Fish endures prolonged fasting periods during migration, while costs of swimming increase in warming environments, which restrains energy budgets. Here, critical and sustained swimming capacities along with metabolic rates and haematological parameters were assessed in Atlantic salmon before and after 4 weeks of fasting at midrange and elevated suboptimal temperatures.
Keywords: tail beat frequency, sustained aerobic swimming, starvation, migration, critical swimming speed, cost of transport
Abstract
Predicted future warming of aquatic environments could make fish vulnerable to naturally occurring fasting periods during migration between feeding and spawning sites, as these endeavours become energetically more expensive. In this study, Atlantic salmon (Salmo salar) acclimated to midrange (9°C) or elevated suboptimal (18°C) temperatures were subjected to critical (Ucrit) and sustained (4 hours at 80% Ucrit) swimming trials before and after 4 weeks of fasting. Fasting caused weight losses of 7.3% and 8.3% at 9°C and 18°C, respectively. The Ucrit was unaffected by fasting, but higher at 18°C. Fatigue was associated with higher plasma cortisol, osmolality, Na+ and Cl− at 18°C, and ionic disturbances were higher in fasted fish. All fish completed the sustained swim trials while maintaining constant oxygen uptake rates (ṀO2), indicating strictly aerobic swimming efforts. At low swimming speeds ṀO2 was downregulated in fasted fish by 23.8% and 15.6% at 9°C and 18°C, respectively, likely as an adaptation to preserve resources. However, at higher speeds ṀO2 became similar to fed fish showing that maximum metabolic rates were maintained. The changes in ṀO2 lowered costs of transport and optimal swimming speeds in fasted fish at both temperatures, but these energetic alterations were smaller at 18°C while routine ṀO2 was 57% higher than at 9°C. As such, this study shows that Atlantic salmon maintain both glycolytic and aerobic swimming capacities after extended fasting, even at elevated suboptimal temperatures, and adaptive metabolic downregulation provides increased swimming efficiency in fasted fish. Although, improved swimming energetics were smaller when fasting at the higher temperature while metabolism becomes elevated. This could affect migration success in warming climates, especially when considering interactions with other costly activities such as coping with parasites obtained when passing aquaculture sites during seaward travel or gonad development while being voluntarily anorexic during upriver travel to spawning grounds.
Introduction
All physiological functions have an energetic cost, and animals therefore require food to obtain energy to offset these costs if they are to forage, grow, migrate, reproduce and survive (Porter and Gates, 1969). How often animals need to eat varies between species group. For instance, fish and other ectotherms may endure weeks or months of food deprivation without suffering detrimental effects owing to lower metabolic demands than in endotherms such as birds and mammals (McCue, 2010; Wang et al., 2006).
In nature, many species of fish will periodically encounter extended fasting periods because of seasonal fluctuations in food supplies in their habitat, migratory behaviours or during reproduction events (Green and Farwell, 1971; Miller et al., 2009; Van Ginneken et al., 2005). To cope during periods of food shortage, fish are able to downregulate metabolic rates to preserve resources (Fu et al., 2005; Hvas et al., 2020; Mehner and Wieser, 1994), which on the biochemical level is facilitated by beneficial changes in gene expressions, enzyme activities and mitochondrial functions in various organs (Bermejo-Nogales et al., 2015; Cassidy et al., 2016; Méndez and Wieser, 1993; Salin et al., 2018). Furthermore, following extended periods of food deprivation, such physiological adjustments can be reversed and stunted growth can be compensated as fish have flexible and indeterminate growth trajectories (Ali et al., 2003; Hvas et al., 2022; Morgan and Metcalfe, 2001).
Although fish are well adapted to temporal variability in feeding opportunities, long-term starvation will eventually jeopardize survival as physiological functions become compromised. The impact of extended fasting periods will depend on species, initial body condition and various environmental factors. Water temperature is a particularly important environmental factor to consider, as increasing temperatures drastically elevates the metabolic demands in fish (Brett, 1971; Eliason and Farrell, 2016). Warming of aquatic habitats from anthropogenic climate change could therefore make species of fish more vulnerable to naturally occurring extended fasting periods as energy stores gets depleted sooner (Morgan et al., 2001).
The Atlantic salmon (Salmo salar) is a relevant species to study with regards to the interactions between fasting and temperature on energetic costs and physiological capacities. It is an anadromous migratory species that endures prolonged fasting periods at various phases in its life cycle (Hendry and Beall, 2004; Kadri et al., 1995; Thorstad et al., 2012). Furthermore, it also encounters variable thermal environments ranging from 0–3°C in its northern distribution (Lacroix, 2013; Reddin, 1985) to presumably above 20°C occasionally during summer river migrations in its southern limits (Valiente et al., 2011). Although, for the majority of its life during the marine phase, it is found in waters below 10°C (Jensen et al., 2014; Lacroix, 2013; Reddin, 1985). Growth of Atlantic salmon post-smolts is maximized at 13°C (Handeland et al., 2003; Handeland et al., 2008) and is reduced above 18°C owing to lower appetite and feed conversion efficiency (Hevrøy et al., 2015; Kullgren et al., 2013; Wade et al., 2019). Behaviorally, Atlantic salmon will actively avoid environments above 16°C (Johansson et al., 2009; Lacroix, 2013) and long-term survival is not possible at chronic temperatures above 22°C (Gamperl et al., 2020; Hvas et al., 2017).
Populations of Atlantic salmon have long been declining as a consequence of habitat destruction and overfishing (Dadswell et al., 2021; Pardo et al., 2021; Parrish et al., 1998), with more recent threats coming from sea cage aquaculture that spreads parasitic copepodids onto wild seaward migrating fish (Johnsen et al., 2020; Krkošek et al., 2007). These parasites exert a substantial increased energetic burden on infected fish (Hvas and Bui, 2022), coinciding with observed reductions in body conditions of salmon returning from sea (Susdorf et al., 2018). Such energetic disadvantages will presumably be exacerbated by increasing temperatures and make completion of life cycles more difficult.
Energetics of fish are most often assessed from oxygen uptake rates (ṀO2) as a proxy of the aerobic metabolic rate (Clark et al., 2013; Nelson, 2016). Using swim tunnel respirometers, the ṀO2 can be measured during swimming at defined activity levels together with maximum swimming capacities (Brett, 1964; Steffensen et al., 1984). From ṀO2 at known swimming speeds the cost of transport (CoT) can be derived, and the optimal swimming speed (Uopt) can be defined as the speed that minimizes the CoT (Claireaux et al., 2006). For general cruising in search of food and migration specifically, fish should swim at their Uopt to maximize energy efficiency, which also seems to be the case when monitoring fish movements in the wild (Drenner et al., 2012; Weihs, 1973).
When assessing maximum swimming capacities, protocols most commonly focus on the highest attainable speed by incrementally increasing water currents until fatigue is reached. These are known as critical swim speed (Ucrit) tests, which involves utilization of both aerobic and anaerobic metabolism (Farrell, 2007; Wilson and Egginton, 1994). Less commonly, protocols may also focus on strictly aerobic fueled metabolism in sustained swimming trials where fish are tested at a constant speed for several hours (Beamish, 1978; Cotterell and Wardle, 2004; Hvas et al., 2021a). In the case of fed Atlantic salmon within midrange temperatures, the transition from sustained aerobic swimming to a mixture of aerobic and anaerobic swimming is at 80–85% of the Ucrit, while the Uopt for minimum CoT tends to be 60–65% of the Ucrit (Beddow and McKinley, 1999; Hvas et al., 2021a; Hvas and Oppedal, 2017). Together, the Ucrit, the Uopt and sustained swimming capacity provides valuable insights into the athleticism and energetics of fish that are reflective of environmental adaptations (Beamish, 1978; Claireaux et al., 2006; Plaut, 2001).
The physiological responses to extended fasting periods in Atlantic salmon have so far mainly been studied at the lower and mid ranges of their thermal niche. For instance, at 12°C Atlantic salmon gradually downregulate resting metabolic rates over a 4-week fasting period as an adaptive response to save energy, while still maintaining the ability to adequately respond and recover from acute stress (Hvas et al., 2020, 2021b). Additionally, weight loss and changes in body composition during up to 12 weeks of fasting have been studied in winter conditions, showing a remarkable resilience to food deprivation at low temperatures (Einen et al., 1998; Lie and Huse, 1992).
