Abstract
Objective
Myasthenia gravis (MG) is a chronic autoimmune neuromuscular disorder. Recent studies report that long non-coding RNAs (lncRNAs) play vital roles in the pathogenesis of various diseases. This study explored the molecular mechanism of lncRNA growth arrest specific 5 (GAS5) in regulating the T helper 17 (Th17)/regulatory T (Treg) cell balance in MG.
Methods
GAS5 and miR-23a expression levels were detected by quantitative reverse transcription polymerase chain reaction. Flow cytometry was performed to examine the proportion of Th17 and Treg cells in CD4+ T cells from MG patients. The interaction between GAS5 and miR-23a was verified by luciferase reporter and RNA immunoprecipitation assays. Levels of Th17 and Treg-related proteins were examined using western blots and enzyme-linked immunosorbent assays.
Results
GAS5 expression levels were significantly decreased in the CD4+ T cells of MG patients, and GAS5 overexpression restrained Th17 differentiation in CD4+ T cells. Moreover, miR-23a was confirmed as a downstream target of GAS5 and negatively regulated by GAS5 through a direct interaction. Further exploration showed that GAS5 can inhibit Th17 differentiation by downregulating miR-23a.
Conclusion
Collectively, our results indicate that GAS5 can regulate the Th17/Treg balance by targeting miR-23a expression, providing a scientific basis for clinical therapeutic development for MG.
Keywords: Myasthenia gravis, growth arrest specific 5, miR-23a, T helper 17, regulatory T cell, CD4+ T cell, long non-coding RNA
Introduction
Myasthenia gravis (MG) is a common B cell-mediated autoimmune disorder that affects neuromuscular connections and is primarily caused by autoantibodies against postsynaptic acetylcholine receptor (AChR), leading to skeletal muscle weakness and fatigue. 1 Accumulating studies have shown that a T helper 17 (Th17)/regulatory T (Treg) cell imbalance is involved in the pathogenesis of autoimmune diseases, including MG.2,3 Th17 and Treg cells are differentiated from CD4+ T cells. 4 Th17 cells expressing RAR-related orphan receptor γt (RORγt) have a vital role in inducing autoimmune tissue injuries and inflammation by producing cytokines, such as interleukin (IL)-17 and tumor necrosis factor (TNF)-α. 5 Treg cells expressing forkhead box P3 (Foxp3) exhibit an anti-inflammatory effect and maintain tolerance to self-components by releasing anti-inflammatory cytokines, such as IL-10. 6 Therefore, it is important to understand the molecular mechanisms of Th17 cell differentiation and develop novel effective therapeutic approaches for MG.
Long non-coding RNAs (lncRNAs) are a group of endogenous RNA molecules greater than 200 nucleotides in length that lack protein-coding ability.7,8 LncRNAs have been identified as vital regulators in MG in different studies. For instance, Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) exerts a protective effect in MG by acting as a competing endogenous RNA (ceRNA) of miR-338-3p to regulate MSL2 expression. 9 LncRNA IFNG-AS1 can decrease Th1 and promote Treg cell proliferation in MG by regulating HLA-DRB1. 10 LncRNA XLOC_003810 facilitates T cell activation and suppresses the programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) pathway in patients with MG-related thymoma. 11 LncRNA growth arrest specific 5 (GAS5), located at chromosome 1q25, was discovered in 1988 by screening overexpressed genes in growth arrest cells.12,13 Liu et al. indicated that GAS5 restrained CD4+ T cell activation in systemic lupus erythematosus by increasing E4 promoter binding protein (E4BP4) expression via sponging of miR-92a-3p. 14 However, the exact function of GAS5 in MG remains unknown.
MicroRNAs (miRNAs) are small non-coding RNAs that are 18 to 25 nucleotides in length and negatively regulate gene expression at the post-transcriptional level by binding to the 3ʹ untranslated region (3ʹ UTR) of particular target mRNAs.15,16 Several studies have reported that miRNAs play a vital role in MG pathogenesis. For example, miR-181a reduced the proportion of Th17 cells and increased the proportion of Treg cells in MG by targeting IL-2. 17 Upregulation of miR-150-5p contributed to the abnormal immune responses in MG by decreasing levels of IL-17 and increasing levels of IL-10. 18 In addition, miR-23a was confirmed to be a downstream target of GAS5 in various diseases, such as non-small cell lung cancer, 19 hepatic fibrosis, 20 and sepsis. 21 However, whether miR-23a is a downstream regulator of GAS in MG needs to be further elucidated.
