Abstract
Hematopoietic stem cell (HSC) transplantation therapy is one of the most effective treatments for life-threatening hematopoietic diseases. Bone marrow (BM) and mobilized peripheral blood are the major sources of HSCs, but these resources are limited by a paucity of human leukocyte antigen (HLA)-matched donors. Umbilical cord blood (UCB) is the most promising alternative to obtain HSCs for transplantation therapy. However, UCB transplantation therapy is limited by low numbers of HSCs per unit of UCB. In vitro HSC expansion is believed to be the most effective and applicable strategy to address this issue. Here we report that a moderate concentration of GSK3 inhibitor promotes HSC expansion by inducing moderate levels of β-catenin activity in HSCs. However, such a concentration of GSK3 inhibitor also stimulates myeloid cells to produce inflammatory cytokines, which attenuate HSC expansion by inducing p38 activation. Thus, when unpurified HSCs were used in culture, inhibition of p38-induced inflammatory cytokine signaling was required to ensure HSC expansion induced by the low concentration of GSK3 inhibitor. Our study suggests that the combination of a moderate concentration of p38 inhibitor plus a GSK3 inhibitor synergistically promotes the expansion of both murine BM HSCs and human UCB HSCs.
Keywords: GSK3, p38, β-catenin, HSCs, UCB
Introduction
Hematopoietic stem cell (HSC) transplantation therapy is the most effective treatment for life-threatening hematopoietic diseases such as bone marrow (BM) failure and leukemia. It is also a very effective supportive therapy for many solid tumors when combined with chemotherapy and/or radiation therapy [1]. Each year, >50,000 patients worldwide receive HSC transplantation therapy, and many more patients are waiting for appropriate HSC donors for such treatment. HSCs isolated from donor BM or mobilized peripheral blood (mPB) are the major resources for HSC transplantation therapy, but this is restricted by a lack of fully matched human leukocyte antigen (HLA) donors [1]. Graft-versus-host disease (GvHD) and nonrecurrence mortality are the major problems for HLA partially matched HSC transplantation despite the present availability of more effective regimens for GvHD prophylaxis [2].
Umbilical cord blood (UCB) is an alternative resource for HSCs. Compared to HSCs collected from adult donors, UCB is readily available, absent donor attrition, and relatively less HLA restricted (due to the significantly lower doses and a more naive repertoire of T cells) [3]. However, UCB HSC transplantation therapy is limited by the low number of HSCs per unit of UCB, which results in slow hematopoietic recovery, increased risk of graft failure, and defective immune reconstitution [4].
Although HSCs in most UCB units are sufficient to reconstitute hematopoiesis in a smaller patient (<20 kg body weight), for larger patients, HSCs equivalent to 2–4 UCB units are required. Combined transplantation of ≥2 UCBs failed to solve this problem because it was found that only one of the UCB units became dominant and contributed to long-term engraftment. Thus, in vitro HSC expansion is believed to be the most effective and applicable strategy to address these hurdles [4–10]. As little as a three- to fivefold expansion of functional HSCs should be sufficient to make single-unit UCB transplantation therapy successful for almost all patients.
Significant efforts have been made during the last 50 years. However, since the key factors that promote self-renewal and maintain the undifferentiated state of HSCs have not been sufficiently identified, our ability to expand HSCs in vitro remains clinically inadequate [4]. Most culture conditions that have been evaluated in clinical trials for the expansion of UCB HSCs can only promote the early recovery of blood cells without significant enhancement of long-term hematopoietic reconstitutive capacity [4,6–10].
Wnt/β-catenin signaling has been identified as a major driving force for self-renewal in many types of tissue stem cells, such as intestinal and hair follicle stem cells. Overactivation of Wnt/β-catenin signaling induces stem cell expansion in these tissues [11]. However, in HSCs, only mildly increased Wnt/β-catenin activity (two- to threefold increase over baseline) enhances self-renewal activity, while greater than fourfold above baseline levels of Wnt/β-catenin signaling induces differentiation or apoptosis, resulting in HSC exhaustion [12,13].
GSK3 is a key negative regulator that represses Wnt/β-catenin signaling by phosphorylation-triggered β-catenin degradation. Inactivation of GSK3 induces constitutive activation of β-catenin. Two submembers of this family of enzymes, GSK3α and GSK3β, have been identified in mammals and share 98% sequence identity within their catalytic domains. CHIR99021 (CHIR here after) is a well-known inhibitor of both GSK3α and β. We found that a moderate concentration of CHIR promotes the expansion of purified HSCs by inducing a moderate level of β-catenin activity. However, in cultures of unpurified HSCs, such a concentration of CHIR also stimulates myeloid cells to produce inflammatory cytokines, such as IL-1β and TNFα. These cytokines attenuate HSC activity by stimulating p38. A moderate concentration of p38 inhibitor is required to enhance HSC expansion when combined with a moderate concentration of GSK3 inhibitor.
Materials and Methods
Mice and genotyping
CD45.1+ and CD45.2+ mice were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. β-cateninflox (exons 2–6; Stock No. 004152) and Mx1Cre mice were purchased from the Jackson Laboratory and crossed to generate Mx1Creβ-cateninflox mice. All mice were maintained in a C57BL6/J background and housed under a 12-h light/dark cycle in microisolator cages contained within a laminar flow ventilation system. β-catenin knockout was induced in mice by five injections of polyI:C as described previously [14,15]. NSG mice were purchased from the Jackson Laboratory. All procedures were conducted in accordance with the approved guidelines provided by the Laboratory Animal Resource Center of Shanghai Normal University and the National Institutes of Health guidelines for the care and use of laboratory animals for research purposes and were approved by the Loyola University Chicago's IACUC (AU#513380).
The polymerase chain reaction (PCR) primers used for the genotyping of experimental mice are as follows: for β-cateninflox, forward: 5′-aaggtagagtgatgaaagttgtt and reverse: 5′-caccatgtcctctgtctattc; for Cre, forward: 5′-gcggtctggcagtaaaaactatc and reverse: 5′-gtgaaacagcattgctgtcactt; and for internal control, forward: 5′-ctaggccacagaattgaaagatct and reverse: 5′-gtaggtggaaattctagcatcatcc.
Flow cytometry and antibodies for HSC purification and analysis
Antibodies used in this study were purchased from eBioscience, Inc. They include anti-murine antibodies: eFluor 450-Lin (including CD3, CD8, Ter119, B220, and Gr1), APC-c-Kit, PE-Sca1, PE-Cyanine7-CD150, APC eFluor® 780-CD48, PE-Mac1, APC-Gr1, APC-B220, and FITC-CD3. Anti-human antibodies: FITC-Lin (including CD3, CD11b, CD11c, CD14, CD19, CD24, CD56, CD66b, and CD235), PE-CD34, and APC-CD38.
For murine HSC purification, mononuclear cells (MNCs) were isolated from BM of mice after red blood cell lysis. Lin− hematopoietic cells were enriched using the EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit (eBioscience, Inc.). Lin−-enriched cells were then stained with FITC-lineage antibodies (Gr1, CD11b, CD3, B220, CD19, and Ter119), PE-c-Kit, APC-Sca1, PE-Cyanine7-CD150, and APC eFluor 780-CD48 surface markers. LSK hematopoietic stem/progenitor cells (HSPCs) and LSK-CD150+48− HSCs were purified by FACS using a BD Aria III. For murine HSC analysis, cultured cells were suspended in FACS buffer (1 × phosphate-buffered saline supplemented with 2% fetal bovine serum) at a concentration of 5 × 106 cells/mL and aliquoted into flow cytometry tubes (100 μL per tube) for indicated antibody staining. Data were collected using a BD LSR Fortessa cell analyzer or LSR II and analyzed by FlowJo10.0 software.
