Abstract
The Lactococcus lactis SK11 cell envelope proteinase is an extracellular, multidomain protein of nearly 2,000 residues consisting of an N-terminal serine protease domain, followed by various other domains of largely unknown function. Using a strategy of deletion mutagenesis, we have analyzed the function of several C-terminal domains of the SK11 proteinase which are absent in cell envelope proteinases of other lactic acid bacteria. The various deletion mutants were functionally expressed in L. lactis and analyzed for enzyme stability, activity, (auto)processing, and specificity toward several substrates. C-terminal deletions of first the cell envelope W (wall) and AN (anchor) domains and then the H (helix) domain leads to fully active, secreted proteinases of unaltered specificity. Gradually increasing the C-terminal deletion into the so-called B domain leads to increasing instability and autoproteolysis and progressively less proteolytic activity. However, the mutant with the largest deletion (838 residues) from the C terminus and lacking the entire B domain still retains proteolytic activity. All truncated enzymes show unaltered proteolytic specificity toward various substrates. This suggests that the main role played by these domains is providing stability or protection from autoproteolysis (B domain), spacing away from the cell (H domain), and anchoring to the cell envelope (W and AN domains). In addition, this study allowed us to more precisely map the main C-terminal autoprocessing site of the SK11 proteinase and the epitope for binding of group IV monoclonal antibodies.
Lactococci are gram-positive bacteria used as starters in a variety of dairy fermentation processes. These bacteria have a complex proteolytic system for the degradation of caseins, the major milk proteins, into small peptides and free amino acids that are subsequently used for cell growth, but they can also contribute to flavor development in fermented milk products (29, 32, 38). A single, cell-wall-bound extracellular proteinase (CEP) is generally considered to be responsible for the initial breakdown of caseins (7, 10, 12, 29, 44, 51, 52). Gene deletion and modification studies have demonstrated that Lactococcus lactis strains grow very poorly in milk in the absence of a functional CEP (28, 29, 44).
Three distinctly different types of genes encoding CEPs, referred to as prtB, prtH, and prtP (47), have been cloned and sequenced from dairy lactic acid bacteria (20, 25, 27, 30, 43, 54). The prtP gene of L. lactis SK11 (54) encodes a pre-pro-protein of 1,962 amino acid residues with a calculated molecular mass of >200 kDa. This precursor is autocatalytically processed at the N terminus and thereby activated during or after membrane translocation. A chaperone or maturation protein, PrtM, is required for this activation of PrtP, and the required prtM gene is located directly upstream of the prtP gene but is oppositely transcribed (22, 55).
A comparative analysis of CEPs from different lactic acid bacteria led to the prediction of a number of different domains, and their homology, characteristics, and putative functions have been described (47). Starting from the N terminus, the PrtP of L. lactis SK11 is predicted to consist of a pre-pro-domain (187 residues) for secretion and activation, a serine protease domain (∼510 residues, including an internal inserted domain of 151 residues), two large middle domains A (∼410 residues) and B (∼480 residues) of predicted regulatory and stabilizing function, a helical spacer domain (∼210 residues), a hydrophilic cell wall spacer domain (∼130 residues), and a cell wall anchor domain (∼40 residues). Not all of these domains are present in the other CEPs, which raises the question as to whether and how the various domains of PrtP contribute to protease activity, specificity, or stability.
The catalytic or protease domain is common to all CEPs and belongs to the superfamily of subtilisin-like serine proteases, also referred to as subtilases (48, 49). Using a homology model for its three-dimensional structure, strategies for protein engineering of the PrtP catalytic domain from L. lactis SK11 were developed and implemented, strategies aimed at modulating either stability, catalytic activity, or substrate specificity (3, 4, 10, 49, 50). Mutations near the substrate binding site mainly led to changes in activity and specificity (4, 50). Deletion of the insert of 151 residues in the protease domain led to a threefold-reduced activity and altered the specificity toward caseins (3). The latter result suggests that through deletion of other domains it may be possible to generate novel PrtP variants with altered properties; these could be useful for mechanistic studies to determine the function of various domains, for application in flavor diversification, or for accelerated cheese ripening but also for facilitated isolation, purification, characterization, and perhaps even crystallization. Proteinases of the kexin family of subtilases also consist of an N-terminal protease domain followed by a number of different C-terminal domains (40). Carboxy-terminal deletion analysis in this family has shown that only the highly conserved middle domain of 140 residues directly coupled to the protease domain is required for full proteolytic activity and specificity, while all other C-terminal extensions such as Cys-rich or Ser-Thr-rich domains, transmembrane domains, and cytosolic domains can be deleted (1, 18, 24).
The 40 most C-terminal residues of PrtP are homologous to A3-type cell wall-membrane anchor sequences identified in a great number of cell envelope proteins from other gram-positive bacteria (37, 41, 54). Initial C-terminal deletion analysis of the prtP gene has demonstrated that the absence of this membrane anchor results in secretion of the proteinase into the medium; furthermore, C-terminal segments up to 403 residues can be deleted without loss of PrtP activity or specificity (2, 8, 31). Alternatively, release from the cell envelope of a fully active, truncated form of PrtP can be induced by treating cells with Ca-free buffer (12, 13, 15, 23, 33, 39). This “released form” of PrtP has a molecular mass of about 145 kDa, implying that ca. 500 C-terminal residues have been removed. This release is believed to result from an intramolecular autoproteolytic event at a site that becomes accessible only after the removal of Ca2+ ions (15, 33); this cleavage site(s) has not been identified yet.