In this study, we found that GAS5 can regulate the Th17/Treg balance by targeting miR-23a in MG. These discoveries could provide a promising treatment strategy for MG patients.
Materials and methods
Patients and CD4+ T cell collection
Thirty patients with MG were enrolled in this study between February 2017 and July 2018 at The Second Affiliated Hospital of Fujian Medical University. Thirty sex- and age-matched healthy donors with no history of autoimmune disease served as controls. Blood was collected from untreated MG patients to isolate peripheral blood mononuclear cells. The collection and culturing of CD4+ T cells were performed following the protocol previously described. 22 The purity of isolated CD4+ T cells was assessed as >95%. The study protocols were approved by the Ethics Committees of the Second Affiliated Hospital of Fujian Medical University (Quanzhou, China, January 2017) and written informed consent was obtained from all participants.
Cell transfection
A GAS5 overexpression plasmid (pcDNA3.1/GAS5) and its corresponding empty vector negative control (pcDNA3.1), as well as a miR-23a mimic and its negative control (NC mimic), were purchased from GenePharma (Shanghai, China). The fresh CD4+ T cells were cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) in six-well plates at a density of 5 × 105 cells per well. All cells were cultured at 37°C with 5% CO2. At 24 hours after seeding, cells were transfected with 100 nM pcDNA3.1, 100 nM pcDNA3.1/GAS5, 100 nM miR-23a mimic, and 100 nM NC mimic using Lipofectamine 2000 (Invitrogen, Waltham, MA, USA). After 48 hours, the transfected cells were stimulated with phorbol PMA (2 μg/mL) and ionomycin (5 μg/mL), and then collected for subsequent experiments.
Flow cytometry
For Th17 analysis, cells were stained with a fluorescein isothiocyanate (FITC)-labeled anti-CD4 antibody for 30 minutes at 4°C in the dark, fixed and permeabilized with Fix/Perm solution (eBioscience, Hatfield, UK) for 30 minutes at 4°C in the dark, washed twice with ice-cold permeabilization buffer (eBioscience), then stained with 10 µL phycoerythrin (PE)-labeled anti-IL-17A antibody. For Treg analysis, cells were stained with an allophycocyanin (APC)-labeled anti-CD25 antibody and FITC-anti-CD4 for 30 minutes at 4°C. After being washed with 2 mL of cold flow cytometry staining buffer, the cells were fixed and permeabilized with Fix/Perm solution for 30 minutes at 4°C in the dark. The cells were washed with ice-cold permeabilization buffer twice and incubated with 5 µL PE-anti-FoxP3 (eBioscience). All antibodies were purchased from eBioscience. Data were obtained from a FACS Canto II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The viability of the cells was examined by staining with propidium iodide.
Bioinformatic analysis and dual-luciferase reporter assay
The StarBase 2.0 database (http://starbase.sysu.edu.cn/) was used to predict the binding sites between GAS5 and miR-23a. Wild-type and mutant GAS5 sequences were subcloned into the pmirGLO vector to establish the GAS5-WT and GAS5-MUT vectors, respectively. Then, GAS5-WT/MUT and miR-23a/NC mimic were co-transfected into 293T cells using Lipofectamine 2000 (Invitrogen). 293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS at 37°C with 5% CO2. After 48 hours, luciferase activity was assessed using a dual-luciferase reporter assay system (Promega Corporation, Madison, WI, USA).
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) assays
Total RNA was isolated from CD4+ T cells using TRIzol® reagent (Invitrogen) and reverse transcribed into cDNA using ReadyScript® cDNA Synthesis Mix (Sigma-Aldrich, St. Louis, MO, USA). Then, RT-qPCR was performed using a SYBR Green Kit (Applied Biosystems, Waltham, MA, USA) on the ABI 7500 Real-time PCR system (Applied Biosystems). The relative expression levels of GAS5 were normalized to GAPDH and miR-23a levels were normalized to U6 using the 2−ΔΔCq method. 23 The following primer sequences were used: GAS5 forward, 5′-CTTCTGGGCTCAAGTGATCCT-3′ and reverse, 5′-TTGTGCCATGAGACTCCATCAG-3′; miR-23a forward, 5′-TAGCTTATCAGACTGA-3′ and reverse, 5′-CTGGAGCAGCACAGCCAATA-3′; GAPDH forward, 5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse, 5ʹ‑AGTCTTCTGGGTGGCAGTGAT-3′; U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′.
Enzyme-linked immunosorbent assays (ELISAs)
The levels of inflammatory cytokines IL-17 and IL-10 were examined with commercially available ELISA kits (eBioscience) following the manufacturer’s protocols.
