare the calcium flux in Jurkat cells, fluo-8 calcium dye (Abcam) was used, and the assay was performed as described previously.15 Briefly, 10,000 HFLS-RA cells were seeded in each well in 96 well plates, and after 72 h incubation, HFLS-RA cells were treated with different concentrations of peptide (5 nM and 200 nM) and incubated for 1 h. Simultaneously Jurkat cells were washed three times with PBS and incubated with Fluo-8 calcium dye for 1 h, followed by washing of Jurkat cells 3 times. These dye-loaded cells were activated by CD3 antibody, and immediately the Jurkat cells were resuspended in PBS supplemented with calcium chloride and added over the peptide treated HFLS-RA cells. The plate was placed into the fluorescence plate reader (Synergy H1 Hybrid plate reader (Biotek)), and fluorescence reading of the Fluo-8 calcium dye (Ex/Em = 490/525 nm) was measured for 2.5 h by using the kinetic parameter. Experiments were repeated three times. A graph of relative change in fluorescence vs. time was plotted. Results were represented as mean ± standard deviation.
2.12. Proximity ligation assay (PLA)
PLA involving two different cells was performed following the protocol published from our laboratory.19 PLA kit (Sigma) was used to carry out the experiment. Initially, 10,000 HFLS-RA cells were treated with diff concentrations of SFTI-DBF and incubated for 2 h. Jurkat cells coated on 8 well chamber slide was co-cultured with HFLS-RA cells treated with different concentrations of SFTI-DBF and incubated for 48 h. After which, the cells were washed and fixed with methanol at −20 °C for 15 min. Then they were incubated in a blocking solution for 90 min followed by the addition of CD2 and CD58 antibodies (Santa Cruz) and incubated overnight in a moist chamber by gentle shaking. This was followed by the addition of positive and negative PLA probes and incubated for 1 h at 37 °C, further washing was done, and ligation solution was added in each well and incubated for another 1-h in a moist chamber. The well chambers were washed with provided wash buffer A, DNA polymerase, and Amplification red were added to the chambers and incubated for 100 min in a moist chamber in the dark. Following the incubation, the wells were washed with wash buffer B for 10 min, after which the walls of chambers were removed, and 10–20 μl of DAPI with mounting medium was added over the slides and covered with a coverslip. The slides were observed on a fluorescence microscope. Images were taken with Olympus BX63 fitted with deconvolution optics using DAPI, FITC, and Texas Red filters at 60× magnification and processed by using CellSens dimension software. PLA dots were quantified using ImageJ software (NIH).
2.13. Collagen induced arthritis- In-Vivo study
All animal studies were conducted according to the University guidelines in addition to the approved protocol by the Institutional Animal Care and User Committee (IACUC) committee, University of Louisiana Monroe, and National Institutes of Health (NIH) guidelines. Seven to eight weeks old, DBA1 mice were purchased from Jacksons Laboratories. Equal number of male and female mice were used in the RA study. Arthritis in mice was induced by following the published protocol for collagen-induced arthritis (CIA).20,21 Bovine type II Collagen, Complete Freunds Adjuvant (CFA), and Incomplete Freunds Adjuvant (IFA) were obtained from Chondrex. Animals were divided into different groups (N = 6). Very fine emulsion of an equal amount of 5 mg/mL collagen and CFA was prepared by homogenization, and the first immunization (Day 0) was done by injecting 100 μl of the emulsion around 1 cm from the base of the tail intradermally. This was followed by 2nd booster dose of an emulsion of IFA and collagen on day 21 for inducing arthritis.21,22 From day 22, the treatment was started with intravenous administration of SFTI-DBF 0.5 mg/kg or 1 mg/kg through the tail vein. Methotrexate (Sigma) at a dose of 1.5 mg/kg was given intraperitoneally, and animals injected with saline by IV were controls. A separate group of naïve mice without injection of collagen and adjuvant was used as healthy control. Arthritis score and body weight were taken on alternate days till the end of the study. Arthritis score was given on the scale of 0–4 by visual inspection of paws and joints; hence the maximum total score of 16 per mouse was given following the published protocol.21,23 At the end of the experiment, the animals were sacrificed, blood, organs, and limbs were harvested for further study. Disease incidence, arthritis score, change in body weight were plotted. Limbs were fixed in 10% formalin and decalcified in 0.5 M EDTA for 15 days. Oxalic acid was used to verify the extent of decalcification by observing the precipitate. Tissue sections were cut into 5 μm thick sections. Hematoxylin and eosin (H&E) staining was performed on these slides and were then studied and scored for joint inflammation, synovial hyperplasia, and cartilage/bone erosion for each section by an experienced pathologist. The total score in each group was calculated based on the grades of the individual parameters.
2.14. Cytokine analysis
Cytokine analysis in CIA mice serum was done by using a customized multiplex Elisa kit (EMD Millipore’s Milliplex® MAP Mouse Cytokine/Chemokine Magnetic Bead kit. This 96 well plate kit can be customized for simultaneous quantification of 32 different analytes in mouse tissues/cell lysates, culture supernatant samples, and serum or plasma samples. We customize the kit for cytokine quantification, which includes inflammatory cytokines like TNF-α, IFN-γ, IL-6, and IL-17 that play a major role in inflammation in rheumatoid arthritis. Serum cytokine levels were determined quantitatively by following the protocol provided with the manufacturer kit. Briefly, the well plate was washed with 200 μl of wash buffer, and the plate was sealed and kept on the shaker for 10 min at room temperature. Wash buffer was decanted, and 25 μl of each standard or control was added to appropriate wells. Then, 25 μl of the serum matrix solution provided with the kit was added to the background, standard, and control wells. 25 μl of the assay buffer was added to the sample wells. In the sample wells, 25 μl of diluted serum samples were added. This was followed by the addition of 25 μl of the magnetic beads in each well (beads corresponding to different analytes were vortexed and mixed in a mixing bottle). The plate was sealed, wrapped in a foil, and incubated with agitation on a plate shaker overnight at 2–8 °C. The following day, the well contents were removed gently and washed two times with 2X wash buffer by resting the plate on a handheld magnet for 30–60 s 25 μl of detection antibodies were added to each well, and the plate was sealed, covered with foil, and incubated with agitation on a plate shaker for 1 h at room temperature. After incubation, 25 μl of Streptavidin-phycoerythrin were added into each well and further incubated for 30 min with agitation on a plate shaker at room temperature. Then, the contents in the wells were removed gently, and the plates were washed two times with 2X wash buffer by using a handheld magnet throughout the process. 150 μl of sheath fluid was added to each well, and the beads were resuspended on a plate shaker for 5 min. Then, the reading was taken using BioRad Bioplex System (Bio-Rad, Hercules, CA). The standard curves were obtained, and the cytokines were quantified for each group and analyzed by using Graph Pad Prism.
2.15. Collagen antibody level
Blood samples collected at the end of the in vivo study were centrifuged at 10,000 rpm for 10 min, and serum was isolated. Collagen antibody level in mice serum was analyzed by ELISA using the manufacturer’s protocol provided with the kit from Chondrex (Woodinville, WA). Briefly, blood samples collected at the end of the in vivo study were centrifuged at 10,000 rpm for 10 min, and serum was isolated. 100 μl of blocking buffer was added to each well and incubated at room temperature for 1 h. The plates were washed with 1X wash buffer at least three times. Different concentrations of standards were prepared as described in the protocol. The serum sample was prepared by centrifuging at 10,000 rpm at room temperature for 3 min to remove insoluble materials and lipids. Then, the serum was diluted at 1:40,000 with provided solution B. This dilution was decided based on the optimization of the antibody levels in CIA mice serum. 100 μl of diluted standards and samples into wells were added and incubated at room temperature for 2 h. This was followed by washing three times with 1X washing buffer. Then, 100 μl of diluted secondary antibody solution into each well was added and incubated at room temperature for 1 h, followed by washing. 100 μl of 3,3′,5,5′-Tetramethylbenzidine (TMB) solution was added to each well and incubated at room temperature for 15 min. The reaction was stopped by the addition of 50 μl of stop solution into wells. Then the absorbance was immediately read at 450 nm. The standard curve was obtained, and the relative level of Bovine type II collagen antibody in serum of different groups of animals was plotted and analyzed by using GraphPad Prism (San Diego CA).
2.16. Immunogenicity study of SFTI-DBF
The group of mice treated with SFTI-DBF 1 mg/kg in the CIA study was used for this study. At the end of the CIA study, the mice were sacrificed, and spleens were harvested. Spleens from each animal were processed to obtain the splenocytes. After isolation of the spleen, it was kept in RPMI 1640 medium, and the spleen was passed through a 70 μm cell strainer (Corning, Durham, NC) to obtain a homogeneous suspension of cells. The suspension was centrifuged, and to the pellets, 2 mL of Ammonium-Chloride-Potassium (ACK) lysing buffer (Gibco, Fisher Scientific) was added to lyse the red blood cells (RBC) and centrifuged. The supernatant was discarded, and the pellet obtained was resuspended in RPMI 1640 complete medium and cultured at 37 °C and 5% CO2. These splenocytes were used for immunogenicity study. 10 million splenocytes/well were cultured in 24 well plates and treated with different concentrations of SFTI-DBF peptide. Concanavalin A was used as the immunogenic agent and as a control (10 μg/mL). The plate was incubated for 48 h, and cell proliferation was assessed by using Cell Titre Glo assay. High proliferation of the splenocytes (two times more than the non-treated group) was considered to be immunogenic.
2.17. Statistical analysis
Statistical significance between the different groups in PLA study, in vivo study, histology analysis, collagen autoantibody and cytokine analysis were carried out by GraphPad Prism using one-way ANOVA with post-hoc Tukey test.