We have undertaken here a more extensive C-terminal truncation analysis by deletion mutagenesis to address the following issues: (i) what is the effect of removal of different domains by progressive C-terminal truncation on the proteolytic activity, specificity and stability of the SK11 proteinase; (ii) what is the minimal size of a stable and active enzyme; and (iii) where is the autocatalytic cleavage site located that leads to “release” of the enzyme in Ca-free medium?
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli MC1061 (5) was grown in L broth-based media and was used as intermediate host for DNA constructions. Strain L. lactis subsp. lactis MG1363 is a plasmid-free, proteinase-deficient derivative of L. lactis subsp. lactis NCDO 712 (19) that was used as a host for all proteinase plasmid transformations. L. lactis strains were generally grown in M17 broth (E. Merck AG, Darmstadt, Germany). For proteinase expression studies, L. lactis cells were grown in 10% (wt/vol) pasteurized, reconstituted skimmed milk or in whey permeate medium (8) containing 1.9% (wt/vol) β-glycerolphosphate and 0.1% (wt/vol) Casitone (Difco Laboratories, Detroit, Mich.). If appropriate, the media contained 0.5% (wt/vol) glucose and chloramphenicol (10 μg/ml).
Molecular cloning.
Isolation of plasmid DNA from E. coli and standard recombinant DNA techniques were performed as described previously (45). All enzymes were purchased from Bethesda Research Laboratories (Breda, The Netherlands), Boehringer Mannheim (Almere, The Netherlands), or New England Biolabs Corp. (Hitchin, Herefordshire, United Kingdom) and were used according to the manufacturer's instructions. Isolation of plasmid DNA from L. lactis and transformation of L. lactis were performed as described previously (8, 55). Recombinant L. lactis strains were analyzed by restriction enzyme analysis and direct sequencing of double-stranded plasmid DNA (21, 46, 57).
Construction of C-terminal deletions of the proteinase gene.
Plasmid pNZ521 contains the complete prtP gene and a functional prtM gene of L. lactis subsp. cremoris strain SK11 resulting in production of an active proteinase located in the cell envelope (8). Plasmid pNZ527 (denoted previously as pNZ521ΔH) was constructed by deletion of a 16-bp HindIII fragment from pNZ521 (2); frameshift readthrough then reaches a stop codon after four codons. This plasmid encodes a proteinase lacking the 190 most C-terminal residues (Δ190), which results in secretion of the truncated proteinase into the growth medium. Plasmid pNZ596 was constructed by deletion of a 1,671-bp KpnI fragment from pNZ521, filling in the sticky ends with Klenow fragment of DNA polymerase I of E. coli, and subsequent ligation of plasmid DNA. Plasmid pPR31 containing the structural prtP gene (54) was completely digested with BglII and BstEII and partially digested with NdeI. The sticky ends were filled in with Klenow and ligated. For the production of mutant proteinases in L. lactis, correctly mutated EcoRI-SacI prtP gene fragments were used to construct derivatives of pNZ521 containing a mutant prtP gene. The resulting plasmids were designated pNZ522 (NdeI), pNZ523 (BstEII), and pNZ524 (BglII). In this way, all constructed plasmids contained a frameshift mutation (resulting in stop codons within 20 codons) in the C-terminal part of the coding region of the prtP gene. Plasmid pNZ574 is a derivative of pNZ527 containing the S433A mutation of the catalytic Ser residue, which leads to an inactive proteinase with the propeptide still attached to the N terminus (9, 50). All constructs were verified by DNA sequence analysis of relevant regions.
Growth studies.
The ability of L. lactis cells to produce a functional proteinase was assayed by growth of these cells in 10% (wt/vol) pasteurized, reconstituted skimmed milk. The maximum specific growth rate (μmax) values of lactococcal strains in milk were determined by measuring the optical density at 600 nm (OD600) of cultures clarified by using a modified EDTA-borate treatment (26, 42).
Proteinase expression studies.
Lactococcal cells were grown in whey-based medium to the mid-log growth phase (OD600 = 0.9), and wild-type proteinase was released from the cell envelope by incubation in Ca2+-free buffer (cell-envelope “release fraction”) as described previously (13, 39). Secreted proteinase was isolated from the culture medium by freeze-drying dialyzed samples (8). Proteinase samples isolated from equal amounts of lactococcal cells (as determined by measuring the OD600) were analyzed on sodium dodecyl sulfate (SDS)-polyacrylamide gels (36) that were stained with Coomassie brilliant blue. Proteinases were detected by Western blotting using polyclonal antibodies raised against SK11 proteinase (8) and monoclonal antibodies (MAbs) of groups I and IV raised against the cell envelope proteinase PrtP of L. lactis strain Wg2 which cross-react with those of strain SK11 (34, 35).
Proteinase activity assays.
The proteolytic activity of secreted SK11 proteinases was measured at pH 6.5 and at 30°C toward α- and β-casein (56) and the cheese peptide α-s1-casein-(1-23) fragment (14, 16). Initial activities toward the chromophoric substrate Suc-Ala-Glu-Pro-Phe-pNA (Bachem AG) were measured at pH 6.8 and 25°C (11).
RESULTS
Analysis of proteinase C-terminal truncation mutants.