Western blot analysis
Total protein was extracted from CD4+ T cells using RIPA buffer (Invitrogen). Protein samples (20 μg) were separated using 10% SDS-PAGE and transferred to a PVDF membrane (EMD Millipore, Burlington, MA, USA). The membranes were blocked with 5% skim milk diluted in 0.1% TBST, and incubated with primary antibodies against RORγt (1:1000, 14-6981-82, eBioscience), Foxp3 (1:1000, ab20034, Abcam, Cambridge, UK), and GAPDH (1:1000, ab8245, Abcam) at 4°C overnight. Next, the membranes were incubated with appropriate secondary antibodies for 1 hour at room temperature. Then, the bands were evaluated with the enhanced chemiluminescence (ECL) Kit (Sigma-Aldrich).
RNA immunoprecipitation (RIP)
The Magna RIP RNA-Binding Protein RIP Kit (Millipore) was used to perform RIP assays. Cells were lysed and incubated with magnetic beads (Millipore) conjugated with anti-Ago2 and IgG antibodies (Millipore). The immunoprecipitated RNA was used for PCR analysis following the previously described methods.
Statistical analysis
All experiments were performed in triplicate. Data are represented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test was used to evaluate the normality of the distribution. The normally distributed data were analyzed by Student's t-tests, while non-normally distributed data were evaluated by Kruskal–Wallis tests. Comparisons among multiple groups were performed by one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
GAS5 and miR-23a expression in CD4+ T cells of MG patients
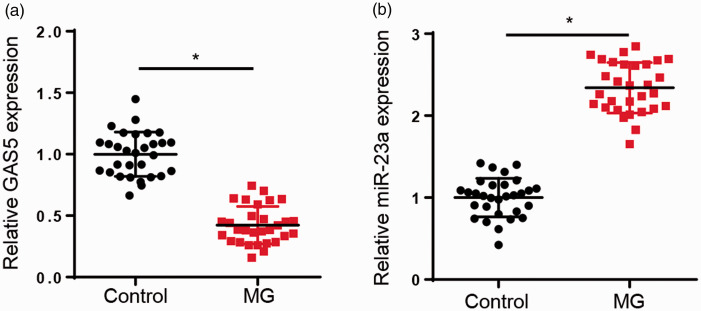
The clinical characteristics of MG patients are presented in Table 1. Initially, we explored the expression levels of GAS5 and miR-23a in CD4+ T cells from healthy controls and MG patients. RT-qPCR analysis showed that GAS5 expression levels were reduced, while miR-23a levels were enhanced, in the CD4+ T cells of the MG group relative to the controls (P < 0.05, Figure 1a and b).
Table 1.
Characteristics of patients with myasthenia gravis (MG).
| Patient ID | Age | Degree of thymic hyperplasia | MGFA score at thymectomy | Corticoid treatment | Cholinesterase inhibitors | Anti-AChR titer (nmol/L) |
|---|---|---|---|---|---|---|
| MG1 | 33 | NS | NS | No | No | 2.48 |
| MG2 | 27 | Low | II b | No | Yes | 1.67 |
| MG3 | 39 | Low | II b | No | Yes | 1.82 |
| MG4 | 19 | High | II b | No | Yes | >100 |
| MG5 | 35 | High | NS | No | Yes | 23.3 |
| MG6 | 25 | Low | II b | No | Yes | 2.31 |
| MG7 | 26 | High | II b | No | Yes | 5.7 |
| MG8 | 34 | Low | II b | No | Yes | 1.04 |
| MG9 | 32 | NS | III b | No | Yes | NS |
| MG10 | 37 | High | NS | No | Yes | 5.82 |
| MG11 | 27 | Low | II b | No | Yes | 1.64 |
| MG12 | 33 | Low | NS | No | Yes | 12.3 |
| MG13 | 28 | Low | NS | No | Yes | 117.8 |
| MG14 | 19 | NS | NS | No | No | >100 |
| MG15 | 33 | Low | NS | No | Yes | 1.82 |
| MG16 | 21 | High | NS | No | Yes | 29.1 |
| MG17 | 36 | NS | NS | No | No | >100 |
| MG18 | 32 | Low | NS | No | Yes | 1.2 |
| MG19 | 31 | Low | NS | No | No | 4.8 |
| MG20 | 20 | Low | NS | No | Yes | 2.2 |
| MG21 | 29 | Low | NS | No | Yes | 117.9 |
| MG22 | 31 | High | III b | No | Yes | 65.8 |
| MG23 | 38 | Low | II a | No | Yes | >100 |
| MG24 | 20 | High | NS | No | Yes | 10.9 |
| MG25 | 35 | High | NS | No | Yes | 8.1 |
| MG26 | 27 | Low | NS | No | Yes | 12.5 |
| MG27 | 32 | Low | NS | No | Yes | 7.1 |
| MG28 | 36 | Low | II b | No | Yes | 48.1 |
| MG29 | 23 | Low | II a | No | Yes | 1.6 |
| MG30 | 26 | NS | NS | No | No | >100 |
MGFA, Myasthenia Gravis Foundation of America; AChR, acetylcholine receptor; NS, not specified.
