Abstract
Objective:
The objective of this study was to assess the associations of abdominal visceral and subcutaneous adipose tissue with blood glucose and beta-cell function.
Methods:
In this study, 11,223 participants without known diabetes were selected for this cross-sectional analysis. Visceral and subcutaneous fat area (VFA and SFA) were measured by magnetic resonance imaging. An oral glucose tolerance test was conducted, and beta-cell function was evaluated.
Results:
Men had significantly larger VFA but smaller SFA than women. After controlling for age, linear regression showed that SFA was adversely associated with 0-minute, 30-minute, and 2-hour plasma glucose (PG) and early-, first- and second-phase disposition indices (DIs). After further adjustment for BMI and VFA, some associations of SFA with PG indices and DIs disappeared, while the other associations became significantly weaker in men (2-hour PG: 0.05 and DI2nd: −0.05) or were reversed in women (0-minute, 30-minute, and 2-hour PG: from −0.07 to −0.04; DI1st: 0.04, P < 0.05). After adjustment for age, BMI, and SFA, VFA was significantly and adversely associated with PG indices and DIs, with the largest standardized regression coefficients with 2-hour PG.
Conclusions:
The associations of SFA with blood glucose and beta-cell function were clinically insignificant in Chinese adults. VFA had the strongest association with 2-hour PG.
Introduction
It has already been established that visceral adipose tissue is adversely associated with hyperglycemia (1–3), impaired glucose regulation (1,2,4), and decreased insulin function (5), whereas the association of abdominal subcutaneous adipose tissue with blood glucose and beta-cell function in human populations is still a controversial topic (1–3,5) in the context of subcutaneous adipose tissue as a natural storage depot of energy (6). Precision estimates of visceral adipose tissue and subcutaneous adipose tissue are dependent on dual-energy x-ray absorptiometry, magnetic resonance imaging (MRI), and computerized tomography (CT), which are difficult to perform in a large population-based study. Gender differences in the associations of visceral adipose tissue and subcutaneous adipose tissue with the blood glucose metabolism have also been reported (1,2). Thus, several previous relevant studies assessing the associations between abdominal adiposity distribution and glucose metabolism had some common limitations, like a very small sample size or a special population, which made them relatively ineffective in controlling confounding and sufficiently comparing gender differences (7–9). We have shown that visceral fat area (VFA) was strongly associated with newly diagnosed diabetes in both genders, whereas subcutaneous fat area (SFA) was negatively associated with newly diagnosed diabetes in women but not in men (P Chen, X Hou, and W Jia, unpublished data). In the present study, we further discussed whether there was any underlying association between adipose distributions and glucose metabolism that supported the above phenomenon.
Type 2 diabetes mellitus is characterized by impaired insulin secretion and beta-cell failure in the setting of insulin resistance (IR). Disposition indices (DIs), comprehensive indices of insulin secretion that are appropriately adjusted for insulin sensitivity, have been proven to be strong predictors of the development of diabetes (10–12). The aim of the present study was to assess the gender-specific associations of VFA and SFA measured by MRI with blood glucose and DIs derived from a three-time-point oral glucose tolerance test (OGTT) in a large population-based study of Chinese adults. To date, relevant studies are still scarce.
Methods
Study population
A population-based prospective study has been designed to investigate the prevalence, incidence, and related factors of cardiometabolic diseases. A baseline survey for this study was completed between April 2013 and August 2014. A total of 21,408 adults 45 to 70 years old living in the Nicheng suburb of Shanghai, China, were invited to participate in the baseline survey, and 17,212 subjects completed the survey, yielding an 80.4% response rate. The present analyses included 11,223 subjects after excluding those without complete analysis data (n = 4,027), those with previously diagnosed diabetes (n = 1,048), and those with negative values of insulin secretion and IR according to their assay values (n = 914) (see Supporting Information Figure S1). The study was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and written informed consent was obtained from each participant before the start of the study.
Baseline measurements
At the local community clinics, participants without any prior diagnosis of diabetes mellitus underwent a 75-g OGTT after an overnight fast of at least 10 hours. Venous blood samples were drawn at 0, 30, and 120 minutes following an OGTT. Fasting plasma glucose (FPG), 30-minute plasma glucose (PG), and 2-hour PG were assayed by a glucose oxidase method, and fasting, 30-minute, and 2-hour serum insulin concentrations were assayed by the electrochemiluminescence immunoassay method.
Information on demographics, history of diseases, medical history, leisure-time physical activity, smoking habits, and alcohol consumption was collected using a standard questionnaire. The participants were grouped into nonsmokers, ex-smokers, and current smokers according to smoking status and into nondrinkers, ex-drinkers, and current drinkers according to alcohol consumption. Leisure-time physical activity was categorized as 0min/d, 1 to 29 min/d, and ≥ 30 min/d. Education level was classified as primary school or less, middle school, and high school or more. Family history of diabetes was defined as having at least a first-degree relative (biological father, mother, siblings, and offspring) with diabetes.
Body weight and height were measured according to standard protocols (13). Height and weight were measured without shoes and with light clothing. BMI was calculated by dividing the weight by height squared. Waist circumference was measured at the horizontal plane between the inferior costal margin and the iliac crest on the midaxillary line (13).
Measurement of abdominal adipose tissue
Abdominal adiposity was detected using an MRI machine with an abdominal coil and a 3.0-T General Electric scanner (GE Healthcare, Milwaukee, Wisconsin). Participants were positioned supine and were scanned in cross-sectional planes. T1 axial images were centered at the navel and obtained with a slice thickness of 10.0 mm for eight slices. Fat area in a single umbilical slice image was separated into VFA and SFA and was manually measured by two trained observers using SliceOmatic image analysis software (version 5; Tomovision Inc., Montreal, Quebec, Canada). If results differed by more than 10%, a third observer who did not know the results reanalyzed the images. The average of two measures was then used in the analysis.