Prolonged fasting may influence aerobic and anaerobic swimming properties differently. Fast white muscle fibres tend to be more affected than slow red muscle fibres in a range of fish species investigated with regards to fibre size, protein synthesis, glycogen levels and capillary supply, and effects are larger on glycolytic than on oxidative functionality regardless of fibre type (Beardall and Johnston, 1983; Johnston and Goldspink, 1973; Loughna and Goldspink, 1984; Martínez et al., 2003; Scarabello et al., 1991). As such, food deprivation should first reduce high-speed short-term swimming capacity (e.g. Ucrit), while sustained aerobic swimming capacities will remain preserved for longer periods. In Atlantic cod (Gadus morhua) fasted for 12 weeks, the Ucrit was indeed reduced due to impairment of anaerobic work (Martínez et al., 2004). Although the Ucrit in Atlantic salmon remained unaffected after 4 weeks of fasting at 12°C (Hvas et al., 2021b), similar resilience to food deprivation is presumably not possible in warmer, less optimal environments. Moreover, it is unknown to what extent Atlantic salmon are able to downregulate metabolic demands at higher temperatures during fasting periods and how sustained swimming, CoT and Uopt are affected. Food-deprived fish may therefore have less capacity for long-term high-intensity swimming within their normal aerobic limit when substrate depletion is imminent.
The purpose of the present study was to compare the impact of a 4-week fasting period at a normal midrange (9°C) and an elevated suboptimal (18°C) temperature with regards to swimming energetics and capacities in Atlantic salmon. Fed and fasted fish at both temperatures were subjected to a typical Ucrit trial as well as a 4-hour sustained swimming trial at 80% of the Ucrit. It was hypothesized that food-deprived Atlantic salmon would be more impaired at the higher temperature because accelerated metabolic rates would deplete resources more quickly. Specifically, it was predicted that fasting at 18°C would reduce Ucrit and that fish would struggle to complete the sustained swimming trial, while fasted fish at 9°C would maintain their swimming capacities as well as benefitting from an improved CoT. Finally, analyses of haematological parameters in fatigued fish were done to assess potential differences in osmotic, endocrine and metabolic disturbances, for instance owing to changes in the functionality of anaerobic muscle fibres in fasted fish.
Materials and methods
Fish husbandry
Atlantic salmon post-smolts produced on site at the Matre Research Station, Institute of Marine Research, Norway, were allocated in six indoor circular holding tanks (diameter, 3 m; volume, 5.3 m3). The holding tanks were supplied with aerated, filtered and UV-C-treated full strength seawater of 34 ppt with a continuous inflow of 120 l min−1, which ensured constant oxygen levels above 85% saturation and prevented accumulation of waste products. Three tanks were maintained at 9°C and the other three tanks at 18°C. The temperature was controlled by automatic mixing of ambient and heated water supplies in large header tanks prior to reaching the holding tanks. The fish were subjected to a simulated natural photoperiod and fed commercial feed in excess each day via automated feeders (pellet size, 4.5 mm; Skretting, Norway). Before starting the experimental trials, the fish had been acclimating in these conditions for approximately 1 month.
This study was made between September and December 2021 following ethical approval by The Norwegian Food Safety Authorities under permit number 254966 for use of animals in scientific research.
Swim tunnel setup
Critical and sustained swim trials were performed using a large Brett-type swim tunnel respirometer that has been described in detail previously (Hvas et al., 2017; Remen et al., 2016). The key dimensions of this system were a 248-cm-long cylindrical swim section with an internal diameter of 36 cm and a total water volume of 1905 l. A camera was fixated behind the rear grid downstream of the swim section so that the fish being tested could be observed remotely. An oxygen sensor (RINKO ARO-FT, JFE Advanced, Japan) was attached next to the camera and was setup via computer software to log oxygen concentrations in 2-second intervals (MiniSoft SD200W, SAIV Environmental Sensors & Systems, Norway). In the downstream end of the swim section the top lid could be removed for access when fish had to be transferred into or from the tunnel. To minimize turbulence and achieve approximately laminar flow conditions, a resting chamber followed by a flow straightener with honeycomb-shaped cells (5 mm in diameter) and a reduction cone was situated upstream of the swim section. Water current speeds were generated with a motor-driven propeller (Flygt 4630, 11° propeller blade, Xylem Water Solutions, Norway) opposite of the swim section, and a flow metre (Höntzsch Flow Measuring Technology, Germany) was used to confirm that desired current speeds were obtained with a given motor output. A large pipe connected to the same header tanks as used for the holding tanks supplied water into the tunnel via an inlet behind the propeller section. Here, a steady open flow through the tunnel ensured stable temperatures and normoxic conditions. However, for measurements of ṀO2 this water supply could be closed off periodically followed by subsequent rapid flushing to re-establish oxygen levels.
Experimental design and protocols
In the first part of the experiment, Ucrit and sustained swim trials were performed on fed fish acclimated to either 9°C or 18°C. After completion of these trials, feeding was stopped, and the fish were fasted in their holding tanks for 4 weeks. In the second part of the experiment, after the 4-week fasting period, Ucrit and sustained swim trials were performed again at both temperatures.
Prior to each Ucrit trial, eight fish were netted from an appropriate holding tank in the afternoon and quickly transferred to the swim tunnel that was located in the same room. The fish were then allowed to acclimate inside the swim tunnel overnight at a low current speed of 15 cm s−1. The following morning the Ucrit trial started and consisted of a stepwise increase in current speed of 15 cm s−1 every 30 minutes. At high speeds the fish would eventually struggle to maintain swimming positions and fall back on the rear grid. Fatigue was then defined as when a fish was unable to resume swimming even after tactile encouragement by the experimenter’s hand. At this point, time was noted, and the fish was removed and euthanized with a blow to the head. Immediately after, a blood sample was drawn via caudal puncture with a heparinized syringe and stored on ice momentarily until further processing. The weight and fork length was then recorded. The Ucrit trial was continued until all eight fish had reached fatigue.
To measure ṀO2 in response to increasing swimming speeds in the Ucrit trials, the water inflow was closed off for 20 minutes during each speed interval and flushed for the remaining 10 minutes, which ensured oxygen saturations above 85% throughout the trial. Closed measurement periods began 5 minutes into a new speed increment to provide some time for the fish to adjust swimming efforts and respiratory requirements, and thus ended 5 minutes prior to the onset of the next speed increment. Once the first fish had been removed due to fatigue, ṀO2 was no longer monitored on the remaining fish. All ṀO2 measurements thereby represented an average value of eight fish at defined activity levels (Hvas and Oppedal, 2019). Background respiration was not corrected as it was found to be undetectable at different temperatures in this large setup (Hvas et al., 2017). Additionally, the tail beat frequency was measured in four random fish at each current speed by using camera observations to count the time required to perform 50 tail beats. Three replicate Ucrit trials were performed at each temperature on both fed and fasted fish meaning that a total of 24 fish were tested per Ucrit treatment group.
The sustained swim trials were performed after the associated Ucrit trials had been completed at a specific temperature and feeding status on novel fish. The Ucrit trials needed to be conducted prior to sustained swimming trials in order to determine the sustained swimming test speed, which was defined as 80% of the mean Ucrit from the same cohort treatment. Similar to the Ucrit trials, eight fish were moved to the tunnel in the afternoon to acclimate overnight at 15 cm s−1. The following morning the sustained swim trial started by increasing current speeds every 5 minutes by 15 cm s−1 until 80% Ucrit had been reached. This speed was then maintained for 4 hours. If a fish became fatigued, it was removed and time was noted, while fish that did not become fatigued after 4 hours were noted to have completed the test. The fish were then euthanized whereafter weight and fork length were recorded. During the sustained swim trials, ṀO2 was measured in 30-minute intervals using 20-minute closed periods followed by 10 minutes of flushing. Furthermore, the tail beat frequency was measured every 30 minutes on four random fish, as in the Ucrit trials. Three replicate sustained swim trials were performed at each temperature on both fed and fasted fish.
