Abstract
The allure of tobacco smoking is linked to the instant gratification provided by inhaled nicotine. Unfortunately, tobacco curing and burning generates many mutagens including more than 70 carcinogens. There are two types of mutagens and carcinogens in tobacco smoke (TS): direct DNA damaging carcinogens and procarcinogens, which require metabolic activation to become DNA damaging. Recent studies provide three new insights on TS-induced DNA damage. First, two major types of TS DNA damage are induced by direct carcinogen aldehydes, cyclic-1,N2-hydroxy-deoxyguanosine (γ- OH-PdG) and α-methyl-1,N2- γ -OH-PdG, rather than by the procarcinogens, polycyclic aromatic amines and aromatic amines. Second, TS reduces DNA repair proteins and activity levels. TS aldehydes also prevent procarcinogen activation. Based on these findings, we propose that aldehydes are major sources of TS induced DNA damage and a driving force for carcinogenesis. E-cigarettes (E-cigs) are designed to deliver nicotine in an aerosol state, without burning tobacco. E-cigarette aerosols (ECAs) contain nicotine, propylene glycol and vegetable glycerin. ECAs induce O6-methyl-deoxyguanosines (O6-medG) and cyclic γ-hydroxy-1,N2--propano-dG (γ -OH-PdG) in mouse lung, heart and bladder tissues and causes a reduction of DNA repair proteins and activity in lungs. Nicotine and nicotine-derived nitrosamine ketone (NNK) induce the same types of DNA adducts and cause DNA repair inhibition in human cells. After long-term exposure, ECAs induce lung adenocarcinoma and bladder urothelial hyperplasia in mice. We propose that E-cig nicotine can be nitrosated in mouse and human cells becoming nitrosamines, thereby causing two carcinogenic effects, induction of DNA damage and inhibition of DNA repair, and that ECA is carcinogenic in mice. Thus, this article reviews the newest literature on DNA adducts and DNA repair inhibition induced by nicotine and ECAs in mice and cultured human cells, and provides insights into ECA carcinogenicity in mice.
Keywords: DNA damage, DNA repair, tobacco smoke, E-cigarette, carcinogenesis
Introduction
Tobacco smoke (TS) delivers the stimulant nicotine, which can reach the brain within seconds and provide instant gratification (1). Unfortunately, TS contains carcinogenic compounds and TS has long been recognized as a human carcinogen (2– 4). TS is responsible for roughly one-third of human cancer incidence (5–7). Lung cancer is the most prevalent TS induced cancer; 85% of lung cancer is TS related (8, 9). Annually there are 1.8 million lung cancer deaths worldwide, with more than 130,000 in the US alone (7, 10). The lung cancer risk of life-long smokers is 23 times higher than non-smokers (7, 11, 12). The major cancer etiological agents in TS come from tobacco curing and burning. More than 7000 chemicals resulting from the incomplete combustion products of tobacco leaves, and among these over 70 chemicals have been proven to be potently carcinogenic in animal models (13–17).
TS compositions have been studied since the early 1950s (14). There has been extensive study on the effects of individual TS compounds. As well, total TS induced DNA damage and TS effects on cellular functions and tumorigenicity have been widely studied (14). Numerous excellent reviews on these topics have been published (2, 11, 16), and the topics of identifying TS carcinogens and their carcinogenic mechanisms have been well-covered. Therefore, this article does not address the known chemical nature of carcinogenic TS agents, such as polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatic amines (AAs), DNA damage induced by these agents, or their carcinogenicity. Rather, this article focuses on the puzzling questions arising from recent results on DNA damage induced by TS in the lung tissues of mice and humans (18–21).
Nicotine is a major component of TS. It is known to be addictive, but it is believed to be non-carcinogenic (2, 12, 22, 23). The lack of strong evidence that nicotine is carcinogenic, as opposed to the 70 known carcinogens in TS, is probably one of the leading reasons, aside from profit, behind the development of stimulant nicotine delivery devices that do not involve burning tobacco. The most notable devices include nicotine patches, nicotine gum, and electronic-cigarettes (E-cigs) (24–26).
E-cigs are designed to deliver nicotine in an aerosol state. Nicotine is dissolved in relatively harmless organic solvents [propylene glycol (PG) and vegetable glycerin (VG)] and aerosolized by controlled electric heating (~250 oC) (27, 28). E-cigs do not use tobacco leaves, do not have curing byproducts, do not contain tobacco specific nitrosamines (TSNAs), and the process of generating E-cig aerosols (ECAs) (or vapor) does not involve burning, Therefore ECAs do not contain incomplete combustion byproducts (29). E-cig aerosol (ECA) contains only aerosolized nicotine and organic solvents, therefore, E-cigs are promoted as delivering a TS ‘high’ without its negative effects (30). The population of E-cig users is rising rapidly. Currently there are more than 11 million E-cig users in the US alone, among them 4 million are middle and high school students (31). The health effects of ECAs, particularly their potential carcinogenicity, deserves careful scrutiny. This article will focus on reviewing the current understanding of DNA damage, DNA repair inhibition and carcinogenic effect induced by nicotine and ECA in mice and in cultured human cells, as well as ECA carcinogenicity in mice.
Although many E-cigs on the market contain flavors besides nicotine and the PG and VG solvents, study on the effect of flavors in the ECA on the DNA damage at molecular level remains lacking (32), and the effect of flavors in E-cig on the cytotoxicity and endothelial dysfunction remain controversial (33, 34). Therefore, the topic of the role flavor on ECA chemistry and biological effect is beyond the scope of this review.
Paradox of tobacco smoke carcinogenicity in humans and mice
It is well established that only 10–15% of life-time heavy smokers go on to develop lung cancer and, on average, it takes two decades for smokers to develop lung cancer (35–37). This low lung cancer incidence among heavy smokers is in stark contrast to many occupational exposure related cancers with high incidence, such as the 40–50% bladder cancer rate among life-time printing shop workers who were exposed to aminobiphenyl (38), and mesothelioma in 23–90% of asbestos workers (39). In addition to the puzzling results of low incidence of cancer among heavy smokers are the findings that TS is not a strong carcinogen in animal models (40, 41). In fact, TS has been classified as a weak carcinogen because it does not significantly enhance spontaneous lung cancer incidence in mouse models (40, 41). Intriguingly, many TS compounds, such as benzo(a)pyrene (BP), and TSNAs, induce mutagenesis and carcinogenesis more effectively than TS in animal models (12, 42–44). To date, the underlying reasons why despite containing 70 carcinogens, TS as a whole is not a strong cancer-causing agent in animal models remain unclear, despite many attempts to address this question (12, 17, 45–48).
TS induces DNA damage in humans and in mice
It is well established that TS contains more than 70 proven carcinogens (13–16).The relatively low lung cancer incidence (10–15%) among life-time tobacco smokers based on human epidemiological study results and from animal experiments (40, 41,49–52) raises three possible scenarios on TS carcinogenesis: first, the carcinogenicity of TS is not additive from the 70 or so carcinogens in TS; second, the bioavailable carcinogens in TS are low; and third, TS components may interfere with the metabolic activation of procarcinogens. To understand the reasons behind the low carcinogenicity of TS and to distinguish the hypothetic aforementioned scenarios, identification of DNA damage induced by TS in vivo is essential. This is a critical way to fully understand the mechanism of TS carcinogenesis and the role of individual TS carcinogens.
TS contains numerous chemicals such as aldehydes, which have been shown to directly bond with naked DNA and cellular genomic DNA to form different isomeric forms of DNA adducts (19, 53, 54). TS also contains numerous compounds, such as polycyclic aromatic hydrocarbons (PAHs), heterocyclic aromatic amines (AAs), nitrosamines and benzene, which via metabolic activation, can be transformed into derivatives that can covalently bond with naked DNA and cellular genomic DNA to form different isomeric forms of DNA adducts (38, 55, 56). Recently, we found that aflatoxin B1 (AFB1) following metabolism can induce oxidative stress and lipid peroxidation, consequently inducing abundant lipid peroxidation byproducts, aldehydes, that induce more DNA adducts than those induced by metabolically activated AFB1 (57). Although AFB1 is rare in TS, many enzymes that metabolize it, such as, CYP1A1, CYP1A2, CYP2A6, and CYP3A4, also have the capability to metabolize many TS compounds and lead to the same aldehyde-dependent outcome as AFB1 (55). Therefore, it is reasonable to assume that many TS components can induce secondary toxicants, which can form oxidative DNA damage and aldehyde-DNA adducts with cellular genomic DNA. On the other hand, it has been found that TS aldehydes, such as acrolein, can inhibit TS indirect DNA damaging agents, such as benzo(a)pyrene (BP) and nicotine-derived nitrosamine ketone (NNK) to induce DNA damage, presumably via their covalently modification of p450 enzymes (20).
The fact that TS contains numerous carcinogens, which by themselves can cause DNA damage, mutations and tumorigenesis, yet TS, as a whole, is a weak mutagen and carcinogen in animal models (28,29), raises the possibility that TS-induced DNA damage is not a sum of potential DNA damage from all TS DNA damaging agents. If this is true, then determining the major DNA damage induced by TS, and not focusing on the minute novel DNA adducts, is crucial for understanding the mechanisms of TS carcinogenesis.
With this in mind this article focuses on reviewing the literature for TS that: 1) identifies the more abundant TS-induced DNA adducts rather than dwelling on finding new and minor DNA adducts; and 2) explores the potential mechanisms of the TS DNA adduct induction and their role in carcinogenesis.
Quantify the total DNA adducts induced by TS
At least four important issues should be considered when interpreting the results from measuring TS-induced DNA damage. First, there are differences in p450 expression in different organs which determines the amount of procarcinogens (such as PAHs TSNAs, and AAs) that become DNA damaging agents (12, 58–60). Second, there are potential interactions among different TS components, which may attenuate DNA adduct formation compared to that observed in studies of individual TS components.Third, TS components may prevent the activation of procarcinogens becoming DNA binding agents (20, 61). Fourth, many DNA adducts can be formed in TS exposed animals and humans, which result from secondary toxicants rather than from TS components directly (57). Therefore, one cannot assume that TS induced DNA adducts are a sum of all DNA damaging agents in TS. To assess TS induced DNA damage in vivo, it is imperative to measure DNA adducts directly from different tissues of animals and humans following exposures to the mixture that is TS.
The analytical method for detecting TS-induced DNA adducts was originally developed by Randerath and his colleagues in the 1980’s, and then improved in the 1990’s. It involves 32P labeling of adducted nucleotide monophosphates and two- dimensional thin layer chromatography/high performance liquid chromatography (2D- TLC/HPLC) separation of the adducted deoxyribonucleoside monophosphate (dNMP) from the unadducted ones. This method remains one of the best methods to detect the totality of the abundant DNA adducts induced by TS (62–67). This method uses various phosphodiesterase to digest DNA to dNMPs, then employs a kinase to label the dNMPs (including those that are adducted) with 32P and nuclease P1 (NP1) to dephosphorylate the unadducted dNMPs. The 32P-labeled and adducted dNMPs are then separated via 2D-TLC and form a so called diagonal radioactive zone (DRZ) (66, 67). The DRZ contains most, if not all, of the TS-induced DNA adducts including PAH-, alkyl-, AA- and aldehyde-dNMP adducts (62, 66, 67). The DNA adducts in the DRZ can be further separated by HPLC using elution conditions tailored based upon the nature of the DNA adducts (20, 68). The amount of DRZ formation has been shown to be related to the level of TS consumption (66). The quantification of DNA adducts is based on the assumption that 32P labelling efficiency of DNA adducts versus deoxyguanosine monophosphate (dGMP) has a constant relationship (69, 70). It is foreseeable that this “constant relationship” could vary for different DNA adducts with different structures. Therefore, the calculation is not “one-size-fits-all” and trial and error are necessary steps for establishing the 32P labelling efficiency of DNA adducts versus dGMP for better quantifications. This calculation is relative rather than absolute, and therefore the assay has been characterized as a “semiquantitative” method (71). Nonetheless, this method is ideal for detecting a broad range of TS induced DNA damage. Furthermore, this method requires less than a few μg of DNA and is able to detect DNA damage in the range of a few adducts per 1010 nucleotides (62). This method uses relatively mild conditions (neutral pH and low temperature) to digest DNA, therefore it is relatively non-destructive with respect to most DNA adducts.
Identify and quantify the individual DNA adducts induced by TS
Two other powerful methods, besides the 32P post-labeling 2D-TLC/HPLC method, have been used to identify individual DNA adducts induced by TS in biological samples, an immunochemical method and liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) (20, 68, 71). The immunochemical method uses primary antibody against specific DNA adducts to bind the DNA damage and then utilizes a secondary antibody labeled with a fluorescent dye to visualize the primary antibody-DNA damage complex. There are two ways to measure the fluorescent intensity of the antibody-DNA damage complex. In the ELISA protocol, the genomic DNAs that are fixed in individual wells are first reacted with the primary antibody. After unbound primary antibodies are washed away, a secondary antibody with a fluorescent dye is added. The unbound secondary antibodies are removed by washing and the fluorescence intensity of the complexes of DNA adducts bound to the primary and secondary antibodies are quantified by fluorescent spectrophotometry (72–75). In the slot blotting method, genomic DNAs are fixed onto a nitrocellulose membrane which is overlaid by the primary antibody followed by a reaction with the fluorescent secondary antibody. After washing away the excessive unbound antibodies, the fluorescence intensity of complexes of DNA adducts bound to the primary and secondary antibodies are quantified (20, 61, 76). This immunochemical method requires only a minute amount of DNA (<1 μg) to detect DNA adducts in the range of a few adducts per 108 nucleotides; it is a simple protocol that can be conducted in clinical settings. Although the fluorescent labeled antibody-DNA adduct complexes can be quantified using an ELISA method, this method requires relatively large amount of DNA compared to the membrane blotting method. One advantage of specific antibody-DNA adduct reaction methods is that they can also be used in situ at the cytological level to locate and visualize DNA adducts inside cells (77, 78). This unique capability of the immunochemical method has been applied to view the dynamics of the DNA damage process in vivo in real time (79–81). For example, this method has been applied to discover the dynamics of UV-induced photoproduct formation and repair in different cellular compartments (81). It should be noted, however, that certain DNA adduct specific antibodies are more specific and efficient in recognizing DNA adducts in single-stranded DNA, which can be generated by the denaturation of double-stranded DNA through heating. Therefore, this method is ideal for quantifying heat-stable DNA adducts in human samples (76, 82). It should be noted that very often the epitope that the antibodies recognize can be shared by different DNA adducts. For example, cyclobutane pyrimidine dimer (CPD) antibodies can recognize all CPD structures formed at -TT-, -TC-, -CT- and -CC- sequences (83, 84), and the monoclonal antibodies against acrolein (Acr)-DNA adducts can recognize all cyclic 1,N2-propano-dG (PdG) with different length side chains (82).
For targeted DNA adduct analysis, the LC-MS/MS method provides unmatched specificity in identifying DNA adduct structures (71). This method uses non-radio- isotopic labeling and has the potential to be at least as sensitive as the 32P post-labeling method, if not more sensitive. Currently, it has been reported that the most improved LC-MS/MS method is able to detect DNA adducts at the level of 10-11, equivalent to the detection of one DNA adduct in 100 human cells (71). This method is widely used by many chemistry laboratories for the identification of DNA adduct structures, particularly for discovery of novel and minor DNA adducts. The LC-MS/MS method requires DNA to be digested into mononucleotide form by phosphodiesterase, the use of non-radioisotopes to label DNA adducts, followed by electro-spray HPLC and mass spectrometry (71). The super-sensitivity of adduct detection by this method requires a relatively large amount of DNA (>10 μg). LC-MS/MS has been useful for identifying the chemical nature of abundant DNA adducts and minor DNA adducts in TS (85–87). Currently, however, the information based on this method for finding the relationship between TS induced DNA damage and tumorigenesis is limited. Nevertheless, with greatly improved data analysis ability, coupled with new state-of-the-art powerful LC-MS/MS instruments, a new field of “tobacco smoke-induced adductomics” is emerging (86, 87).
