Abstract
Paired corticospinal-motoneuronal stimulation (PCMS) elicits spinal synaptic plasticity in humans with chronic spinal cord injury (SCI). Here, we examined whether PCMS-induced plasticity could be potentiated by acute intermittent hypoxia (AIH), a treatment also known to induce spinal synaptic plasticity in humans with chronic incomplete cervical SCI.
During PCMS, we used 180 pairs of stimuli where corticospinal volleys evoked by transcranial magnetic stimulation over the hand representation of the primary motor cortex were timed to arrive at corticospinal-motoneuronal synapses of the first dorsal interosseous (FDI) muscle ~1–2 ms before antidromic potentials were elicited in motoneurons by electrical stimulation of the ulnar nerve. During AIH, participants were exposed to brief alternating episodes of hypoxic inspired gas (1 min episodes of 9% O2) and room air (1 min episodes of 20.9% O2). We examined corticospinal function by measuring motor evoked potentials (MEPs) elicited by cortical and subcortical stimulation of corticospinal axons and voluntary motor output in the FDI muscle before and after 30 min of PCMS combined with AIH (PCMS+AIH) or sham AIH (PCMS+sham-AIH). The amplitude of MEPs evoked by magnetic and electrical stimulation increased after both protocols, but most after PCMS+AIH, consistent with the hypothesis that their combined effects arise from spinal plasticity. Both protocols increased electromyographic activity in the FDI muscle to a similar extent. Thus, PCMS effects on spinal synapses of hand motoneurons can be potentiated by AIH. The possibility of different thresholds for physiological vs behavioral gains needs to be considered during combinatorial treatments.
Keywords: Spinal cord injury, plasticity, acute intermittent hypoxia, paired stimulation
Introduction
Pairing noninvasive stimulation of the primary motor cortex and a peripheral nerve (paired corticospinal-motoneuronal stimulation; PCMS) enhances corticospinal synaptic transmission and voluntary motor output in humans with chronic SCI (Bunday and Perez, 2012; Bunday et al., 2018; Jo and Perez, 2020; Urbin et al., 2017). PCMS-induced plasticity in humans with chronic SCI is enhanced by applying stimulation during mild tonic voluntary activity (Bunday et al., 2018) or by combining stimulation with exercise training (Jo and Perez, 2020). Thus, PCMS may have maximal benefits in motor recovery when paired with other treatments, what it remains unclear which combinatorial treatments would optimize PCMS enhancement of corticospinal synaptic transmission.
The question that we addressed in the present study is how we can enhance the effect of PCMS to maximize recovery in humans with SCI. One possibility is to combine PCMS with a therapy which has its aftereffects mediated through similar neural mechanisms and would possibly boost PCMS-mediated spinal plasticity. PCMS pairs noninvasive stimulation of the primary motor cortex and a peripheral nerve, enhancing corticospinal transmission at the spinal cord level, likely by engaging spike-timing dependent plasticity (STDP) mechanisms that depend on N-methyl-D-aspartate (NMDA) receptor activation (Donges et al., 2017). PCMS predominantly affects low-threshold motoneurons (D’Amico et al., 2017), and its aftereffects in humans with SCI can be potentiated by decreasing the threshold for motoneuron activation (Bunday et al., 2018). STDP-like effects are potentiated by blocking serotonin reuptake (Batsikadze et al., 2013), and are sensitive to BDNF genotype (Cheeran et al., 2008). A non-invasive strategy that induce spinal plasticity and improves voluntary motor output in humans with SCI is acute intermittent hypoxia (AIH; Hayes et al., 2014; Lynch et al., 2016; Navarrete-Opazo et al., 2017a,b; Trumbower et al., 2012, 2017; Sandhu et al., 2019). AIH acts through multiple cellular cascades that are regulated by brainstem or non-brainstem circuits, which have significant cross talk. For example, AIH increases BDNF expression in somatic motoneurons (Lovett-Barr et al., 2012; Satriotomo et al., 2016) and BDNF is necessary for NMDA receptor-dependent synaptic plasticity (Crozier et al., 2008), consistent with the view that these plasticity protocols can have synergistic interactions. It is also possible that spinal serotonin release (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009) plays a role in the facilitatory effect of AIH on STDP-like plasticity since serotonin binding to Gq-metabotropic receptors can amplify NMDA-receptor dependent calcium influx (Gu, 2002; Johnson and Heckman, 2014). In addition to serotonin and BDNF, AIH-mediated potentiation of motoneuronal activity can be related to adenosine through a signaling cascade that competes with serotonin and BDNF signaling pathways (Nichols et al., 2012; Hoffman et al., 2010).
Earlier work showed that AIH (Hayes et al., 2014; Prosser-Loose et al., 2015) and PCMS (Jo and Perez, 2020) effects in motor function in people with chronic SCI are more pronounced when combined with motor practice, suggesting that these plasticity protocols interact with activity-dependent processes (Welch et al., 2020). The synergistic effects of PCMS and AIH are also supported by results showing an additive facilitatory effects on corticospinal excitability when PCMS and AIH are applied in combination (Christiansen et al., 2018). Therefore, AIH is a possible choice for combination with PCMS to target spinal plasticity, thereby maximizing its efficacy for neurorehabilitation (Mateika et al., 2015; Gonzalez-Rothi et al., 2015; Welch et al., 2020). Here, we test the hypothesis that PCMS combined with AIH boosts spinal plasticity more than PCMS combined with sham AIH in humans with chronic incomplete SCI.
Methods
Participants.
Sixteen spinal cord injured participants (mean age 50.2±12.8 years; 4 females) participated in the study. All procedures were approved by the local ethics committee at the University of Miami in accordance with the guidelines established in the Declaration of Helsinki. Participants had a chronic SCI (≥1 year) and were classified as having a C1-C7 SCI based on the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) exam. Three out of 16 individuals were categorized by the American Spinal Cord Injury Impairment Scale (AIS) as AIS A (complete injury) due to the lack of sacral sparing (Marino et al., 2003), despite being able to elicit voluntary force with the targeted muscle. The other 13 individuals were classified as incomplete AIS C (n=8) or D (n=5; Table 1). SCI participants were able to exert maximal voluntary isometric contraction (MVC, measured as the highest mean rectified EMG activity found in 1 s during the MVC burst) into index finger abduction to a lesser extent compared with age-matched control subjects (Note that data in aged-matched control obtained from Bunday and Perez, 2012; controls=0.5±0.3 mV, SCI=0.3±0.1 mV, p<0.001). Each subject underwent all testing sessions at the same time of the day and the majority of subjects were tested in the morning. All subjects (n=16) participated in the two main experimental sessions were we examined the effect of PCMS+AIH and PCMS+sham-AIH on the amplitude of MEPs elicited by TMS. Later, in a subgroup of subjects, we examined the effect of both interventions on the amplitude of MEPs elicited by electrical stimulation (n=9), and small levels of voluntary motor output and MVCs (n=10).
Table 1:
Spinal cord injury participants
| Participants | Age | Sex | Etiology | Level of lesion | AIS | Years since injury |
|---|---|---|---|---|---|---|
| 1 | 67 | M | T | C2 | C | 11 |
| 2 | 50 | M | T | C5 | D | 4 |
| 3 | 53 | F | T | C5 | D | 14 |
| 4 | 39 | F | NT | C1 | D | 25 |
| 5 | 34 | M | T | C7 | C | 2 |
| 6 | 38 | M | T | C7 | C | 3 |
| 7 | 38 | M | T | C5 | C | 11 |
| 8 | 55 | M | T | C7 | A | 18 |
| 9 | 51 | F | T | C2–6 | A | 10 |
| 10 | 33 | M | T | C2 | C | 9 |
| 11 | 66 | M | T | C4 | D | 6 |
| 12 | 69 | M | T | C2 | C | 5 |
| 13 | 48 | F | T | C6 | C | 7 |
| 14 | 60 | M | NT | C1 | D | 3 |
| 15 | 36 | M | T | C5 | C | 2 |
| 16 | 66 | M | NT | C5–7 | A | 6 |
AIS=American Spinal Injury Association Impairment Scale, F=Female, M=Male, T=Traumatic, NT=Non-traumatic
EMG and force recordings.
EMG was recorded from the first dorsal interosseous (FDI) through surface electrodes (Ag–AgCl, 10 mm diameter) in a muscle tendon montage with one electrode placed over the belly of the muscle. All signals were amplified, filtered (30–1000 Hz), and sampled at 2 kHz for off-line analysis (Cambridge Electronic Design 1401 with Signal software v4.1). At the start of the experiment, subjects were instructed to perform 3 brief MVCs for 3–5 s into index finger abduction, separated by 2 min of rest. Participants were verbally cued to commence the contraction by the experimenter whom also provided strong verbal encouragement throughout the contraction. EMG data were processed offline calculating the mean rectified EMG over a 100 ms window centered over peak EMG. In brief, EMG digitized signals were rectified and smoothed using a 4th order Butterworth filter and low-passed at 5 Hz (Trumbower et al., 2012). Peak EMG was found through visual inspection and the mean value within a window ranging from 50 ms before to 50 ms after the peak was noted. Background EMG was subtracted from this value and the average of the 3 trials was noted. Three MVCs were also measured at baseline and 3 MVCs were collected 30 min after each intervention.