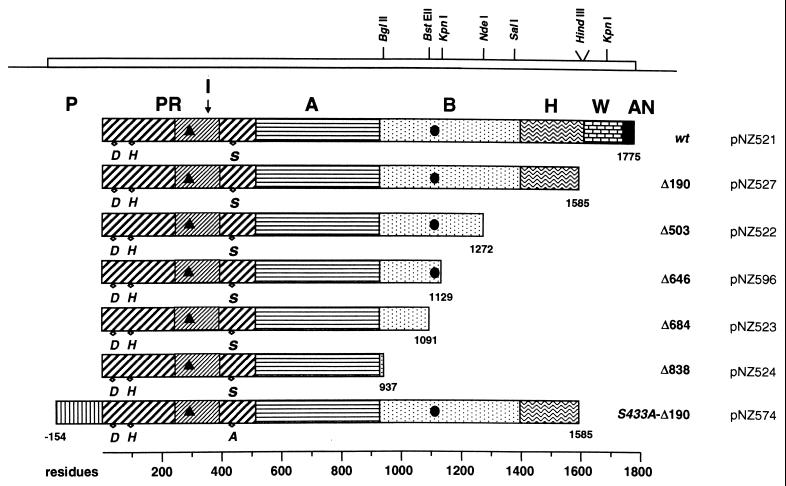
Lactococcal plasmids carrying mutant prtP genes encoding wild-type or truncated SK11 proteinases were introduced into L. lactis MG1363 and functionally expressed. An overview of the resulting C-terminally truncated proteinases is given in Table 1 and Fig. 1.
TABLE 1.
Characteristics of wild-type and truncated L. lactis PrtP
| Plasmid | Truncation at restriction site | Residual N-terminal residues (range) | Deleted C-terminal residues (total no.) | Apparent mass (kDa)
|
Residual activitya | Binding of MAb IV | Comments (reference) | |
|---|---|---|---|---|---|---|---|---|
| Calculated | SDS-PAGEb | |||||||
| pNZ521 | 1–1775 | 0 | 186 | 145 | ++++ | + | Wild type (8) | |
| pNZ527 | HindIII | 1–1585 | 190 | 168 | 145 | ++++ | + | This work (2) |
| pNZ511 | SalI | 1–1372 | 403 | 147 | 145 | ++++ | ND | Reference 8 |
| pNZ522 | NdeI | 1–1272 | 503 | 137 | 145 | +++ | + | This work |
| pNZ596 | KpnI | 1–1129 | 646 | 121 | 125 | ++ | + | This work |
| pNZ523 | BstEII | 1–1091 | 684 | 116 | 110 | + | − | This work |
| pNZ524 | BglII | 1–937 | 838 | 102 | 100 | + | − | This work |
| pNZ574 | HindIII | (−154)–1585 | 190 | 184 | 190 | − | + | Active-site mutant S433A (9) |
Approximate average of activities against various substrates (see text): ++++, 100%; +++, 50 to 100%; ++, 10 to 50%; +, 1 to 10%; and −, <1%.
Apparent mass of the largest component observed.
FIG. 1.
Schematic representation of wild-type and C-terminally truncated SK11 proteinases. Domains: propeptide, P; protease, PR; insert, I; A; B; helix, H; wall, W; anchor, AN (47). Residue numbering begins at the N terminus of the mature enzyme. The number of C terminally deleted residues is indicated at the right. The approximate position of catalytic residues D, H, and S in the PR domain are indicated. Putative MAb I (▴) and IV (●) binding sites are indicated. The positions of relevant restriction sites used in cloning experiments are indicated in the coding region of the prtP gene at the top, and plasmids encoding the proteinases are shown at the far right.
Wild-type and recombinant strains were assayed for their ability to grow in milk (Table 2). Only strain MG1363(pNZ527), which specifies a proteinase lacking the C-terminal 190 residues, showed a μmax in milk identical to that of strain MG1363(pNZ521) expressing wild-type proteinase (Table 2). The μmax of other mutant strains gradually declines as the C-terminal truncation of the proteinase increases. While strain MG1363(pNZ522) encoding PrtP lacking 503 C-terminal residues grew only slightly more slowly than wild type, the MG1363(pNZ524) strain expressing PrtP with the largest C-terminal truncation (838 residues) showed a fourfold reduced μmax in milk. In contrast, strains without proteinase (MG1363) or an inactive proteinase (MG1363 harboring pNZ574) grew extremely slowly and did not reach high cell densities in milk (Table 2).
TABLE 2.
Maximum specific growth rates (μmax) in milk of L. lactis MG1363 harboring different proteinase plasmids
| Plasmid | μmax (h−1)a |
|---|---|
| pNZ521 (wild-type PrtP) | 0.63 |
| pNZ527 | 0.63 |
| pNZ522 | 0.58 |
| pNZ596 | 0.34 |
| pNZ523 | 0.23 |
| pNZ524 | 0.15 |
| pNZ574 | <0.05 |
| Noneb | <0.05 |
μmax values are the average of three experiments (standard deviation, ±0.03).
Strain L. lactis MG1363 is a plasmid-free, proteinase-deficient strain which is unable to grow in milk (19).
