Abstract
Purpose of Review
The purpose of this review is to give an update on chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).
Recent findings
There are several recent developments in CIDP, the major one being the 2021 second revision of the European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Other updates address therapy in CIDP, antibodies, serum neurofilament light chain, chronic immune sensory polyradiculopathy (CISP) and CIDP mimics.
Summary
CIDP criteria continue to be refined and some disorders are now excluded from the classification. Treatment options are expending and promising biomarkers are being studied.

Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), although rare and heterogeneous, is one of the most common immune-mediated neuropathies. CIDP, however, especially CIDP variants, can be difficult to diagnose.1 There is no gold standard testing for CIDP, and although a few specific disease-associated antibodies have been described, these are absent in most patients. Furthermore, many experts consider the presence of these antibodies to be indicative of a separate disease.2 Ultimately, CIDP remains a diagnosis of exclusion. Mimics are ruled out either by history or additional ancillary testing. Following experts' guidelines on the diagnosis of CIDP significantly increases the accuracy of diagnosis. On a different note, although most patients with CIDP respond well to treatment, some may not tolerate therapy and other may be refractory to first-line treatments. In this review, I discuss 5 new things related to CIDP: (1) the updated European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) CIDP guidelines, (2) the expansion of the chronic immune sensory polyradiculopathy (CISP) spectrum, (3) updates in therapy for CIDP, (4) antibodies testing, serum neurofilament light (sNfL) chain and their role, and (5) disorders that can mimic CIDP.
The 2021 EAN/PNS Guideline on Diagnosis and Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy
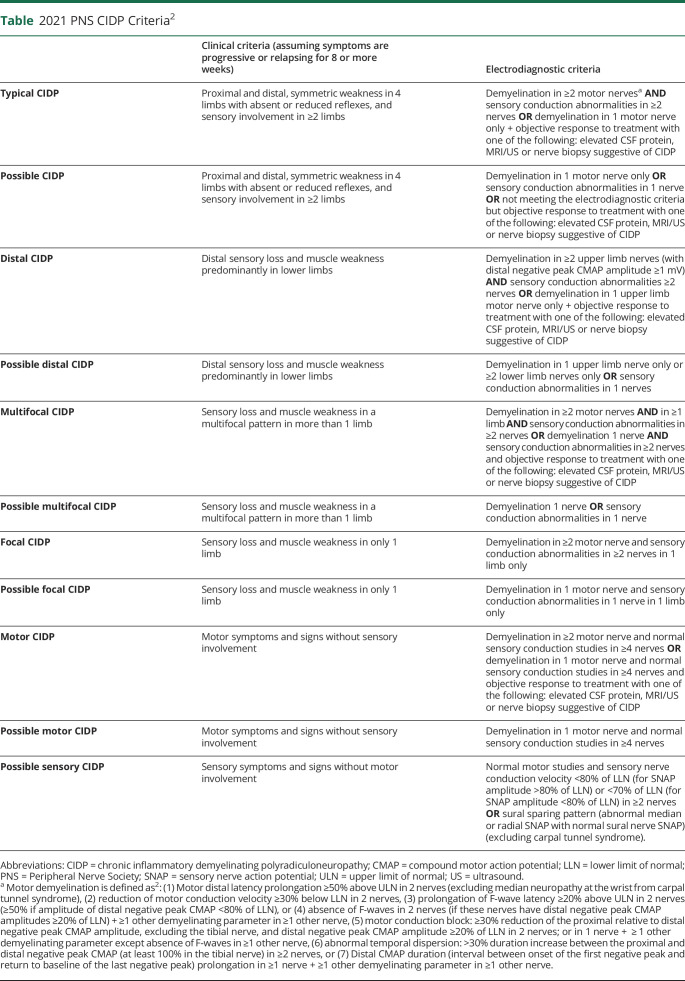
The European Federation of Neurologic Societies/PNS CIDP criteria are widely used because they are the most accurate compared with the 15 other criteria published.3,4 The criteria were revised in 2010 and more recently in 2021.2 In the latest revision, the first change was to refine CIDP into “typical CIDP” and “CIDP variant”. The “atypical CIDP” term was dropped because these “CIDP variants” share the common features of demyelination, response to immune therapy, and are well characterized entities. CIDP variants comprise distal CIDP, multifocal or focal CIDP, motor CIDP, motor predominant CIDP, sensory CIDP, and sensory predominant CIDP. Sensory CIDP can only be classified as possible sensory CIDP given that the sensory conduction study criteria can only fulfill possible CIDP and those cannot be upgraded by supportive criteria and motor conduction must be normal per definition (Table). CISP, discussed below, was removed from the CIDP classification. Furthermore, the autoimmune nodopathies associated with antibodies against nodal-paranodal cell-adhesion molecules are no longer considered a part of CIDP.2 These are also discussed below.