Experimental setup.
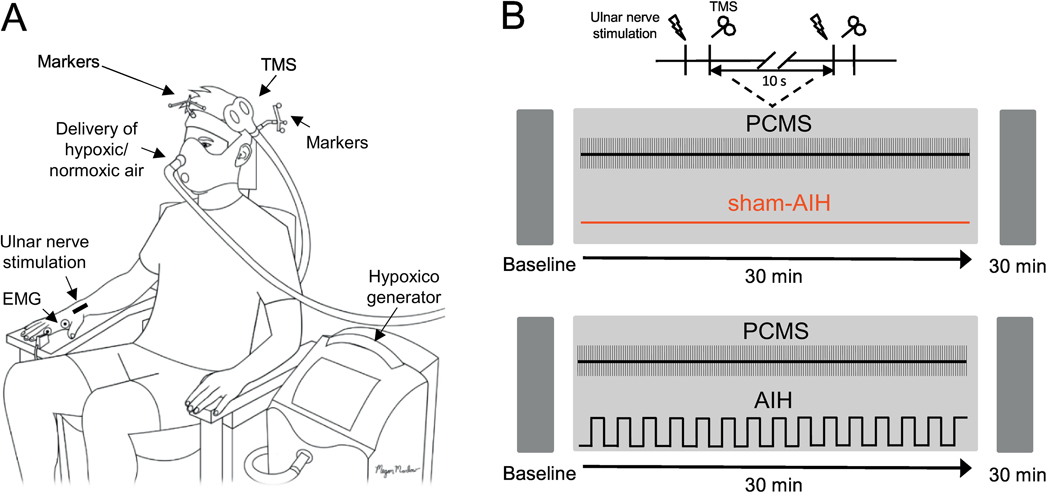
During testing, all participants were seated in an armchair with both arms relaxed and flexed at the elbow by 90° with the forearm pronated and the wrist and forearm restrained by straps (Fig. 1A). Participants participated in two sessions in a randomized order separated by at least one week: PCMS combined with AIH (PCMS+AIH) and PCMS combined with sham AIH (PCMS+sham-AIH). During both sessions, participants remained at rest for all electrophysiological measurements taken before and after each protocol (Fig. 1B). PCMS was applied using a paired pulse paradigm previously described (Bunday and Perez, 2012; Taylor and Martin, 2009; Urbin et al., 2017).
Figure 1. Experimental set-up.
(A) Participants were seated in a customized chair during paired corticospinal-motoneuronal stimulation (PCMS) combined with acute intermittent hypoxia (PCMS+AIH) and sham AIH (PCMS+sham-AIH). PCMS was delivered using transcranial magnetic stimulation (TMS) over the hand representation of the primary motor cortex and supramaximal peripheral nerve stimulation (PNS) over the ulnar nerve with 180 pairs of stimuli delivered every 10 s (0.1 Hz). The position of the TMS coil was monitored using a frameless stereotaxic system and electromyographic (EMG) activity was recorded from the first dorsal interosseous (FDI) muscle to record electrophysiological outcomes. AIH was administered by using the Hypoxico Inc. (EVEREST SUMMIT II, New York) and the protocol consisted of 15 cycles of 1 min of inspiring ambient air with 1 min of hypoxic air. (B) Timeline of the experimental procedures. Electrophysiological outcomes were measured before (Baseline) and 30 min after each protocol.
PCMS.
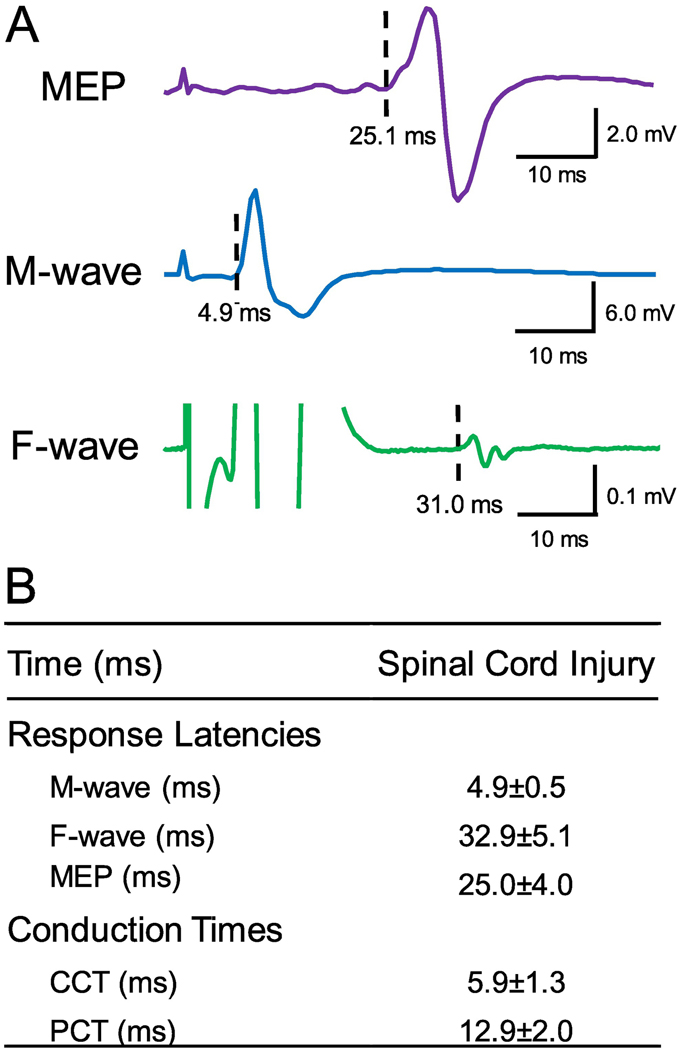
A total of 180 pairs of stimuli were delivered every 10 s (~34 min, 0.1 Hz) where corticospinal volleys evoked by TMS over the hand representation of the primary motor cortex were timed to arrive at corticospinal-motoneuronal synapses of the FDI muscle 1–2 ms before antidromic spikes evoked in motoneurons by supramaximal peripheral nerve stimulation (PNS) of the ulnar nerve. Magnetic stimuli were delivered with 100 % of the maximal stimulator output (MSO). PNS was set at an intensity of 150% of the intensity needed to evoke the maximal motor response (M-max) in the FDI muscle. Inter-stimulus intervals (ISIs) between TMS and PNS were tailored to individual subjects based on central (CCT) and peripheral (PCT) conduction times calculated from latencies of MEPs, C-root, F-wave, and M-max (Fig. 2). In 6 out of 16 participants, the ISI was calculated based on F-waves instead of C-root stimulation. This was done upon participant’s request based on preferred stimulation site and was kept consistent across all sessions. There was no significant difference in ISIs calculated based on F waves (6.1±1.4) and C-root stimulation (5.1±1.0, p=0.2). At ISIs of ~5.5 the F-wave resulting from the antidromic activation of the motoneuron somas and potentials evoked from magnetic stimulation cannot be distinguished. The peak-to-peak amplitude of the combined response was analyzed and expressed in relation to M-max for each subject. For statistical comparisons the responses to the 180 paired pulses were pooled in blocks of 10 resulting in 18 data points for each participant (Fig. 3C; Bunday and Perez, 2012). MEP latencies were recorded during isometric 10% of MVC index finger abduction to determine the shortest and clearest response for our estimations. C-root potentials, F-waves and M-max latencies were investigated during rest. For MEPs, C-root potentials and M-max, the onset latency was defined as the time when each response exceeded 2 SD of the mean rectified pre-stimulus activity (100 ms) in the averaged waveform, whereas the earliest onset latency of 40 F-waves was used. Cervical roots were stimulated using the same figure-of-eight coil with the outer edge of the coil placed between C7 and T1 spinous processes with the handle in a horizontal position pointing away from the cervical spine (Schmid et al., 1990; Inaba et al., 2002). Potentials evoked at the most proximal part of the C-roots were identified by increasing stimulation intensities in 5% increments until a consistent small response appeared. F-waves were measured using supramaximum stimulus intensity to the ulnar nerve at the wrist (200 μs pulse duration, DS7A; Digitimer) with a monopolar bar electrode with the cathode positioned proximally. The stimuli were delivered at 1 Hz at an intensity of 150% of the M-max. The M-max was similar across sessions (PCMS+AIH=13.5 mV, PCMS+sham-AIH=12.9 mV, p=0.4). For each trial, we quantified each F-wave’s latency. F-wave trials were filtered using a 2nd order Bessel high-pass filter (200 Hz) to ‘flatten the tail of the M-wave’ (Khan et al., 2012). M-max was acquired using our regular filter parameters (30–1000 Hz). Sixty F-waves were tested to determine PCMS ISIs. An F-wave was considered to be present if a response with a proper latency (minimum of 20 ms) had an amplitude >20 μV above background. The PCT was calculated using one of the two following calculations: (C-root – M-max+0.5) or (F-wave– M-max) * 0.5 and the CCT was calculated as MEP – C-root +1.5 or MEP - (PCT + M-max). PCT and CCT and ISIs between TMS and PNS are summarized in Figure 2B and were not different between the two testing sessions (all p>0.05).
