Abstract
Aducanumab (Aduhelm), developed by the biotechnology firm Biogen in Cambridge, MA, was approved using the less common accelerated approval pathway by the Federal Drug Administration (FDA) reserved for treatments that fill a significant unmet need.1 Its approval on June 7, 2021, has been met with an outpouring of opinions from prescribers, insurers, advocacy groups, and hospital systems regarding its risk-benefit profile.2-4 Originally approved for all forms of Alzheimer disease (AD), the FDA updated aducanumab's labeling on July 8, 2021, for “treatment in patients with mild cognitive impairment (MCI) or mild dementia stage of disease, the population in which treatment was initiated in clinical trials.”5 With 6 million people nationally in the United States who suffer from AD and an anticipated one-third of those who may now fulfill the criteria under the revised labeling, the implications of aducanumab's approval continue to generate national interest.6

Aducanumab (Aduhelm) is a monoclonal antibody targeting amyloid deposits in individuals with mild AD. Aducanumab is administered in monthly 1-hour infusions starting at 1mg/kg and over 24 weeks, the dose is increased to 10 mg/kg. There are no guidelines for treatment cessation other than development of severe amyloid-related imaging abnormalities (ARIAs). Neurologists will have to consider the availability of Aβ PET scans or lumbar puncture to detect abnormal levels of CSF biomarkers, learn how to infuse their patients, and familiarize themselves with several aspects related to the coordination of care in administering aducanumab. In this study, we outline several aspects of current coverage policies and practical considerations for initiating treatment with aducanumab.
The amyloid hypothesis postulates that deposition of β-amyloid peptide is the primary event in AD pathogenesis.7 Aducanumab is a high-affinity, recombinant, human immunoglobulin G1 monoclonal antibody targeting soluble Aβ aggregates and insoluble fibrils. Aducanumab was evaluated in 3 studies: a phase 1b (PRIME) and 2 phase 3 trials (EMERGE and ENGAGE). The PRIME study showed a time-dependent and dose-dependent reduction in brain Aβ level plaque and comparatively less cognitive decline in the aducanumab (10 mg/kg) arm.8 Both the EMERGE and ENGAGE trials were halted after futility analysis. The ENGAGE trial showed no benefit of aducanumab vs placebo in both doses tested.9 However, a reduction in clinical decline was noted in the EMERGE trial, and this finding was supported by an ad hoc analysis of a subset of patients receiving 10 mg/kg of aducanumab.10 Both studies showed dose-dependent and time-dependent reduction in amyloid pathology.9,10
Patient Eligibility
Cognitive Impairment
Based on the criteria used in clinical trial,9,10 patients with MCI due to AD or mild AD would be candidates to receive aducanumab (Mini-Mental State Examination [MMSE] score between 24 and 30 and clinical dementia rating scale of 0.5).11 Although aducanumab was initially approved for the treatment of any individual with AD, the labeling was modified in July 2021 to reflect this narrower indication. Others have suggested a Montreal Cognitive Assessment (MoCA) score ≥17 or MMSE ≥21 because test-retest reliability of the MMSE score is 3 points; hence, scores of 24 and 21 are virtually identical.12
