Abstract
Hypertension (HTN) is a complex disease influenced by heritable genetic elements and environmental interactions. Dietary salt is among the most influential modifiable factors contributing to increased blood pressure (BP). It is well established that men and women develop BP impairment in different patterns and a recent emphasis has been placed on identifying mechanisms leading to the differences observed between the sexes in HTN development. The current work reported here builds on an extensive genetic mapping experiment that sought to identify genetic determinants of salt-sensitive (SS) HTN using the Dahl SS rat. BTG antiproliferation factor 2 (Btg2) was previously identified by our group as a candidate gene contributing to SS HTN in female rats. In the current study, Btg2 was mutated using transcription activator-like effector nuclease (TALEN)-targeted gene disruption on the SSBN congenic rat background. The Btg2 mutated rats exhibited impaired BP and proteinuria responses to a high-salt diet compared with wild-type rats. Differences in body weight, mutant pup viability, skeletal morphology, and adult nephron density suggest a potential role for Btg2 in developmental signaling pathways. Subsequent cell cycle gene expression assessment provides several additional signaling pathways that Btg2 may function through during salt handling in the kidney. The expression analysis also identified several potential upstream targets that can be explored to further isolate therapeutic approaches for SS HTN.
Keywords: hypertension, renal, salt sensitivity, TALEN
INTRODUCTION
Hypertension (HTN) remains a major health concern in the United States and worldwide, leading to significant and often severe damage to vital organs. Risk in developing HTN is attributed to both genetic and environmental contributions (1, 2). Among modifiable traits, high level of salt consumption has been well established as a detrimental factor in blood pressure (BP) regulation (3). Adding to HTN’s complexity is the recognition that males and females develop hypertension at different rates (4, 5). In response to this, a recent emphasis has been placed on designing studies to explore the origins of sex differences in the development of HTN and renal dysfunction (6, 7). Recent results have pointed to the contribution of novel genetic loci under sex-specific regulation (8–10).
Chromosomal substitution and congenic mapping have proven to be powerful strategies for complex disease-associated gene and genetic element discovery (11). Previous work by our group had utilized the Dahl salt-sensitive rat (SS/JrHsdMcwi) and the Brown Norway rat (BN/NHsdMcwi) to develop congenic strains used to map multiple genetic loci that contributed to female-specific BP and renal phenotypes on rat chromosome 13. Two overlapping congenic strains, line 9E [SS.BN(D13Rat25-D13rs106935835)Mcwi; RGD: 12802363] and line 9 F [SS.BN(D13Rat25-D13rs198199323)Mcwi; RGD: 12802364], were used to identify BTG antiproliferation factor 2 (Btg2) as a candidate gene (8, 9). The overlapping congenic strains contained a common BN region (RNO13: 50,260,357-50,918,009; Line9bp3) which established a female-specific attenuation of salt-induced HTN. The two strains differed in the 23 kb flanking region where Btg2 resides (Line9bp4); line 9E contains BN sequence, whereas line 9 F contains SS sequence. The addition of the BN flanking region in line 9E impaired the female-specific protective effect observed in line 9 F and resembled parental SS renal and BP responses (9).
Although there are no sequence variants predicted to be damaging between SS and BN in the Btg2 gene, we previously found significantly higher female-specific Btg2 mRNA expression in the kidney cortex within the hypertensive susceptible line 9E strain before a high-salt challenge. These results indicated that altered Btg2 regulation leading to higher expression in the kidney cortex may predispose the female line 9E animals to increased BP in response to high-salt diet (9).
Btg2 is a transcriptional coregulator and member of the antiproliferative BTG family. It has known functions in cell-cycle regulation (12, 13), mRNA stability (14), development, differentiation (15, 16), and interaction with estrogen signaling (17, 18). Several studies have implicated its role in the cardiovascular system (9, 19, 20), but its role in BP regulation and renal injury has not been established.
In the present study, we hypothesized that gene disruption of Btg2 using transcription activator-like effector nuclease (TALEN) on a hypertensive rat background strain will result in protection from NaCl-induced BP increase and renal damage in a sex-specific pattern. The findings from our characterization of the Btg2 mutation in rat demonstrate a sex-specific response and provide insight into Btg2’s role in renal development and NaCl impaired hemodynamics.
METHODS
An expanded Materials and Methods section is available in the Supplemental Material; all Supplemental material is available at https://doi.org/10.5281/zenodo.6502875.
Animals
All animal procedures and breeding were performed at the Medical College of Wisconsin following protocols approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. All breeding and experimental rats were weaned at 21 days and maintained on a 0.4% NaCl AIN-76 diet (LS, low salt; Dyets). As part of the experimental protocol, at 9 wk rats were switched to an 8% NaCl AIN-76 modified diet (HS, high salt; Dyets) for 21 days. Rats were maintained in rooms on a standard 12 h light/12 h dark lighting cycle.
Generation of Btg2 Mutant Rat Strain on Congenic SSBN13-Line 9E Background
The line 9E congenic strain [SS.BN(D13Rat25-D13rs106935835)/Mcwi; RGD: 12802363] was used for creation of the mutant rats used in this study. Btg2 mutant rats were generated using transcription activator-like effector nuclease (TALEN) constructs specific for the rat Btg2 gene designed to target exon 1 using the target sequence AGGTTTCCTCACCAGTCtcctgaggactcggggcTGCGTGAGCGAGCAGAGA (Fig. 1). The TALENs were assembled by Transposagen and validated by the Gene Editing Rat Resource Center at the Medical College of Wisconsin. mRNA encoding the Btg2 TALEN constructs were diluted in microinjection buffer (1 mM Tris and 0.1 mmol/L EDTA, pH 7.4) at a concentration of 10 ng/μL and injected into one-cell line 9E rat embryos as described previously (21). At 10–14 days of age, pups were ear-punched, and DNA was extracted and screened for TALEN-induced mutations as described previously (21). Among several mutant founders, one founder animal harboring a 44-bp deletion in exon 1 (RNO13:50,916,769-50,916,812; aaaccttgagtctctgctcgctcacgcagccccgagtcctcagg) was back-crossed to the parental line 9E strain, and heterozygous animals from subsequent generations were intercrossed to generate homozygous mutant and wild-type (WT) animals for phenotyping (Fig. 1). This mutation can be classified as a loss of function mutation, which would likely lead to nonsense-mediated decay of the truncated transcribed product, however, this has not been experimentally verified. This strain is designated as SS.BN-(D13Rat25-rs106935835)-Btg2em7Mcwi (RGD ID: 10054305), hereafter referred to Btg2−/− or Btg2 mutant, and was used for all reported studies.
Figure 1.
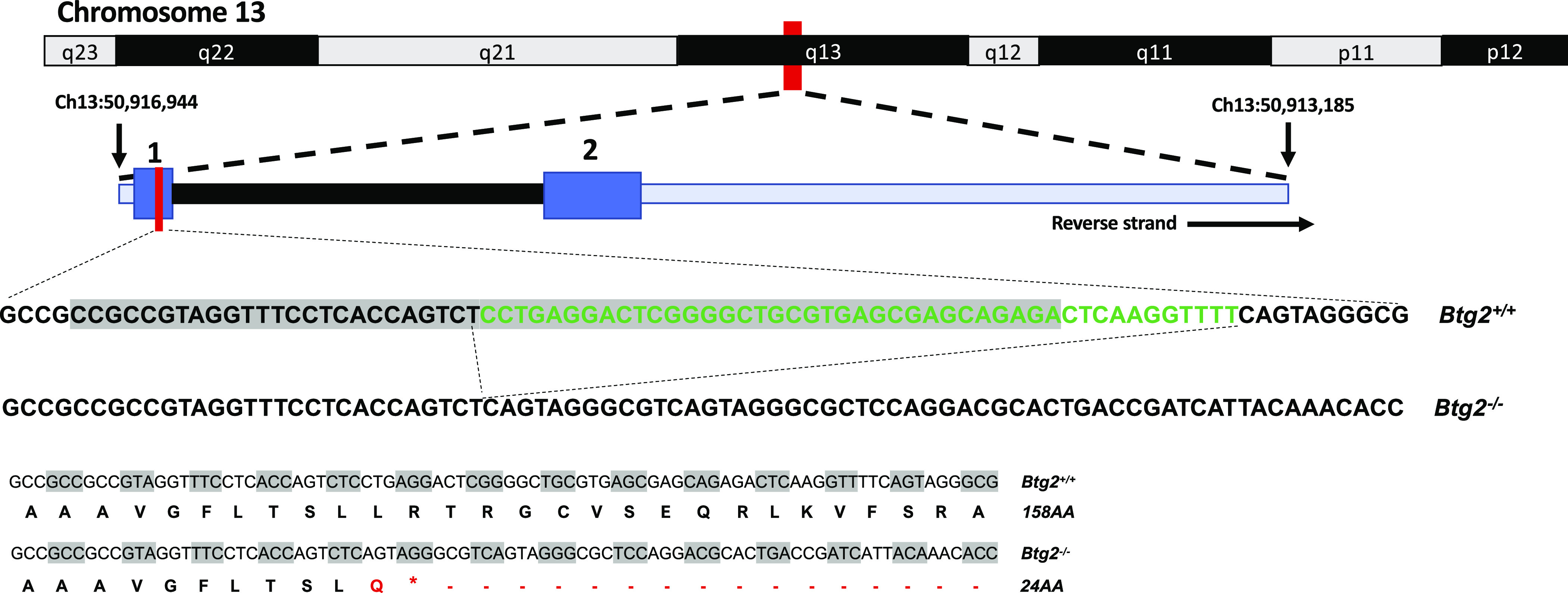
Transcription activator-like effector nuclease (TALEN) targeted exon 1 on rat Btg2 gene. The 10- to 14-day-old pups were ear-punched and DNA was screened for TALEN-induced mutations. Among several mutant founders, one founder animal (m7) harbored a 44-bp deletion (green text) in exon 1 of rat Btg2. Representative sequences showing TALEN target (gray highlight) and editing outcome along with corresponding translated peptide sequences.