After the Ucrit trials, blood sampled from fatigued fish were centrifuged in Eppendorf tubes at 6000 g for 5 minutes. The plasma supernatant was then transferred to new Eppendorf tubes and stored at −80°C until later analyses. Once all swim trials had been completed, plasma osmolality was measured with freeze point determination in 20 μl subsamples using a Fiske 210 Micro-Sample Osmometer (Advanced Instruments). The concentration of plasma cortisol was measured in 20 μl subsamples using an ELISA assay kit (standard range: 20–800 ng ml−1; IBL International GmbH). Finally, the concentration of plasma lactate, Na+ and Cl− were measured in 65 μl subsamples with an ABL90 FLEX blood gas analyzer (Radiometer).
Data analyses
The Ucrit was calculated, according to Brett (1964), as follows:
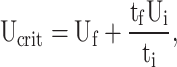 |
where Uf is the highest completed swimming speed, Ui is the increment between speeds (15 cm s−1), tf is time endured at the last speed before reaching fatigue and ti is the time interval between speeds (30 minutes). Since the cross-sectional area of the swim section being large relative to the size of the fish, solid blocking effects were not corrected for in the reported Ucrit values, as this effect would be minimal (Bell and Terhune, 1970; Plaut, 2001).
The ṀO2 was calculated for each measurement period in the critical and sustained swim trials from the decrease in dissolved oxygen over time as follows:
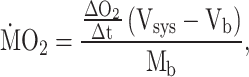 |
where ΔO2/Δt is the change in oxygen over time; Vsys is the volume of the swim tunnel system; Vb is the volume of the fish, assuming a density of 1 kg l−1; and Mb is the biomass of the fish. The CoT at specific swimming speeds could then be derived and was expressed as mg O2 kg−1 km−1. In addition, a quadratic regression was fitted to the U-shaped relationship between CoT and swimming speed in the Ucrit trials, where the minimum of this function was defined as the Uopt for minimum CoT.
To assess the impact of fasting on body morphology, the condition factor of all fish was calculated as 100 (weight (g)/length(cm)3) (Ricker, 1975).
To test for statistical differences between feeding status and acclimation temperature on Ucrit, Uopt, size parameters and haematological parameters, a two-way ANOVA with the Hold–Sidak method for multiple comparison procedures was used after confirming normality and equal variance of the data with the Shapiro–Wilk test and the Brown–Forsythe test, respectively (SigmaPlot 14.5). To adhere to these test assumptions, it was necessary to perform a log transformation of the data for weight, osmolality, Na+ and Cl−. The ṀO2, CoT and tail beat frequency data were similarly analyzed with a two-way ANOVA to assess effects of treatment (feeding status plus temperature) at specific swimming speeds or time points in the Ucrit and sustained swim trials, respectively. A P-value below 0.05 was considered significant in the statistical tests and data are reported as the mean ± s.e.m.
Results
The 4-week fasting period did not result in any mortalities at either 9°C or 18°C, nor were any apparent changes in behaviours or incidences of injuries observed on the fish within the holding tanks.
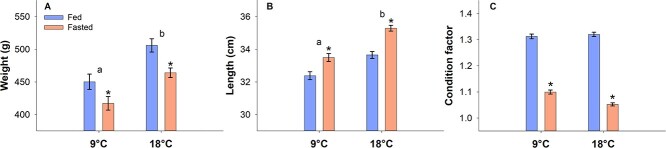
Fish acclimated to 18°C were significantly heavier and longer than at 9°C regardless of feeding status (two-way ANOVA, DF = 191, P < 0.001 for both parameters). Condition factor was similar in fed fish across temperature (P = 0.491), but lower in fasted fish at 18°C compared with fasted fish at 9°C (P < 0.001). The fasting period reduced the weight significantly within both temperatures (9°C, P = 0.017; 18°C, P = 0.009) from 450 ± 12 to 417 ± 10 g at 9°C and from 506 ± 10 to 464 ± 7 g at 18°C, which corresponded to an average percentage weight loss of 7.3% and 8.3%, respectively (Fig. 1A). Meanwhile length increased significantly during fasting within temperature (P < 0.001 in both) from 32.4 ± 0.2 to 33.5 ± 0.2 cm at 9°C and from 33.7 ± 0.2 to 35.3 ± 0.2 cm at 18°C (Fig. 1B). These changes in weight and length resulted in significantly lower condition factors in fasted fish (P < 0.001 within both temperatures), from 1.31 ± 0.01 to 1.10 ± 0.01 at 9°C and from 1.32 ± 0.01 to 1.05 ± 0.01 at 18°C (Fig. 1C). The lowest measured condition factor on an individual fasted fish was 0.97 at 18°C.
Figure 1.
Size parameters. Statistical differences between temperatures regardless of feeding status are indicated with letters, and statistical differences between fed and fasted fish within a specific temperature are indicated with asterisks (two-way ANOVA with Hold–Sidak post-hoc analyses P < 0.05). N = 48 and data are mean ± s.e.m.
The Ucrit was significantly higher at 18°C compared with at 9°C across feeding status both when expressed as cm s−1 and as body length s−1 (two-way ANOVA, DF = 95, P < 0.001) (Fig. 2A,B). However, the Ucrit was unaffected by fasting period within either temperature when expressed in cm s−1 (9°C, P = 0.125; 18°C, P = 0.058) as well as in body length s−1 (9°C, P = 0.199; 18°C, P = 0.197) (Fig. 2A,B). Although not significantly different, fasted fish had a slightly higher Ucrit when expressed in cm s−1 and a slightly lower Ucrit when expressed in body length s−1, which can be ascribed to the increased fork lengths in fasted fish.
Figure 2.
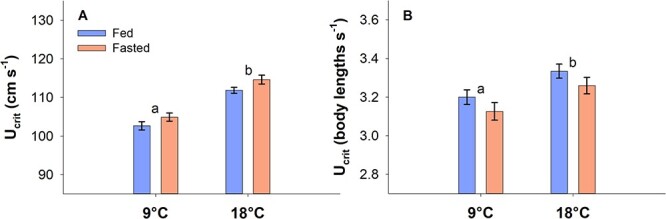
The critical swimming speed (Ucrit) expressed in cm s−1 (A) and body lengths s−1 (B). Statistical differences between temperatures regardless of feeding status are indicated with letters. Feeding status did not cause significant differences within either temperature (two-way ANOVA with Hold–Sidak post-hoc analyses, P < 0.05). N = 24 and data are mean ± s.e.m.
The sustained swim trials were based on the 80% Ucrit of fish from the same temperature and feeding status. The specific sustained test speeds used were 82.1 cm s−1 (2.56 body lengths s−1) on fed fish at 9°C, 83.9 cm s−1 (2.50 body lengths s−1) on fasted fish at 9°C, 89.5 cm s−1 (2.67 body lengths s−1) on fed fish at 18°C and 91.7 cm s−1 (2.61 body lengths s−1) on fasted fish at 18°C. All fed and fasted fish tested at either temperature managed to complete 4 hours of swimming at 80% Ucrit without becoming fatigued.
The ṀO2 increased with swimming speed and differed between all treatment groups in the Ucrit trials (two-way ANOVA, DF = 59, P < 0.001), where fish at 18°C had a higher ṀO2 than at 9°C and fasted fish had a lower ṀO2 within both temperatures (Fig. 3A). Specific differences between fed and fasted fish in ṀO2 were found at 30, 45 and 60 cm s−1 at both temperatures while ṀO2 also differed at 75 cm s−1 within 9°C. At the highest test speeds, as the maximum aerobic metabolic rate was approached, the ṀO2 became similar between fed and fasted fish at both temperatures (Fig. 3A).
Figure 3.
