Abstract
Current knowledge of the link between microbiota and hypertension is limited to the gut. Besides the gut, oral cavity and skin are other locations where sodium chloride (NaCl) is in direct contact with microbiota. Although oral nitrate-reducing bacteria generate nitric oxide, which leads to vasodilation and lowering of blood pressure (BP), the skin excretes sodium via sweat glands and is an important site for sodium and BP homeostasis. However, knowledge on the contributions of oral and skin microbiota to BP regulation, is limited. Therefore, the current study was conducted to compare the tripartite relationship between site, sex, and genetic effects on the composition of oral, skin, and gut microbiota impacting hypertension. Microbiota were profiled from the oral cavity, skin, and feces of both male and female hypertensive Dahl salt-sensitive (S) and congenic rats with genomic substitutions on rat chromosomes (RNO) 1, 5, 9, and 10, demonstrating disparate BP effects. Sex-specific differences in β-diversity were observed only in skin microbiota. The most abundant taxa of the oral and skin microbiota were Actinobacteria and Cyanobacteria, respectively. Oral Actinobacteria were inversely associated with BP. Although the abundance of oral Actinobacteria was upregulated by the BP locus on RNO10 in both sexes, depletion of skin Cyanobacteria decreased the protection from hypertension in the RNO5 female, but not male, congenic strain. In conclusion, to our knowledge this is the first study to identify specific microbiota in sites other than gut as contributors to BP regulation. Notably, both oral Actinobacteria and skin Cyanobacteria were beneficial for lowering BP.
Keywords: blood pressure, cardiovascular, dermal, microbiome, salt-sensitive
INTRODUCTION
Elevated blood pressure (BP) or hypertension, which affects more than 50% of the global population, is the top risk factor for cardiovascular diseases. Cardiovascular diseases are the leading cause for human mortality. Recent investigations indicate that interindividual variation in BP regulation is attributed not only to genetics and environmental factors but also to variations in the composition of commensal microbiota. Such commensal, which are presumably first acquired at birth, are not limited to the gut, but acquired on all exposed sites of the host, such as the oral cavity and skin. In the oral site, nitrate-reducing bacteria are reported to convert nitrate to nitric oxide, which leads to vasodilation and lower blood pressure (1, 2). Interestingly, it is reported that in humans, the use of mouthwash is shown to lower oral nitrate-reducing bacteria and increase BP (3). On the other hand, sodium nitrate supplementation reduces BP in both Wistar and Sprague–Dawley rats (4, 5). The skin is the largest organ in our body (6), the Na+:K+ ratio of the skin has been linked to blood pressure (7, 8). As evidenced by animal (9–11) and human (8) studies, a high-salt diet raises the sodium content of the skin. In patients with primary and secondary hypertension, sodium magnetic resonance imaging revealed a high sodium content in the skin (12, 13). It is also reported that skin-based blood pressure control via sodium and water balance and Na+ could accumulate in an osmotically inactive form, bypassing renal control. Most importantly, the Dahl salt-sensitive (S) rats, which are well-known to develop hypertension in response to a high-salt diet, showed a decreased capacity to store osmotically inactive Na+. This made them prone to fluid retention and resulted in hypertension (14). However, beyond these limited studies, oral and skin microbiota have not been investigated as much as gut microbiota for their potential effects on BP. Furthermore, sex as a factor is reported to impact gut microbiota composition and differences in arterial pressure (15), but such sex effects, if any, imparted by oral and skin microbiota remain unknown. Finally, because microbiota is responsive to host genomic factors, the composition of site- and sex-specific alterations in microbiota can vary depending on interindividual variation in host genetics. Previous research in our laboratory has shown that specific genomic regions of the S rat harbor quantitative trait loci for BP on chromosomes 1, 5, 9, and 10 (16–19). The loci on these specific chromosomes were demonstrated to significantly lower BP in males (16–19), but their impact on BP in females was not examined. Therefore, we first examined the BP effect of these loci in females followed by understanding the potential tripartite relationship between site, sex, and genetic effects on the composition of microbiota affecting hypertension. Using well-defined genetic rat models harboring loci for BP regulation, we tested the hypothesis that commensal in the oral cavity and on the skin are associated with salt-sensitive blood pressure.