Figure 2. Response latencies.
(A) Raw traces showing a motor-evoked potential (MEP), an F-wave, and the maximal motor response (M-max) for representative subject from the FDI muscle. (B) MEP, F-wave, and M-max latencies were used to calculate central (CCT) and peripheral conduction time (PCT) used to estimate time the arrival of pre- and postsynaptic volleys at the cortico-motoneuronal synapses for PCMS in SCI subjects.
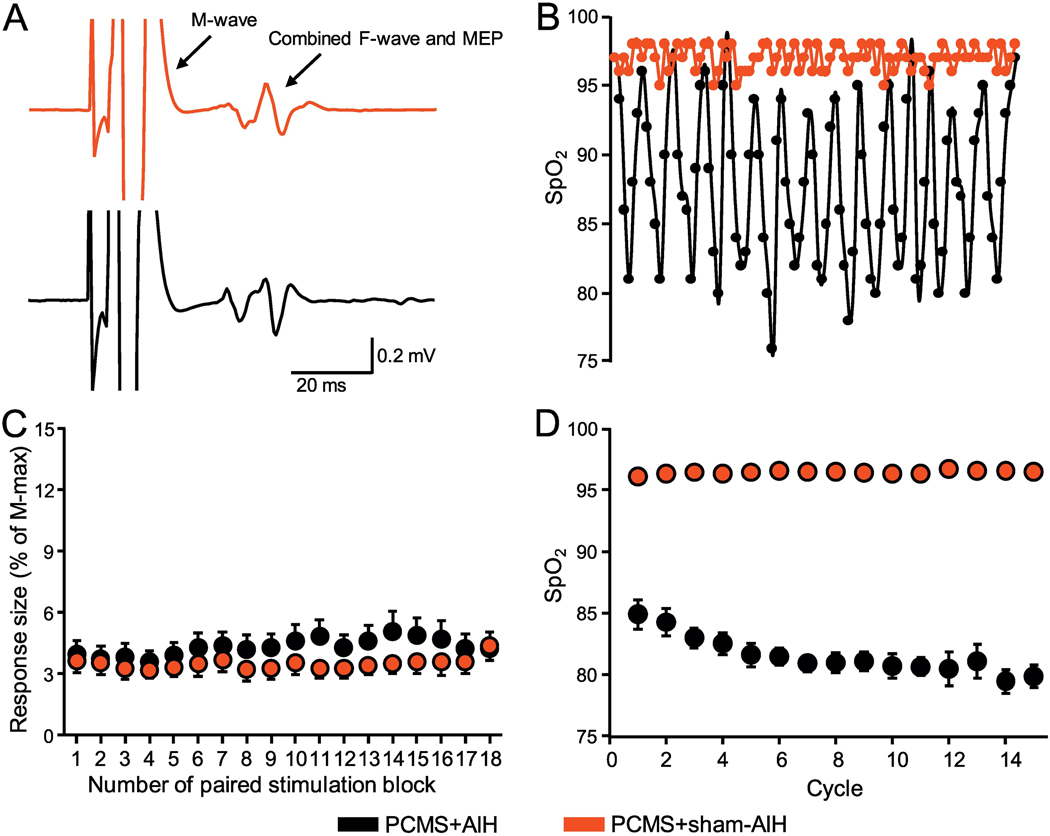
Figure 3. Monitoring during protocols.
(A) Raw traces from the FDI muscle during PCMS+sham-AIH and PCMS+AIH. Note that the M-wave is followed by an F-wave that is likely to be combined with a MEP elicited by TMS. (B) SpO2 from a representative participant during the PCMS+sham-AIH (red) and PCMS+AIH (black) protocols. The AIH protocol consisted of 15 cycles of 1 min of inspiring ambient air (20.9% O2) with 1 min of hypoxic air (9.4% O2). (C) The abscissa shows the number of pairs of stimuli during each protocol (a total of 180 pairs of stimuli). At each point, the average of 10 responses is shown. The ordinate shows the size of the conditioned response expressed as % of the M-max in SCI participants during PCMS+sham-AIH and PCMS+AIH. (D) Group data showing the SpO2 observed during the 15 cycles in the PCMS+sham-AIH (red) and PCMS+AIH (black) protocol.
AIH.
AIH was delivered through a face-mask with 2 one-way valves restricting inspiration to the top valve and expiration to the bottom valve using an oxygen generator (EVEREST SUMMIT II model, Hypoxico Inc, New York, New York; Fig. 1A). The hypoxic (FiO =9±0.1) and normoxic (FiO2=20.9±3%) gas mixture was delivered through the top valve. The protocol consisted of 15 episodes of hypoxia/normoxia interspaced with 1 min inspiring ambient air (gas mixture supply unplugged). We monitored oxygen concentration before during, and after both sessions using a Handi+® oxygen monitor (maxtec, Salt Lake City, Utah, US). Participants breathed through the mask for 2 min before the first cycle to familiarize them with procedures; heart rate and blood pressure were monitored for safety during the AIH protocol and values remained similar to baseline measurements at all times. Normal blood oxygen saturation levels (SpO2) and heart rate were assessed via pulse oximeter (Nonin 3150 WristOx2 ™, Nonin medical Inc, Plymouth, Minnesota, United States). SpO2 nadir after each 60 s exposure was noted and is presented in Figure 3D. During sham AIH, subjects breathed throughout the same mask the protocol consisted of 15 episodes of normoxia/normoxia interspaced with 1 min inspiring ambient air.
TMS.
Transcranial magnetic stimuli were applied using a figure-of-eight coil (loop diameter 70 mm) through a Magstim 2002 magnetic stimulator (Magstim, Whitland, Dyfed, UK) with a monophasic current waveform. Optimal position for eliciting a MEP in the FDI muscle (hot spot) was found by moving the coil with the handle pointing backward and 45° away from the midline in small steps across the hand representation of the contralateral primary motor cortex. With this coil position, the current flowed in a posterior-anterior direction and probably produced predominantly early I wave activation (Sakai et al., 1997). Coil position was monitored using a frameless stereotaxic neuro-navigation system (Brainsight 2, Rogue Research, Montreal, Canada). Two sets of 20 MEPs were collected at baseline and 30 min after each intervention.
MEPs elicited by TMS.
The resting motor threshold (RMT) was defined as the minimal stimulus intensity required to induce MEPs >50μV peak-to-peak amplitude above the background EMG in 5/10 consecutive trials in the relaxed muscle (Rothwell et al., 1999). MEPs were tested at rest at an intensity that evoked a MEP with amplitude of approximately 50% of MEP-max in both sessions (PCMS+AIH=75.5±11.7% of MSO, PCMS+sham-AIH=74.1±13.9% of MSO, p=0.8). MEP-max was defined at rest by increasing stimulus intensities in 5% steps of maximal device output until the MEP amplitude did not show additional increases (PCMS+AIH=1.9±1.7 mV, PCMS+sham-AIH=2.5±3.8 mV, p=0.4). At baseline, TMS pulses were delivered at 4 s intervals in sets of 20 separated by resting periods as needed. MEPs were visually inspected and analyzed trial by trial. Two sets of 20 MEPs were collected at each time point.
MEPs elicited by electrical stimulation.