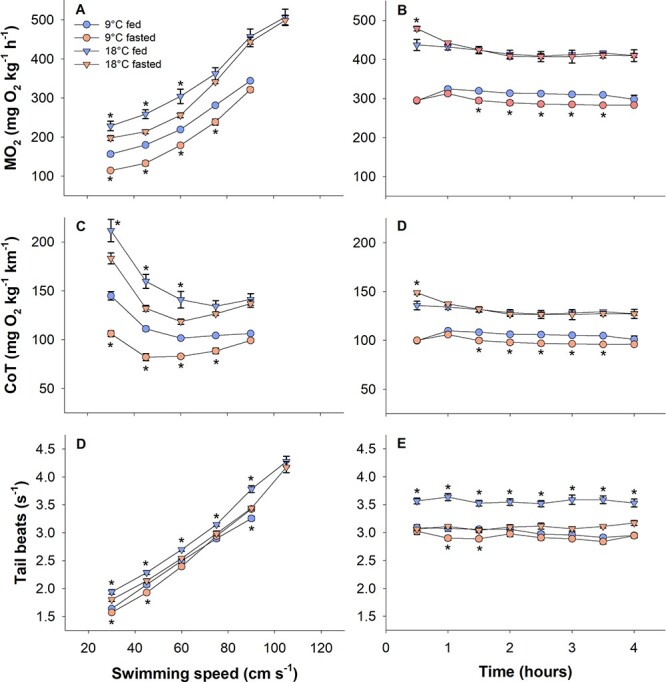
Metabolic rates (ṀO2), CoT and tailbeat frequencies in the Ucrit (left panels) and sustained swim (right panels) trials. Statistical differences between fed and fasted fish within a specific temperature at different swim speeds or time points are indicated with asterisks (two-way ANOVA with Hold–Sidak post-hoc analyses, P < 0.05). N = 3 for ṀO2 and CoT measurements and N = 12 for tail beat frequencies. Data are mean ± s.e.m.
In the sustained swim trials, the ṀO2 differed between treatment groups (two-way ANOVA, DF = 95, P < 0.001). Fish at 18°C had a higher ṀO2 than fish at 9°C (P < 0.001), and within temperature fasted fish had a lower ṀO2 at 9°C (P < 0.001), but not at 18°C (P = 0.247) (Fig. 3B). Significant differences at specific time points between fed and fasted fish within temperature were found at 30 minutes for 18°C and at 90, 120, 150, 180 and 210 minutes for 9°C (Fig. 3B). The ṀO2 was generally constant over time within each treatment group during the 4-hour sustained swim test. One exception to this was in the fasted 18°C group where the 30-minute time point was higher than all other points, while the 60-minute time point differed from the 120-, 150- and 180-minute time points (Fig. 3B).
The CoT derived from the Ucrit trials also differed between treatment groups and swimming speeds (two-way ANOVA, DF 59, P < 0.001) (Fig. 3C). Specifically, fish at 9°C had an overall lower CoT and fasted fish had a lower CoT within temperature. The CoT derived from the sustained swimming trials differed between groups in a similar manner to their ṀO2 response (two-way ANOVA, DF 59, P < 0.001) (Fig. 3D). Notably, CoT was lower at 9°C while fasted fish had lower CoT within 9°C (P < 0.001), but not within 18°C (P = 0.256) (Fig. 3D).
Tail beat frequency increased approximately linearly with swimming speed in the Ucrit trials and remained constant over time during the 4-hour sustained swim trials within all treatment groups (Fig. 3E,F). Significant differences in tail beat frequency between treatments were found in the Ucrit trials (two-way ANOVA, DF = 239, P < 0.001), where the most notable effect was that fed fish at 18°C had higher tail beat frequencies than fasted counterparts at 30, 45, 60, 75 and 90 cm s−1 (Fig. 3E). A similar tendency for higher tail beat frequencies in fed fish within 18°C was also observed in the sustained swim trials (two-way ANOVA, DF = 383, P < 0.001) (Fig. 3F).
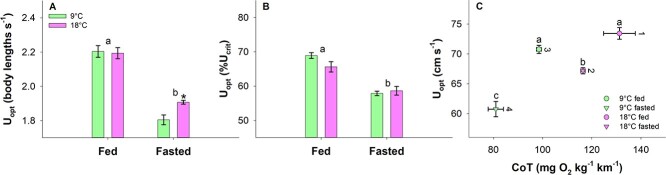
The Uopt expressed in body lengths s−1, as a percentage of the Ucrit, and as cm s−1 versus CoT are shown in Fig. 4. Fasted fish had a significantly lower Uopt (body length s−1) than fed fish within both temperatures (two-way ANOVA, DF = 11, P < 0.001). Fed fish showed similar Uopt across temperatures (P = 0.802) whereas fasted fish were different and had a lower Uopt (body lengths s−1) at 9°C (P = 0.032) (Fig. 4A). Similarly, fasted fish had a lower Uopt (%Ucrit) within temperature (two-way ANOVA, DF = 11; 9°C, P < 0.001; 18°C, P = 0.002); however, both fed and fasted fish showed similar Uopt (%Ucrit) across temperatures (Fed, P = 0.068; Fasted, P = 0.0646) (Fig. 4B). The Uopt (cm s−1) showed the same patterns in statistical differences as the Uopt (body lengths s−1) (Fig. 4C). The minimum CoT differed in all comparisons between the four treatment groups with the fasted 9°C group being lowest and the 18°C fed group being highest (two-way ANOVA, DF = 11, P < 0.05) (Fig. 4C).
Figure 4.
The optimal swimming speed (Uopt) for minimum CoT between groups expressed as body lengths s−1 (A), a percentage of the critical swimming speed (Ucrit) (B) and in cm s−1 versus the CoT (C). Statistical differences in (A) and (B) between fed and fasted fish across temperature are indicated with letters, and statistical differences between 9°C and 18°C within fed or fasted fish are indicated with asterisks. Vertical differences in Uopt in (C) are indicated with letters, while numbers indicate horizontal differences in CoT (two-way ANOVA with Hold–Sidak post-hoc analyses, P < 0.05). N = 3 and data are mean ± s.e.m.
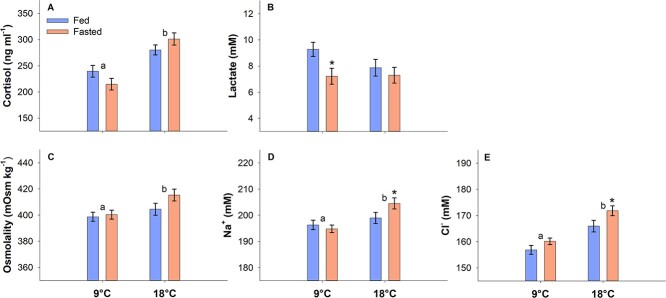
The haematological parameters measured in fatigued fish after the Ucrit trials are shown in Fig. 5. Plasma cortisol was higher at 18°C (two-way ANOVA, DF = 95, P < 0.001) and was unaffected by feeding status within each temperature (P = 0.863) (Fig. 5A). Significant differences in plasma lactate were only observed within 9°C where fed fish had higher lactate concentrations (P = 0.017) (Fig. 5B).
Figure 5.
Haematological parameters. Blood samplings were done on exhausted fish immediately after Ucrit measurements. Statistical differences between temperatures across feeding status are indicated with letters, and statistical differences between fed and fasted fish within a specific temperature are indicated with asterisks (two-way ANOVA with Hold–Sidak post-hoc analyses, P < 0.05). N = 24 and data are mean ± s.e.m.
Plasma osmolality was significantly higher at 18°C (P = 0.013), while it was unaffected by feeding status within temperature (P = 0.122) (Fig. 5C). Both plasma Na+ and Cl− concentrations were significantly higher at 18°C (P = 0.002 and P > 0.001, respectively), and fasted fish within 18°C had higher plasma Na+ and Cl− concentrations (P = 0.041 and P = 0.023) (Fig. 5D,E).
Discussion
Weight loss and body condition
It was hypothesized that extended fasting at 18°C would assert a greater negative impact on body condition in Atlantic salmon owing to accelerated metabolic rates draining resources more quickly compared with at 9°C. Indeed, 4 weeks of fasting did cause a higher average weight loss and a greater reduction in condition factor at 18°C, coinciding with a higher ṀO2 at similar activity levels.