Radiotelemetric Blood Pressure Recording
At 9 wk of age, rats were surgically implanted with a blood pressure (BP) monitoring transmitter (PA-C40; Data Sciences International), residing in the rat’s left flank with catheter secured in the abdominal aorta via the left femoral artery. Following 3 days of recovery, three consecutive days of BP recordings by radio telemetry in conscious, freely moving rats were acquired at 500 Hz for 10 s every 2 min for 24 h each day while the animals remained on the 0.4% NaCl diet (LS). The 24-h BP recordings were averaged into single daily values. On the afternoon of the 3rd day, animal diets were changed to 8% NaCl diet (HS), and BP recordings continued daily for 21 days except for the 16th and 17th day of 8% NaCl diet, although the rats were moved to metabolic cages for acclimation and 24-h urine collection. The recording protocol ended in the afternoon on the 21st day of the 8% NaCl diet, and data from this day was not included in the 24-h analysis. Six to eight animals were included in each group. The second day of LS recording was used for the control period for statistical analysis and figures.
To assess the mutant gene impact on diurnal variation, BP recordings were binned into 12-h segments from 6:00 AM to 6:00 PM and 6:00 PM to 6:00 AM corresponding to the on/off room light cycle. The absolute value of the difference between adjacent 12-h bin values was then normalized by dividing by the mean of the adjacent 12-h bins. BP and heart rate data were analyzed across the same protocol indicated earlier.
Renal Injury Assessment
After 16 days of an 8% NaCl diet, the animals were placed in metabolic cages for overnight acclimation followed by a 24-h urine collection. Urine samples were processed and analyzed by the Medical College of Wisconsin, Department of Physiology Biochemistry Assay Core. Urine electrolytes were measured by flame photometry. Total protein was measured by Coomassie Plus-Better Bradford assay (Pierce, Rockford, IL), albumin was measured using albumin blue 580 dye using a fluorescence plate reader (FL600, Bio-Tek, Winooski, VT), and creatinine was measured using Jaffé reaction assay by autoanalyzer (ACE, Alfa Wassermann). At the end of the 21st day on an 8% NaCl diet, animals were weighed and anesthetized with isoflurane. The kidneys and heart were then harvested, weighed, and stored in 10% formalin or frozen in liquid nitrogen and moved to −80°C freezer for storage.
Renal Histology
Renal histological analysis was performed as described previously (22, 23). Briefly, after 21 days of an 8% NaCl diet, the left kidney was harvested and fixed in 10% buffered formalin. Kidneys were bisected along coronal axis, paraffin embedded with an automated tissue processor, sectioned at 3 µm, mounted on slides, and stained with Masson’s trichrome. The slides were scanned at ×40 magnification and digitized using Hamamatsu NanoZoomer at 227 nm/pixel (112k dots per inch) and assessed with NDP view software (Hamamatsu Photonics, Hamamatsu City, Japan). The digitized images were manually assessed for glomerular injury, tubulointerstitial fibrosis, and cortical glomerular density. Glomerulosclerosis was scored on a scale of 0 (∼0% injury) to 4 (>75% injury; 24, 25), and the data are presented as the mean score of at least 90 glomeruli per animal. At least 20 images of the outer medulla were taken at ×20 magnification in the NDP view software, and the images were analyzed for tubulointerstitial fibrosis using MetaMorph software (Molecular Devices, Sunnyvale, CA). Fibrosis was quantified by selecting for the blue hue of the fibrotic tissue and calculated as the percent of total outer medullary tissue area in the ×20 image (23). Trichrome-stained slides were additionally assessed for glomeruli counts using Image J software (National Institutes of Health) and normalized to cortical area using MetaMorph selection tools. The images used for cortex selection were ×2.5 magnified images in TIFF format with a resolution of 7k dots per inch.
Cell Cycle Quantitative Real-Time Polymerase Chain Reaction Array
Cell cycle gene expression was examined using a rat cell cycle real time-2 profiler PCR array (PARN-020Z; Qiagen, Germantown, MD), according to the manufacturer’s protocol. Animals used in gene expression arrays were weaned to a 0.4% NaCl diet, and tissues were collected at 10 wk of age following 7 days of an 8% NaCl diet or remaining on a 0.4% NaCl diet. Kidney cortex samples from 3 males and 3 females within each salt diet condition (0.4% NaCl, LS; or 8% NaCl, HS) and each genotype [Btg2+/+, WT; Btg2−/−, knockout (KO)] were assessed. Individual samples were included in the assay with four samples run on each 384 well plate using Quantstudio 6 Flex instrument (Applied BioSystems, Foster City, CA). Data were normalized to Ldha (NM_017025), and relative mRNA expression from ΔΔCt method generated low-salt to high-salt comparisons in each genotype within each sex. Follow up comparisons were made between female Btg2+/+ to male Btg2+/+ data and female Btg2−/− to male Btg2−/− data using multiple t test analysis. Discovery gene sets from multiple t test analysis were determined using the Benjamini (26) two-stage linear step-up procedure with a false discovery rate of Q = 5% (Graphpad Prism 8.3.0 software). The discovery gene sets were compared with one another to determine significant differentially expressed genes unique to Btg2+/+ or Btg2−/− animals. The Btg2−/− specific gene set was assessed using Ingenuity Pathway Analysis software (Qiagen, Germantown, MD).
Statistical Analysis
Statistical analyses were performed using Graphpad Prism 8.3.0 software. BP, heart rate, and body weight data were analyzed using two-way ANOVA with Sidak post hoc analysis. Renal values and tissue weights were analyzed using unpaired Student’s t test. Breeding zygosity was assessed using chi-square test. Discovery gene sets from multiple t test analysis were determined using the Benjamini (26) two-stage linear step-up procedure with a false discovery rate of Q = 5%. P values were considered significant at values less than 0.05. Data are presented as means ± SE.