3. Results
3.1. Design and characterization of SFTI-DBF
Our approach was to obtain the conformationally constrained grafted peptide with sequences from the CD2 adhesion domain. In our earlier publication, we successfully grafted peptide 6 into SFTI-framework to obtain a potent, stable peptide SFTI-a.14 Both peptide 6 and SFTI-a exhibited beta-strand/beta-hairpin structure stabilized by a beta-turn-inducing Pro-Pro motif.13,14 SFTI-a exhibited relatively lower cell adhesion inhibition activity (0.051 μM) compared to peptide 6 (0.007 μM).14,24 The peptide AS1(alanine scanning 1) was grafted onto the sunflower trypsin inhibitor (SFTI) template to obtain SFTI-a1 (Table 1), which was more potent in inhibiting the CD2-CD58 protein–protein interaction compared to SFTI-a.24 Our effort was to modify the amino acid residues in SFTI-a1 to obtain a conformationally rigid structure. We incorporated a dibenzofuran moiety (DBF) in the peptide backbone structure instead of the Pro–Pro sequence,13 resulting in peptidomimetic SFTI-DBF (Fig. 1). The peptide was synthesized by solid-phase peptide synthesis and characterized by HPLC, mass spectrometry. The peptide exhibited >95% purity. As a control peptide, we used SFTI-1.
Table 1.
The amino acid sequence of grafted peptides and their cell adhesion inhibition activity (Jurkat T cells and OVACR-3 cells and Jurkat T cells and HFLS-RA cells).
| Peptide Code | Peptide Sequence | Lymphocyte Cell Adhesion Inhibition Assay IC50 (mM) | |
|---|---|---|---|
| OVCAR-3: Jurkat | HFLS-RA: Jurkat | ||
| SFTI-aa | Cyclo[D1G2D3D4C5K6A7S8A9P10P11S12C13Y14] | 0.043 ± 0.025 | 0.051 ± 0.025 |
| SFTI-a1b | Cyclo[D1G2D3D4C5K6S7A8P9P10S11C12A13Y14] | 0.023 ± 0.046 | 0.037 ± 0.001 |
| SFTI-DBF | Cyclo[D1G2D3D4C5K6S7A8DBF9S10C11A12Y13] | 0.0006 ± 0.0003 | 0.0038 ± 0.002 |
| SFTI-1 | Cyclo [D1G2R3C4T5K6S7I8P9P10I11C12F13P14] | >100 | >100 |
Disulfide bonds are indicated by the underline.
Sable et al., ACS Chem. Biol. 2016 11(8):2366–2374.
Parajuli et al., Chem Biol Drug Design, 2020.
3.2. Cell adhesion inhibition assay
Jurkat cells express CD2 protein, whereas OVCAR-3 and HFLS-RA cells express CD58 protein; hence adhesion interaction between CD2 and CD58 proteins can be studied by using these model systems. HFLS-RA cells and OVCAR-3 are adherent cells, whereas Jurkat cells are non-adherent. Various concentrations of peptides (0.00001–10 μM) were incubated with adherent cells expressing CD58 and CD2 expressing Jurkat cells were added over it to assess the adhesion. Inhibition of adhesion between these two-cell models was evaluated by fluorescence of Jurkat cells pre-loaded with BCECF-AM. The fluorescence is proportional to the number of Jurkat cells adhering to the HFLS-RA cells or OVCAR-3 cells, and a concentration–response curve was obtained. The IC50 value for cell adhesion inhibition for SFTI-DBF using Jurkat and OVCAR-3 was 0.6 nM (Table 1, Supporting information Figure S1A) and using Jurkat -HFLS-RA cells 3.8 nM (Supporting information Figure S1B). In comparison, the cell adhesion inhibition activity of SFTI-a1 was 23 nM in the Jurkat-OVCAR-3 model and 37 nM in Jurkat and HFLS-RA model. Thus, the introduction of DBF moiety resulted in the enhancement of cell adhesion inhibition activity of the grafted peptidomimetic. Peptide SFTI-1 having an amino acid sequence of native sunflower trypsin inhibitor was used as control (IC50 > 100 μM).
3.3. Competitive binding experiment
To evaluate the binding of SFTI-DBF to the CD58 protein adhesion domain, a competitive binding experiment using flow-cytometry was performed. OVCAR-3 cells expressing CD58 were fixed with a formaldehyde solution and were treated with fluorescein isothiocyanate (FITC) conjugated CD58 antibody (20 μl, Santa Cruz Biotechnology, Dallas, TX, USA). This FITC conjugated CD58 antibody binds to the adhesion domain of CD58 (CD2-CD58 interaction interface). These cells were further treated with different concentrations of SFTI-DBF for observing the competitive binding of the peptide and FITC conjugated CD58 antibody to CD58. Result suggests the non-treated cell populations showed the lowest fluorescence and were towards the left of the histogram, whereas only the FITC-conjugated antibody cell population showed the highest fluorescence. This fluorescence was decreased in a concentration-dependent manner in the case of SFTI-DBF treated cells, and the histogram was shifted to the left side (Figure S2, Supporting Information). SFTI-DBF was observed to displace the antibody binding from CD58 receptors. This result suggests that SFTI-DBF shows competitive binding with the FITC conjugated CD58 antibody to bind with the CD58 receptor on the epithelial cell surface expressed in OVCAR-3 cells.
3.4. Thermal stability
Peptides can undergo conformational change when subjected to high temperature; in particular, a disulfide-bonded peptide can undergo a conformational change. Furthermore, SFTI-1 is known to exhibit multiple conformations, and as the temperature is increased,14 this interconversion can be rapid. We wanted to investigate whether the SFTI-DBF changes the overall conformation as the temperature is increased. CD spectra of peptides exhibit an average conformation of the peptide but are sensitive to change in the conformation of peptides. The degradation of the peptide is also monitored by the change in conformations.25 The significant change in CD spectra indicates the degradation of peptides at a higher temperature. Hence, CD spectroscopy was used to monitor changes in the conformation of the SFTI-DBF peptide at different temperatures. Furthermore, SFTI peptides exhibit higher thermal and chemical stability compared to linear are backbone cyclized peptides.26,27 Circular dichroism spectroscopy was used to assess the thermal stability of peptides by monitoring the changes in CD spectra in a wide range of temperatures from 25 to 60 °C with an interval of 5 °C increase in temperature. The result indicates there was no significant change in CD spectra of SFTI-DBF peptide, suggesting that it is stable even at temperatures studied (Fig. 2A). The mass spectrometry analysis of the SFTI-DBF samples at 25 and 60 °C also showed that the SFTI-DBF is intact at those temperatures (Fig. 2B and C). This experiment was further validated by HPLC analysis in which SFTI-DBF incubated at 25, 55, and 85 °C was found to have no significant change in chromatogram peaks indicating the high thermal stability of SFTI-DBF (Supporting information Figure S3).
Fig. 2.
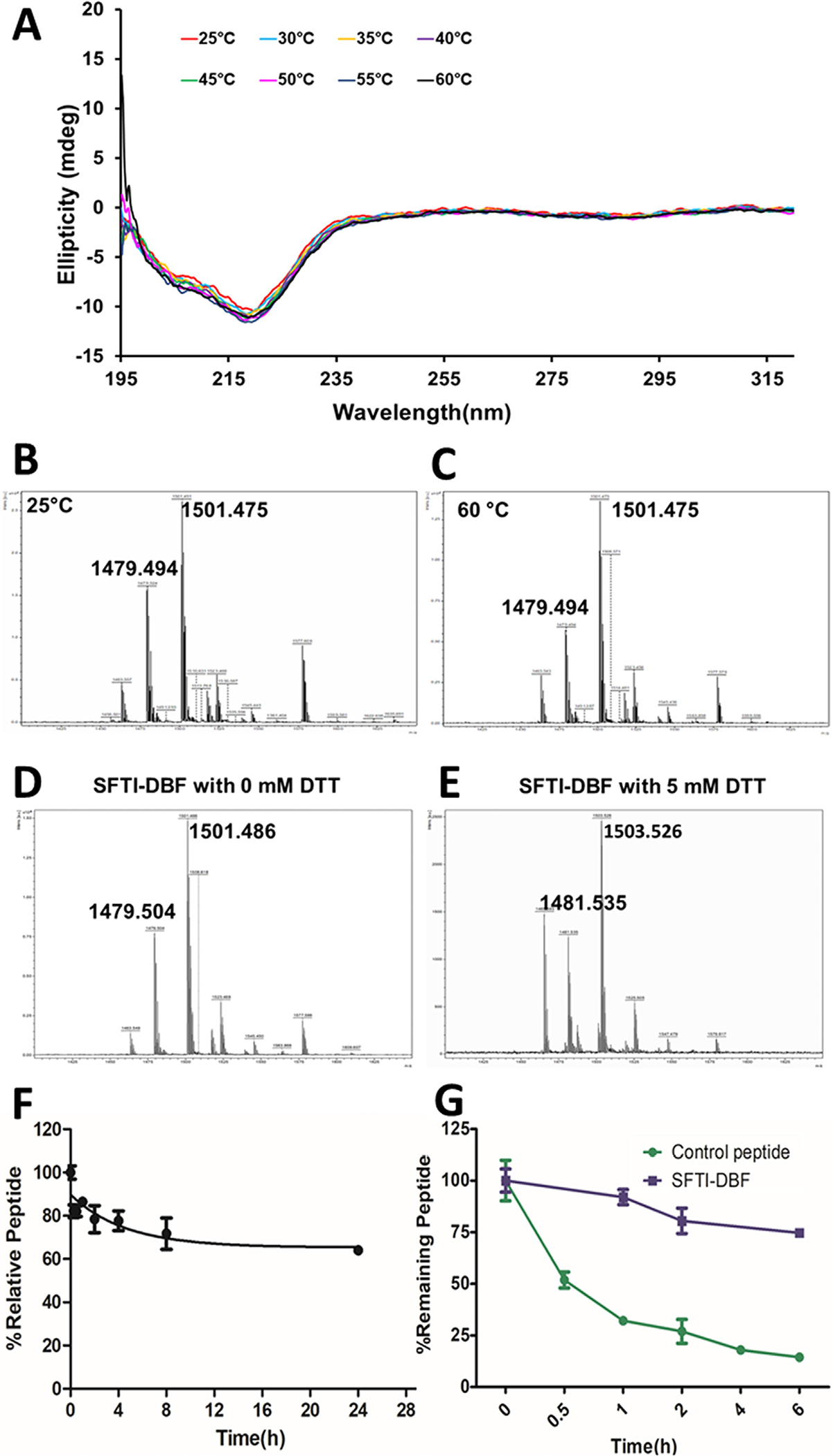
Thermal stability study. A) CD spectra of SFTI-DBF in methanol:water mixture (50%:50%) in temperature ranging from 25 to 60 °C. There was no significant change in CD spectra even at higher temperatures indicating exceptional thermal stability of SFTI-DBF. B&C) Mass spectrometry analysis of SFTI-DBF at 25 and 60 °C. MALDI-TOF mass spectrometry was used for analysis. m/z corresponding to the peptide ion and sodium adduct were seen. D&E) Disulfide bond stability of SFTI- DBF using mass spectrometry. Mass spectra of free peptides indicate m/z 1479 corresponding to intact SFTI-DBF peptides. To the peptide solution, different amount of DTT was added, and changes in mass spectra were observed. Notice that reduction of disulfide bond by addition of DTT results in changes in molecular mass of 2 units–1481. In vitro serum and enzymatic stability of SFTI-DBF studied by HPLC. F) In-vitro serum stability of SFTI-DBF. SFTI-DBF was incubated with pooled human serum, and aliquots were obtained at different intervals and analyzed by HPLC.SFTI-DBF showed exceptional stability in human serum in-vitro. G) Enzymatic stability of SFTI-DBF. SFTI-DBF incubated with trypsin and analyzed by HPLC. Nearly 80% of SFTI-DBF was intact after 6 h with enzyme trypsin in solution. As a control, a linear peptide was also subjected to trypsin assay, and the control peptide degraded to nearly 25% in 1 h.