However, the difference in weight loss was surprisingly minor between the two temperatures being 7.3% and 8.3% at 9°C and 18°C, respectively. Meanwhile, using ṀO2 obtained at the lowest test speed in the Ucrit swim trials as an indicator of routine metabolic costs irrespective of feeding status, fish at 18°C had a 57% higher ṀO2 than fish at 9°C. Factorial changes in functional rates in 10°C intervals are often expressed with the temperature quotient (Q10), and the increase in ṀO2 here corresponded to a Q10 of 1.7. Depending on acclimation history and section of the thermal niche assessed, resting metabolic rates in fish species generally increases with a Q10 of 1–3 (Lefevre, 2016), while the resting metabolic rates of thermally acclimated Atlantic salmon previously was found to increase with a Q10 of 2.1 between 8°C and 18°C (Hvas et al., 2017). As such, if resting and routine energetic costs approximately doubles with a 10°C temperature increase, then weight loss over an extended fasting period should theoretically also double. However, similar proportional effects were clearly not found in the present study (i.e. 57% higher costs, but only a 1% difference in weight loss). This discrepancy may be explained by the ṀO2 only being an indirect measurement of aerobic metabolic rates (Nelson, 2016), and that the relationship between oxygen uptake and energy production can vary between environmental conditions (Salin et al., 2015). Specifically, mitochondrial functioning becomes less efficient at higher temperatures due to impaired phosphorylation caused by increased proton leakage and decreased activity of complex I (Michaelsen et al., 2021; Roussel and Voituron, 2020). For instance, Atlantic salmon had a lower ATP produced to oxygen consumed ratio in cardiac mitochondria when acclimated to 20°C compared with 12°C acclimation (Gerber et al., 2021). Consequently, if less ATP is produced per oxygen at elevated temperatures, a higher ṀO2 is required to support similar metabolic demands as in lower temperatures. In this way temperature can cause a non-proportional relationship between ṀO2 and energy production in fish and other ectotherms (Salin et al., 2015), and thus help explain a lower weight loss than otherwise expected from the proportionally greater increase in ṀO2 at 18°C. Assuming that ṀO2 and energy production were proportional across temperatures, a weight loss of approximately 12% instead of 8.3% would then have been expected at 18°C in the present study.
The reported weight loss of food-deprived Atlantic salmon in previous studies have generally been lower than the present one, likely owing to having used larger size classes, as mass specific energetic requirements decreases with size (Killen et al., 2006; Oldham et al., 2019), combined with lower temperatures. For instance, 8–9 weeks of fasting reduced weights by 6–7% (Hvas et al., 2022; Reimers et al., 1993) and 11–12 weeks of fasting reduced weights by 10–11% (Einen et al., 1998; Lie and Huse, 1992), where these studies used fish ranging from 1.2 to 5 kg.
While losing weight the length increased over the 4-week fasting period. Similar continued length growth has previously been shown in food-deprived Atlantic salmon and indicates that vertebrae and muscle development may work independently of each other (Einen et al., 1998; Hvas et al., 2021b, 2022). Furthermore, continued length increases could prepare fish for accelerated muscle growth when feed becomes available again and thereby help facilitate compensatory growth (Ali et al., 2003).
Exacerbated by continued length growth, the condition factor dropped substantially during fasting from 1.32 across temperature down to 1.10 and 1.05 at 9°C and 18°C, respectively. However, a state of severe starvation and malnourishment in Atlantic salmon is generally first associated with condition factors below 0.9 (Noble et al., 2018). The fish in the present study could therefore have endured a longer fasting period before entering a stage of protein catabolization, which signifies severe starvation (Wang et al., 2006). As such, considering the modest changes in weights and the resulting intermediate condition factors, 4 weeks of fasting was well within the tolerance limit of Atlantic salmon, even at a suboptimal elevated temperature of 18°C.
Swimming capacity
The Ucrit is highest in the upper ranges of the thermal niches in Atlantic salmon and other fish species studied, coinciding with higher maximum metabolic rates (Brett, 1964; Claireaux et al., 2006; Hvas et al., 2017; Yuen et al., 2019). A higher Ucrit at 18°C compared with at 9°C was therefore to be expected in the present study.
Interestingly, the Ucrit was unaffected by the 4-week fasting period within both acclimation temperatures. In a separate study Atlantic salmon also maintained their full Ucrit over a 4-week fasting period at 12°C (Hvas et al., 2021b), and a similar result was therefore expected at 9°C owing to lower temperatures having a lesser impact on metabolic demands. However, an unaffected Ucrit at 18°C was surprising as this elevated temperature represents suboptimal conditions for growth and behavioural preferences in this species (e.g. Johansson et al., 2009; Kullgren et al., 2013). In a study on food-deprived cod the Ucrit was reduced in the food-deprived treatment, but their condition factor had decreased from 1.0 to 0.5 (Martínez et al., 2004). While different species have different morphometrics, a halving of condition factor is a dramatic change compared with only a 20% reduction at 18°C in the present study. Hence, a more modest impact on body condition likely explains why Ucrit remained unaffected in Atlantic salmon.
All fish tested managed to complete 4 hours of sustained swimming at 80% Ucrit without becoming fatigued. The limit of sustained swimming in fed Atlantic salmon have consistently been found to be 80–85% Ucrit at midrange temperatures of 12–13°C where speeds above 85% Ucrit necessitates recruitment of anaerobic white muscle fibres and eventually causes fatigue (Beddow and McKinley, 1999; Hvas et al., 2021a; Hvas and Oppedal, 2017). However, whether lower or higher temperatures would alter this threshold for aerobic swimming was previously unknown. It has been shown in other fish species that white muscles are recruited at lower swimming speeds in lower temperatures, which causes a reduction in sustained swimming capacity (Rome et al., 1990; Sisson and Sidell, 1987; Taylor et al., 1996). Similarly, in Atlantic salmon swimming at 3°C and 8°C, the transition to partially anaerobic burst and glide swimming occurs at lower speeds than at higher temperatures (Hvas et al., 2017). However, in the present study, aerobic swimming limits were not yet impaired at 9°C.
The aetiology of fatigue can either be a failure to supply sufficient metabolites at the required rates (e.g. Ucrit tests) or the depletion of metabolite supplies (e.g. fixed long-term velocity tests) (Jones, 1982). The latter being analogous to collapsing marathon runners ‘hitting the wall’ despite working within their normal aerobic limit. It was therefore hypothesized that fasted fish at 18°C eventually would fatigue in the sustained swim trials as food deprivation results in a greater exhaustion of resources at this temperature. However, as discussed above, the fasting regime at 18°C only caused a moderate reduction in body condition meaning that fish still were able to fuel long-term high intensity aerobic swimming. The 80% Ucrit threshold for sustained swimming in Atlantic salmon can therefore now also be applied to a wider thermal interval and to fish that have experienced extended fasting periods.
The ṀO2 remained stable within each treatment group during 4 hours of sustained swimming, providing evidence for that swimming efforts indeed remained aerobic. If this was not the case, anaerobic metabolism would cause lactate to accumulate in muscles and blood since lactate clearance is slow in salmonids (Hvas et al., 2021b; Schulte et al., 1992). Furthermore, only 3 ATP are produced when glycogen is converted to lactate while 5 ATP are required to convert lactate back to glycogen (Burgetz et al., 1998). Hence, if a significant anaerobic component was present, the increasing need to metabolize lactate at a cost deficit while swimming would drive up the ṀO2 until maximum rates were reached whereafter fatigue would be imminent.
Exhaustive exercise stress in salmonids causes substantial disturbances in osmotic, endocrine, and metabolic balances (Kieffer, 2000; Wood, 1991). The haematological parameters measured in the present study after the Ucrit tests were therefore characteristic of fatigued Atlantic salmon in seawater (Hvas et al., 2018, 2020). However, since the Ucrit was unaffected by food deprivation, only modest variations were found between treatment groups. Fish at 18°C were swimming for longer and at higher final speeds while achieving a substantially higher ṀO2, which facilitated a greater increase in plasma osmolality, Cl− and Na+ compared with at 9°C owing to the osmorespiratory tradeoff of the fish gill in hyperosmotic environments (Hvas et al., 2018; Sardella and Brauner, 2007). Interestingly, fasted fish at 18°C showed a greater ionic disturbance, which may be a subtle indicator that athletic performance was starting to become affected, as this would have some implications for repeat-swimming capabilities since recovery of ion homeostasis is particularly slow in seawater adapted Atlantic salmon (Hvas et al., 2018, 2020). The higher cortisol values at 18°C were likely caused by a combination of increased swimming efforts and temperature, as the cortisol response to a fixed stressor also was elevated at 17°C compared with 9°C in a previous study on Atlantic salmon (Madaro et al., 2018). Plasma lactate was unaffected by temperature, which suggests that similar anaerobic swimming efforts were made prior to fatigue meaning that the improved Ucrit at 18°C was aerobically driven. However, fasted fish within 9°C had lower lactate levels, which could hint at lower functionality of white muscle fibres as shown for other food-deprived fish species (Beardall and Johnston, 1983; Martínez et al., 2003). Although, considering that resting lactate levels should be <1 mM (e.g. Wood, 1991), and all treatment groups had greatly elevated values of >7 mM, this indicates preserved high capacities for anaerobic work following 4 weeks of fasting in Atlantic salmon.