Table.
2021 PNS CIDP Criteria2
Second, electrodiagnostic studies that are essential for typical CIDP diagnosis, no longer have the “definite CIDP criteria.” Electrodiagnostic studies are now classified into supportive of “CIDP” and “possible CIDP”. Furthermore, sensory conduction studies criteria for typical CIDP are now essential. Briefly, for typical CIDP, the following findings should be present: evidence of primary demyelination in 2 motor nerves and sensory conduction abnormalities in 2 nerves. A diagnosis of “possible CIDP” can be made if the patient fulfills clinical criteria but has evidence of demyelination on only 1 nerve or does not meet the electrodiagnostic criteria, but there is objective response to treatment and if one or more of the following supportive criteria is met: specific ultrasound or MRI abnormalities, CSF protein elevation, or there is specific pathology on nerve biopsy.2 The table summarizes the new 2021 EAN/PNS CIDP criteria.
Third, from a treatment perspective, both intravenous immunoglobulin (IVIG) and steroids remain first-line therapy for CIDP. These are preferred over plasma exchange (PLEX) mainly because of ease of administration. For maintenance, subcutaneous IG (SCIG) was added as first-line therapy. When switching from IVIG to SCIG, it is reasonable to start using the same mean dose per week at 1:1 conversion from the IVIG dose. The group suggested that alternative therapy such as azathioprine, mycophenolate mofetil, or cyclosporine may be considered as immunoglobulin or corticosteroid-sparing agents. For refractory patients, they suggested cyclophosphamide, cyclosporine, or rituximab. Finally, for pain which is typically rare in CIDP, they suggested tricyclic antidepressants, pregabalin, gabapentin, or serotonin noradrenaline reuptake inhibitors (duloxetine or venlafaxine) as first-line treatments.2
The Expansion of the CISP Spectrum
In 2004, Sinnreich et al.5 reported on a group of 15 patients with chronic sensory ataxia with normal nerve conduction studies and abnormal somatosensory evoked potential (SSEP), enlarged nerve roots, elevated CSF protein, and inflammatory hypertrophic changes of sensory nerve rootlet tissue. Some of these patients responded to immunomodulating therapies, and the authors coined the term CISP. CISP was initially included as “atypical CIDP” in the 2010 European Federation of Neurologic Societies/PNS.6 As mentioned above, CISP is no longer considered CIDP in the updated 2021 criteria because the group did not find enough evidence to determine whether CISP is demyelinating or related to sensory CIDP. In 2021, Shelly et al.7 expanded the spectrum of CISP by reporting a group of patients who present similar to CISP but in addition have mild distal weakness and mild abnormalities on NCS. They refer to this condition as CISP-plus. These patients respond to immunomodulating drugs typically used for CIDP. It is important to report these patients because they would not fulfill the electrophysiologic criteria for CIDP, given that the abnormalities are mainly at the dorsal nerve root. The electrophysiologic abnormalities in CISP-plus consisted of mildly prolonged F-waves latencies, reduced motor and sensory amplitudes, or mild slowing of conduction velocities. Given these findings, it is important that patients with chronic sensory ataxia and normal or mildly abnormal NCS undergo SSEP, MRI of the spine with IV contrast, and CSF examination for better characterization of the clinical syndrome.7
Current Therapy for CIDP