We measured corticospinal transmission and/or motoneuron excitability by stimulating the corticospinal tract using high voltage electrical current (200 μs duration, Digitimer DS7AH) passed between two small gold-cup electrodes (GRASS Technologies ®, Astro Med ®, Inc., Warwick, Rhode Island, USA) placed behind the mastoid process at the cervicomedullary level (n=3; Taylor and Gandevia, 2004a). We adjusted the stimulation intensity to produce a MEP of ~3% M-max. This response was not contaminated by activation of cervical spinal roots. This was verified by increasing intensity until a latency decrement of ~2 ms indicated that the ventral roots were stimulation directly and then turning it back down with an ensuing increment in latency (Taylor and Gandevia, 2004a). In 6 subjects the corticospinal tract was stimulated through the scalp with the cathode at the vertex and anode 7 cm lateral towards the external meatus acusticus (Day et al., 1989) using a stimulus intensity needed to elicit MEPs of ~3% of M-max in the FDI muscle. The latency of MEPs elicited by cortical electrical stimulation (24.1±2.2 ms) was shorter than the latency of MEPs evoked by TMS (26.0±2.0 ms, p=0.007), suggesting that we stimulated corticospinal axons directly. Because MEPs elicited by cervico-medullary and cortical electrical stimulation showed the same changes across conditions we grouped all results together under MEPs elicited by electrical stimulation. Ten MEPs elicited by electrical stimulation were tested at baseline and 20 MEPs were collected 30 min after each protocol.
Small voluntary contractions.
Index finger abduction force and FDI EMG activity were measured during short, ballistic, isometric contractions into abduction using a custom LabView program (n=10). One cursor showed the target force (10% of MVC) and another cursor showed the force exerted by the subject. Subjects were instructed to perform index finger abduction to move the actual force to the target force as fast as possible without correcting the applied force production during the contraction based on the online visual feedback. After familiarization, four sets of 20 contractions were performed with partially simulated visual feedback of the cursor. Here, subjects controlled the movement of the cursor up to 8% of MVC and the remaining 2% of MVC was simulated by LabView. During the simulation period the speed of the cursor was maintained constant (1 video frame: 15.6ms duration, range=0.61 to 0.67% MVC/ms), while the end point of the cursor was randomly varied within ±0.5% standard deviation of the target (10% of MVC). This strategy was used to avoid online real feedback of the force exerted to help to maintain subjects unaware of the possible effects of the stimulation (Bunday and Perez 2012). Force and EMG during each contraction were measured during a 250 ms window (125 ms before and 125 ms after the peak force and EMG values). Forty contractions were performed before and 30 min after each intervention.
Statistics.
Sphericity was tested using Mauchly’s test. The distribution of the dependent variables was examined using Shapiro Wilkinson’s test and was natural log transformed (ln) in cases where it was not normally distributed. Repeated-measures analysis of variance (ANOVA) was performed to examine the effect of INTERVENTION (PCMS+AIH and PCMS+sham-AIH) and TIME (baseline and 30 min after each intervention) on MEP size, small voluntary contractions, and MVC. Repeated-measures ANOVA was used to examine the effect of BLOCK (from 1 to 18) and INTERVENTION on the response size of measurements acquired during PCMS. Paired t-tests were used to compare the size of the baseline MEPs and MVCs as needed. Bonferroni’s post hoc analysis was used to test for significant comparisons. Changes in SpO2 from baseline over the course of 15 cycles were analyzed using two-way repeated measures ANOVA in a 2 by 16 design. Response amplitudes during the intervention were contrasted to the first block of 20 to reduce the risk of type 2 errors. This resulted in 17 within and 18 between group comparisons. Ln-transformed relative changes in electrically evoked potentials were compared between the two conditions using a paired t-test. Significance was set at p<0.05. Group data are presented as the mean±SD in the text and figures.
Results
SpO2 and evoked responses during PCMS