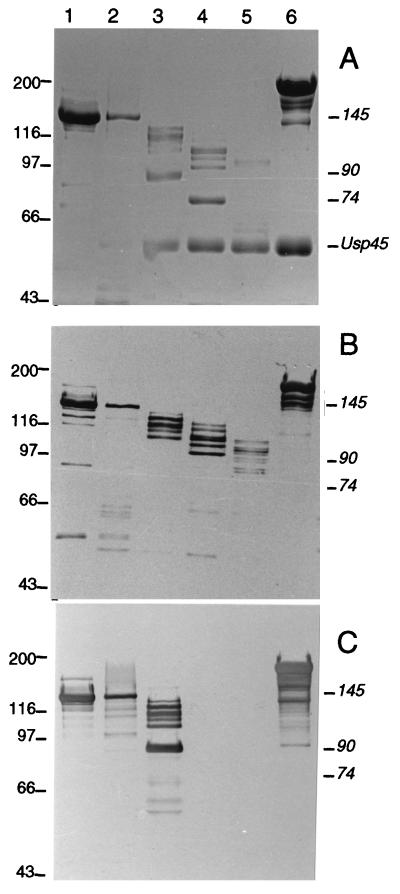
Since all PrtP mutants lack the C-terminally located membrane anchor, the truncated proteinases are secreted into the growth medium. SDS-polyacrylamide gel electrophoresis (PAGE) of supernatant fractions shows that strain MG1363(pNZ527) produces a proteinase with an apparent molecular mass of about 145 kDa (Fig. 2A, lane 1), despite the fact that a truncated proteinase of 168 kDa is encoded (Table 1). It has been shown previously that MG1363(pNZ521) produces wild-type PrtP that remains anchored to the cell but can be released as a 145-kDa autodigestion product upon incubation in Ca-free medium (2, 4, 50). Therefore, it appears that the PrtP(1–1585) encoded by strain MG1363(pNZ527) is further autoprocessed C-terminally to generate the same 145-kDa product as the “released” wild-type PrtP. As a control, the strain with plasmid pNZ574, specifying the inactive S433A-PrtP lacking the last 190 residues (8, 50), secretes a main component with an apparent molecular mass of about 190 kDa (Fig. 2A, lane 6), as expected in the absence of both C-terminal autoprocessing and N-terminal autoprocessing of the propeptide of 154 residues (Table 1).
FIG. 2.
SDS-PAGE and Western blot analysis of proteins secreted into the growth medium of lactococcal cells harboring pNZ527 (lane 1), pNZ522 (lane 2), pNZ596 (lane 3), pNZ523 (lane 4), pNZ524 (lane 5), and pNZ574 (lane 6). (A) Coomassie brilliant blue staining. (B) Western blot with MAbs of group I. (C) Western blot with MAbs of group IV. Molecular mass markers (in kilodaltons) are indicated to the left. Positions of Usp45 and the 145-, 90-, and 74-kDa products of SK11 proteinase are indicated on the right.
In contrast, for L. lactis MG1363 harboring pNZ522, pNZ596, pNZ523, and pNZ524 the slowest-migrating proteinase band corresponded to an apparent molecular mass of ca. 145, 125, 110, and 100 kDa, respectively (Fig. 2A, lanes 2 to 5), which is in good agreement with the theoretical calculated mass (Table 1). In addition, the supernatant from strains MG1363(pNZ596) and MG1363(pNZ523) (Fig. 2A, lanes 3 and 4, respectively) show several PrtP autodegradation products, including two major proteolytic fragments of approximately 90 kDa (from pNZ596) and 74 kDa (from pNZ523). These results indicate that the truncated proteinases PrtP(1–1129) and PrtP(1–1091) are more sensitive to autoproteolysis than PrtP(1–1272) or longer variants. The enzyme with the largest C-terminal truncation, PrtP(1–937), is extremely sensitive to autoproteolysis since very little undegraded protein is detected on the gel (Fig. 2A, lane 5).
Usp45, the secreted 60-kDa protein of L. lactis of unknown function (53), is a natural substrate for the cell envelope proteinase, and it is completely degraded in the supernatant of strains expressing PrtP with a wild-type level of activity, viz., MG1363 harboring pNZ521 (2, 4), pNZ527 (Fig. 2A, lane 1), and pNZ522 (Fig. 2A, lane 2). Analysis of extracellular proteins of strains expressing PrtP with larger C-terminal deletions (Fig. 2A, lanes 3 to 5) or expressing an inactive PrtP (Fig. 2A, lane 6) show significant amounts of undigested Usp45 protein, suggesting that these mutants show reduced proteolytic activity toward this natural substrate.
Identification of (autodegradation) products and mapping of epitope.
Polyclonal antibodies raised against SK11 proteinase and MAbs of groups I and IV raised against Wg2 proteinase, which cross-react with SK11 proteinase (34, 35), were used to identify autodegradation products of the C-terminally truncated SK11 proteinases. Group I MAbs are directed against an epitope of SK11 proteinase between residues 238 and 388, consisting of an external loop of the proteolytic domain (3), also referred to as the I (insert) domain (47). These group I MAbs were found to react with the major 145-kDa protein band of SK11 proteinase secreted by MG1363(pNZ527) and MG1363(pNZ522) (Fig. 2B, lane 1 and 2) and with the major 125-, 110-, and 100-kDa protein bands produced by strains with plasmids pNZ596, pNZ523, and pNZ524, respectively (Fig. 2B, lanes 3, 4, and 5).
Group IV MAbs are directed against an epitope located between Thr816 and Leu1219 of the SK11 and Wg2 proteinases (35). These group IV antibodies reacted with the 145-kDa band from strains MG1363(pNZ527) and MG1363(pNZ522) and the 125-kDa band from MG1363(pNZ596) (Fig. 2C, lanes 1, 2, and 3, respectively) but also with several other PrtP fragments present in these supernatants, particularly from MG1363(pNZ596). However, these group IV MAbs do not bind to any protein bands from strains MG1363(pNZ523) and MG1363(pNZ524) secreting the truncated enzymes PrtP(1–1091) and PrtP(1–937), respectively (Fig. 2C, lanes 4 and 5). These results indicate that the epitope of group IV MAbs is located in a region between Ala1092 and Thr1129 of the SK11 proteinase. The 90- and 74-kDa protein bands found in supernatants of cells with pNZ596 and pNZ523 both react with polyclonal antibodies raised against SK11 proteinase (not shown), identifying these bands as proteolytic fragments derived from SK11 proteinase. Furthermore, the 90-kDa protein reacts with group IV MAbs (Fig. 2C, lane 3) but not with group I MAbs (Fig. 2B, lane 3), while the 74-kDa band binds neither group I MAbs (Fig. 2B, lane 4) nor group IV MAbs (Fig. 2C, lane 4). These results indicate that both the 90-kDa and the 74-kDa fragments represent N-terminal autodegradation products lacking the group I epitope between residues 238 and 388.