Intracellular antibody staining
To analyze the expression and activities of p38, mTor, Jnk, Erk, and Akt, cultured cells were fixed immediately in 4% paraformaldehyde for 5 min to protect the epitopes. The cells were permeabilized with Cytofix/Cytoperm™ (BD Biosciences) and stained with antibodies to specifically recognize pan-p38, p-p38, pan-S6, p-S6, pan-Jnk, p-Jnk, Erk, p-Erk, Akt, and p-Akt for 1 h on ice. Cells were washed with FACS buffer and the expression and activation of p38, mTor, Jnk, Erk, and Akt were measured on a BD LSR Fortessa cell analyzer by examining the intensity of fluorescent antibody staining.
Quantitative reverse transcription PCR for gene expression
RNA was isolated from cells using TRIzol reagent (Invitrogen). Complementary DNAs (cDNAs) were generated from RNA using SuperScript® III Reverse Transcriptase (Life Technologies). Levels of messenger RNA (mRNA) for Axin 2 (Mm00443610_m1), HoxB4 (Mm00657964_m1), Myc (Mm00487804_m1), and Ccnd1 (Mm00432359_m1) were examined by quantitative reverse transcription PCR (qRT-PCR) using TaqMan assay following the instructions provided by the vendors (Thermo Fisher Scientific). Gapdh (Mm99999915_g1) was used as a control. The amount of transcript was determined based on a standard curve specific for each gene and normalized to the amount of Gapdh transcript in the same sample.
Murine HSC culture and phenotype analysis
The stem cell culture medium used in this study was StemSpan™ serum-free expansion medium (SFEM; Stem Cell Technologies) supplemented with 10 μg·mL−1 heparin (Sigma), 50 ng·mL−1 SCF, 20 ng·mL−1 TPO, and 20 ng·mL−1 Flt3 ligand (eBioscience, Inc.). For purified HSC culture, 200 LSK-CD150+48− HSCs or 1,000 LSK HSPCs from CD45.1+ mice were cultured in 150 μL stem cell medium containing the indicated inhibitors in each well of U-bottom 96-well plates for 9 days. For MNC culture, 5 × 105 MNCs were cultured in 1.5 mL stem cell medium containing the indicated inhibitors in each well of a six-well plate. Cells were cultured for 9 days with medium change every other day. At the end of the culture period, total cell numbers were counted, and cells were stained with indicated antibodies and analyzed or sorted by flow cytometry.
Competitive hematopoietic reconstitutive assay and secondary transplantations for functional analysis of expanded HSCs
To study the functional hematopoietic reconstitutive capacity of the expanded HSCs, as described above, all cells from each well were collected separately on day 9 of culturing and suspended into 1 mL of serum-free medium and mixed with 1 mL of support BM cells (2 × 106/mL) for competitive hematopoietic reconstitutive (CHR) assay. Cells from each well were proportionally transplanted into 10 lethally irradiated (10 Gy) recipient mice (CD45.2+), 200 μL per mouse. Therefore, each recipient received expanded cells from 20 input LSK-CD150+48− HSCs, or 100 input LSK HSPCs or 5 × 104 input BM MNCs together with 2 × 105 support BM cells. Two hundred freshly isolated LSK-CD150+48− HSCs, 1,000 freshly isolated LSK HSPCs, or freshly isolated 5 × 104 BM MNCs were mixed together with 1 mL of support BM cells (2 × 105/mL) and transplanted in parallel as controls.
Long-term engraftment of cultured cells was examined by detecting CD45.1+ cells in the PB of recipient mice 4 months after transplantation. The multilineage differentiation capacity of the cultured cells was examined by detecting the percentage of Gr1+Mac1+ granulocytes, CD3+ T cells, and B220+ B cells among CD45.1+ PB MNCs. To examine the durable engraftment capacity of the cultured HSCs, secondary transplantation was conducted to evaluate second engraftment capacity. For each group, BM MNCs were collected from six of the first recipient mice 4 months after transplantation, combined, and then transplanted into lethally irradiated (10 Gy) mice (CD45.2+), 107 MNCs per recipient. Secondary engraftment capacity of the expanded HSCs was examined by detecting CD45.1+ cells in the PB of recipient mice 4 months after transplantation.
Human UCB culturing and transplantation
An IRB protocol for the use of human UCB cells was approved in advance by Loyola University's Institutional Review Board. MNCs of UCB were obtained from the Stem Cell Bank at the Cardinal Bernardin Cancer Center, Loyola University Chicago. The UCB samples had been previously collected and stored under liquid nitrogen. Before use, cells were thawed at 37°C, and the MNCs were purified by a Ficoll-Paque gradient. For flow cytometric analysis, MNCs were cultured in 106/mL StemSpan serum-free medium supplemented with 50 ng each of human (h) SCF, hTPO, and hFLT3L at 37°C, 5% CO2 with or without 0.5 μM/mL CHIR and 0.5 μM/mL SB. Cells were cultured for 12 days with medium change every third day. At the end of the culture period, total cell numbers were counted and cells were stained with indicated antibodies and analyzed or sorted by flow cytometry.
For in vivo transplantation, 2 × 107 MNCs were seeded at a density of 106 cells/mL of medium with or without 0.5 μM/mL CHIR and 0.5 μM/mL SB. All cells from each well were collected separately and suspended into 1 mL of serum-free medium for CHR assay. Cells from each well were equally transplanted into 10 sublethally radiated (3 Gy) NSG mice, 200 μL per mouse. Therefore, each recipient received expanded cells from 2 × 106 input MNCs. Two million uncultured MNCs were transplanted in parallel as controls. Long-term engraftment of cultured cells was examined by detecting human CD45+ cells in the PB of recipient mice 4 months after transplantation. The multilineage differentiation capacity of the cultured cells was examined by detecting the percentage of CD33+ granulocytes, CD3+ T cells, and CD19+ B cells in CD45+ PB MNCs.
Statistical analysis
Data are expressed as mean ± standard deviation. One-way analysis of variance (multiple groups) and Student's t-test (two groups) were performed to determine the statistical significance of differences among and between experimental groups at a significance level of P < 0.05.
Results
A moderate concentration of GSK3 inhibitor promotes expansion of purified HSCs in vitro
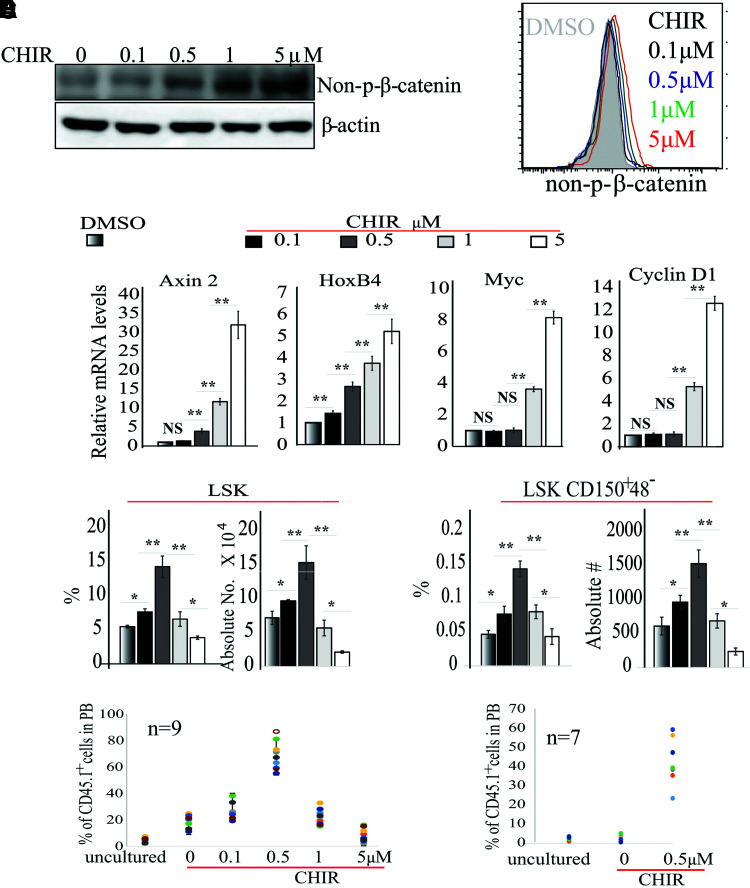
Inhibition of GSK3 to promote in vitro expansion of HSCs has been tested but has achieved minimal success, which can possibly be explained by the high concentration of inhibitor used in previous studies [16,17]. We predicted that a moderate concentration of GSK3 inhibitor, which induces a moderate level of β-catenin activity, may help to promote HSC expansion. To test such an idea, we first determined the correlation of CHIR concentration with β-catenin activity in HSCs. To do so, we isolated c-kit+ HSPCs (a mixture of HSCs and HPCs) from the BM of wild-type (WT) C57Bl57/J mice and treated them with increasing concentrations of CHIR. Following 3 h of treatment, cells were collected for western blotting to detect the nonphosphorylated (active) form of β-catenin (AC-β-catenin).