Figure 1.
Expression levels of growth arrest specific 5 (GAS5) and miR-23a in CD4+ T cells of myasthenia gravis (MG) patients. (a and b) The expression levels of GAS5 and miR-23a were examined in CD4+ T cells of MG patients (n = 30) and healthy controls (n = 30) by RT-qPCR. *P < 0.05.
Upregulation of GAS5 inhibits Th17 cell differentiation
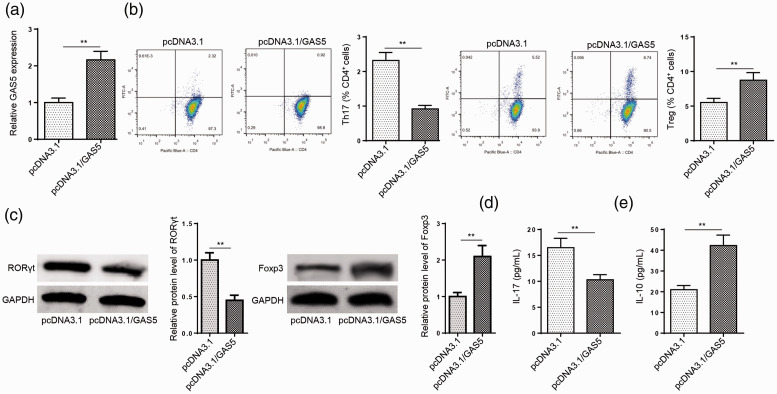
To explore whether GAS5 is involved in Th17 cell differentiation in MG, CD4+ T cells were transfected with pcDNA3.1 and pcDNA3.1/GAS5. As shown in Figure 2a, GAS5 expression was significantly (P < 0.01) upregulated in CD4+ T cells transfected with the GAS5 overexpression plasmid. Flow cytometry analysis indicated that the addition of GAS5 reduced the Th17 cell percentage in CD4+ T cells, but increased the proportion of Treg cells (P < 0.01, Figure 2b). Moreover, western blot analyses indicated that GAS5 overexpression inhibited Th17-associated molecule RORγt protein levels, while the protein expression of Treg-specific Foxp3 was promoted (P < 0.01, Figure 2c). In addition, ELISA results suggested that Th17-associated cytokine IL-17 production was decreased following GAS5 overexpression (P < 0.01, Figure 2d). However, Treg-associated cytokine IL-10 production was enhanced (P < 0.01, Figure 2e). These data indicate that upregulating GAS5 expression can reduce the proportion of Th17 cells in the CD4+ T cells of MG patients.
Figure 2.
Growth arrest specific 5 (GAS5) overexpression inhibits Th17 cell differentiation. (a) RT-qPCR analysis showing GAS5 expression levels in CD4+ T cells transfected with pcDNA3.1 or pcDNA3.1/GAS5. (b) Flow cytometry was used to detect the percentage of Th17 cells and Treg cells in CD4+ T cells transfected with pcDNA3.1 or pcDNA3.1/GAS5. (c) The protein levels of RAR-related orphan receptor γt (RORγt) and forkhead box P3 (Foxp3) were detected by western blot in CD4+ T cells transfected with pcDNA3.1 or pcDNA3.1/GAS5. (d and e) The protein levels of interleukin (IL)-17 and IL-10 in supernatants were measured by enzyme-linked immunosorbent assays (ELISAs). n = 3; **P < 0.01.