TS induced DNA damage in human lung tissues
The formation and compositions of adducts identified in the DRZ using Randerath protocols on lung tissues of tobacco smokers have been extensively studied and assessed by many excellent review articles (88, 89). These studies conclude that the DRZ contains a mixture of DNA adducts induced by PAHs, AAs, nitrosamines, benzene, aldehydes and perhaps other reactive components from TS (18). Formation of DRZ adducts has been shown to correlate strongly with TS exposure (66). Since PAHs and AAs are powerful mutagens and are carcinogenic, the DRZ has been viewed for many years as a collection of DNA adducts, induced by PAHs and AAs (18). Consequently, PAHs and AAs have been believed to be the major TS carcinogens (18, 20). This belief was recently challenged by findings from Gupta’s laboratory, specifically that the two most abundant and characteristic adducts in the TS-induced DRZ are not the result of PAHs and AAs, as is commonly believed. Instead they were found to be induced by aldehydes (18). Gupta et al.’s findings raise important questions. What are the major DNA adducts induced by TS that can lead to carcinogenesis? Is it possible that while the DNA adducts induced by PAHs and AAs are powerfully mutagenic and carcinogenic, the amounts of these DNA adducts in human lung are negligible due to the minute levels of these compounds in TS, and/or are these compounds not activated to become DNA damaging agents in human lung tissues? In this regard it is worth noting the results from Schoket’s group (21) show that while the total bulky DNA adduct formation (total DNA adducts in the DRZ, detected by 32P-postlabeling 2D TLC method) in lung tissues of smokers (n = 47) is higher than in nonsmokers (n = 38), the PAH-DNA adduct levels (2.6–6.2/108 nt), detected by polyclonal antibodies against BPDE-DNA, show no statistical difference between smokers and nonsmokers. These results are similar with the results from Tang’s group (20) which show no statistical significance of the differences of the levels of BPDE-dG adducts (average 0.9–1.3/108 nt, detected by antibody against BPDE-DNA adduct, the same as Schoket) in lungs of smokers (n = 41) compared to nonsmokers (n =13).
To address these questions further, using the same 32P post-labeling method with 2D-TLC separation, our laboratory has further separated the TS-induced DRZ adducts resulted from tobacco smokers’ lung tissues by HPLC and found that Acr-dG and crotonaldehyde (Cro)-dG adducts are the two most abundant adducts in lung tissues of tobacco smokers (20). These results are consistent with previous reports that higher levels of aldehyde-DNA adducts were formed in buccal cells and sputum of tobacco smokers than non-smokers (20, 54).
Detection of tobacco smoke-induced DNA adducts – benzo(a)pyrene diol epoxide (BPDE)-dG adducts versus aldehyde-induced cyclic 1,N2-propano-dG (PdG) adducts – by LC-MS/MS method.
While immunochemical and 32P post-labeling 2D-TLC/HPLC can quantify adducted bases with great sensitivity and separation, both methods rely on comparison with standard materials for quantification and identification. Thus, both applications yield indirect measurements. It is well known that nonspecific agents which cross-react with presumably specific antibodies can occur, consequently introducing errors in immunochemical assay results. With the 2D-TLC/HPLC method, it is likely that different materials can have the same migration and elution profiles, therefore a single peak could contain more than one species of substance. The LC-MS/MS method avoids these potential sources of error; however, a relatively high quantity of sample DNA is needed for this method. For example, using 40 μg of genomic DNA, Hecht’s laboratory was able to detect BPDE-dG adducts at the level of 1 BPDE-dG/1011 nucleotides in the lung tissues of nonsmokers and 3.1 BPDE-dG/1011 nucleotides in the lung tissues of smokers, which is equivalent to 1 and 3 BPDE-dG per 100 lung cells in lung tissues of non-smokers and smokers, respectively (71). It should be noted that this level of BPDE-dG in smokers’ lungs is far lower than the level of 8-oxo-dG adducts and depurination sites that spontaneously occur at one to ten thousand per genome in normal human cells (90). It is difficult to imagine the impact that this low level of TS- induced BPDE-dG adducts would have in cells with a background of more than one thousand-fold higher levels of spontaneously occurring oxidative DNA damage and abasic sites.
Using both 32P post-labeling/3D-TLC analysis and the immunochemical method, we found that there is no significant difference in BPDE-dG levels between lung tissues of smokers compared to non-smokers (20). Due to the strongly carcinogenic and mutagenic effect of BP in both cultured human cells and in animal models, BPDE-dG has long been accepted as a “poster child” for the DNA damage that induces lung carcinogenesis by TS (91–96). However, based on the conflicting results from different groups, along with the extremely low level of BPDE-dG detected by Hecht’s group (71), the role of BPDE-dG in TS lung carcinogenesis needs to be carefully re-evaluated.
On the other hand, we found that the levels of both the Acr- and Cro-DNA adducts, γ -OH-PdG and α-methyl- γ -OH-PdG, in buccal cells, sputum and lung tissues of smokers are significantly higher than in non-smokers (20). We also found that PdG induces G to T and G to A mutations (Table 1), which are similar to the mutations found in the TP53 gene in lung cancer. Furthermore, PdG distribution in the TP53 gene is similar if not identical to the TP53 mutational spectrum in lung cancer (97, 98) (Fig. 1). Based on these results, we have suggested that PdG adducts are the major cause for TS lung carcinogenesis (20). It should be noted that it has been found that Acr is carcinogenic in rodents (99). Acr has recently been classified as class 2 carcinogen by IARC (100). It has long been recognized that Cro can cause liver cancer in animal models (101), and Cro has also been classified as a group 2A carcinogen by IARC (100).
Table 1.
Identification of types of mutations induced by Acr or BPDE in the CpG methylated and unmethylated supF gene compared with types of mutations that occur at CpG mutational hotspots in the TP53 gene in tobacco smoke–related lung cancer (Adapted from Wang et al.(97)).
| Mutations at CpG site | Control | Acrb | BPDEc | p53d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| UMa | Ma | UM | M | UM | M | ||||||
| G to T | 2 (40%) | 1 (6%) | 9 (36%) | 15 (54%) | 10 (48%) | 20 (71%) | 296 (49%) | ||||
| G to A | 2 (40%) | 17 (94%) | 7 (28%) | 11 (39%) | 1 (4%) | 3 (11%) | 219 (36%) | ||||
| Otherse | 1 (20%) | 0 (0%) | 9 (36%) | 2 (7%) | 10 (48%) | 5 (18%) | 96 (15%) | ||||
UM: unmethylated supF; M: methylated supF.
Acr: 0.5 mM at 37°C for 24 h.
BPDE: 2μM at 37°C for 1 h.
CpG sites at codons 157, 158, 175, 245, 248, 273 and 282 of p53 in TS-related lung cancer.
Other types of mutations including G to C, insertion, deletion or tandem mutations.
Fig. 1.
Acr– dG and BPDE– dG distributions in the TP53 gene. (A and B) Acr– dG and BPDE– dG adduct distribution in exons 5, 7, and 8 of the TP53 gene of normal human lung cells treated with Acr (A) and BPDE (B). In A, normal human bronchial epithelial (NHBE) cells and normal human lung fibroblasts (NHLF) were treated with 20 μM Acr for 6 h, and in B, NHBE cells were treated with 1 μM BPDE for 30 min. Genomic DNA was then isolated, the DNA adduct distribution was mapped by the UvrABC-ligation- mediated PCR (LMPCR) method, and the DNA was separated by electrophoresis (211). AG and TC are Maxam and Gilbert sequencing reaction products. (C and D) Comparisons of the frequency of Acr– dG (C) and BPDE-dG distribution along the TP53 gene in NHBE cells with the frequency of the TP53 mutations in TS-related lung cancer (International Agency for Research on Cancer TP53 Mutation database, http://www-p53.iarc.fr ) (Adapted from Feng et al.(98, 211),).
Measurement of cyclized propano-dG adducts can be problematic and many variables can affect the outcome. By adding glutathione to the solution for PdG adduct detection and using the sophisticated LC-MS/MS method, Hecht’s laboratory found that, in general, the α-OH-PdG levels are higher than the γ -OH-PdG levels in human lung tissues and leukocytes, and that smokers and non-smokers have similar levels of both isoforms of PdG adducts (71, 102, 103). Two particular results reported by Hecht’s group, the higher level of α-OH-PdG than γ -OH-PdG, and the lack of difference between smokers and non-smokers for these PdG adducts in lung tissues, are different from the findings of others (20, 54, 61, 68, 76, 97). It appears that differences in DNA isolation, DNA digestion, and the detection methods may contribute to these discrepancies. PdG is sensitive to ring-opening at high pH under reducing conditions (104, 105). Chung’s group has addressed this important issue by a systematic analysis of the effect of different buffer systems used for genomic DNA isolation on the yield the two isomers of Acr-dG adducts and reported that 1) using phosphate buffer for genomic DNA isolation results in not only low yield of PdG but also disproportionally high ratio of α-OH-PdG to γ -OH-PdG; and 2) Tris buffer, buffer with amino acids, and cell lysates can eliminate these artifacts (106). Furthermore, we found that under normal physiological pH 7 conditions the major PdG adducts resulting from Acr-DNA interaction are γ -OH-PdG, with only a few percent of α-OH-PdG (105). Only under high pH conditions was more α-OH-PdG produced than γ -OH-PdG (105). It should be noted that TS contains relatively large quantity Acr which has been shown to induce γ -OH-PdG with a minor amount of α-OH-PdG in cultured human cells (76, 97, 105).
In summary, there are two conflicting results regarding the determination of the type and quantity of DNA adducts in lung tissues of tobacco smokers versus non-smokers. Using the immunochemical and 32P post-labeling 2D-TLC/HPLC methods, the major DNA adducts found in the lung tissues of smokers are γ -OH-PdG and α-methyl- γ - OH-PdG in the range of a few adducts per 107 nucleotides (20, 54, 61, 68, 76, 97). Very low levels of αγ-OH-PdG were detected in lung tissues of smokers and non-smokers (20). There was no difference in the levels of BPDE-dG adduct in lung tissues of smokers versus nonsmokers (20). In contrast, using succinate buffer in the presence of glutathione for DNA isolation and LC-MS/MS analysis method, Hecht’s group found similar levels of α-OH-PdG and γ -OH-PdG at a few adducts per 108 nucleotide range in human lung tissues, with no difference in the level of α- and γ -OH-PdG adducts between smoker and non-smokers (71, 102, 103).
Mainstream TS versus side-stream TS in DNA adduct induction in mice
Very few experiments have been conducted to date on determining the levels of DNA adducts and their chemical nature induced by real time TS exposure in animal models. This may be due to the mechanical and physiological difficulties of “making” animals smoke. Instead, the “tar” collection of “particulate matter” (PM) from tobacco smoke has been widely used as a surrogate for exposure to TS for assessing TS mutagenicity, tumorigenicity and other biological effects in animals (66, 107–111). It has been shown that when topically applied to mouse skin, TS-derived tar induces DRZ adducts in skin and lung tissues (69, 107–111).
When a smoker inhales a lit cigarette two types of cigarette smoke will be generated – mainstream tobacco smoke (MTS) and side-stream tobacco smoke (SSS). SSS results from passive burning of the lit cigarette. The temperature of SSS can be up to 400°C. When a smoker inhales from a lit cigarette, a stream of oxygen passes through the burning cigarette, which elevates the burning temperature up to 400–900°C. While the chemical compositions of MTS and SSS are similar, the ratio of components in each varies significantly (11, 112, 113). For example, SSS is much richer in aldehydes and BP than MTS (114, 115). It has been suggested that in a pound-by- pound comparison, SSS is much more toxic than MTS (114, 116). Epidemiological and animal studies also suggest that second hand smoke is more carcinogenic than MTS (117, 118). These results raise the possibility that SSS and MTS may have different efficiencies in DNA damage induction. This question was recently addressed in mice by determining the DNA damage induced by MTS and SSS using a whole-body exposure approach in mice (19, 20).
We found that SSS induces γ -OH-PdG adducts in the lung and bladder tissues in mice after 16 weeks of chronic exposure (19). Interestingly, under the same experimental conditions, no significant DNA damage was found in different organs of mice exposed to SSS for 8 weeks (19). In contrast, both γ -OH-PdG and α -methyl- γ -OH- PdG adducts were detected in lung tissues of mice exposed to MTS after 12 weeks of exposure (20). However, only γ -OH-PdG adducts, not α -methyl- γ -OH-PdG adducts, were detected in bladder tissues (20). It has been established that γ -OH-PdG is formed by Acr-DNA interaction and that α -methyl- γ -OH-PdG is formed by Cro-DNA interaction (54, 119, 120). Recently, it has been reported that acetaldehyde, via dimerization producing crotonaldehyde, can induce α -methyl- γ -OH-PdG (121). Acetaldehyde is the most abundant aldehyde in TS (2, 11, 16, 122–124). These results raise the possibility that the TS acetaldehyde in the blood stream may be further metabolized into acetates, in which case acetaldehyde might not be excreted in the urine. The level of Cro is one tenth that of Acr in TS; the low concentration of Cro in TS, and lack of validation of acetaldehyde in urine, may explain why only γ -OH-PdG was detected in bladder tissues of MTS exposed mice (2, 11, 16, 20, 60, 68). On the other hand, acetaldehydes and Cro in TS may directly damage lung cells to induce α -methyl- γ -OH-PdG. This scenario may account for the formation of two different PdG in the lungs of MTS exposed mice (20).The boiling point of acetaldehyde is 16 °C, which is lower than ambient temperature (20, 125). Therefore, the concentration of acetaldehyde may be greatly reduced in the process of collecting of SSS. Hence, SSS does not induce α -methyl- γ -OH-PdG in the lung tissues of mice. Consistent with this explanation is the observation that both types of PdG are found in lung tissues of human smokers and that the level of α -methyl- γ -OH- PdG is higher than γ -OH-PdG (20).
It should be noted that the levels of the BPDE-dG adduct in different organs including lungs show no significant difference between TS (MTS and SSS) exposed and control mice (one BPDE per 107 nt) (20). These results are consistent with the findings that there is no significant difference of the level of BPDE-dG adduct in lung tissues of tobacco smokers and non-smokers (20). BP is a potent carcinogen, and it has been found that BPDE, the metabolic activated BP, preferentially causes G to T mutations and binds to the mutational hotspots in the TP53 gene often mutated in lung cancer (91– 96). Therefore, BP is generally believed to be the most important carcinogen in TS (91– 96) (Fig. 1). The observations that MTS and SSS exposure do not enhance BPDE-dG in mouse lung tissues, and that lung tissues of tobacco smokers and non-smokers have similar levels of BPDE-dG are important, albeit negative findings. It is worth noting, however, that the amount of BP per cigarette is relatively minute (~25 ng/cig) (2, 11, 16), compared to the amount of BP in the air from fuel (0.3–9300 ng/m3), heating (2–490 ng/m3) and cooking (6–24 ng/m3) (126, 127). Therefore, it is likely that the amount of BP intake from ambient air and diet is much higher than the BP intake from TS, which consequently offsets the sensitivity of detection of BP from TS. We believe the same rationale could explain the lack of difference in BPDE-dG level in different tissues including lungs between tobacco smokers and non-smokers (21, 128–130).