The PCMS protocol consisted of 180 pairs of stimuli delivered every 10 s (0.1Hz, 10 blocks; Fig. 3C). Two-way repeated measures ANOVA revealed no effect of BLOCK (F3.6,50.8=1.8, p=0.1), INTERVENTION (F1,14=0.3, p=0.5) nor their interaction (F2.3,31.7 =1.3, p=0.2) on response size, suggesting that the stimulus intensity was maintained constant across conditions. The AIH protocol consisted of 15 episodes of hypoxia/normoxia interspaced with 1 minute inspiring ambient air (Fig. 3D). Two-way repeated measures ANOVA revealed an effect of INTERVENTION (F1,15=553.3, p<0.001), CYCLE (F5.8,87.1=45.3, p<0.001) and their interaction (F5.6,83.4=46.8, p<0.001) on SpO2. Post hoc testing revealed between-group differences at all 15 cycles (all p<0.001) but not at baseline (p=0.5). SpO2 was significantly lower during the PCMS+AIH versus PCMS+sham-AIH session in all cycles (all p<0.001) for PCMS+AIH, whereas no differences were observed during PCMS+sham-AIH (all p=1.0). The lowest average SpO2 was observed after cycle 14 during the PCMS+AIH treatment (79.4±0.9) and after the first cycle during PCMS+sham-AIH (96.0±0.4). SpO2 levels were different throughout both sessions (PCMS+AIH=81.5±3.2, PCMS+sham-AIH= 96.4±1.6, p<0.001).
MEPs elicited by TMS
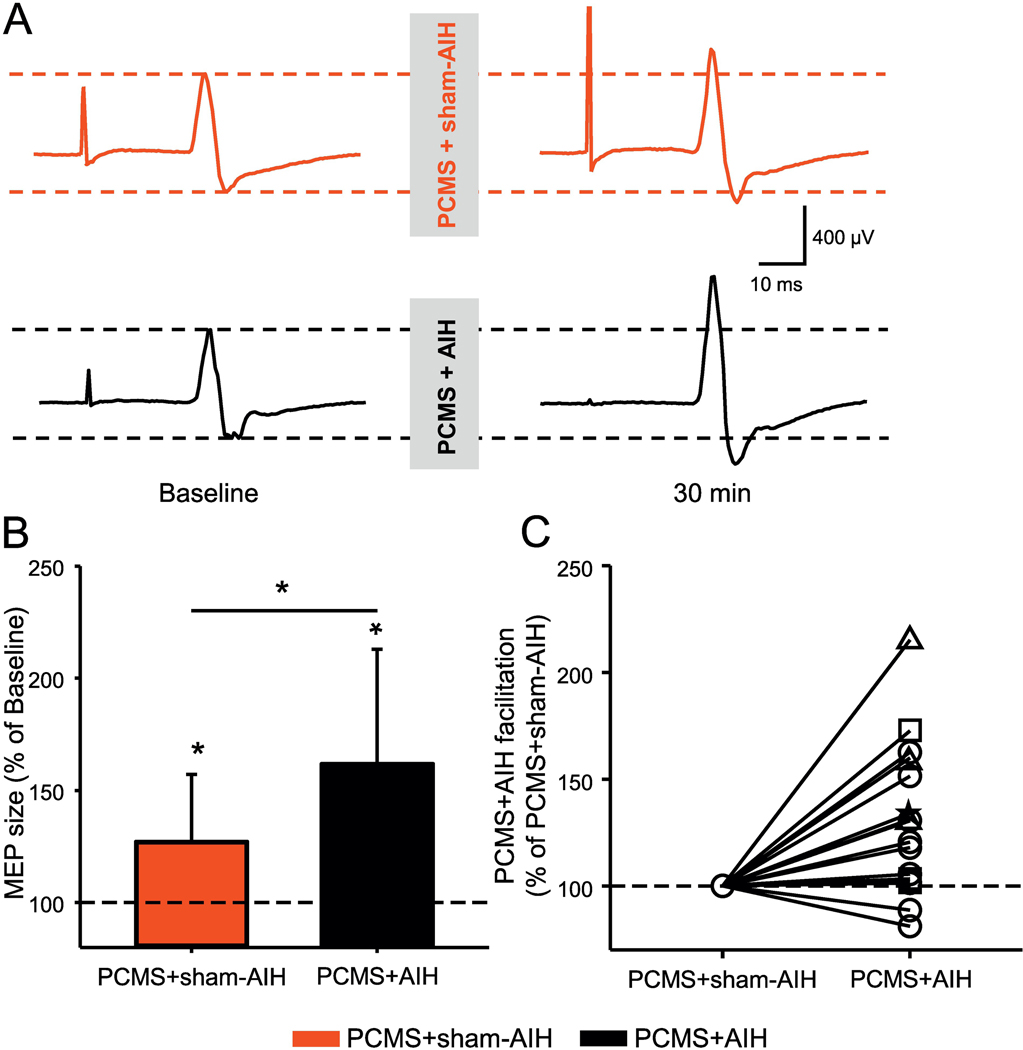
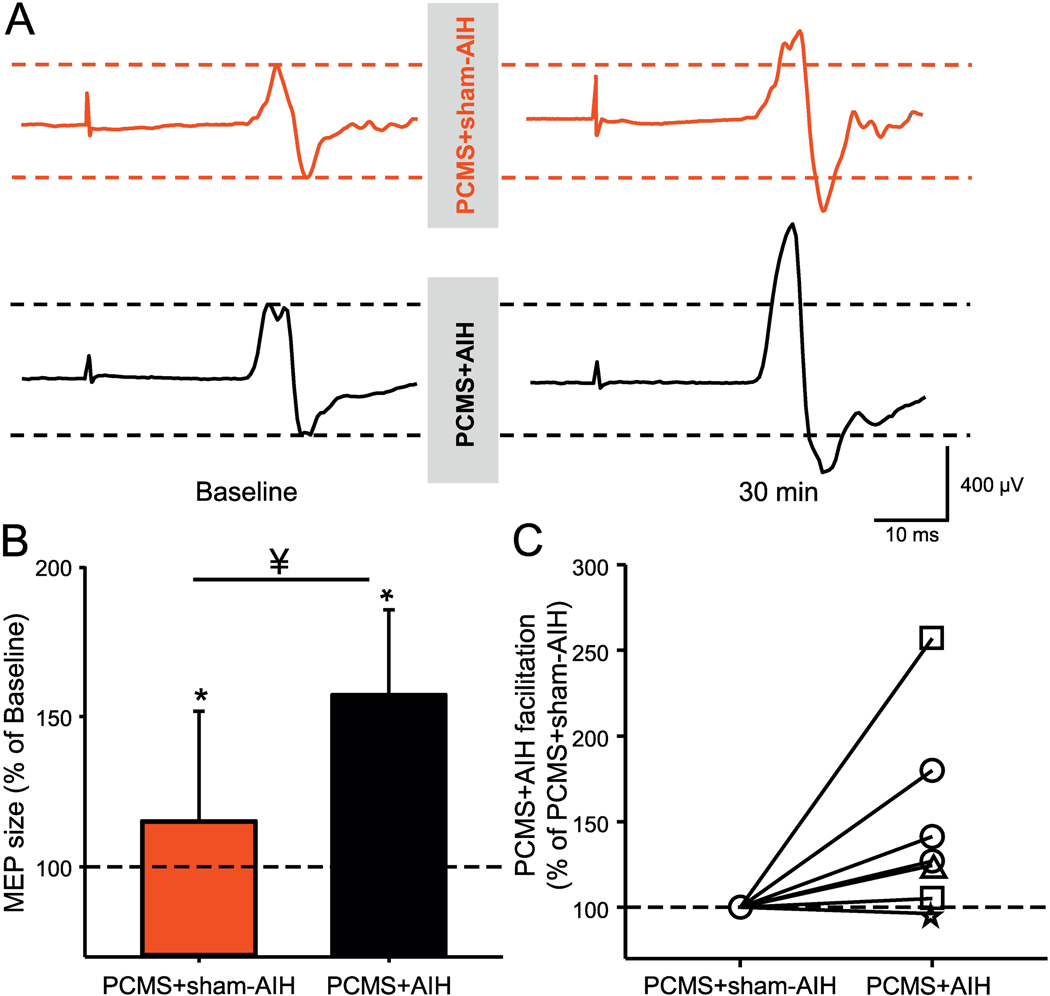
Figure 4A illustrates traces of MEPs elicited by TMS over the primary motor cortex in the FDI muscle from a representative SCI participant during both protocols. Here, the MEP amplitude increased largely after PCMS+AIH compared with PCMS+sham-AIH (Fig. 4B). Two-way repeated measures ANOVA revealed an effect of INTERVENTION (F1,15=26.3, p<0.001), TIME (F1,15=8.8, p=0.01), and in their interaction (F1,15=8.8, p=0.01) on MEP amplitude. Post Hoc comparisons revealed increases in MEP amplitude at 30 min (61.88±50.97%; p<0.001) after PCMS+AIH. This facilitation was significantly higher than following PCMS+sham-AIH (p=0.009). Following PCMS+sham-AIH the MEP amplitude increased by 27.0±30.0% on average. MEPs were facilitated in most subjects after PCMS+AIH (14/16, range from 1.4% to 115.2%) comparing baseline to measurements at 30 min after PCMS+sham-AIH (Fig. 4C).
Figure 4. MEPs elicited by TMS.
(A) Representative MEP traces recorded from the FDI muscle in a SCI participant before and 30 min after PCMS+sham-AIH (red traces) and PCMS+AIH (black traces). Waveforms represent the average of 20 MEPs. (B) Graph shows group data (n=16). The abscissa shows the protocols tested (PCMS+sham-AIH=red bar and PCMS+AIH=black bar) and the ordinate shows the size of MEPs expressed as a % of MEPs at baseline. (C) Graph shows individual data (AIS A=triangle, AIS B=star, AIS C=square, and AIS D=circle). The ordinate shows the % of changes in MEP amplitude after PCMS+AIH expressed as % of the changes after PCMS+sham-AIH. The dotted line indicates the baseline. Error bars indicate SDs. *p<0.05, comparison with baseline; ¥p<0.05, comparison between PCMS+sham-AIH and PCMS+AIH protocols.
MEPs elicited by electrical stimulation
Figure 5A illustrates traces of MEPs elicited by electrical stimulation over the cervicomedullary junction in the FDI muscle from a representative SCI participant during both protocols. MEP amplitude increased more after PCMS+AIH versus PCMS+sham-AIH. Two-way repeated measures ANOVA revealed an effect of INTERVENTION (F1,6=6.9, p=0.03), TIME (F1,6=14.7, p=0.009), and in their interaction (F1,7=6.9, p=0.03) on MEP amplitude. Post hoc comparisons revealed increases in MEP amplitude 30 min (57.2±28.2%) after PCMS+AIH. This facilitation was significantly higher than after PCMS+sham-AIH (15.2±36.5%, p=0.03). MEPs were facilitated in most subjects after PCMS+AIH (6/7, range from 5.05% to 156.9%) versus measurements taken after the PCMS+sham-AIH protocol (Fig. 5C).
Figure 5. MEPs elicited by electrical stimulation at the cervicomedullary level.
(A) Raw MEPs traces recorded from the FDI muscle of a representative SCI participant before and 30 min after PCMS+sham-AIH (red traces) and PCMS+AIH (black traces). Waveforms represent the average of 20 MEPs. (B) The graph shows group data (n=9) with the abscissa showing the protocols tested (PCMS+sham-AIH=red bar and PCMS+AIH=black bar) and the ordinate showing the size of MEPs expressed as % of MEPs at baseline. (C) Graph shows individual data (AIS A=triangle, AIS B=star, AIS C=square, and AIS D=circle). The ordinate shows the % of changes in MEP amplitude after PCMS+AIH expressed as a % of the facilitation after PCMS+sham-AIH. The dotted line indicates the baseline. Error bars indicate SDs. *p<0.05, comparison with baseline; ¥p<0.05, comparison between PCMS+sham-AIH and PCMS+AIH protocols.
Voluntary motor output
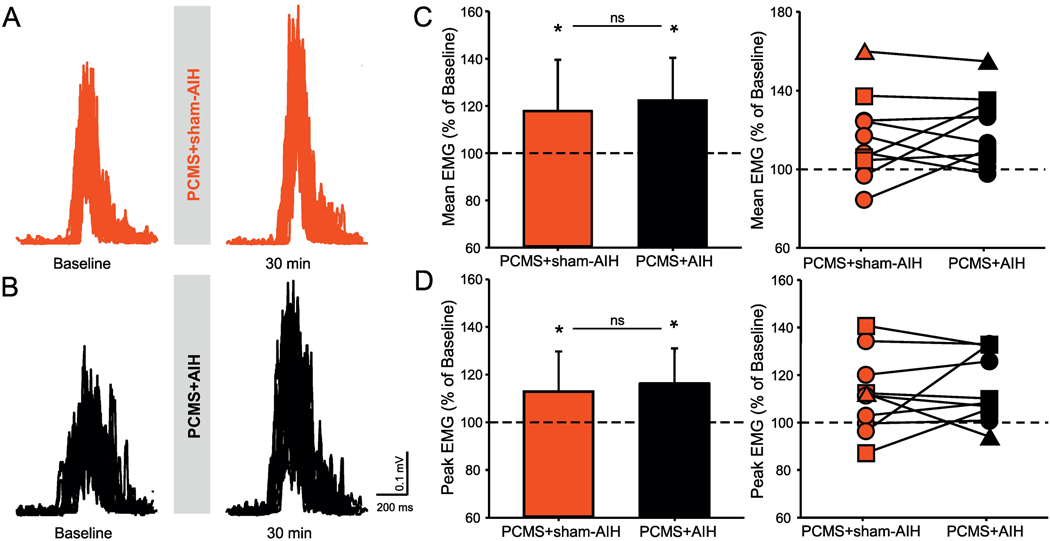
Figure 6 illustrates rectified raw EMG recordings from the FDI muscle from a representative SCI participant. Note that in this participant the magnitude of EMG increased to a similar extent after PCMS combined with AIH or sham-AIH. Repeated measures ANOVA revealed an effect of TIME (F1,9=9.1, p=0.001), but not INTERVENTION (F1, 9=0.2, p=0.6) or their interaction (F1,9=1.4, p=0.2), on mean EMG amplitude during small voluntary contractions. The mean EMG was facilitated in the subjects after PCMS+AIH (5/10, range from 1.5% to 32.3%) versus PCMS+sham-AIH. Similar results were found in peak EMG amplitude after both protocols [TIME (F1,9=8.7, p=0.01, but not INTERVENTION (F1,9=0.2, p=0.6) or their interaction (F1,9=1.5, p=0.2)]. Peak EMG was facilitated after PCMS+AIH (5/10, range from 1.1% to 37.6%), versus PCMS+sham-AIH.