MATERIALS AND METHODS
Animals
All animal research protocols were approved by the University of Toledo’s Institutional Animal Care and Use Committee. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
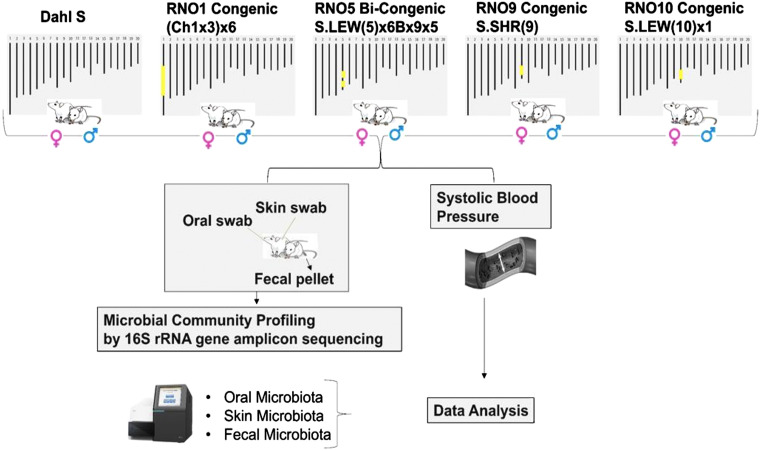
Inbred genetically hypertensive S rats were obtained from our colony. Four congenic strains were used in this study. These four congenic male and female strains are referred to by the rat chromosome (RNO) followed by a number as suffix depicting the RNO number on which the genomic substitution was made as follows: RNO1 Congenic, RNO5 Bi-congenic, RNO9 Congenic, and RNO10 Congenic (Fig. 1). The names of these congenic strains in prior publications are as follows: RNO1 Congenic: (Ch1x3)x6; RNO5 Bi-congenic: S.LEW(5)x6Bx9x5; RNO9 Congenic: S.SHR(9)Prot; and RNO10 Congenic: S.LEW(10)x1 (16–19). The congenic strain (Ch1x3)x6 has a congenic segment positioned at 165,129,767-165,129,939 (Rnor_6.0) on rat chromosome 1. S.LEW(5)x6Bx9x5 is a bi-congenic strain with congenic segments positioned at 116,151,550-120,258,073 (Rnor_6.0) and 122,679,504-133,413,079 (Rnor_6.0) on rat chromosome 5. S.SHR(9)Prot has a congenic segment positioned at 25,819,185-99,041,068 (Rnor_6.0) on rat chromosome 9. S.LEW(10)x1 has a congenic segment positioned at 66,219,766-64,767,118 (Rnor_6.0) on rat chromosome 10.
Figure 1.
Overall study design. This schematic represents the overview of the study described in materials and methods. RNO1 is a congenic strain (Ch1x3)x6 with a congenic region between 165,129,767 and 165,129,939. RNO5 is a bi-congenic strain of S.LEW(5)x6Bx9x5 with congenic regions at 116,151,550-120,258,073 and 122,679,504-133,413,079. RNO9 is a congenic strain S.SHR(9)Prot with a congenic region at 25,819,185-99,041,068. RNO10 is a congenic strain S.LEW(10)x1 with a congenic region at 66,219,766-64,767,118 (Rnor 6.0). RNO, rat chromosomes.
BP Measurements and Housing Conditions
All animals in the study were weaned at 4 wk onto a low-salt diet (0.3% NaCl, Harlan Teklad, TD 7034) and after 2 wk, they were fed with a high salt diet (2% NaCl, Harlan Teklad, TD 94217) for 24–28 days. Radio-telemetry transmitters were surgically implanted and animals were allowed to recover from surgery for 1 wk postimplantation before BP was monitored. Rats (both males and females) were 10–13 wk old when their BP was monitored. They were housed in the Department of Laboratory Animal Resource in the University of Toledo. The animal rooms were maintained at 70° ± 2°F. The humidity in the animal rooms were maintained at 50% ± 20%.
Collection of Microbiota
Microbiota from oral cavity, skin, and feces were collected from a cohort of 80 rats, which were either control S rats or one of the four congenic male and female strains mentioned earlier (7 or 8 rats/group/sex). Oral swabs, skin swabs, and fecal pellets were collected from the same animal. Oral and fecal samples were collected while weaning at 4 wk of age. This time-point was chosen to have the rats stabilize their acquisition of microbiota during the lactation phase. Oral swabs were collected by gently holding the rat and rotating a sterile cotton swab against the posterior aspect of the tongue and roof of the mouth. Skin swab samples were collected from 4- to 6-days-old rats for avoiding interference from fur and for ease of collection of original microbiota on their dermis before they developed their coats/fur. This was done by gently rubbing a sterile cotton swab across the skin of the rat’s back. The cotton end of the swab was removed immediately and transferred to an empty tube and capped. Two oral swabs, two skin swabs, and one fecal pellet were collected for each animal. All samples were stored at −80°C for further processing.
Isolation of DNA from Microbiota
Microbial DNA from oral and skin swabs were extracted by using QIAamp DNA Mini Isolation Kit (Qiagen, No. 51304) following the manufacturer’s protocol. Fecal DNA was extracted from one fecal pellet (∼0.2 g) using QIAamp Power Fecal DNA kit (Qiagen, No. 12830) followed by the manufacturer’s protocol. Extracted DNA was eluted in 50 µL of low TE buffer (0.1 mM EDTA, Tris-HCl buffer, 10 mM, pH 8.5) and stored at −20°C. Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA) was then used to quantify total double-stranded DNA to ensure detectable concentrations of DNA in all samples. Nanodrop 2000 was used to quantify DNA concentrations in all samples. Prior to PCR library preparation, the samples were diluted to 5 ng/µL in low TE buffer.
rRNA Gene Sequencing and Analysis of Microbiota Composition
PCR library preparation, clean-up, normalization, and pooling.
We followed Illumina User Guide: 16S Metagenomic Sequencing Library Preparation-Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System (Part. No. 15044223 Rev. B). The 16S rRNA gene targeting V3-V4 region was amplified by PCR using Illumina sequencing primers: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT. For index PCR, Nextera XT index kit (FC-131–1002) were used to attach dual indices.
Each 25 µL reaction mixture contained 2.5 µL of 10X reaction butter (Invitrogen, Thermo Fisher Scientific, Waltham, MA), 0.5 µL of 10 mM dNTPs, 0.75 µL of 50 mM MgCl2, 0.1 µL of 5 U/µL of HotTaq polymerase (Invitrogen), 1 µL of 5 µM each primer and 2.5 µL of 5 ng/µL DNA for the first PCR for target or purified PCR product for the second PCR for adding index and adapter, water was added up to meet the total volume of 25 µL.