TS effect on DNA repair
It has been long recognized that aldehydes through their carbonyl group and olefin double bond can react with many amino acid moieties, such as, lysine, histidine and arginine, which are found in most proteins (105). These aldehyde modifications very often affect protein functions and structural integrity, and lead to protein degradation (105). At high concentrations, aldehydes can cause protein crosslinks (131, 132). Since TS contains abundant aldehydes it is conceivable that TS may interfere with many cellular functions via interactions of TS aldehydes with various cellular proteins. Two possible effects of aldehyde perturbations associated with cellular functions related to TS carcinogenesis have recently been tested: DNA repair functions and the activation of procarcinogens (19, 20, 53, 61, 76, 98). It has been found that aldehydes can instantaneously inhibit both nucleotide excision repair (NER) and base excision repair (BER) functions in in vitro assay systems (19, 20, 53, 61, 76, 98). In cultured human cells, aldehyde treatments not only can impair three major DNA repair mechanisms, NER, BER and mismatch repair (MMR), but can also cause degradation of repair proteins and enhancement of mutational susceptibility (19, 20, 53, 61, 76, 98). In mice, TS causes a reduction of NER and BER activity and reduces the levels of repair proteins such as, XPC, OGG1, Ref1 and MHL1 in lung tissues (19, 20, 61). Since it has been well-established that aldehydes can cause protein dysfunction and degradation via aldehyde-protein interactions, and that aldehydes are major components in TS, these results, together, strongly suggest that aldehydes contribute greatly to the TS-induced reduction of DNA repair proteins and repair activity.
Aldehydes play a dominant and negative effect in TS-induced DNA adduct formation
It is well established that upon uptake, many procarcinogens in TS, such as PAHs, AAs, benzene, and heterocyclic hydrocarbons, will be “detoxified” and become electrophilic via O-acetylation, N-hydroxylation, N-oxidation, N-acetylation, epoxidation, sulfation and esterification (133–140). It is these electrophilic derivatives that can covalently interact with DNA to form mutagenic and carcinogenic DNA adducts (86, 141–143). These detoxification reactions are mainly carried out by numerous cellular enzymes such as cytochrome p450s, sulfotransferases, and acetyl esterases (133–140). We have recently found that adding Acr to cultured human cells can prevent activation of procarcinogens BP and NNK from becoming DNA damaging agents (20). We have also found that in mouse models the major DNA adducts induced by both MTS and SSS are aldehyde-DNA adducts, such as Acr-dG and Cro-dG adducts, and not the PAH-diol epoxide (PAHDE)-dG and O6-medG adducts (19, 20). These results suggest that the abundant aldehydes in TS not only induce DNA damage but also inhibit the activation of procarcinogens such as PAHs, AAs, and nitrosamines. The same reasoning may explain the fact that aldehyde-DNA adducts are the major types of DNA damage found in buccal cells, sputum and lung tissues of tobacco smokers, and that the levels of BPDE-dG and O6-medG are no different between tobacco smokers and nonsmokers (20).
In summary, most recent findings show that the major DNA adducts induced by TS are the consequence of interactions of direct DNA damaging agents in TS, such as Acr, acetaldehyde, and Cro, and the DNA adducts are not induced by procarcinogens such as PAHs, AAs, and nitrosamines as conventionally believed (19, 20). One important concept derived from the recent findings is that TS induced DNA adducts are not a simple arithmetic sum of all TS DNA damaging agents. Simply put, TS aldehydes are major driving forces to induce TS carcinogenesis via three mechanisms: induction of DNA adducts, inhibition of procarcinogen activation, and lastly, inhibition of DNA repair activity, and enhancement of mutagenesis susceptibility via interactions with activation enzymes and repair proteins (Fig. 2). We wish to point out that our results show that in a short period of TS exposure (8 weeks) in mice, while no significant amounts of Acr-DNA adduct were found in the lung tissues, an elevated level of BPDE-dG was found in bronchial lavage and bladder tissues (p=0.035) (19). These results suggest that the TS aldehyde effect can potentially be greatly reduced at a given stage, and that there are abundant aldehyde-neutralizing agents such as glutathione in the upper aerodigestive system. In this case PAHs, perhaps AAs, and NNK also, may be activated and able to induce DNA adducts.
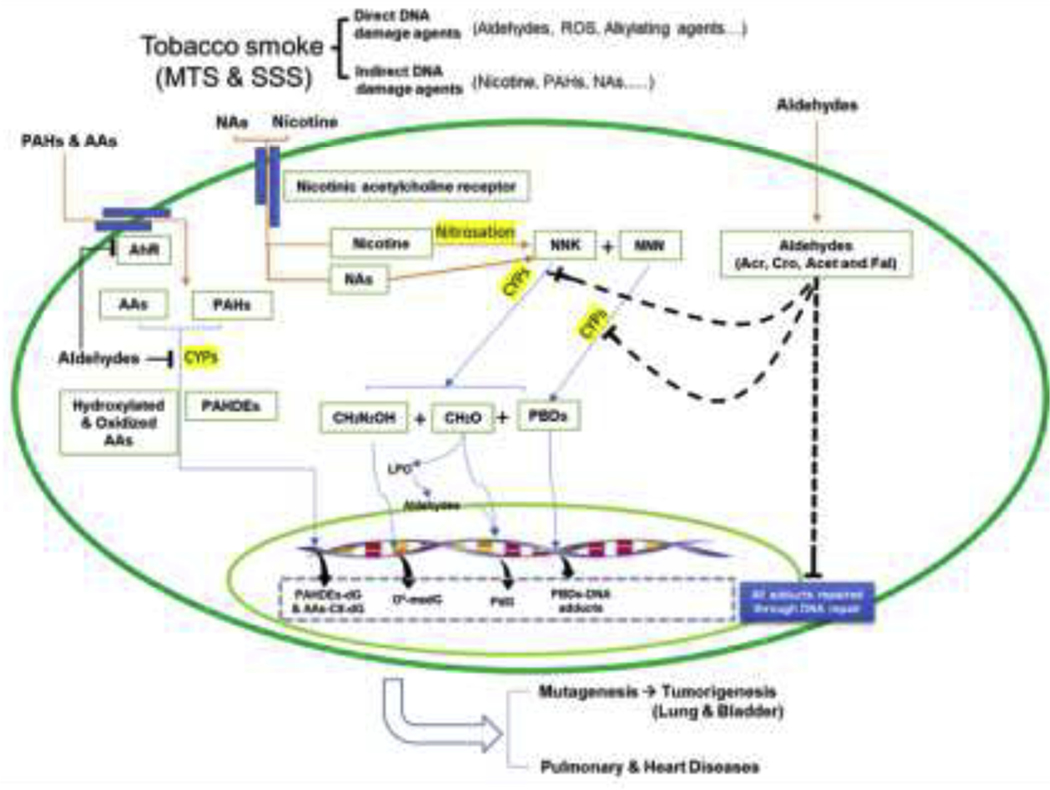
Fig. 2. Summary of TS induced DNA damage and effects on DNA repair.
This scheme is based on the results from DNA adduct detection in lung and bladder tissues of mice exposed to MST and SSS, DNA adduct analysis in lung tissues of tobacco smokers versus non-smokers, and results of analysis of aldehyde effect on DNA adduct induction, DNA repair activity, repair protein levels, and mutagenesis susceptibility in cultured human lung and bladder epithelial cells (19, 20, 61). Symbols: NNK, nicotine- derived nitrosamine ketone; NNN, N-nitrosonornicotine; AhR, aromatic hydrocarbon receptor; AAs, aromatic amines; CYPs, cytochrome p450s; NAs, nitrosamines; Acr, acrolein; Cro, crotonaldehyde; Fal, formaldehyde; LPO, lipid peroxidation; PBDs, pyridylic-butylic derivatives; PAHs, polycyclic aromatic hydrocarbons; PAHDEs, PAH diol epoxides.
Electronic cigarettes
Following the recognition of the harmful effects of tobacco smoke, most notably the carcinogenic nature of the products of the incomplete combustion of the tobacco leaves, alternative devices to deliver nicotine emerged. One of the most notable examples is Herbert Gilbert’s 1963 development of a device that utilizes battery power to heat and aerosolize the “flavor” materials, including nicotine, that are dissolved in propylene glycol (PG) and vegetable glycerin (VG) (144). From 1970 to late 1990 there was a flurry of activity involving similarly designed devices intended to heat and aerosolize nicotine (145). However, Gilbert’s patented design never reached market and the tobacco companies did not seriously market their similar devices (145). The electronic-cigarettes (E-cigs) in current use became popular more than a decade ago only when a pharmacist Han Li started marketing his reinvented device using inhalation- riggered-battery-powered electricity, which allows aerosolization of nicotine containing organic solvents of PG and VG. Han Li’s E-cig gained popular acceptance almost instantaneously (24). For the past decade literally scores of similar devices have emerged on the market, most notably the trademarked JUUL and “Pod” brands, which currently occupy more than 70% of the market (146). The essential design and operation of all these E-cig devices are similar, that is, they contain two major components: a compartment to store E-liquid, or E-juice – (nicotine dissolved in the organic solvents, PG and VG), and a battery powered device that is triggered by inhalation to heat up and aerosolize the E-liquid. The sources of PG and VG are from chemical synthesis, while nicotine is extracted from tobacco leaves, because tobacco leaf nicotine is abundant and nicotine extraction is a simple process. Various flavorants such as spice, fruit and candy-flavorings are typically added to the E-liquid to enhance the product’s appeal (24). Since the boiling points of the solvents (PG,188.2°C and VG, 290°C) and nicotine (247°C) are relatively low, the battery heating temperature is usually designed so it does not cause burning. Consequently, negligible amounts of incomplete combustion products are generated (147, 148). The E-cig is therefore marketed as a device for the delivery of a “nicotine high” without the ill effects of TS incomplete combustion products (24, 30, 149, 150). The focus of the remainder of this review is on the induction of DNA damage and the carcinogenic effect of E-cig aerosols (ECAs) and its major component, nicotine.
The components in ECAs
The compositions of ECAs have been extensively analyzed (147, 148, 151). Due to the lack of regulation, the compositions of E-liquids are not necessarily uniform, and the mechanical designs of the E-cig devices vary among different products (152, 153). The compositions of ECAs and the proportionality of the different components have been found to vary across brands (153). Many brands of E-cig contain a variety of flavors ranging from menthol, peppermint, tobacco, or coffee to fruit flavors such as mango, kiwi, apple and strawberry and much more. The purpose of adding flavors in E-cig juice is for enticing E-cig usage. The health effect of these flavors remains largely unknown. It has been reported that the different methods of generating ECAs, such as the sources of air, ambient air versus filtered air, and the range of voltage, greatly affect the compositions of the ECAs (153). Assessments of the impact of ECA generating methodology on the chemical compositions of ECAs are well documented (151, 153). Findings reported in all publications on ECAs are consistent in four aspects: first, ECA is rich in nicotine, PG and VG; second, the chemicals found commonly in conventional TS, such as PAHs, nitrosamines, carbonyls, aromatic amines and metals are occur at extremely low levels in ECA; third, elevating the voltage of an E-cig device to higher than that recommended (<4.2 V) for aerosolizing the E-liquid results in carbonyls in ECAs; and fourth, adding flavor substances to E-liquid may or may not change the ECA composition (151, 153). Therefore, our discussion on the ECA biological effects will be limited to ECA generated by the basic process recommended for most marketed E-cig brands, that is, aerosolized PG or VG (50:50 ratio) containing nicotine (1 to 3.6%) at low voltage heating (4.0 V,1.96 amp), excluding flavorants.
Methodology for assessing the biological effects of ECA
The methodology for assessing ECA’s biological effects is similar, if not identical, to those used for assessing TS effects (154–162). The most often used technique is collecting the ECA on membranes (158–162). This method can collect ECA particulate materials larger in size than the pore size of the membrane and that originate from puffs at different times, such as early puffs after lighting and later end puffs. The collected materials, often referred as ECA extracts, were then used to treat cultured cells or animals in order to assess their effects. While this method is convenient, there are likely limitations since it is conceivable that the ECA extracts are subjected to aging, and chemical interactions that may not occur in the aerosol state. Furthermore, the total aerosols and condensate do not encompass all the constituents of whole aerosols because some gases and vapors pass through the collection system (i.e., the filter pads). Exposure of animals to ECA generated from E-cig mimic devices in real time is a preferable procedure for assessing ECA effects. To accomplish this, animals are usually housed in a controlled chamber, and inhale only the ECA injected into the chamber. It should be noted that the body of the animals is also exposed to ECA under most exposure conditions using current available exposure chambers. Therefore, the effects measured by this method represent the sum of inhaled ECA, ECA absorbed by the skin and ECA taken in through the digestive system via licking.
ECA cytotoxicity
Compared to many elements in TS, such as aldehydes, PAHs, AAs, and metals, both the solvents found in E-cig, PG and VG, and the nicotine in E-liquid are significantly less cytotoxic (147, 156, 157, 159, 160). Therefore, it was not surprising to find that ECA extracts are much less cytotoxic than TS extracts towards cultured cardiomyocytes and fibroblasts (158, 159, 161, 162). Furthermore, the concentrations of solvents and nicotine seem to have negligible effect on ECA cytotoxicity (161, 162). These studies confirm the non-cytotoxic nature of PG and VG. However, it has been found that the ECA generated by high voltage and electric power were much more cytotoxic than the ECA generated by regular usage settings, and even more cytotoxic than TS extracts (160–165). It has been found that high voltage generates carbonyls, including acrolein, acetaldehyde, and formaldehyde, similarly to TS (151, 163, 164). Since carbonyls are relatively cytotoxic, it is not surprising that the high-voltage- generated ECA is very cytotoxic (53, 76, 98). It should be noticed that acrolein is a potent apoptotic agent that induces release of caspases from mitochondria (105, 166– 168), and that the cytotoxicity of TS is mainly via induction of apoptosis (169). Because of its low cytotoxicity, E-cigs have been suggested to replace tobacco for smoking cessation and therapeutic purposes (170). However, caution is warranted given that the carbonyl content of ECA can be increased easily by slight increase of the E-cig voltage and electric power (163–165).
Induction of DNA damage by ECA
The finding that ECA is much less cytotoxic than TS strengthens the argument that ECA is less carcinogenic than TS to humans. Many studies focused on measuring the harmful materials present in TS, such as PAHs, AAs, TSNAs, benzene, and aldehydes in ECA and rendered the obvious results that these harmful and carcinogenic materials are at significantly lower levels in ECAs than in TS (171–173). In fact, the levels of these materials in ECAs is equivalent to ambient air (171). These results emboldened many studies to conclude that E-cigs are harmless and to recommend E-cigs as an alternative to regular cigarettes (171, 174). However, all these studies conveniently avoid discussing the implications of the crucial fact that there is as much nicotine in ECA as in TS, if not more (175–177). Nicotine by itself has been found to induce sarcomas and leiomyomas in animal models (22, 178). Therefore, measuring ECA’s harmful and carcinogenic effects, rather than measuring the TS carcinogenic components in ECAs, is the proper way to address the question of whether or not ECA is harmful and carcinogenic.