miR-23a is a downstream target of GAS5
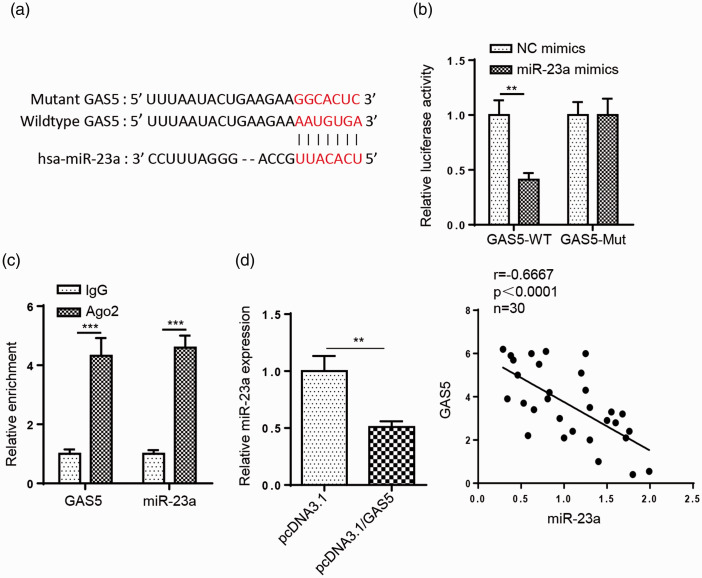
Subsequently, we verified the miR-23a binding sites within the GAS5 sequence using StarBase (Figure 3a). Luciferase reporter assays revealed that miR-23a transfection attenuated the luciferase activity of GAS5-WT, but not that of GAS5-MUT (P < 0.01, Figure 3b). Additionally, RIP assay results demonstrated that GAS5 and miR-23a expression were enriched in the anti-Ago2 group (P < 0.001, Figure 3c). Analysis with RT-qPCR uncovered that miR-23a expression was significantly decreased following GAS5 overexpression (P < 0.01, Figure 3d). Moreover, miR-23a expression levels were negatively correlated with GAS5 expression levels in MG (Figure 3e). Overall, the abovementioned data suggest that GAS5 negatively regulates miR-23a expression by direct interaction.
Figure 3.
miR-23a is a downstream target of growth arrest specific 5 (GAS5). (a) The putative binding sequences of GAS5 and miR-23a was predicted using StarBase. (b) Luciferase reporter assays showed the luciferase activity of wild-type or mutant GAS5 in 293T cells transfected with miR-23a or negative control (NC) mimic and miR-23a. (c) RNA immunoprecipitation (RIP) assays were used to analyze the interaction between GAS5 and miR-23a. (d) qRT-PCR showed the expression levels of miR-23a in CD4+ T cells transfected with pcDNA3.1 or pcDNA3.1/GAS5. (e) Pearson correlation analysis showed the correlation between GAS5 and miR-23a in myasthenia gravis (MG). n = 3; **P < 0.01, ***P < 0.001.
GAS5 attenuates Th17 differentiation by reducing miR-23a expression
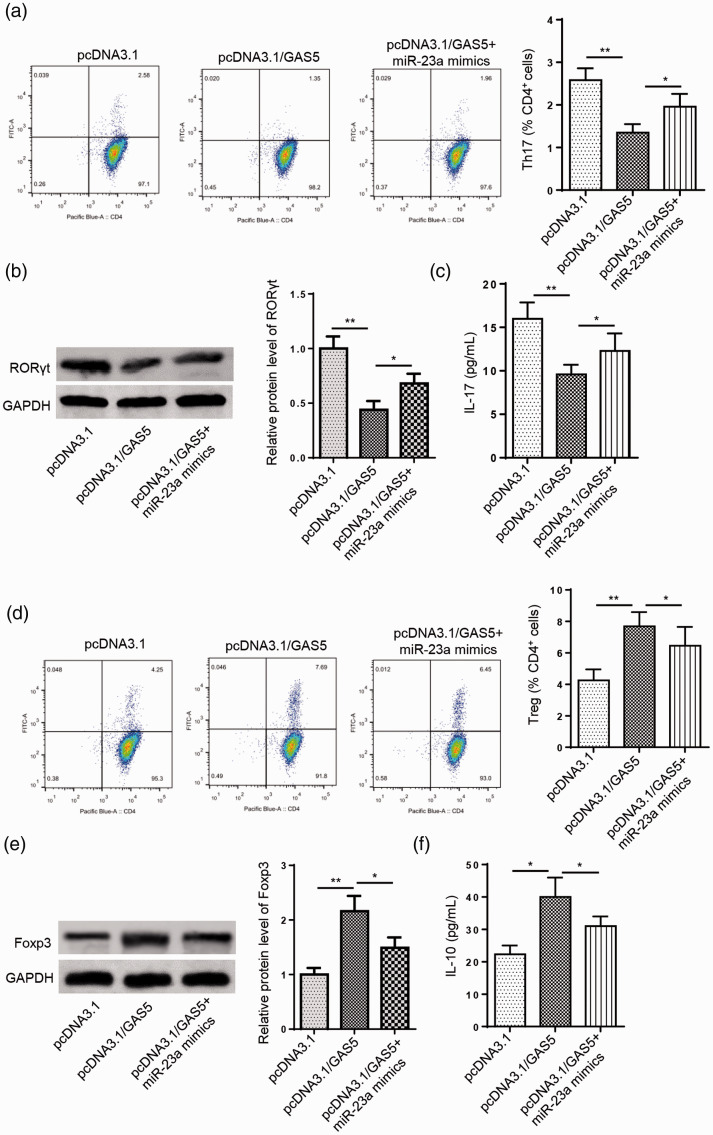
To investigate whether GAS5 can regulate the Th17/Treg balance through miR-23a, CD4+ T cells were transfected with pcDNA3.1, pcDNA3.1/GAS5, or pcDNA3.1/GAS5 + miR-23a mimic. Flow cytometry results suggested that the suppressive effect of GAS5 overexpression on the percentage of Th17 cells was rescued by miR-23a mimic transfection in CD4+ T cells (*P < 0.05, **P < 0.01, Figure 4a). Moreover, the upregulation of GAS5 decreased the protein expression levels of RORγt and IL-17, while co-transfection of pcDNA3.1/GAS5 and the miR-23a mimic reversed these effects (*P < 0.05, **P < 0.01, Figure 4b and c). On the contrary, the percentage of Treg cells was increased by GAS5 overexpression, while miR-23a mimic transfection abrogated this effect (*P < 0.05, **P < 0.01, Figure 4d). Additionally, the miR-23a mimic neutralized the promoting effects of GAS5 overexpression on protein levels of Foxp3 and IL-17 (*P < 0.05, **P < 0.01, Figure 4e and f). Collectively, these results reveal that GAS5 inhibits Th17 differentiation by targeting miR-23a.