The thermocycling was performed in a Bio-Rad T100TM thermal cycler (Hercules, CA) and the cycling conditions were as follows: initial denaturation at 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min for target PCR. Index PCR was performed with eight cycles followed by protocol. Each PCR amplicon sample was purified in two rounds using AMPure XP beads (Beckman Coulter Inc. Brea, CA). Each concentration of purified index PCR products was measured using the Qubit dsDNA HS Assay kit with Qubit 3.0 fluorometer (Life Technologies, Carlsbad, CA). The 4 nM each amplicon was pooled equally. Pooled library was checked for expected product size on a 2100 Bioanalyzer (Agilent, Santa Clara, CA) before sequencing.
Library denaturing and MiSeq sample loading.
Following Illumina User Guide Illumina MiSeq System, the abovementioned amplicon was loaded on the Illumina MiSeq V3 flow cell kit for sequencing over 2 × 300 cycles.
Quality filtering, ASV picking, and data analysis.
Chimeric sequences were identified and filtered using Quantitative Insights Into Microbial Ecology (QIIME II) software package (v. 2021.11) (20). The amplicon sequence variants (ASVs) were subsequently picked using QIIME II, and taxonomy assignment was performed using Silva (21) as the reference database.
Statistical Analysis
Observed features were used as the algorithm to calculate the α-diversity and unpaired t test between the last two points was used to calculate the P value of α-diversity. The ANOSIM (ANalysis Of SIMilarities) statistical method was used to calculate the P value of the β-diversity. Two-way ANOVA followed by Tukey’s post hoc tests was used for microbiota comparison between S rats and congenic strains in males and females.
RESULTS
Skin Microbiota Was Significantly Different between Sexes, but Not Oral or Gut Microbiota
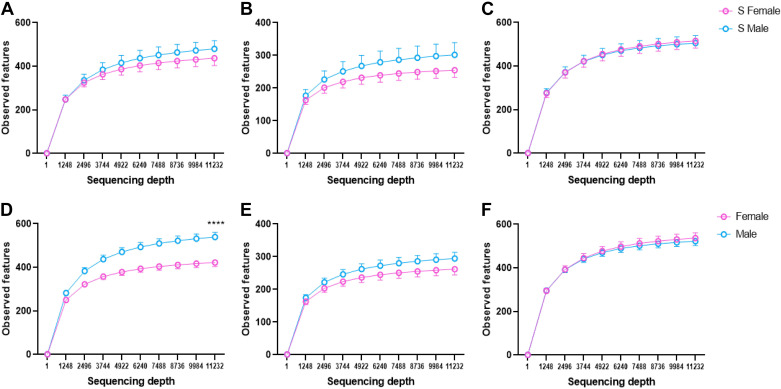
Comparisons of α-diversity revealed that no significant differences of skin, oral, or fecal microbiota were noted between S males and S females (Fig. 2, A–C). However, when we combined all S and congenic rats, skin microbiota of female S and congenic rats had lower α-diversity compared with males, whereas oral and fecal were not significantly different between the sexes (Fig. 2, D–F).
Figure 2.
α-Diversity (observed features) of S rats and four congenic strains (RNO1, RNO5, RNO9, and RNO10) in both sexes. A: skin microbiota from S rats (female, n = 23; male, n = 22). B: oral microbiota from S rats (female, n = 24; male, n = 23). C: fecal microbiota from S rats (female, n = 24; male, n = 24). D: combined skin microbiota from rats (female, n = 23 S+32 congenic; male, n = 22 S+32 congenic). E: combined oral microbiota from rats (female, n = 24 S+31 congenic; male, n = 23 S+32 congenic). F: combined fecal microbiota from rats (female, n = 24 S+32 congenic; male, n = 24 S+32 congenic). P value was calculated at the last data points using unpaired t test, ****P < 0.0001. Line colors: pink, female; blue, male. RNO, rat chromosomes; S, Dahl salt-sensitive.
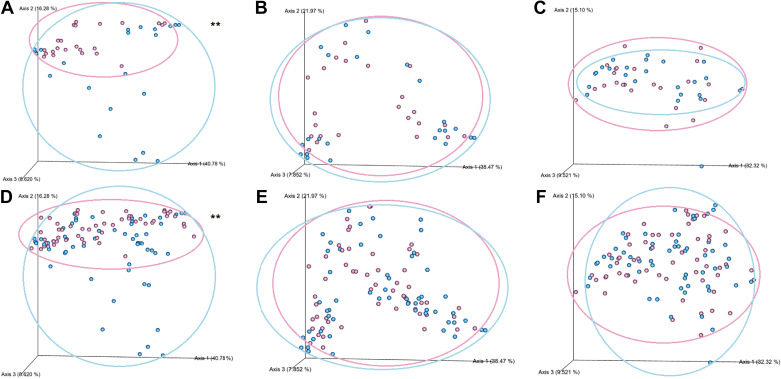
The β-diversity comparisons are shown by the Principal Coordinates Analysis (PCoA) plots in Fig. 3.The weighted β-diversity of skin microbiota were in distinctly separated clusters between the male and female S rats. Such sex-differences were not observed either in oral or fecal microbiota (Fig. 3, A–C). Similarly, when we combined all S and congenic rats, the weighted β-diversity of skin microbiota were segregating in sex-specific clusters, whereas oral and fecal microbiota were not (Fig. 3, D–F). These data indicated that, compared with the oral and gut microbiota, which were not sex-specific, the skin microbiota was prominently different between the sexes.