3.5. Disulfide bond stability
SFTI-DBF is grafted multicyclic peptide in which the disulfide bridge is formed between two Cys amino acid residues. Previous studies have shown that this disulfide bridge must be intact for both stability and activity in SFTI-1.28 Disulfide-deficient analogs of SFTI undergo degradation very quickly in the presence of a proteolytic enzyme, emphasizing the critical role of the cysteine bridge in maintaining proteolytic stability of SFTI peptides.29 To evaluate whether the peptide SFTI-DBF has an intact disulfide bond SFTI-DBF was incubated with various concentrations of reducing agent dithiothreitol (DTT). The reduction of the disulfide bond may result in a change in the CD spectra of the peptide because of change in conformation of SFTI-DBF. SFTI-DBF was incubated with three different concentrations of DTT (0.1, 100 μM, and 5 mM), and CD spectra were acquired. The results indicated that the CD spectra did not change significantly in the presence of 0.1 and 100 μM DTT. However, the CD spectra of SFTI-DBF showed changes on incubation with 5 mM of DTT, indicating a reduction of disulfide bond of SFTI-DBF (Supporting Information Figure S4). Samples of SFTI-DBF incubated with different concentrations of DTT were analyzed by mass spectrometry. Samples treated with 5 mM DTT indicated increase in m/z compared to without DTT suggesting reduction of disulfide bond in SFTI-DBF (Fig. 2D and E).
3.6. In vitro human serum stability
The stability of drugs in serum is of vital importance as the degradation of drugs in serum results in poor drug availability at target sites. Enzymes present in serum have a great role in determining the fate of drugs. Peptides are very vulnerable to degradation by the proteases present in serum.30 The grafted peptides on SFTI have shown a very high degree of stability in human serum in vitro. The stability of the SFTI-DBF in human serum was evaluated by incubating the peptide in a pooled human serum, and samples were analyzed at various time points by HPLC. HPLC peak intensity for SFTI-DBF at zero time point was taken as 100% intact peptide, and the peaks at other time points were represented with respect to zero time point. The results indicated that SFTI-DBF shows significant stability up to 24 h (Fig. 2F) with nearly 80% of peptide intact at 24 h (calculated from the peak area of the chromatogram from HPLC).
3.7. Trypsin stability assay
Trypsin is one of the major proteolytic enzymes secreted by the pancreas. Trypsin is a serine protease that is responsible for protein hydrolysis and absorption. peptides that are orally available should show significant stability against trypsin digestion. SFTI was found to be quite stable towards the tryptic degradation and hence can be used as a framework for the design of stable peptides.29 The trypsin stability assay was done by incubating SFTI-DBF in trypsin solution in ammonium bicarbonate buffer, and the peptide was quantified at various intervals 0, 0.5, 1, 2, 4, and 6 h using HPLC analysis. Stability studies indicated that nearly 70% of the peptide was intact up to 6 h (small intestine pH 6–8, mean residence in the small intestine is 3–5 h)31 of incubation in trypsin (Fig. 2G). As a control, a linear peptide was used, and similar studies were carried out. The linear peptide incubated in the presence of trypsin showed nearly 75% degradation in 1 h (Fig. 2G).
3.8. Calcium flux assay
Calcium flux is a pivotal signaling event in the activation of T cells.32 The interaction between the T-cell receptor (TCR) and antigen-presenting cell (APC) leads to cytoskeletal rearrangement and a profound increase in the intracellular level of calcium in T-cells which is an indication of T-cell activation. The increase in intracellular calcium level is due to the outflux of calcium from the endoplasmic reticulum into the cytoplasm, which occurs within seconds of the interaction of TCR and APC.33,34 Evaluation of intracellular calcium level can provide the state of T -cells; hence we wanted to evaluate if the peptide SFTI-DBF has any effect on calcium level and signaling in T-cells. HFLS-RA cells were pretreated with 5 nM and 200 nM SFTI-DBF and compared the intra-cellular level of calcium on Jurkat cells upon co-culture of peptide treated and non-treated APC (HFLS-RA cells). Co-culture of HFLS-RA cells and Jurkat cells will trigger the influx of calcium, leading to activation of T-cells. After treating the HFLS-RA cells with 5 nM and 200 nM and co-culturing of these HFLS-RA cells with Jurkat cells led to a concentration-dependent decrease in intracellular calcium level in Jurkat cells (Fig. 3A and B). These results clearly suggest that the SFTI-DBF can decrease the calcium influx and inhibits the further activation of T-cells.
Fig. 3.
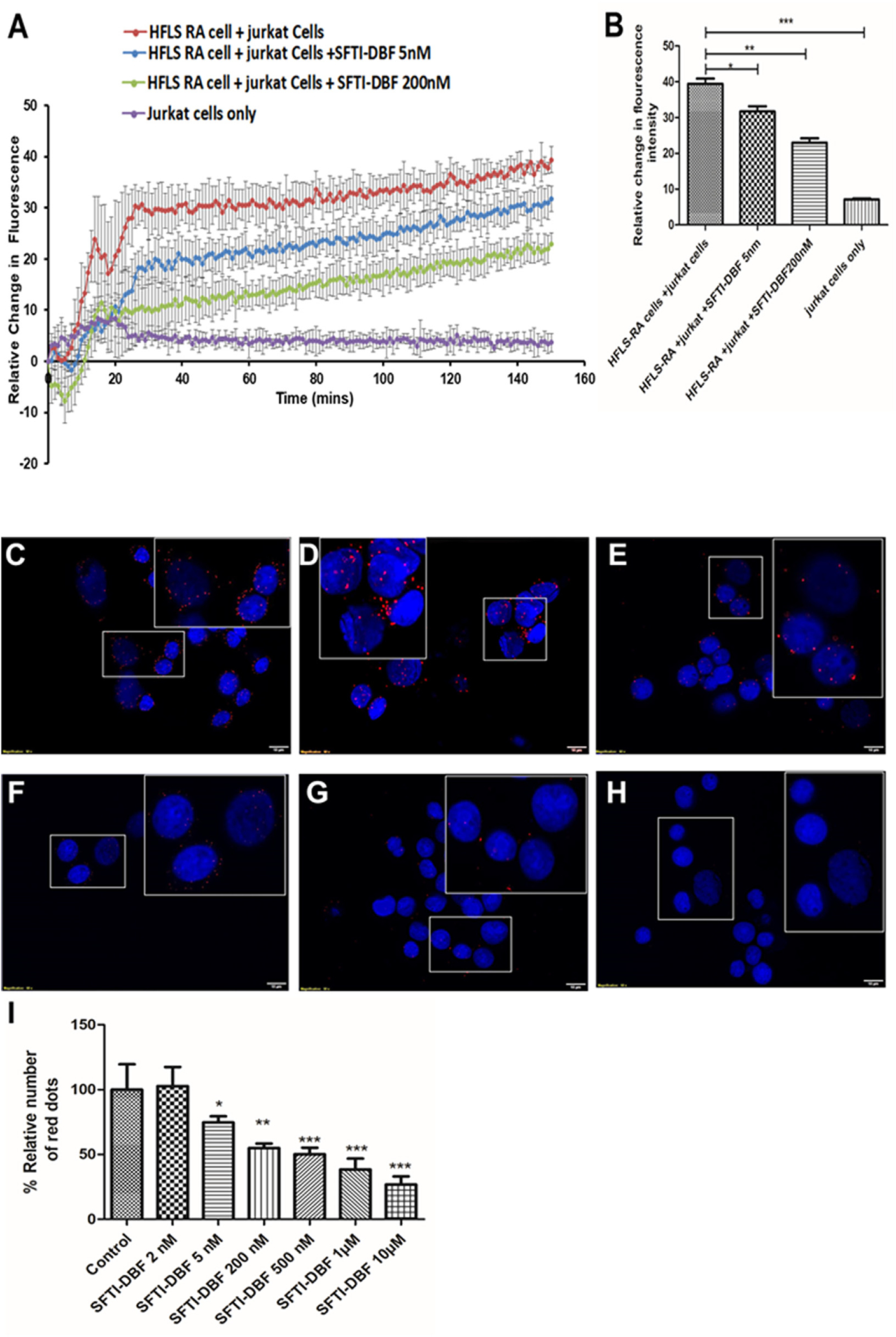
A) Effect of SFTI-DBF on cell signaling measured by calcium flux. B) Quantitative analysis of calcium flux measurement by Jurkat cells. In the presence of CD3 and HFLS-RA cells, there was a drastic increase in calcium flux due to the CD2 signaling mechanism. In the presence of SFTI-DBF at 5 and 200 nM, calcium flux was decreased significantly compared to controls. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Proximity ligation assay in the presence and absence of SFTI-DBF for CD2 and CD58 protein heterodimerization and its inhibition. (HFLS-RA cells and Jurkat cells). Red dots in PLA occur due to the PPI in proximity. Jurkat cells and HFLS-RA pretreated with different peptide concentrations are co-cultured, and PPI between CD2 and CD58 are quantified. C) Without treatment, only HFLS-RA and Jurkat cells D) Inhibition of CD2-CD58 by SFTI-DBF at 2 nM. E) Inhibition of PPI between CD2-CD58 by peptide SFTI-DBF at 5 nM. F) Inhibition of PPI between CD2-CD58 by peptide SFTI-DBF 0.2 μM. G) Inhibition of PPI between CD2-CD58 by peptide SFTI-DBF 1 μM. H) Inhibition of PPI between CD2-CD58 by peptide SFTI-DBF 10 μM. I) Quantification of red dots in PLA. Statistical analysis indicated that p < 0.05(*), p < 0.01 (**), p < 0.001 (***) for different treatment groups compared to control.