Activity and specificity of mutant proteinases.
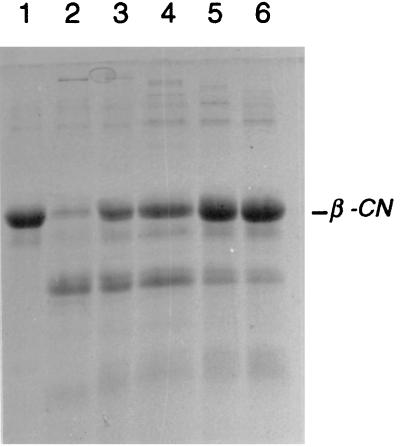
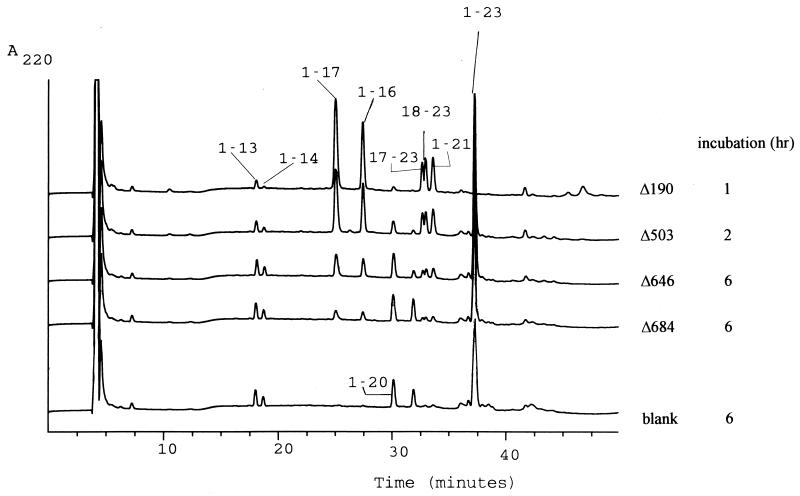
We analyzed the effect of increasing C-terminal deletions on the activity and specificity of the SK11 enzyme toward several substrates. All truncated proteinases were still able to degrade β-casein (Fig. 3) and αs1-casein (data not shown), but less casein degradation was found as C-terminal truncation of PrtP increased (Table 1). Similar results were obtained using the αs1-casein-(1-23) substrate (Fig. 4) and the chromophoric substrate suc-Ala-Glu-Pro-Phe-pNA (data not shown). The specific activity of the PrtP mutants toward these substrates cannot be determined accurately, since the truncated proteinases significantly differ in their stability (see Fig. 2). Based on the incubation times needed for a comparable amount of degradation of various substrates, the supernatant containing SK11 proteinase with the largest truncation (838 residues) has approximately 5% residual activity compared to that of the wild-type enzyme (see, for example, Fig. 4). While the various truncated PrtP species differed considerably in residual activity, no clear differences were found in specificity toward the tested substrates, e.g., the bonds cleaved in αs1-casein-(1-23) were always 16-17, 17-18, and 21-22 (Fig. 4), the same as those cleaved by wild-type PrtP (14, 50).
FIG. 3.
Caseinolytic activity of C terminally truncated SK11 proteinase mutants toward β-casein. Lane 1, no proteinase added; lanes 2 to 6, supernatant fractions added containing truncated proteinases specified by plasmids pNZ527 (lane 2), pNZ522 (lane 3), pNZ596 (lane 4), pNZ523 (lane 5), and pNZ524 (lane 6).
FIG. 4.
Caseinolytic activity and specificity of C terminally truncated SK11 proteinase mutants toward αs1-casein-(1-23). Analytical reversed-phase high-pressure liquid chromatography patterns of the products of αs1-casein-(1-23) degradation. Note that incubation times were varied between 1 and 6 h to allow for the large differences in proteinase activity. The “blank” data refer to S433A/Δ190 (pNZ527), which has no PrtP activity but shows a low background activity toward the substrate due to released intracellular PepO activity (12, 15).
DISCUSSION
Using a strategy of deletion mutagenesis we have analyzed the function of several C-terminal domains of the SK11 proteinase which are absent in other CEPs of lactic acid bacteria (47). The truncations led first to loss of the cell envelope W (wall) and AN (anchor) domains (pNZ527), then to further loss of the H (helix) domain (pNZ511), and then to partial deletions (pNZ522, pNZ596, and pNZ523) or entire deletion of the B domain (pNZ524), as schematically depicted in Fig. 1. The various deletion mutants were functionally expressed in L. lactis and analyzed for enzyme activity, (auto)processing, and specificity toward several substrates. All of the C-terminally truncated enzymes were secreted and exhibited the expected size of normally N-terminally-autoactivated proteinases, except the enzyme specified by pNZ527 which was also C-terminally autoprocessed (Fig. 2), presumably in the same way as wild-type lactococcal proteinase in Ca-free medium (2, 23).