Amyloid Positivity
The presence of amyloid pathology was a criterion for inclusion in clinical trials, but this criterion is not included on the aducanumab label. In the clinical trials, Aβ PET was used to detect the necessary pathology, but other tests, including CSF analysis of Aβ 42, total tau/Aβ 42 ratio, and phosphorylated tau 181/Aβ 42 ratio, have good concordance with results of Aβ PET scans.13 Special handling is required for CSF samples. The sample must be collected in low-binding polypropylene tubes (not polystyrene collection tubes typically included in lumbar puncture collection kits), and the sample must be kept and shipped between 2 and 8°C after collection.14 Biogen has instituted a provider support program for processing of CSF AD biomarkers, which will provide the appropriate tubes for sample collection.18 Blood tests to detect AD biomarkers are also being developed.15
MRI
A baseline MRI with appropriate sequences: fluid-attenuated inversion recovery (FLAIR) T2*-weighted gradient-recalled echo or susceptibility-weighted imaging or quick diffusion-weighted imaging is needed to determine whether there are contraindications to receiving aducanumab.16 Potential contraindications include acute or subacute hemorrhage, macrohemorrhage, and ≥4 microhemorrhages; presence of cortical infarction >1.5 cm or lacunar infarction >1.5 cm; 1 area of siderosis; and diffuse white matter disease.12
Exclusion Criteria
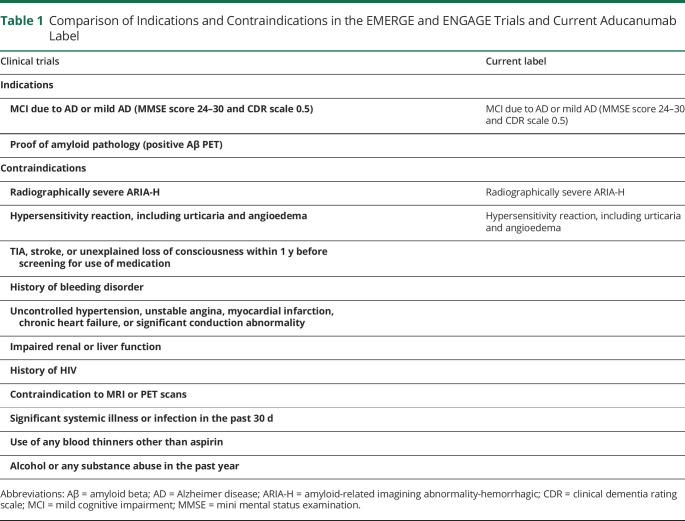
Participants were excluded from the trials for use of anticoagulants or antiplatelets other than aspirin. A complete listing of the exclusion criteria for the phase 3 trials compared with the limitations included on the aducanumab label is detailed in Table 1. Additional practical considerations regarding patient selection may include presence of coagulopathy, unstable psychiatric condition, unstable medical or cardiovascular disease, organ failure, active neoplasia (other than low-grade basal and squamous cell cancer), contraindications to completing PET scans or brain MRIs (including the presence of an MRI-incompatible implanted device), and whether the patient has a reliable caregiver or informant to ensure accurate reporting of their response to intervention.12 Although not a contraindication, others have recommended against treatment in patients with Down syndrome, dementia with Lewy bodies, and cerebral amyloid angiopathy, regardless of amyloid positivity status because data regarding the potential risks and benefits of treatment with antiamyloid therapy are scarce in these patient populations. Caution should be exercised also on patients with autosomal dominant AD and those with atypical syndromes for similar reasons.12
Table 1.
Comparison of Indications and Contraindications in the EMERGE and ENGAGE Trials and Current Aducanumab Label
APOE-4 Testing
Patients heterozygous or homozygous for APOE-4 may have different responses to aducanumab and are at higher risk of developing ARIA. Consider testing when discussing risks and benefits of treatment with patients, including lack of insurance coverage for testing and additional genetic counseling.