Figure 4.
Growth arrest specific 5 (GAS5) attenuates Th17 differentiation by reducing miR-23a expression. (a) Flow cytometry was used to detect the percentage of Th17 cells in CD4+ T cells transfected with pcDNA3.1, pcDNA3.1/GAS5, or pcDNA3.1/GAS5+miR-23a mimic. (b and c) Western blot analysis and enzyme-linked immunosorbent assays (ELISAs) showed the protein expression levels of RAR-related orphan receptor γt (RORγt) and interleukin (IL)-17 in CD4+ T cells transfected with pcDNA3.1, pcDNA3.1/GAS5, or pcDNA3.1/GAS5+miR-23a mimic. (d) Flow cytometry was used to detect the percentage of Treg cells in CD4+ T cells transfected with pcDNA3.1, pcDNA3.1/GAS5, or pcDNA3.1/GAS5+miR-23a mimic. (e and f) Western blot analysis and ELISAs showed the protein expression levels of forkhead box P3 (Foxp3) and IL-10 in CD4+ T cells transfected with pcDNA3.1, pcDNA3.1/GAS5, or pcDNA3.1/GAS5+miR-23a mimic. n = 3; *P < 0.05, **P < 0.01.
Discussion
With the development of second-generation sequencing methods, a large number of genes or non-protein-coding transcripts, such as lncRNAs and miRNAs, have been identified in MG that could possibly serve as biomarkers to assist with diagnosing or treating this disease.24,25 Here, we investigated the molecular mechanism of GAS5 in MG pathogenesis.
Accumulating studies have demonstrated that lncRNAs participate in various physiological processes, such as cell viability, cell differentiation, and immune responses.26,27 GAS5 has been widely reported to participate in the development of diverse diseases. For example, GAS5 can suppress the progression of diabetic nephropathy via the miR-221/sirtuin 1 (SIRT1) axis. 28 Knockdown of GAS5 inhibited atherosclerosis progression by reducing enhancer of zeste homolog 2 (EZH2)-mediated transcription of ATP binding cassette subfamily A member 1 (ABCA1). 29 In addition, Chi et al. revealed that GAS5 can regulate the Th17/Treg balance by sponging miR-217 and regulating signal transducer and activator of transcription 5 (STAT5) expression in childhood pneumonia. 30 GAS5 inhibited Th17 differentiation and alleviated immune thrombocytopenia by decreasing STAT3 levels. 31 In the current study, we showed that GAS5 is downregulated in CD4+ T cells of MG. Moreover, GAS5 overexpression restrained the Th17 cell percentage in CD4+ cells and decreased Th17-related RORγ and IL-17 protein levels.