Classically, the treatment for CIDP included IVIG, steroids, and PLEX. Most patients with CIDP respond well to these therapies.8 In the United States, IVIG is widely used as a first-line treatment for CIDP. IVIG is effective and relatively safe. However, it is associated with frequent side effects.9 These side effects can range from mild to severe and may require premedication with acetaminophen, antihistamine, and corticosteroids. Side effects include headache, rash, flu-like symptoms, and thromboembolic events.10 IVIG is usually given at a loading dose of 2 g/kg, followed by a maintenance dose of 1 g/kg every 3–4 weeks. Once the patient recovered to what is felt to be their baseline, IVIG is typically weaned off—either by slowly decreasing the dose or increasing time between treatments.11 Sometimes, higher doses of IVIG are required to achieve adequate clinical response. The ultimate goal is to give patients the least therapy needed. Many patients require lifelong treatment, but some patients go in remission, so it is important to always assess whether treatment can be weaned. Side effects or treatment-related fluctuation that do not respond to additional premedication or dose adjustments may benefit from SCIG. SCIG has been found effective in CIDP and is typically given on a weekly basis.12 Patients have less side effects and experience less treatment-related fluctuation with this treatment. In addition, many patients appreciate the self-sufficiency and being able to get the treatment when they want it and where they want it.13 Although 2021 guidelines suggest that 1:1 dose conversion is reasonable, SCIG doses of 0.2 and 0.4 g/kg/wk were studied in a clinical trial and were both found to be effective compared with placebo.12 Furthermore, long-term efficacy results from the PATH extension study showed lower relapse rates with the higher dose of 0.4 g/kg/wk.14 SCIG is usually administered once a week but can be given over 2 or even 3 days for larger doses.
When CIDP is refractory to IVIG or steroids, it is important to take a step back and reassess the diagnosis because most patients would respond to these drugs. In case one is certain about the diagnosis, testing for the antibodies as mentioned below is also important. Alternative therapy for refractory patients includes rituximab and cyclophosphamide. Recently, a group reported on a series of 11 patients with refractory CIDP who received rituximab and 10/11 showed significant improvement in strength and function.15 Some of these patients went from quadriparetic to ambulatory despite many years of disease. We had similar experience with some of our patients, but some did not respond to treatment. In our series of 45 patients with CIDP seen in a tertiary referral center, 7 of 10 refractory patients responded to either rituximab or cyclophosphamide. In the 3 patients who did not respond to aggressive treatment, the axonal loss may have been very advanced, so it would be difficult to tell with certainty whether the patients were truly refractory to treatment or whether they were treated too late.16
Antibodies and sNfL Chain
In the last decade, antibodies directed against membrane proteins located at or around the node of Ranvier, neurofascin (anti-NF155, anti NF 140/186), contactin (anti-CNTN1), and contactin-associated protein 1 (anti-Caspr1) have been described. These antibodies are rare, found in less than 5% of patients diagnosed with CIDP.17 Although some consider these conditions as CIDP, more recently, experts have separated them from CIDP and proposed to name these conditions “autoimmune nodopathies” instead of CIDP. The reason was that these autoimmune nodopathies have a specific clinical phenotype (acute onset and pronounced ataxia), have no overt inflammation, or macrophage-mediated demyelination when nerves are biopsied and poorly respond to IVIG. These antibodies are associated with severe autoimmune neuropathies and are usually of IgG3 or IgG4 subtypes. IgM NF144 or NF186 has been described in some patients with CIDP (nonrefractory) and other neuropathies (Charcot Marie Tooth [CMT] and idiopathic). Their role remains unclear at this time.2 Patients with IgG3 and 4 antibodies are refractory to IVIG in general but respond to B cell depletion (usually with rituximab).17