Imaging Recommendations During Course of Therapy
A baseline MRI of the brain should be obtained no more than 12 months before the first infusion. While the labeling comments that the safety of aducanumab in patients with any pretreatment superficial siderosis, 10 or more brain microhemorrhages, and/or a brain hemorrhage greater than 1 cm within 1 year of treatment has not been studied,1 participants were excluded from the PRIME trial if they had more than 4 microhemorrhages at baseline MRI, a cortical infarct, or greater than 1 lacunar infarct.8
Greater than 40% of participants in the phase 3 trials experienced ARIA events.1,17 Most of the participants were asymptomatic (24% symptomatic at the 10 mg/kg dose).18 Thirty-five percent of individuals had edema (ARIA-E), but a significant proportion also experienced hemorrhagic events (ARIA-H). Hemorrhagic events included microhemorrhage (19.7%), superficial siderosis (13.3%), and cerebral hemorrhage (0.5%).1,17 ARIA-E was more common in ApoE-4 carriers (42% vs 20%),1 and most of the events occurred within the first 8 doses. The aducanumab labeling advises MRI imaging before the 7th and 12th aducanumab infusions, which differs from the clinical trials where surveillance MRIs were completed at weeks 14, 22, 30, 42, 54, 66, and 78.1,17 A recent expert consensus recommendation suggested brain MRI before the 5th, 7th, and 12th infusions and imaging before the 10th dose in APOE-4 carriers.12
Possible ARIA-related symptoms include headache, confusion, acute changes in cognition, dizziness, visual changes, and nausea. Seizures, gait changes, and alterations in consciousness have also been reported. Additional brain MRI's should be obtained if any new neurologic symptoms develop while an individual is receiving aducanumab.12 Dosing was suspended in the trials for participants with symptomatic ARIA or moderate-to-severe asymptomatic ARIA and was stopped for any participant with severe ARIA-H1 (moderate ARIA-E: FLAIR hyperintensity of 5–10 cm or more than 1 site of involvement each measuring <10 cm; severe ARIA-E: 1 or more areas of FLAIR hyperintensity >10 cm with significant subcortical white matter and/or sulcal involvement; moderate ARIA-H: 5–9 new microhemorrhages or 2 areas of superficial siderosis; and severe ARIA-H: 10 or more new microhemorrhages or >2 focal areas of superficial siderosis).1
ARIA-E resolved in 68% of the participants in 12 weeks and in 91% of the participants by 20 weeks.1 Currently, monitoring is recommended for any patient continuing infusions with mild-to-moderate ARIA.1 One may wish to consider a monthly MRI in patients with symptomatic ARIA or moderate-to-severe asymptomatic ARIA after dosing is suspended, with resumption after symptom resolution in ARIA-E and after stabilization of imaging changes in ARIA-H. Patients with asymptomatic ARIA that is mild may be able to continue dosing with monthly MRI surveillance.12
Practical Considerations
Patient Selection
Once an individual has been identified as a potential candidate for aducanumab, the provider needs to have a detailed discussion with the patient, their family, and care partner to help manage therapeutic expectations. In post hoc analysis of the trial data, aducanumab did not reverse cognitive dysfunction. Analysis of the data demonstrated only a modest degree of protection against the progression of cognitive decline in a subset of individuals receiving the highest dose of medication.9,10 The burden of Aβ pathology is reduced with the use of this medication, but no correlation between biomarker change and cognitive benefits has been demonstrated.19 The populations included in these trials were not ethnically diverse, and many populations, including Blacks, Hispanics, and indigenous people, were not well represented. Therefore, safety and efficacy in these populations is not known.
Neurologists should be aware of the dosing window and need for monthly infusions and ensure that patients have reliable transportation to infusion and imaging appointments. Patients and care partners should be counseled to inform treating physicians of any symptoms or changes in status that could indicate adverse treatment effects. Shared decision-making and informed consent should occur with the patient, care partner, and physician ensuring that the treatment has the potential to meet the patient's desired goals of care. Patients should also be aware of their potential financial responsibility associated with infusions, diagnostic testing, and clinical and radiographic monitoring.