Previous studies have indicated that certain miRNAs participate in the regulation of the Th17/Treg balance in diverse diseases. Li et al. demonstrated that miR-195 could maintain the Th17/Treg balance by decreasing CD40 expression in non-alcoholic fatty liver disease. 32 You et al. revealed that miR-10a can mediate the Th17/Treg balance and improve renal injury by targeting regenerating family member 3 alpha (REG3A) in lupus nephritis. 33 Wang et al. showed that miR-155 can modulate the Th17/Treg ratio by regulating suppressor of cytokine signaling 1 (SOCS1) in acute pancreatitis. 34 In addition, lncRNAs can act as ceRNAs by sequestering specific miRNAs, then modulate the Treg/Th17 balance in diverse diseases. For example, lncRNA maternally expressed 3 (MEG3) could function as a ceRNA to regulate the Treg/Th17 balance in patients with asthma via the miR-17/RORγt axis. 22 LncRNA H19 suppressed Th17 cell differentiation to relieve endometriosis by acting as a ceRNA of miR-342-3p to regulate immediate early response 3 (IER3) expression. 35 In addition, GAS5 could also function as a ceRNA to sponge miR-21, which promoted Th17 cell differentiation. 36 Therefore, we screened the downstream targets of GAS5. In our study, miR-23a was confirmed as a downstream gene of GAS5 that is negatively regulated by this lncRNA. Moreover, upregulating GAS5 restrained Th17 differentiation in CD4+ T cells, while miR-23a supplementation reversed this effect. These results indicate that GAS5 can suppress Th17 differentiation by sponging miR-23a.
In conclusion, our study illustrates that GAS5 expression is decreased in MG and can inhibit Th17 differentiation by directly targeting miR-23a. These findings may provide novel insights into the pathogenesis of this disease.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was supported by Second Affiliated Hospital of Fujian Medical University.
ORCID iD: Yingying Xu https://orcid.org/0000-0001-6957-3825
References
- 1.Mantegazza R, Cavalcante P. Diagnosis and treatment of myasthenia gravis. Curr Opin Rheumatol 2019; 31: 623–633. [DOI] [PubMed] [Google Scholar]
- 2.Gradolatto A, Nazzal D, Truffault F, et al. Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: roles of IL-17 and TNF-α. J Autoimmun 2014; 52: 53–63. [DOI] [PubMed] [Google Scholar]
- 3.Masuda M, Matsumoto M, Tanaka S, et al. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol 2010; 225: 123–131. [DOI] [PubMed] [Google Scholar]
- 4.Diller ML, Kudchadkar RR, Delman KA, et al. Balancing Inflammation: The Link between Th17 and Regulatory T Cells. Mediators Inflamm 2016; 2016: 6309219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007; 8: 345–350. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev 2006; 212: 8–27. [DOI] [PubMed] [Google Scholar]
- 7.Gao R, Fang C, Xu J, et al. LncRNA CACS15 contributes to oxaliplatin resistance in colorectal cancer by positively regulating ABCC1 through sponging miR-145. Arch Biochem Biophys 2019; 663: 183–191. [DOI] [PubMed] [Google Scholar]
- 8.Ma X, Zhou J, Liu J, et al. LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression. Onco Targets Ther 2018; 11: 8399–8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong X, Wang J, Cao Y, et al. The long noncoding RNA MALAT-1 functions as a competing endogenous RNA to regulate MSL2 expression by sponging miR-338-3p in myasthenia gravis. J Cell Biochem 2019; 120: 5542–5550. [DOI] [PubMed] [Google Scholar]
- 10.Luo M, Liu X, Meng H, et al. IFNA-AS1 regulates CD4(+) T cell activation in myasthenia gravis though HLA-DRB1. Clin Immunol 2017; 183: 121–131. [DOI] [PubMed] [Google Scholar]
- 11.Hu B, Niu L, Jiang Z, et al. LncRNA XLOC_003810 promotes T cell activation and inhibits PD-1/PD-L1 expression in patients with myasthenia gravis-related thymoma. Scand J Immunol 2020; 92: e12886. [DOI] [PubMed] [Google Scholar]
- 12.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta 2013; 1832: 1613–1623. [DOI] [PubMed] [Google Scholar]
- 13.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988; 54: 787–793. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q, Deng Y, Li C, et al. LncRNA GAS5 suppresses CD4(+) T cell activation by upregulating E4BP4 via inhibiting miR-92a-3p in systemic lupus erythematosus. Immunol Lett 2020; 227: 41–47. [DOI] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136: 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight?. Nat Rev Genet 2008; 9: 102–114. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Luo M, Meng H, et al. MiR-181a regulates CD4(+) T cell activation and differentiation by targeting IL-2 in the pathogenesis of myasthenia gravis. Eur J Immunol 2019. [Google Scholar]
