Abstract
Background and Objectives
The effects of the SARS-CoV-2 vaccination and infection on clinical outcomes, including relapse risk, have been insufficiently explored in people with multiple sclerosis (PwMS). The objectives of this study were to determine the incidence of new neurologic symptoms or symptom recrudescence among PwMS who received the SARS-CoV-2 vaccine, characterize outcomes after SARS-CoV-2 infection, and assess MS-specific determinants of vaccine hesitancy.
Methods
Online surveys that assessed incidence and severity of SARS-CoV-2 infection, vaccination status/type, reasons for vaccine deferral, and postvaccination symptoms were administered to PwMS. Medical charts were reviewed for consenting respondents. Associations between infection, postvaccination outcomes, and clinical characteristics were compared using χ2 tests, 2-sample t tests, and adjusted logistic regression models.
Results
In total, 292 of 333 respondents were vaccinated, of whom 58% reported postvaccination side effects, most commonly among mRNA vaccine recipients (p = 0.02), younger patients (p < 0.01), and people with relapsing-remitting MS (p = 0.03). Twelve percent endorsed recrudescence of existing MS symptoms, while 3% endorsed new neurologic symptoms postvaccination. Sixty-two participants reported SARS-CoV-2 infection since the start of the pandemic, more frequent in younger individuals (1-year odds ratio [OR] = 0.958, 10-year OR = 0.649, p < 0.01). Neither disease-modifying therapy nor B-cell therapies specifically were associated with vaccine side effects, neurologic symptoms, or SARS-CoV-2 infection. Twenty-one percent of unvaccinated cited a desire for provider guidance before vaccination.
Discussion
Our findings provide new data to suggest that among PwMS who received SARS-CoV-2 vaccination, clinical disease worsening is rare and mostly associated with symptom recrudescence, as opposed to new relapses. Postvaccination side effects may occur more often among mRNA vaccine recipients and in younger individuals.

Multiple sclerosis (MS) is a chronic autoimmune disease of the CNS that causes demyelination and axonal degeneration. Although disease-modifying therapies (DMTs) effectively reduce the likelihood of MS disease progression,1 some DMTs have the potential to increase vulnerability to infection,2 highlighting the importance of SARS-CoV-2 vaccination (and vaccination in general) in this population.3 That said, data regarding postvaccination outcomes in people with MS (PwMS) are scarce, and SARS-CoV-2 vaccine acceptability remains variable.4,5 An improved understanding of postvaccination neurologic outcomes in PwMS and MS-specific determinants for vaccine hesitancy is necessary to guide informed vaccine discussions between providers and PwMS. Additional data on outcomes after SARS-CoV-2 infection in PwMS on DMT are also needed.
The objectives of this study were to (1) determine the incidence of new neurologic symptoms or recrudescence of MS symptoms among PwMS who received the SARS-CoV-2 vaccine, (2) examine MS-specific determinants of vaccine hesitancy in a cohort of PwMS followed at a tertiary care center, and (3) analyze outcomes after SARS-CoV-2 infection in PwMS.
Methods
Study Design and Participant Description
A 31-item online survey was created and delivered using the Research Electronic Data Capture web-based platform to patients followed in the Michigan Medicine Multiple Sclerosis Clinic. Respondents aged 18 years or older who endorsed a self-reported MS diagnosis, of any subtype, were invited to complete the online questionnaire through smartphone, tablet, or computer. Survey items included questions regarding the prevalence and severity of SARS-CoV-2 infection, vaccination status, vaccine type, reasons for vaccine deferral, and postvaccination symptoms and side effects.
Eligible participants were recruited (Figure 1) from June 18, 2021, to July 20, 2021. The main method of recruitment was through email with our established patients at the Michigan Medicine Multiple Sclerosis Center. Using DataDirect, a self-serve tool from the University of Michigan Office of Research that enables access to clinical data on patients seen at Michigan Medicine, we obtained email addresses of the established patients (defined as at least 1 return MS clinic visit) seen by our neuroimmunology physicians from the start of the SARS-CoV-2 pandemic in March 2020 to June 2021 (n = 1,242). In addition, fliers were distributed to eligible participants during routine face-to-face visits in the MS clinic, and the study was posted on the University of Michigan Health Research online web source at umhealthresearch.org, which allows interested participants to be involved in ongoing studies at Michigan Medicine. Participants received no financial compensation for their participation.