sNfL chain are nonspecific markers of axonal damage and have been studied in different type of peripheral neuropathies. A recent study in patients with CIDP suggested that sNfL levels might be useable as a biomarker of disease activity. Researchers showed that patients with active disease (nonresponders and patients who relapsed after IVIG withdrawal) had higher sNfL levels compared with patients with stable disease (responders and patients who were successfully withdrawn from IVIG treatment). sNfL levels were increased in a third of patients with patients starting immunotherapy and reflected axonal damage.18
Mimics of CIDP
Disorders that mimic CIDP are very few, especially when abiding to the CIDP criteria guidelines discussed above. Most of the time, when patients are misdiagnosed with CIDP, it is because of overinterpretation of the CSF and the electrodiagnostic findings.19 For example, elevation in CSF protein can be seen in many different types of neuropathy including hereditary transthyretin amyloidosis, CMT, and diabetes and is not specific to CIDP. Some researchers have suggested that the upper reference limits for CSF protein should be higher than 45 mg/dL (closer to 60 mg/dL) and should be stratified by age.20
Overinterpretation of conduction velocity slowing is another cause for diagnosis pitfall. Mild conduction slowing can be seen in axonal neuropathy because of loss of fast-conducting large-myelinated fibers. Cold skin temperature also results in slow conduction velocities (under 30°C in lower extremity and <32°C in upper extremity).
On the other hand, certain disorders such as Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal Protein, Skin Changes (POEMS) syndrome and neurolymphomatosis could fulfill the electrophysiologic criteria, and we have seen people misdiagnosed with CIDP by neuromuscular experts because of this.21 However, POEMS is usually associated with a monoclonal protein, pain and swelling, thrombocytopenia, and skin changes that should prompt to the correct diagnosis. Neurolymphomatosis is typically painful and multifocal. One should not just rely on treatment response because some patient may respond well to immunomodulation initially.22 PET scan may be more sensitive than MRI in ruling out neurolymphomatosis. However, CT assessment of bone lesions is more sensitive than PET scan for small bone lesions in patients with POEMS syndrome.23
Future Considerations
CIDP is a rewarding condition for the neuromuscular physician because the patients respond well to treatment and recovery could be complete, especially if diagnosed and treated early. There remain many challenges that will be hopefully resolved as we educate ourselves better about the diagnosis, discover better biomarkers, and develop novel therapies for this condition.
Appendix. Author
Study Funding
No targeted funding reported.
Disclosure
C. Karam has received consulting honoraria from Alnylam, Argenx, CSL Behring, Sanofi, and Ionis. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
FIVE NEW THINGS
The 2021 revision of the EAN/PNS guideline bring significant changes to the diagnosis criteria and treatment of CIDP aiming to optimize diagnostic accuracy and to improve patient outcomes.
CISP and CISP plus can be difficult to diagnose but may respond to immuno-modulating therapy.
SCIG is now recommended for maintenance therapy in CIDP patients.
More studies are needed on the value of serum neurofilament light chain which may help better evaluate disease activity and response to treatment. Autoimmune nodonopathies, no longer considered part of CIDP spectrum, should be suspected in specific clinical phenotypes and the presence of auto-antibodies.
True CIDP mimics are rare and can be suspected in the presence of serum monoclonal protein, lack of response to first line therapy and/or the presence of systemic symptoms and signs.