We previously found that the activity and caseinolytic specificity of two secreted SK11 proteinases specified by plasmids pNZ527 (lacking 190 3′ codons) and pNZ511 (lacking 402 3′ codons) is identical to that of the cell-envelope-bound, wild-type enzyme (2, 8). Therefore, we can now conclude that the three most C-terminal domains, H, W, and AN, are not required for obtaining wild-type activity, specificity, or stability. Their function appears to be tethering to the cell envelope (W and AN domains) and acting as spacers (H and W domains) to position the other N-terminal domains away from the cell (47). Our present study indicates that larger C-terminal deletions ranging from 503 up to 838 codons, corresponding to progressive deletion of the B domain, significantly affect the stability and residual activity of the proteinase toward various tested substrates such as Usp45 (Fig. 2A), β-casein (Fig. 3), αs1-casein-(1-23) (Fig. 4), and total milk protein (Table 2). We have shown earlier that the thermal stability and residual activity of the wild-type SK11 proteinase is directly related to autoproteolysis (50). Therefore, the observed lower stability of C-terminally truncated enzymes presumably makes them increasingly sensitive to autoproteolysis (Fig. 2), leading to loss of active enzyme. Domain B may therefore play a role in the stabilization of the PR and/or A domains, at least in the secreted enzyme. Although the smallest construct PrtP(1–937), consisting only of the PR and A domains (called the PR-A form), still appears to be active, its specificity and specific activity cannot be determined due to its great instability. Suggestions for stabilization could come from studies of the homologous streptococcal proteinases ScpA from Streptococcus pyogenes (6), Csp from S. agalactiae (T. O. Harris and C. E. Rubens, personal communication), and PrtS from S. thermophilus (V. Monnet, personal communication), which are active and stable in the absence of a B domain (47).
While having a profound effect on stability, none of the C-terminal deletions of PrtP altered the specificity of the truncated proteinases. Therefore, the B, H, W, and AN domains do not play a role in determining substrate specificity, and they are presumably not in the vicinity of the substrate binding region of the PR domain. This is clearly in contrast to deletion of the I domain that led to altered specificity in addition to lower activity (3).
We have identified main 90- and 74-kDa components present in the supernatant of MG1363(pNZ596) and MG1363(pNZ523) cells, respectively, as N-terminal autodegradation products of the SK11 proteinase (Fig. 2). Both the 90- and 74-kDa components lack the MAb group I epitope, and their size suggests that both lack ca. 300 N-terminal residues, so autocleavage presumably occurs in the I domain after the group I MAb binding site. As a consequence, these 74- and 90-kDa forms are likely to be inactive since they lack the catalytic Asp and His residues (Fig. 1). If these inactive fragments can be purified they could prove to be useful in the elucidation of the structure and possibly the function of the A domain, but only if correct folding is retained. The epitope of group IV MAbs, previously mapped between residues 816 and 1219 of the Wg2 and SK11 proteinases (35), has now been more precisely mapped between residues Ala1092 and Thr1129 of the SK11 proteinase sequence (Fig. 1 and 2). Therefore, group IV MAb can be used as a specific probe for the B domain of PrtP (and in particular its central part).
Washing of the lactococcal cells in a Ca2+-free buffer results in release of the active 145-kDa proteinase from the lactococcal cell envelope due to autoproteolysis. This deletion mutagenesis study has allowed us to locate this important C-terminal autoprocessing site(s) in the B domain somewhere near residue 1272, since the truncated enzyme PrtP(1–1272) also has an apparent molecular mass of 145 kDa on SDS-PAGE (Fig. 1, Table 1).
In summary, we have answered many of the questions initially posed at the outset but, unfortunately, we were not yet able to generate stable, C terminally truncated proteinases with altered specifity. An alternative approach to elucidate functions and/or necessity of domains could be to construct genes encoding CEPs that lack one or more “internal” domains, for instance, by deleting the A, B, and/or H domains while retaining the W and/or AN domains. Such natural CEPs have already been found in other lactic acid bacteria (47). Novel hybrid enzymes could also be made by recombination of the domains of CEPs from different species of lactic acid bacteria. Previously, such hybrid enzymes were made between two highly homologous PrtP variants and provided insight into residues involved in determining substrate specificity (56). Hybrids formed from more distantly related CEPs could broaden the scope of innovation and application of extracellular proteinases, not only by modulating known functions such as stability, proteolytic activity/specificity, or cell wall attachment but also to introduce entirely new functions, such as adhesion, antibody binding, receptor binding, etc.
ACKNOWLEDGMENTS
We thank Harry Laan (University of Groningen, Groningen, The Netherlands) for the generous gift of monoclonal antibodies. We thank Fred Exterkate, Arno Alting, Michiel Kleerebezem, and Richard van Kranenburg for critical reading of the manuscript. We also acknowledge the technical assistance of Arno Alting, Paul Doesburg, Peter Laverman, and Monique Nijhuis in this study.
This work was partially financed by European Union grants BIOT-CT91-0263 and BIOT-CT96-0016.