RESULTS
Blood Pressure
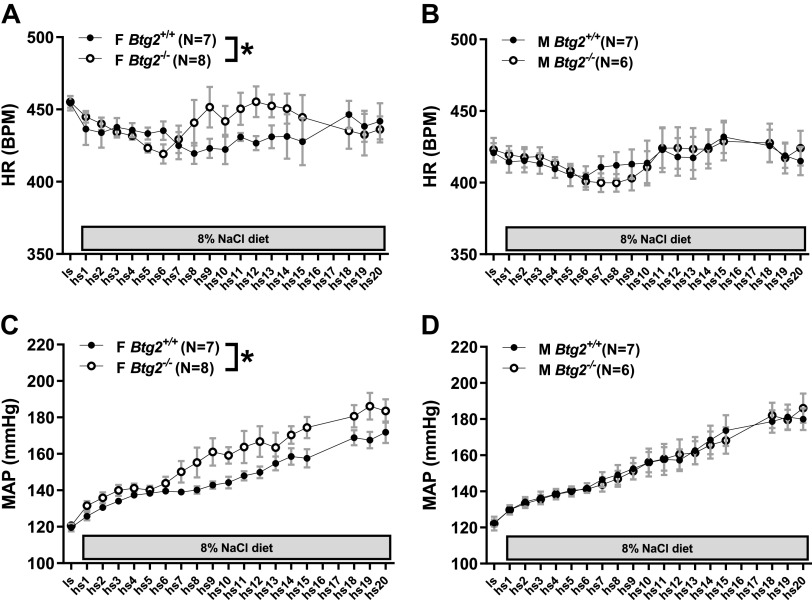
Radiotelemetric BP monitoring of rats fed 0.4% NaCl diet resulted in no significant differences between 24-h mean arterial pressure (MAP) in Btg2+/+ and Btg2−/− females (120 ± 2, 121 ± 1 mmHg) or males (122 ± 2, 122 ± 4 mmHg; Fig. 2, C and D). Two-way ANOVA testing demonstrated no significant difference between male Btg2+/+ and Btg2−/− rats across all timepoints from low salt through high salt day 20 (Fig. 2D). The same assessment in females exhibited a significant difference between Btg2+/+ and Btg2−/− strains (P < 0.0001; Fig. 2C and Table 1). Significant differences between female strains were also observed in systolic BP (P < 0.0001), diastolic BP (P < 0.0001), pulse pressure (P = 0.008), and heart rate (P = 0.015) from 24-h recordings across the 3-wk 8% NaCl diet protocol (Fig. 2, A and B, and Table 1). There were no significant differences between male strains in these measurements (Table 1).
Figure 2.
Blood pressure and heart rate measurements by radiotelemetry. Twenty-four measurements of heart rate in female (A) and male (B) Btg2+/+ and Btg2−/− rats during low-salt diet (LS) and 20 days of high-salt diet (HS). Bottom figures depict 24-h measurements of mean arterial pressure (MAP) in female (C) and male (D) Btg2+/+ and Btg2−/− rats during low-salt diet (LS) and 20 days of high-salt diet (HS). Graphs represent recordings obtained once every 2 min continuously for 24 h. Data are presented as mean shown in circles and SE shown as bars (only depicted in positive direction). Levels of statistical significance were analyzed by two-way ANOVA. *P < 0.05 (strain comparison). BPM, heart rate in beats per minute; F, female; HR, heart rate; M, male.
Table 1.
Statistical analysis results for blood pressure comparison between Btg2+/+ and Btg2−/− rats, within female and male
| Two-Way ANOVA | Female |
Male |
||
|---|---|---|---|---|
| P Values | WT vs. KO | (WT vs. KO) vs. Time | WT vs. KO | (WT vs. KO) vs. Time |
| n | n = 7, 8 | n = 7, 8 | n = 7, 6 | n = 7, 6 |
| 24 h HR | 0.015 | ns | ns | ns |
| 12 h day HR | 0.001 | ns | ns | ns |
| 12 h night HR | ns | ns | ns | ns |
| norm (d-n) HR | <0.0001 | ns | <0.0001 | ns |
| 24 h MAP | <0.0001 | ns | ns | ns |
| 12 h day MAP | <0.0001 | ns | ns | ns |
| 12 h night MAP | <0.0001 | ns | ns | ns |
| norm (d-n) MAP | 0.003 | 0.005 | 0.004 | ns |
| 24 h SBP | <0.0001 | ns | ns | ns |
| 12 h day SBP | <0.0001 | ns | ns | ns |
| 12 h night SBP | <0.0001 | ns | ns | ns |
| norm (d-n) SBP | ns | 0.014 | 0.0006 | ns |
| 24 h DBP | <0.0001 | ns | ns | ns |
| 12 h day DBP | <0.0001 | ns | ns | ns |
| 12 h night DBP | <0.0001 | ns | ns | ns |
| norm (d-n) DBP | <0.0001 | 0.011 | 0.004 | ns |
| 24 h PP | 0.008 | ns | ns | ns |
| 12 h day PP | 0.01 | ns | 0.002 | ns |
| 12 h night PP | ns | ns | 0.001 | ns |
| norm (d-n) PP | <0.0001 | ns | ns | ns |
Values represent P value from two-way ANOVA for 6–8 rats/group. Within each sex the first column represents strain comparison, whereas the second column represents strain by time comparison. DBP, diastolic blood pressure; HR, heart rate; knockout (KO), Btg2−/−; MAP, mean arterial pressure; norm (d-n), (dark-light cycle)|/((dark + light cycle)/2); ns, not significant; PP, pulse pressure; SBP, systolic blood pressure; wild type (WT), Btg2+/+.
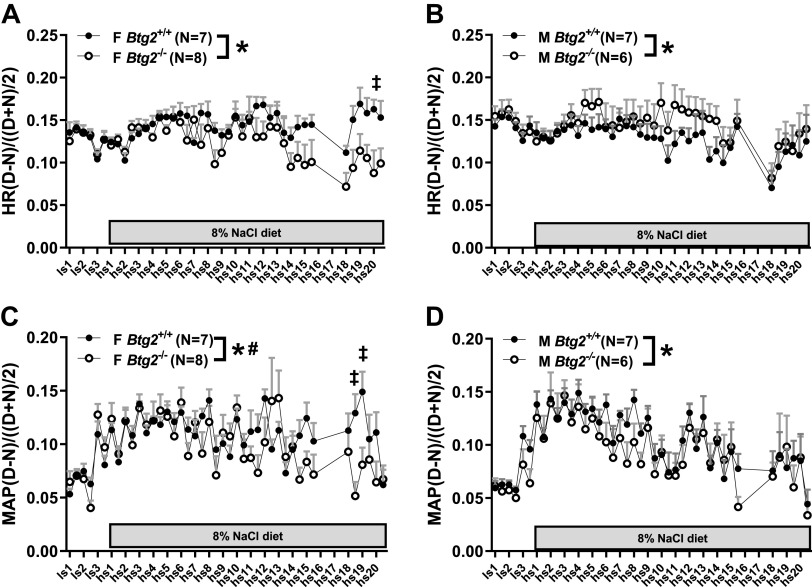
The BP recordings were also split into 12-h light and dark cycle bins to assess potential diurnal impairment in the mutant strains. To generate the comparison, the difference between each 12-h bin and its preceding 12-h bin was calculated. To account for potential differences in magnitude across the salt diet protocol, the light to dark cycle difference was normalized by dividing it by the mean of the 12-h bin and its preceding 12-h bin. This normalization was performed across the time series to generate a set of 42 data points. Two-way ANOVA characterization of Btg2+/+ and Btg2−/− rats identified significant differences in both male and female heart rate (P < 0.0001, P < 0.0001), MAP (P = 0.004, P = 0.003), and diastolic pressure (P = 0.004, P < 0.0001; Fig. 3, A–D, and Table 1). Male only difference was found in systolic pressure (P = 0.006), and female only difference in pulse pressure (P < 0.0001; Table 1). The effect of an 8% NaCl diet on diurnal BP change over time was determined to be significantly different only in females in MAP (P = 0.005), systolic pressure (P = 0.014), and diastolic pressure (P = 0.011; Fig. 3, C and D, and Table 1).
Figure 3.
Day-night difference in blood pressure and heart rate measurements. Telemetry blood pressure measurements were recorded for 15 s every 2 min and were binned into 12-h light and dark cycle bins for diurnal comparison. Differences between adjacent light and dark cycle 12-h bins were normalized to the mean of the same adjacent 12-h segments. The protocol shown covers 3 days of low-salt (LS) and 20 days of high-salt (HS) diet. Heart rate is plotted for female (A) and male (B) Btg2+/+and Btg2−/− rats along with MAP for females (C) and males (D). The plots depict recordings obtained once every 2 min continuously for 12 h. Data are presented as mean shown in circles and SE shown as bars (only depicted in positive direction). Levels of statistical significance were analyzed by two-way ANOVA. *P < 0.05 (strain comparison), #P < 0.05 (strain by time comparison), ‡P < 0.05 (Sidak post hoc). F, female; HR, heart rate; M, male.