It is well established and generally accepted that induction of DNA damage and mutations are necessary steps for genotoxic carcinogens and procarcinogens to initiate carcinogenesis (140–143, 179–181). Therefore, it is reasonable that the first step toward addressing the carcinogenicity of ECAs is to determine whether or not ECAs can induce DNA damage in cells. Since under normal operation conditions (≤4 volts, 1.96 amp), E-cigarette vaping does not generate significant amounts of aldehydes (151, 163, 164, 182), and PG and VG by themselves have never been shown to be able to damage DNA directly, if ECA can induce DNA damage, it is most likely due to nicotine.Therefore, the answer to these questions resides in the results of treating cells and animals with ECA and nicotine. To date, it has been found that ECA extracts can induce DNA strand breaks and oxidative DNA damage (ODD), such as 8-oxo-dG, in cultured cells (183, 184). ECA can induce O6-medG and cyclic γ -OH-PdG adducts in lung, heart and bladder tissues of exposed mice (61). And, nicotine can induce the same type of DNA adducts as ECA in cultured human bronchial epithelial cells and bladder urothelial cells (61). The review will next focus on the causes of the variety of DNA damage induced by ECAs and nicotine.
DNA strand break and oxidative DNA damage induced by ECA
To date, DNA strand breaks and ODD, 8-oxo-dG in particular, are the most often reported DNA damage in cultured human cells resulting from ECA exposure. Based on comet assay results, it has been reported that ECA extracts induced DNA strand breaks (183, 185, 186). Using the ELISA assay, Ganapathy et al., (184) reported that ECA extracts caused an increase of 8-oxo-dG in human oral squamous sarcoma cells. ECA extracts also cause blockage of DNA primer extension, as detected by quantitative PCR (QPCR) indicating that ECA induces ‘bulky” DNA damage. However, the mechanisms by which ECA extracts cause DNA strand breaks and 8-oxo-dG are not clear, and the types of DNA damage that block DNA synthesis were not investigated in these studies. It has been speculated that ECA extracts induce oxidative stress responses and suppression of cellular antioxidant defense mechanisms (184). The underlying mechanisms that lead to induction of oxidative DNA damage by ECA extracts seem to be that ECA extracts negatively affect the cellular homeostasis of oxidative potential and, consequently, excessive ROS are generated from cellular activity causing strand breaks and oxidative DNA damage. It is unlikely that the metabolites of ECA extracts can directly interact with DNA causing DNA strand breaks and 8-oxo-dG. In this regard, it is worth noting that the Fenton reaction is necessary for induction of ROS, which can induce 8-oxo-dG and DNA strand breakage. It has been found that nicotine treatment causes reduction of cellular ROS (187) and that nicotine is a chelating agent for divalent cations, which are necessary for Fenton reactions to generate ROS (188). These contradictory results on 8-oxo-dG and ROS production by ECA extracts and nicotine are difficult to reconcile and indicate that unknown components in ECA extracts cause oxidative stress in cells, which leads to 8-oxo-dG formation. It is worth noting that Yu et al., (183) found that ECA extracts generated from E-liquid with and without nicotine have the same cytotoxicity and clastogenic effect. These results raise the possibility that the steps of condensing ECA may introduce unknown chemical reactions producing new compounds that can cause both cytotoxicity and DNA strand breaks. These results highlight the necessity of using real time ECA for determining E-cig effects.
ECA induces O6-medG and cyclic γ -OH-PdG adducts, inhibits DNA repair, and reduces repair protein level in mice
It is well established that during tobacco curing and burning a small portion of nicotine (0.1%) is converted into so-called tobacco specific nitrosamines (TSNAs) due to nitrosation of nicotine mediated by nitronium ions (189–191). Many of the TSNAs, such as NNK (nicotine-derived nitrosamine ketone) and NNN (N-nitrosonornicotine), are potently carcinogenic in animal models and have been recognized as human carcinogens (12, 17, 192).
Upon uptake, nitrosamines (such as NNK and NNN) are subjected to metabolism by cytochrome p450s, which are abundant in animal and human cells, and the metabolites are eventually degraded into formaldehyde, methyldiazohydroxides (MDOH), and pyridylic-butylic derivatives (PBDs) (12). While all these final products are potential DNA damaging agents, nitrosamine carcinogenicity has been attributed to MDOH, which have been shown to induce mutagenic and carcinogenic O6-medG adducts with minor adducts of medT and medC (193). Because of these findings, the level of NNK, NNAL [(4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol] and NNN in TS, as well as in body fluids, have been widely used to assess the carcinogenic potency of TS from various cigarette brands and to estimate the carcinogenic risks of human exposure (193, 194). Commercially available E-liquid contains a relatively large quantity of nicotine (1 to 5%), which can be converted into carcinogenic nitrosamines by a single nitrosation (Fig. 3). Since the nitronium ions necessary for the nicotine nitrosation reaction are being generated constantly during normal cellular function (195), intuitively, one would think that nicotine nitrosation must take place in vivo. It is surprising, this question has not been carefully scrutinized. Hecht’s group reported the detection of minute amounts of NNAL in saliva of animals exposed to nicotine (196). The lack of finding NNAL and the low level of NNAL detected in animals exposed to relatively large quantities of nicotine raises two questions. Are only minute amounts of exogenous nicotine subjected to nitrosation in vivo? Or, does nicotine nitrosation occur in vivo at all? What is the cause of low NNAL detection in nicotine exposed mice? One possible scenario is that during uptake by cells nicotine is nitrosated and becomes various nitrosamines, which are ultimately metabolized and degraded into DNA damaging agents. Consequently, very low levels of nitrosated nicotine, if any at all, exist inside the cells and the levels are even lower in body fluids. Since animal and human cells have an abundance of P450s which can metabolize nitrosamines resulting in their spontaneous degradation into DNA damaging agents, we propose that the DNA damage induced by these processes can initiate mutagenesis and carcinogenesis. If this is the case, then measuring DNA damage induced by ECA is a direct way to address the carcinogenic effect of nicotine and ECA and measuring nitrosamine levels may underestimate the extent of the nicotine induced carcinogenic effect. The seriousness of this issue has recently surfaced. Shahab et al., (197) have reported that the NNAL level in the urine and saliva in E-cig users is 5% of that in tobacco smokers. Subsequently, Hecht’s group also detected NNAL in the urine samples of E-cig users at much lower levels than in the urine samples of regular tobacco smokers (198, 199).These findings have lead British medical organizations to conclude that E-cig is only 5% as harmful as TS, and to recommend E-cig as a substitute for regular cigarettes and for tobacco cessation therapeutic purposes (200, 201).
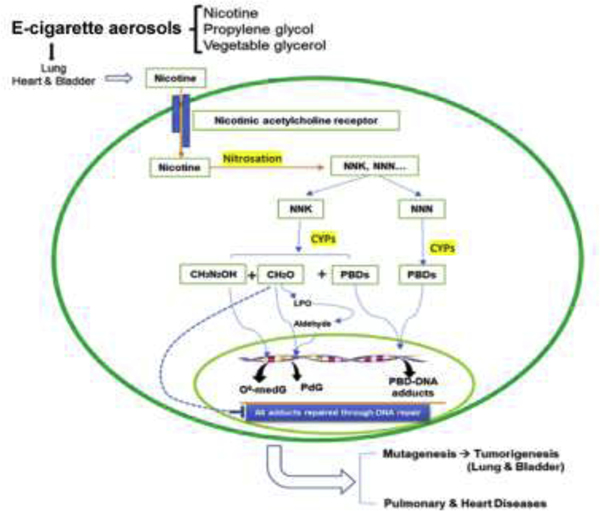
Fig. 3. Schematic representation of the uptake and nitrosation of nicotine in E-cig aerosols, the metabolism, subsequent degradation, the induction of DNA damage that follows, and the inhibition of DNA repair.
This scheme is based on the results from the DNA adduct analysis, DNA repair analysis, and tumorigenesis in lung and bladder tissues of mice exposed to ECAs (61, 105, 206). Symbols are the same as in Fig. 2.
However, a different picture emerges with the approach of detecting nitrosamine related DNA adducts in different organs of mice exposed to nicotine containing ECA versus ECA without containing nicotine. Real time ECA exposure induced three clear effects in mice: induction of mutagenic DNA adducts, γ -OH-PdG and O6-medG, in lung, heart, and bladder tissues; inhibition of nucleotide and base excision repair (NER and BER) function; and reduction of the level of repair proteins XPC and OGG1 in lungs (61). Furthermore, our research also shows that nicotine and NNK induce the same types of DNA adducts and same inhibitory effect on DNA repair in cultured human bronchial epithelial and bladder urothelial cells (61). These results indicate that nicotine, upon uptake by cells, can be nitrosated into nitrosamines, such as NNK (61). These results together indicate that ECA and nicotine are DNA damaging agents in mice and potentially in humans. We wish to point out that these mice were exposed to ECA generated under the vaping conditions of 4.0 volts and 1.96 amp of the E-cig juice containing nicotine 10 mg/ml dissolved in solvents of PG and VG at 1 to 1 ratio without any flavors (57).
Carbonyl production in E-cigarette.
Although the amounts of aldehydes generated from E-cig vaping are minute, some of these aldehydes, particularly acrolein, can induce cyclic propano-dG adducts, the same type of DNA adducts which are also induced by the nitrosamines, the metabolites of nicotine. Therefore, a careful evaluation of the level of aldehydes generated from E-cig vaping, and their possible roles in DNA damage induction, by comparison to the level of nicotine and nicotine’s role on DNA damage induction in ECA is worthwhile. It is well established that ECA contain minute amount of carbonyls such as formaldehyde, acetaldehyde and acrolein (202, 203). Because many aldehydes in ECA are carcinogenic, ample researches have investigated the origin of these carbonyls and the amounts of these carbonyls generated under different vaping conditions. Two major factors that have been found to determine the types and amount of carbonyl production are organic solvents, particularly the ratio of PG versus VG, and the heating coil temperature, which is proportional to the voltage and watt used in the E-cig (163, 204). Results reported from different laboratories, in general, are in a good agreement that the amounts of formaldehyde, acetaldehyde and acrolein generated from E-cig vaping conditions of voltage <4.5 volts, are minute. For example, Kosmider et al (163), report that under vaping conditions of total watt 10 W, the amounts of formaldehyde and acetaldehyde generated are 72 ng/puff and 25 ng/puff, respectively. The average amount of acrolein in an E-cig puff is 3.2 ng (205). It worth noting that the amounts of nicotine generated from regular E-cig are from 0.033 – 1 mg/puff (203), which are 104 −105 -fold of the aldehyde levels in ECA. While acrolein-DNA adducts are detected in the lung tissues of mice exposed to ECA, no acrolein-DNA adducts were detected in lung tissues of mice expose to PG and VG solvent without nicotine. Furthermore, crotonaldehyde-DNA adducts, the acetaldehyde -induced DNA adducts (121) were not detected in these lung tissues (61). Based on these results we have concluded that the acrolein-DNA adducts in these lung tissues are resulted from the nitrosamines of nicotine nitrosation products and not derived from the carbonyls generated in ECA (61).
ECA induces lung adenocarcinoma and bladder urothelial hyperplasia.
The carcinogenicity of ECA in mice has recently been discovered. Our lab recently reported that long-term (54 weeks) exposure to ECA induces lung adenocarcinoma and bladder urothelial hyperplasia in mice (206). While the number of mice used in the tumor experiment is less than ideal (40 for ECA, 20 each for filtered air and solvent aerosol exposure), the results are nonetheless statistically significant (206). The biochemical results and tumorigenesis results individually leave room for doubt about the potency of the carcinogenic effect of ECA and nicotine, but together they constitute a strong case that ECA and nicotine are carcinogens in mice, and thus potentially for humans.
Necessity of using cultured human cells and mouse models to determine ECA carcinogenesis.
The ultimate answers as to whether or not ECA is a human carcinogen will come from epidemiological research. It usually takes between one to two decades for tobacco smokers to develop cancer, including lung cancer (35). Given that E-cig use has only become popular in the past eight years, epidemiological data may not be able to accurately indicate a relationship between ECA and human lung cancer for another decade. Therefore, animal models are necessary as surrogates to study the ECA carcinogenic effects and the molecular changes induced by ECA. Mouse models are the best choice for three reasons. First, most human lung carcinogens, including NNK and PAHs, can induce lung cancer in mice (2, 12, 207, 208). Therefore, if ECA causes lung cancer in mice, it is likely that it will also cause lung cancer in humans. Second, based on the findings that NNK and PAHs induce similar types of DNA damage in mice and humans (2, 12, 207, 209), it is reasonable to expect that the metabolism and processing of ECA in mice and humans are similar. Third, genes that are frequently mutated in human lung cancer, such as TP53 and RAS, are also frequently mutated in mouse lung cancer, indicating that lung carcinogenesis in humans and mice are similar (210). These results suggest that the carcinogenic effects of ECAs can be unraveled, similarly to many human carcinogens, in cultured human cell models and mouse models. Unfortunately, to date only a few such studies on the tumorigenicity of ECA in animal models have been performed.
Hypothetic role of nicotine in TS and ECA carcinogenesis
The role of nicotine in ECA and TS carcinogenicity is a logical conundrum. On one hand, it appears that nicotine and TSNAs in TS do not induce significant amounts of nitrosamine-specific DNA adducts (O6-medG) in lung tissues of mice exposed TS or in the lungs of tobacco smokers, indicating that aldehydes in TS inhibit nicotine nitrosation and subsequent activation of nitrosamines to become DNA damaging agents (20). On the other hand, nicotine in ECA induces not only O6-medG but also γ -OH-PdG adducts, the aldehyde DNA adduct signature in mice (61). Nicotine alone also induces the same types of DNA adducts in cultured human cells (61). Based on these results, we hypothesized that the overwhelming amount of aldehydes, generated during incomplete combustion of tobacco leaves, exerts a dominant and negative role among different TS components in DNA adduct induction and procarcinogen activation (20).However, due to the absence of incomplete combustion of tobacco generated aldehydes, nitrosated nicotine can be metabolized in cells to generate DNA damaging products such as, formaldehyde, MDOH and PBDs, which induce DNA adducts, inhibit DNA repair, and modify DNA repair proteins (Fig. 3).
Which is worse, TS vs ECA?
Although both TS and ECA induce PdG adducts, the mechanisms that lead to PdG formation by these two agents are diametrically different. Aldehydes in TS are the major cause for PdG formation, whereas in ECA nicotine induces PdG. These findings raise a curious but nonetheless serious question of interest to the general population: TS versus ECA, which one is more carcinogenic? The answer to this question requires a well-designed experimental approach using animal models, and only epidemiological studies will render the final conclusion. Nonetheless, biochemical results from TS and ECA exposed mice of the same strain, offer some clues. In the aforementioned studies, the nicotine consumption in TS exposed mice is roughly equivalent to an individual who smokes 2.8 packs per day of cigarette (17,18). The nicotine consumption in ECA exposed mice is roughly equivalent to an individual who smokes 0.4 pack of regular cigarettes per day (56). We found that the amount of PdG formed in lung and bladder urothelial tissues in mice exposed to ECA is higher than in mice exposed to TS (Table 2) (19, 20, 61). While nicotine in ECA induces O6-medG in addition to PdG, nicotine in TS does not. It appears that this nicotine effect is inhibited by aldehydes in TS exposed mice. However, TS contains thousands of chemicals that foreseeably can induce detrimental effects that ECA does not. We found that ECA induces lung adenocarcinoma by 5-fold, which is similar if not higher than the lung cancer induced by TS (Table 2) (206). Based on current results we propose that E-cig can cause lung cancer in mice. However, more animal experiments and epidemiological studies are needed to establish whether or not E-cigs are carcinogenic for humans. On the other hand, epidemiological results have firmly established TS causes cancer in humans (3, 4).