Figure 3.
Weighted β-diversity of S rats and four congenic strains (RNO1, RNO5, RNO9, and RNO10) in both sexes. A: skin microbiota from S rats (female, n = 23; male, n = 22). B: oral microbiota from S rats (female, n = 24; male, n = 23). C: fecal microbiota from S rats (female, n = 24; male, n = 24). D: combined skin microbiota from rats (female, n = 23 S+32 congenic; male, n = 22 S+32 congenic). E: combined oral microbiota from rats (female, n = 24 S+31 congenic; male, n = 23 S+32 congenic). F: combined fecal microbiota from rats (female, n = 24 S+32 congenic; male, n = 24 S+32 congenic). ANOSIM P value was calculated using the QIIME II script, **P < 0.01. QIIME II, Quantitative Insights Into Microbial Ecology II; RNO, rat chromosomes; S, Dahl salt-sensitive.
Site-Specific Enrichment of Different Taxa in Oral, Skin, and Gut Microbiota
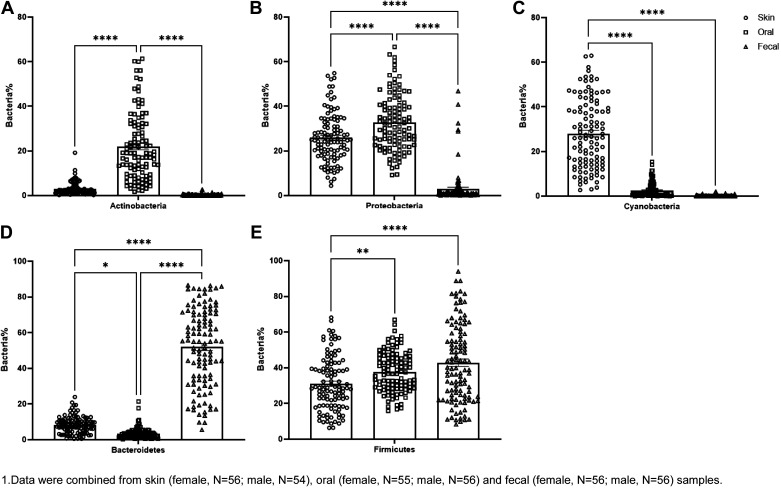
Next, we examined the enrichment of microbiota in the various sites. In all the strains tested, bacteria enriched differently in oral, skin, and fecal. In oral, Actinobacteria and Proteobacteria were the most abundant phyla (Fig. 4, A and B). On the skin, the phylum Cyanobacteria was the most abundant taxon (Fig. 4C). Although in the gut, as we already know, Bacteroidetes and Firmicutes were the most abundant phyla (Fig. 4, D and E).
Figure 4.
The main five bacteria disperse in oral, skin, and fecal: data were combined from skin (female, n = 56; male, n = 54), oral (female, n = 55; male, n = 56), and fecal (female, n = 56; male, n = 56) samples from S and congenic rats. The enriched Actinobacteria, Proteobacteria in oral (A and B), Cyanobacteria on skin (C), and Bacteroidetes and Firmicutes in fecal (D and E) samples in both sexes of S and congenic rats RNO1, RNO5, RNO9, and RNO10. *P < 0.05, **P < 0.01, ****P < 0.0001 determined by one-way ANOVA followed by Tukey’s post hoc tests. RNO, rat chromosomes; S, Dahl salt-sensitive.
Site-Specific Enrichment of Microbiota in Rats with Genetic Disparities
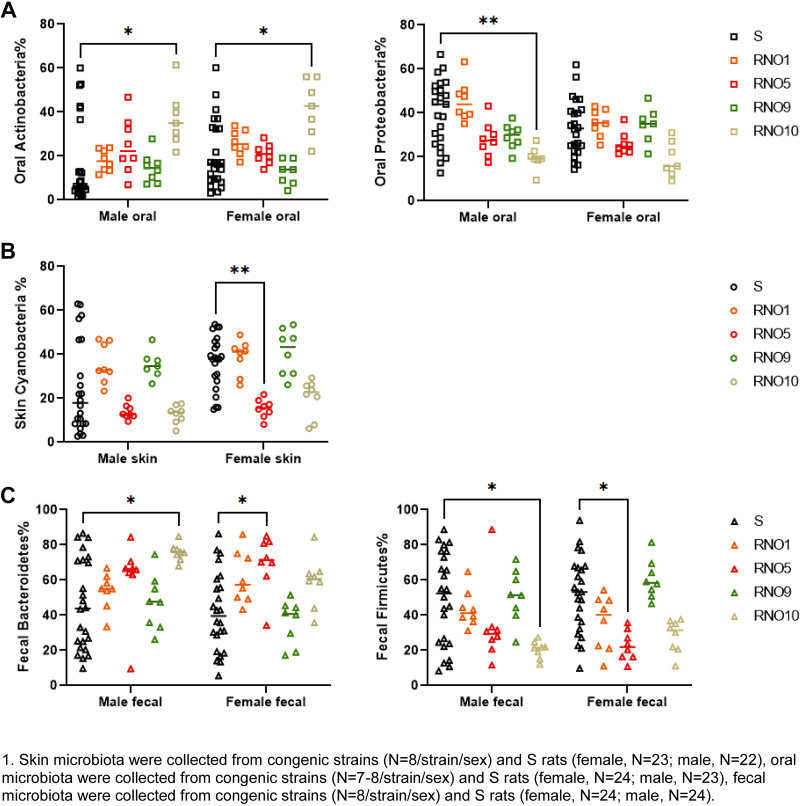
Next, we asked if substitution of select genomic sites of the hypertensive S rats had any influence on the abundances of the abovementioned phyla. Figure 5 shows the comparisons of enrichment in various phyla in both sexes of each of the congenic strains with substitutions on RNO1, RNO5, RNO9, and RNO10. As shown in Fig. 5A, among the oral microbiota, Actinobacteria were strain-specifically higher in the RNO10 congenic strain, whereas Proteobacteria were sex-specifically lower in the RNO10 males. Compared with S, Cyanobacteria were sex-specifically lower in the RNO5 female congenics (Fig. 5B). In contrast to these data from oral and skin microbiota, fecal Bacteroidetes was higher and Firmicutes was lower in both RNO5 females and RNO10 males (Fig. 5C).