Figure 1. Selection of Participants Leading to Responses Analyzed and Charts Reviewed.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the University of Michigan Intuitional Review Board before initiation of any study activities.
Survey Content
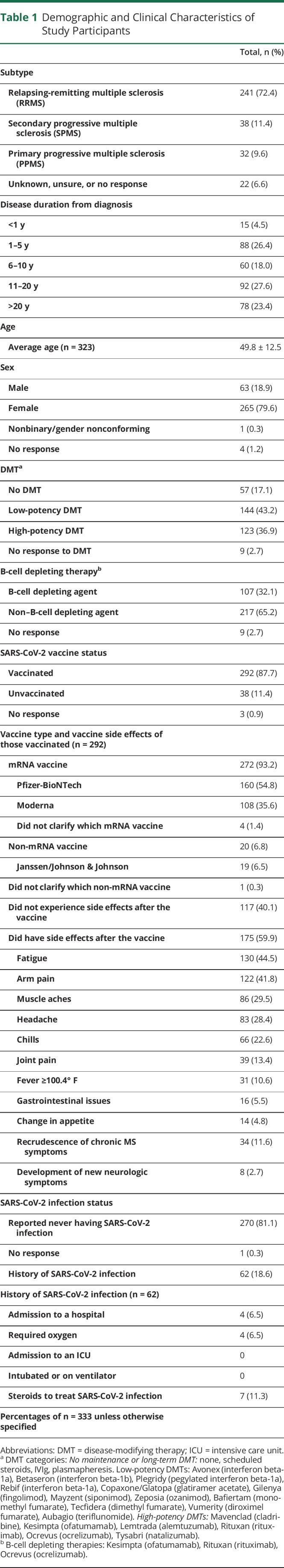
Survey respondents who self-endorsed a diagnosis of MS were allowed to proceed with the remainder of the questionnaire. Information obtained included age, sex, MS subtype (relapsing-remitting, secondary progressive, or primary progressive), disease duration from diagnosis (<1 year, 1–5 years, 6–10 years, 11–20 years, or >20 years), current DMT chosen from the list of all available DMTs (later categorized into no DMT, low-potency DMT, and high-potency DMT, as summarized in Table 1), SARS-CoV-2 vaccination status, vaccine type, and history of SARS-CoV-2 infection. Using branching logic, unvaccinated respondents were prompted to provide a reason. Vaccinated respondents were queried about postvaccination side effects and postvaccination neurologic symptoms including recrudescence of chronic MS symptoms, development of new neurologic symptoms after vaccination, and length of these symptoms (<24 hours, 24–48 hours, 48 hours-1 week, or >1 week). We defined recrudescence as both pseudorelapse (recurrence of prior symptoms) and worsening of a patient's chronic MS symptoms (e.g., fatigue).
Table 1.
Demographic and Clinical Characteristics of Study Participants
The survey was anonymous; however, the last question gave participants the option to provide their personal identifying information and consent to allow the study team to review their medical records for confirmation of results. For those who consented charts were reviewed for patient correspondence regarding postvaccination or infection symptoms, diagnostic testing ordered, and any related changes in DMT for MS treatment.
Statistical Analysis
Summary statistics for demographic and clinical data are presented as mean and SD for continuous variables, and frequency and percentage for categorical variables. T-tests, χ2 tests, and Fisher exact tests (when cells included less than 5 respondents) were used to examine bivariate associations between SARS-CoV-2 vaccination status/type, SARS-CoV-2 infection, demographic, and clinical variables (e.g., DMT use, including B-cell depleting vs non–B-cell depleting therapy). We then constructed 2 logistic regression models with SARS-CoV-2 infection and new neurologic symptoms as separate dependent variables and age, sex, and DMT use as the independent variables. To avoid collinearity, we fitted subsequent models, replacing age with disease duration and treatment with B-cell depleting therapy. All statistical procedures were performed using SAS software, version 9.4.6 Tests were 2-sided with a level of statistical significance set at 0.05.
Data Availability
Further anonymized data can be made available to qualified investigators on request to the corresponding author.