Table 2.
A summary of the quantification of γ-OH-PdG,α-methyl-γ-OH-PdG, O6-medG and BPDE-dG adduct formed in the lungs, heart, liver and bladder of mice exposed to MTS1, SSS2 and E CA3.
| Aerosol | Exposure | Nicotine In the chamber (μg/m3) | Source of Aerosol | Aerosol Level (mg/m3) | Organ | Exposed (n=10)/unexposed (n=10) | |||
|---|---|---|---|---|---|---|---|---|---|
| γ-OH-PdG/105 dG | α-methyl-γ −OH-PdG/105 dG | O6-me-dG/107 dG | BPDE-dG/107 dG | ||||||
| MTS1 | 3 cig4 6 h/d 5 d/wk 12 wks |
871.5 |
2R4F cig 2 puffs/min 35 ml/puff |
75 |
Lung | 0.55/0.05**** | 0.19/ND | 0.05/0.25 ns | 0.75/0.75 ns |
| Heart | 0.15/0.15 ns | ----- | 0.3/0.4 ns | 0.4/0.4 ns | |||||
| Liver | 0.4/0.3 ns | ----- | 0.075/0.07 5 ns |
0.6/0.6 ns | |||||
| Bladder | 0.3/0.05*** | ND/ND | 0.2/0.1 ns | 0.2/0.1 ns | |||||
| SSS2 | 3 cig4 6 h/d 5 d/wk 16 wks |
36 |
2R4F cig 2 puffs/min 35 ml/puff |
0.454 |
Lung | 0.5/0.3* | ND | 2/2 ns | |
| Heart | 0.05/0.05 ns | ----- | 2/2 ns | ||||||
| Liver | 0.4/0.35 ns | ----- | 2/1.75 ns | ||||||
| Bladder | 0.7/0.4** | ND | 1.8/1.7 ns | ||||||
| ECA3 | E-liquid (Nicotine 10 mg/ml +PG and VG) 3 h/d 5 d/wk 12 wks |
129 |
E-juice (nicotine, PG and VG) puffs/min 35 ml/puff |
130 |
Lung | 1/0.2*** | ND | 4/0.2**** | ----- |
| Heart | 0.3/0.05**** | ----- | 0.5/0.1*** | ----- | |||||
| Liver | 0.04/0.035 ns | ----- | 1/1 ns | ---- | |||||
| Bladder | 0.4/0.05*** | ND | 1.25/0.2** | ---- | |||||
Highlights.
Tobacco smoke induces mainly acrolein- and crotonaldehyde-DNA adducts in lungs.
Tobacco smoke reduces DNA repair in lungs and aldehydes do the same in human cells.
Aldehydes in tobacco smoke are the major driving forces of inducing DNA damage.
E-cigarettes induce acrolein-DNA and methyl-dG adducts in the lungs and bladder.
E-cigarettes induce lung adenocarcinoma and bladder urothelial hyperplasia in mice.
ACKNOWLEDGMENTS.
We thank Dr. Cathy Klein, Emily Davison and Mia Nydam for reviewing the manuscript. Research was supported by NIH Grants, RO1190678, 1PO1CA165980, P30CA16087, and ES00260, and an American Lung Association Lung Cancer Discovery Grant.
Abbreviations
- Acr
acrolein
- Acr-dG
Acr-deoxyguanosine
- Acr-DNA
Acr adducted DNA
- AFB1
aflatoxin B1
- AAs
aromatic amines
- BER
base excision repair
- BP
benzo(a)pyrene
- BPDE
benzo(a)pyrene diol epoxide
- BPDE-dG
BPDE-deoxyguanosine
- Cro
crotonaldehyde
- Cro-dG
crotonaldehyde-dG (the same as α-methyl-γ-OH-dG)
- dGMP
deoxyguanosine 5’-monophosphate (dGMP)
- dNMP
deoxyribonucleoside 3’-monophosphate
- DRZ
diagonal radioactive zone
- E-cig
Electronic-cigarette
- E-cigs
Electronic-cigarettes
- ECA
E-cigarette aerosol
- ECAs
E-cigarette aerosols
- LC-MS/MS
liquid chromatography coupled with tandem mass spectrometry (LC- MS/MS)
- medC
methylated cytosine
- medT
methylated thymine
- MDOH
methyldiazohydroxides
- NER
nucleotide excision repair
- NNAL
[(4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol]
- NNK
nicotine-derived nitrosamine ketone
- NP1
nuclease P1
- nt
nucleitide
- ODD
oxidative DNA damage
- O6-medG
O6-methyl-deoxyguanosines
- PAHs
polycyclic aromatic hydrocarbons
- PBDs
pyridylic-butylic derivatives
- PdG
cyclic 1,N2-propano-dG
- PG
propylene glycol
- QPCR
quantitative PCR
- TS
tobacco smoke
- TSNAs
tobacco specific nitrosamines
- VG
vegetable glycerin
- α-methyl- γ -OH-dG
α-methyl- γ -hydroxy-1,N2-propano-dG
- α-OH-PdG
cyclic α−hydroxy-1,N2--propano-dG
- γ -OH-PdG
cyclic γ −hydroxy-1,N2--propano-dG
- 2D-TLC/HPLC
two-dimensional thin layer chromatography/high performance liquid chromatography
- 8-oxo-dG
8-oxo-deoxyguanosine
Footnotes
Conflict of interests
The authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362(24):2295–303. Epub 2010/06/18. doi: 10.1056/NEJMra0809890. PubMed PMID: 20554984; PMCID: PMC2928221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91(14):1194–210. doi: 10.1093/jnci/91.14.1194. PubMed PMID: 10413421. [DOI] [PubMed] [Google Scholar]
- 3.United States Public Health Service. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC. US Department of Health, Education, and Welfare; 1964. [Google Scholar]
- 4.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Tobacco Smoke and Involuntary Smoking. 2002, Vol. 83. [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. PubMed PMID: 17237035. [DOI] [PubMed] [Google Scholar]
- 6.Hammond EC, Seidman H. Smoking and cancer in the United States. Prev Med. 1980;9(2):169–74. doi: 10.1016/0091-7435(80)90071-7. PubMed PMID: 7383981. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7– 30. doi: 10.3322/caac.21590. PubMed PMID: 31912902. [DOI] [PubMed] [Google Scholar]
- 8.Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2(6051):1525–36. doi: 10.1136/bmj.2.6051.1525. PubMed PMID: 1009386; PMCID: PMC1690096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Division of Cancer Prevention and Control. Centers for Disease Control and Prevention. Updated 2020. Access at https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm.
- 10.Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: a review. Circulation. 2010;121(13):1518–22. doi: 10.1161/CIRCULATIONAHA.109.904235. PubMed PMID: 20368531; PMCID: PMC2865193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14(7):767–90. Epub 2001/07/17. doi: 10.1021/tx000260u. PubMed PMID: 11453723. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–44. doi: 10.1038/nrc1190. PubMed PMID: 14570033. [DOI] [PubMed] [Google Scholar]
- 13.Khariwala SS, Hatsukami D, Hecht SS. Tobacco carcinogen metabolites and DNA adducts as biomarkers in head and neck cancer: potential screening tools and prognostic indicators. Head Neck. 2012;34(3):441–7. Epub 2011/05/28. doi: 10.1002/hed.21705. PubMed PMID: 21618325; PMCID: PMC5536330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc. 1950;143(4):329–36. doi: 10.1001/jama.1950.02910390001001. PubMed PMID: 15415260. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis. 1988;9(6):875–84. doi: 10.1093/carcin/9.6.875. PubMed PMID: 3286030. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health. 1997;50(4):307–64. Epub 1997/03/01. doi: 10.1080/009841097160393. PubMed PMID: 9120872. [DOI] [PubMed] [Google Scholar]
- 17.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2004) Tobacco Smoke and Involuntary Smoking, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (WHO International Agency for Research on Cancer, Lyon, France: ), Vol 83. [PMC free article] [PubMed] [Google Scholar]
- 18.Arif JM, Dresler C, Clapper ML, Gairola CG, Srinivasan C, Lubet RA, Gupta RC. Lung DNA adducts detected in human smokers are unrelated to typical polyaromatic carcinogens. Chem Res Toxicol. 2006;19(2):295–9. doi: 10.1021/tx0502443. PubMed PMID: 16485906. [DOI] [PubMed] [Google Scholar]
- 19.Lee HW, Wang HT, Weng MW, Chin C, Huang W, Lepor H, Wu XR, Rom WN, Chen LC, Tang MS. Cigarette side-stream smoke lung and bladder carcinogenesis: inducing mutagenic acrolein-DNA adducts, inhibiting DNA repair and enhancing anchorage-independent-growth cell transformation. Oncotarget. 2015;6(32):33226–36. doi: 10.18632/oncotarget.5429. PubMed PMID: 26431382; PMCID: PMC4741761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng MW, Lee HW, Park SH, Hu Y, Wang HT, Chen LC, Rom WN, Huang WC, Lepor H, Wu XR, Yang CS, Tang MS. Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc Natl Acad Sci U S A. 2018;115(27):E6152–E61. doi: 10.1073/pnas.1804869115. PubMed PMID: 29915082; PMCID: PMC6142211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyorffy E, Anna L, Gyori Z, Segesdi J, Minarovits J, Soltesz I, Kostic S, Csekeo A, Poirier MC, Schoket B. DNA adducts in tumour, normal peripheral lung and bronchus, and peripheral blood lymphocytes from smoking and non-smoking lung cancer patients: correlations between tissues and detection by 32P-postlabelling and immunoassay. Carcinogenesis. 2004;25(7):1201–9. doi: 10.1093/carcin/bgh131. PubMed PMID: 15001535. [DOI] [PubMed] [Google Scholar]
- 22.Sanner T, Grimsrud TK. Nicotine: Carcinogenicity and Effects on Response to Cancer Treatment - A Review. Front Oncol. 2015;5:196. doi: 10.3389/fonc.2015.00196. PubMed PMID: 26380225; PMCID: PMC4553893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haussmann HJ, Fariss MW. Comprehensive review of epidemiological and animal studies on the potential carcinogenic effects of nicotine per se. Crit Rev Toxicol. 2016;46(8):701–34. doi: 10.1080/10408444.2016.1182116. PubMed PMID: 27278157; PMCID: PMC5020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glantz SA, Bareham DW. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu Rev Public Health. 2018;39:215–35. Epub 2018/01/13. doi: 10.1146/annurev-publhealth-040617-013757. PubMed PMID: 29323609; PMCID: PMC6251310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska JJ, Benowitz NL. Current advances in research in treatment and recovery: Nicotine addiction. Sci Adv. 2019;5(10):eaay9763. Epub 2019/10/31. doi: 10.1126/sciadv.aay9763. PubMed PMID: 31663029; PMCID: PMC6795520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri MA, Morris PB, Ratchford EV, Sarna L, Stecker EC, Wiggins BS. 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332–65. Epub 2018/12/12. doi: 10.1016/j.jacc.2018.10.027. PubMed PMID: 30527452. [DOI] [PubMed] [Google Scholar]
- 27.Rowell TR, Tarran R. Will chronic e-cigarette use cause lung disease? Am J Physiol Lung Cell Mol Physiol. 2015;309(12):L1398–409. Epub 2015/09/27. doi: 10.1152/ajplung.00272.2015. PubMed PMID: 26408554; PMCID: PMC4683316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health. 2016;219(3):268–77. Epub 2016/02/06. doi: 10.1016/j.ijheh.2016.01.004. PubMed PMID: 26847410. [DOI] [PubMed] [Google Scholar]
- 29.Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med. 2012;6(1):63–74. Epub 2012/01/31. doi: 10.1586/ers.11.92. PubMed PMID: 22283580. [DOI] [PubMed] [Google Scholar]
- 30.Drummond MB, Upson D. Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc. 2014;11(2):236–42. Epub 2014/03/01. doi: 10.1513/AnnalsATS.201311-391FR. PubMed PMID: 24575993; PMCID: PMC5469426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1276–7. Epub 2018/11/16. doi: 10.15585/mmwr.mm6745a5. PubMed PMID: 30439875; PMCID: PMC6290807 potential conflicts of interest. No potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthumalage T, Lamb T, Friedman MR, Rahman I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep. 2019;9(1):19035. Epub 2019/12/15. doi: 10.1038/s41598-019-51643-6. PubMed PMID: 31836726; PMCID: PMC6910911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee WH, Ong SG, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, Springer ML, Schick SF, Bhatnagar A, Wu JC. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J Am Coll Cardiol. 2019;73(21):2722–37. Epub 2019/05/31. doi: 10.1016/j.jacc.2019.03.476. PubMed PMID: 31146818; PMCID: PMC6637948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolkart G, Kollau A, Stessel H, Russwurm M, Koesling D, Schrammel A, Schmidt K, Mayer B. Effects of flavoring compounds used in electronic cigarette refill liquids on endothelial and vascular function. PLoS One. 2019;14(9):e0222152. Epub 2019/09/10. doi: 10.1371/journal.pone.0222152. PubMed PMID: 31498828; PMCID: PMC6733504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howlader N NA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 36.Villeneuve PJ, Mao Y. Lifetime probability of developing lung cancer, by smoking status, Canada. Can J Public Health. 1994;85(6):385–8. PubMed PMID: 7895211. [PubMed] [Google Scholar]
- 37.National Cancer Institute Division of Cancer Control and Population Sciences., Cancer stat facts: Lung and bronchus cancer. Updated 2020. Access at https://seer.cancer.gov/statfacts/html/lungb.html.