Figure 5.
The comparison of oral Actinobacteria and Proteobacteria (A), skin Cyanobacteria (B), and fecal Bacteroidetes and Firmicutes (C) in four congenic strains with S rats in both sexes: skin microbiota were collected from congenic strains (n = 8/strain/sex) and S rats (female, n = 23; male, n = 22); oral microbiota were collected from congenic strains (n = 7 or 8/strain/sex) and S rats (female, n = 24; male, n = 23); and fecal microbiota were collected from congenic strains (n = 8/strain/sex) and S rats (female, n = 24; male, n = 24). *P < 0.05, **P < 0.01 determined by two-way ANOVA followed by Tukey’s post hoc tests. S, Dahl salt-sensitive.
Association of Oral Actinobacteria and Skin Cyanobacteria with Blood Pressure Regulation
Next, we sought to address the association between oral and skin microbiota and BP regulation. To do so, we required BP data from both sexes, but such data were previously collected only in males. Therefore, we first collected blood pressure data from female congenic strains. As shown in Table 1 and Supplemental Fig. S1 (https://doi.org/10.6084/m9.figshare.19606144), similar to the male rats, three of the female congenic strains, RNO1, RNO9, and RNO10 also demonstrated significant BP lowering effects. However, in contrast to the male RNO5 congenic strain, which demonstrated a BP lowering effect, there was no BP lowering effect observed in the female RNO5 congenic strain compared with S females (Table 1 and Supplemental Fig. S1).
Table 1.
The congenic strains and their blood pressure comparison in both males and females
| Location of the Congenic Segment on the Rat Chromosomein Megabases | Congenic Strain | BP Effect in Females (Congenic minus S, mmHg) | BP Effect in Males (Congenic minus S, mmHg) |
|---|---|---|---|
| 165,129,767-165,129,939 | RNO1 | −10* | −31*a |
| 116,151,550-120,258,073 & 122,679,504-133,413,079 | RNO5 | −7ns | −39*b |
| 25,819,185-99,041,068 | RNO9 | −21* | −27*c |
| 66,219,766-64,767,118 | RNO10 | −37* | −43*d |
*P < 0.05 as determined by unpaired t test; ns, not significant. aSaad et al. Physiological Genomics 2001 (16). bPillai et al. Physiological Genomics 2013 (17). cToland et al. Journal of Hypertension 2008 (18). dGarrett et al. Hypertension 2001 (19). BP, blood pressure; RNO, rat chromosomes; S, Dahl salt-sensitive.
The BP data were in alignment with specific phyla of microbiota. The abundance of oral Actinobacteria was specifically increased in the RNO10 congenic of males and females, both of which had BP lowering effects (Fig. 5A, Supplemental Table S1, and Supplemental Fig. S1). These data indicate that the BP locus on RNO10 regulates the abundance of oral Actinobacteria independent of sex. In each of the strains, which demonstrated a BP lowering effect compared with the S rat, the composition of skin Cyanobacteria were similar. However, a depletion of skin Cyanobacteria was observed exclusively in the female RNO5 congenic strain compared with the female S rat, which failed to elicit BP lowering effect (Fig. 5B, Supplemental Table S1, and Supplemental Fig. S1). These data indicated that depletion of skin Cyanobacteria decreased the protection from hypertension in the RNO5 female congenic strain. Overall, these observations suggest that oral Actinobacteria and skin Cyanobacteria can be prioritized as beneficial microbiota, which were associated with lowering of BP.
In contrast to the data obtained from oral and skin microbiota, fecal Bacteroidetes and Firmicutes were inconsistent for their associations with BP in the S and congenic strains. Bacteroidetes were higher both in the RNO5 female congenic strain, which did not demonstrate a BP lowering effect and in the RNO10 male congenic strain, which did demonstrate a BP lowering effect. Similarly, fecal Firmicutes were lower both in the RNO5 female congenic strain, which did not have a BP lowering effect as well as in the RNO10 male congenic strain, which demonstrated a lower BP compared with S.