Assessment of beta-cell function based on OGTT test
Early-phase insulin secretion was evaluated using the insulinogenic index (14), the ratio of incremental insulin to incremental glucose responses during the first 30 minutes of the OGTT (Ins30 – Ins0 / Gluc30 – Gluc0). First and second phases of insulin secretion were estimated using the Stumvoll formula as follows: first-phase secretion (pmol/L) =1,283 +1.829 × Ins30 (mU/L) × 6.965 −138.7 ×Gluc30 (mmol/L) +3.772×Ins0 (mU/L)×6.965, and second-phase secretion (pmol/L) =393 +1.163 ×Ins0 (mU/L) ×6.965 −40.72 ×Gluc120 (mmol/L) +0.313 ×Ins120 (mU/L) ×6.965 (15,16). IR was evaluated using the homeostatic model assessment of IR (HOMA-IR): HOMA-IR=Gluc0 × Ins0 /22.5 (17). Gluc0, Ins0, Gluc30, Ins30, Gluc120, and Ins120 refer to glucose and insulin levels at 0, 30, and 120 minutes, respectively. The early-phase DI (DIearly), first-phase DI (DI1st), and second-phase DI (DI2nd) were calculated, respectively, as insulin secretion indices of each phase (early-, first- and second-phase secretion), adjusted for HOMA-IR, to estimate relative insulin secretion.
Definitions of glucose regulation status
Glucose regulation statuses were categorized according to the World Health Organization criteria. Newly diagnosed diabetes was defined as FPG ≥7.0 mmol/L (126 mg/dL) or 2-hour PG ≥11.1 mmol/L (200 mg/dL); impaired glucose regulation was defined as FPG ≥6.1 mmol/L (110 mg/dL) and <7.0 mmol/L or 2-hour PG ≥7.8 mmol/L (140 mg/dL) and <11.1 mmol/L; and normal glucose was defined as FPG <6.1 mmol/L and 2-hour PG <7.8 mmol/L during an OGTT and without any diagnosis of diabetes prior to the time of this survey (18).
Statistical analysis
Descriptive statistics were reported as means (95% CIs), medians (interquartile ranges), geometric means (95% CIs), or frequencies (percentages). The differences in means, medians, or proportions between two groups were tested with the independent-sample t test, the Mann–Whitney U test, and the χ2 test, as appropriate.
The adjusted means (95% CIs) were estimated using the general linear model, in which the covariates included age, BMI, and SFA or VFA, as appropriate. A linear trend test for adjusted means among multiple groups was tested by linear regression. The multiple linear regression analysis was used to assess the associations between the dependent variable and two or more independent variables. Here, the dependent variable was each one of blood glucose indices (FPG, 30-minute PG, and 2-hour PG) or log-transformed DIs (lgDIearly, lgDI1st, and lgDI2nd), and independent variables included two variables of age and VFA or SFA (in Model 1) or four variables of age, BMI, VFA, and SFA simultaneously (in Model 2). All statistical analyses were performed with SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, New York). P <0.05 (two-tailed) was considered to be statistically significant.
Results
The characteristics of the study population are presented in Table 1. Out of the 11,223 middle-aged and elderly participants, 4,835 were men and 6,388 were women. Men and women had similar mean values of age. Men had very slightly higher mean values of FPG and 30-minute PG but had lower mean values of 2-hour PG than women. Men also had comparable median levels of DIearly and DI1st but had a higher median DI2nd level. There were significant gender differences in adipose distribution. Men had significantly larger mean values of VFA (122.0 cm2 vs. 107.7 cm2, P <0.001), BMI (24.9 kg/m2 vs. 24.8 kg/m2, P=0.032), and waist circumference (86.3 cm vs. 82.1 cm, P <0.001) but had a smaller mean value of SFA (127.1 cm2 vs. 167.8 cm2, P <0.001) than women. There was a difference in proportions of glucose regulation status between men and women. In addition, men were much more likely to be current smokers or drinkers compared with women.
TABLE 1.
Characteristics of study participants by gender
| Men | Women | P a | |
|---|---|---|---|
|
| |||
| No. of participants | 4,835 | 6,388 | |