- 38.International Agency for Research on Cancer (2016) Source aromatic amines and related compounds (International Agency for Research on Cancer, Lyon, France: ), Vol 127. [Google Scholar]
- 39.Steenland K, Burnett C, Lalich N, Ward E, Hurrell J. Dying for work: The magnitude of US mortality from selected causes of death associated with occupation. Am J Ind Med. 2003;43(5):461–82. Epub 2003/04/22. doi: 10.1002/ajim.10216. PubMed PMID: 12704620. [DOI] [PubMed] [Google Scholar]
- 40.Witschi H, Espiritu I, Yu M, Willits NH. The effects of phenethyl isothiocyanate, N- acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis. 1998;19(10):1789–94. doi: 10.1093/carcin/19.10.1789. PubMed PMID: 9806160. [DOI] [PubMed] [Google Scholar]
- 41.Witschi H. A/J mouse as a model for lung tumorigenesis caused by tobacco smoke: strengths and weaknesses. Exp Lung Res. 2005;31(1):3–18. doi: 10.1080/01902140490494959. PubMed PMID: 15765916. [DOI] [PubMed] [Google Scholar]
- 42.International Agency for Research on Cancer (2007) Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer, Lyon, France: ), Vol 89. [PMC free article] [PubMed] [Google Scholar]
- 43.Levin W, Wood AW, Yagi H, Dansette PM, Jerina DM, Conney AH. Carcinogenicity of benzo[a]pyrene 4,5-, 7,8-, and 9,10-oxides on mouse skin. Proc Natl Acad Sci U S A. 1976;73(1):243–7. doi: 10.1073/pnas.73.1.243. PubMed PMID: 1061121; PMCID: PMC335877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levin W, Wood AW, Yagi H, Jerina DM, Conney AH. (+/−)-trans-7,8-dihydroxy-7,8- dihydrobenzo (a)pyrene: a potent skin carcinogen when applied topically to mice. Proc Natl Acad Sci U S A. 1976;73(11):3867–71. doi: 10.1073/pnas.73.11.3867. PubMed PMID: 1069271; PMCID: PMC431245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller MF, Goodson WH, Manjili MH, Kleinstreuer N, Bisson WH, Lowe L. Low-Dose Mixture Hypothesis of Carcinogenesis Workshop: Scientific Underpinnings and Research Recommendations. Environ Health Perspect. 2017;125(2):163–9. Epub 2016/08/16. doi: 10.1289/EHP411. PubMed PMID: 27517672; PMCID: PMC5289915 interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis. 2001;22(12):1903–30. Epub 2001/12/26. doi: 10.1093/carcin/22.12.1903. PubMed PMID: 11751421. [DOI] [PubMed] [Google Scholar]
- 47.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. Lyon (FR): International Agency for Research on Cancer; 1987. (IARC MONOGRAPHS ON THE EVALUATION OF CARCINOGENIC RISKS TO HUMANS, No. Supplement 7). [PubMed] [Google Scholar]
- 48.International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, volume 38. Tobacco smoking. Lyon, France: IARC; 1986. [Google Scholar]
- 49.Mattson ME, Pollack ES, Cullen JW. What are the odds that smoking will kill you? Am J Public Health. 1987;77(4):425–31. doi: 10.2105/ajph.77.4.425. PubMed PMID: 3826460; PMCID: PMC1646951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–9. doi: 10.1136/bmj.321.7257.323. PubMed PMID: 10926586; PMCID: PMC27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz AG, Prysak GM, Bock CH, Cote ML. The molecular epidemiology of lung cancer. Carcinogenesis. 2007;28(3):507–18. doi: 10.1093/carcin/bgl253. PubMed PMID: 17183062. [DOI] [PubMed] [Google Scholar]
- 52.Mauderly JL, Gigliotti AP, Barr EB, Bechtold WE, Belinsky SA, Hahn FF, Hobbs CA, March TH, Seilkop SK, Finch GL. Chronic inhalation exposure to mainstream cigarette smoke increases lung and nasal tumor incidence in rats. Toxicol Sci. 2004;81(2):280–92. doi: 10.1093/toxsci/kfh203. PubMed PMID: 15213336. [DOI] [PubMed] [Google Scholar]
- 53.Wang HT, Zhang S, Hu Y, Tang MS. Mutagenicity and sequence specificity of acrolein- DNA adducts. Chem Res Toxicol. 2009;22(3):511–7. doi: 10.1021/tx800369y. PubMed PMID: 19146376; PMCID: PMC4606861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nath RG, Ocando JE, Guttenplan JB, Chung FL. 1,N2-propanodeoxyguanosine adducts: potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998;58(4):581–4. PubMed PMID: 9485001. [PubMed] [Google Scholar]
- 55.Diaz GJ, Murcia HW, Cepeda SM, Boermans HJ. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010;39(4):279–85. doi: 10.1080/03079457.2010.495109. PubMed PMID: 20706884. [DOI] [PubMed] [Google Scholar]
- 56.International Agency for Research on Cancer (2010) Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (International Agency for Research on Cancer, Lyon, France: ). Vol 92. [PMC free article] [PubMed] [Google Scholar]
- 57.Weng MW, Lee HW, Choi B, Wang HT, Hu Y, Mehta M, Desai D, Amin S, Zheng Y, Tang MS. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde-DNA adducts at p53 mutational hotspot codon 249. Oncotarget. 2017;8(11):18213–26. doi: 10.18632/oncotarget.15313. PubMed PMID: 28212554; PMCID: PMC5392321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol Sci. 2015;145(1):5–15. doi: 10.1093/toxsci/kfv040. PubMed PMID: 25911656; PMCID: PMC4408964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sankhwar M, Sankhwar SN. Variations in CYP isoforms and bladder cancer: a superfamily paradigm. Urol Oncol. 2014;32(1):28 e33–40. doi: 10.1016/j.urolonc.2012.10.005. PubMed PMID: 23428537. [DOI] [PubMed] [Google Scholar]
- 60.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23(6):907–22. doi: 10.1093/carcin/23.6.907. PubMed PMID: 12082012. [DOI] [PubMed] [Google Scholar]
- 61.Lee HW, Park SH, Weng MW, Wang HT, Huang WC, Lepor H, Wu XR, Chen LC, Tang MS. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci U S A. 2018;115(7):E1560–E9. doi: 10.1073/pnas.1718185115. PubMed PMID: 29378943; PMCID: PMC5816191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klaene JJ, Sharma VK, Glick J, Vouros P. The analysis of DNA adducts: the transition from (32)P-postlabeling to mass spectrometry. Cancer Lett. 2013;334(1):10–9. doi: 10.1016/j.canlet.2012.08.007. PubMed PMID: 22960573; PMCID: PMC3706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta RC, Reddy MV, Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–92. doi: 10.1093/carcin/3.9.1081. PubMed PMID: 7139866. [DOI] [PubMed] [Google Scholar]
- 64.Everson RB, Randerath E, Santella RM, Cefalo RC, Avitts TA, Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986;231(4733):54–7. doi: 10.1126/science.3941892. PubMed PMID: 3941892. [DOI] [PubMed] [Google Scholar]
- 65.Randerath K, Reddy MV, Gupta RC. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981;78(10):6126–9. doi: 10.1073/pnas.78.10.6126. PubMed PMID: 7031643; PMCID: PMC348990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randerath E, Avitts TA, Reddy MV, Miller RH, Everson RB, Randerath K. Comparative 32P-analysis of cigarette smoke-induced DNA damage in human tissues and mouse skin. Cancer Res. 1986;46(11):5869–77. PubMed PMID: 3756927. [PubMed] [Google Scholar]
- 67.Randerath K, Randerath E, Agrawal HP, Gupta RC, Schurdak ME, Reddy MV. Postlabeling methods for carcinogen-DNA adduct analysis. Environ Health Perspect. 1985;62:57–65. doi: 10.1289/ehp.856257. PubMed PMID: 3910421; PMCID: PMC1568676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee HW, Wang HT, Weng MW, Hu Y, Chen WS, Chou D, Liu Y, Donin N, Huang WC, Lepor H, Wu XR, Wang H, Beland FA, Tang MS. Acrolein- and 4-Aminobiphenyl-DNA adducts in human bladder mucosa and tumor tissue and their mutagenicity in human urothelial cells. Oncotarget. 2014;5(11):3526–40. doi: 10.18632/oncotarget.1954. PubMed PMID: 24939871; PMCID: PMC4116500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hemminki Kari Yang Ke Rajaniemi Heli Tyndyk Margarita Alexei L. Postlabelling HPLC analysis of lipophilic DNA adducts from human lung. Biomarkers. 1997;2(6):341–8. doi: 10.1080/135475097231427. PubMed PMID: 23889151. [DOI] [PubMed] [Google Scholar]
- 70.Randerath K, Randerath E, Danna TF, van Golen L, Putman KL. A new sensitive 32P- postlabeling assay based on the specific enzymatic conversion of bulky DNA lesions to radiolabeled dinucleotides and nucleoside 5’-monophosphates. Carcinogenesis. 1989;10(7):1231–9. doi: 10.1093/carcin/10.7.1231. PubMed PMID: 2544310. [DOI] [PubMed] [Google Scholar]
- 71.Ma B, Stepanov I, Hecht SS. Recent Studies on DNA Adducts Resulting from Human Exposure to Tobacco Smoke. Toxics. 2019;7(1). Epub 2019/03/22. doi: 10.3390/toxics7010016. PubMed PMID: 30893918; PMCID: PMC6468371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boguszewska K, Szewczuk M, Urbaniak S, Karwowski BT. Review: immunoassays in DNA damage and instability detection. Cell Mol Life Sci. 2019;76(23):4689–704. Epub 2019/07/26. doi: 10.1007/s00018-019-03239-6. PubMed PMID: 31342119; PMCID: PMC6858475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santella RM. Immunological methods for detection of carcinogen-DNA damage in humans. Cancer Epidemiol Biomarkers Prev. 1999;8(9):733–9. Epub 1999/09/25. PubMed PMID: 10498391. [PubMed] [Google Scholar]
- 74.Kow YW, Dare A. Detection of abasic sites and oxidative DNA base damage using an ELISA-like assay. Methods. 2000;22(2):164–9. Epub 2000/10/06. doi: 10.1006/meth.2000.1057. PubMed PMID: 11020331. [DOI] [PubMed] [Google Scholar]
- 75.Figueroa-Gonzalez G, Perez-Plasencia C. Strategies for the evaluation of DNA damage and repair mechanisms in cancer. Oncol Lett. 2017;13(6):3982–8. Epub 2017/06/08. doi: 10.3892/ol.2017.6002. PubMed PMID: 28588692; PMCID: PMC5452911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang HT, Hu Y, Tong D, Huang J, Gu L, Wu XR, Chung FL, Li GM, Tang MS. Effect of carcinogenic acrolein on DNA repair and mutagenic susceptibility. J Biol Chem. 2012;287(15):12379–86. Epub 2012/01/26. doi: 10.1074/jbc.M111.329623. PubMed PMID: 22275365; PMCID: PMC3320987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CH, Chen YT, Lin JH, Wang HT. Acrolein induces ribotoxic stress in human cancer cells regardless of p53 status. Toxicol In Vitro. 2018;52:265–71. Epub 2018/07/03. doi: 10.1016/j.tiv.2018.06.022. PubMed PMID: 29964147. [DOI] [PubMed] [Google Scholar]
- 78.Liu JH, Wang TW, Lin YY, Ho WC, Tsai HC, Chen SP, Lin AM, Liu TY, Wang HT. Acrolein is involved in ischemic stroke-induced neurotoxicity through spermidine/spermine-N1- acetyltransferase activation. Exp Neurol. 2020;323:113066. Epub 2019/10/21. doi: 10.1016/j.expneurol.2019.113066. PubMed PMID: 31629858. [DOI] [PubMed] [Google Scholar]
- 79.McCready S. A dot-blot immunoassay for measuring repair of ultraviolet photoproducts. Methods Mol Biol. 2006;314:229–38. Epub 2006/05/06. doi: 10.1385/1-59259-973-7:229. PubMed PMID: 16673885. [DOI] [PubMed] [Google Scholar]
- 80.Garinis GA, Mitchell JR, Moorhouse MJ, Hanada K, de Waard H, Vandeputte D, Jans J, Brand K, Smid M, van der Spek PJ, Hoeijmakers JH, Kanaar R, van der Horst GT. Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J. 2005;24(22):3952–62. Epub 2005/10/28. doi: 10.1038/sj.emboj.7600849. PubMed PMID: 16252008; PMCID: PMC1283948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steurer B, Turkyilmaz Y, van Toorn M, van Leeuwen W, Escudero-Ferruz P, Marteijn JA. Fluorescently-labelled CPD and 6–4PP photolyases: new tools for live-cell DNA damage quantification and laser-assisted repair. Nucleic Acids Res. 2019;47(7):3536–49. Epub 2019/01/31. doi: 10.1093/nar/gkz035. PubMed PMID: 30698791; PMCID: PMC6468286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan J, Awoyemi B, Xuan Z, Vohra P, Wang HT, Dyba M, Greenspan E, Fu Y, Creswell K, Zhang L, Berry D, Tang MS, Chung FL. Detection of acrolein-derived cyclic DNA adducts in human cells by monoclonal antibodies. Chem Res Toxicol. 2012;25(12):2788–95. doi: 10.1021/tx3004104. PubMed PMID: 23126278; PMCID: PMC3561715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Komatsu Y, Tsujino T, Suzuki T, Nikaido O, Ohtsuka E. Antigen structural requirements for recognition by a cyclobutane thymine dimer-specific monoclonal antibody. Nucleic Acids Res. 1997;25(19):3889–94. doi: 10.1093/nar/25.19.3889. PubMed PMID: 9380513; PMCID: PMC146980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung CH, Kim JH, Chung BH. Detection of UV-induced mutagenic thymine dimer using graphene oxide. Anal Chem. 2014;86(23):11586–91. doi: 10.1021/ac503577t. PubMed PMID: 25375800. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Lovell MA, Lynn BC. Development of a method for quantification of acrolein- deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal Chem. 2005;77(18):5982–9. doi: 10.1021/ac050624t. PubMed PMID: 16159131. [DOI] [PubMed] [Google Scholar]
- 86.Balbo S, Turesky RJ, Villalta PW. DNA adductomics. Chem Res Toxicol. 2014;27(3):356–66. doi: 10.1021/tx4004352. PubMed PMID: 24437709; PMCID: PMC3997222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo J, Turesky RJ. Emerging Technologies in Mass Spectrometry-Based DNA Adductomics. High Throughput. 2019;8(2). doi: 10.3390/ht8020013. PubMed PMID: 31091740; PMCID: PMC6630665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer. 2012;131(12):2733–53. doi: 10.1002/ijc.27827. PubMed PMID: 22961407. [DOI] [PubMed] [Google Scholar]
- 89.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23(12):1979–2004. doi: 10.1093/carcin/23.12.1979. PubMed PMID: 12507921. [DOI] [PubMed] [Google Scholar]
- 90.Friedberg C, Errol GCW, Siede Wolfram, Wood Richard D, Schultz Roger A, Ellenberger Tom. DNA Repair and Mutagenesis. 2. ASM Press; 2005. p. 1118. [Google Scholar]
- 91.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–2. doi: 10.1126/science.274.5286.430. PubMed PMID: 8832894. [DOI] [PubMed] [Google Scholar]
- 92.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435–51. doi: 10.1038/sj.onc.1205803. PubMed PMID: 12379884. [DOI] [PubMed] [Google Scholar]