Organ and Body Weight
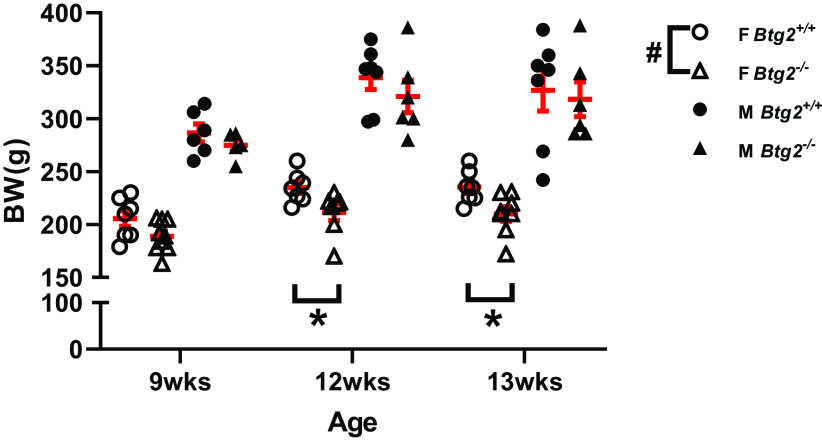
A two-way ANOVA comparison of body weight between female Btg2+/+ and Btg2−/− animals demonstrated a significant difference between strains and follow up post hoc test found differences at 12 wk (211 ± 8, 235 ± 5 g; P = 0.048) and 13 wk (210 ± 7, 235 ± 6 g; P = 0.023; Fig. 4). In contrast, there was no difference in body weight between male Btg2+/+ and Btg2−/− animals (Fig. 4). There were no differences in heart weight or heart weight normalized to body weight between Btg2+/+ and Btg2−/− female or male rats (Table 2). Raw kidney weights were also not different, however female Btg2−/− exhibited significantly increased kidney weights normalized to body weight than Btg2+/+ (12.60 ± 0.29, 11.64 ± 0.15 mg/g; P = 0.015), whereas males were not different (12.52 ± 0.72, 12.76 ± 0.47 mg/g) when assessed by t test (Table 2).
Figure 4.
Decreased body weight in female Btg2−/− compared with Btg2+/+ rats. Nine-week-old animals were switched to high-salt diet and measured after 2 (12 wk) and 3 (13 wk) wk. Individual data presented with means ± SE in red. Statistical significance was analyzed separately within each sex by two-way ANOVA. #P < 0.05 (strain comparison), *P < 0.05 (Sidak post hoc). BW, body weight; F, female; M, male; n = 6–8/group.
Table 2.
Renal measurements of female and male Btg2+/+ and Btg2−/− rats following high-salt diet
| Female |
Male |
|||
|---|---|---|---|---|
| Btg2+/+ | Btg2−/− | Btg2+/+ | Btg2−/− | |
| n | 7 | 8 | 7 | 6 |
| LS MAP, mmHg | 120 ± 2 | 121 ± 1 | 122 ± 2 | 122 ± 4 |
| HS19 MAP, mmHg | 168 ± 4 | 186 ± 7* | 181 ± 7 | 180 ± 6 |
| UPro, mg/day | 129 ± 16 | 238 ± 40* | 509 ± 94 | 479 ± 99 |
| UAlb, mg/day | 98 ± 18 | 180 ± 33 | 328 ± 81 | 278 ± 68 |
| UPro/UCr | 16 ± 2 | 39 ± 12* | 45 ± 12 | 38 ± 8 |
| UAlb/UCr | 12 ± 2 | 29 ± 9 | 31 ± 11 | 22 ± 5 |
| UNa, mmol/L | 372 ± 20 | 335 ± 20 | 284 ± 17 | 352 ± 60 |
| UK, mmol/L | 26 ± 2 | 26 ± 3 | 21 ± 2 | 28 ± 4 |
| Body Wt, g | 235 ± 6 | 210 ± 7* | 327 ± 19 | 318 ± 17 |
| Heart Wt, g | 1.15 ± 0.03 | 1.10 ± 0.04 | 1.39 ± 0.05 | 1.36 ± 0.02 |
| Kidney Wt, g | 1.38 ± 0.05 | 1.30 ± 0.06 | 2.02 ± 0.08 | 1.91 ± 0.05 |
| HW/BW, g/kg | 4.9 ± 0.1 | 5.2 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.2 |
| KW/BW, g/kg | 11.6 ± 0.2 | 12.6 ± 0.3* | 12.8 ± 0.5 | 12.5 ± 0.7 |
| Glom. Density | 3.7 ± 0.2 | 3.3 ± 0.1* | 2.6 ± 0.2 | 2.6 ± 0.2 |
| Glom. Scl. | 3.26 ± 0.05 | 2.70 ± 0.10* | 3.09 ± 0.15 | 3.14 ± 0.15 |
Values represent means ± SE for 6–8 rats per group. *P < 0.05 between Btg2−/− and Btg2+/+ within sex, using an unpaired t test. BW, body weight; Glom. Scl, glomerular damage score; HS, high-salt diet; HS19, HS diet day 19; HW, heart weight; KW, kidney weight; LS, low-salt diet; MAP, mean arterial pressure; UAlb, urine albumin; UCr, urine creatinine; UK, urine potassium; UNa, urine sodium; UPro, urine protein.
Renal Injury
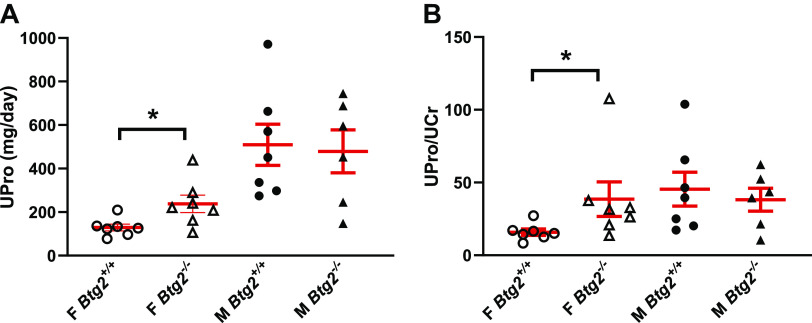
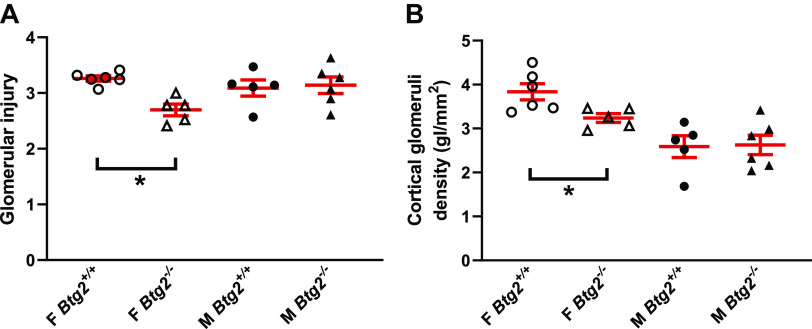
Urine was collected on day 17 of 8% NaCl diet to examine the effect of Btg2 mutation on renal injury under high-salt conditions. Urinary protein excretion was significantly higher in Btg2−/− females (238 ± 40 mg/day, P = 0.027) than Btg2+/+ females (135 ± 17 mg/day), and urine creatinine excretion was lower, but not significant (7.1 ± 0.5, 8.4 ± 0.5 mg/day). No difference was observed between male Btg2+/+ and Btg2−/− animals in urinary protein or creatinine excretion rate (Fig. 5A and Table 2). Urinary protein to urinary creatinine ratio was also significantly higher in female Btg2−/− compared with Btg2+/+ females but not different in males (Fig. 5B and Table 2). Urinary albumin excretion (UAE) and albumin creatinine ratio (ACR) trended higher in Btg2−/− females compared with Btg2+/+ (UAE: 180 ± 33, 106 ± 20 mg/day and ACR: 29.3 ± 8.8, 12.3 ± 2.5 mg/day), whereas males displayed no significant differences (UAE: 278 ± 68, 328 ± 81 mg/day and ACR: 22.0 ± 5.1, 30.6 ± 10.6 mg/day; Table 2). Cortical glomerular density was used as a metric to estimate nephron numbers within the kidney. The female Btg2−/− had significantly lower cortical area normalized glomerular density than Btg2+/+ kidneys (3.72 ± 0.15, 3.26 ± 0.14 glomeruli/mm2, P = 0.02), whereas males displayed no difference between groups (2.59 ± 0.25, 2.63 ± 0.22 glomeruli/mm2; Fig. 6A and Table 2). The female Btg2−/− had significantly reduced glomerular damage compared with Btg2+/+ kidneys (2.70 ± 0.10, 3.26 ± 0.05 glomeruli/mm2, P < 0.0001), whereas males displayed no difference between strains (3.14 ± 0.15, 3.09 ± 0.15 glomeruli/mm2; Fig. 6B and Table 2).