Swimming energetics and U opt
While the capacities for both aerobic and anaerobic swimming were preserved following extended fasting, some temperature-dependent changes in swimming energetics were observed. At low and intermediate swimming speeds from 30 to 60 cm s−1, fasted fish within both temperatures had a lower ṀO2. At 9°C this corresponded to an average reduction of 43.1 mg O2 kg−1 h−1 or 23.8% across speeds, and at 18°C a comparable absolute reduction of 41.4 mg O2 kg−1 h−1 was found, but a lower percentage reduction of 15.6% caused by higher basal costs. Similarly, the resting metabolic rate of Atlantic salmon after 3–4 weeks of fasting at 12°C was reduced by 22% (Hvas et al., 2020), while other fish species also downregulate metabolic rates in response to food deprivation (Fu et al., 2005; Mehner and Wieser, 1994).
These reductions in ṀO2 in fasted fish were unlikely to be an artefact of elevated ṀO2 in the fed control fish caused by specific dynamic action effects, as these fish had been fasting overnight for a minimum of 20 hours prior to measurements, which should have allowed for completion of most digestive processes (Handeland et al., 2008; Storebakken et al., 1999). Hence, lower ṀO2 in food-deprived fish should represent real adaptive responses to minimize energetic requirements that may involve a range of biochemical adjustments in gene expressions, decreased enzyme activities, reduced protein syntheses and improved efficiency of mitochondrial functioning in appropriate organs (Bermejo-Nogales et al., 2015; Cassidy et al., 2016; Méndez and Wieser, 1993; Salin et al., 2018).
Interestingly, the ability to save energy during food deprivation was less pronounced at higher activity levels where the ṀO2 between fed and fasted fish converged, and this pattern occurred earlier at 18°C. As such, the ability to preserve energy appeared to be less efficient when fasting at higher temperatures in Atlantic salmon. In addition, the similar ṀO2 between fed and fasted fish at high activity levels suggests that maximum aerobic metabolic rats were unaffected, in agreement with an unaffected Ucrit. This observation is advantageous for food-deprived Atlantic salmon, as they can use less resources during resting and routine conditions while still being able to perform at their highest athletic ability, if needed.
The reduced ṀO2 in fasted fish resulted in lower CoT and Uopt at both temperatures, and these effects were greater at 9°C. This means that fasted Atlantic salmon in colder waters, in theory, will swim slower and at lower costs when foraging or migrating. For instance, based on the Uopt of the present study, a hypothetical 1000 km migration would increase from 16.3 to 19.1 days between fed and fasted fish at 9°C, and similarly increase from 15.7 to 17.2 days at 18°C. While using longer time to migrate a given distance, the overall energetic requirement will still be reduced in food-deprived Atlantic salmon, with 17.8% and 11.3% lower total costs at 9°C and 18°C, respectively. Moreover, regardless of feeding status, cruising at Uopt was 27.5% cheaper at 9°C compared with at 18°C. The maximum distance that can be covered by food restricted migratory Atlantic salmon will consequently be lower in warming marine environments.
Conclusion
The Atlantic salmon is an environmentally flexible species that is able to handle and adapt to many of the diverse challenges it may encounter during its life cycle. This study exemplifies this flexibility as extended fasting was successfully endured with moderate effects on weight and body condition because of the ability to beneficially downregulate metabolic requirements, even at an elevated suboptimal temperature. Furthermore, despite of 4 weeks of food deprivation, Atlantic salmon maintained their full capacity for both aerobic and anaerobic swimming across temperatures as shown by a preserved Ucrit and completion of the sustained swim trials.
However, some ecological implications may be considered with regards to swimming energetics in a warming climate in conjunction with other stressors. Elevated temperatures reduced the fasting-induced energetic savings, while inherently also driving up basal maintenance costs and thus draining resources more quickly. This could restrain migratory limits and have implications for survival, both during initial seaward travel in newly smoltified fish and upriver return in spawning adults. Specifically, seaward-bound juvenile Atlantic salmon are under substantial predation pressure owing to their small sizes and are also at high risk of parasite infections dispersed from aquaculture farms when travelling through the fjords and coastal zones (Johnsen et al., 2020; Thorstad et al., 2012). Rapid growth at sea is therefore crucial to ensure survival, but this task could become more difficult in warmer marine environments, particularly if fish also have to cope with the energetic burden of parasites (Hvas and Bui, 2022; Susdorf et al., 2018). Furthermore, Atlantic salmon become anorexic when migrating back to their spawning grounds (Kadri et al., 1995). Provided lower initial body conditions (e.g. Susdorf et al., 2018) together with increased travel costs at higher temperatures (present study), while diverting substantial resources on gonad development (Hendry and Beall, 2004), this could lead to shorter and less prolific spawning events as well as reducing the number of surviving repeat-spawning adult Atlantic salmon.
Acknowledgements
The author thanks the technical staff at the Matre Research station for excellent animal husbandry, Karen Anita Kvestad for performing haematological analyses and Tone Vågseth for assisting with the swim tunnel setup.
Funding
This work was funded by the Research Council of Norway through the projects SFI Exposed (237790) and Fastwell (295200).
Supplementary material
Supplementary material is available at Conservation Physiology online.
Contributions
M.H. conceived and performed the experiment, analyzed the data and wrote the manuscript.
Data availability
Raw data is available in a supplementary file.
Conflict of Interest
The authors declare that they have no competing interests.
Supplementary Material
References
- Ali M, Nicieza A, Wootton RJ (2003) Compensatory growth in fishes: a response to growth depression. Fish Fish 4: 147–190. [Google Scholar]
- Beamish FWH (1978) Swimming capacity. In Hoar WS, Randall DJ, eds, Fish Physiology. Academic Press, New York, NY, pp. 101–187 [Google Scholar]
- Beardall CH, Johnston IA (1983) Muscle atrophy during starvation in a marine teleost. Eur J Cell Biol 29: 209–217. [PubMed] [Google Scholar]
- Beddow TA, McKinley RS (1999) Importance of electrode positioning in biotelemetry studies estimating muscle activity in fish. J Fish Biol 54: 819–831. [Google Scholar]
- Bell WH, Terhune LDB (1970) Water tunnel design for fisheries research. Fisheries Research Board of Canada, Technical Report No. 195.