| Age (y) | 56.6 ± 6.5 | 56.6 ± 6.6 | 0.76 |
| Glucose and insulin indices | |||
| Fasting plasma glucose (mmol/L) | 5.84 ± 0.66 | 5.81 ± 0.68 | 0.019 |
| 30-min plasma glucose (mmol/L) | 10.2 ± 1.73 | 10.0 ± 1.82 | < 0.001 |
| 2-h plasma glucose (mmol/L) | 7.43 ± 2.42 | 8.01 ± 2.46 | < 0.001 |
| Fasting serum insulin (uU/mL) | 6.36 (4.38–9.13) | 7.52 (5.47–10.5) | < 0.001 |
| 30-min serum insulin (uU/mL) | 47.4 (32.3–71.4) | 54.3 (37.9–77.5) | < 0.001 |
| 2-h serum insulin (uU/mL) | 35.4 (20.2–59.8) | 49.9 (32.2–78.1) | < 0.001 |
| HOMA-IR | 1.63 (1.10–2.41) | 1.92 (1.36–2.74) | < 0.001 |
| Insulinogenic index | 9.65 (6.08–16.0) | 11.4 (7.33–17.8) | < 0.001 |
| First-phase secretion | 693 (408–1,046) | 822 (541–1,174) | < 0.001 |
| Second-phase secretion | 208 (149–290) | 239 (178–319) | < 0.001 |
| Early-phase disposition index | 6.18 (3.79–10.1) | 6.03 (3.64–9.56) | 0.21 |
| First-phase disposition index | 420 (251–634) | 429 (272–612) | 0.20 |
| Second-phase disposition index | 130 (91.5–183) | 126 (89.1–171) | 0.018 |
| Variables from MRI | |||
| Visceral fat area (cm2) | 122.0 ± 53.1 | 107.7 ± 41.2 | < 0.001 |
| Subcutaneous fat area (cm2) | 127.1 ± 46.3 | 167.8 ± 57.2 | < 0.001 |
| Anthropometric variables | |||
| BMI (kg/m2) | 24.9 ± 3.0 | 24.8 ± 3.2 | 0.032 |
| Waist circumference (cm) | 86.3 ± 9.0 | 82.1 ± 9.0 | < 0.001 |
| Glucose regulation status | < 0.001 | ||
| Normal glucose regulation, n (%) | 2,491 (51.5) | 3,046 (47.7) | |
| Impaired glucose regulation, n (%) | 1,873 (38.7) | 2,581 (40.4) | |
| Newly diagnosed diabetes, n (%) | 471 (9.7) | 761 (11.9) | |
| Demographic variables | |||
| Education levels, n (%) | < 0.001 | ||
| Primary school or less | 1,921 (40.3) | 3,405 (53.9) | |
| Middle school | 2,343 (49.1) | 2,420 (38.3) | |
| High school or more | 508 (10.6) | 491 (7.8) | |
| Smoking status, n (%) | < 0.001 | ||
| Nonsmokers | 2,055 (42.5) | 6,372 (99.7) | |
| Ex-smokers | 290 (6.0) | 7 (0.1) | |
| Current smokers | 2,490 (51.5) | 9 (0.1) | |
| Alcohol consumption, n (%) | < 0.001 | ||
| Nondrinkers | 3,142 (65) | 6,352 (99.4) | |
| Ex-drinkers | 124 (2.6) | 3 (0.05) | |
| Current drinkers | 1,569 (32.5) | 33 (0.5) | |
| Leisure-time physical activity, n (%) | 0.12 | ||
| 0 min/d | 4,577 (94.7) | 5,999 (93.9) | |
| 1–29 min/d | 117 (2.4) | 158 (2.5) | |
| ≥ 30 min/d | 141 (2.9) | 231 (3.6) | |
| Family history of diabetes, n (%) | 603 (12.5) | 869 (13.6) | 0.08 |
Data are presented as means ± SD, medians (interquartile range), or frequencies (percentage). To convert values for insulin to pmol/L, multiply by 6.965.
P values from t test for difference in means, Mann–Whitney U test for difference in medians, or χ2 test for difference in proportions between two groups. HOMA-IR, homeostatic model assessment of insulin resistance; MRI, magnetic resonance imaging.
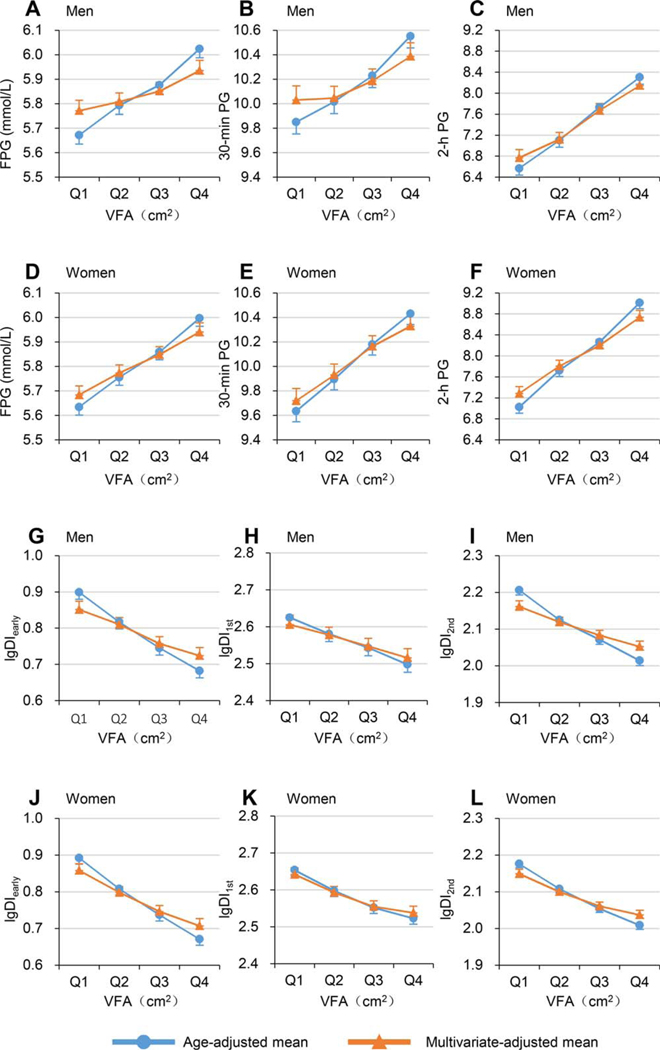
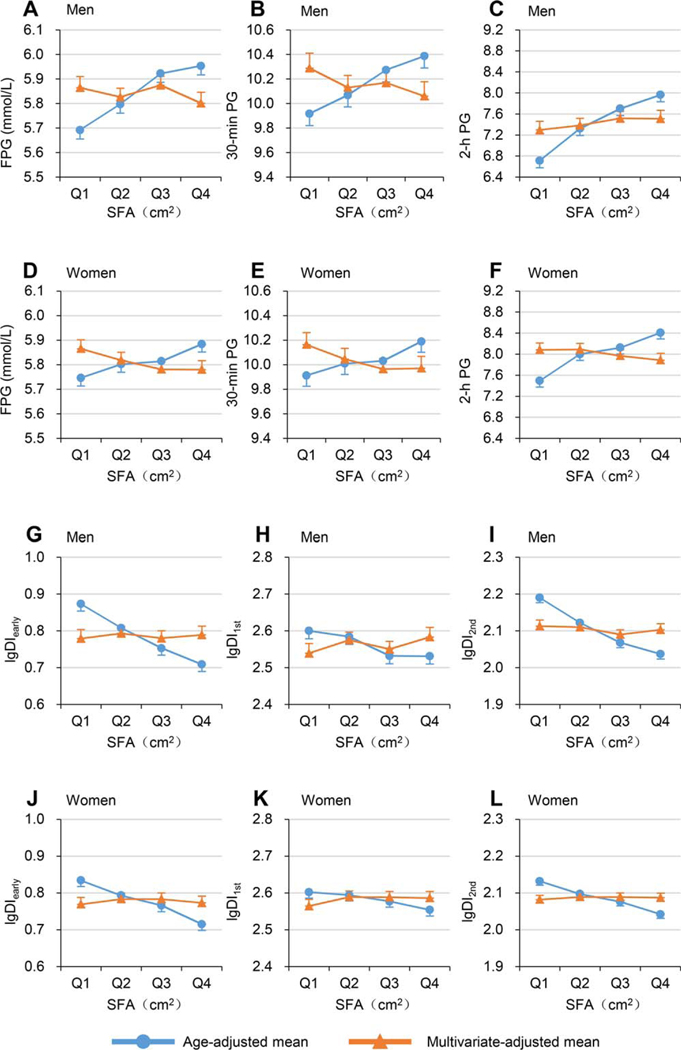
Table 2 and Table 3 present the age-adjusted and multivariable-adjusted mean values (95% CIs) of blood glucose and log-transformed DIs across quartiles of VFA or SFA (also shown in Figures 1 and 2). The age-adjusted mean levels of FPG, 30-minute PG, and 2-hour PG all significantly linearly increased, whereas age-adjusted mean values of log-transformed DIearly, DI1st, and DI2nd significantly linearly decreased across quartiles of either VFA or SFA in both men and women (all P <0.001 for linear trend). After adjustment for age, BMI, and SFA, the increasing linear trends of FPG, 30-minute PG, and 2-hour PG and the decreasing linear trends of log-transformed DIearly, DI1st, and DI2nd across VFA remained significant, with a slightly flatter slope among both men and women (all P <0.001 for linear trend). However, after adjustment for age, BMI, and VFA, the increasing linear trends of FPG, 30-minute PG, and 2-hour PG with SFA and the decreasing linear trends of log-transformed DIearly, DI1st, and DI2nd with SFA disappeared or even changed directions in men and women.