3.9. Proximity ligation assay (PLA)
PLA is one of the best in-vitro assays to visualize the protein–protein interaction. Duolink® PLA technology allows one to detect protein–protein interactions at endogenous protein levels with high sensitivity and specificity.35 One of the limitations of PLA is that it does not confirm direct protein–protein interaction; instead, the presence of the PLA signal suggests the existence of two proteins in proximity. Protein–protein interaction can be visualized by fluorescence signal and quantified by fluorescent microscopy. PLA for proteins on the cell surface of two different cells was reported earlier from our laboratory.36 The interaction between CD2 and CD58 proteins involving two different cells was observed using the same protocol with slight modification in the procedure. CD2 expressing T-cells coated on the 8 well chambers, and it was co-cultured with different concentrations of peptide treated and non-treated HFLS-RA cells (express CD58) and PLA was performed. Co-cultured Jurkat cells and HFLS-RA cells without SFTI-DBF exhibited red fluorescence intensity suggesting proximity of CD2-CD58 proteins. When SFTI-DBF was added at various concentrations (2 nM–10 μM), the fluorescence intensity or the number of fluorescence red dots decreased in a concentration-dependent manner (Fig. 3D–H, Supporting Information Figure S5) and the fluorescence red dots from the images were quantified by Image J software (Fig. 3I). In the presence of SFTI-DBF at 5 nM, there was a significant decrease in the fluorescence, and it further decreased in the presence of increased concentration of SFTI-DBF. These results suggest that SFTI-DBF binds to CD58 protein on HFLS-RA cells and prevents the interaction between HFLS-RA cells and Jurkat cells in a concentration-dependent manner.
3.10. Collagen induced arthritis- in vivo study
Collagen-induced arthritis (CIA) is one of the most widely used preclinical models for arthritis. Susceptibility to CIA is highly linked to major histocompatibility complex class II genes. The development of arthritis occurs through robust T cell and B cell response to type II collagen.21 Because of the involvement of T-cells and B-cells immune response in the development of CIA, it has become a widely used model to evaluate the therapeutic efficacy of a drug for rheumatoid arthritis.37 Our study involved the development of CIA in DBA1/J mice by transdermal injection of an emulsion of type II bovine collagen and Complete Freunds Adjuvant followed by booster injection of an emulsion of type II collagen and Incomplete Freunds Adjuvant on day 21. Animals were divided into different groups; group 1: vehicle-treated group; group 2: SFTI-DBF peptide treated group (0.5 mg/kg); group 3: SFTI-DBF peptide treated group (1 mg/kg); group 4: methotrexate (MTX) group, and group 5: naïve group (healthy control). SFTI-DBF was administered via i.v. route through the tail vein starting day 22, and a total of six doses (on alternate days) were administered during the study. MTX was given at a dose of 1.5 mg/kg intraperitoneally starting on day 22 on alternate days during the study. Scoring and body weight measurement were performed from day 18 on every alternate day till the end of the study. A visual inspection of the limbs for redness and the swelling was scored on a scale of 0–4 according to the previously reported work12,20,23 (Supporting information Figure S6). The % disease incidence in each group was calculated (Fig. 4A), and it was observed that SFTI-DBF treated group has significantly lower disease incidence compared to the vehicle-treated group and other group through most of the study. Arthritis score results indicated that the mean arthritis score was decreased significantly in SFTI-DBF treated mice compared with untreated mice (Fig. 4B). Also, the arthritic score significantly decreased in MTX-treated mice compared to the control vehicle-treated group. SFTI-DBF at a dose of 1 mg/kg decreased the arthritic score significantly compared to the 0.5 mg/kg SFTI-DBF and MTX (1.5 mg/kg, IP) treated group (Fig. 4C). The statistical significance between the groups was calculated by GraphPad Prism using one-way ANOVA with a post hoc Tuckey test (Fig. 4D). The bodyweight of peptide-treated groups animals did not change significantly throughout the study, indicating the non-toxic nature of SFTI-DBF (Supporting information Figure S7). In vitro cytotoxicity of SFTI-DBF in OVCAR-3 and HFLS-RA cells indicated that it is non-toxic to cells studied (Supporting information Figure S8 A–B).
Fig. 4.
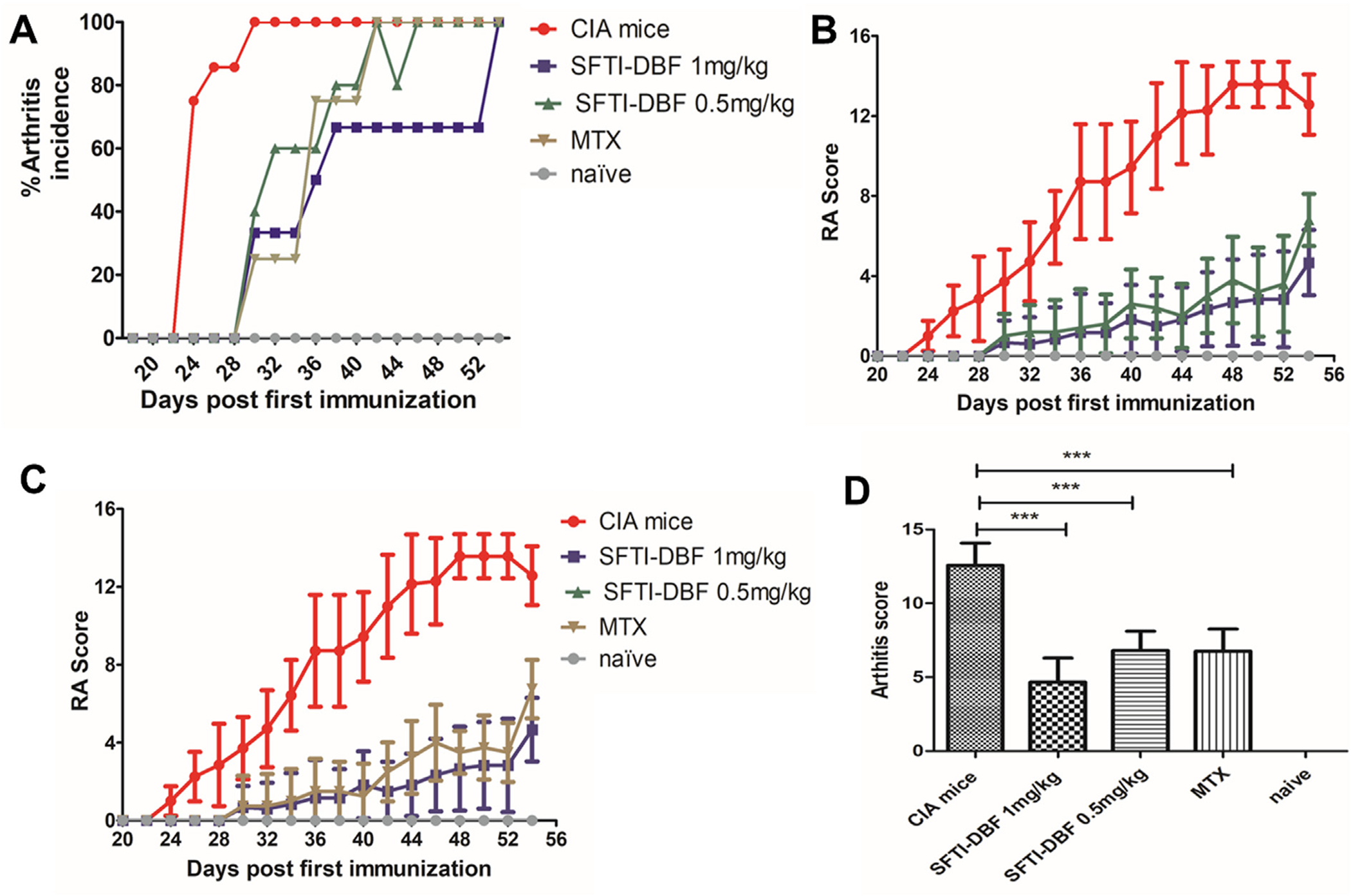
Efficacy of SFTI-DBF in Collagen-induced arthritis model of DBA/1 J mice. A) Arthritis incidence in different groups of peptides treated and non-treated collagen-induced arthritis mice. B&C) Mice were induced with arthritis by injection of Type II collagen and complete Freunds adjuvant emulsion intradermally at the base of the tail. Treatment with SFTI-DBF and control was started the next day after the booster dose of an emulsion of Type II collagen and incomplete Freunds adjuvant. SFTI-DBF was given thrice a week for two weeks on alternate days, and arthritis progression was monitored by visual scoring (N = 6, 3 male, 3 female). SFTI-DBF was dissolved in PBS in 100 μL and given at doses: 1 mg/kg and 0.5 mg/kg. Methotrexate (MTX) was used as a positive control and, PBS as vehicle control (CIA). Naïve is without arthritis. MTX was given via IP dose of 1.5 mg/kg. D) Quantitative analysis of arthritis score. There was a significant difference between mice treated with SFTI-DBF (1 mg/kg and 0.5 mg/kg as well as MTX compared to the control group.