Figure 6. Small level of voluntary contraction.
Representative EMG traces recorded during small level of voluntary contraction in a participant with SCI before and after (A) PCMS+sham-AIH (red traces) and (B) PCMS+AIH (black traces). Graphs show the group (n=10) and individual (AIS A=triangle, AIS C=square, and AIS D=circle) data. The abscissa shows the protocols tested (PCMS+sham-AIH=red bars and PCMS+AIH=black bars) and the ordinate shows the mean (C) and peak (D) EMG activity expressed as % of baseline EMG in the FDI muscle during small level of voluntary contractions (10% MVC) tested. The dotted line indicates the baseline. Error bars indicate SDs. *p<0.05, comparison with baseline.
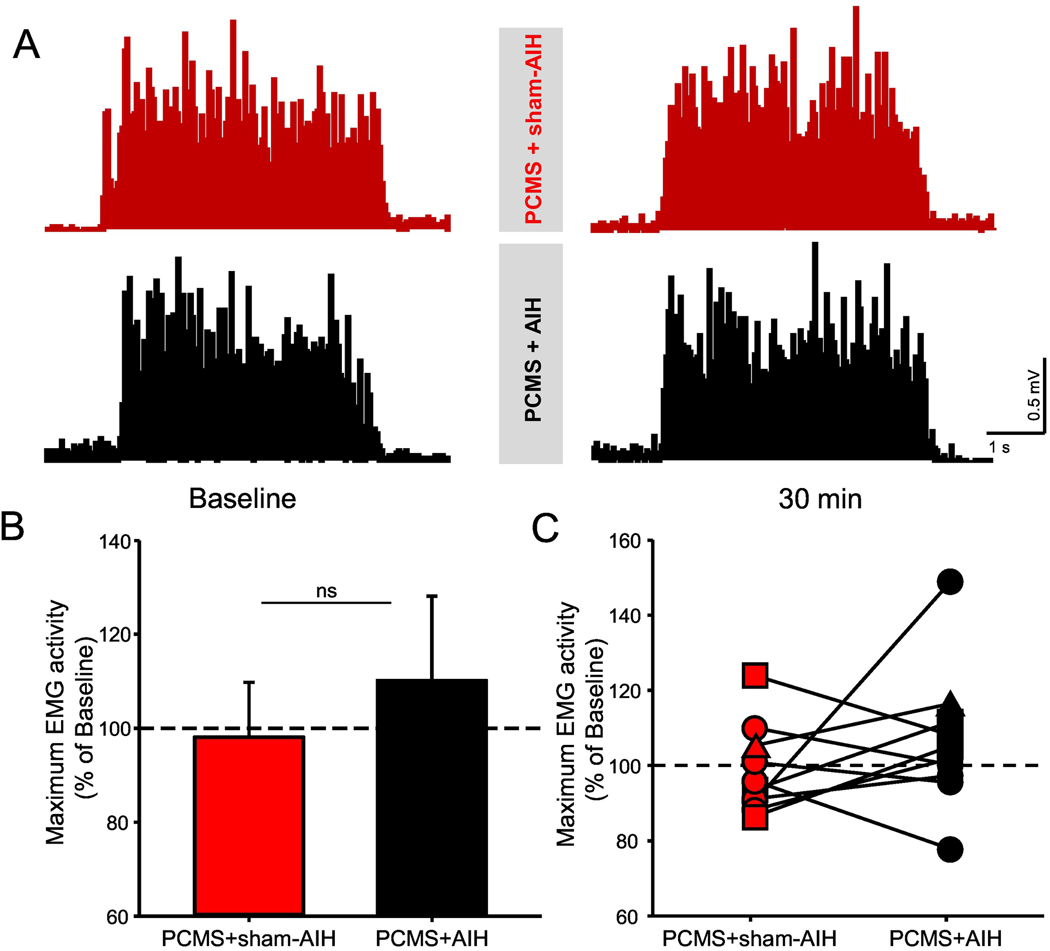
Figure 7 illustrates MVCs in the FDI muscle tested before and after PCMS+AIH and PCMS+sham-AIH in a representative SCI participant. Baseline MVC measurements were similar between the two protocols (PCMS+AIH=0.3±0.2 mV, PCMS+sham-AIH=0.3±0.1 mV, p=0.1). A two-way repeated measures ANOVA revealed no effect of INTERVENTION (F1,9=1.7, p=0.2), TIME (F1,9=1.7, p=0.2) or their interaction (F1,9=0.01, p=0.9) on MVC.
Figure 7. Maximum voluntary contraction (MVC).
(A) Representative EMG traces recorded during MVC (measured as the highest mean rectified EMG activity found in 1 s during the MVC burst) in a participant with SCI before and after PCMS+sham-AIH (red traces) and PCMS+AIH (black traces). (B) Graph shows the group data (n=10). The abscissa shows the protocols tested (PCMS+sham-AIH=red bars and PCMS+AIH=black bars) and the ordinate shows the mean EMG activity expressed as % of baseline during MVC. (C) Graph shows individual data (AIS A=triangle, AIS C=square, and AIS D=circle). The ordinate shows the magnitude change in MVC after 30 min of PCMS+sham-AIH (red circles) and PCMS+AIH (black circles). The dotted line indicates the baseline. Error bars indicate SDs. *p<0.05, comparison with baseline.
Discussion
Our findings support the view that AIH is an effective strategy to enhance PCMS-induced spinal synaptic plasticity in humans with chronic incomplete SCI. The amplitude of MEPs evoked by magnetic and electrical stimulation were further increased when PCMS was applied in combination with AIH, suggesting a spinal origin for these effects. This is consistent with previous results showing that both protocols elicit spinal plasticity. Combined PCMS with AIH or sham-AIH increased EMG activity to a similar extent in a finger muscle during brief, ballistic voluntary finger contractions. In contrast, neither protocol resulted in MVC changes. We argue that the fact that combined PCMS plus AIH potentiated physiological outcomes, but not voluntary motor output suggests that physiological effects have a lower threshold compared with effects on voluntary motor output, which needs to be considered when using combinatorial treatment approaches in humans with SCI.
Physiological effects of PCMS and AIH
Our results agree with previous findings that PCMS increases corticospinal excitability in upper limb muscles in normal subjects (Christiansen et al., 2018; D’Amico et al., 2017; Donges et al., 2017; Fitzpatrick et al., 2016; Taylor and Martin, 2009) and in humans with chronic SCI (Bunday and Perez, 2012; Bunday et al., 2018; Jo and Perez, 2020). The magnitude of PCMS effects on MEP amplitude elicited by magnetic and electrical stimulation (~20% increase) in our SCI participants was similar our previous studies (Bunday and Perez, 2012; Bunday et al., 2018; Jo and Perez, 2020). Because MEPs elicited by anodal electrical stimulation of the primary motor cortex activates axons of pyramidal tract neurons at the axon initial segment (Day et al., 1989), and stimulation at the cervicomedullary junction activates corticospinal axons at the pyramidal decussation (Taylor and Gandevia, 2004), increases in MEP amplitude likely have a subcortical origin.
The synergistic effects of PCMS and AIH on MEP amplitude are consistent with previous results in control subjects showing that combined PCMS and AIH had additive facilitatory effects on corticospinal excitability (Christiansen et al., 2018). PCMS enhances corticospinal transmission at the spinal cord level, likely engaging STDP mechanisms that depend on NMDA receptor activation (Donges et al., 2017). NMDA receptors are often critical to generate persistent activity arising from neural associations (Welch et al., 2020). One possibility is that the synergistic effects of PCMS and AIH on spinal synaptic plasticity relate to new BDNF synthesis (Welch et al., 2020). AIH increases BDNF expression in somatic motoneurons (Lovett-Barr et al., 2012; Satriotomo et al., 2016) and BDNF is necessary for NMDA receptor-dependent synaptic plasticity (Crozier et al., 2008). Motoneuron TrkB protein expression is necessary for AIH-induced, BDNF-dependent long-term facilitation of motor neurons (Dale et al., 2017), and TrkB plays an important role in neuroplasticity following SCI (Garraway and Huie, 2016). BDNF effects on spinal synaptic transmission can be either presynaptic or postsynaptic at the same connection (Arvanian and Mendell, 2001). For example, BDNF facilitates monosynaptic excitatory postsynaptic potentials in neonatal rat motoneurons via postsynaptic NMDA receptors (Arvanian and Mendell, 2001). In fact, PCMS is contingent upon the timely somatic/dendritic invasion of the antidromic volley in the motoneurons causing a depolarization of the postsynaptic membrane 1–2 ms after presynaptic end-boutons are depolarized by descending volleys triggered by the magnetic stimulation. Thus, the postsynaptic terminal is a likely site for potentiating AIH/PCMS combinatorial plasticity.