We found a concentration-dependent β-catenin activation function, which is stimulated by CHIR (Fig. 1A). To study whether CHIR induces β-catenin activation in HSCs, we treated Lin−Sca1+c-kit+ CD48−CD150+ (LSK-150) LT-HSCs isolated from mouse BM with various concentrations of CHIR, and then evaluated β-catenin activity after 3 h of treatment using flow cytometry for AC-β-catenin (Fig. 1B) and qRT-PCR assay for the expression of β-catenin target genes, including Axin2, HoxB4, Ccnd1, and c-Myc (Fig. 1C). We found a CHIR concentration-dependent activation function for β-catenin in HSCs. Moreover, we determined that, compared with vehicle-treated controls, 0.1 μM CHIR only slightly induced HoxB4 expression but did not induce Axin2, Myc, and Ccnd1 at all, while 0.5 μM CHIR induced a two- to fourfold increase in both Axin 2 and HoxB4 expressions but not in Ccnd1 and Myc. Significantly increased expression of all four target genes was detected when higher concentrations of CHIR were used (Fig. 1C).
FIG. 1.
Determining the optimal concentration of CHIR99021 (CHIR) for HSC expansion. (A) c-Kit+ HSPCs were isolated from BM of WT mice and treated with increasing concentrations of CHIR for 3 h. β-catenin activity was examined by western blotting for non-p-β-catenin. (B, C) LSK-CD150+48− HSCs were isolated from BM of WT mice and treated with indicated concentrations of CHIR for 3 h. β-catenin activity was assessed by FACS for intracellular non-p-β-catenin staining (B) and Axin 2, HoxB4 cyclin D1, and c-myc expression (C). (D–F) LSK-CD150+48− HSCs were cultured in stem cell medium with indicated concentrations of CHIR. Two hundred HSCs were sorted for each group by FACS. Cells were cultured for 9 days with medium change every other day. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (D); functional HSCs were evaluated by CHR assay (E) and serial transplantation (F). In (E, F), freshly isolated (uncultured) LSK-CD150+48− HSCs were studied as controls. BM cells used for second transplantations in (F) were mixtures of BM cells collected from all nine recipient mice from the first transplantation in each group in (E). The percentage of CD45.1+ cells among mixed BM cells was 5.7% for the uncultured group, 19.2% for the 0 μM CHIR group, and 67.5% for the 0.5 μM CHIR group, respectively. Data in (A–D) are representative of three independent experiments. *P < 0.05; **P < 0.01. BM, bone marrow; CHR, competitive hematopoietic reconstitutive; HSC, hematopoietic stem cell; HSPCs, hematopoietic stem/progenitor cells; NS, not significant; WT, wild type. Color images are available online.
To test which concentration of CHIR best enhances HSC expansion, we sorted LSK-150 (Lin−Sca1+Kit+CD48−CD150+) HSCs (as described in Supplementary Fig. S1) into a 96-well plate, 200 cells per well, containing 150 μL of stem cell culture medium [StemSpan serum-free medium supplemented with 50 ng of murine (m) SCF, mTPO, and mFLT3L]. Cells were incubated at 37°C, 5% CO2 with various concentrations of CHIR and half medium change every 3 days for 9 days (Fig. 1D). The percentages and numbers of phenotypic HSCs were examined by flow cytometry for LSK HSPCs and LSK-150 HSCs (Supplementary Fig. S1b). We found that the numbers of both types of cells were significantly increased in the 0.1 and 0.5 μM CHIR groups with the greatest increase in the 0.5 μM CHIR group (greater than threefold increase of LSK-150 HSCs compared with vehicle group), while such numbers were reduced by further increases in CHIR concentration (Fig. 1D). To determine which concentration of CHIR best promotes the expansion of functional HSCs, we sorted LSK-150 HSCs (CD45.1+ background) directly into a 96-well plate at 200 cells per well.
Cells were treated with various concentrations of CHIR as described above. On day 9 of treatment, all cells from each well were collected and mixed with 2 × 106 freshly isolated support BM cells (CD45.2+ background). These mixed cells were equally transplanted into lethally irradiated mice (CD45.2+ background). Thus, each recipient received cultured cells from exactly 20 input HSCs and 2 × 105 support BM cells. Two hundred freshly isolated LSK-150 LT-HSCs (CD45.1+ background) were mixed with 2 × 106 support BM cells (CD45.2+ background) and were transplanted into 10 recipient mice in parallel as controls (Fig. 1E). The percentage of donor cells was analyzed 4 months post-transplantation by examining the percentage of CD45.1+ cells in the PB of recipient mice. Compared with freshly isolated HSCs, the % of donor cells in the vehicle-treated group was increased by threefold; such values were further increased in groups with low-concentration CHIR.
Among all groups, the % of donor cells was highest in 0.5 μM CHIR (11-fold compared with the uncultured group and 3.5-fold compared with the vehicle-only group) (Fig. 1E). However, further increases in CHIR concentration resulted in reduced % of donor cells. In addition, the cultured HSCs showed a multipotent differentiation ability as demonstrated by examining the percentages of Gr1+/CD11b+ myeloid cells, CD3+ T lymphocytes, and B220+ B lymphocytes in the CD45.1+ population 4 months post-transplantation (Supplementary Figs. S2 and S3). Most importantly, HSCs in the vehicle-treated group only subtly improved hematopoietic reconstitution capacity (HRC) in the second transplantation, while a 20-fold increase in secondary HRC was observed in the 0.5 μM CHIR group compared with the uncultured group (Fig. 1F). In addition, we found that the numbers of LSK HSPCs and LSK-150 HSCs were accurately correlated to the % of donor cells in the first transplantation. These data suggest that 0.5 μM CHIR induces a moderate level of β-catenin activity and promotes the expansion of durable functional HSCs.