3.11. Histopathology study of limbs
Slides of H and E stained sections of the limbs were studied and evaluated for inflammation, synovial hyperplasia, cartilage, and/or bone erosion in all groups (Fig. 5). The evaluation was performed by a certified pathologist. The evaluation was based on the severity of the histological findings, with 0 being no pathology and 4 being major destruction of the studied tissues.38 A total score was calculated by adding the scores of the individual parameters evaluated with Score 0; normal joint, 1; mild inflammation with synovial lining hyperplasia with minimal cartilage and bone erosion and 2–4; severe inflammation and synovial lining hyperplasia with pannus formation and cartilage erosion. In the CIA group, significant inflammation, synovial hyperplasia, and cartilage erosion were observed, and the total score was 4. Histopathological changes were significantly reduced in SFTI-DBF (1 mg/kg) and SFTI-DBF (0.5 mg/kg) groups. Also, MTX treated group showed a reduced occurrence of the changes. SFTI-DBF (1 mg/kg) groups showed the least total score among the treated groups with minimal inflammation, minimal hyperplasia, and minimal bone erosion (Fig. 6A–D).
Fig. 5.
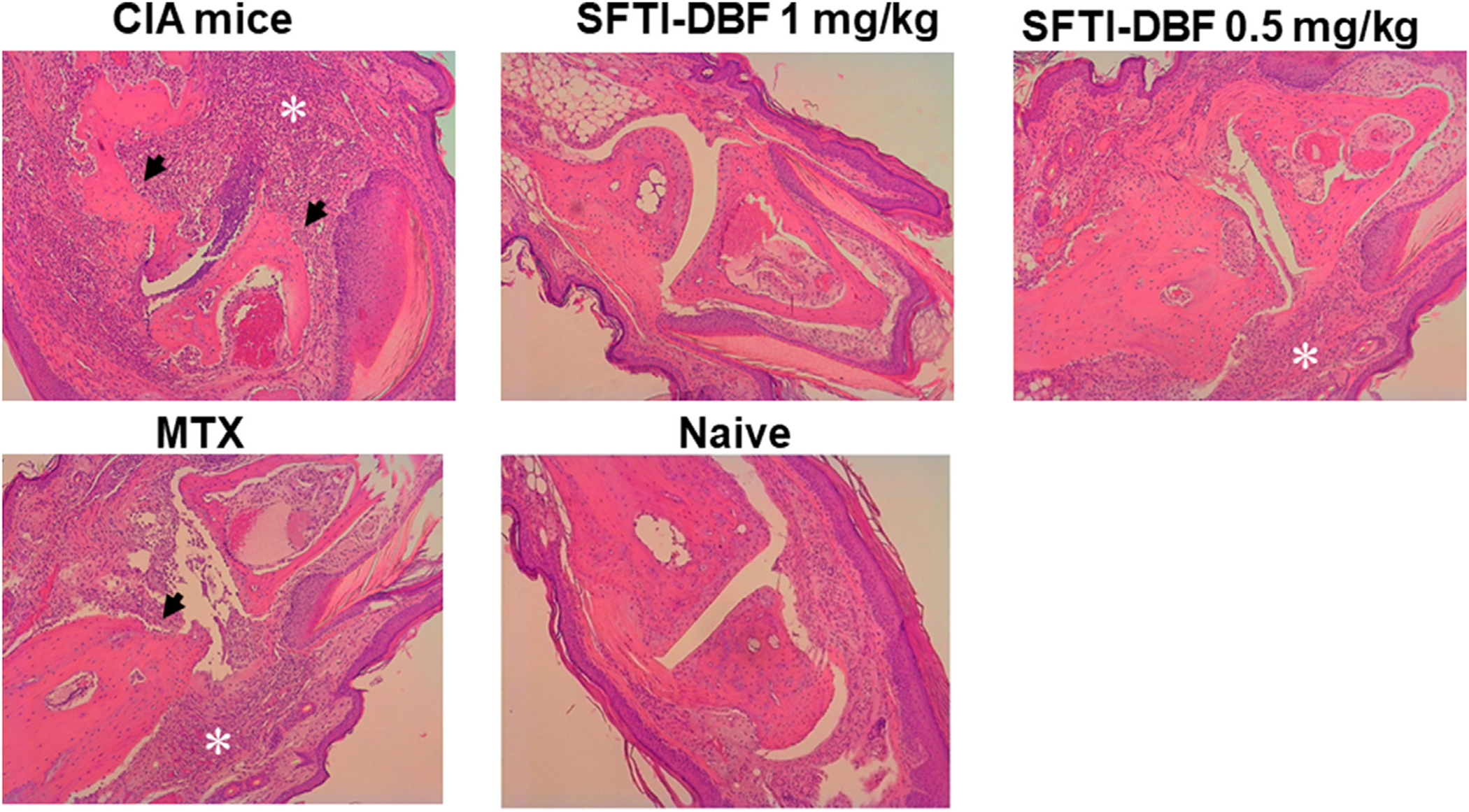
H&E-stained section of a CIA joint treated with PBS, 1 mg/kg of SFTI-DBF, 0.5 mg/kg of SFTI-DBF, Methotrexate, and a naïve healthy control (Numbers of limbs tissue analyzed (N) = 4). Arthritic mice limbs showed inflammatory infiltration in the synovium (asterisks), cartilage, and bone erosion (arrows). Treatment group limbs showed drastic improvement, and SFTI-DBF 1 mg/kg treated group limbs resembled naïve healthy limbs. Images obtained at 10X magnification.
Fig. 6.
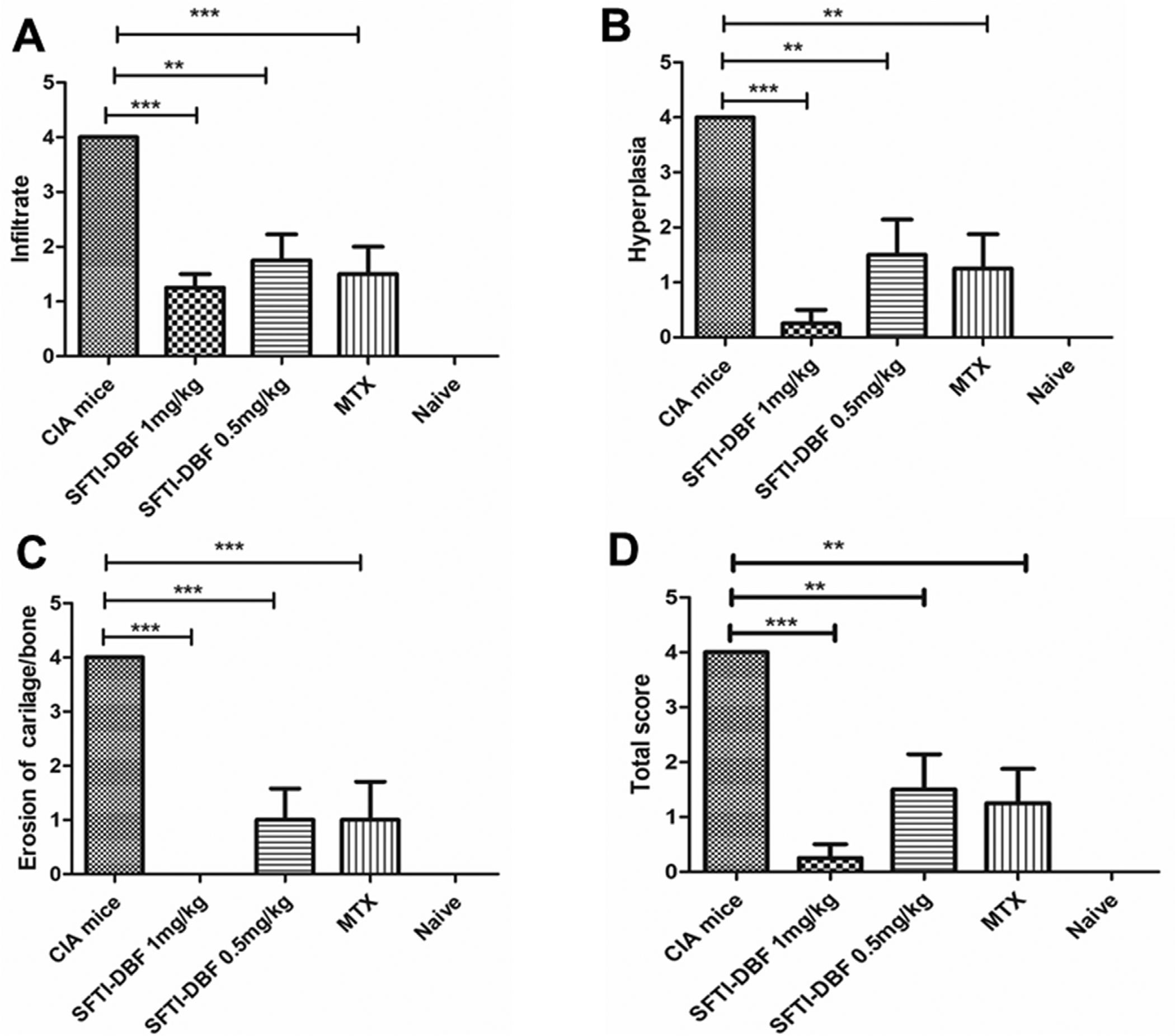
A-D). Evaluation of inflammation, synovial hyperplasia, and erosion of bone and cartilage in joint sections in CIA mice (Numbers of limb tissues analyzed (N) = 4). A score of 0–4 was assigned depending on the severity of the histological findings. Each joint was given a total score by observing the above changes with score 0; normal joint, score 1; mild inflammation and synovial hyperplasia without damage to cartilage/bone and score 2–4; severe inflammation and synovial lining hyperplasia with pannus formation and cartilage. Scores are compared within different groups, and values represent the mean ± SEM. Statistical analysis indicated that p < 0.01 (**), p < 0.001 (***) for different treatment groups compared to control (CIA) mice.
3.12. Inflammatory cytokine analysis
Cytokines and chemokines are low molecular weight potent signaling molecules involved in mediating intercellular communications, especially in immune systems. Cytokines are secreted mainly by immune cells like macrophages and lymphocytes. Cytokines regulate inflammation and play a significant role in the regulation of immune response. There are two different types of cytokines pro-inflammatory and anti-inflammatory cytokines. In auto-immune disease, there is a significant imbalance in the homeostasis in the immune system affecting these pro and anti-inflammatory cytokines levels.39
In rheumatoid arthritis, cytokines play a major role in the pathogenesis and progression of the diseases. The major pro-inflammatory cytokines that are involved in auto-immune diseases include TNF-α, IFN-γ, IL-2, IL-6, and IL-17. Serum and joint cytokines levels can indicate disease progression and severity.40 Hence in our study, we have determined the serum cytokine level in CIA and peptide-treated mice to see the effect of the SFTI-DBF on the cytokine levels. Since SFTI-DBF significantly suppressed arthritis in CIA, we expect the major pro-inflammatory cytokines levels to be lower than the CIA mice.