Figure 5.
Impaired renal function in female Btg2−/− compared with Btg2+/+ rats. Urinary protein and creatinine were measured in male and female Btg2+/+ and Btg2−/− rats following 17 days of high-salt diet. Figures depict female and male urinary protein excretion (A) and urinary protein-to-creatinine ratio (B). Individual data are presented with means ± SE shown in red. Statistical significance was analyzed by unpaired t test within each sex separately. *P < 0.05. F, female; M, male; UCr, Urinary creatinine excretion; UPro, urinary protein excretion.
Figure 6.
Female Btg2−/− rats exhibit lower nephron density with protected glomeruli compared with Btg2+/+ rats. Following 3 wk of high-salt diet, rat kidneys were processed and imaged for assessment of glomerulus damage and are presented for comparison within female and male rat strains shown in A. Cortical area normalized glomeruli counts in female and male rats were also determined and results are depicted in B. Individual data are presented with means ± SE shown in red. Statistical significance was analyzed by unpaired t test within each sex separately. *P < 0.05. F, female; M, male; gl, glomeruli.
Skeletal Morphology
Radiographic images were taken of male and female Btg2+/+ and Btg2−/− rats to assess axial skeletal morphology. Each animal was imaged in dorsal to ventral and lateral orientations. Assessment of the images revealed signature differences between Btg2+/+ and Btg2−/− rats. Both male and female Btg2−/− rats demonstrated lumbar to sacral transition changes compared with Btg2+/+. The two features common between Btg2−/− males and females were the reduction of intervertebral space caudal to the 6th lumbar vertebra, and the cranial shift of the lumbar spine relative to the pelvis as assessed by vertebral position lateral to the cranial aspect of the iliac crest (n = 6/6 rats). Unique to the female Btg2−/− rats were the partial or complete transformation of the L6 to S1 vertebra. Although not clearly depicted in the radiographs, the apparent partial transformation was observed as a possible unilateral extension of transverse process nearing proximity of the ilium without loss of sacral vertebrae (partial; n = 2/3 rats). Complete transformation was observed as complete loss of L6 or complete fusion of L6 to the ilium and S1 with loss of S4 (complete; n = 1/3 rats; Supplemental Fig. S1). Follow-up histological staining with digital image assessment of P1 pups confirmed malformations observed in both male and female Btg2−/− compared with Btg2+/+ rats from radiographic images (Supplemental Fig. S2).
Breeding Outcomes
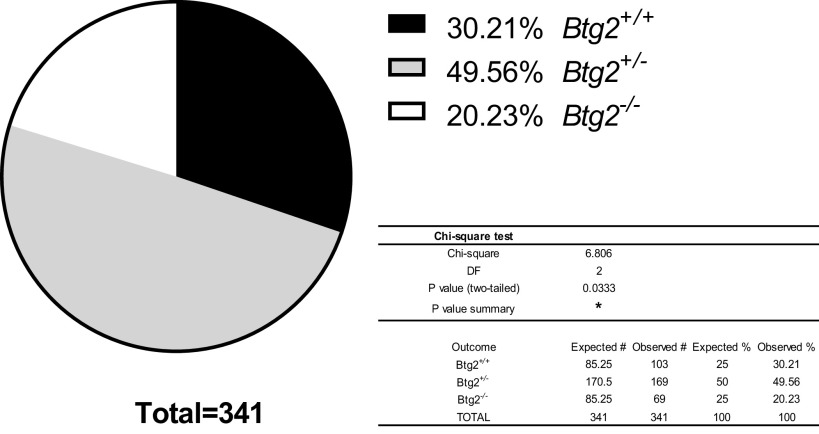
Genotyping and sex identification of litters born from heterozygous breeders were performed between 10 and 17-days old. Forty-nine litters containing 341 pups were born in the breeding and experimental colony over the course of this project. The mean litter size was 7.6 pups/litter with 0.6 pups/litter not developing to weaning age (21 days). The Btg2+/+ pups accounted for 30% (2.10 ± 0.24/litter) of all pups genotyped, whereas the Btg2−/− pups accounted for 20% (1.41 ± 0.19/litter) and Btg2+/− pups accounted for 50% (3.45 ± 0.25/litter; Fig. 7). A significant chi-square value of 6.81 with 2 degrees of freedom (P = 0.03) was determined using a 1:2:1 expected relationship of pup zygosity (Fig. 7). Using a similar assessment of sex within genotype, the chi-square produced (8.48, 5 df) was not significant (P = 0.87) from expected values using a 1:1:2:2:1:1 ratio. The 30% contribution of Btg2+/+ pups compared with 20% Btg2−/− pups and 50% Btg2+/− pups suggests that both Btg2−/− and Btg2+/− pups had reduced survival rates.
Figure 7.
Btg2 zygosity distribution in genotyped litters. Heterozygous breeders produced 49 litters with 371 pups that were genotyped before wean. Pup genotype distribution is presented in the chart. Data presented as % of total litter count. Statistical significance was determined by χ2-test. *P < 0.05.
Cell Cycle qRT-PCR Array
To explore potential cell cycle regulatory impact in our mutant animals, renal cortex samples were collected from wild type (WT) and mutant (KO) male and female animals on a 0.4% NaCl diet (LS) or 7 days of 8% NaCl diet (HS). All gene expression products were compared with the control gene Ldha, NM_017025. ΔCT expression values compared within female samples between WT LS versus KO LS, WT HS versus KO HS, WT LS versus WT HS, KO LS versus KO HS, did not yield any genes with Q value less than 5% false discovery rate (FDR). Within male samples, no genes were identified with Q value less than 5% FDR in WT LS versus KO LS or WT HS versus KO HS. However, a comparison within male samples between WT LS versus WT HS identified a list of 58 of the 84 genes in the array with significantly different expression using a 5% Q value cutoff (Supplemental Table S2). Within the 58 differentially expressed genes, 46 represented decreased expression under HS conditions. Comparison within male KO LS versus KO HS identified a list of 56 genes with significantly different expression using a 5% Q value cutoff (Supplemental Table S1). Within this list of 56 differentially expressed genes, 48 represented decreased expression under HS conditions. A comparison between these gene sets showed 7 genes that were unique to WT males (Skp2, Mki67, Aurka, Chek1, Msh2, Aurkb, and Inha; Supplemental Table S1), and 5 genes that were unique to KO males (Brca2, Cdkn2a, E2f1, Mcm4, and Rbl1; Supplemental Table S1). A comparison between ΔΔCT values from male versus female, where differences between LS and HS conditions within each sex and strain were first generated, produced two sets of genes from the array using the 5% Q value cutoff. A comparison between female WT LS to HS and male WT LS to HS identified a list of 53 genes (Supplemental Table S2), whereas comparing female KO LS to HS and male KO LS to HS identified a list of 60 genes (Supplemental Table S2). Unique gene sets were generated for WT (Ccne1, Cdc6, Chek1, Mcm2, and Skp2) and KO (Brca2, Ccna2, Cdc20, Cdc7, Cdkn2b, Ddit3, Inha, Rad17, Rbl1, Sfn, Stmn1, Tsg101, and Wee1) when the two ΔΔCT male versus female lists were compared (Supplemental Table S2).
Pathway Analysis
Following the identification of cell cycle regulatory genes unique to the kidney cortex of the Btg2−/− rats, the KO specific list of 13 genes was uploaded to Ingenuity Pathway Analysis software (IPA) for gene set enrichment analysis (Table S2). We identified four enriched toxicological functions containing at least 3 genes from our gene set. These functions were liver tumor (P = 0.0421), liver carcinoma (P = 0.0476), cell death of kidney cells (P = 0.0026), and hepatocellular carcinoma (P = 0.0353; Supplemental Table S3). An enrichment analysis of upstream regulation for the gene set identified many potential targets for future studies. The top ten upstream regulators are discodermolide (P = 1.77E-11), TP53 (P = 1.40E-10), ROR2 (P = 6.42E-10), LY294002 (P = 6.75E-09), adrenoreceptor beta (ADRB) (P = 1.34E-08), BRCA1 (P = 4.39E-08), sulindac (P = 5.33E-08), NAE1 (P = 5.98E-08), TP73 (P = 6.21E-08), and CDKN1A (P = 1.22E-07; Supplemental Table S4). The top five canonical pathways identified were mitotic roles of polo-like kinase (P = 6.88E-06), cyclins and cell cycle regulation (P = 1.28E-05), DNA damage-induced 14-3-3σ signaling (P = 5.31E-05), hereditary breast cancer signaling (P = 6.57E-05), and estrogen-mediated S-phase entry (P = 1.01E-04; Supplemental Table S5).