REFERENCES
- 1.Brenner C, Fuller R S. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci USA. 1992;89:922–926. doi: 10.1073/pnas.89.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruinenberg P G, Vos P, de Vos W M. Proteinase overproduction in Lactococcus lactis strains: regulation and effect on growth and acidification in milk. Appl Environ Microbiol. 1992;58:78–84. doi: 10.1128/aem.58.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruinenberg P G, Doesburg P, Alting A C, Exterkate F A, de Vos W M, Siezen R J. Evidence for a large dispensable segment in the subtilisin-like catalytic domain of the Lactococcus lactis cell-envelope proteinase. Protein Eng. 1994a;7:991–996. doi: 10.1093/protein/7.8.991. [DOI] [PubMed] [Google Scholar]
- 4.Bruinenberg P G, de Vos W M, Siezen R J. Prevention of C-terminal autoprocessing of Lactococcus lactis SK11 cell-envelope proteinase by engineering of an essential surface loop. Biochem J. 1994b;302:957–963. doi: 10.1042/bj3020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban M J, Chou J, Cohen S N. In vitro fusions that join an enzymatically active β-galactosidase segment to aminoterminal fragments of exogenous proteins in Escherichia coli: plasmid vectors for the detection of translation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C C, Cleary P P. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J Biol Chem. 1990;265:3161–3167. [PubMed] [Google Scholar]
- 7.Coolbear T, Reid J R, Pritchard G G. Stability and specificity of the cell wall-associated proteinase from Lactococcus lactis subsp. cremoris H2 released by treatment with lysozyme in the presence of calcium. Appl Environ Microbiol. 1992;58:3263–3270. doi: 10.1128/aem.58.10.3263-3270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos W M, Vos P, de Haard H, Boerrigter I. Cloning and expression of the Lactococcus lactis subsp. cremoris SK11 gene encoding an extracellular serine proteinase. Gene. 1989;85:169–176. doi: 10.1016/0378-1119(89)90477-0. [DOI] [PubMed] [Google Scholar]
- 9.de Vos W M, Boerrigter I, Vos P, Bruinenberg P, Siezen R J. Production, processing, and engineering of the Lactococcus lactis SK11 proteinase. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 115–119. [Google Scholar]
- 10.de Vos W M, Siezen R J. Engineering pivotal proteins for lactococcal proteolysis. In: Andrews A T, Varley J, editors. Biochemistry of milk products. Cambridge, United Kingdom: Royal Society of Chemistry; 1994. pp. 56–71. [Google Scholar]
- 11.Exterkate F A. Differences in short peptide-substrate cleavage by two cell-envelope-located serine proteinases of Lactococcus lactis subsp. cremoris are related to secondary binding specificity. Appl Microbiol Biotechnol. 1990;33:401–406. doi: 10.1007/BF00176654. [DOI] [PubMed] [Google Scholar]
- 12.Exterkate F A. The lactococcal cell envelope proteinases: differences, calcium-binding effects and role in cheese ripening. Int Dairy J. 1995;5:995–1018. [Google Scholar]
- 13.Exterkate F A, de Veer G J C M. Partial isolation and degradation of caseins by cell wall proteinase(s) of Streptococcus cremoris HP. Appl Environ Microbiol. 1985;49:328–332. doi: 10.1128/aem.49.2.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exterkate F A, Alting A C. The conversion of the αs1-casein-(1-23)-fragment by the free and bound forms of the cell-envelope proteinase of Lactococcus lactis subsp. cremoris under conditions prevailing in cheese. Syst Appl Microbiol. 1993;16:1–8. [Google Scholar]
- 15.Exterkate F A, Alting A C. Role of calcium in activity and stability of the Lactococcus lactis cell envelope proteinase. Appl Environ Microbiol. 1999;65:1390–1396. doi: 10.1128/aem.65.4.1390-1396.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Exterkate F A, Alting A C, Slangen C J. Specificity of two genetically related cell-envelope proteinases of Lactococcus lactis subsp. cremoris towards αs1-casein-(1-23)-fragment. Biochem J. 1991;273:135–139. doi: 10.1042/bj2730135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Exterkate F A, Alting A C, Bruinenberg P G. Diversity of cell envelope proteinase specificity among strains of Lactococcus lactis and its relationship to charge characteristics of the substrate-binding region. Appl Environ Microbiol. 1993;59:3640–3647. doi: 10.1128/aem.59.11.3640-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller R S, Brake A, Thorner J. Yeast prohormone processing enzyme (Kex2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci USA. 1989;86:1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasson M J. Plasmid complements of Streptococcus lactis NCDO712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert C, Atlan D, Portalier R, Germond G J, Lapierre L, Mollet B. A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. J Bacteriol. 1996;78:3059–3065. doi: 10.1128/jb.178.11.3059-3065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo L H, Yang R C A, Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983;11:5521–5539. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haandrikman A J, Kok J, Laan H, Soemitro S, Ledeboer A M, Konings W N, Venema G. Identification of a gene required for maturation of an extracellular lactococcal serine proteinase. J Bacteriol. 1989;171:2789–2794. doi: 10.1128/jb.171.5.2789-2794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haandrikman A J, Meesters R, Laan H, Konings W N, Kok J, Venema G. Processing of the lactococcal extracellular serine proteinase. Appl Environ Microbiol. 1991;57:1899–1904. doi: 10.1128/aem.57.7.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatsuzawa K, Murakami M, Nakayama K. Molecular and enzymatic properties of furin, a Kex2-like endoprotease involved in precursor cleavage at Arg-X-Lys/Arg-Arg sites. J Biochem. 1992;111:296–301. doi: 10.1093/oxfordjournals.jbchem.a123753. [DOI] [PubMed] [Google Scholar]