GSK3 inhibitor-induced HSC expansion is dependent upon β-catenin
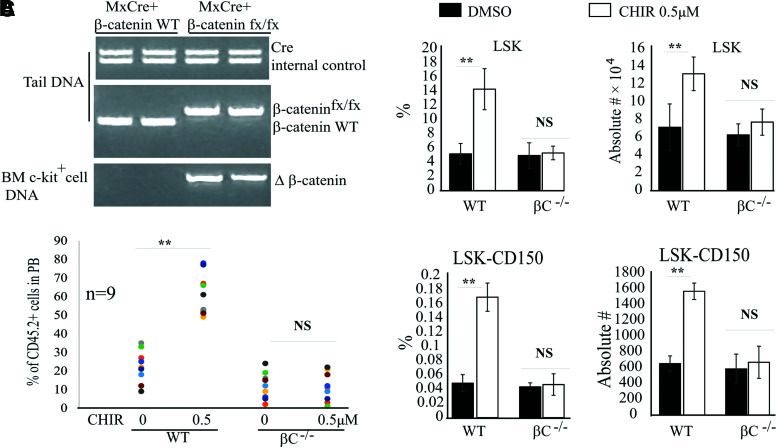
To determine whether CHIR-induced HSC expansion is dependent upon β-catenin, we treated Mx1Cre+β-cateninflox mice with poly I:C every other day for 5 days to induce β-catenin deletion. One month following induction, after verifying β-catenin deletion in HSCs by PCR (Fig. 2A), LSK-150 HSCs were purified from the BM of β-catenin knockout mice (β-catenin−/−) and incubated in stem cell medium with 0.5 μM CHIR as described above. LSK-150 HSCs isolated from WT mice were studied in parallel as controls. After 9 days of culturing, we found a significant expansion of WT HSCs as shown by significantly increased LSK and LSK-150 populations treated with 0.5 μM CHIR compared with the vehicle-treated control group (Fig. 2B). However, both β-catenin−/− LSK HSPCs and LSK-150 LT-HSCs were comparable between the control group and the CHIR group (Fig. 2B). In addition, 0.5 μM CHIR did not induce the expansion of functional β-catenin−/− HSCs as demonstrated by transplantation assay (Fig. 2C). This suggested that GSK3 inhibitor-induced HSC expansion is dependent upon β-catenin.
FIG. 2.
CHIR-induced HSC expansion is dependent upon β-catenin. (A) c-Kit+ HSPCs were isolated from BM of β-catenin−/− and WT mice. β-catenin knockout was examined by PCR assay for Δ-β-catenin. DNA from tail tissue was collected from the same mice for genotype analysis by PCR for Cre gene and β-cateninfx/fx. (B, C) LSK-CD150+48− HSCs were isolated from BM of WT and β-catenin−/− mice and cultured in stem cell medium with or without 0.5 μM CHIR. Two hundred HSCs were sorted in each group by FACS. Cells were cultured for 9 days with medium change every other day. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (B) and functional HSCs were evaluated by CHR assay (C). Freshly isolated (uncultured) LSK-CD150+48− HSCs were studied as controls. Data in (B) are representative results of three independent experiments. **P < 0.01. PCR, polymerase chain reaction. Color images are available online.
GSK3 inhibitor-induced HSC expansion is attenuated by inflammatory cytokines in unpurified BM cells
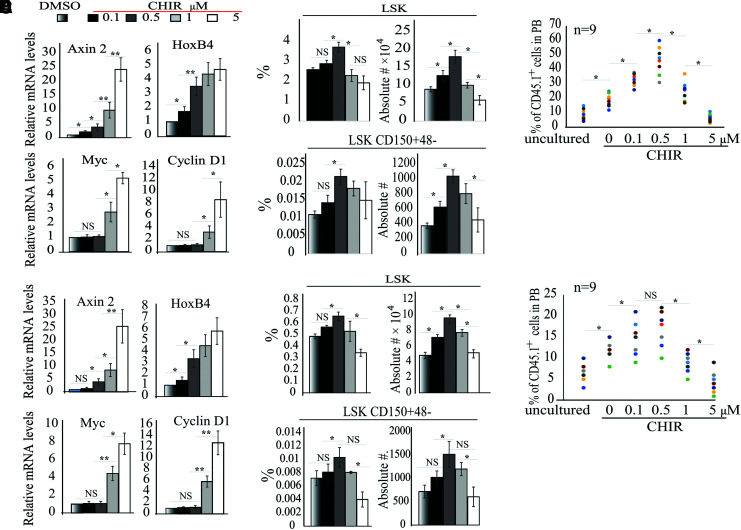
Since significant numbers of HSCs are usually lost during the purification procedures, we asked whether we could expand HSCs in the absence of the purification process. To do so, we first tested whether 0.5 μM CHIR can still induce a two- to fourfold increase of Axin 2 and HoxB4 expression in HSCs when they are mixed with other progenitor cells and/or mature hematopoietic cells. We treated LSK HSPCs (Fig. 3A) and BM MNCs (Fig. 3E) with the indicated concentrations of CHIR for 3 h, followed by purification of LSK-150 HSCs from the cultured cells by FACS. β-catenin activity was evaluated by qRT-PCR for β-catenin target genes. We observed a CHIR concentration-dependent activation of β-catenin in HSCs regardless of their purification status.
FIG. 3.
GSK3 inhibitor-induced HSC expansion is attenuated when unpurified BM cells were used in culture. (A, B) LSK HSPCs (A) or BM MNCs (B) were cultured for 6 h in stem cell medium with indicated concentrations of CHIR. LSK-CD150+ HSCs were purified by FACS. β-catenin activity was examined by qRT-PCR for target gene expression. (C–F) LSK HSPCs (1,000 cells per group) (C, D) or BM MNCs (500,000 cells per group) (E, F) were cultured for 9 days in stem cell medium with indicated concentrations of CHIR with medium change every other day. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (C); HSPCs were examined by CFU assay (E). Functional HSCs were evaluated by CHR assay (D, F). *P < 0.05; **P < 0.01. MNCs, mononuclear cells; qRT-PCR, quantitative reverse transcription polymerase chain reaction. Color images are available online.
In addition, we detected a consistent 2-4-fold increase in Axin 2 and HoxB4 expression but without induction of Myc and Ccnd1 in 0.5 μM CHIR-treated groups (Fig. 3A, B). Further increased CHIR concentration induced expression of all 4 target genes. However, compared with the purified LSK-150 HSC culture (Fig. 1), HSC expansion was attenuated in the LSK cell cultures (Fig. 3C, D) and was further compromised in BM MNC cultures (Fig. 3E, F). In LSK cultures, ∼2- and ∼2.5-fold increases in LSK HSPCs and LSK-150 HSCs were observed in the 0.5 μM CHIR group compared with the control group (Fig. 3C, D). The % of donor cells in the control group was increased by 2.1-fold compared with the uncultured group, while the % of donor cells in the 0.5 μM CHIR group was increased by 5.3- and 2.5-fold compared with the uncultured group and the control group, respectively (Fig. 3D). However, in BM MNC culture, less than twofold increases in LSK HSPCs and LSK-150 HSCs were observed in the 0.5 μM CHIR-treated group (Fig. 3E, F), which correlated to a 1.5-fold increase in the % of donor cells compared with the vehicle-only group (Fig. 3F). Thus, we concluded that some factors produced by mature cells repress HSC expansion.
Several inflammatory cytokines, such as TNFα, IL-6, IL-1β, IFN-α, and IFN-γ, have been implicated as negative regulators of HSC self-renewal [18]. To identify which of these cytokines repress HSC expansion in our culture system, we compared the cytokine profile in the supernatants of our cultures using the cytokine 23-Plex assay [19]. We found that, compared with the supernatant collected from purified HSC culture, the supernatant from our BM MNC culture contained significantly higher concentrations of TNFα and IL-1β, which were further increased in CHIR-treated BM MNCs (Supplementary Table S1).