Different pro-inflammatory cytokines levels were observed. CIA groups show marked elevation of pro-inflammatory cytokine levels like IL-6, TNF-a, and IL-17 compared to naive; the serum levels of these cytokines were decreased significantly in SFTI-DBF and methotrexate treated group (Fig. 7). These results suggested that CIA in mice was associated with higher levels of pro-inflammatory cytokines, and the SFTI-DBF was effective in lowering these pro-inflammatory cytokines by inhibiting hyperactivation of T-cell.
Fig. 7.
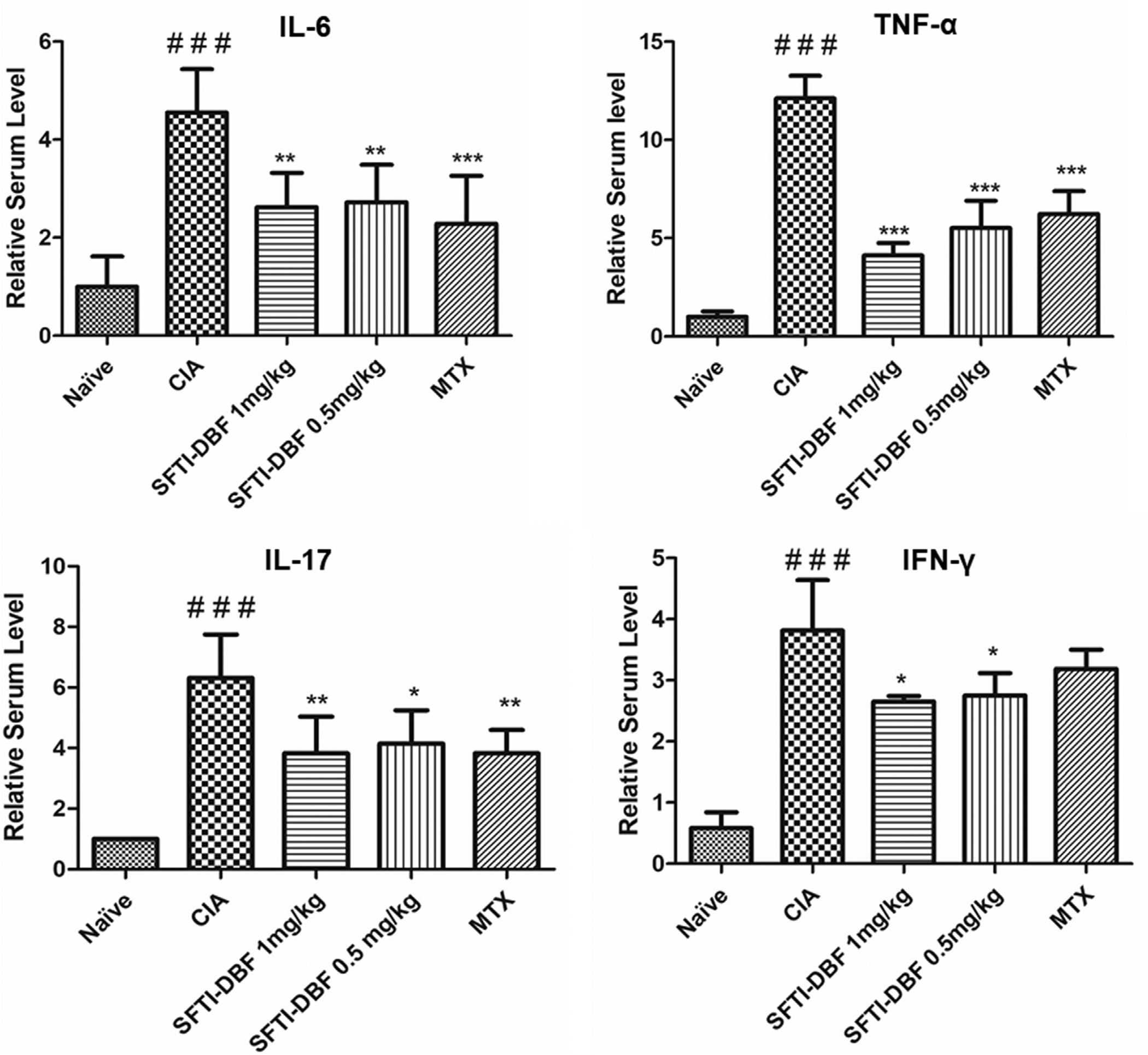
Levels of pro-inflammatory cytokines like IL-6, TNF-a, IL-17, and IFN-γ were significantly higher in the CIA group compared to the treatment group. (N = 3) ###p < 0.001 compared to naïve mice, p < 0.05 (*), p < 0.01 (**), p < 0.001(***) compared to CIA group.
3.13. Collagen antibody level
Development of arthritis in CIA is associated with robust T-cell and B-cell immune response, which leads to the generation of high levels of antibody against type II collagen. This antibody plays a major role in the pathogenesis and severity of arthritis in DBA1/J mice.41 We wanted to investigate whether treatment with SFTI-DBF reduces the collagen antibody level in the CIA model. At the end of the CIA in-vivo study, animals were sacrificed, and the blood sample was collected. The blood was processed, and serum from mice was obtained. Bovine II collagen antibody level in serum was determined by ELISA following the manufacturer’s protocol provided in a kit from Chondrex. Results showed that the bovine collagen antibody level in SFTI-DBF treated group is significantly lower than the vehicle control group. Also, the antibody level in MTX treated group was found lower than the vehicle-treated group. Antibody level in SFTI-DBF 1 mg/kg was found to be lower than SFTI-DBF 0.5 mg/kg and MTX treated group (Fig. 8A). Collagen antibody production is known to damage the cartilage at the joints. From in vivo studies, it is evident that SFTI-DBF was able to decrease the antibody level in mice serum and hence stop the progression of cartilage damage of arthritis mice suggesting mechanistic aspect of SFTI-DBF in modulating the signaling for immune response in autoimmune disease.
Fig. 8.
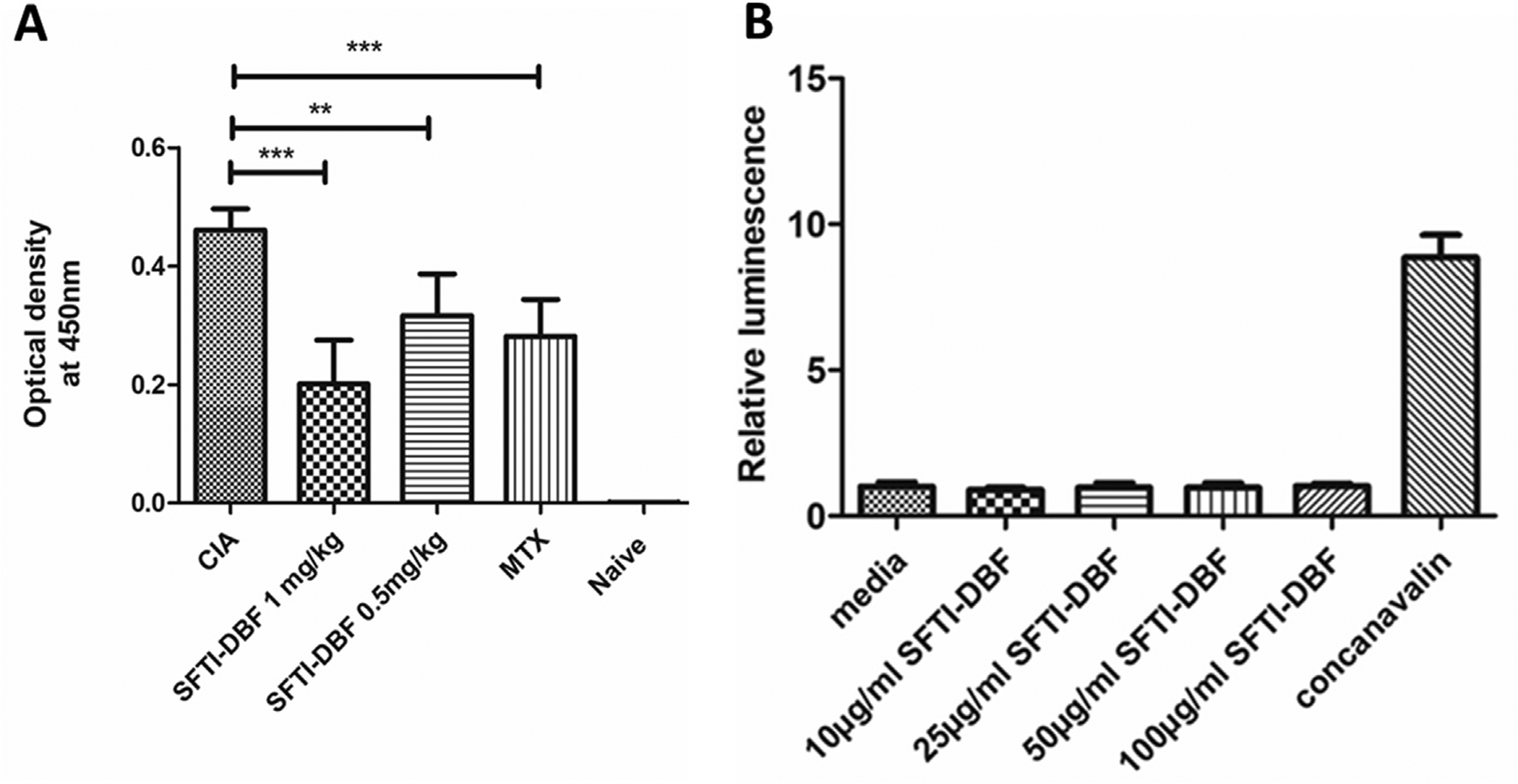
A) Reduction of circulating anti-CII Ab titer in serum of mice with CIA measured by ELISA. (N = 6). Relative levels of anti-CII Ab titer were observed with SFTI-DBF-treated mice compared to the control group (CIA). Values represent the mean ± SEM. Statistical analysis indicated that p < 0.01 (**), p < 0.001 (***) for SFTI-DBF treated mice compared to control (CIA). B) Immunogenicity study of SFTI-DBF. Splenocytes obtained from SFTI-DBF 1 mg/kg injected DBA1/J mice (N = 3) are cultured in the presence of a different concentration of SFTI-DBF (10, 25, 50, and 100 μg/mL SFTI-DBF) and T-cell mitogenic activator Concanavalin A (10 μg/mL) for 48 h. Cell proliferation is then measured by using CellTiter-Glo assay.