- 25.Holck A, Naes H. Cloning, sequencing and expression of the gene encoding the cell-envelope-associated proteinase from Lactobacillus paracasei subsp. paracasei NCDO 151. J Gen Microbiol. 1992;138:1353–1364. doi: 10.1099/00221287-138-7-1353. [DOI] [PubMed] [Google Scholar]
- 26.Kanasaki M, Breheny S, Hillier A J, Jago G R. Effect of temperature on the growth and acid production of lactic acid bacteria. 1. A rapid method for the estimation of bacterial populations in milk. Aust J Dairy Technol. 1975;30:142–144. [Google Scholar]
- 27.Kiwaki M, Ikemura H, Shimizu-Kadota M, Hirashima A. Molecular characterization of a cell wall-associated proteinase gene from Streptococcus lactis NCDO763. Mol Microbiol. 1989;3:359–369. doi: 10.1111/j.1365-2958.1989.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 28.Kok J. Genetics of the proteolytic system of lactic acid bacteria. FEMS Microbiol Rev. 1990;87:15–42. doi: 10.1111/j.1574-6968.1990.tb04877.x. [DOI] [PubMed] [Google Scholar]
- 29.Kok J, de Vos W M. The proteolytic system of lactic acid bacteria. In: Gasson M J, de Vos W M, editors. Genetics and biotechnology of lactic acid bacteria. London, England: Blackie and Professional; 1994. pp. 169–210. [Google Scholar]
- 30.Kok J, Leenhouts K J, Haandrikman A J, Ledeboer A M, Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988;54:231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kok J, Hill D, Haandrikman A J, de Reuver M J B, Laan H, Venema G. Deletion analysis of the proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol. 1988;54:239–244. doi: 10.1128/aem.54.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunji E R S, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 33.Laan H, Konings W N. Mechanism of proteinase release from Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1989;55:3103–3106. doi: 10.1128/aem.55.12.3101-3106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laan H, Smid E J, de Leij L, Schwander E, Konings W N. Monoclonal antibodies to the cell-wall-associated proteinase of Lactococcus lactis subsp. cremoris Wg2. Appl Environ Microbiol. 1988;54:2250–2256. doi: 10.1128/aem.54.9.2250-2256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laan H, Kok J, Haandrikman A J, Venema G, Konings W N. Localization and accessibility of antigenic sites of the extracellular serine proteinase of Lactococcus lactis. Eur J Biochem. 1992;204:815–820. doi: 10.1111/j.1432-1033.1992.tb16700.x. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Leenhouts K, Buist G, Kok J. Anchoring of proteins to lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:367–376. [PubMed] [Google Scholar]
- 38.Mierau I, Kunji E R S, Venema G, Kok J. Casein and peptide degradation in lactic acid bacteria. Biotechnol Genet Eng Rev. 1997;14:279–301. doi: 10.1080/02648725.1997.10647945. [DOI] [PubMed] [Google Scholar]
- 39.Mills O E, Thomas T D. Nitrogen sources for growth of lactic streptococci in milk. N Z J Dairy Sci Technol. 1981;16:43–55. [Google Scholar]
- 40.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarre W W, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otto R. An ecophysiological study of starter streptococci. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1981. [Google Scholar]
- 43.Pederson J A, Mileski G J, Weimer B C, Steele J L. Genetic characterization of a cell envelope-associated proteinase from Lactobacillus helveticus CNRZ32. J Bacteriol. 1999;181:4592–4597. doi: 10.1128/jb.181.15.4592-4597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pritchard G G, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:179–206. doi: 10.1111/j.1574-6976.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 46.Sanger F, Nicklen S, Coulson R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siezen R J. Multi-domain cell-envelope proteinases of lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:139–155. [PubMed] [Google Scholar]
- 48.Siezen R J, Leunissen J A M. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siezen R J, de Vos W M, Leunissen J A M, Dijkstra B W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteases. Protein Eng. 1991;4:719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- 50.Siezen R J, Bruinenberg P G, Vos P, van Alen-Boerrigter I J, Nijhuis M, Alting A C, Exterkate F A, de Vos W M. Engineering of the substrate binding region of the subtilisin-like, cell-envelope proteinase of Lactococcus lactis. Protein Eng. 1993;6:927–937. doi: 10.1093/protein/6.8.927. [DOI] [PubMed] [Google Scholar]
- 51.Smid E J, Poolman B, Konings W N. Casein utilization by lactococci. Appl Environ Microbiol. 1991;57:2447–2452. doi: 10.1128/aem.57.9.2447-2452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan P S T, Poolman B, Konings W N. Proteolytic enzymes of Lactococcus lactis. J Dairy Res. 1993;60:269–286. doi: 10.1017/s0022029900027606. [DOI] [PubMed] [Google Scholar]
- 53.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis ssp. lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 54.Vos P, Simons G, Siezen R J, de Vos W M. Primary structure and organization of the gene for a prokaryotic cell envelope-located serine proteinase. J Biol Chem. 1989;264:13579–13585. [PubMed] [Google Scholar]
- 55.Vos P, van Asseldonk M, van Jeveren F, Siezen R J, Simons G, de Vos W M. A maturation protein is essential for the production of active forms of Lactococcus lactis SK11 serine proteinase located in or secreted from the cell envelope. J Bacteriol. 1989;171:2795–2802. doi: 10.1128/jb.171.5.2795-2802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos P, Boerrigter I J, Buist G, Haandrikman A J, Nijhuis M, de Reuver M B, Siezen R J, Venema G, de Vos W M, Kok J. Engineering of the Lactococcus lactis serine proteinase by construction of hybrid enzymes. Protein Eng. 1991;4:479–484. doi: 10.1093/protein/4.4.479. [DOI] [PubMed] [Google Scholar]
- 57.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]