An assessment of disease and functional enrichment through IPA and utilization of curated gene sets available through Gene Set Enrichment Analysis (GSEA) application provide more insight into cell cycle regulatory molecules in the Btg2−/− unique gene set and their roles in specific cell cycle phases as shown in Supplemental Tables S6 and S7.
DISCUSSION
The results generated in this study follow an extensive rat genetic mapping experiment that had previously isolated a locus on rat chromosome 13 that contributes to salt-sensitive HTN (8, 9, 11, 27–31). Btg2 was the lone gene within the narrowed 23 kb region separating two overlapping congenic strains that exhibited a sex-specific BP response. Gene expression within the kidney cortex of the female HTN susceptible strain showed a higher level of Btg2 than the protected female strain. We hypothesized that mutating this gene on the HTN susceptible rat strain would attenuate the pathophysiologic BP and proteinuria phenotypes. The results of the current work demonstrate that rats with mutated Btg2 reproduce a female-specific response in cardiovascular and renal phenotypes. Contrary to our hypothesis, however, the response in our female Btg2−/− rats showed greater BP and proteinuria impairment than wild-type animals (Table 2).
The sex-specific response in cardiovascular, renal, and developmental phenotypes in the Btg2−/− rats suggest an interaction between Btg2 and estrogen signaling. Supporting evidence from research in other laboratories has shown that BTG2 contains two LxxLL motifs that facilitate interaction with nuclear receptors including estrogen receptor α (ERα) and androgen receptor (AR; 18, 32). In addition, repression of Btg2 has been demonstrated during estrogen-mediated proliferation (33, 34), and estrogen receptor (ER) response elements have exhibited regulation of the BTG2 promoter (12, 35). When combined, the evidence for interactions with estrogen signaling suggest that alteration of Btg2 may result in sex-specific phenotypes. Our current research supports this idea and builds on our previous work that had identified female-specific phenotypes within a refined quantitative trait locus (QTL) containing Btg2 (9).
In addition to the sex-specific response in our cardiovascular results, we also observed circadian differences between wild-type and knockout animals in BP and heart rate during an elevated NaCl diet. The circadian difference was present in both male and female rats, but the females demonstrated a difference that also increased with longer exposure to the high-salt diet. The circadian differences that we observed may be due to Btg2’s previously reported participation in circadian rhythm mRNA turnover (36). A key feature of Btg2 function has been attributed to its ability to assist in deadenylation during posttranscriptional regulation (37). A microRNA circadian regulation molecule, miR-132, has been shown in the mouse to directly target Btg2 within the suprachiasmatic nucleus of the brain and exert a circadian response by stimulating the decay of mPer1 and mPer2, both well-established circadian associated genes (36). The interaction between these molecules may provide a direction for future exploration to define a potential mechanism that Btg2 works through in rats to exhibit altered circadian cardiovascular results such as those we have reported here.
The work presented here has also shown skeletal morphology differences in the lumbar spine from some of our mutant Btg2 rats. The conserved BTG domain can function through protein-protein interaction, and Btg2, in particular, has been shown to interact with developmental cell fate regulatory gene Hoxb9 (38). Hoxb9 knockout mice have demonstrated skeletal abnormalities in rib structure and at transition locations between vertebral types within the axial column (39). Btg2 has also demonstrated an interaction with the developmental pathway signaling of bone morphogenic protein (BMP; 16). The association with this signaling pathway in Btg2-null mice resulted in altered skeletal vertebral patterning (16). Subsequent work using Btg2 mutant mice demonstrated an effect on vertebral transformation at the sacral-lumbar transition (40). In addition to skeletal abnormalities in our mutant rats, we also observed lower body mass, decreased nephron numbers in female Btg2−/− rats, and fewer Btg2−/− and Btg2+/− pups born per litter. Taken together, we suggest that the morphological changes observed in our Btg2−/− rats could be a result of altered HoxB and BMP developmental signaling pathways.
Building on this idea, we explored evidence from published literature in an attempt to explain a potential alteration in nephrogenesis that may lead to the decreased nephron density that we observed. In the early stages of embryonic nephron development, impaired molecular signaling can lead to a number of disorders including decreased nephron number, renal hypoplasia, ectopic ureter, and renal tubular dysgenesis among others anomalies (41–46). Btg2 expression during ureteric bud induction in embryonic development has been demonstrated within adjacent tissue and interacts with BMP signaling to alter somite development (16). In addition, Bmp4 has been shown to play an important role in signaling during ureteric bud branching in mouse (47) and impaired renal development resulting in renal hypoplasia in human (44). Assessment of the results reported in this paper demonstrating altered nephron number and impairment of BP and renal regulation in Btg2−/− rats offer a potential novel role for Btg2 in nephron development through interaction with BMPs.
Our original hypothesis suggested that mutating Btg2 on the line 9E congenic background would attenuate salt-induced BP increase and renal damage in females, however, we saw the opposite response. Based on the results reported here, we begin to reveal multiple pathways that this gene may participate in to influence BP and renal outcomes. We propose that the morphological changes incurred during kidney development produce an exacerbated response that may mask any protective response that might be observed in the adult exposed to the NaCl diet.
A result that does not fit with our renal data is a modest, yet significant, protection from glomerulosclerosis observed in the female Btg2−/− rats compared with Btg2+/+ rats. Although we have no additional data to support a mechanism that explains this observation, there have been numerous reports demonstrating an interaction between immune response and renal damage in HTN susceptible rats (22, 48–52). Moreover, using gene-edited mouse models (c-mybh/h, Baff-r−/−) investigators have begun to explore targeted B cell deficiency and its protective response in vascular and endothelial dysfunction during HTN development (53–56). In addition, a human Genome-Wide Association Study (GWAS) has implicated BTG2 in blood cell traits (57) and work in mouse has also shown a role for estradiol stimulated Btg2 in hematopoietic stem cell expansion in bone marrow (58). Still, others have identified Btg2’s role in the regulation of pre-B cell differentiation and B cell development through cell cycle pathway interaction (59). Adding to this idea, Btg2−/− mouse models have demonstrated decreased B cell fraction in early progenitors taken from bone marrow samples (60). The well-established immune and inflammation mediator ΝFκΒ has also been associated with Btg2 through its ability to bind the Btg2 promoter and regulate expression in response to stimuli such as oxidative stress (17, 61). When assessing our glomerular protective results in the context of the aforementioned information, we suggest that future studies may be justified to reveal the impact of decreased Btg2 expression in immune cell development and immune signaling that may contribute to our observation of attenuated salt-induced damage.
To further explore the potential signaling mechanisms that were impacted by the loss of this gene before and during an environmental stressor, a preliminary pathway analysis focused on sex-specific and dietary salt differences. The glomerular damage comparison between Btg2−/− and Btg2+/+ rats suggests a potential sex-specific protective role, and this gene is a well-documented contributor to cell cycle regulation and antiproliferation (62–66). With these ideas in mind, we sought to identify a potential protective pathway by isolating cell cycle regulatory genes that were expressed differentially in the kidney cortex of Btg2−/− and Btg2+/+ rats on low-salt or high-salt diet. Using cell cycle gene expression arrays, we generated lists of candidate cell cycle regulatory genes unique to Btg2−/− and Btg2+/+ that represented an altered sex-specific gene expression response between low- and high-salt conditions (Supplemental Tables S1 and S4).
The gene list unique to Btg2−/− was further explored using gene enrichment software. The top enriched canonical pathways and toxicological functions indicated an estrogen-mediated cell cycle role and potential role in kidney cell viability (Supplemental Tables S3 and S5). Top upstream regulators such as the pharmacological agents discodermolide, LY294002, and sulindac present options for follow-up research to identify Btg2’s potential protective mechanism in the kidney (Supplemental Table S3).
The scope of the work included in this project and the interpretation of its results have been limited by a number of factors. First, the loss of function mutation created by TALEN editing most likely leads to nonsense-mediated decay of the truncated transcribed product, but this has not been experimentally verified. In addition, the results of this study do not include measurement of the mutant or wild-type transcript and this should be included in future studies. The low sample sizes used in the cell cycle array limit the strength of the gene expression experiment. Future work should include additional samples in each group and a more comprehensive set of genes should be included for interpretation. The selection of a cell cycle-specific array has also narrowed the ability of pathway and enrichment analyses to offer novel insight outside of cell cycle genes.