Results
Demographics of Survey Participants
A total of n = 333 responses were included in the analytic sample. The average age was 49.8 ± 12.5 years, 79.6% were female, and 72.4% had relapsing-remitting multiple sclerosis. Approximately 43% reported using a low-potency DMT, 36.9% used high-potency DMT, and 17.1% reported no maintenance or long-term DMT use (Table 1). Of the 333 respondents, 292 were vaccinated, 38 were unvaccinated, and 3 did not respond.
Among vaccinated individuals, the average age was 50.4 ± 12.4 years and 81.4% were female. Ninety-three percent of the participants received an mRNA vaccine (either Pfizer-BioNTech or Moderna) compared with 6.8% who received a non-mRNA vaccine (Janssen/Johnson & Johnson).
Postvaccination Outcomes
Average time interval between vaccine and survey administration was 4 months. Of the 292 who were vaccinated, 175 (60%) reported side effects (Table 1), with an average of 3 side effects per individual. The most common side effects included fatigue, arm pain, and myalgias. Postvaccination side effects were most common among those who received mRNA vaccines (p = 0.02), younger patients (p < 0.01), and people with relapsing-remitting MS (p = 0.03).
Thirty-four participants (11.6% of those vaccinated) reported recrudescence of their chronic MS symptoms after vaccination (Table 2). The most commonly reported symptoms were worsening MS fatigue and pain. Most symptoms lasted 24–28 hours or greater than 1 week. Eight participants reported talking with their doctor about these symptoms. Of the 25 participants in this group who consented to chart review, no participant required updated imaging, high-dose steroid use, or change in their DMT because of worsening MS symptoms.
Table 2.
Symptoms of Recrudescence or Worsening of Chronic MS Symptoms Reported by 34 People With MS After SARS-CoV-2 Vaccination

Eight participants (2.7%) reported the development of new neurologic symptoms after vaccination (Table 3). The most common symptoms were weakness, dizziness/vertigo, and paresthesias. Three reported new neurologic symptoms within 2 days of vaccination, 2 reported within 3 weeks of vaccination, and 1 reported within 2 months of vaccination. On average, these new neurologic symptoms lasted for greater than 1 week, although 1 participant reported symptoms for less than 1 day. Of the 5 participants in this group who consented to chart review, no participant required updated imaging, laboratory testing (including blood, urine, or cerebral spinal fluid), high-dose steroid use, or change in their DMT because of new neurologic symptoms.
Table 3.
New Neurologic Symptoms Reported by 8 People With Multiple Sclerosis After SARS-CoV-2 Vaccination

Neither MS subtype, DMT use in general, nor B-cell based therapy use was associated with recrudescence of MS symptoms or new neurologic symptoms. Similarly, vaccine type was not significantly associated with MS-specific outcomes.
SARS-CoV-2 Infection
A total of 62 participants reported SARS-CoV-2 infection since the start of the pandemic. The average age was 45.4 ± 11.9 years, and 77.8% were female. Most reported SARS-CoV-2 symptoms lasted less than 1 week or a few weeks to a month. Four participants reported admission to the hospital, and all 4 indicated the need for oxygen. No participants reported admission to the intensive care unit (ICU) or need for intubation. Eight patients reported SARS-CoV-2 infection despite vaccination, although not every participant provided time of infection in comparison with time of vaccination. Not all comorbidities were captured, but among the 4 participants who reported hospital admission and oxygen, 2 consented to chart review. Of these, 1 had no comorbidities, and 1 had a history of diabetes, coronary artery disease, hypothyroidism, and ovarian cancer.
In adjusted logistic regression models, for every 10-year increase in age, the odds of infection decreased by 35% (10-year odds ratio estimate 0.649, confidence interval = 0.507–0.832, p = 0.0006). There was no significant association between SARS-CoV-2 infection and MS subtype, DMT use, B-cell depleting therapy use, disease duration, or sex.