It is also possible that it is the serotonin induced BNDF synthesis (Baker-Herman et al., 2004) that might have contributed to our findings. Since spinal serotonin release and postsynaptic receptor activation is necessary and sufficient for AIH-induced phrenic motor plasticity (Baker-Herman and Mitchell, 2002; MacFarlane and Mitchell, 2009), it may play a similar role in the facilitatory effect of AIH on STDP-like plasticity. Serotonin modulates the excitability of motoneurons through both presynaptic and postsynaptic actions (Rekling et al., 2000). AIH triggers phasic activity in serotonergic neurons from the raphe nuclei projecting to the cervical spinal cord (Hayashi et al., 1993; Morris et al., 2008) where various 5-HT receptor subtypes are expressed in the active zone of postsynaptic ventral horn neurons (Ridet et al., 1994). Specifically, AIH-induced long-term facilitation in cervical motoneurons is contingent on 5-HT2 receptor activity (Tadjalli and Mitchell, 2019). Binding of 5-HT to both 5-HT2 receptors triggers intracellular cascades that combine to elicit long-term facilitation (Baker-Herman and Mitchell, 2002; Devinney et al., 2015; Tadjalli and Mitchell, 2019). Serotonin binding to Gq-metabotropic receptors also inhibits outward potassium currents, thereby amplifying NMDA-receptor dependent calcium influx (Gu, 2002; Johnson and Heckman, 2014). Consistent with the idea that both serotonin and BDNF modulate STDP-like plasticity in humans (Batsikadze et al., 2013; Cheeran et al., 2008). It is important to consider that we used a fixed FiO2 of 9% in all SCI participants; regardless of how responsive each individual was to hypoxia. Since individuals with SCI have low blood pressures (Krassioukov and Claydon, 2006), it is certainly possible that the same arterial oxygen saturation could lower spinal tissue PO2 more than in control subjects and, thus, alter the expression of plasticity.
Lower tissue PO2 is expected to shift the balance between serotonin-dependent versus adenosinergic mechanisms in motor circuitry, undermining, cancelling or even replacing serotonin-dependent effects (depending on the tissue PO2; Perim and Mitchell, 2020). Thus, variations in the degree of facilitation in response to PCMS+AIH could be, at least in part, related to different degrees of between-subject tissue hypoxia in response to the same AIH protocol. It is less likely that these variations were related to the degree of spared corticospinal tract fibers since similar effects were found in participants with larger and smaller MEP amplitudes and/or more or less impaired AIS levels.
Effects of PCMS and AIH on Voluntary Motor Output
Consistent with previous findings, PCMS combined with sham-AIH increased small levels of voluntary motor output in individuals with SCI (Bunday and Perez, 2012; Urbin et al., 2017). One critical question is why combined PCMS and AIH did not further increase MVCs and/or small level voluntary output. Using a similar AIH protocol, Trumbower and collaborators (2012) showed increased voluntary ankle strength in a similar group of SCI participants. It is possible that AIH exerts differential actions on upper vs lower limb muscles. Hand muscles, as tested in our study, are under a strong cortical control, and even a single AIH session induces changes at subcortical (not cortical) levels (Christiansen et al., 2018). However, AIH also improves hand function in people with SCI (Trumbower et al., 2017). It is also possible that PCMS prevented AIH-induced increases in maximal voluntary output and/or that the lack of sufficient synergy to change voluntary motor output may reflect the fact that AIH and PAS involve similar pathways. This possibility is unlikely since AIH potentiated PCMS effects on corticospinal excitability in this and an earlier publication from our group (Christiansen et al., 2018), suggesting additive effects between these plasticity protocols.
A parsimonious explanation is that PCMS and AIH target different sizes of motoneurons, consistent with evidence that motoneurons of different size have different sensitivities to different plasticity mechanisms (Krishnan, 1983). This concept is supported by findings that a single PCMS session increases the magnitude of small levels of voluntary motor output (likely mediated by small motor units), but not MVCs (likely mediated by small and large motor units) in humans with and without SCI (Taylor and Martin, 2009; Bunday and Perez, 2012; D’Amico et al., 2018; Donges et al., 2019; Jo and Perez, 2020). Further support for this idea is provided by our observation that larger MEPs showed greater increases in amplitude versus smaller MEPs with combined PCMS and AIH. Since TMS recruits contralateral motor units in sequence from small to large, small and large motor units likely contribute to larger MEPs (Bawa and Lemon, 1993; Gandevia and Rothwell, 1987; Mills, 1991). Thus, if PCMS favors low threshold motoneurons whereas AIH favors high threshold motoneurons, we might expect that their combination increases motor output during small and large contractions as with physiological outcomes. These differential actions might be explained by threshold differences in physiological versus motor output gains. The responsiveness of motoneurons right before (Federico and Perez, 2017) and during (Vastano and Perez, 2020) voluntary activity decreases in humans with SCI versus control participants, which could contribute to the different thresholds between physiological vs motor output outcomes.
The lack of increases in voluntary motor output may suggest that changes in serotonin release and/or BDNF levels, which are needed to promote synaptic plasticity (Cotman et al., 2007; Baker-Herman et al., 2004), were not adequate. This idea is supported by observations that chronic protocols (1 week) of more intense intermittent hypoxia elicit greater and/or more enduring BDNF-related plasticity (Fuller et al., 2003; Navarette-Opazo et al., 2017) and that AIH can result in aberrant plasticity under some conditions (Warren et al., 2018). Multiple PCMS sessions are also needed to increase MVCs in humans with SCI (Jo and Perez, 2020). In the present study, we only measured EMG activity during maximal voluntary contraction. Previous studies demonstrated parallel changes in torque and EMG activity following PCMS and AIH (Bunday and Perez, 2012; Trumbower et al., 2012; Sandhu et al., 2019), but, considering the complex relationship between EMG-force output (Paquin and Power, 2018), future studies will benefit from including both outcomes. It is less likely that other factors such as the time of measurements contributed to our findings. In the study by Trumbower and collaborators (2012), MVC in plantarflexor muscles was increased 30 and 60 minutes after AIH; our measurements were completed 30 min after AIH. Similarly, PCMS effects on small levels of voluntary contraction in upper and lower limb muscles were present 30 min post-stimulation, and returned to baseline ~60–80 min after the end of the stimulation (Bunday and Perez, 2012; Bunday et al., 2018; Urbin et al., 2017). We also highlight that either PCMS or AIH alone increases corticospinal excitability and voluntary motor output in people with SCI. Although we did not test the effect of AIH alone on corticospinal excitability in the present study, unpublished data from our laboratory indicates that the amplitude of corticospinal responses elicited by TMS increases after a single PCMS and AIH session in people with SCI. A direct comparison of the physiological and motor output magnitudes between protocols is difficult since the nature of protocols differs. Our results indicate that the beneficial effects of these protocols are complementary on corticospinal excitability, but this does not extrapolate to voluntary motor outcomes, suggesting it is difficult to determine which protocol might represent the best therapy.