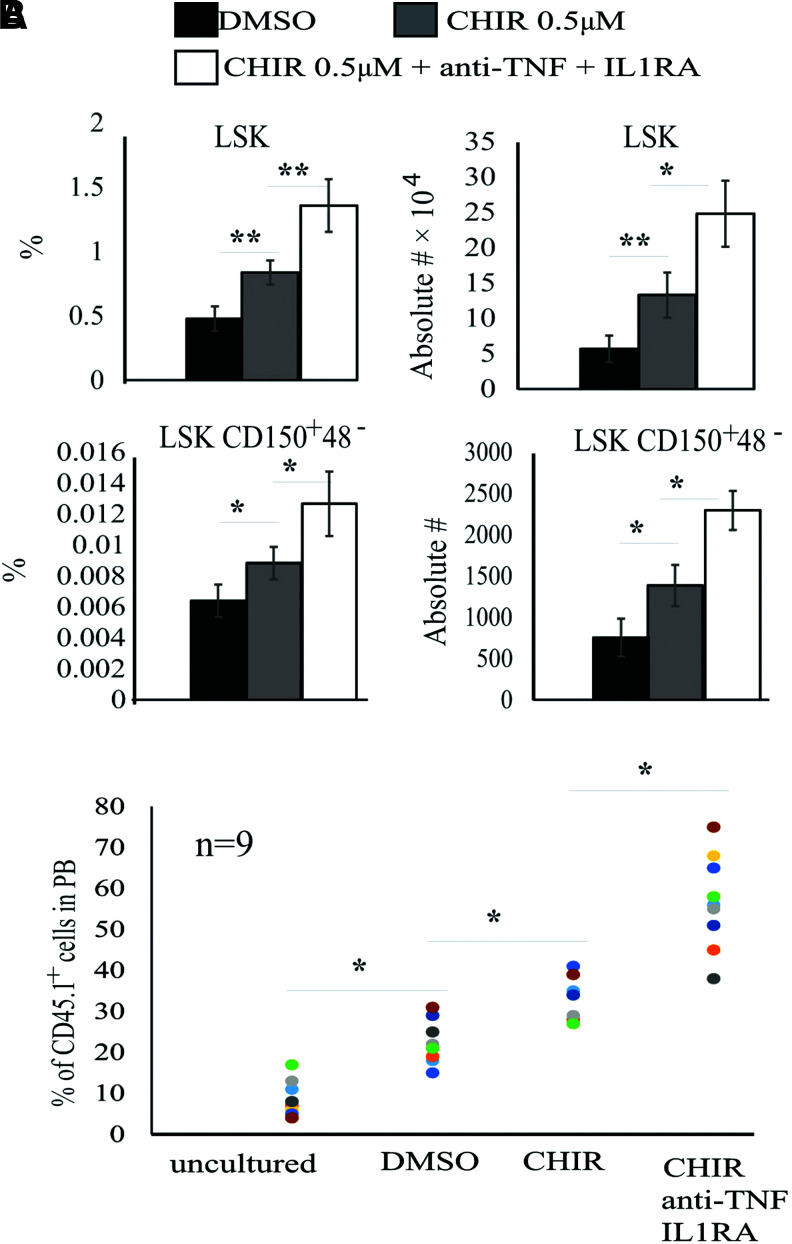
To examine whether TNFα and IL-1β repress HSC expansion in our BM MNC culture, we blocked TNFα and IL-1β signaling using the anti-TNF antibody and an IL-1 blocker, IL-1RA, respectively. We found that inhibition of either TNFα or IL-1β signaling failed to restore the HSC expansion induced by CHIR (data not shown). However, inhibition of both cytokine signals largely restored HSC expansion capacity (Fig. 4A and B). Furthermore, the addition of conditioned medium collected from the BM MNC culture, recombinant TNFα, or recombinant IL-1β attenuated the expansion of the purified HSCs (Supplementary Fig. S4). These data suggested that both TNFα and IL-1β produced by mature cells repressed HSC expansion in our cultures.
FIG. 4.
GSK3 inhibitor-induced HSC expansion is attenuated in unpurified BM MNCs by inflammatory cytokines. BM MNCs (300,000 cells in each group) were cultured for 9 days in stem cell medium containing 0.5 μM CHIR with or without anti-TNF and IL-1RA with medium change every other day. DMSO-treated cells were studied in parallel as a control. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (A). Functional HSCs were evaluated by CHR assay (B). Data in (A) are representative results from three independent experiments. *P < 0.05; **P < 0.01. DMSO, dimethyl sulfoxide. Color images are available online.
Inflammatory cytokines repress HSC expansion by inducing p38 signaling
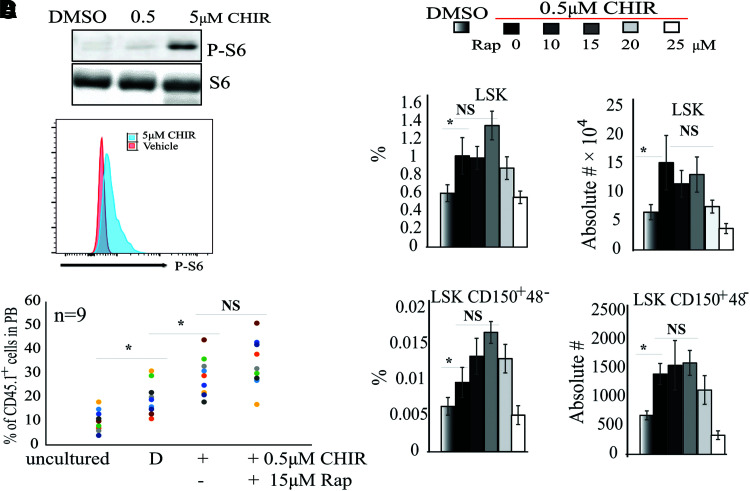
Akt-mTor signaling negatively regulates HSC self-renewal by inducing proliferation and differentiation [20]. It was reported that genetic inactivation of Gsk3β not only induces β-catenin activation but also stimulates mTor signaling. Thus, HSCs in Gsk3β-knockout mice are only transiently expanded, followed by their exhaustion [21]. We first tested whether CHIR induces mTor signaling in HSCs in our cultures. We found that a high concentration of CHIR induces the activation of mTor signaling in HSCs as shown by an increased p-S6 level, while 0.5 μM CHIR did not do so, regardless of whether purified or unpurified HSCs were used (Fig. 5A, B). We also found that a combination of the mTor inhibitor, rapamycin, plus 0.5 μM CHIR did not enhance HSC expansion in MNC culture (Fig. 5C, D), suggesting that the compromised HSC expansion in MNC culture is not due to the activation of mTor signaling.
FIG. 5.
Inflammatory cytokines repress GSK3 inhibitor-induced HSC expansion independent of Akt-mTor signaling. (A, B) BM MNCs were cultured for 2 days in stem cell medium at the indicated CHIR concentrations. DMSO-treated cells were used as a control. Protein lysates were collected for western blotting for p-S6 (A). LSK HSPCs were sorted and stained with p-S6 antibodies. mTor activity was examined by FACS for p-S6 (B). (C, D) BM MNCs were cultured for 9 days in stem cell medium with 0.5 μM CHIR99021 plus indicated concentrations of rapamycin with medium change every other day. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (C) and functional HSCs were evaluated by CHR assay (A). *P < 0.05. Color images are available online.