DISCUSSION
To our knowledge, our work published in 2015 was the first to report an association between gut microbiota and hypertension in the S rat, which is a genetic model of hypertension selectively bred to develop salt-sensitive hypertension. This report was affirmed with multiple publications from other laboratories using rats (22–24) and mice (25–27) as well as in humans (28–32). However, microbiota reside in locations on the body other than the gut, including the oral cavity and on the skin, both of which are sites potentially participating in sodium homeostasis. This is because of reports that oral microbiota generate the potent vasodilator nitric oxide and that the skin Na+ K+ ratio is associated with BP (33). Both in animal models (9–11) and humans (8), consumption of a high-salt diet raises skin sodium content. Also, patients with primary and secondary hypertension are reported to present with a higher skin sodium content (12, 13). Furthermore, the compositions of microbiota in the oral cavity and on the skin could vary depending on host sex and genetics, however, oral and skin microbiota have not been investigated as much as gut microbiota for their potential effects on BP regulation. Therefore, we examined both oral and skin microbiota composition in a group of hypertensive strains with a common genetic lineage belonging to the S rat. The results of the current study are the first to report the following: 1) there is a sex difference on the diversity of microbiota on the skin compared with gut and oral microbiota; 2) there are site-specific variations in relative abundances of different phyla located on the skin, in the oral cavity, and gut; 3) oral Actinobacteria are beneficial, and the abundance of oral Actinobacteria is regulated by the BP locus on RNO 10; and 4) skin Cyanobacteria were associated with BP as site- and sex-specific microbiota and could therefore represent a new phylum of beneficial microbiota for curbing hypertension.
Among the three factors studied, there were clear differences between the compositions of microbiota on the skin versus the oral cavity and gut. Cyanobacteria in the skin, Actinobacteria and Proteobacteria in the oral cavity, and Bacteroidetes and Firmicutes in fecal samples were site-specific taxa in all animals regardless of strain and sex (Fig. 4). Second, genetic effects were explored in our study using four different males and females congenic strains built on the genomic background of the S rat. Although this is a limited study testing only four out of the >400 known rat BP quantitative trait loci (https://rgd.mcw.edu/rgdweb/elasticResults.html?term=blood+pressure&chr=ALL&start=&stop=&species=Rat&category=QTL&objectSearch=true), the data readily identified at least one, RNO10, that harbor genomic elements regulating the abundances of oral Actinobacteria (Fig. 5A and Supplemental Table S1). In the case of skin microbiota, lower Cyanobacteria was exclusively noted in the RNO5 female congenic strain, which failed to maintain a BP lowering effect (Fig. 5B and Supplemental Table S1). This suggests that Cyanobacteria confer protection from the development of hypertension. The mechanisms by which an increased abundance of skin Cyanobacteria protects from hypertension needs further studies.
In comparison with Cyanobacteria, which, to our knowledge, are not mentioned in the literature for any links to BP regulation, oral Actinobacteria could be beneficial to lower BP by some of the known mechanisms. For example, the nitrate-reducing bacteria in the mouth convert nitrate to nitric oxide, which causes vasodilation and lowers blood pressure even further (1, 2). The use of mouthwash in humans reduces oral nitrate-reducing bacteria while increasing blood pressure (3). Sodium nitrate supplementation, on the other hand, lowers blood pressure in rats (4, 5). It is reported that Actinobacteria were nitrate-reducing bacteria (34). What is new and requires further study is that an unknown genetic element on RNO10 regulates the abundance of oral Actinobacteria.
Cyanobacteria are an evolutionarily important group of aerobic microbiota (35), which have been studied as environmental microbiota rather than as commensal microbiota. Interestingly, Cyanobacteria are salt tolerant (36) and possess sodium-dependent chloride transporters (37, 38), whereby they are an interesting taxa to be prioritized for further study in the context of BP regulation by the skin. Titze and colleagues (33) propose that in salt-sensitive hypertension, the skin interstitium sequesters excess Na+ and Cl−. They propose that local, yet unknown extrarenal regulatory mechanisms may function for electrolyte clearance of interstitial fluid under the skin to maintain extracellular electrolyte clearance to maintain BP homeostasis. Also, a recent human study indicates that skin Na+: K+ ratio correlated with blood pressure in a sex-biased manner with higher skin Na+: K+ ratio associated with high BP in men but not in women (8, 39), but the reasons for these associations are unknown. None of these studies considered skin microbiota as a potential factor. In addition, Cyanobacterial acyl-acyl carrier protein synthetases are predicted to be involved in fatty acid activation and production of acetate and butyrate (40–43). Both acetate and butyrate are major metabolic products of bacteria fermentation in the intestine, which converge on the host citric acid cycle and contribute to blood pressure regulation (44). Based on the recently recognized skin-gut axis, all these short-chain fatty acids that are derived from the gut, could interact with skin receptors and modify the composition of dermal commensal microbiota (45). Further research is however needed to reveal if skin Cyanobacteria-related short-chain fatty acid metabolism contributes to blood pressure regulation or not.
Perspectives
To our knowledge, this study is the first to report oral and skin microbiota as potential modulators associated with BP. Specifically, we compared skin, oral, and gut microbiota for the regulation of blood pressure in S rats and discovered that oral Actinobacteria and skin Cyanobacteria are specific groups of beneficial commensal to lower BP. Compositions of Actinobacteria were strain-specific, with higher abundance in RNO10 congenic strain while Cyanobacteria were sex-specific, with lower abundance noted in RNO5 females. Based on the known properties of Actinobacteria as nitrate-reducing bacteria, and Cyanobacteria as salt-tolerant bacteria with genes for sodium-dependent chloride transporters as well as short-chain fatty acid regulators, both Actinobacteria and Cyanobacteria are beneficial commensal to be prioritized for mechanistic studies for alleviating salt-sensitive hypertension.