- 18.Ao W, Tian C, He X, et al. Upregulation of miR150-5p in generalized myasthenia gravis patients is associated with decreased serum levels of IL-17 and increased serum levels of IL-10. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2020; 164: 57–62. [DOI] [PubMed] [Google Scholar]
- 19.Mei Y, Si J, Wang Y, et al. Long Noncoding RNA GAS5 Suppresses Tumorigenesis by Inhibiting miR-23a Expression in Non-Small Cell Lung Cancer. Oncol Res 2017; 25: 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Z, Li S, Wang X, et al. lncRNA GAS5 restrains CCl(4)-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol 2019; 316: G539–G550. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z, Huang D. lncRNA GAS5‑mediated miR‑23a‑3p promotes inflammation and cell apoptosis by targeting TLR4 in a cell model of sepsis. Mol Med Rep 2021; 24: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu YY, Wu Y, Lin MJ, et al. LncRNA-MEG3 functions as a competing endogenous RNA to regulate Treg/Th17 balance in patients with asthma by targeting microRNA-17/RORgammat. Biomed Pharmacother 2019; 111: 386–394. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Zheng S, Xin N, et al. Identification of novel MicroRNA signatures linked to experimental autoimmune myasthenia gravis pathogenesis: down-regulated miR-145 promotes pathogenetic Th17 cell response. J Neuroimmune Pharmacol 2013; 8: 1287–1302. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta M, Wang BD, Lee NH, et al. MicroRNA and mRNA expression associated with ectopic germinal centers in thymus of myasthenia gravis. PLoS One 2018; 13: e0205464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Xu X, Wang D, et al. Hypermethylation-mediated downregulation of long non-coding RNA MEG3 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells and promotes pediatric aplastic anemia. Int Immunopharmacol 2021; 93: 107292. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Shen X, Ma J, et al. LncRNA NEAT1 participates in inflammatory response in macrophages infected by mycobacterium tuberculosis through targeted regulation of miR-377-3p. Microb Pathog 2021; 150: 104674. [DOI] [PubMed] [Google Scholar]
- 28.Ge X, Xu B, Xu W, et al. Long noncoding RNA GAS5 inhibits cell proliferation and fibrosis in diabetic nephropathy by sponging miR-221 and modulating SIRT1 expression. Aging (Albany NY) 2019; 11: 8745–8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng XD, Yao HH, Wang LM, et al. Knockdown of GAS5 Inhibits Atherosclerosis Progression via Reducing EZH2-Mediated ABCA1 Transcription in ApoE(-/-) Mice. Mol Ther Nucleic Acids 2020; 19: 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chi X, Guo Y, Zhang L, et al. Long non-coding RNA GAS5 regulates Th17/Treg imbalance in childhood pneumonia by targeting miR-217/STAT5. Cell Immunol 2021; 364: 104357. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Tian J, Lu J, et al. LncRNA GAS5 inhibits Th17 differentiation and alleviates immune thrombocytopenia via promoting the ubiquitination of STAT3. Int Immunopharmacol 2020; 80: 106127. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Jiang HT, Han LB, et al. MiR-195 regulates CD40 to maintain Th17/Treg balance in rats with non-alcoholic fatty liver disease. Biomed Pharmacother 2020; 124: 109930. [DOI] [PubMed] [Google Scholar]
- 33.You G, Cao H, Yan L, et al. MicroRNA-10a-3p mediates Th17/Treg cell balance and improves renal injury by inhibiting REG3A in lupus nephritis. Int Immunopharmacol 2020; 88: 106891. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Tang M, Zong P, et al. MiRNA-155 Regulates the Th17/Treg Ratio by Targeting SOCS1 in Severe Acute Pancreatitis. Front Physiol 2018; 9: 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Liu L, Zhong Y, et al. LncRNA H19 over-expression inhibited Th17 cell differentiation to relieve endometriosis through miR-342-3p/IER3 pathway. Cell Biosci 2019; 9: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Wan X, Ruan Q. The MicroRNA-21 in Autoimmune Diseases. Int J Mol Sci 2016; 17: 864. [DOI] [PMC free article] [PubMed] [Google Scholar]