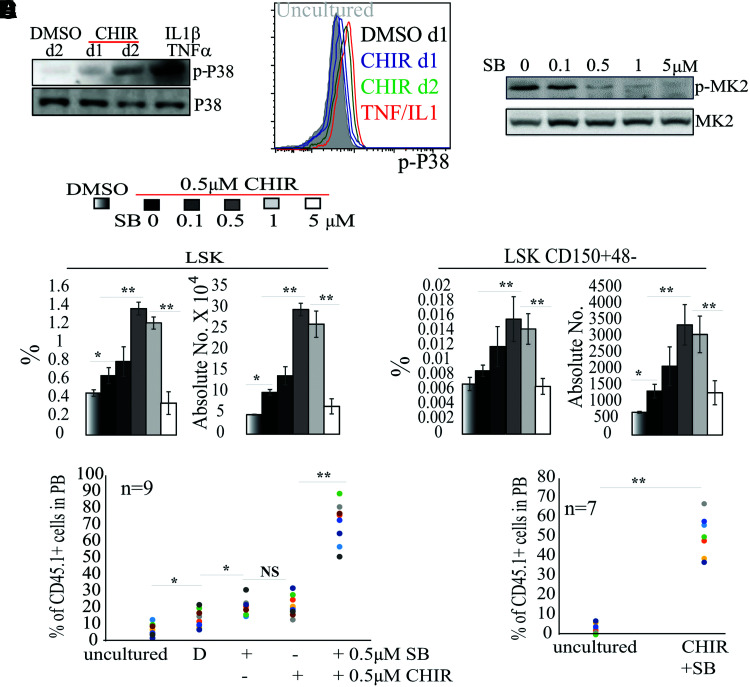
To study the downstream pathway that mediates inflammatory cytokine signaling, we examined the activities of the major cytokine signaling pathways, including ERK, JNK, NF-κB, p38, and JAK-STAT. We found that only p38 was increased in CHIR-treated BM MNC culture compared with vehicle-only treatment (Fig. 6A, B). To test whether activation of p38 compromises HSC expansion in MNC culture, we added increasing concentrations of the p38 inhibitor SB203580 (SB hereafter) to the cultures. We found that SB at ≥0.5 μM can repress p38 activity as demonstrated by a reduction of p-MK2, the substrate for p38 (Fig. 6C). However, SB treatment did not alter the levels of TNFα and IL-1β in the supernatant of the culture (Supplementary Fig. S5), supporting the idea that p38 is downstream of TNFα and IL-1β signaling. Consistently, 0.5–1 μM SB showed synergistic effects on HSC expansion when used in combination with 0.5 μM CHIR in MNC culture (Fig. 6D–F). The combination of 0.5–1 μM SB and 0.5 μM CHIR resulted in 3-fold, 2.8-fold, and 3.1-fold expansion of LSK HSPCs, LSK-150 HSCs, and functional HSCs, respectively, when compared with a 0.5 μM CHIR-only group (Fig. 6D, E). More importantly, HSCs in 0.5 μM SB plus 0.5 μM CHIR1 were able to reconstitute hematopoiesis in secondary transplant recipients, suggesting an expansion of long-term, durable HSCs (Fig. 6F). However, any further increase in SB concentration repressed HSC expansion (Fig. 6D).
FIG. 6.
Moderate concentration of GSK3 inhibitor plus p38 inhibitor promotes HSC expansion in MNC culture. (A, B) BM MNCs were cultured in stem cell medium with 0.5 μM CHIR for either 1 or 2 days. DMSO treatment was used as a negative control. TNFα (50 ng)- plus IL-1β (50 ng)-stimulated BM MNCs (30-min stimulation) were used as positive controls. p38 activity was examined by western blotting for p-p38 levels (A). LSK HSPCs from the culture were then purified by FACS. p38 activity was examined by FACS for p-p38 (B). (C) BM MNCs were cultured for 2 days in stem cell medium containing 0.5 μM CHIR with indicated concentrations of SB203580 (SB). p38 activity was examined by western blotting for p-p38 levels. (D–F) BM MNCs were cultured for 9 days in stem cell medium with indicated concentrations of SB plus 0.5 μM CHIR with medium change every other day. The percentages and numbers of LSK HSPCs and LSK-CD150+48− HSCs were examined by FACS (D) and functional HSCs were evaluated by CHR assay (E) and second transplantation (F). The BM cells used for the second transplantation in (F) were mixtures of BM cells collected from all nine recipient mice from the first transplantation in each group in (E). The percentage of CD45.1+ cells among mixed BM cells was 4.9% for the uncultured group and 56.6% for the CHIR+SB group, respectively. *P < 0.05; **P < 0.01. Color images are available online.
The combination of a low concentration of GSK3 inhibitor and p38 inhibitor promoted the expansion of human UCB HSCs
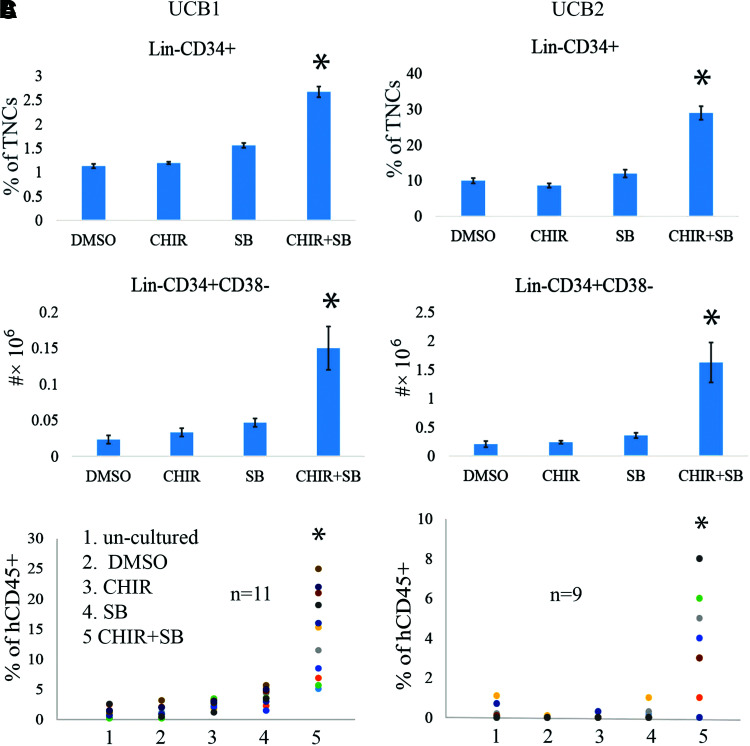
To study whether GSK3 inhibitor+p38 inhibitor combination treatment could also promote the expansion of human UCB HSCs, we collected MNCs from human UCB and cultured them in StemSpan serum-free medium supplemented with 50 ng each of human (h)SCF, hTPO, and hFLT3. Equal numbers of MNCs (106) were seeded into each well of six-well plates. These were treated with 0.5 μM SB, 0.5 μM CHIR, or both in combination, with splitting and medium change every 3 days. After 12 days of culturing, cells were collected for HSC analysis by flow cytometry to detect Lin−CD34+ HSPCs and Lin−CD34+CD38− HSCs. We found that HSPC and HSC numbers were only slightly increased in the 0.5 μM CHIR-treated group compared with the vehicle-only group, while a significant increase in HSPC and HSC numbers was detected in the SB-treated group (Fig. 7A, B). The number of HSPCs and HSCs in the combination treatment group was significantly greater than all other groups (Fig. 7A, B), suggesting a synergistic effect of both p38 and GSK3 inhibitors in promoting HSC expansion.
FIG. 7.
Modest concentration of GSK3 inhibitor plus p38 inhibitor promotes the expansion of human UCB HSCs. MNCs were collected from 2 U of UCB separately and were cultured in stem cell medium with 0.5 μM SB, 0.5 μM CHIR, or a combination of both for 12 days with medium change every 3 days. (A, B) The percentages and absolute numbers of CD34+ and CD34+CD38− HSCs were determined by FACS. (C) The LT-HRC of the cultured cells was analyzed by CHR assay. UCB, umbilical cord blood. Color images are available online.
To study whether the combination of inhibitors also promotes functional HSCs, we transplanted the cultured cells into sublethally irradiated (3 Gy) NSG mice. Each mouse received cultured cells derived from 106 initial MNCs (Fig. 7C). An equal number of uncultured MNCs from the same UCB unit was transplanted as a control. Compared with uncultured controls, the % of donor cells of UCB cells in the vehicle-treated group was slightly increased. p38 inhibitor or GSK3 inhibitor treatment only subtly enhanced the % of donor cells of UCB cells. However, the p38+GSK3 inhibitor combination treatment significantly enhanced the % of multilineage donor cells by 9- to 11-fold in the first transplantation (Fig. 7C). In secondary transplantation, only 2/8 recipients of uncultured cells demonstrated low levels of human hematopoietic cells (0.7% and 1.1%); no HRC were detected in mice that received vehicle- or individual inhibitor- treated cells. However, 7/8 of mice that received combined inhibitor cells showed >1% human hematopoietic cells.