Dosing for Aducanumab
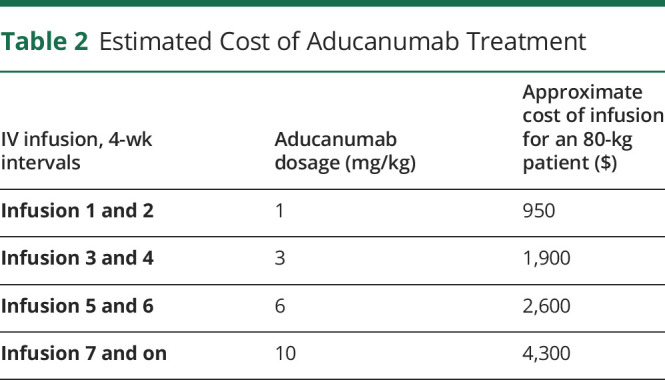
The titration schedule for aducanumab is listed in Table 2. The recommended dosage of aducanumab is 10 mg/kg given intravenously over approximately 1 hour every 4 weeks.1 Aducanumab is available as 170 mg/1.7 mL (100 mg/mL) and 300 mg/3 mL (100 mg/mL) solutions in single-dose vials.1 The wholesale acquisition cost of aducanumab is $952 for the 1.7-mL package size and $1,680 for the 3-mL package size, resulting in an estimated cost of $4,300 per infusion for an 80-kg patient.20 The yearly cost at the maintenance dose of 10 mg/kg is estimated at an average of $56,000 by the manufacturer, which will vary based on the patient's weight and the first-year titration costs.
Table 2.
Estimated Cost of Aducanumab Treatment
The actual costs of administering aducanumab go beyond the cost of the drug and include infusion-related administration costs, nursing time, and clinical and imaging monitoring. Both clinical and MRI monitoring will increase in frequency should ARIA be detected. The drug pricing watchdog group, the Institute for Clinical and Economic Review (ICER)'s health-benefit price benchmark range for aducanumab is $3,000-$8,400 per year for patients with early AD—which would require an 85%–95% discount off the treatment's US list price of $56,000.21 Members of the California Technology Assessment Forum, an independent appraisal committee of ICER, voted 15-0 against both safety and efficacy of aducanumab.22
Treatment Monitoring and Treatment Cessation
Monitoring of cognitive and functional status should use objective assessment tools such as the MMSE, MoCA, Dementia Screening Interview, Neuropsychiatric Inventory, and the Functional Activities Questionnaire.12 Other than the development of severe ARIA-H, it is unclear when and how to stop treatment. An honest discussion with patients and care partners should ensue when a patient progresses to moderate or severe stages of Alzheimer because aducanumab was not tested in these patient populations. Difficulties adhering to treatment protocols or other adverse effects may also result in discontinuation of this medication.12
Imaging
Patients must be referred to facilities able to perform MRIs with appropriate sequences to detect microhemorrhages and ARIA and that have radiologists with experience and comfort in ARIA identification. Quick and reliable communication of abnormal results between radiologist, neurologist, and patient is important to ensure patient safety.
Infusion Considerations
When choosing an infusion center, consider proximity of the infusion site to the patient's home or workplace, participation with the patient's insurance plan, environment, responsiveness of the infusion nurses, and efficiency of the scheduling. Infusion locator services provide the most convenient way to locate an external infusion center. Outreach to a few local external infusion centers to gather more detailed information about these factors will provide a smoother transition for patients and providers when using an external infusion site.
Workflow
Patients will need not only baseline MRI and CSF analyses or Aβ PET imaging to establish treatment eligibility but also repeat surveillance and possible unscheduled MRI examinations. Neurologists should ensure that a system is in place to track overdue and missing test results and communicate quickly with patients in cases of ARIA. Offices will need to consider whether to provide infusion services in-house or use an outside facility; factors to consider include drug purchase and storage, patient monitoring, and infusion chair capacity and turnover.
Coverage for Aducanumab
While the debate about aducanumab's efficacy, safety profile, and mechanism of action shows no signs of abating, some health insurers and hospitals are moving forward with coverage and access-related decisions. Most notably, on July 12, 2021, the Center for Medicare and Medicaid Services (CMS) opened its National Coverage Determination (NCD) analysis period for aducanumab.23 The NCD is a process that allows CMS to determine whether Medicare will establish a national coverage policy for monoclonal antibodies targeting amyloid for the treatment of AD, including aducanumab and future class-related therapies. The first of 2 public listening and commentary sessions were held on July 22, and CMS anticipates the final NCD policy to be published sometime in April 2022.24
Some hospitals have taken a definitive approach regarding access to aducanumab. At the time of this writing, the Cleveland Clinic, Mount Sinai Health System in New York, and Providence Hospital in Renton, Washington State, have announced that they will not be administering aducanumab in their facilities.25 Several Blue Cross Blue Shield Health plans have already adopted the position that aducanumab is considered investigational due to the insufficient evidence of clinical benefit and is therefore not eligible for coverage under medical necessity.26 Most recently, the US Veterans Administration has decided not to cover aducanumab on its National Formulary.27 In summary, few health-care institutions have announced their policies concerning access to aducanumab, and several insurers have made public their plans related to coverage before CMS has finalized its coverage policy.