DATA AVAILABILITY
The data that support the findings of this study will be made available upon reasonable request from the corresponding author.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.19606144.
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.19708993.
GRANTS
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute Grant R01HL1430820 (to B. Joe).
DISCLOSURES
Xi Cheng is an editor of Physiological Genomics and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
B.J. conceived and designed research; X.M., B.M., J.-Y.Y., and N.C. performed experiments; X.M., B.M., X.C., J.-Y.Y., and B.J. analyzed data; X.M., B.M., X.C., T.Y., and B.J. interpreted results of experiments; X.M., B.M., and X.C. prepared figures; B.J. drafted manuscript; X.M., B.M., X.C., T.Y., and B.J. edited and revised manuscript; X.M., B.M., X.C., J.-Y.Y., T.Y., N.C., and B.J. approved final version of manuscript.
REFERENCES
- 1.Hezel M, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis 21: 7–16, 2015. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 2.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93–100, 2013. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, Hodgson JM. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens 28: 572–575, 2015. doi: 10.1093/ajh/hpu192. [DOI] [PubMed] [Google Scholar]
- 4.Hyde ER, Luk B, Cron S, Kusic L, McCue T, Bauch T, Kaplan H, Tribble G, Petrosino JF, Bryan NS. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic Biol Med 77: 249–257, 2014. doi: 10.1016/j.freeradbiomed.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Petersson J, Carlström M, Schreiber O, Phillipson M, Christoffersson G, Jägare A, Roos S, Jansson EA, Persson AEG, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med 46: 1068–1075, 2009. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Yanez DA, Lacher RK, Vidyarthi A, Colegio OR. The role of macrophages in skin homeostasis. Pflugers Arch 469: 455–463, 2017. doi: 10.1007/s00424-017-1953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvarajah V, Connolly K, McEniery C, Wilkinson I. Skin sodium and hypertension: a paradigm shift?. Curr Hypertens Rep 20: 94, 2018. doi: 10.1007/s11906-018-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SF, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension 70: 930–937, 2017. doi: 10.1161/HYPERTENSIONAHA.117.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 285: F1108–F1117, 2003. doi: 10.1152/ajprenal.00200.2003. [DOI] [PubMed] [Google Scholar]
- 10.Ivanova L, Archibasova V, Shterental' ISh. Sodium-depositing function of the skin in white rats. Fiziol Zh SSSR Im IM Sechenova 64: 358–363, 1978. [PubMed] [Google Scholar]
- 11.Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol 287: H203–H208, 2004. [Erratum in Am J Physiol Heart Circ Physiol 287: H1433, 2004]. doi: 10.1152/ajpheart.01237.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Müller DN, Schmieder RE, Cavallaro A, Eckardt K-U, Uder M, Luft FC, Titze J. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 61: 635–640, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00566. [DOI] [PubMed] [Google Scholar]
- 13.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schöfl C, Renz W, Santoro D, Niendorf T, Müller DN, Neininger M, Cavallaro A, Eckardt K-U, Schmieder RE, Luft FC, Uder M, Titze J. 23Na magnetic resonance imaging of tissue sodium. Hypertension 59: 167–172, 2012. doi: 10.1161/HYPERTENSIONAHA.111.183517. [DOI] [PubMed] [Google Scholar]
- 14.Titze J, Krause H, Hecht H, Dietsch P, Rittweger J, Lang R, Kirsch KA, Hilgers KF. Reduced osmotically inactive Na storage capacity and hypertension in the Dahl model. Am J Physiol Renal Physiol 283: F134–F141, 2002. doi: 10.1152/ajprenal.00323.2001. [DOI] [PubMed] [Google Scholar]
- 15.Beale AL, Kaye DM, Marques FZ. The role of the gut microbiome in sex differences in arterial pressure. Biol Sex Differ 10: 22, 2019. doi: 10.1186/s13293-019-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saad Y, Garrett MR, Rapp JP. Multiple blood pressure QTL on rat chromosome 1 defined by Dahl rat congenic strains. Physiol Genomics 4: 201–214, 2001. doi: 10.1152/physiolgenomics.2001.4.3.201. [DOI] [PubMed] [Google Scholar]
- 17.Pillai R, Waghulde H, Nie Y, Gopalakrishnan K, Kumarasamy S, Farms P, Garrett MR, Atanur SS, Maratou K, Aitman TJ, Joe B. Isolation and high-throughput sequencing of two closely linked epistatic hypertension susceptibility loci with a panel of bicongenic strains. Physiol Genomics 45: 729–736, 2013. doi: 10.1152/physiolgenomics.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toland EJ, Yerga-Woolwine S, Farms P, Cicila GT, Saad Y, Joe B. Blood pressure and proteinuria effects of multiple quantitative trait loci on rat chromosome 9 that differentiate the spontaneously hypertensive rat from the Dahl salt-sensitive rat. J Hypertens 26: 2134–2141, 2008. doi: 10.1097/HJH.0b013e32830ef95c. [DOI] [PubMed] [Google Scholar]
- 19.Garrett MR, Zhang X, Dukhanina OI, Deng AY, Rapp JP. Two linked blood pressure quantitative trait loci on chromosome 10 defined by Dahl rat congenic strains. Hypertension 38: 779–785, 2001. doi: 10.1161/hy1001.091503. [DOI] [PubMed] [Google Scholar]