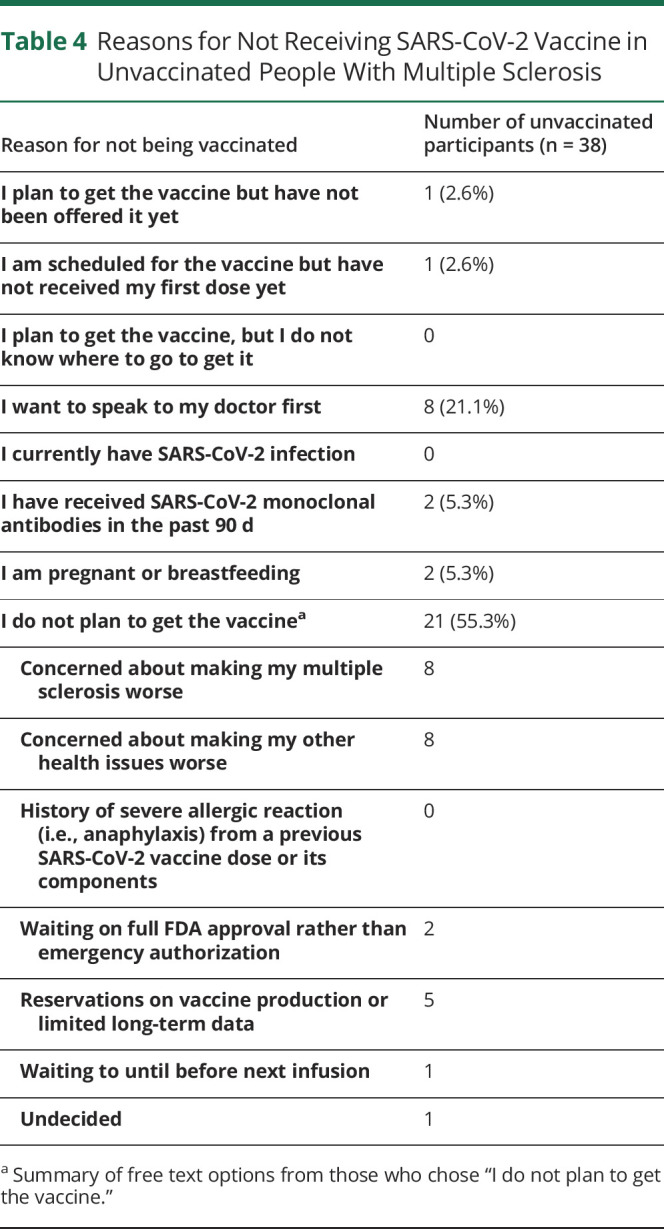
Unvaccinated PwMS
Thirty-eight participants reported they were not vaccinated. Descriptive responses are reported in Table 4. Fifty-five percent of these respondents indicated no plan to get the vaccine, citing concerns about making their multiple sclerosis or other medical conditions worse. Of note, 21% reported a desire to speak to their doctor before receiving the SARS-CoV-2 vaccine.
Table 4.
Reasons for Not Receiving SARS-CoV-2 Vaccine in Unvaccinated People With Multiple Sclerosis
Discussion
In this study of PwMS vaccinated and unvaccinated against SARS-CoV-2, postvaccination neurologic symptoms were rare and not associated with change in clinical care or vaccine subtype. Neither MS subtype nor DMT use was associated with postvaccination neurologic symptoms. Younger adults with MS were more likely to report SARS-CoV-2 infection than older adults. Although vaccine acceptance was high among respondents, concerns regarding vaccine-related worsening of MS or other health issues and further need for provider guidance on vaccination were overarching reasons for vaccine avoidance. These data obtained from a representative sample of PwMS address recent knowledge gaps regarding the differential effects of SARS-CoV-2 vaccination on MS outcomes and highlight the importance of healthcare provider input on this issue.
Respondents in our study who received the SARS-CoV-2 vaccine had similar incidence of typical systemic side effects compared with those included in the vaccine clinical trials,7-9 and similar to these trials, younger patients were more susceptible to these side effects. PwMS were not explicitly included in the SARS-CoV-2 vaccine trials, and our data suggest that the MS population does not fare worse than the general population regarding immediate postvaccination symptoms.