Practice and Patient Expenses
The FDA approval of aducanumab could present multiple challenges to clinical practice. One immediate challenge is the increased demand for screening using cognitive tools and AD biomarker testing to provide a formal diagnosis of AD dementia for an undiagnosed, symptomatic population and older adults worried about their subjective memory losses. The capacity of academic memory disorder centers to absorb this demand may be stretched as indicated by a recent analysis.28 Similarly, the increased demands on diagnostic Aβ PET imaging and MRI-based surveillance monitoring may further burden imaging facilities.29 For private practices entering into the world of infusible therapies for the first time, consideration of how insurers reimburse infusible therapies, which infusion sites are covered under a patient's insurance benefit, and familiarizing themselves with infusion billing codes are critical.
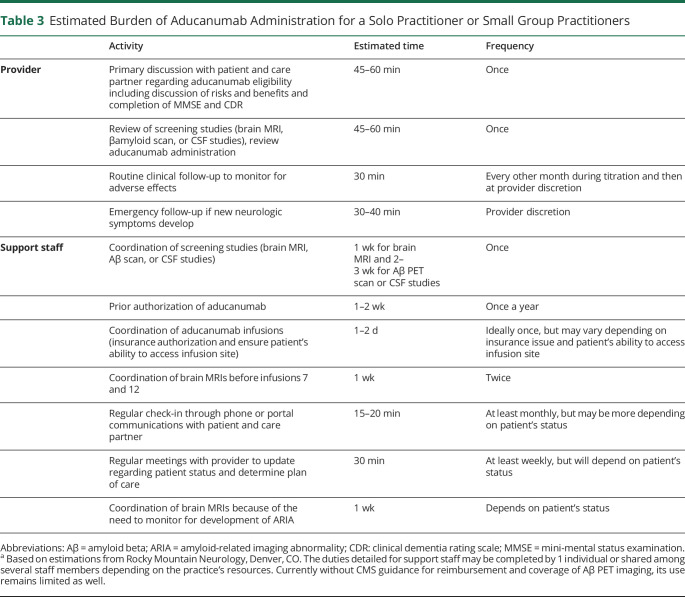
The decision to prescribe aducanumab may be especially difficult for small group and solo practitioners. Before the medication is even prescribed, appropriate resources need to be identified. The coordination of the many tests required to identify and monitor patients before and during therapy, scheduling monthly infusions, and arranging for the frequent required clinical visits will place an undue burden on practices that may not have extra patient care and administrative resources to devote to a select patient population. Table 3 summarizes an estimate of additional time both a practitioner in solo or a small group practice and their support staff may need to incorporate into their clinical schedule if a patient is determined to be a candidate for aducanumab therapy. Small group and solo practitioners may also face an unanticipated financial burden because both CMS and private payers are still determining coverage for not only the medication itself but also the multiple additional imaging studies and clinical visits required to monitor patients who are taking aducanumab. Coordination of care for the patient between the neurologist's practice, the patient's insurer, and the external infusion site may present unforeseen challenges as well.
Table 3.