The results presented here suggest that Btg2 is a multifaceted gene that exhibits its effect at different stages and within different tissues during rat growth and development. The congenic mapping experiment that sought to identify novel genes contributing to salt-sensitive HTN, had narrowed a candidate locus to a small region that included a lone gene, Btg2. Although there is no established BP role implicating Btg2, based on previous mapping work we hypothesized that mutating this gene would lead to BP and renal responses like we had seen in an adjacent protected congenic strain. Our results show that the mutated gene on the hypertensive congenic strain demonstrates a sex-specific response leading to amplified BP and renal injury compared with wild-type animals. A result that did not appear to fit the damaged renal pathology was the protection of female Btg2−/− glomeruli. We have presented our findings from original results and published literature to support pathways that may be altered in our mutant rat model. We propose that developmental abnormalities, particularly in nephrogenesis, generate a damaging obstacle that cannot be overcome by a modest glomerular protection during salt exposure in females. The results from our cell cycle array point to several potential pharmacological agents that target upstream signaling and present options for future exploration of Btg2 interactions utilizing rat models with nonaltered renal development.
DATA AVAILABILITY
Data are available upon request. Strain and phenotype data are available at the Rat Genome Database (https://rgd.mcw.edu).
SUPPLEMENTAL DATA
Supplemental Tables S1–S7 and Supplemental Figs. S1 and S2: https://doi.org/10.5281/zenodo.6502875.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health Grant R24HL11447.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.H., A.M.G., and M.R.D. conceived and designed research; M.J.H., A.T., E.S.J., R.S., and M.G. performed experiments; M.J.H., A.T., E.S.J., R.S., M.G., and A.M.G. analyzed data; M.J.H., A.T., E.S.J., R.S., M.G., A.M.G., and M.R.D. interpreted results of experiments; M.J.H., A.T., and E.S.J. prepared figures; M.J.H. and M.R.D. drafted manuscript; M.J.H., A.T., E.S.J., R.S., M.G., A.M.G., and M.R.D. edited and revised manuscript; M.J.H., A.T., E.S.J., R.S., M.G., A.M.G., and M.R.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Angela Lemke, Allison Zappa, Lynn Lazcares, Anne Temple, Lisa Henderson, Camille Taylor, and Jenifer Phillips for excellent technical support. Our deepest gratitude is extended to the Rat Genome Database group for insight and guidance within the genomic landscape.
REFERENCES
- 1.Padmanabhan S, Joe B. Towards precision medicine for hypertension: a review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev 97: 1469–1528, 2017. doi: 10.1152/physrev.00035.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loscalzo J. Precision medicine. Circ Res 124: 987–989, 2019. doi: 10.1161/CIRCRESAHA.119.314403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease–a delicate balance. N Engl J Med 368: 1229–1237, 2013. doi: 10.1056/NEJMra1212606. [DOI] [PubMed] [Google Scholar]
- 4.Reckelhoff JF, Romero DG, Yanes Cardozo LL. Sex, oxidative stress, and hypertension: insights from animal models. Physiology (Bethesda) 34: 178–188, 2019. doi: 10.1152/physiol.00035.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 14: 185–201, 2018. doi: 10.1038/nrneph.2017.189. [DOI] [PubMed] [Google Scholar]
- 6.Maric-Bilkan C, Arnold AP, Taylor DA, Dwinell M, Howlett SE, Wenger N, Reckelhoff JF, Sandberg K, Churchill G, Levin E, Lundberg MS. Report of the National Heart, Lung, and Blood Institute Working Group on sex differences research in cardiovascular disease: scientific questions and challenges. Hypertension 67: 802–807, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannon EC, Ray SC, Ryan MJ, Sullivan JC. Does sex matter?: an update on the implementation of sex as a biological variable in research. Am J Physiol Renal Physiol 318: F329–F331, 2020. doi: 10.1152/ajprenal.00575.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW Jr. Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics 31: 228–235, 2007. doi: 10.1152/physiolgenomics.00280.2006. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman MJ, Flister MJ, Nunez L, Xiao B, Greene AS, Jacob HJ, Moreno C. Female-specific hypertension loci on rat chromosome 13. Hypertension 62: 557–563, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol 37: 746–756, 2017. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowley AW Jr, Dwinell MR. Chromosomal substitution strategies to localize genomic regions related to complex traits. Compr Physiol 10: 365–388, 2020. doi: 10.1002/cphy.c180029. [DOI] [PubMed] [Google Scholar]
- 12.Paruthiyil S, Cvoro A, Tagliaferri M, Cohen I, Shtivelman E, Leitman DC. Estrogen receptor β causes a G2 cell cycle arrest by inhibiting CDK1 activity through the regulation of cyclin B1, GADD45A, and BTG2. Breast Cancer Res Treat 129: 777–784, 2011. doi: 10.1007/s10549-010-1273-5. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Liu J, Park ES, Jo M, Curry TE Jr.. The B cell translocation gene (BTG) family in the rat ovary: hormonal induction, regulation, and impact on cell cycle kinetics. Endocrinology 150: 3894–3902, 2009. doi: 10.1210/en.2008-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauxion F, Faux C, Séraphin B. The BTG2 protein is a general activator of mRNA deadenylation. EMBO J 27: 1039–1048, 2008. doi: 10.1038/emboj.2008.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelisti C, Astolfi A, Gaboardi GC, Tazzari P, Pession A, Goto K, Martelli AM. TIS21/BTG2/PC3 and cyclin D1 are key determinants of nuclear diacylglycerol kinase-zeta-dependent cell cycle arrest. Cell Signal 21: 801–809, 2009. doi: 10.1016/j.cellsig.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Lee YJ, Lee HJ, Seki T, Hong KH, Park J, Beppu H, Lim IK, Yoon JW, Li E, Kim SJ, Oh SP. B-cell translocation gene 2 (Btg2) regulates vertebral patterning by modulating bone morphogenetic protein/smad signaling. Mol Cell Biol 24: 10256–10262, 2004. doi: 10.1128/MCB.24.23.10256-10262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakubo H, Carey JL, Brachtel E, Gupta V, Green JE, Walden PD, Maheswaran S. Expression of the NF-κB-responsive gene BTG2 is aberrantly regulated in breast cancer. Oncogene 23: 8310–8319, 2004. doi: 10.1038/sj.onc.1208008. [DOI] [PubMed] [Google Scholar]
- 18.Prévôt D, Morel AP, Voeltzel T, Rostan MC, Rimokh R, Magaud JP, Corbo L. Relationships of the antiproliferative proteins BTG1 and BTG2 with CAF1, the human homolog of a component of the yeast CCR4 transcriptional complex: involvement in estrogen receptor alpha signaling pathway. J Biol Chem 276: 9640–9648, 2001. doi: 10.1074/jbc.M008201200. [DOI] [PubMed] [Google Scholar]
- 19.Masumura Y, Higo S, Asano Y, Kato H, Yan Y, Ishino S, Tsukamoto O, Kioka H, Hayashi T, Shintani Y, Yamazaki S, Minamino T, Kitakaze M, Komuro I, Takashima S, Sakata Y. Btg2 is a negative regulator of cardiomyocyte hypertrophy through a decrease in cytosolic RNA. Sci Rep 6: 28592, 2016. doi: 10.1038/srep28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang FL, Guo M, Yutzey KE. Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation 133: 1081–1092, 2016. doi: 10.1161/CIRCULATIONAHA.115.019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geurts AM, Cost GJ, Rémy S, Cui X, Tesson L, Usal C, Ménoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 597: 211–225, 2010. doi: 10.1007/978-1-60327-389-3_15. [DOI] [PubMed] [Google Scholar]
- 22.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr.. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prisco SZ, Prokop JW, Sarkis AB, Yeo NC, Hoffman MJ, Hansen CC, Jacob HJ, Flister MJ, Lazar J. Refined mapping of a hypertension susceptibility locus on rat chromosome 12. Hypertension 64: 883–890, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 25.Packard M, Saad Y, Gunning WT, Gupta S, Shapiro J, Garrett MR. Investigating the effect of genetic background on proteinuria and renal injury using two hypertensive strains. Am J Physiol Renal Physiol 296: F839–F846, 2009. doi: 10.1152/ajprenal.90370.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93: 491–507, 2006. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 27.Cowley AW Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456–461, 2001. doi: 10.1161/01.HYP.37.2.456. [DOI] [PubMed] [Google Scholar]