- 93.Pfeifer GP, Denissenko MF, Tang MS. PCR-based approaches to adduct analysis. Toxicol Lett. 1998;102–103:447–51. doi: 10.1016/s0378-4274(98)00337-3. PubMed PMID: 10022294. [DOI] [PubMed] [Google Scholar]
- 94.Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R. Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol Rep. 2015;67(5):996–1009. doi: 10.1016/j.pharep.2015.03.004. PubMed PMID: 26398396. [DOI] [PubMed] [Google Scholar]
- 95.Alexandrov K, Rojas M, Satarug S. The critical DNA damage by benzo(a)pyrene in lung tissues of smokers and approaches to preventing its formation. Toxicol Lett. 2010;198(1):63–8. doi: 10.1016/j.toxlet.2010.04.009. PubMed PMID: 20399842. [DOI] [PubMed] [Google Scholar]
- 96.Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131(12):2724–32. doi: 10.1002/ijc.27816. PubMed PMID: 22945513; PMCID: PMC3479369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang HT, Weng MW, Chen WC, Yobin M, Pan J, Chung FL, Wu XR, Rom W, Tang MS. Effect of CpG methylation at different sequence context on acrolein- and BPDE-DNA binding and mutagenesis. Carcinogenesis. 2013;34(1):220–7. doi: 10.1093/carcin/bgs323. PubMed PMID: 23042304; PMCID: PMC3534198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103(42):15404–9. doi: 10.1073/pnas.0607031103. PubMed PMID: 17030796; PMCID: PMC1592536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsumoto M, Yamano S, Senoh H, Umeda Y, Hirai S, Saito A, Kasai T, Aiso S. Carcinogenicity and chronic toxicity of acrolein in rats and mice by two-year inhalation study. Regul Toxicol Pharmacol. 2021;121:104863. doi: 10.1016/j.yrtph.2021.104863. PubMed PMID: 33465397. [DOI] [PubMed] [Google Scholar]
- 100.Marques MM, Beland FA, Lachenmeier DW, Phillips DH, Chung FL, Dorman DC, Elmore SE, Hammond SK, Krstev S, Linhart I, Long AS, Mandrioli D, Ogawa K, Pappas JJ, Parra Morte JM, Talaska G, Tang M-s, Thakur N, van Tongeren M, Vineis P, Grosse Y, Benbrahim-Tallaa L, Suonio E, Turner MC, El Ghissassi F, Middleton D, Miranda-Filho A, Chung F, Liu Y, Vega S, Mattock H, Schubauer-Berigan MK, Guyton KZ. Carcinogenicity of acrolein, crotonaldehyde, and arecoline. The Lancet Oncology. 2021;22(1):19–20. doi: 10.1016/S1470-2045(20)30727-0. [DOI] [PubMed] [Google Scholar]
- 101.Chung FL, Tanaka T, Hecht SS. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986;46(3):1285–9. PubMed PMID: 3002613. [PubMed] [Google Scholar]
- 102.Yang J, Balbo S, Villalta PW, Hecht SS. Analysis of Acrolein-Derived 1, N(2)- Propanodeoxyguanosine Adducts in Human Lung DNA from Smokers and Nonsmokers. Chem Res Toxicol. 2019;32(2):318–25. doi: 10.1021/acs.chemrestox.8b00326. PubMed PMID: 30644728; PMCID: PMC6644703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S, Balbo S, Wang M, Hecht SS. Analysis of acrolein-derived 1,N2- propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol. 2011;24(1):119–24. doi: 10.1021/tx100321y. PubMed PMID: 21090699; PMCID: PMC3064499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kurtz AJ, Lloyd RS. 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J Biol Chem. 2003;278(8):5970–6. doi: 10.1074/jbc.M212012200. PubMed PMID: 12502710. [DOI] [PubMed] [Google Scholar]
- 105.Tang MS, Wang HT, Hu Y, Chen WS, Akao M, Feng Z, Hu W. Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol Nutr Food Res. 2011;55(9):1291–300. Epub 2011/06/30. doi: 10.1002/mnfr.201100148. PubMed PMID: 21714128; PMCID: PMC4606864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chung FL, Wu MY, Basudan A, Dyba M, Nath RG. Regioselective formation of acrolein-derived cyclic 1,N(2)-propanodeoxyguanosine adducts mediated by amino acids, proteins, and cell lysates. Chem Res Toxicol. 2012;25(9):1921–8. doi: 10.1021/tx3002252. PubMed PMID: 22853434; PMCID: PMC3535281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Randerath E, Mittal D, Randerath K. Tissue distribution of covalent DNA damage in mice treated dermally with cigarette ‘tar’: preference for lung and heart DNA. Carcinogenesis. 1988;9(1):75–80. doi: 10.1093/carcin/9.1.75. PubMed PMID: 2826034. [DOI] [PubMed] [Google Scholar]
- 108.Wynder EL, Graham EA, Croninger AB. Experimental production of carcinoma with cigarette tar. Cancer Res. 1953;13(12):855–64. PubMed PMID: 13116124. [PubMed] [Google Scholar]
- 109.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195(1):31–52. doi: 10.1016/j.tox.2003.08.006. PubMed PMID: 14698566. [DOI] [PubMed] [Google Scholar]
- 110.Rickert WS, Trivedi AH, Momin RA, Wright WG, Lauterbach JH. Effect of smoking conditions and methods of collection on the mutagenicity and cytotoxicity of cigarette mainstream smoke. Toxicol Sci. 2007;96(2):285–93. doi: 10.1093/toxsci/kfl195. PubMed PMID: 17189562. [DOI] [PubMed] [Google Scholar]
- 111.Hoffmann D. and Wynder EL (1986) Chemical constituents and bioactivity of tobacco smoke. In Zaridze D. and Peto R. (eds), Tobacco: A Major International Health Hazard, IARC Scientific Publication no. 74. IARC, Lyon, pp. 145–16. [PubMed] [Google Scholar]
- 112.Guerin MR, Jenkins RA, Tomkins BA. the chemistry of environmental tobacco smoke: composition and management. Chelsea (MA): Lewis Publishers; 1992. [Google Scholar]
- 113.Jenkins RA, Guerin MR, Tomkins BA. Chemistry of Environmental Tobacco Smoke: Composition and Measurement. 2. Boca Raton (FL): CRC Press LLC; 2000. Mainstream and sidestream cigarette smoke; pp. 49–75. [Google Scholar]
- 114.Schick S, Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: more toxic than mainstream smoke. Tob Control. 2005;14(6):396–404. doi: 10.1136/tc.2005.011288. PubMed PMID: 16319363; PMCID: PMC1748121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res C Embryo Today. 2008;84(1):61–72. doi: 10.1002/bdrc.20120. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 116.Diethelm PA, Rielle J-C, McKee M. The whole truth and nothing but the truth? The research that Philip Morris did not want you to see. The Lancet. 2005;366(9479):86–92. doi: 10.1016/S0140-6736(05)66474-4. [DOI] [PubMed] [Google Scholar]
- 117.Mohtashamipur E, Mohtashamipur A, Germann PG, Ernst H, Norpoth K, Mohr U. Comparative carcinogenicity of cigarette mainstream and sidestream smoke condensates on the mouse skin. J Cancer Res Clin Oncol. 1990;116(6):604–8. doi: 10.1007/BF01637081. PubMed PMID: 2254379. [DOI] [PubMed] [Google Scholar]
- 118.Office on Smoking and Health (US). The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2006. 2, Toxicology of Secondhand Smoke. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44321/. [PubMed] [Google Scholar]
- 119.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,n(2)-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry. Chem Res Toxicol. 2006;19(10):1386–92. doi: 10.1021/tx060154d. PubMed PMID: 17040109; PMCID: PMC2596066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009;22(5):759–78. doi: 10.1021/tx9000489. PubMed PMID: 19397281; PMCID: PMC2685875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garcia CC, Angeli JP, Freitas FP, Gomes OF, de Oliveira TF, Loureiro AP, Di Mascio P, Medeiros MH. [13C2]-Acetaldehyde promotes unequivocal formation of 1,N2-propano-2’- deoxyguanosine in human cells. J Am Chem Soc. 2011;133(24):9140–3. doi: 10.1021/ja2004686. PubMed PMID: 21604744. [DOI] [PubMed] [Google Scholar]
- 122.Seeman JI, Dixon M, Haussmann HJ. Acetaldehyde in mainstream tobacco smoke: formation and occurrence in smoke and bioavailability in the smoker. Chem Res Toxicol. 2002;15(11):1331–50. doi: 10.1021/tx020069f. PubMed PMID: 12437324. [DOI] [PubMed] [Google Scholar]
- 123.Counts ME, Morton MJ, Laffoon SW, Cox RH, Lipowicz PJ. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine- smoking conditions. Regul Toxicol Pharmacol. 2005;41(3):185–227. doi: 10.1016/j.yrtph.2004.12.002. PubMed PMID: 15748796. [DOI] [PubMed] [Google Scholar]
- 124.International Agency for Research on Cancer (1983) Polynuclear Aromatic Compounds, Part 1: Chemical, Environmental and Experimental Data, IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans (International Agency for Research on Cancer, Lyon, France: ), Vol 32, pp 33–451. [PubMed] [Google Scholar]
- 125.Salthammer T. Very volatile organic compounds: an understudied class of indoor air pollutants. Indoor Air. 2016;26(1):25–38. doi: 10.1111/ina.12173. PubMed PMID: 25471461. [DOI] [PubMed] [Google Scholar]
- 126.Choi H, Harrison R, Komulainen H, et al. Polycyclic aromatic hydrocarbons. In: WHO Guidelines for Indoor Air Quality: Selected Pollutants. Geneva: World Health Organization; 2010. 6. Available from:https://www.ncbi.nlm.nih.gov/books/NBK138709/. [PubMed] [Google Scholar]
- 127.Zhu L, Wang J. Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air, China. Chemosphere. 2003;50(5):611–8. Epub 2003/04/11. doi: 10.1016/s0045-6535(02)00668-9. PubMed PMID: 12685737. [DOI] [PubMed] [Google Scholar]
- 128.Pratt MM, King LC, Adams LD, John K, Sirajuddin P, Olivero OA, Manchester DK, Sram RJ, DeMarini DM, Poirier MC. Assessment of multiple types of DNA damage in human placentas from smoking and nonsmoking women in the Czech Republic. Environ Mol Mutagen. 2011;52(1):58–68. doi: 10.1002/em.20581. PubMed PMID: 20839217; PMCID: PMC3003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gyorffy E, Anna L, Kovacs K, Rudnai P, Schoket B. Correlation between biomarkers of human exposure to genotoxins with focus on carcinogen-DNA adducts. Mutagenesis. 2008;23(1):1–18. doi: 10.1093/mutage/gem043. PubMed PMID: 17989146. [DOI] [PubMed] [Google Scholar]
- 130.Pratt MM, Sirajuddin P, Poirier MC, Schiffman M, Glass AG, Scott DR, Rush BB, Olivero OA, Castle PE. Polycyclic aromatic hydrocarbon-DNA adducts in cervix of women infected with carcinogenic human papillomavirus types: an immunohistochemistry study. Mutat Res. 2007;624(1–2):114–23. doi: 10.1016/j.mrfmmm.2007.04.008. PubMed PMID: 17583755; PMCID: PMC4383290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37(5):790–6, 8–802. doi: 10.2144/04375RV01. PubMed PMID: 15560135. [DOI] [PubMed] [Google Scholar]
- 132.LoPachin RM, Gavin T. Molecular mechanisms of aldehyde toxicity: a chemical perspective. Chem Res Toxicol. 2014;27(7):1081–91. doi: 10.1021/tx5001046. PubMed PMID: 24911545; PMCID: PMC4106693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guengerich FP, Shimada T. Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res. 1998;400(1–2):201–13. doi: 10.1016/s0027-5107(98)00037-2. PubMed PMID: 9685642. [DOI] [PubMed] [Google Scholar]
- 134.Banoglu E. Current status of the cytosolic sulfotransferases in the metabolic activation of promutagens and procarcinogens. Curr Drug Metab. 2000;1(1):1–30. doi: 10.2174/1389200003339234. PubMed PMID: 11467078. [DOI] [PubMed] [Google Scholar]
- 135.Rendic S, Guengerich FP. Summary of information on the effects of ionizing and non- ionizing radiation on cytochrome P450 and other drug metabolizing enzymes and transporters. Curr Drug Metab. 2012;13(6):787–814. doi: 10.2174/138920012800840356. PubMed PMID: 22571481; PMCID: PMC4007673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6(12):947–60. doi: 10.1038/nrc2015. PubMed PMID: 17128211. [DOI] [PubMed] [Google Scholar]
- 137.Adris P, Chung KT. Metabolic activation of bladder procarcinogens, 2-aminofluorene, 4- aminobiphenyl, and benzidine by Pseudomonas aeruginosa and other human endogenous bacteria. Toxicol In Vitro. 2006;20(3):367–74. doi: 10.1016/j.tiv.2005.08.017. PubMed PMID: 16203120. [DOI] [PubMed] [Google Scholar]
- 138.Bellamri M, Yao L, Bonala R, Johnson F, Von Weymarn LB, Turesky RJ. Bioactivation of the tobacco carcinogens 4-aminobiphenyl (4-ABP) and 2-amino-9H-pyrido[2,3-b]indole (AalphaC) in human bladder RT4 cells. Arch Toxicol. 2019;93(7):1893–902. doi: 10.1007/s00204-019-02486-7. PubMed PMID: 31203411; PMCID: PMC6714057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kadlubar FF, Butler MA, Kaderlik KR, Chou HC, Lang NP. Polymorphisms for aromatic amine metabolism in humans: relevance for human carcinogenesis. Environ Health Perspect. 1992;98:69–74. doi: 10.1289/ehp.929869. PubMed PMID: 1486865; PMCID: PMC1519630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beland FA, Kadlubar FF (1990) Metabolic activation and DNA adducts of aromatic amines. In: Cooper CS, Grover PL (eds) Chemical carcinogenesis and mutagenesis. I. Springer, Berlin; Heidelberg New York, pp 267–325. [Google Scholar]
- 141.Klaunig JE, Kamendulis LM. 3.09 - Carcinogenicity*. In: McQueen CA, editor. Comprehensive Toxicology (Second Edition). Oxford: Elsevier; 2010. p. 117–38. [Google Scholar]
- 142.National Research Council (US) Safe Drinking Water Committee. Drinking Water and Health: Volume 9: Selected Issues in Risk Assessment. Washington (DC): National Academies Press (US); 1989. 1, Biologic Significance of DNA Adducts and Protein Adducts. Available from: https://www.ncbi.nlm.nih.gov/books/NBK218897/. [PubMed] [Google Scholar]
- 143.Beland FA, Poirier MC (1989) DNA Adducts and Carcinogenesis. In: Sirica AE (eds) The Pathobiology of Neoplasia. Springer, Boston, MA. [Google Scholar]
- 144.Gilbert AH. U.S. Patent No. 3,200,819. 1965. Brown & Williamson Collection. Bates No. 570328916–570328920. <http://industrydocuments.library.ucsf.edu/tobacco/docs/hzxb0140>.
- 145.The Consumer Advocates for Smoke-free Alternatives Association. 2020. Historical Timeline of Electronic Cigarettes. <http://www.casaa.org/historical-timeline-of-electronic-cigarettes/>.
- 146.JUUL Market Share in 2019: Dominating the US E-cigarette Market. 2019. <https://blog.technavio.com/blog/juul-market-share-dominating-e-cigarettes-market>.