Despite evidence to the contrary and published guidelines,10 potential for neurologic deterioration after vaccination remains a pernicious concern among some PwMS. As seen in our sample, one-fifth of PwMS who had not yet received the vaccine reported concerns that the SARS-CoV-2 vaccination could make their MS worse. Our findings provide additional evidence to allay this concern. Among worsening chronic MS symptoms postvaccination, the most frequently reported symptom was fatigue. However, fatigue was also the most frequently reported side effect of the vaccine, independent of neurologic symptom recrudescence, in both our sample and the mRNA clinical trials. These collective findings suggest that occurrence of this symptom is not unique to PwMS.7,8
Of the 8 participants with MS who reported new neurologic symptoms after SARS-CoV-2 vaccination, none underwent diagnostic testing, acute treatment with steroids, or change in DMT per their medical record. These findings are consistent with literature reviews and previous studies, which did not show an increased risk of MS relapse after influenza vaccination.11 This highlights the importance of the distinction between MS relapse and MS symptom recrudescence (i.e., pseudorelapse), which is characterized by an unmasking of prior relapse symptoms as opposed to new inflammatory activity. Common triggers for recrudescence include metabolic derangements and infection.12 Conduction blocks in demyelinated axons are a postulated cause. Even in cases of axonal repair, the myelin remains thinner with fewer folds and shorter intermodal distances, making it more susceptible to dysfunction with potential triggers.13
Our findings build on this evidence to inform provider-patient discussions regarding the safety of SARS-CoV-2 vaccination in PwMS and provide additional data about risk of neurologic worsening based on MS subtype and DMT use. Although our findings also suggest no association between type of DMT use and postvaccination outcomes (neurologic worsening), given the low number of participants who reported new neurologic symptoms and low power to detect associations in this small group, the findings should be interpreted with caution.
On submission of this paper, 2 studies have been published on SARS-CoV-2 vaccination in PwMS.14,15 Data from Achiron et al. studied the Pfizer-BioNTech vaccination in PwMS at Sheba Medical Center in Israel. Although the investigators used objective demographic data from medical records and face-to-face follow-up neurologic examinations, this study did not stratify findings based on DMT usage, disease duration, MS subtype, nor did they define what constituted an acute MS relapse or recrudescence. In addition, there was a relatively short follow-up period between first (20 days) or second (38 days) vaccine dose and side effect reporting.14 Findings from Allen-Philbey et al. regarding the AstraZeneca vaccine in the United Kingdom included data from a small sample (n = 33) of PwMS. However, this study focused primarily on systemic side effects from vaccination rather than neurologic outcomes. In addition, this study did not include objective clinical data.15 Our study expands on these prior findings with comparison of all 3 Food and Drug Administration-approved or emergency-authorized approved vaccines in relation to MS, specifically focusing on DMT significance and MS clinical characteristics (disease duration and subtype) supported by objective data from medical chart review.
Reassuringly, no respondents who had SARS-CoV-2 infection required ICU admission or intubation. Somewhat surprisingly, older age was associated with reduced incidence of reported SARS-CoV-2 infection. This finding remained significant after adjusting for high-potency DMT use, which would be expected to be used more often for younger patients with relapsing MS and could increase susceptibility to infection. Although reasons for this observation are speculative, it is possible that younger patients in the sample had less access or later access to vaccination during the survey period or may have been less adherent to social distancing and public safety precautions in comparison with their older counterparts.16,17 Additional work is necessary to understand the relationship between age and SARS-CoV-2 vulnerability in PwMS. Of note, findings from a recently published systemic review18 suggest an association between increasing age and adverse outcomes in PwMS who experience SARS-CoV-2 infection.
Our study also adds new information to a growing body of the literature focused on vaccine willingness and hesitancy among PwMS during the SARS-CoV-2 pandemic.4,5,19,20 Our data suggest an underlying desire for provider guidance among PwMS who are considering SARS-CoV-2 vaccination. Recent data from Edhe et al. suggest that vaccine willingness in PwMS is in part influenced by information source and that advice is more likely to be accepted from healthcare providers as opposed to other sources.4,5 Providers should be prepared to counsel patients on vaccine timing and selection, incorporating available data regarding postvaccination relapse risk into these discussions. In particular, PwMS receiving infusion therapies will need guidance on optimal timing of vaccination.