References
- 1.Broers MC, Bunschoten C, Drenthen J, et al. Misdiagnosis and diagnostic pitfalls of chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2021;28(6):2065-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Bergh PYK, van Doorn PA, Hadden RDM, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a Joint Task Force-Second revision. Eur J Neurol. 2021;28(11):3556-3583. [DOI] [PubMed] [Google Scholar]
- 3.Breiner A, Brannagan TH III. Comparison of sensitivity and specificity among 15 criteria for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2014;50(1):40-46. [DOI] [PubMed] [Google Scholar]
- 4.Hughes RA, Bouche P, Cornblath DR, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2006;13(4):326-332. [DOI] [PubMed] [Google Scholar]
- 5.Sinnreich M, Klein CJ, Daube JR, Engelstad J, Spinner RJ, Dyck PJ. Chronic immune sensory polyradiculopathy: a possibly treatable sensory ataxia. Neurology. 2004;63(9):1662-1669. [DOI] [PubMed] [Google Scholar]
- 6.Joint Task Force of the EFNS and the PNS. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(3):185-195. [DOI] [PubMed] [Google Scholar]
- 7.Shelly S, Shouman K, Paul P, et al. Expanding the spectrum of chronic immune sensory polyradiculopathy: CISP-plus. Neurology. 2021;96(16):e2078-e2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bus SRM, Broers MC, Lucke IM, et al. Clinical outcome of CIDP one year after start of treatment: a prospective cohort study. J Neurol. Epub 2021 Jun 26. [DOI] [PMC free article] [PubMed]
- 9.Racosta JM, Sposato LA, Kimpinski K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: a meta-analysis. Muscle Nerve. 2017;55(6):802-809. [DOI] [PubMed] [Google Scholar]
- 10.Donofrio PD, Bril V, Dalakas MC, et al. Safety and tolerability of immune globulin intravenous in chronic inflammatory demyelinating polyradiculoneuropathy. Arch Neurol. 2010;67(9):1082-1088. [DOI] [PubMed] [Google Scholar]
- 11.Rajabally YA, Afzal S. Clinical and economic comparison of an individualised immunoglobulin protocol vs. standard dosing for chronic inflammatory demyelinating polyneuropathy. J Neurol. 2019;266(2):461-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35-46. [DOI] [PubMed] [Google Scholar]
- 13.Goyal NA, Karam C, Sheikh KA, Dimachkie MM. Subcutaneous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2021;64(3):243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Schaik IN, Mielke O, Bril V, et al. Long-term safety and efficacy of subcutaneous immunoglobulin IgPro20 in CIDP: PATH extension study. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muley SA, Jacobsen B, Parry G, et al. Rituximab in refractory chronic inflammatory demyelinating polyneuropathy. Muscle Nerve. 2020;61(5):575-579. [DOI] [PubMed] [Google Scholar]
- 16.Godil J, Barrett MJ, Ensrud E, Chahin N, Karam C. Refractory CIDP: Clinical characteristics, antibodies and response to alternative treatment. J Neurol Sci. 2020;418:117098. [DOI] [PubMed] [Google Scholar]
- 17.Delmont E, Brodovitch A, Kouton L, et al. Antibodies against the node of Ranvier: a real-life evaluation of incidence, clinical features and response to treatment based on a prospective analysis of 1500 sera. J Neurol. 2020;267(12):3664-3672. [DOI] [PubMed] [Google Scholar]
- 18.van Lieverloo GGA, Wieske L, Verhamme C, et al. Serum neurofilament light chain in chronic inflammatory demyelinating polyneuropathy. J Peripher Nerv Syst. 2019;24(2):187-194. [DOI] [PubMed] [Google Scholar]
- 19.Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498-504. [DOI] [PubMed] [Google Scholar]
- 20.Breiner A, Bourque PR, Allen JA. Updated cerebrospinal fluid total protein reference values improve chronic inflammatory demyelinating polyneuropathy diagnosis. Muscle Nerve. 2019;60(2):180-183. [DOI] [PubMed] [Google Scholar]
- 21.Moshe-Lilie O, Ensrud E, Ragole T, Nizar C, Dimitrova D, Karam C. CIDP mimics: a case series. BMC Neurol. 2021;21(1):94-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomita M, Koike H, Kawagashira Y, et al. Clinicopathological features of neuropathy associated with lymphoma. Brain. 2013;136(pt 8):2563-2578. [DOI] [PubMed] [Google Scholar]
- 23.Glazebrook K, Guerra Bonilla FL, Johnson A, Leng S, Dispenzieri A. Computed tomography assessment of bone lesions in patients with POEMS syndrome. Eur Radiol. 2015;25(2):497-504. [DOI] [PubMed] [Google Scholar]