- 28.Kwitek-Black AE, Jacob HJ. The use of designer rats in the genetic dissection of hypertension. Curr Hypertens Rep 3: 12–18, 2001. doi: 10.1007/s11906-001-0072-0. [DOI] [PubMed] [Google Scholar]
- 29.Moreno C, Dumas P, Kaldunski ML, Tonellato PJ, Greene AS, Roman RJ, Cheng Q, Wang Z, Jacob HJ, Cowley AW Jr.. Genomic map of cardiovascular phenotypes of hypertension in female Dahl S rats. Physiol Genomics 15: 243–257, 2003. doi: 10.1152/physiolgenomics.00105.2003. [DOI] [PubMed] [Google Scholar]
- 30.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr.. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flister MJ, Hoffman MJ, Reddy P, Jacob HJ, Moreno C. Congenic mapping and sequence analysis of the Renin locus. Hypertension 61: 850–856, 2013. doi: 10.1161/HYPERTENSIONAHA.111.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu XD, Meng QH, Xu JY, Jiao Y, Ge CM, Jacob A, Wang P, Rosen EM, Fan S. BTG2 is an LXXLL-dependent co-repressor for androgen receptor transcriptional activity. Biochem Biophys Res Commun 404: 903–909, 2011. doi: 10.1016/j.bbrc.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 33.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144: 4562–4574, 2003. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 34.Karmakar S, Foster EA, Smith CL. Estradiol downregulation of the tumor suppressor gene BTG2 requires estrogen receptor-alpha and the REA corepressor. Int J Cancer 124: 1841–1851, 2009. doi: 10.1002/ijc.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38: 1289–1297, 2006. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Saavedra M, Antoun G, Yanagiya A, Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N, Cheng HY. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum Mol Genet 20: 731–751, 2011. doi: 10.1093/hmg/ddq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol 222: 66–72, 2010. doi: 10.1002/jcp.21919. [DOI] [PubMed] [Google Scholar]
- 38.Prévôt D, Voeltzel T, Birot AM, Morel AP, Rostan MC, Magaud JP, Corbo L. The leukemia-associated protein Btg1 and the p53-regulated protein Btg2 interact with the homeoprotein Hoxb9 and enhance its transcriptional activation. J Biol Chem 275: 147–153, 2000. doi: 10.1074/jbc.275.1.147. [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev Biol 181: 186–196, 1997. doi: 10.1006/dbio.1996.8440. [DOI] [PubMed] [Google Scholar]
- 40.Tijchon E, van Ingen Schenau D, van Opzeeland F, Tirone F, Hoogerbrugge PM, Van Leeuwen FN, Scheijen B. Targeted deletion of Btg1 and Btg2 results in homeotic transformation of the axial skeleton. PLoS One 10: e0131481, 2015. doi: 10.1371/journal.pone.0131481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Garrett MR. Nephron number, hypertension, and CKD: physiological and genetic insight from humans and animal models. Physiol Genomics 49: 180–192, 2017. doi: 10.1152/physiolgenomics.00098.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol 293: F494–F500, 2007. doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- 44.Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, Mehls O, Antignac C, Schaefer F, Weber S; ESCAPE Trial Group. Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol 24: 2361–2368, 2009. doi: 10.1007/s00467-009-1287-6. [DOI] [PubMed] [Google Scholar]
- 45.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78, 2001. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 46.Beck BB, Trachtman H, Gitman M, Miller I, Sayer JA, Pannes A, Baasner A, Hildebrandt F, Wolf MT. Autosomal dominant mutation in the signal peptide of renin in a kindred with anemia, hyperuricemia, and CKD. Am J Kidney Dis 58: 821–825, 2011. doi: 10.1053/j.ajkd.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michos O, Gonçalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134: 2397–2405, 2007. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- 48.Mattson DL. Immune mechanisms of salt-sensitive hypertension and renal end-organ damage. Nat Rev Nephrol 15: 290–300, 2019. doi: 10.1038/s41581-019-0121-z. [DOI] [PubMed] [Google Scholar]
- 49.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.0590062222.x. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez V, Quiroz Y, Nava M, Pons H, Rodriguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am J Physiol Renal Physiol 283: F1132–F1141, 2002. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 53.Drummond GR, Vinh A, Guzik TJ, Sobey CG. Immune mechanisms of hypertension. Nat Rev Immunol 19: 517–532, 2019. doi: 10.1038/s41577-019-0160-5. [DOI] [PubMed] [Google Scholar]
- 54.Mikolajczyk TP, Guzik TJ. Adaptive immunity in hypertension. Curr Hypertens Rep 21: 68, 2019. doi: 10.1007/s11906-019-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory Role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 66: 1023–1033, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
- 56.Dingwell LS, Shikatani EA, Besla R, Levy AS, Dinh DD, Momen A, Zhang H, Afroze T, Chen MB, Chiu F, Simmons CA, Billia F, Gommerman JL, John R, Heximer S, Scholey JW, Bolz SS, Robbins CS, Husain MB. Cell deficiency lowers blood pressure in mice. Hypertension 73: 561–570, 2019. doi: 10.1161/HYPERTENSIONAHA.118.11828. [DOI] [PubMed] [Google Scholar]
- 57.Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL , et al. The Allelic landscape of human blood cell trait variation and links to common complex disease. Cell 167: 1415–1429.e19, 2016. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim BC, Ryu MS, Oh SP, Lim IK. TIS21/(BTG2) negatively regulates estradiol-stimulated expansion of hematopoietic stem cells by derepressing Akt phosphorylation and inhibiting mTOR signal transduction. Stem Cells 26: 2339–2348, 2008. [Erratum in Stem Cells 28: 842, 2010]. doi: 10.1634/stemcells.2008-0327. [DOI] [PubMed] [Google Scholar]
- 59.Dolezal E, Infantino S, Drepper F, Borsig T, Singh A, Wossning T, Fiala GJ, Minguet S, Warscheid B, Tarlinton DM, Jumaa H, Medgyesi D, Reth M. The BTG2-PRMT1 module limits pre-B cell expansion by regulating the CDK4-Cyclin-D3 complex. Nat Immunol 18: 911–920, 2017. doi: 10.1038/ni.3774. [DOI] [PubMed] [Google Scholar]
- 60.Tijchon E, van Emst L, Yuniati L, van Ingen Schenau D, Havinga J, Rouault JP, Hoogerbrugge PM, van Leeuwen FN, Scheijen B. Tumor suppressors BTG1 and BTG2 regulate early mouse B-cell development. Haematologica 101: e272–e276, 2016. doi: 10.3324/haematol.2015.139675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imran M, Lim IK. Regulation of Btg2(/TIS21/PC3) expression via reactive oxygen species-protein kinase C-NFκβ pathway under stress conditions. Cell Signal 25: 2400–2412, 2013. doi: 10.1016/j.cellsig.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 62.Lim IK, Lee MS, Ryu MS, Park TJ, Fujiki H, Eguchi H, Paik WK. Induction of growth inhibition of 293 cells by downregulation of the cyclin E and cyclin-dependent kinase 4 proteins due to overexpression of TIS21. Mol Carcinog 23: 25–35, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 63.Montagnoli A, Guardavaccaro D, Starace G, Tirone F. Overexpression of the nerve growth factor-inducible PC3 immediate early gene is associated with growth inhibition. Cell Growth Differ 7: 1327–1336, 1996. [PubMed] [Google Scholar]
- 64.Micheli L, D'Andrea G, Leonardi L, Tirone F. HDAC1, HDAC4, and HDAC9 bind to PC3/Tis21/Btg2 and are required for its inhibition of cell cycle progression and cyclin D1 expression. J Cell Physiol 232: 1696–1707, 2017. doi: 10.1002/jcp.25467. [DOI] [PubMed] [Google Scholar]
- 65.Guardavaccaro D, Corrente G, Covone F, Micheli L, D'Agnano I, Starace G, Caruso M, Tirone F. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol 20: 1797–1815, 2000. doi: 10.1128/MCB.20.5.1797-1815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wheaton K, Muir J, Ma W, Benchimol S. BTG2 antagonizes Pin1 in response to mitogens and telomere disruption during replicative senescence. Aging Cell 9: 747–760, 2010. doi: 10.1111/j.1474-9726.2010.00601.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1–S7 and Supplemental Figs. S1 and S2: https://doi.org/10.5281/zenodo.6502875.
Data Availability Statement
Data are available upon request. Strain and phenotype data are available at the Rat Genome Database (https://rgd.mcw.edu).