Some study limitations should be acknowledged. Because data were based on self-report, responses could have been subject to recall bias or memory decay. That said, more than half of responses were verified by chart review. Referral bias is also a possibility because patients who view the SARS-CoV-2 vaccine favorably, or those who were more dissatisfied with the vaccine experience, have selectively volunteered to complete the survey. In addition, this study included follow-up less than 6 months after vaccination; however, we do not anticipate that a longer follow-up period would have substantively affected our findings because the most vaccine studies in MS focused on follow-up periods of approximately 90 days postvaccination. Finally, our study required the usage of internet and electronic device which could have excluded those of lower socioeconomic status and/or health literacy.
Our findings provide relevant new safety information regarding SARS-CoV-2 vaccination, including the novel mRNA vaccines, indicating minimal changes to short-term MS disease course and without change to MS clinical care or treatment. We also provide patient-centered data on MS-specific determinants of vaccine hesitancy, which will be essential for healthcare providers to use in counseling those PwMS who have deferred vaccination. Our findings promote and support the recommendation for vaccination of PwMS during the SARS-CoV-2 pandemic.21
Appendix. Authors

Footnotes
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
TAKE-HOME POINTS
→ Post-SARS-CoV-2 vaccination side effects experienced by people with MS are similar to those reported in the SARS-CoV-2 vaccine clinical trials, and most common among mRNA vaccine recipients and younger patients.
→ Development of new neurologic symptoms after SARS-CoV-2 vaccine administration are infrequent and not associated with a change in clinical care.
→ Concerns regarding worsening of MS or other health issues and a desire to seek more guidance from medical providers were primary reasons for vaccine deferral, highlighting the importance of patient-provider communication in decision-making regarding vaccine timing and selection.
References
- 1.Rollot F, Casey R, Leray E, et al. Cumulative effects of therapies on disability in relapsing multiple sclerosis. Mult Scler. 2021;27(11):1760-1770. [DOI] [PubMed] [Google Scholar]
- 2.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehde DM, Roberts MK, Herring TE, Alschuler KN. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the United States. Mult Scler Relat Disord. 2021;49:102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehde DM, Roberts MK, Humbert AT, Herring TE, Alschuler KN. COVID-19 vaccine hesitancy in adults with multiple sclerosis in the United States: a follow up survey during the initial vaccine rollout in 2021. Mult Scler Relat Disord. 2021;54:103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SAS Institute Inc. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. SAS Institute Inc; 2013. [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021;384(23):2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farez MF, Correale J, Armstrong MJ, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019;93(13):584-594. [DOI] [PubMed] [Google Scholar]
- 11.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol. 2011;258(7):1197-1206. [DOI] [PubMed] [Google Scholar]
- 12.Bevan C, Gelfand JM. Therapeutic management of severe relapses in multiple sclerosis. Curr Treat Options Neurol. 2015;17(4):345. [DOI] [PubMed] [Google Scholar]
- 13.Repovic P. Management of multiple sclerosis relapses. Continuum (Minneap Minn). 2019;25(3):655-669. [DOI] [PubMed] [Google Scholar]
- 14.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen-Philbey K, Stennett A, Begum T, et al. Experience with the COVID-19 AstraZeneca vaccination in people with multiple sclerosis. Mult Scler Relat Disord. 2021;52:103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nivette A, Ribeaud D, Murray A, et al. Non-compliance with COVID-19-related public health measures among young adults in Switzerland: insights from a longitudinal cohort study. Soc Sci Med. 2021;268:113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coroiu A, Moran C, Campbell T, Geller AC. Barriers and facilitators of adherence to social distancing recommendations during COVID-19 among a large international sample of adults. PLoS One. 2020;15(10):e0239795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrie RA, Kosowan L, Cutter GR, Fox R, Salter A. Uptake and attitudes about immunizations in people with multiple sclerosis. Neurol Clin Pract. 2021;11(4):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang XM, Hollen C, Yang Qet al. COVID-19 vaccination willingness among people with multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211017159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covid-19 Vaccine Guidance for People Living With MS. National Multiple Sclerosis Society. Accessed October 27, 2021. nationalmssociety.org/coronavirus-covid-19-information/multiple-sclerosis-and-coronavirus/covid-19-vaccine-guidance. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further anonymized data can be made available to qualified investigators on request to the corresponding author.