TABLE 2.
Adjusted means (95% CIs) of blood glucose and log-transformed disposition indices across increasing VFA quartiles
| VFA (cm2) |
P for linear trend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|
| |||||
| Men | |||||
| Age-adjusted means | |||||
| Fasting plasma glucose (mmol/L) | 5.67 (5.64–5.71) | 5.79 (5.76–5.83) | 5.88 (5.84–5.91) | 6.02 (5.99–6.06) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 9.85 (9.75–9.95) | 10.0 (9.92–10.1) | 10.2 (10.1–10.3) | 10.6 (10.5–10.6) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 6.57 (6.44–6.70) | 7.10 (6.97–7.23) | 7.73 (7.60–7.86) | 8.30 (8.17–8.43) | < 0.001 |
| lgDIearly | 0.90 (0.88–0.92) | 0.82 (0.80–0.84) | 0.75 (0.73–0.76) | 0.68 (0.66–0.70) | < 0.001 |
| lgDI1st | 2.63 (2.60–2.65) | 2.58 (2.56–2.60) | 2.54 (2.52–2.56) | 2.50 (2.48–2.52) | < 0.001 |
| lgDI2nd | 2.21 (2.19–2.22) | 2.13 (2.11–2.14) | 2.07 (2.06–2.08) | 2.01 (2.00–2.03) | < 0.001 |
| Multivariable-adjusted meansa | |||||
| Fasting plasma glucose (mmol/L) | 5.77 (5.73–5.81) | 5.81 (5.77–5.84) | 5.85 (5.81–5.89) | 5.94 (5.89–5.98) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 10.0 (9.91–10.1) | 10.1 (9.95–10.1) | 10.2 (10.1–10.3) | 10.4 (10.3–10.5) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 6.77 (6.61–6.92) | 7.12 (6.99–7.25) | 7.67 (7.54–7.80) | 8.15 (7.99–8.30) | < 0.001 |
| lgDIearly | 0.85 (0.83–0.87) | 0.81 (0.79–0.83) | 0.76 (0.74–0.78) | 0.72 (0.70–0.75) | < 0.001 |
| lgDI1st | 2.61 (2.58–2.63) | 2.58 (2.56–2.60) | 2.55 (2.53–2.57) | 2.52 (2.49–2.54) | < 0.001 |
| lgDI2nd | 2.16 (2.15–2.18) | 2.12 (2.11–2.13) | 2.08 (2.07–2.10) | 2.05 (2.04–2.07) | < 0.001 |
| Women | |||||
| Age-adjusted means | |||||
| Fasting plasma glucose (mmol/L) | 5.63 (5.60–5.67) | 5.76 (5.72–5.79) | 5.86 (5.83–5.89) | 6.00 (5.96–6.03) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 9.64 (9.55–9.72) | 9.90 (9.81–9.98) | 10.2 (10.1–10.3) | 10.4 (10.3–10.5) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 7.02 (6.91–7.14) | 7.72 (7.61–7.83) | 8.26 (8.15–8.38) | 9.01 (8.90–9.13) | < 0.001 |
| lgDIearly | 0.89 (0.88–0.91) | 0.81 (0.79–0.82) | 0.74 (0.72–0.75) | 0.67 (0.65–0.69) | < 0.001 |
| lgDI1st | 2.65 (2.64–2.67) | 2.60 (2.58–2.61) | 2.55 (2.54–2.57) | 2.52 (2.51–2.54) | < 0.001 |
| lgDI2nd | 2.18 (2.17–2.19) | 2.11 (2.10–2.12) | 2.05 (2.04–2.07) | 2.01 (2.00–2.02) | < 0.001 |
| Multivariable-adjusted meansa | |||||
| Fasting plasma glucose (mmol/L) | 5.68 (5.65–5.72) | 5.77 (5.74–5.81) | 5.85 (5.82–5.88) | 5.94 (5.90–5.98) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 9.72 (9.62–9.82) | 9.93 (9.84–10.0) | 10.2 (10.1–10.3) | 10.3 (10.2–10.4) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 7.28 (7.15–7.41) | 7.80 (7.69–7.92) | 8.20 (8.09–8.32) | 8.74 (8.60–8.87) | < 0.001 |
| lgDIearly | 0.86 (0.84–0.88) | 0.80 (0.78–0.81) | 0.75 (0.73–0.76) | 0.71 (0.69–0.73) | < 0.001 |
| lgDI1st | 2.64 (2.62–2.66) | 2.59 (2.58–2.61) | 2.56 (2.54–2.57) | 2.54 (2.52–2.56) | < 0.001 |
| lgDI2nd | 2.15 (2.14–2.16) | 2.10 (2.09–2.11) | 2.06 (2.05–2.07) | 2.04 (2.02–2.05) | < 0.001 |
Cut points of VFA (cm2) are 83.5, 117.8, and 155.5 in men and 78.4, 103.3, and 131.8 in women. Linear trend test tested by linear regression.