- Bermejo-Nogales A, Calduch-Giner JA, Pérez-Sánchez J (2015) Unraveling the molecular signatures of oxidative phosphorylation to cope with the nutritionally changing metabolic capabilities of liver and muscle tissues in farmed fish. PLoS One 10: e0122889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21: 1183–1226. [Google Scholar]
- Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am Zool 11: 99–113. [Google Scholar]
- Burgetz IJ, Rojas-Vargas A, Hinch SG, Randall DJ (1998) Initial recruitment of anaerobic metabolism during submaximal swimming in rainbow trout (Oncorhynchus mykiss). J Exp Biol 201: 2711–2721. [DOI] [PubMed] [Google Scholar]
- Cassidy AK, Saulnier RJ, Lamarre SG (2016) Adjustments of protein metabolism in fasting Arctic Charr, Salvelinus alpinus. PLoS One 11: e0153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G, Couturier C, Groison A-L (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 209: 3420–3428. [DOI] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- Cotterell SP, Wardle CS (2004) Endurance swimming of diploid and triploid Atlantic salmon. J Fish Biol 65: 55–68. [Google Scholar]
- Dadswell M, Spares A, Reader J, McLean M, McDermott T, Samways K, Lilly J (2021) The decline and impending collapse of the Atlantic salmon (Salmo salar) population in the North Atlantic Ocean: a review of possible causes. Rev Fish Sci Aquac 30: 215–258. [Google Scholar]
- Drenner SM, Clark TD, Whitney CK, Martins EG, Cooke SJ, Hinch SG (2012) A synthesis of tagging studies examining the behaviour and survival of anadromous salmonids in marine environments. PLoS One 7: e31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einen O, Waagan B, Thomassen MS (1998) Starvation prior to slaughter in Atlantic salmon (Salmo salar). Aquaculture 166: 85–104. [Google Scholar]
- Eliason EJ, Farrell AP (2016) Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J Fish Biol 88: 359–388. [DOI] [PubMed] [Google Scholar]
- Farrell AP (2007) Cardiorespiratory performance during prolonged swimming tests with salmonids: a perspective on temperature effects and potential analytical pitfalls. Philos Trans R Soc Lond B Biol Sci 362: 2017–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SJ, Xie XJ, Cao ZD (2005) Effect of fasting on resting metabolic rate and postprandial metabolic response in Silurus meridionalis. J Fish Biol 67: 279–285. [Google Scholar]
- Gamperl AK, Ajiboye OO, Zanuzzo FS, Sandrelli RM, Peroni EFC, Beemelmanns A (2020) The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic salmon (Salmo salar). Aquaculture 519: 734874. [Google Scholar]
- Gerber L, Clow KA, Gamperl AK (2021) Acclimation to warm temperatures has important implications for mitochondrial function in Atlantic salmon (Salmo salar). J Exp Biol 224: jeb236257. [DOI] [PubMed] [Google Scholar]
- Green J, Farwell M (1971) Winter habits of the cunner,Tautogolabrus adspersus(Walbaum 1792), in Newfoundland. Can J Res Sect D Zool Sci 49: 1497–1499. [Google Scholar]
- Handeland SO, Björnsson BT, Arnesen AM, Stefansson SO (2003) Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture 220: 367–384. [Google Scholar]
- Handeland SO, Imsland AK, Stefansson SO (2008) The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 283: 36–42. [Google Scholar]
- Hendry AP, Beall E (2004) Energy use in spawning Atlantic salmon. Ecol Freshw Fish 13: 185–196. [Google Scholar]
- Hevrøy EM, Tipsmark CK, Remø SC, Hansen T, Fukuda M, Torgersen T, Vikeså V, Olsvik PA, Waagbø R, Shimizu M (2015) Role of the GHIGF-1 system in Atlantic salmon and rainbow trout postsmolts at elevated water temperature. Comp Biochem Physiol A Mol Integr Physiol 188: 127–138. [DOI] [PubMed] [Google Scholar]
- Hvas M, Bui S (2022) Energetic costs of ectoparasite infection in Atlantic salmon. J Exp Biol 225: jeb243300. [DOI] [PubMed] [Google Scholar]
- Hvas M, Folkedal O, Imsland A, Oppedal F (2017) The effect of thermal acclimation on aerobic scope and critical swimming speed in Atlantic salmon Salmo salar. J Exp Biol 220: 2757–2764. [DOI] [PubMed] [Google Scholar]
- Hvas M, Folkedal O, Oppedal F (2021a) What is the limit of sustained swimming in Atlantic salmon post smolts? Aquacult Environ Interact 13: 189–198. [Google Scholar]
- Hvas M, Nilsen TO, Oppedal F (2018) Oxygen uptake and osmotic balance of Atlantic salmon in relation to exercise and salinity acclimation. Front Mar Sci 5: 368. [Google Scholar]
- Hvas M, Nilsson J, Vågseth T, Nola V, Fjelldal PG, Hansen TJ, Oppedal F, Stien LH, Folkedal O (2022) Full compensatory growth before harvest and no impact on fish welfare in Atlantic salmon after an 8-week fasting period. Aquaculture 546: 737415. [Google Scholar]
- Hvas M, Oppedal F (2017) Sustained swimming capacity of Atlantic salmon. Aquacult Environ Interact 9: 361–369. [Google Scholar]
- Hvas M, Oppedal F (2019) Influence of experimental set-up and methodology for measurements of metabolic rates and critical swimming speed in Atlantic salmon Salmo salar. J Fish Biol 95: 893–902. [DOI] [PubMed] [Google Scholar]
- Hvas M, Stien LH, Oppedal F (2020) The metabolic rate response to feed withdrawal in Atlantic salmon post-smolts. Aquaculture 529: 735690. [Google Scholar]
- Hvas M, Stien LH, Oppedal F (2021b) The effect of fasting period on swimming performance, blood parameters and stress recovery in Atlantic salmon post smolts. Comp Biochem Physiol A Physiol 255: 110913. [DOI] [PubMed] [Google Scholar]
- Jensen AJ, Karlsson PF, Hansen LP, Østborg GM, Hindar K (2014) Origin and life history of Atlantic salmon (Salmo salar) near their northernmost oceanic limit. Can J Fish Aquat Sci 71: 1740–1746. [Google Scholar]
- Johansson D, Ruohonen K, Juell J-E, Oppedal F (2009) Swimming depth and thermal history of individual Atlantic salmon (Salmo salar L.) in production cages under different ambient temperature conditions. Aquaculture 290: 296–303. [Google Scholar]
- Johnsen IA, Harvey A, Sævik PN, Sandvik AD, Ugedal O, Ådlandsvik B, Wennevik V, Glover KA, Karlsen Ø (2020) Salmon lice-induced mortality of Atlantic salmon during post-smolt migration in Norway. ICES J Mar Sci 78: 142–154. [Google Scholar]
- Johnston IA, Goldspink G (1973) Some effects of prolonged starvation on the metabolism of the red and white myotomal muscles of the plaice Pleuronectes platessa. Mar Biol 19: 348–353. [Google Scholar]
- Jones DR (1982) Anaerobic exercise in teleost fish. Can J Zool 60: 1131–1134. [Google Scholar]
- Kadri S, Metcalfe NB, Huntingford FA, Thorpe JE (1995) What controls the onset of anorexia in maturing adult female Atlantic salmon? Funct Ecol 9: 790–797. [Google Scholar]
- Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol A 126: 161–179. [DOI] [PubMed] [Google Scholar]
- Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc Biol Sci 274: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA (2007) Declining wild salmon populations in relation to parasites from farm salmon. Science 318: 1772–1775. [DOI] [PubMed] [Google Scholar]
- Kullgren A, Jutfelt F, Fontanillas R, Sundell K, Samuelsson L, Wiklander K, Kling P, Koppe W, Larson DGJ, Björnsson BT et al. (2013) The impact of temperature on the metabolome and endocrine metabolic signals in Atlantic salmon (Salmo salar). Comp Biochem Physiol A 164: 44–53. [DOI] [PubMed] [Google Scholar]
- Lacroix GL (2013) Population-specific ranges of oceanic migration for adult Atlantic salmon (Salmo salar) documented using pop-up satellite archival tags. Can J Fish Aquat Sci 70: 1011–1030. [Google Scholar]
- Lefevre S (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol 4: cow009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie Ø, Huse J (1992) The effect of starvation on the composition of Atlantic salmon (Salmo salar). Fisk Dir Skr Ernæring 5: 11–16. [Google Scholar]
- Loughna PT, Goldspink G (1984) The effects of starvation upon protein turnover in red and white myotomal muscle of rainbow trout, Salmo gairdneri Richardson. J Fish Biol 25: 223–230. [Google Scholar]
- Madaro A, Folkedal O, Maiolo S, Alvanopoulou M, Olsen RE (2018) Effects of acclimation temperature on cortisol and oxygen consumption in Atlantic salmon (Salmo salar) post-smolt exposed to acute stress. Aquaculture 497: 331–335. [Google Scholar]
- Martínez M, Bédard M, Dutil J-D, Guderley H (2004) Does condition of Atlantic cod (Gadus morhua) have a greater impact upon swimming performance atUcrit or sprint speeds? J Exp Biol 207: 2979–2990. [DOI] [PubMed] [Google Scholar]
- Martínez M, Guderley H, Dutil J-D, Winger PD, Walsh SJ (2003) Condition, prolonged swimming performance and muscle metabolic capacities of cod (Gadus morhua). J Exp Biol 206: 503–511. [DOI] [PubMed] [Google Scholar]
- McCue MD (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A Physiol 156: 1–18. [DOI] [PubMed] [Google Scholar]
- Mehner T, Wieser W (1994) Energetics and metabolic correlates of starvation in juvenile perch (Perca fluviatilis). J Fish Biol 45: 325–333. [Google Scholar]
- Méndez G, Wieser W (1993) Metabolic responses to food deprivation and refeeding in juveniles of Rutilus rutilus (Teleostei: Cyprinidae). Environ Biol Fishes 36: 73–81. [Google Scholar]
- Michaelsen J, Fago A, Bundgaard A (2021) High temperature impairs mitochondrial function in rainbow trout cardiac mitochondria. J Exp Biol 224: jeb242382. [DOI] [PubMed] [Google Scholar]
- Miller KM, Schulze AD, Ginther N, Li S, Patterson DA, Farrell AP, Hinch SG (2009) Salmon spawning migration: metabolic shifts and environmental triggers. Comp Biochem Physiol 4: 75–89. [DOI] [PubMed] [Google Scholar]
- Morgan IJ, McDonald DG, Wood CM (2001) The cost of living for freshwater fish in a warmer, more polluted world. Glob Chang Biol 7: 345–355. [Google Scholar]
- Morgan IJ, Metcalfe NB (2001) Deferred costs of compensatory growth after autumnal food shortage in juvenile salmon. Proc R Soc B 268: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JA (2016) Oxygen consumption rate v. rate of energy utilization of fishes: a comparison and brief history of the two measurements. J Fish Biol 88: 10–25. [DOI] [PubMed] [Google Scholar]
- Noble C, Gismervik K, Iversen MH, Kolarevic J, Nilsson J, Stien LH, Turnbull JF (2018) Welfare indicators for farmed Atlantic Salmon: tools for assessing fish welfare. 351.