Discussion
Although GSK3 inhibitors had been tested individually for in vitro HSC expansion, previous studies used relatively high concentrations of these inhibitors, which almost completely repressed GSK3 activity [16,22–24]. We found that a moderate concentration of GSK3 inhibitor is required to enhance HSC expansion. Such a concentration of GSK3 inhibitor only partially represses GSK3 activity and stimulates a precisely appropriate level of β-catenin activity, resulting in the regulation of a special slow-cycle type of self-renewal proliferation in HSCs. However, myeloid cells in the culture produced inflammatory cytokines, including TNF and IL-1. The GSK3 inhibitor promotes production of such cytokines, compromising HSC expansion by stimulating p38 signaling. Our study suggested that inhibition of inflammatory cytokine-stimulated p38 signaling is required to promote the HSC expansion induced by a modest concentration of GSK3 inhibitor.
Wnt/β-catenin signaling plays critical roles in both normal and disease hematopoiesis by regulating HSC self-renewal. The role of Wnt/β-catenin signaling in the initiation of early embryonic hematopoiesis has been well documented. Inactivation of Wnt/β-catenin signaling during fetal life leads to defective HSCs [25–29]. A detailed study has elucidated that a transient activation of Wnt/β-catenin signaling is essential for the emergence of HSCs from aorta-gonad-mesonephros (AGM) endothelial precursors [30]. However, in adult BM hematopoiesis, there might be a dose-dependent effect of Wnt/β-catenin signaling on HSC activity. Studies suggested that a two- to threefold increase in Wnt/β-catenin signaling enhances HSC expansion, while a further elevated Wnt/β-catenin signaling will drive HSCs toward myeloid differentiation, T cell differentiation, or apoptosis [13].
Consistent with such a conclusion, we found that 0.5 μM CHIR promotes HSC expansion when purified HSCs were used. Such a concentration of CHIR selectively induces a two- to fourfold increase of expression of β-catenin target genes Axin2 and HoxB4 in HSCs. However, a further increase in CHIR concentration compromises HSC expansion, which is correlated with increased expression of other β-catenin target genes such as Ccnd1 and Myc.
GSK3 is a negative regulator of β-catenin signaling and plays a pivotal role in HSC homeostasis and self-renewal [21]. Inhibition of GSK-3β activates Wnt/β-catenin signaling and preserves functional HSCs in long-term culture when cultured on a BM stromal feeder layer [16]. Pretreatment of in vitro-expanded HSCs with a GSK3 inhibitor 24 h before transplantation restores Wnt/β-catenin signaling and enhances HRC [17,22,31]. In vivo administration of GSK-3β inhibitors improves the early HRC of donor HSCs in recipient mice [32]. However, these studies used a high concentration of GSK3 inhibitor, which not only induces Wnt/β-catenin-mediated self-renewal signaling but also induces the activation of Akt/mTOR signaling [21,33]. Akt/mTOR signaling attenuates HSC activity by promoting proliferation and differentiation. Thus, a high concentration of GSK3 inhibitor might stimulate only a short-term expansion of HSCs followed by their exhaustion. This hypothesis is supported by hematopoietic-specific GSK3β−/− mouse studies [32,34]. Interestingly, full inhibition of both GSK3 and mTor signaling preserves HSCs for at least 7 days in a cytokine- and serum-free medium, but does not enhance HSC expansion [33].
We found that addition of a moderate concentration of GSK3 inhibitor in serum-free medium together with hematopoietic cytokines promoted the expansion of purified HSCs for up to 9 days. However, such HSC expansion was repressed by myeloid cells that produce TNFα and IL-1, which stimulate p38 signaling. Thus, the use of a GSK3 inhibitor alone promotes the expansion of purified HSCs over the short term. In long-term culture, due to the production of myeloid cells, HSC expansion is attenuated (data not shown). In addition, because the procedure for HSC purification is complicated, which increases the risk of contamination and loss of HSCs, it is clinically more practical to expand HSCs using unpurified MNCs. Our study demonstrated that blocking TNF/IL-1 signaling or inhibition of p38 signaling should allow us to effectively expand HSCs without purification when combined with a precise concentration of GSK3 inhibitor.
p38 signaling is a well-established negative regulator of HSC self-renewal. It is activated in response to inflammatory cytokines and oxygen stress, which repress HSC self-renewal by inducing cell cycle entry, differentiation, apoptosis, and/or senescence. Inhibition of p38 can largely prevent oxygen stress-induced HSC exhaustion as shown by Atm−/− and FoxO3−/− animal studies [35]. Inhibition of p38 may also promote the expansion of murine and human HSCs in vitro [36,37].
We found that in our serum-free culture system, p38 is stimulated by inflammatory cytokines produced by myeloid cells. We also found that a proper concentration of p38 inhibitor is required to facilitate GSK3 inhibitor-induced HSC expansion in MNC culture by protecting HSCs from inflammatory cytokine-stimulated stress, while a high concentration of p38 inhibitor represses HSC proliferation and attenuates HSC expansion. In support of this notion, Karigane et al. recently demonstrated that p38α is required for HSC cycle entry by regulating purine metabolism. p38α deletion significantly compromises the long-term hematopoietic reconstitution capacity of HSCs [38].
The mammalian GSK3 isoforms GSK-3α and GSK-3β are ubiquitously expressed. Despite their structural redundancy, GSK3α and GSK3β have distinct functional activities, as shown by knockout studies. Gsk3β-deficient embryos die in midgestation due to severe liver degeneration, whereas Gsk3α−/− mice are viable [39]. Hematopoietic-specific GSK3β−/− mice develop a myelodysplastic syndrome (MDS) phenotype due to the activation of both β-catenin and mTor signaling in HSCs. Deletion of GSK3α results in defects in mitochondrial metabolism in HSCs. Genetic deletion of both Gsk3α and Gsk3β leads to aggressive acute myeloid leukemia. CHIR was used in this study because it is an inhibitor of both Gsk3α and Gsk3β. It will be critical to determine in the future whether such an inhibitor induces genetic mutations in HSCs and whether isoform-specific inhibitors can also promote HSC expansion. Furthermore, in MDS patients, p38 is constitutively activated in hematopoietic cells, contributing to increased HSC apoptosis and TNF/IFN-induced hematopoietic repression [40–43]. Inhibition of p38 signaling can at least partially restore normal hematopoiesis in MDS mice and showed promising treatment effects for MDS patients. It will be important to determine whether p38 is also activated in the hematopoietic cells of Gsk3β−/− mice, and if so, whether it contributes to the observed hematopoietic defects.
In this study, although only the competitive transplantation assay was used to compare functional HSCs among the experimental groups, such experiments fully addressed our questions and hypothesis. In the future, to compare the frequency and fold changes of functional HSCs between the treatment group and the control group, more detailed limiting dilutions and competitive assays will be performed.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Basic Research Program of China (project 2013CB966803), the National Natural Science Foundation of China (project 81670151 and 81700141), and the Program for Basic Research of Shanghai Municipal Science and Technology Commission (grant no. 13JC1406403). It was also supported by the Leukemia Research Foundation New Investigator Award to J.L.