Adjusted for age, BMI, and subcutaneous fat area.
DIearly, early-phase disposition index; DI1st, first-phase disposition index; DI2nd, second-phase disposition index; lg, log-transformed; VFA, visceral fat area.
TABLE 3.
Adjusted means (95%CIs) of blood glucose and log-transformed disposition indices across increasing SFA quartiles
| Abdominal SFA |
P for linear trend | ||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
|
| |||||
| Men | |||||
| Age-adjusted means | |||||
| Fasting plasma glucose (mmol/L) | 5.69 (5.66–5.73) | 5.80 (5.76–5.83) | 5.92 (5.89–5.96) | 5.95 (5.92–5.99) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 9.92 (9.82–10.0) | 10.1 (9.97–10.2) | 10.3 (10.2–10.4) | 10.4 (10.3–10.5) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 6.71 (6.58–6.85) | 7.32 (7.19–7.45) | 7.70 (7.57–7.84) | 7.96 (7.83–8.10) | < 0.001 |
| lgDIearly | 0.87 (0.85–0.89) | 0.81 (0.79–0.83) | 0.75 (0.73–0.77) | 0.71 (0.69–0.73) | < 0.001 |
| lgDI1st | 2.60 (2.58–2.62) | 2.58 (2.56–2.61) | 2.53 (2.51–2.55) | 2.53 (2.51–2.55) | < 0.001 |
| lgDI2nd | 2.19 (2.18–2.20) | 2.12 (2.11–2.14) | 2.07 (2.05–2.08) | 2.04 (2.02–2.05) | < 0.001 |
| Multivariable-adjusted meansa | |||||
| Fasting plasma glucose (mmol/L) | 5.86 (5.82–5.91) | 5.83 (5.79–5.86) | 5.87 (5.84–5.91) | 5.80 (5.76–5.85) | 0.040 |
| 30-min PG (mmol/L) | 10.3 (10.2–10.4) | 10.1 (10.0–10.2) | 10.2 (10.1–10.3) | 10.1 (9.94–10.2) | 0.10 |
| 2-h plasma glucose (mmol/L) | 7.30 (7.13–7.46) | 7.38 (7.25–7.52) | 7.52 (7.38–7.65) | 7.51 (7.35–7.67) | 0.24 |
| lgDIearly | 0.78 (0.76–0.80) | 0.79 (0.77–0.81) | 0.78 (0.76–0.80) | 0.79 (0.77–0.81) | 0.74 |
| lgDI1st | 2.54 (2.51–2.57) | 2.58 (2.55–2.60) | 2.55 (2.53–2.57) | 2.58 (2.56–2.61) | 0.043 |
| lgDI2nd | 2.11 (2.10–2.13) | 2.11 (2.10–2.12) | 2.09 (2.08–2.10) | 2.10 (2.09–2.12) | 0.12 |
| Women | |||||
| Age-adjusted means | |||||
| Fasting plasma glucose (mmol/L) | 5.75 (5.71–5.78) | 5.80 (5.77–5.83) | 5.81 (5.78–5.85) | 5.88 (5.85–5.92) | < 0.001 |
| 30-min plasma glucose (mmol/L) | 9.91 (9.82–10.0) | 10.0 (9.92–10.1) | 10.0 (9.94–10.1) | 10.2 (10.1–10.3) | < 0.001 |
| 2-h plasma glucose (mmol/L) | 7.49 (7.38–7.61) | 8.00 (7.88–8.12) | 8.12 (8.01–8.24) | 8.41 (8.29–8.53) | < 0.001 |
| lgDIearly | 0.83 (0.82–0.85) | 0.79 (0.78–0.81) | 0.77 (0.75–0.78) | 0.72 (0.70–0.73) | < 0.001 |
| lgDI1st | 2.60 (2.59–2.62) | 2.59 (2.58–2.61) | 2.58 (2.56–2.59) | 2.55 (2.54–2.57) | < 0.001 |
| lgDI2nd | 2.13 (2.12–2.14) | 2.10 (2.09–2.11) | 2.08 (2.07–2.09) | 2.04 (2.03–2.05) | < 0.001 |
| Multivariable-adjusted meansa | |||||
| Fasting plasma glucose (mmol/L) | 5.87 (5.83–5.90) | 5.82 (5.79–5.85) | 5.78 (5.75–5.81) | 5.78 (5.74–5.82) | 0.006 |
| 30-min plasma glucose (mmol/L) | 10.2 (10.1–10.3) | 10.1 (9.96–10.1) | 9.96 (9.88–10.1) | 9.97 (9.87–10.1) | 0.027 |
| 2-h plasma glucose (mmol/L) | 8.08 (7.96–8.21) | 8.09 (7.97–8.20) | 7.97 (7.85–8.08) | 7.89 (7.76–8.01) | 0.12 |
| lgDIearly | 0.77 (0.75–0.79) | 0.78 (0.77–0.80) | 0.78 (0.77–0.80) | 0.77 (0.75–0.79) | 0.52 |
| lgDI1st | 2.56 (2.55–2.58) | 2.59 (2.57–2.61) | 2.59 (2.57–2.60) | 2.59 (2.57–2.60) | 0.17 |
| lgDI2nd | 2.08 (2.07–2.09) | 2.09 (2.08–2.10) | 2.09 (2.08–2.10) | 2.09 (2.07–2.10) | 0.78 |
Cut points of SFA (cm2) are 94.9, 124.0, and 155.6 in men and 127.5, 162.0, and 201.6 in women. Linear trend test tested by linear regression.