Estimated Burden of Aducanumab Administration for a Solo Practitioner or Small Group Practitioners
Because insurers are still grappling with coverage decisions, potential out-of-pocket expenses for patients insured under CMS can be hefty due to the cost of their deductible and the 25% coinsurance associated with costs in the initial coverage period. Patients will have to accrue up to $7,050 out-of-pocket costs before they reach the catastrophic benefits period in 2022 when their out-of-pocket expenses dramatically decrease. Currently, Medicare beneficiaries in Part D plans can only access copay assistance programs known as Pharmaceutical Assistance Programs or PAPs by operating outside the Part D benefit.30 Eligibility is based on several determinations and may be restricted to low-income enrollees who have been defined all other options.
In summary, the decision by the FDA to approve aducanumab remains controversial, and its administration will pose many challenges that will be new to neurologists, despite extensive experience with prior intravenous medications.
Acknowledgment
The authors thank Amanda Becker and Rebecca Seale for their editorial assistance and Dr. Victoria Pelak and Mr. Donald Shook for their valuable insights.
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
K. Coerver is a consultant for GE Healthcare Systems. M. Yu has received research support from Biogen. A. D'Abreu and M. Wasserman report no disclosures relevant to the manuscript. K.V. Nair has received institutional grants/research supports from Genentech, Bristol Meyers Squib, Novartis, and Biogen; honoraria or consultation fees from Bristol Meyers Squib, Novartis, and Biogen; and other institutional support from the Pharmaceutical Research and Manufacturers of American Association Center Grant. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Prescribing Information. Biogen; 2021. Accessed July 18, 2021. biogencdn.com/us/aduhelm-pi.pdf. [Google Scholar]
- 2.Salloway S, Cummings J. Aducanumab, amyloid lowering, and slowing of Alzheimer's disease. Neurology. 2021;97(11):543-544. doi: 10.1212/WNL.0000000000012451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knopman DS, Permutter JS. Prescribing aducanumab in the face of meager efficacy and real risks. Neurology. 2021;97(11):545-547. doi: 10.1212/WNL.0000000000012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershey LA, Tarawneh R. Clinical efficacy, drug safety and surrogate endpoints: has aducanumab met all of its expectations? Neurology. 2021;97(11):517-518. doi: 10.1212/WNL.0000000000012453. [DOI] [PubMed] [Google Scholar]
- 5.Aducanumab label revised: initiate treatment for mild cognitive impairment or mild dementia of Alzheimer disease. Accessed July 21, 2021. practicalneurology.com/news/aducanumab-label-revised-treatment-should-be-initiated-for-mild-cognitive-impairment-or-mild-dementia?utm_campaign=Neurologywire&utm_medium=email&_hsmi=139058041&_hsenc=p2ANqtz-9pNl7a2nti20PagljqO0geAhCN-YD049JVdqvfA6bZECMasSqiLaTVlA3pfTpiQ2Qt5l4NDV1NwuVOxDjPhJ9_F6gjUeVsgETtt1G-McjdsK-wbhA&utm_content=139058041&utm_source=hs_email.
- 6.FDA's approval of Biogen's new Alzheimer's drug has huge cost implications for Medicare and beneficiaries. Accessed July 21, 2021. kff.org/medicare/issue-brief/fdas-approval-of-biogens-new-alzheimers-drug-has-huge-cost-implications-for-medicare-and-beneficiaries/.
- 7.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8(6):595-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Multiple Dose Study of Aducanumab (BIIB037) (Recombinant, Fully Human Anti-Ab IgG1 mAb) in Participants With Prodromal or Mild Alzheimer's Disease (PRIME). U.S. National Library of Medicine; 2021. Accessed July 18, 2021. clinicaltrials.gov/ct2/show/NCT01677572. [Google Scholar]
- 9.221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (ENGAGE). ClinicalTrials.gov identifier: NCT02477800. Updated September 2, 2021. Accessed February 9, 2022. https://clinicaltrials.gov/ct2/show/NCT02477800.
- 10.221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer's Disease (EMERGE). ClinicalTrials.gov identifier: NCT02484547. Updated September 2, 2021. Accessed February 9, 2022. https://clinicaltrials.gov/ct2/show/NCT02484547.