3.14. Immunogenicity study of SFTI-DBF
Peptides and proteins can be immunogenic and can elicit unwanted immune responses.42 To determine SFTI-DBF immunogenicity, SFTI-DBF (1 mg/kg) peptide treated group mice from the CIA- in vivo study were used. At the end of the CIA study, spleens from these group of mice were harvested, and they were processed, red blood cells (RBC) was lysed, and mononuclear suspension of splenocytes was obtained. These splenocytes were cultured and treated with different concentrations of SFTI-DBF and incubated for 48 h. Cell Titre Glo assay was performed to evaluate the proliferation of T-cells in peptide treated, non-treated, and concanavalin A treated group of cells. If the peptide is immunogenic, the splenocytes will proliferate aggressively, as in the concanavalin A (10 μg/mL) treated group of splenocytes. Proliferation results indicated that different concentrations of the SFTI-DBF (25, 50, and 100 μg/mL) had no effect on the proliferation of splenocytes compared to the non-treated group, which provides evidence of the non-immunogenic nature of peptide SFTI-DBF (Fig. 8B).
4. Discussion
Generally, linear peptides suffer from low in vivo stability as they are cleaved by proteases.30,43 Various modification approaches, including cyclization, stapling, substitution by unnatural amino acid and small molecule scaffold (peptidomimetic), and grafting of peptides to a stable framework, are employed to address this problem.44–47 Peptidomimetic and grafted peptides have improved stability towards proteolytic degradation.48,49 In this study, we have engineered SFTI-DBF, a grafted peptidomimetic designed by replacing the Pro–Pro residues with dibenzofuran moiety (DBF) in the SFTI-1 framework.50,51 DBF, a small molecule scaffold, is also a beta-turn inducer and has been used in the design of various peptidomimetics.13,52 The designed peptide used CD2 protein epitope to target CD58 protein and inhibit CD2-CD58 interactions resulting in immunomodulation. The peptide we reported in our previous studies; namely, SFTI-a1, contains two prolines. SFTI-1 and some of its analogs are known to exhibit multiple conformations in solution, probably arising from Xaa-Pro cis/trans isomerization that is slow on the NMR time scale.14,53 SFTI-DBF with constrained structure exhibited enhanced activity in inhibiting the APC–T cell adhesion with an IC50 of 3.8 nM with a 9-fold improvement in activity compared to SFTI-a1(IC50 = 37 nM).15 To evaluate the effect of SFTI-DBF on the labeling of Jurkat cells by BCECF-AM, we incubated Jurkat cells with different concentrations of DBF for 1 and 3 h and monitored the fluorescence compared to labeled cells without SFTI-DBF. SFTI-DBF did not have any effect on the labeling of Jurkat cells (Supporting Information Figure S9).
In our work, we have used grafting technology to graft the peptide epitope on to stable SFTI framework. Studies have shown that SFTI peptides exhibit higher thermal and chemical stability than linear and backbone cyclized peptides.44,54 Hence, stability studies of SFTI-DBF were performed to ensure these stability attributes of SFTI are retained in our designed grafted peptidomimetic. SFTI-DBF also exhibited high thermal, chemical and enzymatic stability. This suggests that the SFTI-DBF, a grafted peptide is significantly stable towards the serum proteases and overcomes the limitation of low in vivo stability of conventional linear peptides. Furthermore, disulfide bond stability is important for grafted peptides as the disulfide bond provides exceptional stability to the grafted peptides.28 There was no change in the disulfide bond when the peptide was heated to 60 °C. When dithiothreitol (DTT) was added to the peptide at 100 μM, there was no change in the CD spectra of SFTI-DBF compared to without the addition of DTT. Mass spectrometry data showed that when DTT was added >1 mM, the disulfide bond of SFTI-DBF was reduced, suggesting the stability of the overall structure of the peptide.
Peptide SFTI-DBF with CD2 peptide epitope is presumed to bind to CD58 protein and inhibit PPI of CD2-CD58 and hence cell adhesion that results in a change in the CD2 signaling immune response. Using flow-cytometry analysis we have shown that SFTI-DBF competitively binds to cells overexpressing CD58 with CD58 FITC Ab. The antibody to CD58 binds to the adhesion domain of CD58 and hence SFTI-DBF binds to the adhesion domain of CD58 that binds to CD2. To understand the molecular mechanism of SFTI-DBF to bind to cells that express CD58 and inhibit PPI, PLA was carried out on mixed cells. When T-cells that express CD2 and HFLS-RA cells that express CD58 were mixed, and PLA was performed, it was evident that SFTI-DBF inhibits the CD2:CD58 PPI by binding to the CD58 adhesion domain. PLA experiment provided strong support for our hypothesis that the designed SFTI-DBF binds to the CD58 receptor and could inhibit the CD2:CD58 PPI. Another key signaling mechanism in T cell activation is calcium signaling. Upon CD2-CD58 interaction, the cytoplasmic tail of CD2 generates the signal by calcium flux in the cytoplasm via Zap-70 protein phosphorylation. Monitoring the calcium influx in T-cells in the presence of APC can provide insight into T-cell activation. Calcium flux in T-cells was decreased when HFLS-RA cells were treated with SFTI-DBF, suggesting the inactivation of T-cells by inhibition of PPI of CD2 and CD58 by the peptide.
In vivo efficacy of SFTI-DBF was assessed in T-cell and B cell-mediated collagen-induced arthritis (CIA) mice model that resembles more closely to human RA pathogenesis.55 CIA pathogenesis involves the collagen antigen presentation by APC to T-cells, leading to activation of T-cells and subsequently the generation of anti-collagen II antibody. SFTI-DBF at a dose of 0.5 and 1 mg/kg was able to suppress arthritis in the CIA mice, and its effect was comparable to methotrexate. Suppression of CIA by the peptide strongly supports that SFTI-DBF inhibits the activation of T-cells by inhibiting CD2:CD58 interaction. SFTI-DBF treatment led to the reduction of anti-collagen antibody level, one of the main drivers of cartilage erosion and destruction in the CIA. SFTI-DBF may reduce the anti-collagen II antibody by inhibition of activation of T-cells. Histopathology analysis of limbs showed the improvement of pathological features such as infiltration, synovial hyperplasia, bone and cartilage erosion in the SFTI-DBF treated group. Furthermore, SFTI-DBF also significantly reduced the inflammatory cytokines in the mice model of RA. Inflammatory cytokines such as TNF-alpha, IL-6, IL-17, IFN-gamma play a major role in the pathogenesis of arthritis,56 and the CIA model resembles several pathological features of RA.55,57 These cytokines are released upon T-cell activation by helper T-cells, macrophages, synoviocytes, causing inflammatory reactions. SFTI-DBF treated mice had lower serum levels of the inflammatory cytokines that may be attributed to T-cell inactivation. Due to the lower inflammatory cytokines and collagen II antibody levels in peptide-treated mice, the arthritis progression was significantly reduced.
Other stable peptides such as rhesus theta defensin-1 (RTD-1) have been used as a template to design peptides to target RA. An RTD-1 based peptide reduces the inflammation in the RA model.58,59 However, the action of the mechanism is entirely different. Compared to other peptide template frameworks, the SFTI framework provides a suitable peptide template for designing peptides. It is one of the shortest peptides (14 amino acids) that exhibits exceptional stability against enzymatic and chemical degradation. Qui et al. have shown that the SFTI framework can be used to generate an orally active peptide targeting bradykinin receptor.60 Thus, peptides that target CD2-CD58 to modulate the immune response can be made stable and orally available with proper modification in the design.
5. Conclusion
A conformationally constrained grafted immunomodulating peptidomimetic with a single major conformer by the introduction of dibenzofuran moiety (DBF) in the structure was successfully designed and developed. The peptidomimetic exhibited cell adhesion inhibition activity in the low nanomolar range. This peptidomimetic is thermally and enzymatically stable and could bind to CD58 protein in-vitro. The peptide was also able to significantly suppress the development of arthritis in an in vivo model of collagen-induced arthritis in mice. The peptidomimetic was non-immunogenic and non-toxic. Such potent immunomodulating peptidomimetics can be used as a therapeutic agent in the treatment of autoimmune diseases such as rheumatoid arthritis.
Supplementary Material
Acknowledgments
Funding for this project was supported by the National Institute of General Medical Sciences of the National Institute of Health under grant number P20 GM103424-20. Part of this research work was supported by the National Cancer Institute of the National Institutes of Health (1R01CA255176-01) to SJ.
The authors would like to thank the Microscopy facility, Biology ULM, and mass spectrometry facility at Louisiana State University, Baton Rouge. The authors would also like to thank Dr. Ted Gauthier, LSU Ag Center, Louisiana State University, Baton Rouge, for help with synthesis of SFTI-DBF. Cytokine multiplex assay was carried out at School of Veterenary medicine Louisiana State University at Baton Rouge. Authors thank GeneLab Core at the LSU Vet Med facility for assistance with multiplex experiment.
Footnotes
Declaration of competing interest
The authors declare no competing financial interest. The molecule (SFTI-DBF) dsigned and reported in this publication and data related to SFTI-DBF work was submitted as a US patent application No. 16/865295.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jphs.2022.04.005.