Adjusted for age, BMI, and visceral fat area.
DIearly, early-phase disposition index; DI1st, first-phase disposition index; DI2nd, second-phase disposition index; lg, log-transformed; SFA, subcutaneous fat area.
Figure 1.
Age-adjusted and multivariable-adjusted means (95% CIs) of blood glucose and log-transformed disposition indices across increasing VFA quartiles. Dots or triangles represent means, and bars represent 95% CI of the upper or lower bound. Line with dots indicates age-adjusted means. Line with triangles indicates multivariable-adjusted means (adjusted for age, SFA, and BMI). Mean values of FPG, 30-minute PG, and 2-hour PG, in (A-C) men and (D-F) women. Mean values of lgDIearly, lgDI1st, and lgDI2nd in (G-I) men and (J-L) women. DIearly, early-phase disposition index; DI1st, first-phase disposition index; DI2nd, second-phase disposition index; FPG, fasting plasma glucose; lg, log-transformed; PG, plasma glucose; SFA, subcutaneous fat area; VFA, visceral fat area. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
Age-adjusted and multivariable-adjusted means (95% CIs) of blood glucose and log-transformed disposition indices across increasing SFA quartiles. Dots or triangles represent means, and bars represent 95% CI of the upper or lower bound. Line with dots indicates age-adjusted means. Line with triangles indicates multivariable-adjusted means (adjusted for age, VFA, and BMI). Mean values of FPG, 30-minute PG, and 2-hour PG in (A-C) men and (D-F) women. Mean values of lgDIearly, lgDI1st, and lgDI2nd in (G-I) men and (J-L) women. DIearly, early-phase disposition index; DI1st, first-phase disposition index; DI2nd, second-phase disposition index; FPG, fasting plasma glucose; lg, log-transformed; PG, plasma glucose; SFA, subcutaneous fat area; VFA, visceral fat area. [Color figure can be viewed at wileyonlinelibrary.com]
Adjusted standardized linear regression coefficients of blood glucose and DIs with VFA and SFA are shown in Table 4. After controlling for age, either VFA or SFA was significantly and positively associated with all three blood glucose indices (the coefficients ranged from 0.16 to 0.31 for VFA and from 0.05 to 0.20 for SFA, all P <0.001) but inversely associated with the DIs (the coefficients ranged from −0.30 to −0.13 for VFA and −0.26 to −0.06 for SFA, all P <0.001) among men and women. After further adjustment for BMI and SFA, the positive associations of VFA with blood glucose indices and inverse associations of VFA with DIs in both genders still remained significant but attenuated in association strength (the regression coefficients ranged from 0.10 to 0.25 and from −0.21 to −0.11, respectively, all P <0.001). After further adjustment for BMI and VFA, the positive association of SFA with blood glucose indices and the inverse association of SFA with DIs in men mostly disappeared or were still statistically significant but not clinically significant, with the absolute values of regression coefficients less than 0.1; in women, the associations mostly reversed and became favorable, but not to clinical significance (the absolute values of regression coefficients being less than 0.1). Further adjustment for smoking, drinking, physical activity, and family history of diabetes made little difference in the above results (data not shown).
TABLE 4.
Adjusted regression coefficients of blood glucose and log-transformed disposition indices with visceral fat area, subcutaneous fat area, and BMI
| Independent variable | Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a |
Model 2b |
Model 1a |
Model 2b |
|||||
| β | P | β | P | β | P | β | P | |
|
| ||||||||
| Blood glucose | ||||||||
| Fasting plasma glucose | ||||||||
| Visceral fat area | 0.22 | < 0.001 | 0.13 | < 0.001 | 0.23 | < 0.001 | 0.19 | < 0.001 |
| Subcutaneous fat area | 0.18 | < 0.001 | −0.002 | 0.92 | 0.07 | < 0.001 | −0.07 | < 0.001 |
| BMI | 0.22 | < 0.001 | 0.13 | < 0.001 | 0.17 | < 0.001 | 0.10 | < 0.001 |
| 30-min plasma glucose | ||||||||
| Visceral fat area | 0.16 | < 0.001 | 0.10 | < 0.001 | 0.17 | < 0.001 | 0.14 | < 0.001 |
| Subcutaneous fat area | 0.12 | < 0.001 | −0.02 | 0.38 | 0.05 | < 0.001 | −0.06 | < 0.001 |
| BMI | 0.16 | < 0.001 | 0.11 | < 0.001 | 0.13 | < 0.001 | 0.08 | < 0.001 |
| 2-h plasma glucose | ||||||||
| Visceral fat area | 0.28 | < 0.001 | 0.25 | < 0.001 | 0.31 | < 0.001 | 0.24 | < 0.001 |
| Subcutaneous fat area | 0.20 | < 0.001 | 0.05 | 0.015 | 0.14 | < 0.001 | −0.04 | 0.008 |
| BMI | 0.22 | < 0.001 | 0.001 | 0.97 | 0.25 | < 0.001 | 0.13 | < 0.001 |
| Disposition indices | ||||||||
| lgDIearly | ||||||||
| Visceral fat area | −0.24 | < 0.001 | −0.15 | < 0.001 | −0.25 | < 0.001 | −0.19 | < 0.001 |
| Subcutaneous fat area | −0.20 | < 0.001 | −0.02 | 0.40 | −0.13 | < 0.001 | 0.01 | 0.37 |
| BMI | −0.23 | < 0.001 | −0.11 | < 0.001 | –0.21 | < 0.001 | −0.10 | < 0.001 |
| lgDI1st | ||||||||
| Visceral fat area | −0.13 | < 0.001 | −0.11 | < 0.001 | −0.15 | < 0.001 | −0.13 | < 0.001 |
| Subcutaneous fat area | −0.09 | < 0.001 | 0.02 | 0.42 | −0.06 | < 0.001 | 0.04 | 0.030 |
| BMI | −0.11 | < 0.001 | −0.05 | 0.030 | −0.12 | < 0.001 | −0.06 | 0.004 |
| lgDI2nd | ||||||||
| Visceral fat area | −0.30 | < 0.001 | −0.19 | < 0.001 | −0.29 | < 0.001 | −0.21 | < 0.001 |
| Subcutaneous fat area | −0.26 | < 0.001 | −0.05 | 0.030 | −0.15 | < 0.001 | 0.02 | 0.21 |
| BMI | −0.29 | < 0.001 | −0.13 | < 0.001 | −0.25 | < 0.001 | −0.13 | < 0.001 |
In Model 1, dependent variables were each one of blood glucose indices or log-transformed DIs; independent variables included age and a single one of the obesity indices (visceral fat area, subcutaneous fat area, or BMI).