- 20.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA , et al. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. Peer J Prepr 6: e27295v2, 2018. doi: 10.7287/peerj.preprints.27295v2. [DOI] [Google Scholar]
- 21.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic acids Res 41: D590–D596, 2013. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coutinho E, Prescott M, Marshall C, Hessler S, Herbison A, Campbell R. SUN-LB083 Functional Role of Arcuate Nucleus NPY/AgRP Neurons in the GnRH Circuit Regulating LH Secretion. J Endocr Soc 3: SUN-LB083, 2019. doi: 10.1210/js.2019-SUN-LB083. [DOI] [Google Scholar]
- 24.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin II–induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5: e003698, 2016. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T-h, Tao W-C, Liang Q-e, Tu W-Q, Xiao Y, Chen L-G. Gut microbiota-related evidence provides new insights into the association between activating transcription factor 4 and development of salt-induced hypertension in mice. Front Cell Dev Biol 8: 585995, 2020. doi: 10.3389/fcell.2020.585995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan J, Moeller R, Chakraborty S, Vijay-Kumar M, Joe B. Pressure from the bugs within: gut microbiota and human arterial hypertension. Hypertension 73: 977–979, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14, 2017. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, Salido RA, Sanders K, Brennan C, Humphrey GC, Sanders JG, Vartiainen E, Laatikainen T, Jousilahti P, Salomaa V, Knight R, Lahti L, Niiranen TJ. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc 9: e016641, 2020. doi: 10.1161/JAHA.120.016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR, Shikany JM, Lloyd-Jones DM, Launer LJ, Fodor AA, Meyer KA. Gut microbiota composition and blood pressure: the CARDIA study. Hypertension 73: 998–1006, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmu J, Lahti L, Niiranen T. Targeting gut microbiota to treat hypertension: a systematic review. Int J Environ Res Public Health 18: 1248, 2021. doi: 10.3390/ijerph18031248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiig H, Luft F, Titze JM. The interstitium conducts extrarenal storage of sodium and represents a third compartment essential for extracellular volume and blood pressure homeostasis. Acta Physiol (Oxf) 222: e13006, 2018. doi: 10.1111/apha.13006. [DOI] [PubMed] [Google Scholar]
- 34.Kanaparthi D, Pommerenke B, Casper P, Dumont MG. Chemolithotrophic nitrate-dependent Fe (II)-oxidizing nature of actinobacterial subdivision lineage TM3. ISME J 7: 1582–1594, 2013. doi: 10.1038/ismej.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soo RM, Hemp J, Parks DH, Fischer WW, Hugenholtz P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science 355: 1436–1440, 2017. doi: 10.1126/science.aal3794. [DOI] [PubMed] [Google Scholar]
- 36.Apte SK, Reddy BR, Thomas J. Relationship between sodium influx and salt tolerance of nitrogen-fixing cyanobacteria. Appl Environ Microbiol 53: 1934–1939, 1987. doi: 10.1128/aem.53.8.1934-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apte SK, Thomas J. Sodium transport in filamentous nitrogen fixing cyanobacteria. J Biosci 5: 225–233, 1983. doi: 10.1007/BF02716605. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie R. The cyanobacterium Synechococcus R‐2 (Anacystis nidulans, S. leopoliensis) PCC 7942 has a sodium‐dependent chloride transporter. Plant Cell Environ 15: 163–177, 1992. doi: 10.1111/j.1365-3040.1992.tb01470.x. [DOI] [Google Scholar]
- 39.Bayorh M, Bayorh MA, Socci R, Eatman D, Wang M, Thierry-Palmer M. The role of gender in salt-induced hypertension. Clin Exp Hypertens 23: 241–255, 2001. doi: 10.1081/ceh-100102663. [DOI] [PubMed] [Google Scholar]
- 40.Kaczmarzyk D, Fulda M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol 152: 1598–1610, 2010. doi: 10.1104/pp.109.148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi Y-N, Lee JW, Kim JW, Park JM. Acetyl-CoA-derived biofuel and biochemical production in cyanobacteria: a mini review. J Appl Phycol 32: 1643–1653, 2020. doi: 10.1007/s10811-020-02128-x. [DOI] [Google Scholar]
- 42.Heyer H, Krumbein WE. Excretion of fermentation products in dark and anaerobically incubated cyanobacteria. Arch Microbiol 155: 284–287, 1991. doi: 10.1007/BF00252213. [DOI] [Google Scholar]
- 43.Lai MJ, Lan EI. Photoautotrophic synthesis of butyrate by metabolically engineered cyanobacteria. Biotechnol Bioeng 116: 893–903, 2019. doi: 10.1002/bit.26903. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty S, Mandal J, Yang T, Cheng X, Yeo J-Y, McCarthy CG, Wenceslau CF, Koch LG, Hill JW, Vijay-Kumar M, Joe B. Metabolites and hypertension: insights into hypertension as a metabolic disorder: 2019 Harriet Dustan Award. Hypertension 75: 1386–1396, 2020. doi: 10.1161/HYPERTENSIONAHA.120.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pessemier BD, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 9: 353, 2021. doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.19606144.
Supplemental Table S1: https://doi.org/10.6084/m9.figshare.19708993.
Data Availability Statement
The data that support the findings of this study will be made available upon reasonable request from the corresponding author.