- Oldham T, Nowak B, Hvas M, Oppedal F (2019) Metabolic and functional impacts of hypoxia vary with size in Atlantic salmon. Comp Biochem Physiol A Mol Integr Physiol 231: 30–38. [DOI] [PubMed] [Google Scholar]
- Pardo SA, Bolstad GH, Dempson JB, April J, Jones JA, Raab D, Hutchings JA (2021) Trends in marine survival of Atlantic salmon populations in eastern Canada. ICES J Mar Sci 78: 2460–2473. [Google Scholar]
- Parrish DL, Behnke RJ, Gephard SR, McCormick SD, Reeves GH (1998) Why aren’t there more Atlantic salmon (Salmo salar)? Can J Fish Aquat Sci 55: 281–287. [Google Scholar]
- Plaut I (2001) Critical swimming speed: its ecological relevance. Comp Biochem Physiol A Mol Integr Physiol 131: 41–50. [DOI] [PubMed] [Google Scholar]
- Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39: 227–244. [Google Scholar]
- Reddin DG (1985) Atlantic salmon (Salmo salar) on and east of the grand bank. J Northwest Atl Fish Sci 6: 157–164. [Google Scholar]
- Reimers E, Kjørrefjord AG, Stavøstrand SM (1993) Compensatory growth and reduced maturation in second sea winter farmed Atlantic salmon following starvation in February and march. J Fish Biol 43: 805–810. [Google Scholar]
- Remen M, Solstorm F, Bui S, Klebert P, Vågseth T, Solstorm D, Hvas M, Oppedal F (2016) Critical swimming speed in groups of Atlantic salmon Salmo salar. Aquacult Environ Interact 8: 659–664. [Google Scholar]
- Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191: 1–382. [Google Scholar]
- Rome LC, Funke RP, Alexander RL (1990) The influence of temperature on muscle velocity and sustained performance in swimming carp. J Exp Biol 154: 163–178. [DOI] [PubMed] [Google Scholar]
- Roussel D, Voituron Y (2020) Mitochondrial costs of being hot: effects of acute thermal change on liver bioenergetics in toads (Bufo bufo). Front Physiol 11: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin K, Auer SK, Rey B, Selman C, Metcalfe NB (2015) Variation in the link between oxygen consumption and ATP production, and its relevance for animal performance. Proc R Soc B 282: 20151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin K, Villasevil EM, Anderson GJ, Auer SK, Selman C, Hartley RC, Mullen W, Chinopoulos C, Metcalfe NB (2018) Decreased mitochondrial metabolic requirements in fasting animals carry an oxidative cost. Funct Ecol 32: 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella BA, Brauner CJ (2007) The osmo-respiratory compromise in fish. In Fernandes MN, Rantin FT, Glass ML, Kapoor BG, eds, Fish Respiration and Environment. CRC Press, Boca Raton, FL, pp. 147–165 [Google Scholar]
- Scarabello M, Wood CM, Heigenhauser GJF (1991) Glycogen depletion in juvenile rainbow trout as an experimental test of the oxygen debt hypothesis. Can J Zool 69: 2562–2568. [Google Scholar]
- Schulte PM, Moyes CD, Hochachka PW (1992) Integrating metabolic pathways in post-exercise recovery of white muscle. J Exp Biol 166: 181–195. [DOI] [PubMed] [Google Scholar]
- Sisson JEIII, Sidell BD (1987) Effect of thermal acclimation on muscle fiber recruitment of swimming striped bass (Morone saxatilis). Physiol Zool 60: 310–320. [Google Scholar]
- Steffensen JF, Johansen K, Bushnell PG (1984) An automated swimming respirometer. Comp Biochem Physiol A Physiol 79: 437–440. [Google Scholar]
- Storebakken T, Kvien IS, Shearer KD, Grisdale-Helland B, Helland SJ (1999) Estimation of gastrointestinal evacuation rate in Atlantic salmon (Salmo salar) using inert markers and collection of faeces by sieving: evacuation of diets with fish meal, soybean meal or bacterial meal. Aquaculture 172: 291–299. [Google Scholar]
- Susdorf R, Salama NKG, Todd CD, Hillman RJ, Elsmere P, Lusseau D (2018) Context-dependent reduction in somatic condition of wild Atlantic salmon infested with sea lice. Mar Ecol Prog Ser 606: 91–104. [Google Scholar]
- Taylor SE, Egginton S, Taylor EW (1996) Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximal sustainable exercise level. J Exp Biol 199: 835–845. [DOI] [PubMed] [Google Scholar]
- Thorstad EB, Whoriskey F, Uglem I, Moore A, Rikardsen AH, Finstad B (2012) A critical life stage of the Atlantic salmon Salmo salar: behaviour and survival during the smolt and initial post-smolt migration. J Fish Biol 81: 500–542. [DOI] [PubMed] [Google Scholar]
- Valiente AG, Juanes F, Garcia-Vazquez EG (2011) Increasing regional temperatures associated with delays in Atlantic salmon sea-run timing at the southern edge of the European distribution. Trans Am Fish Soc 140: 367–373. [Google Scholar]
- Van Ginneken VJT, Antonissen E, Müller UK, Booms R, Eding E et al. (2005) Eel migration to the Sargasso: remarkably high swimming efficiency and low energy costs. J Exp Biol 208: 1329–1335. [DOI] [PubMed] [Google Scholar]
- Wade NM, Clark TD, Maynard BT, Atherton S, Wilkinson RJ, Smullen RP, Taylor RS (2019) Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J Therm Biol 80: 64–74. [DOI] [PubMed] [Google Scholar]
- Wang T, Hung CCU, Randall DJ (2006) The comparative physiology of food deprivation: from feast to famine. Annu Rev Physiol 68: 223–251. [DOI] [PubMed] [Google Scholar]
- Weihs D (1973) Optimal fish cruising speed. Nature 245: 48–50. [Google Scholar]
- Wilson RW, Egginton S (1994) Assessment of maximum sustainable swimming performance in rainbow trout (Oncorhynchus mykiss). J Exp Biol 192: 299–305. [DOI] [PubMed] [Google Scholar]
- Wood CM (1991) Acid-base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J Exp Biol 160: 285–308. [Google Scholar]
- Yuen JW, Dempster T, Oppedal F, Hvas M (2019) Physiological performance of ballan wrasse (Labrus bergylta) at different temperatures and its implication for cleaner fish usage in salmon aquaculture. Biol Control 135: 117–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data is available in a supplementary file.