In Model 2, dependent variables were each one of blood glucose indices or log-transformed DIs; independent variables included age, visceral fat area, subcutaneous fat area, and BMI simultaneously.
DIearly, early-phase disposition index; DI1st, first-phase disposition index; DI2nd, second-phase disposition index; lg, log-transformed.
Discussion
The main findings of the present study in Chinese men and women included the following aspects. (1) After adjustment for age, BMI, and VFA, some adverse associations of SFA with blood glucose indices and DIs disappeared, whereas in the rest, the associations became significantly weaker in men (2-hour PG: 0.05 and DI2nd: −0.05) or were even reversed in women (PG: from −0.07 to −0.04; DI1st: 0.04) (all P <0.05). (2) After adjustment for age, BMI, and SFA, VFA was positively associated with indicators of blood glucose at three time points and inversely associated with DIs of three phases; of note, VFA had the strongest positive association with 2-hour PG (0.25 in men and 0.24 in women) and the strongest inverse association with DI2nd (−0.19 in men and −0.21 in women), far beyond BMI.
Some studies have suggested that there are some limitations in reporting beta-cell function in isolation (19) and that insulin secretion should be adjusted by insulin sensitivity (20). Under normal physiological conditions, circulating insulin concentrations are reciprocally related to insulin sensitivity, expressed as the body’s capacity for glucose disposal and ability to suppress hepatic glucose production in response to insulin (12,21). DIs (11,12,20,21), which are derived from an OGTT and incorporate a measurement of insulin sensitivity into assessments of beta-cell responses, have been widely used. Both basic and population studies have proven that defects in early- or first-phase insulin secretion lead to hyperglycemia (22) and type 2 diabetes (10,11,23–25). In addition, DIearly has been reported as the strongest metabolic predictor of subsequent diabetes (10,11). Thus, in the present study, DIs were used to reflect insulin function instead.
The positive associations of visceral adipose tissue with FPG and 2-hour PG have been consistently found in multiple ethnicities, such as white Americans (1), African Americans (2), Greenland Inuit (26), and Caucasians in Denmark (27) (visceral adipose tissue was measured by CT in the former two populations and by ultrasound in the latter two populations). To our knowledge, no population-based studies have examined the associations of visceral adipose tissue with DIs evaluated by a three-time-point OGTT. The inverse association of visceral adipose tissue (measured by MRI or CT) with insulin-mediated glucose disposal rate (measured during euglycemic, hyperinsulinemic, and glucose clamp) has been uniformly reported in some small sample studies reviewed by Garg (28). The present study first reported a positive association of VFA with 0-minute, 30-minute, and 2-hour blood glucose and an inverse association of VFA with the early-, first-, and second-phase DIs in Chinese men and women, independent of SFA and BMI. Moreover, VFA showed a stronger positive association with 2-hour PG than with FPG and 30-minute PG and had a stronger inverse association with DI2nd than with DIearly and DI1st, far beyond BMI, which is supported by the viewpoint that obesity is more intimately associated with post-prandial blood glucose.
The above studies conducted in multiple ethnicities (1,2,26,27) have also examined the association of subcutaneous adipose tissue with FPG and 2-hour PG, but the results are inconsistent, mainly due to different confounding factors being controlled for in each study. The study in African Americans (2) has elucidated a positive association of subcutaneous adipose tissue with FPG without adjustment for BMI and waist circumference, and the other three studies (1,26,27) indicated that the positive association of subcutaneous adipose tissue with glucose intolerance and IR tended toward the inverse or even disappeared after adjustment for BMI and/or waist circumference. Meanwhile, one review (28) including several studies with small study sample sizes indicated that there were inconsistent conclusions regarding the association of subcutaneous adipose tissue with glucose disposal rate and that no significant association of subcutaneous adipose tissue with glucose disposal rate was found in men and women with obesity (7–9).
The present study found that after controlling for age, SFA was positively associated with three blood glucose indices (the standardized coefficients ranged from 0.05 to 0.20, all P <0.001) and inversely associated with DIs (the standardized coefficients ranged from −0.26 to −0.06, all P <0.001) among men and women. After further adjustment for BMI and VFA, most of the positive associations of SFA with blood glucose indices and the inverse associations of SFA with DIs disappeared, the strength of the other associations became significantly weaker in men (2-hour PG: 0.05 and DI2nd: −0.05), or the associations were even reversed in women (PG: from −0.07 to −0.04; DI1st: 0.04) (all P <0.05). The reason for this gender-specific difference in associations of SFA with blood glucose and beta-cell function is unclear, but the significant gender differences in abdominal adipose distribution, in which men had significantly higher VFA (122.0 cm2 vs. 107.7 cm2, P < 0.001) but significantly lower SFA (127.1 cm2 vs. 167.8 cm2, P <0.001), may be one explanation. In addition, estrogen receptors expressed in adipose tissue with variation in density may be another reason (6). The clinically insignificant but favorable association between SFA and glucose metabolism may to some extent explain the inverse association between SFA and newly diagnosed diabetes in women (P Chen, X Hou, and W Jia, unpublished data). The present study provided strong evidence that VFA and BMI severely confounded the associations of SFA with blood glucose and DIs, according to the changes in regression coefficients before and after additional adjustment for BMI and VFA (29). The associations of SFA with blood glucose and DIs were not independent of VFA and BMI. The study conducted by Ross et al. also showed that the correlations between subcutaneous adipose tissue and glucose disposal were close to zero (0.06 in men and 0.02 in women) (7,8), which is in agreement with our findings regarding associations between SFA and DIs.