- 147.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem Res Toxicol. 2016;29(10):1662–78. Epub 2016/09/20. doi: 10.1021/acs.chemrestox.6b00188. PubMed PMID: 27641760. [DOI] [PubMed] [Google Scholar]
- 148.Burstyn I. Peering through the mist: systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health. 2014;14:18. Epub 2014/01/11. doi: 10.1186/1471-2458-14-18. PubMed PMID: 24406205; PMCID: PMC3937158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Henningfield JE, Zaatari GS. Electronic nicotine delivery systems: emerging science foundation for policy. Tob Control. 2010;19(2):89–90. Epub 2010/04/10. doi: 10.1136/tc.2009.035279. PubMed PMID: 20378582. [DOI] [PubMed] [Google Scholar]
- 150.Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310(7):685–6. Epub 2013/07/17. doi: 10.1001/jama.2013.109501. PubMed PMID: 23856948. [DOI] [PubMed] [Google Scholar]
- 151.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Eaton DL, Kwan LY, Stratton K, editors. Public Health Consequences of E-Cigarettes. Washington (DC): National Academies Press (US); 2018. Jan 23. 5, Toxicology of E-Cigarette Constituents. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507184/. [PubMed] [Google Scholar]
- 152.Gottlieb MA. Regulation of E-Cigarettes in the United States and Its Role in a Youth Epidemic. Children (Basel). 2019;6(3). Epub 2019/03/07. doi: 10.3390/children6030040. PubMed PMID: 30836677; PMCID: PMC6463025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control. 2014;23 Suppl 2:ii11–7. Epub 2014/04/16. doi: 10.1136/tobaccocontrol-2013-051482. PubMed PMID: 24732157; PMCID: PMC3995255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Glynos C, Bibli S-I, Pavlidou A, Katsaounou P, Kalomenidis I, Zakynthinos S, Papapetropoulos A. Comparison of the effects of e-cigarette vapor vs cigarette smoke on lung function and inflammation in mice. European Respiratory Journal. 2015;46(suppl 59):OA279. doi: 10.1183/13993003.congress-2015.OA279. [DOI] [PubMed] [Google Scholar]
- 155.Czekala L, Simms L, Stevenson M, Tschierske N, Maione AG, Walele T. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regulatory Toxicology and Pharmacology. 2019;103:314–24. doi: 10.1016/j.yrtph.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 156.Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, Wang L, Christensen C, Ambrose B, Borek N, van Bemmel D, Konkel K, Erives G, Stanton CA, Lambert E, Kimmel HL, Hatsukami D, Hecht SS, Niaura RS, Travers M, Lawrence C, Hyland AJ. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Network Open. 2018;1(8):e185937-e. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stephens WE. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tobacco Control. 2018;27(1):10–7. doi: 10.1136/tobaccocontrol-2017-053808. [DOI] [PubMed] [Google Scholar]
- 158.Taylor M, Carr T, Oke O, Jaunky T, Breheny D, Lowe F, Gaca M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol Mech Methods. 2016;26(6):465–76. doi: 10.1080/15376516.2016.1222473. PubMed PMID: 27690198. [DOI] [PubMed] [Google Scholar]
- 159.Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, Glish GL, Tarran R. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16(3):e2003904. doi: 10.1371/journal.pbio.2003904. PubMed PMID: 29584716; PMCID: PMC5870948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wieczorek R, Phillips G, Czekala L, Trelles Sticken E, O’Connell G, Simms L, Rudd K, Stevenson M, Walele T. A comparative in vitro toxicity assessment of electronic vaping producte-liquids and aerosols with tobacco cigarette smoke. Toxicol In Vitro. 2020;66:104866. doi: 10.1016/j.tiv.2020.104866. PubMed PMID: 32353510. [DOI] [PubMed] [Google Scholar]
- 161.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol. 2013;25(6):354–61. doi: 10.3109/08958378.2013.793439. PubMed PMID: 23742112. [DOI] [PubMed] [Google Scholar]
- 162.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health. 2013;10(10):5146–62. doi: 10.3390/ijerph10105146. PubMed PMID: 24135821; PMCID: PMC3823305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–26. Epub 2014/05/17. doi: 10.1093/ntr/ntu078. PubMed PMID: 24832759; PMCID: PMC4838028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e- cigarette aerosols. N Engl J Med. 2015;372(4):392–4. doi: 10.1056/NEJMc1413069. PubMed PMID: 25607446. [DOI] [PubMed] [Google Scholar]
- 165.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. doi: 10.1371/journal.pone.0057987. PubMed PMID: 23526962; PMCID: PMC3603976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci. 2015;143(2):242–55. doi: 10.1093/toxsci/kfu233. PubMed PMID: 25628402; PMCID: PMC4306719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mohammad MK, Avila D, Zhang J, Barve S, Arteel G, McClain C, Joshi-Barve S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2012;265(1):73–82. doi: 10.1016/j.taap.2012.09.021. PubMed PMID: 23026831; PMCID: PMC3501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singh M, Murthy V, Ramassamy C. Modulation of hydrogen peroxide and acrolein- induced oxidative stress, mitochondrial dysfunctions and redox regulated pathways by the Bacopa monniera extract: potential implication in Alzheimer’s disease. J Alzheimers Dis. 2010;21(1):229–47. doi: 10.3233/JAD-2010-091729. PubMed PMID: 20421692. [DOI] [PubMed] [Google Scholar]
- 169.Comer DM, Elborn JS, Ennis M. Inflammatory and cytotoxic effects of acrolein, nicotine, acetylaldehyde and cigarette smoke extract on human nasal epithelial cells. BMC Pulm Med. 2014;14:32. doi: 10.1186/1471-2466-14-32. PubMed PMID: 24581246; PMCID: PMC3945717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ. A Randomized Trial of E- Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med. 2019;380(7):629–37. doi: 10.1056/NEJMoa1808779. PubMed PMID: 30699054. [DOI] [PubMed] [Google Scholar]
- 171.Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul Toxicol Pharmacol. 2014;70(3):704–10. Epub 2014/12/03. doi: 10.1016/j.yrtph.2014.10.010. PubMed PMID: 25444997. [DOI] [PubMed] [Google Scholar]
- 172.Goniewicz ML, Knysak J, Gawro M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control. 2014;23(2):133–9. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lauterbach JH, and Laugesen M. (2012) Comparison of toxicant levels in mainstream aerosols generated by Ruyan® electronic nicotine delivery systems (ENDS) and conventional cigarette products. Poster 1861, Society of Toxicology, San Francisco, March 11–15. [Google Scholar]
- 174.Lohler J, Wollenberg B. Are electronic cigarettes a healthier alternative to conventional tobacco smoking? Eur Arch Otorhinolaryngol. 2019;276(1):17–25. doi: 10.1007/s00405-018- 5185-z. PubMed PMID: 30392025. [DOI] [PubMed] [Google Scholar]
- 175.El-Hellani A, Salman R, El-Hage R, Talih S, Malek N, Baalbaki R, Karaoghlanian N, Nakkash R, Shihadeh A, Saliba NA. Nicotine and Carbonyl Emissions From Popular Electronic Cigarette Products: Correlation to Liquid Composition and Design Characteristics. Nicotine Tob Res. 2018;20(2):215–23. Epub 2016/11/01. doi: 10.1093/ntr/ntw280. PubMed PMID: 27798087; PMCID: PMC5896517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Geiss O, Bianchi I, Barahona F, Barrero-Moreno J. Characterisation of mainstream and passive vapours emitted by selected electronic cigarettes. Int J Hyg Environ Health. 2015;218(1):169–80. Epub 2014/12/03. doi: 10.1016/j.ijheh.2014.10.001. PubMed PMID: 25455424. [DOI] [PubMed] [Google Scholar]
- 177.DeVito EE, Krishnan-Sarin S. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr Neuropharmacol. 2018;16(4):438–59. Epub 2017/10/20. doi: 10.2174/1570159X15666171016164430. PubMed PMID: 29046158; PMCID: PMC6018193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grando SA. Connections of nicotine to cancer. Nat Rev Cancer. 2014;14(6):419–29. Epub 2014/05/16. doi: 10.1038/nrc3725. PubMed PMID: 24827506. [DOI] [PubMed] [Google Scholar]
- 179.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–214. Epub 2003/07/02. doi: 10.1096/fj.02-0752rev. PubMed PMID: 12832285. [DOI] [PubMed] [Google Scholar]
- 180.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. Epub 2000/01/27. doi: 10.1016/s0092-8674(00)81683-9. PubMed PMID: 10647931. [DOI] [PubMed] [Google Scholar]
- 181.Loeb KR, Loeb LA. Significance of multiple mutations in cancer. Carcinogenesis. 2000;21(3):379–85. Epub 2000/02/26. doi: 10.1093/carcin/21.3.379. PubMed PMID: 10688858. [DOI] [PubMed] [Google Scholar]
- 182.Corbett K. Development of aerosol generator for electronic nicotine delivery system. Thesis for master degree at Department of Environmental Medicine, New York University School of Medicine. 2015. [Google Scholar]
- 183.Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, Tsui T, Aguilera JA, Advani S, Crotty Alexander LE, Brumund KT, Wang-Rodriguez J, Ongkeko WM. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. PubMed PMID: 26547127; PMCID: PMC4891196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, Queimado L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One. 2017;12(5):e0177780. doi: 10.1371/journal.pone.0177780. PubMed PMID: 28542301; PMCID: PMC5436899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;7(47):77196–204. doi: 10.18632/oncotarget.12857. PubMed PMID: 27791204; PMCID: PMC5363580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Anderson C, Majeste A, Hanus J, Wang S. E-Cigarette Aerosol Exposure Induces Reactive Oxygen Species, DNA Damage, and Cell Death in Vascular Endothelial Cells. Toxicol Sci. 2016;154(2):332–40. doi: 10.1093/toxsci/kfw166. PubMed PMID: 27613717. [DOI] [PubMed] [Google Scholar]
- 187.Kacham R (2013) Role of nicotine in oxidative stress. Thesis submitted to the faculty of Missouri University of science and technology in partial fulfillment of the requirements for the degree master of science in chemistry. Masters Theses. 5447. https://scholarsmine.mst.edu/masters_theses/5447.. [Google Scholar]
- 188.Manfredi C, Vero S, Vasca E, Perrotta D, Trifuoggi M, Sorrentino I, Ferri D. On the Interaction Between Nicotine and Metal(II) Ions in Aqueous Solutions. Journal of Solution Chemistry. 2016;45(7):971–89. doi: 10.1007/s10953-016-0483-9. [DOI] [Google Scholar]
- 189.Hecht SS, Chen CB, Ornaf RM, Jacobs E, Adams JD, Hoffmann D. Reaction of nicotine and sodium nitrite: formation of nitrosamines and fragmentation of the pyrrolidine ring. J Org Chem. 1978;43(1):72–6. doi: 10.1021/jo00395a017. PubMed PMID: 619049. [DOI] [PubMed] [Google Scholar]
- 190.Hecht SS, Tricker AR. Chapter 11 - Nitrosamines derived from nicotine and other tobacco alkaloids. In: Gorrod JW, Jacob P, editors. Analytical Determination of Nicotine and Related Compounds and their Metabolites. Amsterdam: Elsevier Science; 1999. p. 421–88. [Google Scholar]
- 191.Xue J, Yang S, Seng S. Mechanisms of Cancer Induction by Tobacco-Specific NNK and NNN. Cancers (Basel). 2014;6(2):1138–56. doi: 10.3390/cancers6021138. PubMed PMID: 24830349; PMCID: PMC4074821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hoffmann D, Brunnemann KD, Prokopczyk B, Djordjevic MV. Tobacco-specific N- nitrosamines and Areca-derived N-nitrosamines: chemistry, biochemistry, carcinogenicity, and relevance to humans. J Toxicol Environ Health. 1994;41(1):1–52. doi: 10.1080/15287399409531825. PubMed PMID: 8277523. [DOI] [PubMed] [Google Scholar]
- 193.Hecht SS, Carmella SG, Foiles PG, Murphy SE, Peterson LA. Tobacco-specific nitrosamine adducts: studies in laboratory animals and humans. Environ Health Perspect. 1993;99:57–63. doi: 10.1289/ehp.939957. PubMed PMID: 8319660; PMCID: PMC1567052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutation research. 1999;424(1–2):127–42. doi: 10.1016/s0027-5107(99)00014-7. PubMed PMID: 10064856. [DOI] [PubMed] [Google Scholar]
- 195.Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA, Darley-Usmar VM. Biological aspects of reactive nitrogen species. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1999;1411(2):385–400. doi: 10.1016/S0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 196.Knezevich A, Muzic J, Hatsukami DK, Hecht SS, Stepanov I. Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine Tob Res. 2013;15(2):591–5. doi: 10.1093/ntr/nts172. PubMed PMID: 22923602; PMCID: PMC3611998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, Feng J, Wang L, West R. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann Intern Med. 2017;166(6):390–400. doi: 10.7326/M16-1107. PubMed PMID: 28166548; PMCID: PMC5362067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bustamante G, Ma B, Yakovlev G, Yershova K, Le C, Jensen J, Hatsukami DK, Stepanov I. Presence of the Carcinogen N’-Nitrosonornicotine in Saliva of E-cigarette Users. Chem Res Toxicol. 2018;31(8):731–8. doi: 10.1021/acs.chemrestox.8b00089. PubMed PMID: 30019582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, Vogel RI, Thompson E, Murphy SE, Hatsukami DK. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17(6):704–9. doi: 10.1093/ntr/ntu218. PubMed PMID: 25335945; PMCID: PMC4481723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McNeill A, Brose LS, Calder R, Bauld L & Robson D (2018). Evidence review of e- cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. London: Public Health England. [Google Scholar]
- 201.McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H (2015). E-cigarttes: an evidence update. A report commissioned by Public Health England. London: Public Health England. London: Public Health England. [Google Scholar]
- 202.Farsalinos KE, Gillman G. Carbonyl Emissions in E-cigarette Aerosol: A Systematic Review and Methodological Considerations. Front Physiol. 2017;8:1119. Epub 2018/01/30. doi: 10.3389/fphys.2017.01119. PubMed PMID: 29375395; PMCID: PMC5769337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Son Y, Bhattarai C, Samburova V, Khlystov A. Carbonyls and Carbon Monoxide Emissions from Electronic Cigarettes Affected by Device Type and Use Patterns. Int J Environ Res Public Health. 2020;17(8). Epub 2020/04/23. doi: 10.3390/ijerph17082767. PubMed PMID: 32316435; PMCID: PMC7215697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.L Y, Burns AE, Tran LN, Abellar KA, Poindexter M, Li X, Madl AK, Pinkerton KE, Nguyen TB. Impact of e-Liquid Composition, Coil Temperature, and Puff Topography on the Aerosol Chemistry of Electronic Cigarettes. Chem Res Toxicol. 2021;34(6):1640–54. Epub 2021/05/06. doi: 10.1021/acs.chemrestox.1c00070. PubMed PMID: 33949191. [DOI] [PubMed] [Google Scholar]
- 205.Mayer B. Acrolein exposure from electronic cigarettes. Eur Heart J. 2020;41(15):1523. Epub 2020/03/27. doi: 10.1093/eurheartj/ehaa002. PubMed PMID: 32211887; PMCID: PMC7154799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tang MS, Wu XR, Lee HW, Xia Y, Deng FM, Moreira AL, Chen LC, Huang WC, Lepor H. Electronic-cigarette smoke induces lung adenocarcinoma and bladder urothelial hyperplasia in mice. Proc Natl Acad Sci U S A. 2019;116(43):21727–31. doi: 10.1073/pnas.1911321116. PubMed PMID: 31591243; PMCID: PMC6815158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ding YS, Zhang L, Jain RB, Jain N, Wang RY, Ashley DL, Watson CH. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3366–71. doi: 10.1158/1055-9965.EPI-08-0320. PubMed PMID: 19064552. [DOI] [PubMed] [Google Scholar]
- 208.Witschi H, Espiritu I, Dance ST, Miller MS. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol Sci. 2002;68(2):322–30. doi: 10.1093/toxsci/68.2.322. PubMed PMID: 12151628. [DOI] [PubMed] [Google Scholar]
- 209.Straif K, Weiland SK, Bungers M, Holthenrich D, Taeger D, Yi S, Keil U. Exposure to high concentrations of nitrosamines and cancer mortality among a cohort of rubber workers. Occup Environ Med. 2000;57(3):180–7. doi: 10.1136/oem.57.3.180. PubMed PMID: 10810100; PMCID: PMC1739921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW Cancer genome landscapes. Science. 2013;339(6127):1546–58. doi: 10.1126/science.1235122. PubMed PMID: 23539594; PMCID: PMC3749880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tang MS, Pfeifer GP, Denissenko MF, Feng Z, Hu W, Pao A, Zheng Y, Zheng JB, Li H, Chen JX. Mapping polycyclic aromatic hydrocarbon and aromatic amine-induced DNA damage in cancer-related genes at the sequence level. Int J Hyg Environ Health. 2002;205(1-2):103–13. doi: 10.1078/1438-4639-00135. PubMed PMID: 12018002. [DOI] [PubMed] [Google Scholar]