References
- 1.Ryan DP, Matthews JM. Protein-protein interactions in human disease. Curr Opin Struct Biol. 2005;15(4):441–446. [DOI] [PubMed] [Google Scholar]
- 2.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450(7172):1001–1009. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. [DOI] [PubMed] [Google Scholar]
- 6.Davis SJ, Ikemizu S, Evans EJ, Fugger L, Bakker TR, van der Merwe PA. The nature of molecular recognition by T cells. Nat Immunol. 2003;4(3):217–224. [DOI] [PubMed] [Google Scholar]
- 7.Balanescu A, Radu E, Nat R, et al. Co-stimulatory and adhesion molecules of dendritic cells in rheumatoid arthritis. J Cell Mol Med. 2002;6(3):415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341(6243):619–624. [DOI] [PubMed] [Google Scholar]
- 9.Wingren AG, Parra E, Varga M, et al. T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit Rev Immunol. 1995;15(3–4):235–253. [DOI] [PubMed] [Google Scholar]
- 10.Chamian F, Lowes MA, Lin SL, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 2005;102(6):2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91(10):1057–1064. [DOI] [PubMed] [Google Scholar]
- 12.Gokhale A, Kanthala S, Latendresse J, Taneja V, Satyanarayanajois S. Immunosuppression by co-stimulatory molecules: inhibition of CD2-CD48/CD58 interaction by peptides from CD2 to suppress progression of collagen-induced arthritis in mice. Chem Biol Drug Des. 2013;82(1):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokhale A, Weldeghiorghis TK, Taneja V, Satyanarayanajois SD. Conformationally constrained peptides from CD2 to modulate protein-protein interactions between CD2 and CD58. J Med Chem. 2011;54(15):5307–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sable R, Durek T, Taneja V, et al. Constrained cyclic peptides as immunomodulatory inhibitors of the CD2:CD58 protein-protein interaction. ACS Chem Biol. 2016;11(8):2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parajuli P, Sable R, Shrestha L, et al. Modulation of co-stimulatory signal from CD2-CD58 proteins by a grafted peptide. Chem Biol Drug Des. 2021;97(3): 607–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheneval O, Schroeder CI, Durek T, et al. Fmoc-based synthesis of disulfide-rich cyclic peptides. J Org Chem. 2014;79(12):5538–5544. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Chow VT, Jois SD. A novel, rapid and sensitive heterotypic cell adhesion assay for CD2-CD58 interaction, and its application for testing inhibitory peptides. J Immunol Methods. 2004;291(1–2):39–49. [DOI] [PubMed] [Google Scholar]
- 18.Svenson J, Stensen W, Brandsdal B-O, Haug BE, Monrad J, Svendsen JS. Anti-microbial peptides with stability toward tryptic degradation. Biochemistry. 2008;47(12):3777–3788. [DOI] [PubMed] [Google Scholar]
- 19.Sable R, Parajuli P, Jois S. Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar Drugs. 2017;15(4):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrosimone KM, Jin M, Poston B, Liu P. Collagen-induced arthritis: a model for murine autoimmune. Arthritis, Bio Protoc. 2015;5(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. [DOI] [PubMed] [Google Scholar]
- 22.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum. 2007;56(1):69–78. [DOI] [PubMed] [Google Scholar]
- 23.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1(5): 2512–2516. [DOI] [PubMed] [Google Scholar]
- 24.Parajuli P, Sable R, Shrestha L, et al. Modulation of costimulatory signal from CD2-CD58 proteins by a grafted peptide. Chem Biol Drug Des. 2021;97(3): 607–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopal R, Park JS, Seo CH, Park Y. Applications of circular dichroism for structural analysis of gelatin and antimicrobial peptides. Int J Mol Sci. 2012;13(3): 3229–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang CK, Gruber CW, Cemazar M, et al. Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis. ACS Chem Biol. 2014;9(1):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craik DJ, Swedberg JE, Mylne JS, Cemazar M. Cyclotides as a basis for drug design. Expet Opin Drug Discov. 2012;7(3):179–194. [DOI] [PubMed] [Google Scholar]
- 28.Poth AG, Chan LY, Craik DJ. Cyclotides as grafting frameworks for protein engineering and drug design applications. Biopolymers. 2013;100(5):480–491. [DOI] [PubMed] [Google Scholar]
- 29.Colgrave ML, Korsinczky MJ, Clark RJ, Foley F, Craik DJ. Sunflower trypsin inhibitor-1, proteolytic studies on a trypsin inhibitor peptide and its analogs. Biopolymers. 2010;94(5):665–672. [DOI] [PubMed] [Google Scholar]
- 30.Jenssen H, Aspmo SI. Serum stability of peptides. Methods Mol Biol. 2008;494: 177–186. [DOI] [PubMed] [Google Scholar]
- 31.Fuhrmann G, Leroux JC. Improving the stability and activity of oral therapeutic enzymes-recent advances and perspectives. Pharm Res (N Y). 2014;31(5): 1099–1105. [DOI] [PubMed] [Google Scholar]
- 32.Christo SN, Diener KR, Nordon RE, et al. Scrutinizing calcium flux oscillations in T lymphocytes to deduce the strength of stimulus. Sci Rep. 2015;5:7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph N, Reicher B, Barda-Saad M. The calcium feedback loop and T cell activation: how cytoskeleton networks control intracellular calcium flux. Bio-chim Biophys Acta Biomembr. 2014;1838(2):557–568. [DOI] [PubMed] [Google Scholar]
- 34.Trebak M, Kinet JP. Calcium signalling in T cells. Nat Rev Immunol. 2019;19(3): 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam MS. Proximity ligation assay (PLA). Curr Protoc Im. 2018;123(1):e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sable R, Jambunathan N, Singh S, Pallerla S, Kousoulas KG, Jois S. Proximity ligation assay to study protein-protein interactions of proteins on two different cells. Biotechniques. 2018;65(3):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yap HY, Tee SZ, Wong MM, Chow SK, Peh SC, Teow SY. Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells. 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael L, Lixen Z. Amelioration of inflammatory arthritis by targeting the preligand assembly domain (PLAD) of tumor necrosis factor receptors. US GOVERNMENT; 2006. [Google Scholar]
- 39.Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. [DOI] [PubMed] [Google Scholar]
- 41.Bajtner E, Nandakumar KS, Engstrom A, Holmdahl R. Chronic development of collagen-induced arthritis is associated with arthritogenic antibodies against specific epitopes on type II collagen. Arthritis Res Ther. 2005;7(5): R1148–R1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunot. 2014;11(2):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010;15(1–2):40–56. [DOI] [PubMed] [Google Scholar]
- 44.de Veer SJ, White AM, Craik DJ. Sunflower trypsin inhibitor-1 (SFTI-1): sowing seeds in the fields of chemistry and Biology. Angew Chem Int Ed Engl. 2021;60(15):8050–8071. [DOI] [PubMed] [Google Scholar]
- 45.Hruby VJ, Cai M. Design of peptide and peptidomimetic ligands with novel pharmacological activity profiles. Annu Rev Pharmacol Toxicol. 2013;53:557–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hess S, Ovadia O, Shalev DE, et al. Effect of structural and conformation modifications, including backbone cyclization, of hydrophilic hexapeptides on their intestinal permeability and enzymatic stability. J Med Chem. 2007;50(24): 6201–6211. [DOI] [PubMed] [Google Scholar]
- 47.Tugyi R, Uray K, Ivan D, Fellinger E, Perkins A, Hudecz F. Partial D-amino acid substitution: improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc Natl Acad Sci U S A. 2005;102(2):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin SJS, Tal-Gan Y, Gilon C, Qvit N. Conversion of protein active regions into peptidomimetic therapeutic leads using backbone cyclization and cycloscan - how to do it yourself. Curr Top Med Chem. 2018;18(7):556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry SP, Fernandez TJ, Anand JP, Griggs NW, Traynor JR, Mosberg HI. Structural simplification of a tetrahydroquinoline-core peptidomimetic mu-opioid receptor (MOR) agonist/delta-opioid receptor (DOR) antagonist produces improved metabolic stability. J Med Chem. 2019;62(8):4142–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesner A, Legowska A, Wysocka M, Rolka K. Sunflower trypsin inhibitor 1 as a molecular scaffold for drug discovery. Curr Pharmaceut Des. 2011;17(38): 4308–4317. [DOI] [PubMed] [Google Scholar]
- 51.Korsinczky ML, Schirra HJ, Rosengren KJ, et al. Solution structures by 1H NMR of the novel cyclic trypsin inhibitor SFTI-1 from sunflower seeds and an acyclic permutant. J Mol Biol. 2001;311(3):579–591. [DOI] [PubMed] [Google Scholar]
- 52.Mayo KH, Dings RP, Flader C, et al. Design of a partial peptide mimetic of anginex with antiangiogenic and anticancer activity. J Biol Chem. 2003;278(46): 45746–45752. [DOI] [PubMed] [Google Scholar]
- 53.Brauer AB, Domingo GJ, Cooke RM, Matthews SJ, Leatherbarrow RJ. A conserved cis peptide bond is necessary for the activity of Bowman-Birk inhibitor protein. Biochemistry. 2002;41(34):10608–10615. [DOI] [PubMed] [Google Scholar]
- 54.Craik DJ, Clark RJ, Daly NL. Potential therapeutic applications of the cyclotides and related cystine knot mini-proteins. Expet Opin Invest Drugs. 2007;16(5): 595–604. [DOI] [PubMed] [Google Scholar]
- 55.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2(5):1269–1275. [DOI] [PubMed] [Google Scholar]
- 56.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. [DOI] [PubMed] [Google Scholar]
- 57.Miyoshi M, Liu S. Collagen-induced arthritis models. Methods Mol Biol. 2018;1868:3–7. [DOI] [PubMed] [Google Scholar]
- 58.Tongaonkar P, Punj V, Subramanian A, et al. RTD-1 therapeutically normalizes synovial gene signatures in rat autoimmune arthritis and suppresses proinflammatory mediators in RA synovial fibroblasts. Physiol Genom. 2019;51(12): 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaal JB, Tran DQ, Subramanian A, et al. Suppression and resolution of auto-immune arthritis by rhesus theta-defensin-1, an immunomodulatory macro-cyclic peptide. PLoS One. 2017;12(11), e0187868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu Y, Taichi M, Wei N, Yang H, Luo KQ, Tam JP. An orally active bradykinin B1 receptor antagonist engineered as a bifunctional chimera of sunflower trypsin inhibitor. J Med Chem. 2017;60(1):504–510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