There are many differences between visceral adipose tissue and subcutaneous adipose tissue, including anatomical (subcutaneous vs. abdominal cavity), cellular (different number of larger adipocytes), molecular (receptor and adipokines), physiological, and metabolic differences (6). Subcutaneous adipose tissue is a natural depot for energy, whereas visceral adipose tissue is considered to be a more pathogenic adipose tissue compartment (6). Compared with those in subcutaneous adipose tissue, adipocytes in visceral adipose tissue are more metabolically active, more sensitive to lipolysis, and are more insulin resistant. Subcutaneous adipose tissue secretes or expresses more favorable adipokines than visceral adipose tissue, and excess visceral adipose tissue is correlated with higher levels of free fatty acid overflow, inflammatory biomarkers, adipocytokines, and proteins (6). These differences may partly explain the different associations of VFA and SFA with glucose metabolism.
Our present study has some advantages. First, to our knowledge, this was the first population-based study with large samples, which let us explore gender-specific weak associations after controlling for confounders. Second, VFA and SFA were accurately measured by MRI, with a higher resolution, and can thus more clearly show fat depots than when measured by CT. Third, blood glucose and insulin were measured at three time points (0 minutes, 30 minutes, and 2 hours) after an OGTT test, which allowed us to have a more comprehensive estimation of beta-cell function in different phases. The limitations were the cross-sectional study format and the single-slice estimates of VFA and SFA by MRI.
Conclusion
The associations of SFA with blood glucose and beta-cell function were clinically insignificant (slightly favorable in women but not in men); VFA was adversely associated with blood glucose and beta-cell function and was most strongly associated with 2-hour PG. Our population-based findings support the hypothesis that SFA is a natural fat depot, but VFA is a pathogenic fat depot and mainly affects 2-hour PG; these findings are very important in guiding the population toward a healthy weight.
Supplementary Material
Acknowledgments
We thank all the investigators for their contributions (listed in the Supporting Information) to this study.
Funding agencies:
This work was supported by the Shanghai Health and Family Planning Commission (grant number 2013ZYJB1001), the Biomedical Engineering Cross Research Foundation of Shanghai Jiao Tong University (grant number YG2015MS18), and the National Key Research and Development Program (grant number 2016YFC0903303).
Footnotes
Disclosure: The authors declared no conflict of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010;95:5419–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oka R, Miura K, Sakurai M, et al. Impacts of visceral adipose tissue and subcutaneous adipose tissue on metabolic risk factors in middle-aged Japanese. Obesity (Silver Spring) 2010;18:153–160. [DOI] [PubMed] [Google Scholar]
- 4.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 2000;23:465–471. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011;96:E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11–18. [DOI] [PubMed] [Google Scholar]
- 7.Ross R, Aru J, Freeman J, Hudson R, Janssen I. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab 2002;282:E657–E663. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Freeman J, Hudson R, Janssen I. Abdominal obesity, muscle composition, and insulin resistance in premenopausal women. J Clin Endocrinol Metab 2002;87: 5044–5051. [DOI] [PubMed] [Google Scholar]
- 9.Brochu M, Starling RD, Tchernof A, et al. Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab 2000;85:2378–2384. [DOI] [PubMed] [Google Scholar]
- 10.Lyssenko V, Almgren P, Anevski D, et al. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005; 54:166–174. [DOI] [PubMed] [Google Scholar]
- 11.Lin Z, Zhou J, Li X, et al. High-normal 2h glucose is associated with defects of insulin secretion and predispose to diabetes in Chinese adults. Endocrine 2015;48:179–186. [DOI] [PubMed] [Google Scholar]
- 12.Hannon TS, Kahn SE, Utschneider KM, et al. A review of methods for measuring beta-cell function: design considerations from the Restoring Insulin Secretion (RISE) consortium. Diabetes Obes Metab 2018;20:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luepker RV, Evans A, McKeigue P, Reddy KS. Cardiovascular Survey Methods. 3rd ed. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 14.Seltzer HS, Allen EW, Herron AL Jr., Brennan MT. Insulin secretion in responseto glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301. [DOI] [PubMed] [Google Scholar]
- 16.Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care 2001;24:796–797. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 19.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 20.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of betacell function: the hyperbolic correction. Diabetes 2002;51 (suppl 1):S212–S220. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationshipbetween insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672. [DOI] [PubMed] [Google Scholar]
- 22.Luzi L, DeFronzo RA. Effect of loss of first-phase insulin secretion on hepaticglucose production and tissue glucose disposal in humans. Am J Physiol 1989;257: E241–E246. [DOI] [PubMed] [Google Scholar]
- 23.Porte D, Kahn SE. Beta-cell dysfunction and failure in type 2 diabetes: potentialmechanisms. Diabetes 2001;50 (suppl 1):S160–S163. [DOI] [PubMed] [Google Scholar]
- 24.Cali AMG, Man CD, Cobelli C, et al. Primary defects in b-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care 2009;32:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulinsecretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen ME, Borch-Johnsen K, Stolk R, Bjerregaard P. Fat distribution andglucose intolerance among Greenland Inuit. Diabetes Care 2013;36:2988–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philipsen A, Jorgensen ME, Vistisen D, et al. Associations between ultrasoundmeasures of abdominal fat distribution and indices of glucose metabolism in a population at high risk of type 2 diabetes: the ADDITION-PRO study. PLoS One 2015;10:e0123062. doi: 10.1371/journal.pone.0123062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab 2004; 89:4206–4210. [DOI] [PubMed] [Google Scholar]
- 29.Pearce N, Greenland S. Confounding and interaction. In: Ahrens W, Pigeot I,eds. Handbook of Epidemiology. Berlin, Germany: Springer-Verlag; 2005:371–398. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.