- 11.Huang HC, Tseng YM, Chen YC, et al. Diagnostic accuracy of the Clinical Dementia Rating Scale for detecting mild cognitive impairment and dementia: a bivariate meta-analysis. Int J Geriatr Psychiatry. 2021;36(2) 239-251. [DOI] [PubMed] [Google Scholar]
- 12.Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: appropriate use recommendations. J Prev Alzheimers Dis. 2021;8(4):398-410. doi: 10.14283/jpad.2021.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays on BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansson O, Rutz S, Zetterberg H, et al. Pre-analytical protocol for measuring Alzheimer's disease biomarkers in fresh CSF. Alzheimers Dement (Amst). 2020;12(1):e12137. doi: 10.1002/dad2.12137. Erratum in: Alzheimers Dement (Amst). 2021;13(1):e12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabers A, Perna L, Lange J, et al. Amyloid blood biomarker detects Alzheimer's disease. EMBO Mol Med. 2018;10(5):e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sperling R, Jack C Jr, Black S, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krudys KM. Aducanumab for the Treatment of Alzheimer's Disease: Clinical Overview of Efficacy. U.S. Food and Drug Administration; 2020. Accessed July 18, 2021. fda.gov/media/143504/download. [Google Scholar]
- 18.Biogen's Alzheimer's disease CSF biomarker testing at no charge. Accessed July 21, 2021. amyloidbetaconfirmed.com/en_us/home.html.
- 19.Knopman D, Jones DT, Greicius MD. Failure to demonstrate efficacy of aducanumab: an analysis of the EMERGE and ENGAGE trials as reported by Biogen. Alzheimers Dement. 2021;17(4):696-701. [DOI] [PubMed] [Google Scholar]
- 20.The Redbook: wholesale acquisition price. Accessed July 21, 2021. micromedexsolutions.com/micromedex2/librarian/ssl/true.
- 21.Lin GA, Whittington MD, Synnott PG, et al. Aducanumab for Alzheimer's Disease: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review; 2021. Accessed July 21, 2021. icer.org/assessment/alzheimers-disease-2021/. [Google Scholar]
- 22.CTAF voting panel on Aducanumab. Accessed July 21, 2021. youtube.com/watch?v=CHf_LMjgHag.
- 23.CMS opens national coverage determination analysis on treatment for Alzheimer's disease. Accessed July 21, 2021. cms.gov/medicare-coverage-database/details/nca-trackingsheet.aspx?NCAId=305&bc=AAAAAAAACAAAAAAA&.
- 24.CMS seeks public comments on coverage for aducanumab. Accessed July 21, 2021. journals.lww.com/neurotodayonline/blog/breakingnews/pages/post.aspx?PostID=1140.
- 25.Cleveland Clinic, Mount Sinai and Providence won't give Biogen's new Alzheimer's drug. Accessed July 21, 2021. wsj.com/articles/cleveland-clinic-mount-sinai-wont-give-biogens-newalzheimers-drug-11626366968.
- 26.Some blues plans won't cover new Alzheimer's therapy aduhelm. Accessed July 21, 2021. formularywatch.com/view/some-blues-plans-won-t-cover-new-alzheimer-stherapy-aduhelm.
- 27.Veterans Administration declines coverage for aduhelm. Veterans Administration Declines Coverage for Aduhelm. Accessed July 21, 2021. formularywatch.com.
- 28.Musiek ES, Morris JC. Possible consequences of the approval of a disease-modifying therapy for Alzheimer disease. JAMA Neurol. 2021;78(2):141-142. doi: 10.1001/jamaneurol.2020.4478. [DOI] [PubMed] [Google Scholar]
- 29.Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer's Treatment. RAND Corporation; 2017. doi: 10.7249/RR2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pharmaceutical manufacturer patient assistance program information. Accessed July 21, 2021. cms.gov/Medicare/Prescription-DrugCoverage/PrescriptionDrugCovGenIn/PAPData.