Supplementary Material
References
- 1. Li HW. and Sykes M. (2012). Emerging concepts in haematopoietic cell transplantation. Nat Rev Immunol 12:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piemontese S, Ciceri F, Labopin M, Arcese W, Kyrcz-Krzemien S, Santarone S, Huang H, Beelen D, Gorin NC, et al. (2017). A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol 10:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D. and Maiers M. (2014). HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 371:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballen KK,. Gluckman E. and Broxmeyer HE. (2013). Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stiff P, Chen B, Franklin W, Oldenberg D, Hsi E, Bayer R, Shpall E, Douville J, Mandalam R, et al. (2000). Autologous transplantation of ex vivo expanded bone marrow cells grown from small aliquots after high-dose chemotherapy for breast cancer. Blood 95:2169–2174. [PubMed] [Google Scholar]
- 6. de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD, et al. (2012). Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 367:2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dahlberg A, Delaney C. and Bernstein ID. (2011). Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood 117:6083–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL. and Bernstein ID. (2010). Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 16:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flores-Guzman P, Fernandez-Sanchez V. and Mayani H. (2013). Concise review: ex vivo expansion of cord blood-derived hematopoietic stem and progenitor cells: basic principles, experimental approaches, and impact in regenerative medicine. Stem Cell Transl Med 2:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walasek MA,. van Os R. and de Haan G. (2012). Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci 1266:138–150. [DOI] [PubMed] [Google Scholar]
- 11. He X. and Axelrod JD. (2006). A WNTer wonderland in Snowbird. Development 133:2597–2603. [DOI] [PubMed] [Google Scholar]
- 12. Famili F, Brugman MH, Taskesen E, Naber BE, Fodde R. and Staal FJ. (2016). High levels of canonical Wnt signaling lead to loss of stemness and increased differentiation in hematopoietic stem cells. Stem Cell Rep 6:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luis TC,. Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, Fibbe WE, van Dongen JJ, Fodde R. and Staal FJ. (2011). Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9:345–356. [DOI] [PubMed] [Google Scholar]
- 14. Xiao Y, Li H, Zhang J, Volk A, Zhang S, Wei W, Zhang S, Breslin P. and Zhang J. (2011). TNF-alpha/Fas-RIP-1-induced cell death signaling separates murine hematopoietic stem cells/progenitors into 2 distinct populations. Blood 118:6057–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang M, Wei X, Guo Y, Breslin P, Zhang S, Zhang S, Wei W, Xia Z, Diaz M, Akira S. and Zhang J. (2008). TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice. J Exp Med 205:1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holmes T, O'Brien TA, Knight R, Lindeman R, Shen S, Song E, Symonds G. and Dolnikov A. (2008). Glycogen synthase kinase-3beta inhibition preserves hematopoietic stem cell activity and inhibits leukemic cell growth. Stem Cells 26:1288–1297. [DOI] [PubMed] [Google Scholar]
- 17. Dolnikov A, Xu N, Shen S, Song E, Holmes T, Klamer G. and O'Brien TA. (2014). GSK-3beta inhibition promotes early engraftment of ex vivo-expanded haematopoietic stem cells. Cell Prolif 47:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baldridge MT,. King KY. and Goodell MA. (2010). Inflammatory signals regulate hematopoietic stem cells. Trends Immunol 32:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xin J, You D, Breslin P, Li J, Zhang J, Wei W, Cannova J, Volk A, Gutierrez R, et al. (2017). Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia 31:1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JY,. Nakada D, Yilmaz OH, Tothova Z, Joseph NM, Lim MS, Gilliland DG. and Morrison SJ. (2010). mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell 7:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang J, Zhang Y, Bersenev A, O'Brien WT, Tong W, Emerson SG. and Klein PS. (2009). Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J Clin Invest 119:3519–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ko KH,. Holmes T, Palladinetti P, Song E, Nordon R, O'Brien TA. and Dolnikov A. (2011). GSK-3beta inhibition promotes engraftment of ex vivo-expanded hematopoietic stem cells and modulates gene expression. Stem Cells 29:108–118. [DOI] [PubMed] [Google Scholar]
- 23. Jiang J, Zhao M, Zhang A, Yu M, Lin X, Wu M, Wang X, Lu H, Zhu S, et al. (2010). Characterization of a GSK-3 inhibitor in culture of human cord blood primitive hematopoietic cells. Biomed Pharmacother 64:482–486. [DOI] [PubMed] [Google Scholar]
- 24. Shen S, Xu N, Klamer G, Ko KH, Khoo M, Ma D, Moore J, O'Brien TA. and Dolnikov A. (2015). Small-molecule inhibitor of glycogen synthase kinase 3beta 6-Bromoindirubin-3-oxime inhibits hematopoietic regeneration in stem cell recipient mice. Stem Cells Dev 24:724–736. [DOI] [PubMed] [Google Scholar]
- 25. Fleming HE,. Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM. and Scadden DT. (2008). Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2:274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luis TC,. Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ. and Staal FJ. (2009). Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood 113:546–554. [DOI] [PubMed] [Google Scholar]
- 27. Lento W, Ito T, Zhao C, Harris JR, Huang W, Jiang C, Owzar K, Piryani S, Racioppi L, Chao N. and Reya T. (2014). Loss of beta-catenin triggers oxidative stress and impairs hematopoietic regeneration. Genes Dev 28:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A. and Reya T. (2007). Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duncan AW,. Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N. and Reya T. (2005). Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 6:314–322. [DOI] [PubMed] [Google Scholar]
- 30. Ruiz-Herguido C, Guiu J, D'Altri T, Ingles-Esteve J, Dzierzak E, Espinosa L. and Bigas A. (2012). Hematopoietic stem cell development requires transient Wnt/beta-catenin activity. J Exp Med 209:1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holmes T, Yan F, Ko KH, Nordon R, Song E, O'Brien TA. and Dolnikov A. (2012). Ex vivo expansion of cord blood progenitors impairs their short-term and long-term repopulating activity associated with transcriptional dysregulation of signalling networks. Cell Prolif 45:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trowbridge JJ,. Xenocostas A, Moon RT. and Bhatia M. (2006). Glycogen synthase kinase-3 is an in vivo regulator of hematopoietic stem cell repopulation. Nat Med 12:89–98. [DOI] [PubMed] [Google Scholar]
- 33. Huang J, Nguyen-McCarty M, Hexner EO, Danet-Desnoyers G. and Klein PS. (2012). Maintenance of hematopoietic stem cells through regulation of Wnt and mTOR pathways. Nat Med 18:1778–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT. and Zon LI. (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136:1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y. and Suda T. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12:446–451. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Kellner J, Liu L. and Zhou D. (2011). Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev 20:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou J, Zou P, Wang J, Li L, Wang Y, Zhou D. and Liu L. (2012). Inhibition of p38 MAPK activity promotes ex vivo expansion of human cord blood hematopoietic stem cells. Ann Hematol 91:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karigane D, Kobayashi H, Morikawa T, Ootomo Y, Sakai M, Nagamatsu G, Kubota Y, Goda N, Matsumoto M, et al. (2016). p38alpha Activates purine metabolism to initiate hematopoietic stem/progenitor cell cycling in response to stress. Cell Stem Cell 19:192–204. [DOI] [PubMed] [Google Scholar]
- 39. Doble BW,. Patel S, Wood GA, Kockeritz LK. and Woodgett JR. (2007). Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell 12:957–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verma A, Deb DK, Sassano A, Kambhampati S, Wickrema A, Uddin S, Mohindru M, Van Besien K. and Platanias LC. (2002). Cutting edge: activation of the p38 mitogen-activated protein kinase signaling pathway mediates cytokine-induced hemopoietic suppression in aplastic anemia. J Immunol 168:5984–5988. [DOI] [PubMed] [Google Scholar]
- 41. Navas T, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, Parmar S, Haghnazari E, Zhou L, et al. (2006). Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood 108:4170–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Navas T, Zhou L, Estes M, Haghnazari E, Nguyen AN, Mo Y, Pahanish P, Mohindru M, Cao T, et al. (2008). Inhibition of p38alpha MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenvironment. Leuk Lymphoma 49:1963–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katsoulidis E, Li Y, Yoon P, Sassano A, Altman J, Kannan-Thulasiraman P, Balasubramanian L, Parmar S, Varga J, et al. (2005). Role of the p38 mitogen-activated protein kinase pathway in cytokine-mediated hematopoietic suppression in myelodysplastic syndromes. Cancer Res 65:9029–9037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.