Functional considerations
We combined PCMS with AIH to engage and facilitate residual corticospinal-motoneuronal connections in humans with SCI. This approach is supported by a number of studies showing that AIH combined with activity-dependent plasticity protocols triggers recovery of motor function in humans with incomplete SCI (Lynch et al., 2016; Navarrete-Opazo et al., 2016; Navarrete-Opazo et al., 2017; Trumbower et al., 2012; Hayes et al., 2014; Sandhu et al., 2019). This idea agrees with multiple combinatorial, non-invasive neurostimulation strategies that successfully potentiated outcomes in humans with SCI (Angeli et al., 2014; Belci et al., 2004; Gad et al., 2018; Gerasimenko et al., 2015; Gomes-Osman and Field-Fote, 2015; Harkema et al., 2011). An important observation is that, whereas combined AIH and PCMS potentiated physiological outcomes, they failed to impact volitional motor behaviors. Similarly, after STDP-like plasticity protocols, physiological outcomes that rely on excitability changes are modulated without changes in voluntary motor output (Elahi et al., 2014; Hussain et al., 2016). Additional studies demonstrate disparities between physiological and behavioral changes after STDP-like plasticity (Rajji et al., 2011; Stefan et al., 2006). Future studies of plasticity-inducing protocols that prime activity-dependent plasticity in controls (Hurley and Machado, 2017) and participants with SCI (Jo and Perez, 2020; Potter-Baker et al., 2018) should incorporate outcomes that reflect both physiological and voluntary motor output improvements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ, 2014. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137, 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM, 2001. Removal of NMDA receptor Mg(2+) block extends the action of NT-3 on synaptic transmission in neonatal rat motoneurons. J Neurophysiol 86, 123–129. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respiration physiology 104, 251–260. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS, 2002. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 6239–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T, Mitchell G, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. The Journal of Physiology 529, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Paulus W, Kuo MF, Nitsche MA, 2013. Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmacology 38, 2260–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Lemon RN, 1993. Recruitment of motor units in response to transcranial magnetic stimulation in man. J Physiol 471, 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ, 2004. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 42, 417–419. [DOI] [PubMed] [Google Scholar]
- Benavides FD, Jo HJ, Lundell H, Edgerton VR, Gerasimenko Y, Perez MA, 2020. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. The Journal of neuroscience : the official journal of the Society for Neuroscience 40, 2633–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Perez MA, 2012. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol 22, 2355–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunday KL, Urbin MA, Perez MA, 2018. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul 11, 1083–1092. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC, 2008. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol 586, 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Urbin MA, Mitchell GS, Perez MA, 2018. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. Elife 7, e34304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA, 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Bi C, Han YR, Plummer MR, 2008. BDNF modulation of NMDA receptors is activity dependent. J Neurophysiol 100, 3264–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Donges SC, Taylor JL, 2018. Paired corticospinal-motoneuronal stimulation increases maximal voluntary activation of human adductor pollicis. J Neurophysiol 119, 369–376. [DOI] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS, 2017. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Experimental neurology 287, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD, 1989. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412, 449–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS, 2015. Phrenic long-term facilitation requires PKCtheta activity within phrenic motor neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 8107–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donges SC, Boswell-Ruys CL, Butler JE, Taylor JL, 2019. The effect of paired corticospinal-motoneuronal stimulation on maximal voluntary elbow flexion in cervical spinal cord injury: an experimental study. Spinal Cord 57, 796–804. [DOI] [PubMed] [Google Scholar]
- Donges SC, D’Amico JM, Butler JE, Taylor JL, 2018. Involvement of N-methyl-d-aspartate receptors in plasticity induced by paired corticospinal-motoneuronal stimulation in humans. J Neurophysiol 119, 652–661. [DOI] [PubMed] [Google Scholar]
- Elahi B, Hutchison WD, Daskalakis ZJ, Gunraj C, Chen R, 2014. Dose-response curve of associative plasticity in human motor cortex and interactions with motor practice. J Neurophysiol 111, 594–601. [DOI] [PubMed] [Google Scholar]
- Federico P, and Perez MA. Altered corticospinal function during movement preparation in humans with spinal cord injury. J Physiology, 595:233–245, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SC, Luu BL, Butler JE, Taylor JL, 2016. More conditioning stimuli enhance synaptic plasticity in the human spinal cord. Clin Neurophysiol 127, 724–731. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS, 2000. Long term facilitation of phrenic motor output. Respir Physiol 121, 135–146. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr., Mitchell GS, 2003. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 2993–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS, 2001. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90, 2001–2006; discussion 2000. [DOI] [PubMed] [Google Scholar]
- Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, Edgerton VR, 2018. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J Neurotrauma 35, 2145–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Rothwell JC, 1987. Knowledge of motor commands and the recruitment of human motoneurons. Brain 110 ( Pt 5), 1117–1130. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Huie JR, 2016. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast 2016, 9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie Edgerton V, 2015. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med 58, 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Osman J, Field-Fote EC, 2015. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil 29, 771–782. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119, 1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, 2002. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR, 2011. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR, 1993. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol 265, R811–819. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine 2A receptor inhibition enhances phrenic long term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley R, Machado L, 2017. Using tDCS priming to improve brain function: Can metaplasticity provide the key to boosting outcomes? Neurosci Biobehav Rev 83, 155–159. [DOI] [PubMed] [Google Scholar]
- Hussain SJ, Darling WG, Cole KJ, 2016. Recent History of Effector Use Modulates Practice-Dependent Changes in Corticospinal Excitability but Not Motor Learning. Brain Stimul 9, 584–593. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Perez MA, 2020. Corticospinal-Motoneuronal Plasticity Further Promotes Exercise-Mediated Recovery in Humans with Spinal Cord Injury Brain in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ, 2014. Gain control mechanisms in spinal motoneurons. Front Neural Circuits 8, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassioukov A, and Claydon VE 2006. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res 152, 223–229. [DOI] [PubMed] [Google Scholar]
- Krishnan RV, 1983. A theory on the lability and stability of spinal motoneuron soma size and induction of synaptogenesis in the adult spinal cord. Int J Neurosci 21, 279–292. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ, 2017. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med 40, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B, 2015. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985) 118, 520–532. [DOI] [PubMed] [Google Scholar]
- Mills KR, 1991. Magnetic brain stimulation: a tool to explore the action of the motor cortex on single human spinal motoneurones. Trends Neurosci 14, 401–405. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C, 2017a. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J Neurotrauma 34, 1803–1812. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Dougherty BJ, Mitchell GS, 2017b. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp Neurol 287, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin J, Power GA. History dependence of the EMG-torque relationship. J Electromyogr Kinesiol 2018; 41: 109–15. [DOI] [PubMed] [Google Scholar]
- Potter-Baker KA, Janini DP, Lin YL, Sankarasubramanian V, Cunningham DA, Varnerin NM, Chabra P, Kilgore KL, Richmond MA, Frost FS, Plow EB, 2018. Transcranial direct current stimulation (tDCS) paired with massed practice training to promote adaptive plasticity and motor recovery in chronic incomplete tetraplegia: A pilot study. J Spinal Cord Med 41, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser-Loose EJ, Hassan A, Mitchell GS, Muir GD, 2015. Delayed Intervention with Intermittent Hypoxia and Task Training Improves Forelimb Function in a Rat Model of Cervical Spinal Injury. J Neurotrauma 32, 1403–1412. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Liu SK, Frantseva MV, Mulsant BH, Thoma J, Chen R, Fitzgerald PB, Daskalakis ZJ, 2011. Exploring the effect of inducing long-term potentiation in the human motor cortex on motor learning. Brain Stimul 4, 137–144. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL, 2000. Synaptic control of motoneuronal excitability. Physiol Rev 80, 767–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Tamir H, Privat A, 1994. Direct immunocytochemical localization of 5-hydroxytryptamine receptors in the adult rat spinal cord: a light and electron microscopic study using an anti-idiotypic antiserum. J Neurosci Res 38, 109–121. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W, 1999. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52, 97–103. [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I, 1997. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113, 24–32. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Gray E, Kocherginsky M, Jayaraman A, Mitchell GS, Rymer WZ, 2019. Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons With Chronic Incomplete Spinal Cord Injury. Neurorehabil Neural Repair 33, 911–921. [DOI] [PubMed] [Google Scholar]
- Satriotomo I, Nichols NL, Dale EA, Emery AT, Dahlberg JM, Mitchell GS, 2016. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience 322, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J, 2006. Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex 16, 376–385. [DOI] [PubMed] [Google Scholar]
- Tadjalli A, Mitchell GS, 2019. Cervical spinal 5-HT2A and 5-HT2B receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. J Appl Physiol (1985) 127, 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC, 2004. Noninvasive stimulation of the human corticospinal tract. J Appl Physiol (1985) 96, 1496–1503. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Martin PG, 2009. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 11708–11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR, 2013. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, 2012. Exposure to Acute Intermittent Hypoxia Augments Somatic Motor Function in Humans With Incomplete Spinal Cord Injury. Neurorehabilitation and Neural Repair 26, 163–172. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA, 2017. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology 89, 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbin MA, Ozdemir RA, Tazoe T, Perez MA, 2017. Spike-timing-dependent plasticity in lower-limb motoneurons after human spinal cord injury. J Neurophysiol 118, 2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastano R, Perez MA. Changes in Motoneuron Excitability during Voluntary Muscle Activity in Humans with Spinal Cord Injury. J Neurophysiology, 123:454–461, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren PM, Steiger SC, Dick TE, MacFarlane PM, Alilain WJ, Silver J, 2018. Rapid and robust restoration of breathing long after spinal cord injury. Nat Commun 9, 4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch Sutor T., Vose AK, Perim RR, Fox EJ, Mitchell GS, 2020. Synergy Between Acute Intermittent Hypoxia and Task-Specific Training. Exerc Sport Sci Rev doi: 10.1249/JES.0000000000000222. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]